Abstract
Individuals with fragile X mental retardation 1 (FMR1) gene premutations are at increased risk for fragile X associated tremor/ataxia syndrome (FXTAS) during aging. However, it is unknown whether older FMR1 premutation carriers, with or without FXTAS, exhibit functional motor control deficits compared with healthy individuals. The purpose of this study, therefore, was to determine whether older FMR1 premutation carriers exhibit impaired ability to perform functional motor tasks. Eight FMR1 premutation carriers (age: 58.88±8.36 yrs) and eight age-and sex-matched healthy individuals (60.13±9.25 yrs) performed 1) a steady isometric force control task with the index finger at 20% of their maximum voluntary contraction (MVC) and; 2) a single step task. During the finger abduction task, firing rate of multiple motor units of the first dorsal interosseous (FDI) muscle were recorded. Compared with healthy controls, FMR1 premutation carriers exhibited 1) greater force variability (coefficient of variation of force) during isometric force (1.48 ± 1.02 vs. 0.63 ± 0.37 %; p = 0.04); 2) reduced firing rate of multiple motor units during steady force, and; 3) reduced velocity of their weight transfer during stepping (156.62 ± 26.24 vs. 191.86 ± 18.83 cm/s; P = 0.01). These findings suggest that older FMR1 premutation carriers exhibit functional motor control deficits that either reflect subclinical issues associated with premutations independent of FXTAS, or prodromal markers of the development of FXTAS.
Keywords: FMR1 premutation, force control, motor unit activity, stepping
1. INTRODUCTION
Individuals with fragile X mental retardation 1 (FMR1) gene premutations, defined as having 55–200 CGG repeats at the 5’ untranslated region of the FMR1 gene, are at risk for fragile X associated tremor/ataxia syndrome (FXTAS), a neurodegenerative disorder with a median age of onset of approximately 60 years (Hagerman and Hagerman 2004; Leehey et al. 2008). 45% of male and 16% of female carriers >50 years develop FXTAS, with penetrance increasing with age (Rodriguez-Revenga et al. 2009). FXTAS is characterized by loss of motor coordination, intention tremor, and cognitive and psychiatric issues in addition to peripheral neuropathy (Hagerman and Hagerman 2004; Jacquemont et al. 2004b; Hall and O’keefe 2012). However, little is known about whether older FMR1 premutation carriers, with or without FXTAS, exhibit functional motor control deficits compared with healthy individuals. Here, we aim to understand whether FMR1 premutation carriers exhibit an impaired ability to perform functional motor control tasks.
Pathology associated with FMR1 premutations is thought to be the consequence of high levels of FMR1 mRNA seen with increased numbers of CGG repeats (Diener et al. 1984; Wang et al. 2004; Hocking et al. 2017). FMR1 premutations contribute to multiple subclinical neurodevelopmental issues, including mild intellectual disability, social deficits, and increased rates of learning disabilities (Kenneson et al. 2001). FMR1 premutations also are associated with sensorimotor issues, though sensorimotor dysfunctions are believed to occur primarily after the onset of FXTAS (Jacquemont et al. 2004b). About one-third of individuals with FMR1 premutations develop FXTAS and associated cortical-cerebellar and movement deterioration during aging (Brunberg et al. 2002). However, it is unknown whether older individuals with FMR1 premutations who do not have FXTAS also exhibit functional motor control deficits, though they exhibit more severe tremor than healthy aging adults (Brown et al. 2018).
Force control is essential in many activities of daily living (Darling et al. 1988; Kornatz et al. 2005; Diermayr et al. 2011). It can be quantified with force variability, or the unintentional variation in the output of voluntary contractions (Christou 2011). The functional significance of variability in force output is that it is related to reduced stability of movement during everyday activities such as walking (Nakamura et al. 1996; Schniepp et al. 2014). For instance, greater postural variability is associated with an increased risk of falls in both healthy older individuals and individuals with neurodegenerative conditions (Nakamura et al. 1996; Kouzaki and Masani 2012; Schniepp et al. 2014). We recently demonstrated that older individuals with FMR1 premutations show greater variability of their center of pressure (COP) during standing (Wang et al. 2019), though force variability during precision motor behaviors and its relation to gross motor behaviors in older FMR1 premutation carriers have not been examined.
The physiological processes associated with motor behavior in aging premutation carriers also are not well understood. Motor units are the final common pathway of the central voluntary command to the muscle, and characteristics of motor unit discharge rates are the primary factors that affect force variability (Enoka and Fuglevand 2001). For instance, lower motor unit discharge rate contributes to increased force variability in younger and older adults (Roos et al. 1997; Enoka et al. 2003; Tracy et al. 2005; Park et al. 2016). There also is evidence that force variability is strongly related to the modulation of multiple motor units in healthy individuals (Farina et al. 2002; Shinohara 2011; Onushko et al. 2013; Moon et al. 2014; Park et al. 2016) and individuals with neurological disorders (Wang et al. 2017b). Interestingly, motor unit discharge rates have been associated with clinically-rated deficits in individuals with neurological diseases (Wang et al. 2017b). In this study, we examined the activation of multiple motor units during steady force contractions to understand underlying neuromuscular mechanisms of force variability in older FMR1 premutation carriers.
The goals of this study were to determine whether older FMR1 premutation carriers, with or without FXTAS, exhibit impaired force control relative to healthy controls, and whether force control abilities were associated with movement control during stepping. We tested the following two hypotheses: 1) force variability in individuals with FMR1 premutations is greater than that in healthy controls and 2) greater force variability in FMR1 premutation carriers is associated with atypical movement control during stepping. We also aimed to determine neuromuscular processes associated with force variability in FMR1 premutation carriers. We predicted that greater force variability in premutation carriers would be associated with reduced discharge rate of multiple motor units.
2. MATERIALS AND METHODS
2.1. Participants
Eight FMR1 premutation carriers (age: 58.88 ± 9.25 yrs) and eight age-and sex-matched healthy individuals (60.13 ± 9.25 yrs) volunteered for this study (Table 1). FMR1 premutation carriers were recruited through our fragile X clinics. During an initial screening interview, none of the premutation carriers reported any clinical issues associated with FXTAS including memory loss, kinetic tremor, gait ataxia, or postural instability. Both FMR1 premutation carriers and control participants reported no limb orthopedic surgery within the past year, neurological, neurodegenerative, or musculoskeletal disorder, or a history of medications that could potentially affect motor control (Reilly et al. 2008). The local IRB approved all procedures and all participants signed informed consent.
Table 1.
Demographic and clinical characteristics of FMR1 gene premutation carriers and healthy control participants
| Premutation carriers (n=8) | Controls (n=8) | F | p | |
|---|---|---|---|---|
| Age in years1 | 59 (8) | 60 (9) | .08 | 0.78 |
| % Male | 50% | 50% | - | 1.00┼ |
| Full-scale IQ1 | 103 (7) | 111 (9) | 4.61 | .05 |
Represents mean (SD)
Chi-square statistics
2.1.1. Genetic and Neurological Characterization
FMR1 CGG repeat length was quantified for all premutation carriers (Table 2). Molecular testing was conducted at Dr. Berry-Kravis’ Molecular Diagnostic Laboratory at Rush University. Genomic DNA was isolated from peripheral blood leukocytes samples. The FMR1 polymerase chain reaction (PCR) test with quantification of allele-specific CGG repeat length was performed using commercially available kits (Asuragen, Inc., Austin, TX).
Table 2.
Clinical and radiological features for each FMR1 premutation carrier
| ID | Clinical Findings | Radiological Findings | Speech | Kinetic | Oculomotor | Posture/gait | Total | CGG repeats |
|---|---|---|---|---|---|---|---|---|
| 1* | Kinetic tremor, mild cerebellar features | ns | 0 | 2 | 0 | 5 | 7 | 99 |
| 2*# | Kinetic tremor, gait ataxia | MCP sign, generalized WM lesion, cerebral atrophy (type 3) | 1 | 2 | 2 | 7 | 12 | 85 |
| 3# | ns | ns | 0 | 0 | 1 | 3 | 4 | 60 |
| 4# | Kinetic tremor | ns | 0 | 0 | 0 | 2 | 2 | 63 |
| 5* | Kinetic tremor, gait ataxia | Cerebral atrophy (type 1) | 0 | 1 | 0 | 4 | 5 | 81 |
| 6 | Kinetic tremor | Cerebral WM hyperintensity | 0 | 1 | 0 | 1 | 2 | 62 |
| 7*# | Kinetic tremor, gait ataxia | Did not receive MRI | 0 | 3 | 0 | 5 | 8 | 58 |
| 8 | ns | Cerebral WM hyperintensity | 0 | 0 | 0 | 1 | 1 | 68 |
-showed clinical/radiological indications of possible, probable, or definite FXTAS
-male participants
Total: total score for speech, kinetic, oculomotor, and posture/gait
ns: no significant findings; MCP: middle cerebellar peduncle; WM: white matter
FMR1 premutation carriers underwent a T2-weighted MRI scan (repetition time = 6350 msec; echo time = 100 msec; flip angle = 120°; field of view = 256 ×156 ×256 mm3; 78 axial slices; voxel size = 1 × 2 × 2 mm; no gap) to test for the presence of hyperintensities within the middle cerebellar peduncle (i.e., the MCP sign), cerebral atrophy or other cerebral or cerebellar-brainstem alterations associated with FXTAS (Jacquemont et al. 2003; Shelton et al. 2017; Wang et al. 2017a). T2-weighted scans were analyzed by a trained neuroradiologist with expertise in diseases of aging.
FMR1 premutation carriers completed a structured neurological exam administered by a neurologist with expertise in ataxia and movement disorders in aging. This exam included evaluations of movement and gait as well as administration of the International Cooperative Ataxia Rating Scale (ICARS) (Schmitz-Hübsch et al. 2006). The ICARS is comprised of 19 sections examining postural and gait disturbances, ataxia, dysarthria and oculomotor functions. Higher scores indicate more severe neuromotor issues. The ICARS has been validated previously for diagnosis in patients with focal cerebellar lesions (Schoch et al. 2007) and Friedrich’s ataxia (Schmitz-Hübsch et al. 2006).
2.2. Experimental protocol
In addition to clinical and genetic testing, all participants performed 1) a steady isometric force control task with the index finger at 20% MVC during which surface EMG recordings were acquired and 2) a single step initiation task with two adjacent and synchronized AMTI force platforms. The experimental session lasted ~1.5 hours. Before the experimental session, we familiarized participants with the tasks and asked them to perform practice trials.
2.3. Experimental arrangement
2.3.1. Force Control Task.
Participants sat on a chair in a darkened room facing a 27-inch Dell LCD monitor (Dell Inc., Dallas, TX, USA; resolution: 1920 × 1080; refresh rate: 120 Hz) located 0.6 m away at eye level. The monitor displayed lines representing the target force and the force produced by the index finger abduction (Figure 1a). All participants affirmed that they could see the display clearly. The shoulder of the participant was abducted at ~45° with ~90° elbow flexion. The forearm rested on a customized arm brace. The dominant hand rested on a custom hand device with an adjustable plate and the middle, ring, and little digits secured (Figure 1a). This arrangement allowed only isometric abduction of the index finger, which was produced primarily by the contraction of the first dorsal interosseous (FDI) muscle (Chao 1989; Li et al. 2003). Participants performed 1) a maximal voluntary contraction (MVC) task and 2) an isometric force task. Detailed information about these tasks is as follows.
Fig. 1.
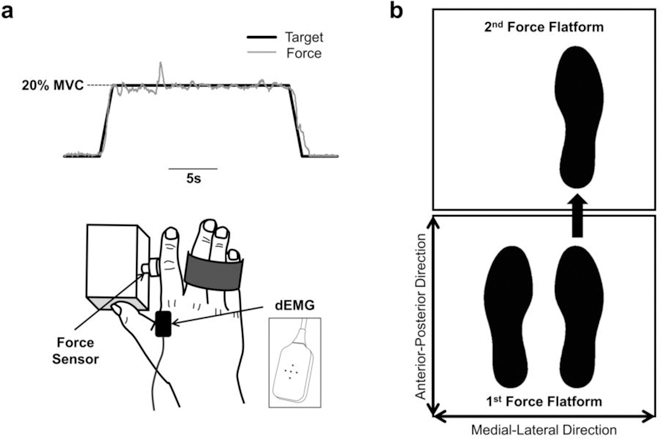
Schematic of the experimental setup for the force control and step initiation tasks. a) Force control task. Participants performed isometric contractions with their index finger. The solid black line represents the force exerted by the participant, whereas the solid grey line represents the target force. We isolated the participants’ middle, ring, and little finger to allow only abduction of the index finger. We recorded the force output and muscle activity from the FDI muscle. b) Step initiation task. Participants stepped forward with their right or left foot from one force platform to the second force platform at a comfortable speed and distance. We recorded COP data to calculate the step length, duration, and velocity.
2.3.1.1. MVC Task
Prior to the isometric force task, we measured each participant’s index finger MVC for their dominant hand. During the MVC task, participants increased their index finger force from baseline to their maximum and then maintained the maximal force for ~5 s. Participants completed three trials with one minute rest between trials.
2.3.1.2. Isometric Force Task
The goal of the force control task was to trace a target with the force output of index finger abduction (Figure 1a). The target force for each participant was normalized to 20% of his or her MVC value. The visual feedback displayed on the monitor was the participant’s force (represented by solid grey line in Figure 1a) relative to the target (represented by solid black line in Figure 1a). We instructed participants to gradually increase their force to match the target force within 2 s and maintain their force on the target (20% MVC) as accurately and as consistently as possible for 17 s. Participants performed 3 trials. The rest time between each trial was 1 min.
2.3.1.3. Force Measurement
The abduction force of the index finger was recorded with a one-dimensional force transducer (100 lb Miniature Beam Load Cell, Interface Inc., PA, USA), which was installed on the hand plate (Figure 1a). The force signal was sampled at 20 KHz with the Bagnoli-16 sEMG System (Delsys, Inc., Boston, MA, USA).
2.3.1.4. Motor Unit Recording
To determine the modulation of the motor neuron pool, we used the Delsys decomposition system that could identify and record the action potentials from multiple motor units using a specialized surface EMG electrode (dEMG; Delsys, Boston, MA, USA). The dEMG is made up of five pins (0.5 mm diameter; Figure 1a) and records four surface EMG signals. The EMG signals were sampled at 20 KHz and stored on a computer. The EMG signals were then decomposed into single motor unit action potentials using the Delsys decomposition algorithm, which has been shown to be reliable during isometric contractions (Nawab et al. 2010).
2.3.2. Single Step Initiation Task
Participants completed trials during which they took one full step forward from one force platform to an adjacent force platform (Figure 1b). Data collection started by requesting participants to stand side-by-side on the posterior force platform. After three to five seconds quiet stance, individuals received an auditory cue of either “right” or “left” to prompt them to lead the step with their right or left foot. Individuals then stepped foward onto the anterior force platform at a comfortable speed and magnitude. To finish the trial, participants needed to have both feet resting on the anterior plate in a side-by-side foot position for three additional seconds. The direction of the auditory cue was randomized across trials, and the timing was randomly presented within three to five seconds after the experimenter confirmed that the participant was neutrally standing on the posterior platform. Three successful trials were collected for each participant. Each trial was followed by 10-seconds of rest. To characterize stepping behavior, we focused on the transition period beginning with the heel strike of the lead leg on the anterior platform and ending with the lifting of the toe of the trail leg from the posterior force platform. This transition phase was examined to determine the rate at which individuals propel their body movement in the AP direction during stepping (Gantchev et al. 1996; Burleigh‐Jacobs et al. 1997).
2.4. Data analysis
We analyzed data using custom-written programs in MATLAB.
2.4.1. Force variability
Force data were analyzed over a segment of 8 s (11–19 s) in the middle of each 17 s trial. Variability was quantified as the coefficient variation (CV) of force, which is calculated by dividing the standard deviation of the time series by the mean force output. CV was measured using the detrended force signal. Detrending the force signal removes the linear trend from the force output and eliminates any drift (i.e., as a participant fatigues over the course of a trial), which could influence the quantification of force variability.
2.4.2. EMG signal decomposition
EMG data were analyzed over a segment of 8 s (11–19 s) in the middle of each 17 s trial. The dEMG electrode recorded four surface EMG signals that were decomposed into action potentials of single motor units using the dEMG decomposition algorithm (Nawab et al. 2010). The outcome of this algorithm provided motor unit trains that included: 1) the number of motor units; 2) the action potential shapes of each identified motor unit; and 3) the firing instances (spikes) of action potentials of each identified motor unit. This outcome was validated (95% accuracy) using the Decompose-Synthesize-Decompose-Compare test (Nawab et al. 2010). Detailed information of the algorithm is described elsewhere (Nawab et al. 2010).
2.4.3. Motor unit selection
We selected five motor units to normalize the number of motor units that would be compared across individuals (Park et al. 2016). The number of spikes, which relates to the number of motor units recorded, influences the quantification of parameters of interest (e.g. mean discharge rate). Therefore, from each trial the following five recorded motor units were selected: 1) the first recruited motor unit (MU1), which represents the smallest motor unit in the pool; 2) the last recruited motor unit (MU5), which represents the largest motor unit in the pool; 3) the motor unit recruited in the middle of the pool (MU3, middle between MU1 and MU5), which represents the average motor unit recruited during the task; 4) the motor unit in the middle between MU1 and MU3 (MU2), which represents the lower-threshold motor units; and 5) the motor unit in the middle between MU3 and MU5 (MU4), which represents the higher-threshold motor units. The selection of these five motor units was essential because the number of motor units recorded varies randomly with trial. Thus, selecting five motor units is an essential methodological adjustment to compare the motor neuron pool activity across trials and populations.
2.4.4. Mean discharge rate of multiple motor units
The mean discharge rate of multiple motor units (motor neuron pool) was quantified from the selected five motor units. The mean discharge rate was quantified as the average of the inter-spike interval, which reflects the time between two consecutive spikes.
2.4.5. COP velocity during the transition phase of stepping
All kinetic data were down sampled to 100 Hz and low pass filtered using a 4th-order double pass Butterworth filter with a cutoff frequency of 6 Hz. The COP time series for each force platform were derived from force and moment data consistent with prior studies (Winter 1995). The net COP time series in the AP direction were derived from COPs and the vertical ground reaction force (vGRF) of both plates (Winter 1995; Wang et al. 2012). The “body weight transfer” phase was examined and defined as the interval between the first heel strike of the lead leg on the anterior force plate and the lifting of the toe of the trail leg off of the posterior plate. The heel strike of individuals’ stepping leg was marked as the time point at which vGRF of the anterior plate first exceeded and remained above zero for ≥ 0.2 sec. The lifting of the toe of the trail leg was marked as the time point at which vGRF of the posterior (starting) plate dropped to and remained at zero for ≥ 0.2 sec. To determine the maximum velocity of the body weight transfer, the first derivative of the net COPAP time series was examined and the maximum value was selected. Therefore, peak velocity was analyzed to determine the rate at which individuals could shift their body weight during stepping movements that often are compromised in patients with ataxia (Burleigh‐Jacobs et al. 1997; Fernandez et al. 2013).
2.5. Statistical analysis
We used an independent t-test to compare CV of force, mean discharge rate of multiple motor units, and COP velocity between the FMR1 premuation and control groups. We used linear regression models to determine the following: 1) the association between the CV of force and mean discharge rate of multiple motor units; and 2) the association between the CV of force/mean discharge rate of multiple motor units and COP velocity during stepping transition. The goodness-of-fit of the model was given by the squared multiple correlations (R2), Durbin Watson statistic (DW), and part correlation coefficients that demonstrate the unique contribution of each predictor to the criterion variable. Analyses were performed with the IBM SPSS Statistics 25.0 statistical package (IBM Corp., Armonk, NY, USA). The alpha level for all statistical tests was set at 0.05 after Bonferroni correction. Data are reported as mean ± SD in the text and mean ± standard error of the mean in the figures.
3. RESULTS
3.1. Motor Control and Motor Unit Activity
FMR1 premutation carriers showed greater force variability (1.79 ± 1.36 vs. 0.66 ± 0.36 %; t = 2.26, P = 0.04; Figure 2a) and a lower mean discharge rate of multiple motor units (92.72 ± 11.40 vs. 125.49 ± 19.91 %; t = −4.04, P = 0.001; Figure 2b) during index finger force contractions compared with healthy controls. Greater force variability was associated with reduced mean discharge rate of multiple motor units across all participants (R2 = 0.50, DW = 0.85, P = 0.002; Figure 3a). Force variability (1.82 ± 1.33 vs. 1.76 ± 1.59 %; t = −0.05, P = 0.96; Figure 3b) and mean discharge rate (92.17 ± 15.72 vs. 93.28 ± 7.42 %; t = −0.13, P = 0.90; Figure 3c) were not significantly different between FMR1 premutation carriers with FXTAS and those without FXTAS. Difference in force variability between FMR1 premutation carriers with FXTAS and healthy controls was significant (t = 2.38, P = 0.04) and that between FMR1 premutation carriers without FXTAS and healthy controls was at the margin of statistical significance (t = 1.94, P = 0.08). Mean discharge rate was significantly different between FMR1 premutation carriers with FXTAS and healthy controls (t = −2.90, P = 0.02) and between FMR1 premutation carriers without FXTAS and healthy controls (t = −3.07, P = 0.01). Force variability was greater in older premutation carriers (r = 0.50, P = 0.21), and mean discharge rate was lower in older carriers (r = −0.56, P = 0.15), though neither of these relationships were significant.
Fig. 2.
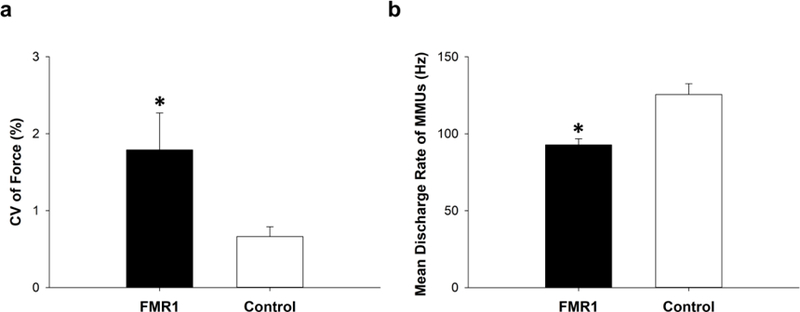
Force control and motor unit activity. a) FMR1 premutation carriers exhibited greater force variability during steady force contractions compared with healthy controls (P = 0.04). b) FMR1 premutation carriers exhibited lower mean discharge rates of multiple motor units compared with healthy controls (P = 0.001).
Fig. 3.
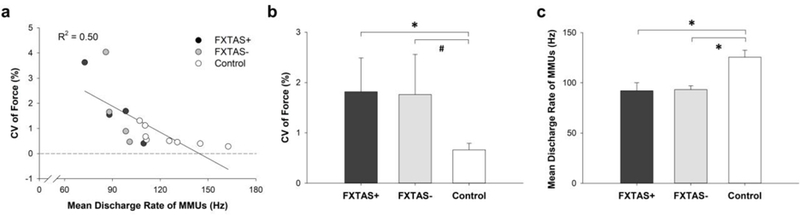
Correlation between force control and motor unit activity. a) Greater force variability was significantly related to lower mean discharge rates of multiple motor units (R2 = .50, P = 0.002). b) There was no significant difference in force variability between FMR1 premutation carriers with FXTAS (+) and those without FXTAS (−) (P = 0.96). Difference in force variability between FMR1 premutation carriers with FXTAS (+) and controls was significant (P = 0.04) and that between FMR1 premutation carriers without FXTAS (−) showed a certain trend toward significance (P = 0.08). c) There was no significant difference in motor unit discharge rate between FMR1 premutation carriers with FXTAS (+) and those without FXTAS (−) (P = 0.90). Difference in motor unit discharge rate between FMR1 premutation carriers with FXTAS (+) and controls (P = 0.02) and between FMR1 premutation carriers without FXTAS (−) and controls (P = 0.01) was significant. * indicates siginificant difference between the groups (P < 0.05). # indicates a significance level of 0.08.
3.2. Single Step Initiation Task
FMR1 premutation carriers exhibited reduced velocity of their body weight transfer during stepping compared with healthy controls (156.62 ± 26.24 vs. 191.86 ± 18.83 cm/s; t = −2.95, P = 0.01; Figure 4a). Body weight transfer velocity was not significantly different between FMR1 premutation carriers with FXTAS and those without FXTAS (152.85 ± 25.12 vs. 160.40 ± 30.61 cm/s; t = −0.38, P = 0.72; Figure 4b). Difference in body weight transfer velocity between FMR1 premutation carriers with FXTAS and healthy controls was significant (t = −2.95, P = 0.02) and that between FMR1 premutation carriers without FXTAS and healthy controls approached significance (t = −2.14, P = 0.06). There were no significant correlations between force variability (P = 0.67) or motor unit properties (P = 0.72) and body weight transfer velocity during stepping. None of our motor control measures were associated with CGG repeat length (CV of force: P = 0.82; mean discharge rate: P = 0.90) or ICARS scores (CV of force: P = 0.57; mean discharge rate: P = 0.49). Body weight transfer velocity was not significantly correlated with age (r = −0.20, P = 0.64). However, subtle effects of age may be present due to limited power (relatively smaller samples).
Fig. 4.
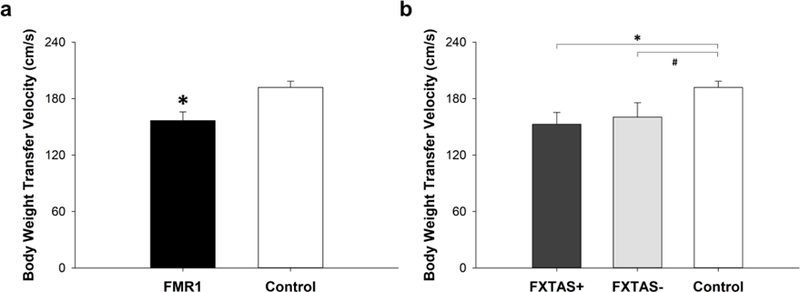
COP velocity during the transition phase of stepping. a) FMR1 premutation carriers exhibited lower COP velocity during the transition phase of stepping compared with healthy controls (P = 0.01). b) There was no significant difference in stepping COP velocity between FMR1 premutation carriers with FXTAS (+) and those without FXTAS (−) (P = .72). Difference in stepping COP velocity between FMR1 premutation carriers with FXTAS (+) and controls was significant (P = 0.02) and that between FMR1 premutation carriers without FXTAS (−) showed a certain trend toward significance (P = 0.06). * indicates siginificant difference between the groups (P < 0.05). # indicates a significance level of 0.06.
4. DISCUSSION
In this study, we aimed to determine whether older FMR1 premutation carriers exhibit impaired force control during finger abduction and during stepping compared with healthy individuals. We found that finger force variability was greater in FMR1 premutation carriers than in healthy controls and was not associated with ICARS scores suggesting that motor issues are present in older premutation carriers regardless of the degree to which they demonstrate clinical features of FXTAS. FMR1 premutation carriers also exhibited reduced COP velocity during stepping compared with healthy controls indicating that multiple motor systems are impacted during aging in premutation carriers. These results provide novel evidence that older individuals with FMR1 premutations exhibit impaired functional motor control abilities regardless of whether clinical or radiological signs of FXTAS are present and independent of CGG repeat length.
4.1. Force Control in FMR1 Premutation Carriers
A major finding in this study was that individuals with FMR1 premutations exhibited greater finger abduction force variability compared with healthy individuals. These results provide new quantitative data indicating that sensorimotor processes are affected in older premutation carriers. In the context of our finding that force control is not associated with ICARS scores, these results also suggest that functional deficits of force control emerge independent of the presence of clinical signs of FXTAS. This hypothesis is further supported by our finding that force control and CGG repeat length were not associated in premutation carriers despite increased CGG repeat length conferring greater risk susceptibility for FXTAS (Tassone et al. 2007). Few known studies have examined force control in older FMR1 premutation carriers, though subclinical cognitive issues have been documented in older premutation carriers (Hagerman 2002). Our finding that FMR1 premutations are associated with deficits in steady force control during aging indicates that sensorimotor systems also are disrupted and they may deteriorate regardless of the presence of FXTAS. Our findings also indicate that reduced force control may serve as an important indicator of FXTAS prior to the disease process becoming clinically detectable. It is possible that motor control differences between FMR1 premutation carriers with FXTAS and those without FXTAS are evident, but that they were not detected based on our relatively smaller sample. In the context of the significant sensorimotor and clinical heterogeneity of FXTAS, larger studies of aging premutation carriers are needed in order to determine the extent to which our measures may represent general or more disease-specific markers in FMR1 premutation carriers.
Our finding of increased force variability in premutation carriers raises the important question of what are the underlying central and peripheral mechanisms contributing to greater force variability in FMR1 premutation carriers. Force variability may be greater in FMR1 premutation carriers because the motor command that activates the muscle may be different. Our results demonstrate that FMR1 premutation carriers exhibit greater force variability likely because the mean discharge rate of multiple motor units is slower compared with healthy controls (Onushko et al. 2013; Park et al. 2016), as demonstrated in Figure 2. The significance of a slower discharge rate of motor units is that it makes signals less deterministic (Person and Kudina 1972; Matthews 1996; Vaillancourt et al. 2002), which increases force variability (Taylor et al. 2003; Park et al. 2016). Our results showing a strong relationship between reduced multiple motor unit discharge rate and greater force variability is consistent with prior findings on healthy adults and indicates that greater force variability in FMR1 premutation carriers reflects a less deterministic motor unit signal implicating altered central-to-peripheral mechanisms of force control (Figure 2 & 3). The altered characteristics of motor unit discharage rate in FMR1 premuation carriers could be clinically important as a target for therapies aimed at addressing deterioration of motor control in aging premutation carriers, and potentially as a sensitive marker of degeneration.
4.2. Single Step Initiation in FMR1 Premutation Carriers
Another important finding was that, independent of ICARS rated features and CGG repeat length, individuals with FMR1 premutation alleles exhibited impaired performance during single step initiation. Specifically, FMR1 premutation carriers showed reduced velocity of their body weight transfer during the transition phase of walking. While prior studies have indicated that gait disturbances are evident in patients with FXTAS (Brunberg et al. 2002; Jacquemont et al. 2004a; Jacquemont et al. 2004b; Zühlke et al. 2004), we demonstrate that stepping issues are associated with aging in premutation carriers prior to or independent of FXTAS. Longitudinal studies are needed to determine whether these measures of stepping may reflect prodromal indicators of FXTAS.
One of the interests of this study was to determine whether altered force control properties (force control or motor unit activity) relate to impaired stepping performance. However, we did not find any significant association between reduced ability to control force variability and stepping issues in premutation carriers or controls. These findings suggest that individuals use different muscles and different control mechanisms to support manual motor force control and stepping performance, and that these distinct mechanisms each are affected in older premutation carriers. For instance, our index finger force control task simply required a single-dimensional control of force because the arrangement of the task (see the method section) allowed only abduction of the index finger, which was produced primarily by the FDI muscle contraction (Chao 1989; Li et al. 2003). However, relative to the force control task, stepping performance requires more complex control mechanisms involving the coordination of multiple distinct joints and muscles as well as the integration of distinct sensorimotor processes, including anticipatory postural adjustments and sensory feedback guided adjustments (Duysens et al. 2000). Our findings indicate that central motor commands or multiple motor unit mechanisms controlling single-dimensional force control are disrupted in FMR1. Further, the coordination of distinct motor control processes that is required for more complex gross motor tasks such as stepping also are compromised. These distinct motor control processes appear to be independently affected, suggesting that FMR1 premutations contribute to the deterioration of multiple motor control systems during aging.
4.4. Limitations
Our participants were limited to middle-aged and older adults. Thus, it is not clear whether our findings extend to younger premutation carriers. The small sample size and cross-sectional design limit our ability to determine if any participants have gone on to develop FXTAS. Larger cohort longitudinal studies are needed to assess the value of functional motor control measurements for prognosis and disease tracking. Since both penetrance and severity of FXTAS are lower in women carriers, studies of sex effects on functional motor abilities in aging premutation carriers are warranted.
5. CONCLUSION
In summary, we provide novel evidence that individuals with FMR1 premutations exhibit deficits in force control and stepping. To our knowledge, the findings from this study present the first evidence that older FMR1 gene premutation carriers, with or without FXTAS, exhibit impaired abilities to perform functional motor control tasks.
Acknowledgement
This study was supported by NIMH R01 Research Project Grant Program (MH 112734), Once Upon a Time Foundation Award, the Kansas Center for Autism Research and Training (K-CART) Research Investment Council Strategic Initiative Grant to Dr. Mosconi, and the NICHD U54 Kansas Intellectual and Developmental Disabilities Research Center Award (U54HD090216).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Brown SS, Basu S, Whalley HC, Kind PC, Stanfield AC (2018) Age-related functional brain changes in FMR1 premutation carriers. NeuroImage: Clinical 17:761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunberg JA, Jacquemont S, Hagerman RJ, et al. (2002) Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. American Journal of Neuroradiology 23:1757–1766 [PMC free article] [PubMed] [Google Scholar]
- Burleigh‐Jacobs A, Horak FB, Nutt JG, Obeso JA (1997) Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Movement Disorders 12:206–215 [DOI] [PubMed] [Google Scholar]
- Chao EY (1989) Biomechanics of the hand: a basic research study. World Scientific [Google Scholar]
- Christou EA (2011) Aging and variability of voluntary contractions. Exercise and Sport Sciences Reviews 39:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling W, Cole K, Abbs J (1988) Kinematic variability of grasp movements as a function of practice and movement speed. Experimental Brain Research 73:225–235 [DOI] [PubMed] [Google Scholar]
- Diener H-C, Dichgans J, Bacher M, Gompf B (1984) Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalography and Clinical Neurophysiology 57:134–142 [DOI] [PubMed] [Google Scholar]
- Diermayr G, McIsaac TL, Gordon AM (2011) Finger force coordination underlying object manipulation in the elderly–a mini-review. Gerontology 57:217–227 [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H (2000) Load-regulating mechanisms in gait and posture: comparative aspects. Physiological Reviews 80:83–133 [DOI] [PubMed] [Google Scholar]
- Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL (2003) Mechanisms that contribute to differences in motor performance between young and old adults. Journal of Electromyography and Kinesiology 13:1–12 [DOI] [PubMed] [Google Scholar]
- Enoka RM, Fuglevand AJ (2001) Motor unit physiology: some unresolved issues. Muscle & nerve 24:4–17 [DOI] [PubMed] [Google Scholar]
- Farina D, Fosci M, Merletti R (2002) Motor unit recruitment strategies investigated by surface EMG variables. Journal of Applied Physiology 92:235–247 [DOI] [PubMed] [Google Scholar]
- Fernandez KM, Roemmich RT, Stegemöller EL, Amano S, Thompson A, Okun MS, Hass CJ (2013) Gait initiation impairments in both Essential Tremor and Parkinson’s disease. Gait & posture 38:956–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantchev N, Viallet F, Aurenty R, Massion J (1996) Impairment of posturo-kinetic coordination during initiation of forward oriented stepping movements in parkinsonian patients. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control 101:110–120 [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ (2004) Fragile X‐associated tremor/ataxia syndrome (FXTAS). Mental Retardation and Developmental Disabilities Research Reviews 10:25–30 [DOI] [PubMed] [Google Scholar]
- Hagerman RJ (2002) The physical and behavioral phenotype. Fragile X syndrome: Diagnosis, treatment, and research 3:206–248 [Google Scholar]
- Hagerman RJ, Protic DD, Rajaratnam A, Salcedo-Arellano MJ, Aydin EY, Schneider A (2018) Fragile X-associated Neuropsychiatric Disorders (FXAND). Frontiers in Psychiatry 9:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, O’keefe JA (2012) Fragile x-associated tremor ataxia syndrome: the expanding clinical picture, pathophysiology, epidemiology, and update on treatment. Tremor and Other Hyperkinetic Movements 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking DR, Birch RC, Bui QM, et al. (2017) Cerebellar volume mediates the relationship between FMR1 mRNA levels and voluntary step initiation in males with the premutation. Neurobiology of Aging 50:5–12 [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Farzin F, Hall D, et al. (2004a) Aging in individuals with the FMR1 mutation. American journal on mental retardation 109:154–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, et al. (2003) Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. The American Journal of Human Genetics 72:869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey MA, et al. (2004b) Penetrance of the fragile X– associated tremor/ataxia syndrome in a premutation carrier population. JAMA 291:460–469 [DOI] [PubMed] [Google Scholar]
- Kenneson A, Zhang F, Hagedorn CH, Warren ST (2001) Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Human Molecular Genetics 10:1449–1454 [DOI] [PubMed] [Google Scholar]
- Kornatz KW, Christou EA, Enoka RM (2005) Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. Journal of Applied Physiology 98:2072–2080 [DOI] [PubMed] [Google Scholar]
- Kouzaki M, Masani K (2012) Postural sway during quiet standing is related to physiological tremor and muscle volume in young and elderly adults. Gait & posture 35:11–17 [DOI] [PubMed] [Google Scholar]
- Leehey M, Berry-Kravis E, Goetz C, et al. (2008) FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology 70:1397–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z-M, Pfaeffle HJ, Sotereanos DG, Goitz RJ, Woo SL-Y (2003) Multi-directional strength and force envelope of the index finger. Clinical Biomechanics 18:908–915 [DOI] [PubMed] [Google Scholar]
- Matthews P (1996) Relationship of firing intervals of human motor units to the trajectory of post‐spike after‐hyperpolarization and synaptic noise. The Journal of physiology 492:597–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H, Kim C, Kwon M, Chen YT, Onushko T, Lodha N, Christou EA (2014) Force control is related to low-frequency oscillations in force and surface EMG. PLoS ONE 9:e109202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Meguro K, Sasaki H (1996) Relationship between falls and stride length variability in senile dementia of the Alzheimer type. Gerontology 42:108–113 [DOI] [PubMed] [Google Scholar]
- Nawab SH, Chang SS, De Luca CJ (2010) High-yield decomposition of surface EMG signals. Clin Neurophysiol 121:1602–1615 doi: 10.1016/j.clinph.2009.11.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onushko T, Baweja HS, Christou EA (2013) Practice improves motor control in older adults by increasing the motor unit modulation from 13 to 30 Hz. Journal of Neurophysiology 110:2393–2401 doi: 10.1152/jn.00345.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Kwon M, Solis D, Lodha N, Christou EA (2016) Motor control differs for increasing and releasing force. Journal of neurophysiology 115:2924–2930 doi: 10.1152/jn.00715.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person R, Kudina L (1972) Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalography and Clinical Neurophysiology 32:471–483 [DOI] [PubMed] [Google Scholar]
- Reilly JL, Lencer R, Bishop JR, Keedy S, Sweeney JA (2008) Pharmacological treatment effects on eye movement control. Brain and Cognition 68:415–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, et al. (2009) Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. European Journal of Human Genetics 17:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Vandervoort AA (1997) Age-related changes in motor unit function. Muscle & Nerve 20:679–690 [DOI] [PubMed] [Google Scholar]
- Schmitz-Hübsch T, Du Montcel ST, Baliko L, et al. (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66:1717–1720 [DOI] [PubMed] [Google Scholar]
- Schniepp R, Wuehr M, Schlick C, et al. (2014) Increased gait variability is associated with the history of falls in patients with cerebellar ataxia. Journal of neurology 261:213–223 [DOI] [PubMed] [Google Scholar]
- Schoch B, Regel JP, Frings M, Gerwig M, Maschke M, Neuhäuser M, Timmann D (2007) Reliability and validity of ICARS in focal cerebellar lesions. Movement disorders: official journal of the Movement Disorder Society 22:2162–2169 [DOI] [PubMed] [Google Scholar]
- Shelton AL, Cornish KM, Godler D, Bui QM, Kolbe S, Fielding J (2017) White matter microstructure, cognition, and molecular markers in fragile X premutation females. Neurology 88:2080–2088 [DOI] [PubMed] [Google Scholar]
- Shinohara M (2011) Adaptations in motor unit behavior in elderly adults. Current aging science 4:200–208 [DOI] [PubMed] [Google Scholar]
- Tassone F, Adams J, Berry‐Kravis EM, et al. (2007) CGG repeat length correlates with age of onset of motor signs of the fragile X‐associated tremor/ataxia syndrome (FXTAS). American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 144:566–569 [DOI] [PubMed] [Google Scholar]
- Taylor AM, Christou EA, Enoka RM (2003) Multiple features of motor-unit activity influence force fluctuations during isometric contractions. Journal of neurophysiology 90:1350–1361 [DOI] [PubMed] [Google Scholar]
- Tracy BL, Maluf KS, Stephenson JL, Hunter SK, Enoka RM (2005) Variability of motor unit discharge and force fluctuations across a range of muscle forces in older adults. Muscle & Nerve 32:533–540 doi: Doi 10.1002/Mus.20392 [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Larsson L, Newell KM (2002) Time-dependent structure in the discharge rate of human motor units. Clinical Neurophysiology 113:1325–1338 [DOI] [PubMed] [Google Scholar]
- Wang H, Ku L, Osterhout DJ, Li W, Ahmadian A, Liang Z, Feng Y (2004) Developmentally-programmed FMRP expression in oligodendrocytes: a potential role of FMRP in regulating translation in oligodendroglia progenitors. Human Molecular Genetics 13:79–89 [DOI] [PubMed] [Google Scholar]
- Wang J, Ethridge LE, Mosconi MW, et al. (2017a) A resting EEG study of neocortical hyperexcitability and altered functional connectivity in fragile X syndrome. Journal of neurodevelopmental disorders 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jordan K, Newell KM (2012) Coordination patterns of foot dynamics in the control of upright standing. Motor Control 16:425–443 [DOI] [PubMed] [Google Scholar]
- Wang Z, Khemani P, Schmitt LM, Lui S, Mosconi MW (2019) Static and dynamic postural control deficits in aging fragile X mental retardation 1 (FMR1) gene premutation carriers. Journal of neurodevelopmental disorders 11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kwon M, Mohanty S, Schmitt LM, White SP, Christou EA, Mosconi MW (2017b) Increased Force Variability Is Associated with Altered Modulation of the Motorneuron Pool Activity in Autism Spectrum Disorder (ASD). International journal of molecular sciences 18:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA (1995) Human balance and posture control during standing and walking. Gait & posture 3:193–214 [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, et al. (2003) The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 112:317–327 [DOI] [PubMed] [Google Scholar]
- Zühlke C, Budnik A, Gehlken U, et al. (2004) FMR1 premutation as a rare cause of late onset ataxia. Journal of Neurology 251:1418–1419 [DOI] [PubMed] [Google Scholar]


