Abstract
Background:
Early childhood social reticence (SR) and preadolescent social anxiety (SA) symptoms increase risk for more severe SA in later adolescence. Yet, not all at-risk youth develop more severe SA. The emergence of distinct patterns of neural response to socially evocative contexts during pivotal points in development may help explain this discontinuity. We tested the extent to which brain function during social interactions in preadolescence influenced the effects of SA and early childhood SR on predicting SA symptoms in mid-adolescence.
Methods:
Participants (N=53) were assessed for SR from ages 2–7. At age 11, SA symptoms were assessed and brain function was measured using fMRI as participants anticipated social evaluation from purported peers with a reputation for being unpredictable, nice, and mean. At age 13, SA symptoms were re-assessed. Moderated-mediation models tested the extent to which early childhood SR, preadolescent SA and preadolescent brain function predicted mid-adolescent SA.
Results:
In individuals with preadolescent SA, the presence of early childhood SR and SR-linked differences in brain activation predicted more severe SA in mid-adolescence. Specifically, in those who exhibited preadolescent SA, greater early childhood SR was associated with enhanced bilateral insula engagement while anticipating unpredictable-vs-nice social evaluation in preadolescence, and more severe SA in mid-adolescence.
Conclusions:
SR-linked neural responses to socially evocative peer interactions may predict more severe SA symptoms in mid-adolescence among individuals with greater preadolescent SA symptoms and childhood SR. This same pattern of neural response may not be associated with more severe SA symptoms in youth with only one risk-factor.
Social anxiety (SA) disorder is characterized by fear of negative evaluation that prompts avoidance and distress in social situations (DSM-5; American Psychiatric Association, 2013). Typical onset of SA disorder occurs in mid-adolescence (M=13.1 years; Beesdo-Baum et al., 2012) with the highest onset rate occurring between 11 and 13 years of age (DeWit et al., 2005). While symptoms often remit in later adolescence, some individuals experience more severe and intractable symptoms persisting into adulthood (Beesdo-Baum et al., 2012; Bruce, Yonkers, Otto, & Eisen, 2005; Reilly-Harrington & Sachs, 2006). Given limited intervention resources (Katzelnick et al., 2001), early identification of individuals likely to experience continued symptoms is imperative (Heiser, Turner, & Beidel, 2003; Kessler, Chiu, Demler, Merikangas, & Walters, 2005; Reilly-Harrington & Sachs, 2006).
Our prior work demonstrates that early childhood social reticence (SR), a characteristic that reflects conflicting drives to interact and withdraw from peers, is associated with heightened insula and dorsal anterior cingulate (dACC) engagement to socially evocative situations during preadolescence (Jarcho et. al., 2016). This suggests a lasting influence of SR on subsequent brain function during social engagement in youth at risk for developing SA disorder. However, this study did not examine whether these neural mechanisms related to preadolescent SA symptoms. Additionally, because participants were tested at 11 years of age, two years prior to the typical age of SA disorder onset, it is unclear whether this pattern of response reflects risk for, or resilience against, subsequent expression of SA symptoms. The current study tests these relations by determining the extent to which early childhood SR, preadolescent brain function, and preadolescent SA symptoms predict expression of SA symptoms at age 13.
Early individual difference risk factors often precede adolescent onset of SA disorder (Clauss & Blackford, 2012; Essex, Klein, Slattery, Goldsmith, & Kalin, 2010; Henderson, Pine, & Fox, 2015). Early childhood SR is associated with behaviorally inhibited temperament (r=.71; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Rubin, Burgess, & Hastings, 2002), a trait-like characteristic that presents in infancy as a predisposition for heightened vigilance, negative affect and fearful responses to novelty (Fox, Henderson, Marshall, Nichols, & Ghera, 2005; Kagan & Snidman, 1991). Infants with stable expression of these temperamental characteristics often go on to exhibit higher levels of SR in early childhood, which in turn is often associated with developing SA disorder in adolescence and young adulthood (OR=2.37 to 3.15; Chronis-Tuscano et al., 2009; Essex et al., 2010; Fox & Pine, 2012; Hirshfeld-Becker et al., 2007; Pérez-Edgar & Guyer, 2014; Rubin, Chen, Mcdougall, Bowker, & Mckinnon, 1995; Schwartz, Snidman, & Kagan, 1999). Greater expression of behavioral inhibition and the closely related characteristic of SR are associated with dysregulated neural response to novel and emotional faces (Blackford, Allen, Cowan, & Avery, 2013; Blackford, Avery, Cowan, Shelton, & Zald, 2011; Pérez-Edgar, et al., 2007; Schwartz, Wright, Shin, Kagan, & Rauch, 2003), threat sensitivity and errors (Buzzell et al., 2017; Jacqueline Alexandra Clauss, Benningfield, Rao, & Blackford, 2016; Fu, Taber-Thomas, & Pérez-Edgar, 2017; Hardee et al., 2013a; McDermott et al., 2009), emotion-based cognition (Jarcho, Fox, et al., 2013; Jarcho et al., 2014), reward processing (Bar-Haim et al., 2009; Guyer et al., 2012; Helfinstein et al., 2011; Lahat, Benson, Pine, Fox, & Ernst, 2016; Pérez-Edgar et al., 2014), and social evaluation (Guyer et al., 2014; Jarcho et al., 2016). Similar patterns of dysregulation are observed in SA disorder (see Caouette & Guyer, 2014; Freitas-Ferrari et al., 2010a for review), which may indicate shared neural mechanisms that could explain the enhanced risk for SA disorder in those with SR and early-onset SA.
Despite this similarity, nearly 50% of children with elevated SR or early SA symptoms remit or experience sub-threshold symptoms by age 14 (Clauss & Blackford, 2012; Beesdo-Baum et al., 2012). One plausible explanation for differences in the development of persistent SA symptoms could be the timing and potency of early SR. Higher levels of early SR may influence neural responses to social interactions and promote maladaptive or anxious thought patterns in subsequent social interactions. These influences are particularly potent during adolescence, when neural networks implicated in social processes undergo developmental changes in response to more complex peer relationships (Blakemore, 2008; Nelson, Jarcho, & Guyer, 2016; Nelson, Leibenluft, McClure, & Pine, 2005). For example, Fu (2017) found that associations between greater dlPFC function and anxiety were linked to an early-emerging biologically-based temperamental vulnerability, which shaped the development of threat-related attention bias and anxiety over time. Thus, higher levels of SR were associated with increased engagement of maladaptive brain response in evocative situations. This combination of greater SR and aberrant brain response may enhance risk for persistent SA. Yet, we know of no study that uses fMRI to test the extent to which brain function predicts the development of SA symptoms. Isolating neural mechanisms of risk for SA in children with greater SR may facilitate the identification of individuals who most need intervention.
The “brain as predictor” approach utilizes neural response in brain regions of interest (ROIs) that are implicated in supporting a psychological construct (such as SA), in conjunction with traditional behavioral or self-report measures of that construct, to predict later psychological functioning (Berkman & Falk, 2013). We focused on insula and dACC ROIs as they are often linked with altered processing in SA. The insula is implicated in relaying interoceptive responses to threat to brain regions necessary for allocating attention and action (see Paulus & Stein, 2006; Uddin, 2015 for review). Heightened engagement of the insula is common in SA disorder (see Etkin & Wager, 2007), and children with greater SR exhibit hyperactive insula responses to social provocation (Clauss et al., 2014; Jarcho et al., 2016; Taber-Thomas, Morales, Hillary, & Pérez-Edgar, 2017). The dACC is implicated in various cognitive processes including salience detection (Uddin, 2015) and threat monitoring (Andreescu et al., 2009). Heightened dACC engagement is common in SA disorder (Blair et al., 2008; see Freitas-Ferrari et al., 2010 for review) and is associated with higher levels of childhood SR (Jarcho et al., 2016; although see Clauss et al., 2011). Anticipating unpredictable peer evaluation is highly salient and threatening for socially anxious preadolescents (Boelen & Reijntjes, 2009; Jackson, Nelson, & Proudfit, 2014; Jarcho et al., 2016; Jarcho, Leibenluft, et al., 2013). Thus, insula and dACC engagement as preadolescents anticipate unpredictable peer evaluation are well-suited candidates for predicting subsequent expression of SA.
The current study examines insula and dACC engagement measured in a context-relevant paradigm, in conjunction with longitudinally assessed early risk factors for SA, to predict symptom expression at its peak age of onset. Brain function was measured during the Virtual School Paradigm (Jarcho et al., 2016; Jarcho, Leibenluft, et al., 2013), which models real-world social interactions with unpredictable and predictable peers. We previously found that preadolescents with childhood SR exhibited heightened dACC and bilateral insula activation while anticipating unpredictable-vs-predictable mean or nice peer evaluation (Jarcho et al., 2016). This study follows the same sample into mid-adolescence to test the extent to which SR-linked insula and dACC dysregulation and SA in preadolescence predict subsequent SA severity. Using moderated mediation models, we hypothesize that SR-linked insula and dACC activation while anticipating unpredictable-vs-predictable peer evaluation will be associated with greater SA in mid-adolescence (age 13) in those who experience early SA (age 11). This study is novel in its use of multiple risk factors, measured across development, that highlight SR-linked neural mechanisms of adolescent SA.
Method
Participants
This study was completed in the context of a larger program of longitudinal research conducted at the National Institute of Mental Health (NIMH) and University of Maryland (UMD). The data described in the present manuscript were obtained from participants who were randomly recruited from the community at 2 years of age. All participants who were successfully recruited were then enrolled in the study; they were not enrolled based on any temperament-based characteristics. The full sample of 384 participants were recruited at random from the District of Columbia metro area. SR was assessed from ages 2–7. During preadolescence (age 11), a subset of participants was invited to complete the current study. Participants were not invited if they had turned 12 years old by this wave of data collection due to age constraints set by the broader longitudinal research program (N = 159), were no longer living in the area (N = 12), had dropped out of the larger study (N = 30), or no longer had valid contact information (N = 17). Among youth invited to participate in the study, a subset was not interested in doing so (N = 49), whereas others were ineligible due to neuroimaging contraindications and exclusion criteria (braces, N = 15; medication use, N = 10; severely impaired mental health, N = 6), scheduling conflicts (N = 4), or did not respond to recruitment attempts (N = 8). Of the remaining potential participants, 70 were recruited (36 males; 60% Caucasian, 10% African American, 6% Hispanic, 20% Mixed/Other, 4% missing data). Data from 17 participants were excluded from analyses due to missing SR data (N = 3) low IQ (N = 1), excessive head motion during the fMRI scan (N = 5), failure to complete the fMRI scan (N = 5), technical failure (N = 2), and a structural brain abnormality (N = 1). This resulted in a final sample of 53 participants who completed self-report measures of SA and underwent fMRI with the Virtual School Paradigm (see Table 1 for demographics). The 17 excluded participants did not differ from those included in the final sample based on age (M = 10.92, SD = 0.33; t(68)=1.43, p > .05), SR (M = 0.14, SD = 0.66; t(64)=−0.42, p > .05), or gender (Male N = 11, Female N = 9; χ2(1) = 0.02, p > .05). During mid-adolescence (age 13), 44 participants completed follow-up self-report measures of SA. Because no significant differences in gender, early childhood SR, preadolescent SA or brain function emerged between youth with missing and sampled data, missing data were interpolated to retain statistical power. Results were largely consistent without interpolated data. The proportion of youth with clinically relevant SA symptoms is comparative to population incidence rates of SAD at both age-points (Table 1). Correlations between SR, SA and brain function can be found in Table 2. Despite the relatively small sample size, careful consideration of sample power for the planned analyses was conducted based on a review of the literature (see Supplemental Material for discussion on power).
Table 1.
Demographic information and social anxiety levels of included participants.
| Childhood (N=53) M (SD) |
Pre-adolescence (N=53) M (SD) |
Mid-adolescence (N=44) M (SD) |
|
|---|---|---|---|
| Age | 11.08 (0.43) | 13.36 (0.60) | |
| IQ | 116.76 (10.68) | ||
| Gender (M/F) | 29/24 | ||
| SR | 0.11 (.60) | ||
| SA | 0.0001 (0.96) | 0.0001 (0.98) | |
| SCARED | Clinically Elevated in Social Anxiety | 25.00% | 17.00% |
| Clinically Elevated in School Phobia | 11.50% | 17.00% | |
| SAS | Clinically Elevated in Total Social Anxiety | 13.70% | 11.80% |
Table 2.
Correlations between SR, SA, and Brain Function
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. SR | 1 | −0.04 | −0.14 | 0.28* | 0.12 | 0.25 | .40** | 0.22 | .28* | 0.19 | 0.14 | 0.04 |
| 2. SA age 11 | 1 | .39** | 0.26 | 0.20 | −0.03 | 0.18 | −0.01 | −0.07 | −0.13 | −0.06 | −0.19 | |
| 3. SA age 13 | 1 | 0.00 | 0.14 | 0.02 | −0.10 | −0.08 | −0.12 | −0.06 | −0.13 | −0.18 | ||
| 4. rINS unpredictable-vs-nice | 1 | .47** | .28* | .70** | .40** | .28* | .47** | .43** | 0.21 | |||
| 5. rINS unpredictable-vs-mean | 1 | −0.25 | .32* | .62** | −0.17 | 0.25 | .28* | −0.17 | ||||
| 6. rINS mean-vs-nice | 1 | .28* | −0.17 | .55** | .28* | 0.02 | .55** | |||||
| 7. lINS unpredictable-vs-nice | 1 | .55** | .36** | .70** | .59** | 0.13 | ||||||
| 8. lINS unpredictable-vs-mean | 1 | −0.09 | .40** | .59** | −0.25 | |||||||
| 9. lINS mean-vs-nice | 1 | .28* | 0.09 | .47** | ||||||||
| 10. dACC unpredictable-vs-nice | 1 | .59** | .28* | |||||||||
| 11. dACC unpredictable-vs-nice | 1 | −0.13 | ||||||||||
| 12. dACC unpredictable-vs-mean | 1 |
p < .10
p < .05;
p < .01 (two-tailed).
SR = composite social reticence; SA =composite social anxiety symptoms; rINS = right insula; lINS = left insula; dACC = dorsal anterior cingulate cortex.
Measures
Social Reticence.
A SR composite was computed based on parent-report questionnaires (Rothbart, Ahadi, Hershey, & Fisher, 2001; Rowe & Plomin, 1977) and behavioral observations of standardized laboratory interactions with unfamiliar age- and gender-matched peers (Degnan et al., 2014) collected between 2–7 years of age (Hane & Fox, 2006; A Lahat et al., 2012; K. Pérez-Edgar et al., 2010). Combining parental and observational data best captures the behavioral and motivational components that characterize SR as a construct. Specifically, observational measures capture approach and avoidance behaviors, whereas maternal report provides motivational information about these behaviors thereby helping to distinguish SR from social disinterest (Rubin, Coplan, & Bowker, 2009). This composite has been used in previous studies from our group (e.g., Degnan et al., 2014; Degnan et al., 2015; Lamm et al., 2014; Perez-Edgar et al., 2011), and has excellent internal consistency (α = 0.81) despite the modest correlation between maternal and observational report data (r=0.245, p=.08).
Although the SR composite is a continuous variable, our prior work took a dichotomous approach such that participants were categorized as high or low in SR based on a cutoff value. In the present paper, the SR composite is treated as a continuous variable. This choice was motivated by methodological and conceptual considerations. Methodologically, moderated mediation analyses require a continuous rather than dichotomous variable. Conceptually, our methods are now more consistent with a shift towards a dimensional, rather than categorical approach to the study of risk for and expression of mental health symptoms (Insel et al., 2010).
Anxiety Measures.
Anxiety was measured in pre- and mid-adolescence. The Screen for Child Anxiety Related Emotional Disorders (SCARED; (Muris, Merckelbach, Schmidt, Mayer, & Birgit, 1999) contains five reliable (alpha=.90) and valid (Birmaher et al., 1999) subscales including social anxiety, school phobia, generalized anxiety, separation anxiety, panic, and total anxiety symptoms. The Social Anxiety Scales (SAS; Grecal & Lopezl, 1998) contain two reliable (alpha=.78) and valid (Storch, Masia-Warner, Dent, Roberti, & Fisher, 2004) subscales including fear of negative evaluation and social avoidance and distress. Higher scores on both scales indicate more severe symptoms.
Virtual School Paradigm.
The fMRI-based Virtual School paradigm (Figure 1) measures brain function as participants anticipate and receive social evaluation from two purported gender-matched peers with reputations for being nice (100% positive evaluations), mean (100% negative evaluations), or unpredictable (50% positive 50% negative evaluations) (see Jarcho, et al., 2013, 2016, for details). Prior to fMRI, participants were told they would be the “new kid” and other students had already been to the Virtual School. While in the scanner, participants engaged in 24 interactions with each peer type. After each interaction, participants made a person-based response (“You’re Nice”, “You’re Mean”), situation-based response (“That’s Nice”, “That’s Mean”), no response (Avoidant), or a sarcastic response (“Thanks!!!”). All participants were deceived by the task and no adverse events occurred.
Figure 1.
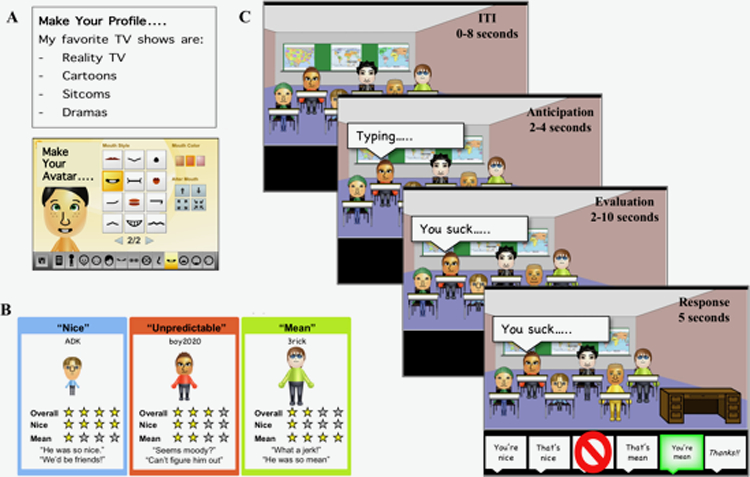
The virtual school paradigm: A. prior to scanning, participants create a personal profile and cartoon avatar of themselves to be shared with the other students. B. then, through yelp-like reviews, they learn the reputations of their purported peers to be nice, mean or unpredictable. C. while undergoing fMRI, participants enter different classrooms across three 9-minute runs for a total of 24 social interactions for each reputation. Following an inter-trial interval, each interaction includes an anticipation phase in which a typing bubble appears above a peer, an evaluation phase in which the participant receives social evaluation, and a response phase in which they select a response option.
fMRI Acquisition.
After undergoing a mock scanning session to familiarize participants with the fMRI environment and reduce motion, data were acquired on a GE 750 3T-scanner (Waukesha, WI) at the NIH. Each functional run included 231 functional image volumes with 24 contiguous axial slices (in-plane resolution=2.6×2.6 mm) obtained with a T2*-weighted echo-planar sequence (repetition time/echo time ([TR/TE])=2,300/25 ms, flip=50◦; field of view (FOV)=240mm, matrix=96×96). A high-resolution structural scan was acquired (axial plane) with a T1-weighted magnetization-prepared spoiled gradient-recalled echo sequence (echo time/inversion time (TE/TI)=min full/425 ms, flip=7◦; FOV=220mm, matrix=256 ×256, in-plane resolution, 1.2×1.2 mm) for anatomical localization and co-registration of functional data.
Data Analysis
fMRI Analysis.
Preprocessing, individual, and group level analyses were completed with AFNI (Cox, 1996). ROIs were defined as functional clusters that emerged from a previously reported whole brain SR (high, low) x Reputation (nice, mean, unpredictable) repeated measures ANOVA performed on data collected as preadolescents anticipated peer evaluation: bilateral insula (right insula 49, −4, 4; ke = 138; left insula −44, −1, 4; ke = 170) and dACC (−1, −1, 39; ke = 215; Jarcho et al., 2016). Data were extracted from each ROI, and all subsequent analyses were performed in SPSS (IBM SPSS Statistics for Mac, Version 25.0. Armonk, NY: IBM Corp).
Social Anxiety EFA Composite.
A SA composite was created using exploratory factor analysis (EFA) from subscales of the SCARED and SAS. Unlike average-based composites, EFA allows for measured indices to contribute unequally to the composite to best represent the latent SA variable. SA composites for pre- and mid-adolescence were created with the MLR estimator and oblique Geomin rotation in Mplus version 8.1.5 (http://www.statmodel.com/) to extract factor scores for use in subsequent moderated mediation models (Muthén & Muthén, 2010). The MLR estimator was selected because it is better for small sample sizes as it is more robust to outliers, therefore is less influenced by a single participant within smaller sample (Curran, West, & Finch, 1996; L. Hu, Bentler, & Kano, 1992). I n accordance with guidelines from Preacher & Maccallum (2002), studies with smaller sample sizes (e.g. N=50) can be used in EFA if communalities are high (h=.4-.6), model error is low, and few factors are retained. Such considerations maximize interpretability of resulting models by minimizing type I and II errors. Evidence from the scree test, available fit indices, and factor interpretability were used to determine dimensionality. Fit indices used for model evaluation were the Comparative Fit Index (CFI), Tucker-Lewis Index (TLI), root mean squared error of approximation (RMSEA), standardized root mean square residual (SRMR), and chi-square. Given that TLI and RMSEA tend to falsely reject models for small samples (Hu & Bentler, 1999), these indices were given less emphasis when determining model fit. CFI and TLI values of .90–.95 are indicative of acceptable model fit (e.g., Bentler, 1990), particularly when used in tandem with other fit parameters (Hu & Bentler, 1999). SRMR values closer to 0 indicate better model fit. Resulting factors were used in the subsequent analyses.
Moderated Mediation Analysis.
Although ROIs were defined based on dichotomized SR data (Jarcho et al., 2016), continuous values were needed to implement moderated-mediation models. These models, conducted using PROCESS Model 14 (Hayes, 2013), examined effects of SR, SA and neural measures in preadolescence on SA symptoms at mid-adolescence. This approach was chosen to determine if SR-linked neural mechanisms engaged during social interactions predict greater SA in mid-adolescence among those who experience early SA. All models included SR as the predictor (X), neural measures in preadolescence as the mediator (M), SA in preadolescence as the moderator (V) of Y~M, and SA in mid-adolescence as the outcome (Y; Figure 2). IQ and gender were used as covariates as they often relate to SA, however, gender did not account for significant variance. Thus, gender was removed from the models in order to maximize power for analyses with smaller sample sizes. Separate models for each ROI (dACC, bilateral insula) and each contrast (anticipation of unpredictable-vs-nice, unpredictable-vs-mean peer evaluation, and mean-vs-nice evaluation) were analyzed.
Figure 2.
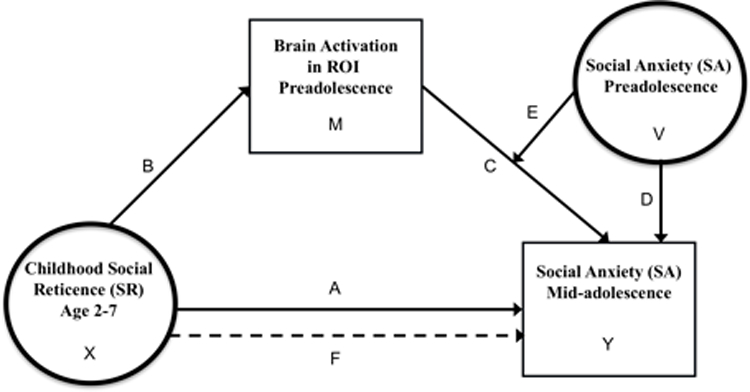
Moderated mediation model showing the relation between early childhood social reticence, brain activation during pre-adolescence, social anxiety during pre-adolescence, and subsequent expression of social anxiety during mid-adolescence. Separate models assessed brain activation in each ROI (bilateral insula and dACC) during the anticipation of social evaluation for each contrast (unpredictable-vs-nice. unpredictable-vs-mean, mean-vs-nice). Letters A through F denote direct or indirect effects reported in Table 2. Paths A: direct effect of SR. Path B: SR to brain, C: direct effect of brain; D: direct effect of preadolescent SA, E: moderation of preadolescent SA on brain to SA mid-adolescence; F: indirect effect of SR on mid-adolescence SA via brain and preadolescent SA. X is the independent variable, M is the mediator, V is the moderator, and Y is the dependent variable.
The direct effect of SR on brain function across conditions was the primary focus of our prior report (Jarcho et al., 2016). Given our prior report used a dichotomous approach to test relations between brain function and SR, to more fully describe the data we provide a depiction of relations between the SR composite (treated as a continuous variable) and brain function in each ROI, separated by gender (see Supplementary Materials).
Results
Social Anxiety EFA Composite
For both pre- and mid-adolescence, a one-factor solution with four indicators (SCARED school avoidance, SCARED social anxiety, SAS fear of negative evaluation, and SAS social avoidance and distress) showed acceptable fit indices (preadolescence: χ2= 25.60, p<0.01, CFI=.93, TLI=.80, RMSEA=0.30, SRMR=.06; mid-adolescence: χ2=11.20, p<0.01, CFI=.94, TLI=.81, RMSEA=0.17, SRMR=.07) and high communalities (preadolescence: h’s>0.44; mid-adolescence: h’s>0.85). Strong CFI model fit indices, high communalities, and single eigenvalue elbow-shape observed on the scree plot suggested a one-factor structure was appropriate. Thus, SA factor scores were extracted to represent SA at each age-point.
Moderated Mediation Analysis
Anticipation of Social Evaluation from Unpredictable-vs-Nice Peers.
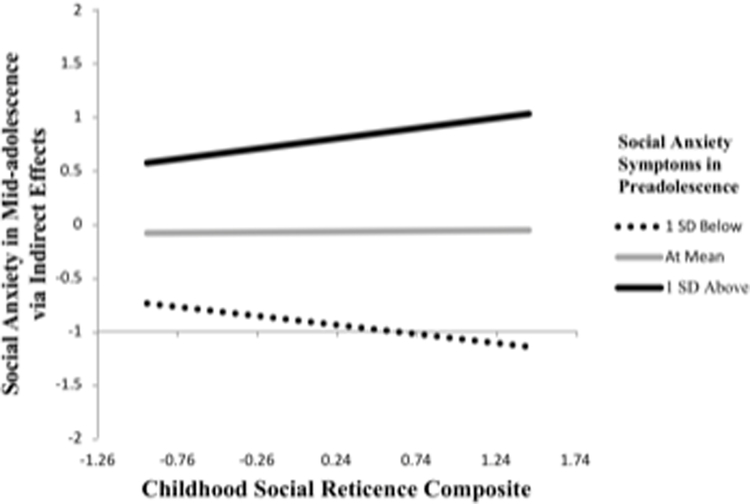
All effects are reported in Table 3. The overall model was significant when right insula activation was treated as a mediator (R2=.31, p=.02). SA in preadolescence moderated the effect of SR on SA in mid-adolescence via right insula activity (B=.22, 95% CI[0.01, 0.53], path F). Specifically, greater SR predicted more severe SA in mid-adolescence when preadolescent SA and brain responses during the anticipation of unpredictable-vs-nice evaluation were elevated (Figure 3). Greater SR was associated with heightened engagement of the right insula (B=.04, p=.02, path B), while more severe SA interacted with heightened engagement to predict mid-adolescent SA (B=5.33, p=.05, path E). The overall model was significant when left insula activation was treated as a mediator (R2=.31, p=.01), and an identical pattern of moderated mediation emerged (B=.25, 95% CI[0.04, 0.56], path F). Although the overall model for dACC was significant (R2=.26, p=.04), there was no evidence of moderated mediation (B=.08, 95% CI[−0.03, 0.40], path F).
Table 3.
| Contrasts | ||||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region of Interest | Path Label | Path Description | Unpredictable-vs-Nice | Unpredictable-vs-Mean | Mean-vs-Nice | |||
| B | p-value/ 95% CI | B | p-value/ 95% CI | B | p-value/ 95% CI | |||
| rINS | A | Direct Effect of SR | −0.19 | 0.40 | −0.10 | 0.64 | −0.22 | 0.32 |
| C | Direct Effect of Brain | 0.65 | 0.80 | −1.41 | 0.54 | −0.12 | 0.97 | |
| D | Direct Effect of Preadolescent SA | 0.28 | 0.15 | 0.44 | 0.02 | 0.33 | 0.11 | |
| B | SR to Brain | 0.04 | 0.02 | 0.02 | 0.13 | 0.02 | 0.14 | |
| E | Moderation of Preadolescent SA on Brain to SA Mid-adolescence |
5.33 | 0.05 | 6.14 | 0.05 | 4.92 | 0.20 | |
| F | Indirect Effect of SR on Mid- adolescence SA via Brain and Preadolescent SA |
0.22 | [.01,0.53] | 0.14 | [0.00,0.51] | 0.09 | [−0.02,0.39] | |
| Overall Model Fit | R2=0.31 | 0.02 | R2=0.30 | 0.02 | R2=0.10 | 0.13 | ||
| lINS | A | Direct Effect of SR | −0.14 | 0.53 | −0.09 | 0.68 | 0.22 | 0.43 |
| C | Direct Effect of Brain | 0.83 | 0.76 | −2.49 | 0.37 | −0.43 | 0.89 | |
| D | Direct Effect of Preadolescent SA | 0.32 | 0.09 | 0.18 | 0.02 | 0.40 | 0.03 | |
| B | SR to Brain | 0.04 | <0.001 | 0.02 | 0.18 | 0.02 | 0.04 | |
| E | Moderation of Preadolescent SA on Brain to SA Mid-adolescence |
6.35 | 0.03 | 5.61 | 0.13 | 6.37 | 0.08 | |
| F | Indirect Effect of SR on Mid- adolescence SA via Brain and Preadolescent SA |
0.25 | [0.04,0.56] | 0.10 | [−0.02,0.42] | 0.14 | [−0.02,0.44] | |
| Overall Model Fit | R2=0.31 | 0.01 | R2=0.26 | 0.04 | R2=0.39 | 0.03 | ||
| dACC | A | Direct Effect of SR | −0.24 | 0.27 | −0.16 | 0.48 | −0.25 | 0.27 |
| C | Direct Effect of Brain | 0.30 | 0.90 | −0.30 | 0.99 | −1.17 | 0.66 | |
| D | Direct Effect of Preadolescent SA | 0.34 | 0.08 | 0.49 | 0.02 | 0.40 | 0.04 | |
| B | SR to Brain | 0.02 | 0.22 | 0.01 | 0.30 | <0.01 | 0.71 | |
| E | Moderation of Preadolescent SA on Brain to SA Mid-adolescence |
5.61 | 0.13 | 4.50 | 0.20 | 2.62 | 0.35 | |
| F | Indirect Effect of SR on Mid- adolescence SA via Brain and Preadolescent SA |
0.08 | [−0.03,0.40] | 0.07 | [−0.04,0.37] | 0.01 | [−0.05,0.19] | |
| Overall Model Fit | R2=0.26 | 0.04 | R2=0.24 | 0.07 | R2=0.47 | 0.09 | ||
Moderated-Mediation effects for each ROI and contrast. Path labels correspond to those depicted in Figure 1. SR = composite social reticence; SA =composite social anxiety symptoms; rINS = right insula; lINS = left insula; dACC = dorsal anterior cingulate cortex. Given that an individual has high social anxiety in preadolescence, the B coefficient is associated with some predicted change in social anxiety symptoms at follow-up. Contrasts reflect percent signal change in ROIs while anticipating distinct types of peer evaluation.
Figure 3.
Higher scores on the x axis indicate greater childhood SR. Higher scores on they y axis indicate more serve SA in mid-adolescence. The relation between SR and SA in mid-adolescence via percent signal change in the right insula are depicted above. The three lines indicate when SA in preadolescence are elevated (I SD above), average (at mean), or below average (I SD below).
Anticipation of Social Evaluation from Unpredictable-vs-Mean Peers.
Although overall models were significant for right insula (R2=0.30, p=0.02), left insula (R2=0.26, p=0.04), and at trend level for dACC (R2=0.24, p=0.07), moderated mediation effects did not emerge for any ROI.
Anticipation of Social Evaluation from Mean-vs-Nice Peers.
Although overall models were significant for right insula (R2=0.26, p=0.005), left insula (R2= 0.27, p=0.01), and approached significance for dACC (R2=0.17 p=0.054), moderated mediation effects did not emerge for any ROI.
Discussion
Our results suggest that youth with childhood SR who have greater preadolescent SA and insula hyperactivation in socially evocative contexts are more likely to exhibit severe SA in mid-adolescence. Thus, integrating brain-based measures may help explain which at-risk youth are likely to have more severe or persistent SA and may benefit most from intervention. Given the relatively limited sample size, results must be interpreted tentatively. However, because this is the first fMRI report we are aware of to demonstrate that brain function plays a role in predicting the development of SA symptoms in at risk youth, even tentatively interpreted results make an important contribution to our understanding of risk for SA.
SR, preadolescent SA symptoms, and brain function predict SA in mid-adolescence
Consistent with our predictions and previous findings (Jarcho et al., 2016), greater childhood SR was associated with increased activation in the bilateral insula while anticipating unpredictable-vs-nice peer evaluation in preadolescence, even after controlling for SA in preadolescence. Additionally, more severe SA and greater activation in bilateral insula while anticipating unpredictable-vs-nice peer evaluation in preadolescence predicted more severe SA in mid-adolescence. These findings are consistent with work showing greater childhood SR (Chronis-Tuscano et al., 2009; J. Clauss & Blackford, 2012; Fox & Pine, 2012), early and severe SA (Beesdo-Baum et al., 2012), and hyperactivation in the insula in adulthood (Boehme et al., 2013; Etkin & Wager, 2007; Klumpp, Angstadt, & Phan, 2012) relate to SA. In the present study, results were specific to anticipating unpredictable-vs-nice peer evaluation. We speculate that this relation emerged for two reasons. First, unpredictable social contexts may be particularly salient for those with higher levels of SR and SA symptoms. A primary characteristic of SA is a prospective fear of encounters that have the potential for negative social outcomes (DSM-5; American Psychiatric Association, 2013). It is noteworthy that in the present paradigm, an unpredictable outcome may be a more potent anxiogenic condition than one that is predictably negative. Uncertainty during anticipation has been highlighted as an anxiety provoking context (Grupe & Nitschke, 2013; Williams et al., 2015), which may relate to aberrant patterns of social learning observed in social anxiety (Jarcho et al., 2015) and represent an important risk factor of SR. Thus, the brain’s propensity to respond to unpredictable social contexts during preadolescence may set the stage for greater expression of this key symptom in mid-adolescence. However, like unpredictable social contexts, predictably negative social contexts may remain relatively salient for pre-adolescents with higher levels of SR and SA. Thus, relations with mid-adolescent SA symptoms may be more difficult to detect when contrasting brain function engaged by anticipating unpredictable- or predictably positive-vs-negative social evaluation. Because of the potency of uncertainty, we believe the most provocative anticipatory context is uncertainty followed by certain negative outcomes, and lastly by predictably positive outcomes. Indeed, our initial report on preadolescent data demonstrate the largest effects of SR on anticipatory brain function in the unpredictable-vs-predictably positive condition. A relatively blunted neural response to anticipating predictably positive social evaluation is consistent with a blunted affective response to positive experiences in those with more severe SA symptoms (Eisner, Johnson, & Carver, 2009; Kashdan, 2007; Kashdan & Steger, 2006). Together, this suggests the anticipation of unpredictable-vs-predictably positive peer evaluation may provide the most meaningful difference in psychosocial contexts in relation to SR and SA. Finally, unlike previous findings, the association between dACC activation and SR (see Jarcho et al., 2016, Figure 3 and individual-level data in Supplementary Figure, plots 2 & 8) were not significant in any contrast. This may be partially due to the fact that our prior analyses did not control for preadolescent SA. However, given the small sample size, caution should be used in interpreting this null result.
This study is the first to examine the role of contextually-relevant SR-linked neural mechanisms in the continuation of longitudinally assessed SA in at-risk youth. Our results suggest that more serve or persistent SA is best predicted by a confluence of three factors: early SR, early SA, and specific brain response patterns when anticipating unpredictable peers. Elevated preadolescent SA symptoms, in the absence of SR-linked alterations in neural responses to social situations, may not predict greater or persistent SA in mid-adolescence. Thus, early SR may be primarily associated with mid-adolescent SA when dysregulated neural responses to social situations occur in the presence of preadolescent SA symptoms. These results are consistent with prior work demonstrating that greater childhood SR and insula dysregulation are associated with more severe concurrent SA symptoms (Hardee et al., 2013b; Taber-Thomas et al., 2017).
Our results are novel in that we demonstrate that the social withdrawal that characterizes individuals with greater childhood SR, may be linked to alterations in the brain that promote anxiety-prone thinking. When this pattern of neural engagement occurs in conjunction with preadolescent SA, SA may be more likely to persist into mid-adolescence. One possible explanation for this mechanism is that children with greater SR, whose withdrawal from social situations result in fewer opportunities for social interaction, develop anxiety sensitivity in social contexts (Reiss, Peterson, Gursky, & McNally, 1986). Increased anxiety sensitivity is associated with hypersensitive emotion processing and monitoring of internal sensations, both processes associated with insula activation (Paulus & Stein, 2006). Therefore, heightened insula engagement during unpredictable social interactions may reflect increased emotional sensitivity and heightened monitoring of internal sensations in children with SR that perpetuate SA. Such dysregulated response to unpredictable social interaction may capture neural mechanisms of SA that could cement neural circuitry and SA expression in later adolescence. These results are also consistent with studies that demonstrate childhood SR generates lasting ‘scars’ that imprint on neural circuitry to confer risk for later anxiety symptoms (dlPFC: Fu et al., 2017; amygdala: Guyer et al., 2014; striatum: Pérez-Edgar et al., 2007). This is in line with theories that suggest early social behavior can influence the development of neural circuits, which then shape social behavior later in life (Jarcho & Guyer, 2018; Nelson, Jarcho, & Guyer, 2016).
Limitations
The current study has several limitations. Although data were collected at multiple points across development (i.e., 2–7, 11, and 13 years), neuroimaging data were only obtained during preadolescence. Thus, we are unable to determine whether differences in insula activation was sustained during mid-adolescence and if sustained activity would be most predictive of SA severity. Additionally, teasing apart whether differences in insula activation precede childhood SR or are a result or ‘scar’ from childhood SR cannot be assessed. We did not find that insula activation while anticipating unpredictable-vs-mean peer evaluation in conjunction with other measures predicted SA in mid-adolescence. This may be due to the small sample size of the current study, reflecting a type II error. An alternative explanation may be that anticipating unpredictable and mean peers may be too contextually similar, and therefore a less meaningful contrast to compare. Indeed, children with high childhood SR not only have a high intolerance for uncertainty (Coplan, Rubin, Fox, Calkins, & Stewart, 1994; Fox et al., 1995), but also are more reactive to predictably aversive feedback (Kambouropoulos & Staiger, 2004). Another explanation is that the absence of effects may reflect a true null result. However, given that greater statistical power is needed to conclusively interpret a null result (Rossi, 1990), this cannot be determined in the current sample (see Supplemental Material). Moreover, it is also possible that our positive results may reflect type I errors, due to a relatively small sample size. Therefore, replication of these analyses in future studies would be useful for interpretations of positive and null results.
Despite the small sample size, various statistical methodologies were employed to improve results’ robustness to low power and non-normality and decrease type I & II errors. One strategy was careful experimental design that increased task effects by enhancing the social evocativeness of the task. Specifically, we utilized a within subject design for assessing brain function during the Virtual School, which compared to between subjects designs, has greater power to detect effects across conditions by better estimating error. We also removed covariates that did not contribute to significant variability to reduce degrees of freedom thereby optimizing power. Additionally, we used a MLR estimator that improves robustness to non-normality to decrease the chance of any highly variable participant within a small sample from skewing results. We also selected model-fit parameters that are less biased by smaller sample sizes (such as the CFI and SRMR, whereas TLI and RMSEA tend to falsely reject models for small samples) to evaluate EFA models. While none of these methods substitute for the power derived from more participants, together they address several potential concerns raised by studying a sample that is moderate in size. However, we believe the unique longitudinal sample combining subjective report, behavioral observation, and fMRI-based data to predict expression of SA symptoms in mid-adolescence provides valuable contributions to the field that outweigh the potential risk for type I and II errors. Additionally, given this is a community sample, results may generalize to larger samples. Nevertheless, future studies in larger samples are needed to replicate these findings.
Finally, in contrast to our previous report (Jarcho et al., 2016), we examined SR dimensionally. Utilizing continuous measures often add to the challenge of interpreting complex interactions in neuroimaging data. However, a continuous approach is more sensitive to detecting nuanced relations (Irwin & McClelland, 2003; Rucker, McShane, & Preacher, 2015; Selvin, 1987), and was required to perform moderated mediation analyses carried out in the present report. From a conceptual perspective, such an approach is consistent with shifts toward using dimensions rather than categories in the study of mental health (Insel et al., 2010). The expression of SR, like the expression anxiety symptom severity, may be better understood using this dimensional framework. We quantified SR as a composite of both maternal report and behavioral observation, which is useful for understanding the construct of SR across contexts. However, there was a relatively low correlation between maternal report and behavioral observation measures. Thus, future studies could benefit from independent examination of maternal report and observational measures in the study of SR.
Clinical Implications and Future Directions
These data highlight the importance of concurrent heightened engagement in the insula during socially evocative situations and early SA in those with childhood SR for predicting more severe or persistent SA. We provide novel evidence for neural mechanisms by which SR develops into SA throughout development. This may help identify the children with high SR that may benefit most from targeted SA interventions rather than other interventions that prevent different psychological disorders for which they are also at risk. Our results stress the need for early identification and intervention for individuals who are likely to experience persistent SA and may develop neural ‘scars’ prior to the onset of more severe SA. Future studies should examine the sensitivity and specificity of hyperactivation of the insula during socially evocative tasks in individuals at risk for SA disorder compared to other disorders. Further studies can examine the role that dysregulation in the insula has on producing SA. Specifically, hyperactivation in the insula could lead to hypersensitivity in interoception, preventing accurate determination of threat or impairing executive functioning skills during social interactions (Paulus & Stein, 2006). Further understanding of such relations could easily be incorporated into psychological assessments and potentially provide a measure of risk that is easily measured in an office setting.
Supplementary Material
Acknowledgments
Source of Funding:
Johanna Jarcho, Ph.D. was generously supported by Stony Brook University and the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD) Young Investigator Award: Ellen Schapiro & Gerald Axelbaum Investigator during the preparation of this manuscript. Daniel S. Pine, Ph.D. was supported by the Intramural Research Program at the National Institute of Mental Health. Nathan A. Fox was supported by grants from the National Institute of Child Health and Human Development (R37HD17899) and National Institute of Mental Health (R01MH093349).
Footnotes
Data availability statement
The data used in the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
None of the authors have any conflicts of interest to disclose.
Contributor Information
Tessa Clarkson, Department of Psychology, Temple University, Philadelphia, PA
Nicholas R. Eaton, Department of Psychology, Stony Brook University, Stony Brook, NY
Eric E. Nelson, Center for Biobehavioral Health, Nationwide Children’s Hospital, Columbus, Ohio Department of Pediatrics, Ohio State University
Nathan A. Fox, Department of Human Development and Quantitative Methodology, University of Maryland
Ellen Leibenluft, Emotion and Development Branch, National Institute of Mental Health
Daniel S. Pine, Emotion and Development Branch, National Institute of Mental Health
Adina C. Heckelman, Mailman School of Public Health, Columbia University, New York, NY
Stefanie L. Sequeira, Dietrich School of Arts & Sciences, University of Pittsburgh
Johanna Jarcho, Department of Psychology, Temple University, Philadelphia, PA
References
- American Psychiatric Association., A. A., & American Psychiatric Association. DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders: DSM-5 : American Psychiatric Publishing; 10.1016/B978-1-4377-2242-0.00016-X [DOI] [Google Scholar]
- Andreescu C, Butters M, Lenze EJ, Venkatraman VK, Nable M, Reynolds CF, & Aizenstein HJ (2009). fMRI activation in late-life anxious depression: A potential biomarker. International Journal of Geriatric Psychiatry, 24(8), 820–828. 10.1002/gps.2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, Perez-Edgar K, Pine DS, & Ernst M: Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci 2009, 20(8):1009–1018.50 10.1111/j.1467-9280.2009.02401.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo-Baum K, Knappe S, Fehm L, Höfler M, Lieb R, Hofmann SG, & Wittchen HU (2012). The natural course of social anxiety disorder among adolescents and young adults. Acta Psychiatrica Scandinavica, 126(6), 411–425. 10.1111/j.1600-0447.2012.01886.x [DOI] [PubMed] [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural equation modeling. Psychology Bulletin, 107(2), 238–246. [DOI] [PubMed] [Google Scholar]
- Berkman ET, & Falk EB (2013). Beyond Brain Mapping: Using Neural Measures to Predict Real-World Outcomes. Current Directions in Psychological Science, 22(1), 45–50. 10.1177/0963721412469394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, & Baugher M (1999). Psychometric properties of the screen for child anxiety related emotional disorders (SCARED): A replication study. Journal of the American Academy of Child and Adolescent Psychiatry, 38(10), 1230–1236. 10.1097/00004583-199910000-00011 [DOI] [PubMed] [Google Scholar]
- Blackford JU, Allen AH, Cowan RL, & Avery SN (2013). Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience, 8(2), 143–150. 10.1093/scan/nsr078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, & Zald DH (2011). Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Social Cognitive and Affective Neuroscience, 6(5), 621–629. 10.1093/scan/nsq073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, Ng P, Hollon N, Jones M, Blair RJR, & Pine DS (2008). Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry, 65(10), 1176–1184. 10.1001/archpsyc.65.10.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ (2008). The social brain in adolescence. Nature Reviews Neuroscience 10.1038/nrn2353 [DOI] [PubMed]
- Boehme S, Ritter V, Tefikow S, Stangier U, Strauss B, Miltner WHR, & Straube T (2013). Brain activation during anticipatory anxiety in social anxiety disorder. Social Cognitive and Affective Neuroscience, 9(9), 1413–1418. 10.1093/scan/nst129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelen PA, & Reijntjes A (2009). Intolerance of uncertainty and social anxiety. Journal of Anxiety Disorders, 23(1), 130–135. 10.1016/j.janxdis.2008.04.007 [DOI] [PubMed] [Google Scholar]
- Bruce SE, Yonkers K. a, Otto MW, & Eisen JL (2005). Influence of Psychiatric Comorbidity on Recovery and Recurrence in Generalize Anxiety Disorder, Social Phobia, and Panic Disorder: A 12-Year prospective Study. The American Journal of Psychiatry, 162(6), 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, Kagan J Pine DS, & Fox NA (2017). A Neurobehavioral Mechanism Linking Behaviorally Inhibited Temperament and Later Adolescent Social Anxiety. Journal of the American Academy of Child and Adolescent Psychiatry, 56(12), 1097–1105. 10.1016/j.jaac.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caouette JD, & Guyer AE (2014). Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Developmental Cognitive Neuroscience, 8, 65–76. 10.1016/j.dcn.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, Raggi VL, & Fox NA (2009). Stable Early Maternal Report of Behavioral Inhibition Predicts Lifetime Social Anxiety Disorder in Adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 48(9), 928–935. 10.1097/CHI.0b013e3181ae09df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, VanDerKlok RM, Rogers BP, Cowan RL, Benningfield MM, & Blackford JU (2014). Neurocircuitry underlying risk and resilience to social anxiety disorder. Depress Anxiety, 31(10), 822–833. 10.1002/da.22265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Benningfield MM, Rao U, & Blackford JU (2016). Altered Prefrontal Cortex Function Marks Heightened Anxiety Risk in Children. Journal of the American Academy of Child and Adolescent Psychiatry, 55(9), 809–816. 10.1016/j.jaac.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Cowan RL, & Blackford JU (2011). Expectation and temperament moderate amygdala and dorsal anterior cingulate cortex responses to fear faces. Cognitive, Affective and Behavioral Neuroscience, 11(1), 13–21. 10.3758/s13415-010-0007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J, & Blackford J (2012). Behavioral Inhibition and Risk for Developing Social Anxiety Disorder: A Meta-Analytic Study. Journal of the American Academy of Child and Adolescent Psychiatry, 51(10), 1–13. 10.1016/j.jaac.2012.08.002.Behavioral [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, & Stewart SL (1994). Being alone, playing alone, and acting alone: distinguishing among reticence and passive and active solitude in young children. Child Development, 65(1), 129–137. [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Curran PJ, West SG, & Finch JF (1996). The Robustness of Test Statistics to Nonnormality and Specification Errors in Confirmatory Factor Analysis. Psychological Methods, 1(1), 16–29. Retrieved from https://pdfs.semanticscholar.org/32a0/3274ac149753d38e2f5ca442577e085f5099.pdf [Google Scholar]
- Degnan KA, Almas AN, Henderson HA, Hane AA, Walker OL, & Fox NA (2014). Longitudinal trajectories of social reticence with unfamiliar peers across early childhood. Developmental Psychology, 50(10), 2311–2323. 10.1037/a0037751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Chandler-Coutts M, Offord DR, King G, McDougall J, Specht J, & Stewart S (2005). Gender differences in the effects of family adversity on the risk of onset of DSM-III-R social phobia. Journal of Anxiety Disorders, 19(5), 479–502. 10.1016/j.janxdis.2004.04.010 [DOI] [PubMed] [Google Scholar]
- Eisner LR, Johnson SL, & Carver CS (2009). Positive affect regulation in anxiety disorders. Journal of Anxiety Disorders, 23(5), 645–649. 10.1016/j.janxdis.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, & Kalin NH (2010). Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. American Journal of Psychiatry, 167(1), 40–46. 10.1176/appi.ajp.2009.07010051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: A meta-ana lysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, & Schmidt LA (2001). Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development, 72(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Fox NA, & Pine DS (2012). Temperament and the emergence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry 10.1016/j.jaac.2011.10.006 [DOI] [PMC free article] [PubMed]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, … Stewart S (1995). Frontal Activation Asymmetry and Social Competence at Four Years of Age Published by: Wiley on behalf of the Society for Research in Child Development Stable URL: http://www.jstor.org/stable/1131909 REFERENCES Linked references are available on JSTOR fo. Child Development, 66(6), 1770–1784. [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JEC, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MHN, Nardi AE, & Crippa JAS (2010a). Neuroimaging in social anxiety disorder: A systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34(4), 565–580. 10.1016/J.PNPBP.2010.02.028 [DOI] [PubMed] [Google Scholar]
- Fu X, Taber-Thomas BC, & Pérez-Edgar K (2017). Frontolimbic functioning during threat-related attention: Relations to early behavioral inhibition and anxiety in children. Biological Psychology, 122, 98–109. 10.1016/j.biopsycho.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecal A. M. La, & Lopezl N (1998). Social Anxiety AInong Adolescents: Relations and Friendships. Journal of Abnonnal Child Psychology, 26(2), 83–94. Retrieved from http://www.as.miami.edu/media/college-of-arts-and-sciences/content-assets/psychology/documents/faculty/publications/lagreca_lopez_1998.pdf [DOI] [PubMed] [Google Scholar]
- Grupe DW, & Nitschke JB (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14(7), 488–501. 10.1038/nrn3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A, Choate V, Detloff A, Benson B, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA, & Ernst M (2012). Striatal function alteration during incentive anticipation in pediatric anxiety disorders. American Journal of Psychiatry, 169, 205–212. 10.1176/appi.ajp.2011.11010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Benson B, Choate VR, Bar-Haim Y, Perez-Edgar K, Jarcho JM, Pine DS, Ernst M, Fox NA, & Nelson EE (2014). Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and Psychopathology, 26(1), 229–243. 10.1017/S0954579413000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane AA, & Fox NA (2006). Natural variations in maternal caregiving of human infants influence stress reactivity. Psychological Science, 17, 550–556. Retrieved from http://journals.sagepub.com/doi/pdf/10.1111/j.1467-9280.2006.01742.x [DOI] [PubMed] [Google Scholar]
- Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, Britton JC, Enrst M, Fox NA, Pine DS, & Pérez-Edgar K (2013a). Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biological Psychiatry, 74(4), 273–279. 10.1016/j.biopsych.2013.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Model Templates for PROCESS for SPSS and SAS. Model Templates for PROCESS for SPSS and SAS, 42(1), 185–227. https://doi.org/http://afhayes.com/public/templates.pdf [Google Scholar]
- Heiser NA, Turner SM, & Beidel DC (2003). Shyness: Relationship to social phobia and other psychiatric disorders. Behaviour Research and Therapy, 41(2), 209–221. 10.1016/S0005-7967(02)00003-7 [DOI] [PubMed] [Google Scholar]
- Helfinstein SM, Benson B, Perez-Edgar K, Bar-Haim Y, Detloff A, Pine DS, Fox NA, & Ernst M (2011). Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia, 49(3), 479–485. 10.1016/j.neuropsychologia.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Pine DS, & Fox NA (2015). Behavioral Inhibition and Developmental Risk: A Dual-Processing Perspective. Neuropsychopharmacology, 40(1), 207–224. 10.1038/npp.2014.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Davis S, Harrington K, & Rosenbaum JF (2007). Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: A five-year follow-up. Journal of Developmental and Behavioral Pediatrics, 28(3), 225–233. 10.1097/01.DBP.0000268559.34463.d0 [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM, & Kano Y (1992). Can test statistics in covariance structure analysis be trusted. Quatitative Methods in Psychology, 112(2), 351–362. 10.1017/CBO9781107415324.004 [DOI] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C & Wang P (2010). Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. American Journal of Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Irwin JR, & McClelland GH (2003). Negative Consequences of Dichotomizing Continuous Predictor Variables. Journal of Marketing Research, 40(3), 366–371. 10.1509/jmkr.40.3.366.19237 [DOI] [Google Scholar]
- Jackson F, Nelson BD, & Proudfit GH (2014). In an Uncertain World, Errors Are More Aversive: Evidence From the Error-Related Negativity. Emotion (Washington, D.C.), 14(5), 6–10. 10.1037/emo0000020 [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Davis MM, Shechner T, Degnan KA, Henderson HA, Stoddard J, Fox NA, Leibenluft E, Pine DS, & Nelson EE (2016). Early-Childhood Social Reticence Predicts Brain Function in Preadolescent Youths During Distinct Forms of Peer Evaluation. Psychological Science, 27(6), 821–835. 10.1177/0956797616638319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Etkin A, Leibenluft E, Shechner T, & Ernst M (2013). The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biological Psychology, 92(2), 306–314. 10.1016/j.biopsycho.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Leibenluft E, Shechner T, Degnan KA, Perez-Edgar K, & Ernst M (2014). Enduring influence of early temperament on neural mechanisms mediating attention-emotion conflict in adults. Depression and Anxiety, 31(1), 53–62. 10.1002/da.22140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Leibenluft E, Walker O, Fox NA, Pine DS, & Nelson EE (2013). Neuroimaging studies of pediatric social anxiety: paradigms, pitfalls and a new direction for investigating the neural mechanisms. Biology of Mood & Anxiety Disorders, 3(1), 14 10.1186/2045-5380-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Romer AL, Shechner T, Galvan A, Guyer AE, Leibenluft E, Pine DS, & Nelson EE (2015). Forgetting the best when predicting the worst: Preliminary observations on neural circuit function in adolescent social anxiety. Developmental Cognitive Neuroscience, 13, 21–31. 10.1016/j.dcn.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambouropoulos N, & Staiger PK (2004). Personality and responses to appetitive and aversive stimuli: the joint influence of behavioural approach and behavioural inhibition systems. Personality and Individual Differences, 37(6), 1153–1165. 10.1016/j.paid.2003.11.019 [DOI] [Google Scholar]
- Kashdan TB (2007). Social anxiety spectrum and diminished positive experiences: Theoretical synthesis and meta-analysis. Clinical Psychology Review 10.1016/j.cpr.2006.12.003 [DOI] [PubMed]
- Kashdan TB, & Steger MF (2006). Expanding the topography of social anxiety an experience-sampling assessment of positive emotions positive events, and emotion suppression. Psychological Science 10.1111/j.1467-9280.2006.01674.x [DOI] [PubMed]
- Katzelnick DJ, Kobak KA, Deleire T, Henk HJ, Greist JH, Davidson JRT, Schneier FR, Stein MB, & Helstad CP (2001). Impact of generalized social anxiety disorder in managed care. American Journal of Psychiatry, 158(12), 1999–2007. 10.1176/appi.ajp.158.12.1999 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry Doi101001archpsyc626617, 62(6 SRC-GoogleScholar FG-0), 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, & Phan KL (2012). Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biological Psychology, 89(1), 273–276. 10.1016/j.biopsycho.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Benson BE, Pine DS, Fox NA, & Ernst M (2018). Neural responses to reward in childhood: Relations to early behavioral inhibition and social anxiety. Social Cognitive and Affective Neuroscience, 13(3), 281–289. 10.1093/scan/nsw122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Pérez-Edgar K, Degnan KA, Guyer AE, Lejuez CW, Ernst M, Pine DS, & Fox NA (2012). Early childhood temperament predicts substance use in young adults. Translational Psychiatry, 2, 157 10.1038/tp.2012.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, & Fox NA (2009). A History of Childhood Behavioral Inhibition and Enhanced Response Monitoring in Adolescence Are Linked to Clinical Anxiety. Biological Psychiatry, 65(5), 445–448. 10.1016/j.biopsych.2008.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, Schmidt H, Mayer B, & Birgit M (1999). The revised version of the screen for child anxiety related emotional disorders (SCARED-R): factor structure in normal children. Personality and Individual Differences Individ Dif, 26, 99–112doi10. [Google Scholar]
- Nelson EE, Jarcho JM, & Guyer AE (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. 10.1016/j.dcn.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, & Pine DS (2005). The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine 10.1017/S0033291704003915 [DOI] [PubMed]
- Paulus MP, & Stein MB (2006). An Insular View of Anxiety. Biological Psychiatry 10.1016/j.biopsych.2006.03.042 [DOI] [PubMed]
- Pérez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, & Fox NA (2010). Attention Biases to Threat and Behavioral Inhibition in Early Childhood Shape Adolescent Social Withdrawal. Emotion, 10(3), 349–357. 10.1037/a0018486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar KE, & Guyer AE (2014). Behavioral Inhibition: Temperament or Prodrome? Current Behavioral Neuroscience Reports, 1(3), 182–190. 10.1007/s40473-014-0019-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Hardee JE, Guyer AE, Benson BE, Nelson EE, Gorodetsky E, Goldman D, Fox NA, Pine DS, & Ernst M (2014). DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Social Cognitive and Affective Neuroscience, 9(4), 445–453. 10.1093/scan/nst001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, McClure EB, Henderson HA, Fox NA, Pine DS, & Ernst M (2007). Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage, 35(4), 1538–1546. 10.1016/j.neuroimage.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Maccallum RC (2002). Exploratory Factor Analysis in Behavior Genetics Research: Factor Recovery with Small Sample Sizes. Behavior Genetics, 32(2). [DOI] [PubMed] [Google Scholar]
- Reilly-Harrington N, & Sachs GS (2006). Psychosocial strategies to improve concordance and adherence in bipolar disorder. The Journal of Clinical Psychiatry, 67(7), 14–19. 10.4088/JCP.0706e04 [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, & McNally RJ (1986). Anxiety sensitivity, anxiety frequency and the predictions of fearfulness. Behaviour Research and Therapy (Vol. 24). 10.1016/0005-7967(86)90143-9 [DOI] [PubMed] [Google Scholar]
- Rossi JS (1990). Statistical power of psychological research: What have we gained in 20 years? Journal of Consulting and Clinical Psychology, 58(5), 646–656. 10.1037/0022-006X.58.5.646 [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, & Fisher P (2001). Investigations of temperament at three to seven years: The children’s behavior questionnaire. Child Development, 72(5), 1394–1408. 10.1111/1467-8624.00355 [DOI] [PubMed] [Google Scholar]
- Rowe DC, & Plomin R (1977). Temperament in Early Childhood. Journal of Personality Assessment, 41(2), 150–156. 10.1207/s15327752jpa4102_5 [DOI] [PubMed] [Google Scholar]
- Rubin KH, Burgess KB, & Hastings PD (2002). Stability and social-behavioral consequences of toddlers’ inhibited temperament and parenting behaviors. Child Development, 73(2), 483–495. 10.1111/1467-8624.00419 [DOI] [PubMed] [Google Scholar]
- Rubin KH, Chen X, Mcdougall P, Bowker A, & Mckinnon J (1995). The Waterloo Longitudinal Project: Predicting internalizing and externalizing problems in adolescence. Development and Psychopathology, 7(4), 751–764. 10.1017/S0954579400006829 [DOI] [Google Scholar]
- Rucker DD, McShane BB, & Preacher KJ (2015). A researcher’s guide to regression, discretization, and median splits of continuous variables. Journal of Consumer Psychology, 25(4), 666–678. 10.1016/j.jcps.2015.04.004 [DOI] [Google Scholar]
- Schwartz CE, Snidman N, & Kagan J (1999). Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry, 38(8), 1008–1015. 10.1097/00004583-199908000-00017 [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, & Rauch SL (2003). Inhibited and uninhibited infants "grown up": adult amygdalar response to novelty. Science (New York, N.Y.), 300(5627), 1952–1953. 10.1126/science.1083703 [DOI] [PubMed] [Google Scholar]
- Selvin S (1987). Two issues concerning the analysis of grouped data. European Journal of Epidemiology, 3(3), 284–287. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3653356 [DOI] [PubMed] [Google Scholar]
- Storch EA, Masia-Warner C, Dent HC, Roberti JW, & Fisher PH (2004). Psychometric evaluation of the Social Anxiety Scale for Adolescents and the Social Phobia and Anxiety Inventory for Children: Construct validity and normative data. Journal of Anxiety Disorders, 18(5), 665–679. 10.1016/j.janxdis.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Taber-Thomas BC, Morales S, Hillary FG, & Pérez-Edgar KE (2017). Altered topography of intrinsic functional connectivity in childhood risk for social anxiety HHS Public Access [DOI] [PMC free article] [PubMed]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience 10.1038/nrn3857 [DOI] [PubMed]
- Williams LE, Oler JA, Fox AS, McFarlin DR, Rogers GM, Jesson MAL, … Kalin NH (2015). Fear of the Unknown: Uncertain Anticipation Reveals Amygdala Alterations in Childhood Anxiety Disorders. Neuropsychopharmacology, 40(6), 1428–1435. 10.1038/npp.2014.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.