Abstract
Purpose
Early detection of risky behaviors involving prescription opioids can assist prescribers in implementing safer prescribing. Patient‐to‐prescriber travel patterns may indicate potential opioid misuse. We introduce doctor hopping, patients bypassing nearby prescribers in favor of more distant ones, as a new spatial estimation of potentially risky behavior, and compare with traditional doctor shopping metrics.
Methods
We examined all filled opioid prescriptions between 2015 and 2016 from the Arkansas Prescription Drug Monitoring Program. We calculated patient‐to‐prescriber travel times and number of prescribers bypassed for each prescription, adjusted for payment method. Opioid recipients traveling further than the nearest urban area and bypassing more prescribers than 99% of other recipients from the same zip code were identified as doctor hoppers. We calculated odds ratios to evaluate how doctor hopping and doctor shopping correspond to high‐risk opioid uses.
Results
Approximately 0.72% of all opioid recipients in Arkansas engaged in doctor hopping two or more times during the study period. Rates of doctor hopping varied spatially but were more common in rural areas. Doctor shopping was more common in urban areas. Both hopping and shopping were significantly associated with higher odds of engaging in high‐risk opioid use. The combination of doctor hopping and doctor shopping metrics can predict high‐risk use better than either metric alone and may allow for earlier detection than doctor shopping alone.
Conclusions
Doctor hopping is positively associated with high‐risk opioid use and is distinct from and complementary to doctor shopping. We recommend Prescription Drug Monitoring Program (PDMP) vendors incorporate similar spatial analyses into their systems.
Keywords: geography, health risk behaviors, opioids, pharmacoepidemiology, prescription drug monitoring program, spatial analysis
KEY POINTS.
Spatial analyses of patient travel patterns offer opportunities for early‐warning indicators of potentially risky opioid use behavior.
We introduce a new indicator called doctor hopping based on patient‐to‐prescriber travel patterns.
Doctor hopping in Arkansas is associated with increased odds of high‐risk opioid use.
Doctor hopping is more common in rural areas, while doctor shopping is more common in urban areas.
We recommend PDMP vendors incorporate spatial analyses into their systems.
1. INTRODUCTION
Between 2015 and 2016, enough opioids were dispensed to Arkansas recipients for every man, woman, and child in the state to take more than 2000 morphine milligram equivalents (MMEs). The Opioid Epidemic is a major health crisis in the United States with over 42 000 deaths related to opioids in 2016 alone.1 In fact, the number of opioid‐related deaths has surpassed fatalities related to motor vehicle accidents2 that were 37 461 in 2016. Prescription opioids play an important role in the ongoing Opioid Epidemic in the United States; the incidence of heroin use is 19 times higher among patients with prior nonmedical opioid use.3 Furthermore, a qualitative study with young, intravenous drug users found that almost all (86%) heroin users had used prescription opioids prior to their heroin use, and in most cases, opioids were obtained from family, friends, or personal prescriptions.4 It is, therefore, imperative that detection of nonmedical use of prescription opioids be identified early.
With prescription claims data, one method of detecting nonmedical use of prescription opioids is identifying doctor shopping behavior, traditionally characterized as patients seeking out multiple prescribers and multiple dispensers in order to obtain larger amounts of opioids.5, 6, 7 Patient‐to‐prescriber travel patterns may also be a useful indicator of potential misuse.8 A national study of opioid doctor shopping behavior found shoppers traveled greater distances than nonshoppers and more often crossed state borders to fill prescriptions.9 A study in Maine found increasing patient‐to‐prescriber travel distance was associated with increased relative odds of a prescriber being subject to severe disciplinary actions by the state medical board.10 Here we introduce doctor hopping behavior, characterized by above average patient‐to‐prescriber travel distances and patients bypassing nearby prescribers in favor of more distant ones. Distinct from doctor shopping, doctor hopping focuses on patient travel patterns, and provides an early‐warning indicator of high‐risk behavior. For a recipient to be identified as engaging in doctor shopping, they would need to have already received opioid prescriptions from several prescribers and pharmacies. In contrast, doctor hopping can be identified after only two prescriptions.
One of the major limitations of using most administrative claims databases is the inability to capture claims that are paid for by cash or by other insurance systems. This limitation can be overcome with the help of a statewide Prescription Drug Monitoring Program (PDMP). PDMPs are databases that contain the records for prescriptions for a controlled substance (primarily opioids, benzodiazepines, eg, Xanax, and stimulants, eg, Ritalin) dispensed from a retail pharmacy for patients within their respective jurisdictions, which in most cases are states. In fact for Arkansas, the legal requirement to submit information to the Arkansas PDMP (AR PDMP) extends to all pharmacies licensed by the Arkansas State Board of Pharmacy, even out‐of‐state mail order pharmacies that ship drugs to Arkansas residents.11 The AR PDMP uses quality checks to ensure the completeness and accuracy of prescription data and complies with standards set by the American Society for Automation in Pharmacy. Unlike payment databases, which are often limited to prescriptions paid for by a particular insurance company, PDMPs include all prescriptions for controlled substances, including those paid for with cash, Medicaid or Medicare. The purpose of PDMPs is to allow prescribers to see what drugs a patient has already been prescribed by other clinicians before writing a new prescription.
Clinicians are constantly looking for ways to turn data into evidence‐based care for their patients. The epidemic of prescription drug abuse afflicts patients in all medical subspecialties. There is evidence that data provided by PDMPs can lead to decreased opioid prescribing and use.12 The AR PDMP seeks to make this information easily accessible to prescribing clinicians. Prescribers using the AR PDMP in 2015 reported access to the program caused positive changes in their prescribing behavior, including a decrease in the number of prescriptions and quantity of dosage, and an increase in patient education and counseling provided.13 In Arkansas, as in many states, prescribers are now required by state law to check the PDMP before writing most new prescriptions for controlled substances. PDMPs also aide pharmacies in detecting forgery and diversion of controlled substances. Besides being a valuable tool for improving the use of controlled substances in health care, PDMPs provide population‐level information about drug use to researchers and public health practitioners. PDMPs can be used to identify inappropriate prescribing trends and inform public health interventions.14 These data and changes in prescribing habits and patient care may allow clinicians to make a meaningful difference in patient prescription drug abuse.
To our knowledge, this is the first study to evaluate the association between patient‐to‐prescriber travel patterns and patients' high‐risk opioid use. We characterize opioid prescribing in the state of Arkansas from 2015 to 2016 using data from the AR PDMP, estimate doctor hopping for opioid prescriptions, compare doctor hopping to doctor shopping, and determine how doctor hopping and doctor shopping are associated with established indicators of high‐risk use. This and other efforts to assist prescribers in implementing safer opioid prescribing could help in combatting the Opioid Epidemic.15, 16, 17
2. METHODS
We obtained data on all filled prescriptions for controlled substances between 2015 and 2016 from the AR PDMP (n = 13 767 084). Prescriptions in the AR PDMP database were merged with the CDC National Drug Code (NDC) Conversion Reference Table for controlled substances (2016 version, www.pdmpassist.org/pdf/Conversion%20Reference%20Table.xlsx) using NDC numbers.18 Those prescriptions that did not merge with the reference table were manually assessed by CJH to determine whether the medication was controlled, if a data error had occurred, or if NDC was missing from the reference table. After manual assessment of these nonmerged medications, only prescriptions for opioids were retained (n = 7 264 003). For each NDC, the CDC reference table contains the morphine milligram conversion factor for each opioid. Using this morphine MME conversion factor, days' supply of the prescription, and the strength of each unit, the MME per day was calculated using the following two equations:
High‐risk opioid use was defined based on Seal et al: (a) high‐dose opioid use (more than or equal to 90 MME per day) and (b) temporal overlap with other centrally acting medications (benzodiazepines and skeletal muscle relaxants [SMR]).19 CDC guidelines urge clinicians to reconsider prescribing more than 90 MME per day, because the risks of such high‐dose prescriptions often outweigh the benefits.15 Temporal overlap with other centrally acting medications has been previously shown to increase the risk of opioid overdose and CDC guidelines recommend such overlap be avoided.15, 20, 21, 22, 23 In our study, temporal overlap in prescriptions was determined according to dispensed days' supply, under the conservative assumption that all prescribed substances were used within the prescribed days' supply. We chose not to include a lag/cushion time to the overlap determination to avoid artificially inflating the number of recipients engaging in high‐risk use. Thus, if a 30‐day supply of opioids was followed by a SMR prescription to the same recipient more than or equal to 31 days later, no overlap was indicated.
To model travel distances from recipient zip codes to prescriber zip codes, an Origin‐Destination Cost Matrix was calculated in a geographic information system (GIS) using road network distances. Origins were all zip code centroids in Arkansas (n = 701) and Destinations included Arkansas zip codes and all zip codes within 200 km of the Arkansas border (n = 2932) to include prescriptions received outside of the state. A distance of 200 km was determined to be sufficient to allow recipients from any zip code in Arkansas to reach an urban area, where more than 90% of prescribers practice.24 We used prescribers present in the PDMP as having written one or more opioid prescriptions in 2015‐2016 to determine the distribution of potential prescribers. We also used the method of payment recorded in the PDMP to determine the distribution of prescribers known to accept each payment method: Commercial Insurance, Private Pay (eg, cash), Medicare, Medicaid, Military Installations and Veterans Affairs (VA), Workers Compensation, and Other. Based on road network travel distances, the number of opioid prescribers (as identified in AR PDMP) per zip code were summed for every zip code closer to the recipient's zip code than the chosen prescriber's zip code. Other prescribers in the destination zip code were not included in the sum. If a recipient received a prescription in their home zip code, the number of prescribers “hopped” was zero. If the prescriber was located more than 200 km from the state of Arkansas, they fell outside the study area, and the calculation was not performed. Results were tabulated for zip codes and for individual recipients. This calculation was performed after adjusting for the method of payment recorded for each prescription by only counting prescribers as hopped if they had written opioid prescriptions for other patients using the same method of payment during the study period. Since not all prescribers accept every method of payment, not all prescribers can be considered available to recipients. Adjusting for method of payment ensures that recipients who must travel long distances just to find a prescriber who accepts their insurance will not be incorrectly identified as voluntarily hopping.
Doctor hopping behavior was defined by three criteria: (a) exceeding a threshold distance of travel from home zip code to prescriber zip code, (b) being in the upper percentile of recipients from the same zip code in terms of the number of prescribers hopped, and (c) meeting the first two criteria two or more times during the study period. Thresholds for these criteria were selected to balance differences in zip code rurality and to reflect real‐world patient travel within the state of Arkansas. Rurality was determined using the Rural‐Urban Commuting Area (RUCA) codes from the USDA Economic Research Service based on classification guidelines from the Washington State Department of Health.25, 26 We used a floating threshold for travel distance that was unique for each zip code by calculating the distance from each zip code to the nearest neighboring urban zip code. Only patient‐to‐prescriber travel exceeding this threshold was considered as potential doctor hopping. For each opioid prescription the number of prescribers hopped was counted, then ranked compared with others from the same zip code. Only those in the top 1% that also exceeded the travel threshold were considered instances of doctor hopping.
Thresholds used for identifying doctor shopping behavior vary greatly between studies but generally include prescriptions from more than one prescriber and/or filled at more than one pharmacy within a given window of time, anywhere from one to 18 months.5, 8, 9, 27 Some use highly restrictive criteria (eg, six or more prescribers and six or more pharmacies within 3 months) that benefit from specificity but likely underestimate the extent of the problem.5 We examined a range of thresholds for doctor shopping behavior, ranging from four or more prescribers and four or more pharmacies within a 90‐day period (4 × 4 × 90), to six or more prescribers and six or more pharmacies within 90 days (6 × 6 × 90). We compared results between different thresholds, as well as when used in combination with the doctor hopping metric.
We calculated odds ratios to determine how engaging in doctor hopping and/or doctor shopping was associated with the likelihood of high‐risk use among opioid recipients, using the Woolf method with Haldane and Anscombe correction for determining 95% confidence intervals.28, 29 Chi‐square tests were used to evaluate associations between recipient covariates and doctor hopping, with an alpha of 0.01. Data management and statistical calculations were performed in SAS 9.3 (SAS Institute Inc., Cary, North Carolina) and R 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
From 2015 to 2016, a total of 7 264 003 opioid prescriptions were reported in the AR PDMP, dispensed to 1 370 272 recipients. After removing prescriptions with missing or invalid zip codes (n = 18 929), veterinary prescriptions (n = 5933), recipients outside Arkansas, and prescribers further than 200 km from Arkansas (n = 122 322), a total of 7 116 819 opioid prescriptions were included in the analysis, dispensed to 1 344 866 recipients for an average of 5.3 opioid prescriptions per recipient. The mean number of prescriptions per recipient at the zip code scale ranged from 1 to 9. Approximately 6.2 billion MMEs were dispensed, with an average of 46.8 MME per day prescribed.
Average distance traveled from recipient zip code to prescriber zip code was 41 km across the state, ranging from a low of 11 km in Little Rock, AR, to a high of 143 km for the small town of Brickeys, AR, located along the Mississippi river in Lee County (see Figure 1). While approximately 93.2% of recipients obtained their prescriptions within the state, in certain zip codes in the southwest corner of the state and one along the eastern border the percentage of opioid prescriptions obtained outside the state was as high as 65%.
Figure 1.
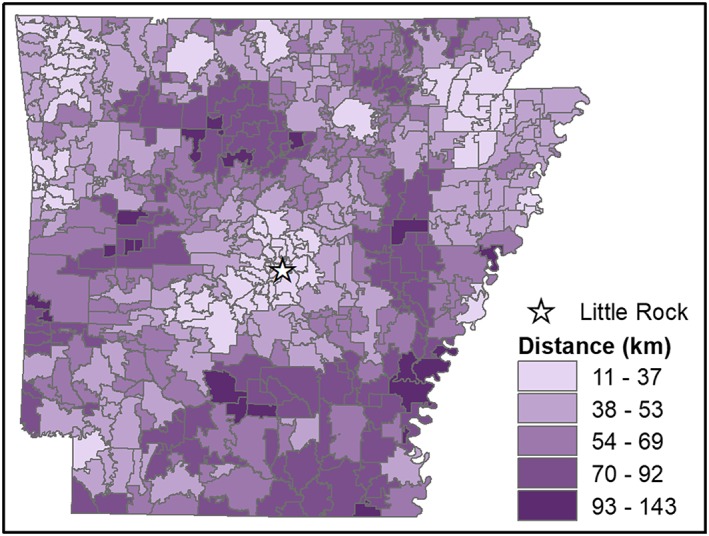
Mean distance (in km) traveled by opioid prescription recipients from each zip code to visit their prescribers [Colour figure can be viewed at wileyonlinelibrary.com]
Across all opioid recipients, approximately 0.72% engaged in doctor hopping two or more times during the study period. Fewer recipients engaged in doctor shopping with 0.39% visiting four or more prescribers and four or more pharmacies in 90 days (4 × 4 × 90), 0.11% visiting five or more prescribers and five or more pharmacies in 90 days (5 × 5 × 90), and 0.04% visiting 6 or more prescribers and six or more pharmacies in 90 days (6 × 6 × 90). The number and proportion of opioid prescription recipients engaging in doctor hopping and/or doctor shopping is shown in Table 1, broken down by recipient covariates. Doctor hopping was more common in rural areas compared with urban zip codes, in contrast with doctor shopping that was slightly higher in urban areas. Recipients traveling out‐of‐state had the highest proportion of doctor hopping at 5.02%, and doctor shopping was consistently higher out‐of‐state than in‐state, ranging from 1.37% (4 × 4 × 90) to 0.18% (6 × 6 × 90). Differences by sex were relatively minor. Younger recipients (age less than or equal to 20) were unlikely to engage in hopping or shopping, with hopping most common between the ages of 51 and 65, and shopping most common between the ages of 36 and 50. Among the four most common payment methods (Commercial Insurance, Private Pay, Medicare, and Medicaid), hopping was most common with Medicare, while shopping was consistently most common with Private Pay. Chi‐square tests indicated significant associations between doctor hopping and rurality, out‐of‐state travel, age, sex, and method of payment (p < .001). Chi‐square tests for doctor shopping varied by the restrictiveness of detection criteria but generally indicated significant associations between doctor shopping and out‐of‐state travel, and method of payment (p < .001).
Table 1.
Recipients engaging in doctor hopping (DH) and/or doctor shopping (DS) by covariate
| DH | DS (4 × 4 × 90) | DH and DS (4 × 4 × 90) | DS (5 × 5 × 90) | DH and DS (5 × 5 × 90) | DS (6 × 6 × 90) | DH and DS (6 × 6 × 90) | All Opioid Recipients | ||
|---|---|---|---|---|---|---|---|---|---|
| Origin | Urban | 4304 (0.67%) | 2724 (0.42%) | 229 (0.04%) | 764 (0.12%) | 89 (0.01%) | 257 (0.04%) | 38 (0.01%) | 645 920 |
| Rural | 5329 (0.76%) | 2561 (0.37%) | 227 (0.03%) | 654 (0.09%) | 80 (0.01%) | 224 (0.03%) | 37 (0.01%) | 698 961 | |
| Travel | In‐state | 5047 (0.4%) | 4033 (0.32%) | 183 (0.01%) | 1015 (0.08%) | 67 (0.01%) | 319 (0.03%) | 27 (<0.01%) | 1 253 485 |
| Out‐of‐state | 4586 (5.02%) | 1252 (1.37%) | 273 (0.30%) | 403 (0.44%) | 102 (0.11%) | 162 (0.18%) | 48 (0.05%) | 91 397 | |
| Sex | Male | 4294 (0.73%) | 2184 (0.37%) | 188 (0.03%) | 590 (0.1%) | 71 (0.01%) | 201 (0.03%) | 34 (0.01%) | 584 885 |
| Female | 5334 (0.7%) | 3097 (0.41%) | 268 (0.04%) | 827 (0.11%) | 98 (0.01%) | 280 (0.04%) | 41 (0.01%) | 758 215 | |
| Age | ≤20 | 96 (0.14%) | 16 (0.02%) | 0 (0%) | 2 (<0.01%) | 0 (0%) | 0 (0%) | 0 (0%) | 69 383 |
| 21‐35 | 1444 (0.49%) | 1310 (0.44%) | 113 (0.04%) | 406 (0.14%) | 49 (0.02%) | 147 (0.05%) | 22 (0.01%) | 296 062 | |
| 36‐50 | 2610 (0.82%) | 2209 (0.7%) | 215 (0.07%) | 642 (0.2%) | 83 (0.03%) | 228 (0.07%) | 36 (0.01%) | 317 629 | |
| 51‐65 | 3278 (0.97%) | 1297 (0.38%) | 104 (0.03%) | 296 (0.09%) | 30 (0.01%) | 93 (0.03%) | 17 (0.01%) | 339 644 | |
| >65 | 2205 (0.68%) | 453 (0.14%) | 24 (0.01%) | 72 (0.02%) | 7 (<0.01%) | 13 (<0.01%) | 0 (0%) | 322 164 | |
| Payment | Commercial insurance | 6984 (0.79%) | 4932 (0.56%) | 430 (0.05%) | 1374 (0.16%) | 164 (0.02%) | 472 (0.05%) | 75 (0.01%) | 878 534 |
| Private pay | 2920 (1.03%) | 3777 (1.33%) | 342 (0.12%) | 1149 (0.4%) | 143 (0.05%) | 411 (0.14%) | 66 (0.02%) | 284 850 | |
| Medicaid | 1515 (0.96%) | 1720 (1.08%) | 161 (0.10%) | 518 (0.33%) | 74 (0.05%) | 170 (0.11%) | 34 (0.02%) | 158 551 | |
| Medicare | 2566 (1.17%) | 1208 (0.55%) | 125 (0.06%) | 302 (0.14%) | 46 (0.02%) | 99 (0.04%) | 21 (0.01%) | 220 184 | |
| Military installations/VA | 505 (1.61%) | 223 (0.71%) | 20 (0.06%) | 49 (0.16%) | 8 (0.03%) | 16 (0.05%) | 3 (0.01%) | 31 364 | |
| Workers compensation | 64 (0.88%) | 69 (0.95%) | 3 (0.04%) | 17 (0.23%) | 2 (0.03%) | 8 (0.11%) | 2 (0.03%) | 7235 | |
| Other | 783 (1.44%) | 824 (1.51%) | 83 (0.15%) | 283 (0.52%) | 37 (0.07%) | 128 (0.23%) | 21 (0.04%) | 54 552 | |
| Total | 9633 (0.72%) | 5285 (0.39%) | 456 (0.03%) | 1418 (0.11%) | 169 (0.01%) | 481 (0.04%) | 75 (0.01%) | 1 344 882 | |
Note. 4 × 4 × 90, 5 × 5 × 90, and 6 × 6 × 90 indicate recipients visited at least four, five, or six prescribers and four, five, or six pharmacies for opioids within 90 d.
We mapped the rate of recipients engaging in doctor hopping per 10 000 people at the zip code scale (see Figure 2). Rates were low in the southwest corner of the state and generally high in the southeast corner. Urban areas tended to have low to moderate rates of doctor hopping, while rural areas ranged from low to high rates.
Figure 2.
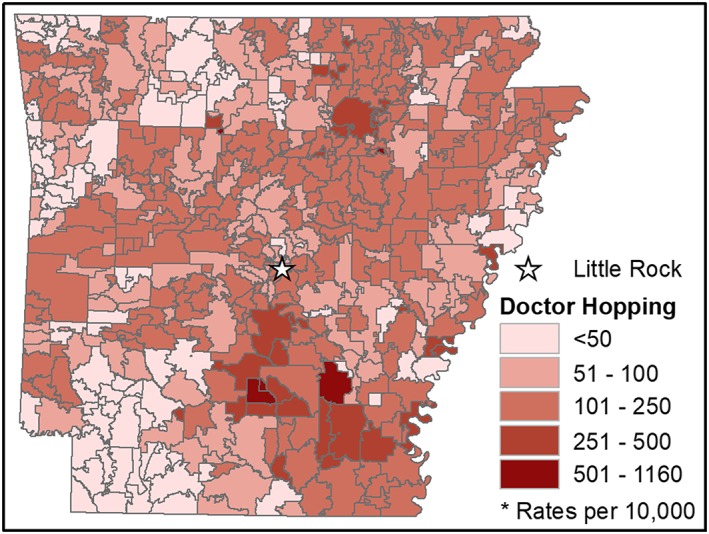
Number of individuals per 10 000 engaging in doctor hopping by zip code [Colour figure can be viewed at wileyonlinelibrary.com]
Nearly a quarter of all recipients (24.4%) exhibited some form of high‐risk use one or more times between 2015 and 2016. Table 2 lists the odds ratio results for patients engaging in doctor hopping, doctor shopping, or both on specific high‐risk uses. Doctor hopping was associated with increased odds of high‐risk use (OR: 3.29) with odds ratios for specific uses ranging from 2.72 to 3.24. Odds ratios for doctor shopping and high‐risk use were much higher, ranging from 11.48 to 19.06. The odds ratios for both doctor hopping and doctor shopping with high‐risk use ranged from 18.55 to 20.33 but in most instances were not significantly different from corresponding odds ratios for doctor shopping alone. Doctor hopping and doctor shopping (4 × 4 × 90) was the exception, with significantly higher odds than shopping (4 × 4 × 90) alone for greater than 90 MMEs per day, overlapping prescriptions for benzodiazepines and opioids, and any high‐risk use.
Table 2.
Odds ratios (with 95% confidence intervals) of doctor hopping (DH) and doctor shopping (DS) and specific high‐risk uses
| >90 Morphine Milligram Equivalents Per Day | Opioid and Benzodiazepine Overlap | Opioid and Skeletal Muscle Relaxant Overlap | Opioid, Benzodiazepine, and Skeletal Muscle Relaxant Overlap | Any High‐Risk Use | |
|---|---|---|---|---|---|
| DH only | 3.12 (2.96‐3.29) | 3.02 (2.90‐3.15) | 2.72 (2.51‐2.93) | 3.24 (2.92‐3.59) | 3.29 (3.16‐3.42) |
| DS (4 × 4 × 90) only | 8.60 (8.13‐9.09) | 8.17 (7.72‐8.65) | 7.18 (6.68‐7.72) | 8.89 (8.13‐9.74) | 11.48 (10.75‐12.26) |
| DH & DS (4 × 4 × 90) | 11.59 (9.65‐13.94) | 11.42 (9.29‐14.05) | 9.00 (7.18‐11.27) | 11.01 (8.35‐14.52) | 18.55 (14.28‐24.10) |
| DS (5 × 5 × 90) only | 10.75 (9.68‐11.94) | 10.10 (9.01‐11.32) | 9.23 (8.13‐10.49) | 10.58 (9.01‐12.42) | 15.96 (13.86‐18.37) |
| DH & DS (5 × 5 × 90) | 13.72 (10.16‐18.54) | 12.29 (8.71‐17.35) | 10.98 (7.74‐15.59) | 14.74 (9.80‐22.18) | 20.33 (13.05‐31.68) |
| DS (6 × 6 × 90) only | 12.45 (10.41‐14.88) | 11.40 (9.32‐13.93) | 10.01 (8.09‐12.39) | 11.61 (8.91‐15.12) | 19.06 (14.74‐24.65) |
| DH & DS (6 × 6 × 90) | 18.14 (11.52‐28.58) | 11.44 (6.89‐18.99) | 8.71 (4.98‐15.21) | 15.02 (8.19‐27.56) | 19.34 (10.08‐37.11) |
Note. 4 × 4 × 90, 5 × 5 × 90, and 6 × 6 × 90 indicate recipients visited at least four, five, or six prescribers and four, five, or six pharmacies for opioids within 90 d.
4. DISCUSSION
Both doctor hopping and doctor shopping were quite variable across the state. This variability is indicative of the importance geographic location plays in obtaining and using opioids. Beyond geographic location alone, doctor hopping was shown to be associated with rurality. While small population sizes in rural areas may make some rates at the zip code scale unreliable, overall trends (shown in Table 1) indicate that doctor hopping is more common in rural areas. Arkansas is a largely rural state with a population of approximately three million people. Several states with high opioid prescribing rates (Alabama, Louisiana, Tennessee, and Oklahoma) are also largely rural. It is imperative that rurality be thoroughly explored as a possible contributor to the opioid epidemic and high levels of opioid prescribing in many rural states. Although recipients from rural zip codes generally must travel farther than recipients from urban zip codes in order to obtain prescriptions, our use of a floating travel distance threshold was designed to ensure rural recipients were not identified as doctor hopping solely due to their rurality.
In addition to urban/rural differences, there appears to be a regional pattern of doctor hopping within Arkansas, with some clustering of low rates in the southwest corner of the state and more diffuse clustering of high rates in the southeast corner, both predominantly rural areas. The low rates in the southwest corner are notable considering the high percentage of opioid recipients from that region who travel to Texas for prescriptions; in some zip codes near the border, nearly two‐thirds of recipients travel out‐of‐state. The low rates of doctor hopping in this area seem to demonstrate that rurality and out‐of‐state travel do not unduly influence the hopping metric. It is unclear if the relatively high rates of doctor hopping in the southeast corner of the state represent a meaningful regional pattern or are the result of smaller populations leading to less stable rate estimates.
The comparison of combined doctor hopping and doctor shopping (4 × 4 × 90) with doctor shopping (4 × 4 × 90) alone in both Tables 1 and 2 is informative. Less than 10% of those flagged as shoppers remain in the group when hopping is included, yet most of the odds ratios are significantly higher. In fact, odds ratios and group size for combined hopping and shopping (4 × 4 × 90) were not significantly different from shopping (6 × 6 × 90) alone. This shows that including doctor hopping allows for less‐restrictive shopping criteria and can therefore be an earlier warning indicator while maintaining efficacy levels comparable to doctor shopping (6 × 6 × 90).
The number of recipients engaging in both doctor hopping and shopping was only about 9% to 16% of those engaging in doctor shopping only, indicating the two metrics capture largely distinct populations of high‐risk opioid recipients (see Table 1). The inclusion of patient travel pattern analyses into risk prediction algorithms offers many interesting opportunities and future directions. Spatial analyses of doctor hopping rates could be linked to demographic information to examine population‐level trends or regional patterns in opioid recipient behavior. Along with predicting opioid recipients who may engage in high‐risk use, doctor hopping could be used to identify opioid prescribers frequented by long‐distance or high‐risk patients, who may need to reevaluate their prescribing behaviors. Chang et al noted that doctor shoppers across multiple states received more opioid prescriptions per person from low‐volume prescribers than high‐volume prescribers, so examining travel patterns may offer insights into prescriber behaviors not captured by traditional methods that focus on volume.30
A limitation of the current study was the lack of specialty information for the prescribers or any diagnosis information for the recipients. For example, patients traveling to specialists, including cancer patients and those receiving opioids for end‐of‐life palliative care could not be evaluated separately from other recipients in the current study. Prescriber specialty information is not included in the data extracts from the AR PDMP vendor and is therefore not available to researchers. Just as not all “doctor shoppers” are engaged in diversion or misuse, many opioid recipients will have legitimate reasons to visit prescribers far from their zip code of residence. Doctor hopping is not intended as confirmation of inappropriate behavior but merely as an indicator of potentially risky behavior. Importantly, these analyses have shown that doctor hopping is positively associated with high‐risk opioid use. High‐risk opioid use has been shown to increase the risk of overdoses from opioids.22 Each of the high‐risk opioid use measures evaluated in this study showed an increased association with doctor hopping, ranging from 272% to 324% higher odds. Therefore, the measure demonstrated here for doctor hopping appears to be a viable instrument for assessing the potential for high‐risk opioid use, particularly when used in combination with doctor shopping metrics. Further, because the addition of doctor hopping to doctor shopping metrics strengthens the association with high‐risk use even with less restrictive shopping criteria, it has the potential to be an early‐warning indicator of risk that does not require as many prior prescriptions before risky behavior is identified. More work needs to be done to determine if doctor hopping, as presently defined, is generalizable to other states. We anticipate minor modifications (such as the distance beyond state boundaries included in the analysis) may be necessary, but the use of floating thresholds and percentiles should be easily transferrable.
5. CONCLUSION
Doctor hopping (defined as both exceeding a threshold distance of travel from home zip code to prescriber zip code and being in the upper percentile of recipients from the same zip code in terms of the number of prescribers hopped) is positively associated with high‐risk opioid use and largely distinct from doctor shopping metrics. Clinicians should be made aware of doctor hopping behavior so they can closely evaluate those patients, preferably before writing additional prescriptions. We recommend PDMP vendors incorporate spatial analyses such as doctor hopping into their systems, in connection with doctor shopping metrics and other automatable algorithms to assist prescribers in implementing safer opioid prescribing.
ETHICS STATEMENT
Study protocol was approved by the Scientific Advisory Committee of the Arkansas Department of Health.
CONFLICT OF INTEREST
The authors declare no competing interests. C.J.H. was supported by the National Institute on Drug Abuse Translational Training in Addiction grant (1T32 DA 022981). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the assistance of the Arkansas Department of Health Scientific Advisory Committee. The views expressed in this paper are not necessarily those of the Arkansas Department of Health. C.J.H. was supported by the National Institute on Drug Abuse Translational Training in Addiction grant (1T32 DA 022981). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Young SG, Hayes CJ, Aram J, Tait MA. Doctor hopping and doctor shopping for prescription opioids associated with increased odds of high‐risk use. Pharmacoepidemiol Drug Saf. 2019;28:1117–1124. 10.1002/pds.4838
The copyright line for this article was changed on 20 August 2019 after original online publication.
REFERENCES
- 1. Centers for Disease Control and Prevention Opioid Overdose|Drug Overdose|CDC Injury Center Available online: https://www.cdc.gov/drugoverdose/index.html (accessed on Jul 2, 2018).
- 2. NHTSA's National Center for Statistics and Analysis 2016 Fatal motor vehicle crashes: overview; National Highway Traffic Safety Administration, US Department of Transportation: Washington, D.C., 2017.
- 3. Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States; Center for Behavioral Health Statistics and Quality, SAMHSA, 2013; p. 17
- 4. Lankenau SE, Teti M, Silva K, Bloom JJ, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simeone R. Doctor shopping behavior and the diversion of prescription opioids. Subst Abuse Res Treat. 2017;11 10.1177/1178221817696077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cepeda MS, Fife D, Chow W, Mastrogiovanni G, Henderson SC. Assessing opioid shopping behaviour: a large cohort study from a medication dispensing database in the US. Drug Saf. 2012;35(4):325‐334. [DOI] [PubMed] [Google Scholar]
- 7. Pradel V, Frauger E, Thirion X, et al. Impact of a prescription monitoring program on doctor‐shopping for high dosage buprenorphine. Pharmacoepidemiol Drug Saf. 2009;18(1):36‐43. [DOI] [PubMed] [Google Scholar]
- 8. Ringwalt C, Schiro S, Shanahan M, et al. The use of a prescription drug monitoring program to develop algorithms to identify providers with unusual prescribing practices for controlled substances. J Prim Prev. 2015;36(5):287‐299. [DOI] [PubMed] [Google Scholar]
- 9. Cepeda MS, Fife D, Yuan Y, Mastrogiovanni G. Distance traveled and frequency of interstate opioid dispensing in opioid shoppers and nonshoppers. J Pain. 2013;14:1158‐1161. [DOI] [PubMed] [Google Scholar]
- 10. Kreiner PW, Strickler GK, Undurraga EA, Torres ME, Nikitin RV, Rogers A. Validation of prescriber risk indicators obtained from prescription drug monitoring program data. Drug Alcohol Depend. 2017;173:S31‐S38. [DOI] [PubMed] [Google Scholar]
- 11. Malone P, Summers T. An act to establish a prescription drug monitoring program; and for other purposes; 2011.
- 12. Rutkow L, Chang H‐Y, Daubresse M, Webster DW, Stuart EA, Alexander GC. Effect of Florida's Prescription Drug Monitoring Program and pill mill laws on opioid prescribing and use. JAMA Intern Med. 2015;175(10):1642. [DOI] [PubMed] [Google Scholar]
- 13. Rittenhouse R, Wei F, Robertson D, Ryan K. Utilization of the Arkansas Prescription Monitoring Program to combat prescription drug abuse. Prev Med Rep. 2015;2:524‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for disease control and prevention what states need to know about PDMPs Available online: https://www.cdc.gov/drugoverdose/pdmp/states.html (accessed on Jul 31, 2018).
- 15. Dowell D, Haegerich TM, Chou R. CDC guidelines for prescribing opioids for chronic pain—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1‐49. [DOI] [PubMed] [Google Scholar]
- 16. O'Donnell JK, Gladden RM, Seth P. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census regions—United States, 2006‐2015. MMWR Morb Mortal Wkly Rep. 2017;66:897‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Canan C, Polinski JM, Alexander GC, Kowal MK, Brennan TA, Shrank WH. Automatable algorithms to identify nonmedical opioid use using electronic data: a systematic review. J Am Med Inform Assoc. 2017;24(6):1204‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Kane N, Hallvik SE, Marino M, et al. Preparing a prescription drug monitoring program data set for research purposes. Pharmacoepidemiol Drug Saf. 2016;25(9):993‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high‐risk opioid use in us veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940‐947. [DOI] [PubMed] [Google Scholar]
- 20. Hernandez I, He M, Brooks MM, Zhang Y. Exposure‐response association between concurrent opioid and benzodiazepine use and risk of opioid‐related overdose in Medicare part D beneficiaries. JAMA Netw Open. 2018;1(2):e180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gressler LE, Martin BC, Hudson TJ, Painter JT. The relationship between concomitant benzodiazepine‐opioid use and adverse outcomes among U.S. veterans. Pain. 2018;159:451‐459. [DOI] [PubMed] [Google Scholar]
- 22. Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garg RK, Fulton‐Kehoe D, Franklin GM. Patterns of opioid use and risk of opioid overdose death among Medicaid patients. Med Care. 2017;55(7):661‐668. [DOI] [PubMed] [Google Scholar]
- 24. Rosenblatt RA, Andrilla CHA, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13:23‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Washington State Department of Health Guidelines for using rural‐urban classification systems for community health assessment 2016.
- 26. Rural‐urban commuting area codes Available online: https://www.ers.usda.gov/data‐products/rural‐urban‐commuting‐area‐codes.aspx (accessed on Jun 21, 2018).
- 27. Walker AM, Weatherby LB, Cepeda MS, Bradford D, Yuan Y. Possible opioid shopping and its correlates. Clin J Pain. 2017;33(11):976‐982. [DOI] [PubMed] [Google Scholar]
- 28. Lawson R. Small sample confidence intervals for the odds ratio. Commun Stat Simul Comput. 2004;33(4):1095‐1113. [Google Scholar]
- 29. Ruxton GD, Neuhäuser M. Review of alternative approaches to calculation of a confidence interval for the odds ratio of a 2 × 2 contingency table. Methods Ecol Evol. 2013;4(1):9‐13. [Google Scholar]
- 30. Chang H‐Y, Murimi IB, Jones CM, Alexander GC. Relationship between high‐risk patients receiving prescription opioids and high‐volume opioid prescribers. Addiction. 2018;113(4):677‐686. [DOI] [PubMed] [Google Scholar]


