SUMMARY
Hematopoietic cell transplantation can correct hematological and immunological disorders by replacing a diseased blood system with a healthy one, but currently requires depleting a patient’s existing hematopoietic system with toxic and non-specific chemotherapy and/or radiation. Here we report an antibody-based conditioning protocol, with reduced toxicity and enhanced specificity, for robust hematopoietic stem cell (HSC) transplantation and engraftment in recipient mice. Host pre-treatment with six monoclonal antibodies targeting CD47, T cells, NK cells, and HSCs followed by donor HSC transplantation enabled stable hematopoietic system reconstitution in recipients with mismatches at half (haploidentical) or all major histocompatibility complex (MHC) genes. This approach allowed tolerance of heart tissue from HSC donor strains in haploidentical recipients, showing potential applications for solid organ transplantation without immune suppression. Fully mismatched chimeric mice developed antibody responses to nominal antigens, showing preserved functional immunity. These findings suggest approaches for transplanting immunologically-mismatched HSCs and solid organs, with limited toxicity.
Graphical Abstract
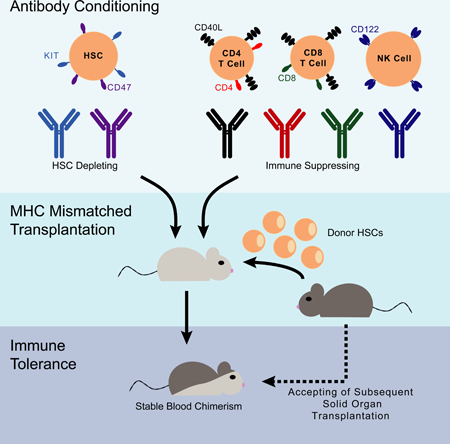
eTOC:
Treatment of hematological disorders via donor cell transplantation requires harsh and non-specific conditioning protocols. George et al. report an antibody-based conditioning protocol for HSC engraftment which does not require chemotherapy or irradiation, and allows robust hematopoietic reconstitution even with fully mismatched MHC donor cells.
INTRODUCTION
Hematopoietic stem cells (HSCs) can self-renew and give rise to all blood cell lineages when transplanted into a recipient (Spangrude et al., 1988, Baum et al., 1992; Uchida et al., 1998, Majeti et al., 2007, Müller et al., 2012). For these reasons, hematopoietic cell transplantation (HCT) can be used to replace an individual’s diseased blood and immune system. While HCT is most commonly performed to treat malignancies, it can be a curative approach for other disorders, such as thalassemia, sickle cell anemia, inherited immunodeficiencies, autoimmune diseases, and metabolic storage disorders (Lucarelli et al., 1990, Hoogerbrugge et al., 1995, Weissman, 2000, Neven et al., 2009, Bolaños-Meade et al., 2012, Ly et al., 2017). HCT can also induce immunological tolerance wherein tissues from an HSC donor can be transplanted without rejection (Billingham et al., 1953, Weissman, 1967, Weissman, 1973, Gandy and Weissman, 1998). Therefore, HCT can facilitate transplantation of immunologically-mismatched organs without the need for lifelong immune suppression, which is associated with the development of malignancy, disordered hematopoiesis, and life-threatening infection (Engels et al., 2011). However, despite the seemingly diverse applicability of HCT, a lack of suitable donors and the toxicities associated with its conventional administration limit its use. Addressing these barriers could allow practitioners to use HCT much more widely in clinical practice and extend its reach into regenerative medicine.
In most transplant situations, donors and recipients are immunologically matched for the major histocompatibility complex (MHC) genes, as they govern rejection of foreign cells (Bix et al., 1991). However, MHC matching of siblings occurs in only 25% of cases, contributing to why many patients do not have a match. Haploidentical transplantation, where donors are matched at half of the MHC loci, is becoming more common but is limited by increased rejection, often requiring high-dose immune suppression to sustain donor grafts (Beatty et al., 1985). If it were possible to perform haploidentical transplantation with limited toxicity and consistent engraftment, this would significantly expand the availability of donors, theoretically allowing any individual to receive HCT from their parent, child, or half of their siblings. Beyond this, the ability to form mixed donor-host chimeras (Sachs, Kawai and Sykes, 2014) without MHC matching would enable nearly universal application of HSC transplants and donor specific organ transplant tolerance.
To perform HCT, a recipient’s blood system is ablated through a process known as conditioning, which provides both immune suppression and makes HSC niches available for donor cell engraftment. Currently, HCT conditioning requires chemotherapy and/or radiation, which can induce life-threatening side effects, such as a period of profound immune suppression during which the patient is at risk of severe infection, irreversible organ toxicity, veno-occlusive disease, mucositis, and secondary malignancy (Michel et al., 1997, Hartman et al., 1998). Therefore, HCT is used to predominantly treat hematologic malignancies (Passweg et al., 2017), where the benefits of HCT outweigh the associated, potentially fatal, risks. Due to the non-specific nature of conventional conditioning regimens, the safety and risk-benefit ratio of HCT for non-malignant diseases could be considerably improved if more specific agents, such as monoclonal antibodies, could be utilized for conditioning. Various studies and clinical protocols have explored the use of antibodies to condition patients for HCT (Cobbold et al., 1986, Sharabi et al., 1989, Nikolic et al., 2000, Spitzer et al., 2003, Czechowicz et al., 2007, Straathof et al., 2009, Worth et al., 2013, Racine et al., 2014, Chhabra et al., 2016). However, these studies still required the use of chemotherapy/radiation or were limited to MHC matched combinations.
In response to these two major barriers, here we report a strategy to safely engraft MHC-mismatched HSCs without the use of chemotherapy/radiation into immune-competent recipient mice. In our previous work, we showed that antibody-mediated depletion of host HSCs and T cells could facilitate transplantation of HSCs mismatched for minor histocompatibility antigens (Chhabra et al., 2016). However, the transplantation of MHC mismatched tissues is much more difficult than in MHC matched situations, partly due to the higher frequency of anti-MHC T cells than minor histocompatibility reactive T cells, plus the activity of alloreactive NK cells (Lindhal and Wilson, 1977, Karre et al., 1986). Therefore, to enable major histocompatibility-mismatched HSC engraftment, we hypothesized that blocking host T cell activation and depleting NK cells would be necessary. We demonstrate that conditioning using six monoclonal antibodies enables mice to receive partially (haploidentical) or fully MHC-mismatched HSCs, thus permitting blood system replacement and induction of tolerance to mismatched donor organs without dependence on chemotherapy or radiation.
RESULTS
We reasoned that expansion of previously demonstrated antibody-conditioning regimens to deplete critical immune subsets would enable more effective HSC transplantation. To deplete HSCs we chose an antibody targeting KIT (anti-CD117), a transmembrane protein expressed on HSCs and hematopoietic progenitor cells, and anti-CD47 which blocks a dominant anti-phagocytic signal. This combination results in macrophage assisted depletion of HSCs (Chhabra et al., 2016). To eliminate host NK cells we used a depleting antibody targeting CD122/Il2Rβ (Tanaka et al. 1993, Seung et al., 2003), which is expressed throughout human and mouse NK cell development (Fathman et al., 2011). To further inhibit T cell mediated rejection we used an antibody that blocks the CD40-CD40L axis; CD40L is a co-stimulatory cell surface molecule expressed by activated T cells whose blockade has been shown to aid in tolerance induction (Lederman et al., 1992, Noelle et al., 1992, Markees et al., 1997, Durham et al., 2000, Wekerle et al., 2000). We tested whether combinations of antibodies to these targets (herein referred to as 4Ab; Fig 1a), would open HSC niches and provide immunosuppression for donor haploidentical or full MHC mismatch HSC engraftment.
Figure 1. A monoclonal antibody cocktail can induce long-term multi-lineage hematopoietic reconstitution.
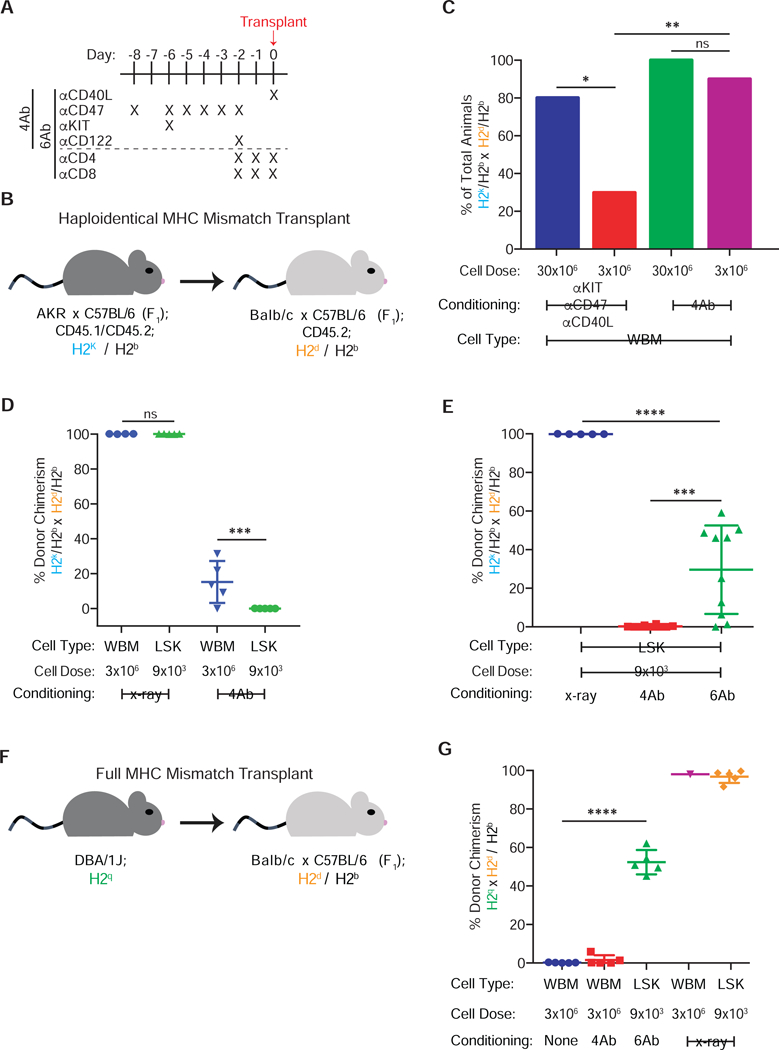
(a) Dosing schedule for 4Ab and 6Ab conditioning regimen, where α refers to antibody. (b) Haploidentical transplantation schema using AKRB6F1 donors and CB6F1 recipients. (c) Percentage of animals which are chimeric at 14–16 weeks after transplanted 30×106 or 3×106 haploidentical WBM cells, with or without NK cell depletion (pooled data from two replicate experiments, pooled n=5–10). (d) Donor granulocyte chimerism at 16 weeks following haploidentical transplantation of 3×106 WBM or 9×103 LSK in the setting of 4Ab conditioning or irradiation (n=4–5). (e) Donor granulocyte chimerism following 9×103 haploidentical LSK transplantation at 16 weeks in irradiated, 4Ab- and 6Ab-conditioned animals (pooled data from two replicate experiments, pooled n=5–10). (f) Transplantation schematic using DBA/1J donors and CB6F1 recipients. (g) Donor granulocyte chimerism at 8 weeks following WBM and LSK fully MHC-mismatched transplantation in irradiated, 4Ab- and 6Ab-conditioned animals (n=5). Data in (c) represent total percentage; one way ANOVA was performed where *P≤0.05 and **P≤0.01. Data and error bars in (d), (e) and (g) represent means ± SD; one way ANOVA was performed where *P≤0.05, **P≤0.01, and ***P≤0.001.
Recipient mice were conditioned with 4Ab using a nine-day treatment scheme (Fig 1a). Haploidentical transplantation was performed using AKR x C57BL/6 F1 mice (hereafter referred to as AB6F1) as donors and BALB/C x C57BL/6 F1 (CB6F1) mice as recipients (Fig. 1b); these mouse strains are matched at the H2b haplotype but mismatched for H2k and H2d. We first transplanted 30×106 haploidentical whole bone marrow (WBM) cells into 4Ab-conditioned mice, given that WBM contains T cells and facilitator CD8+ cells that diminish host rejection of the graft (Shizuru et al., 1996; Gandy et al., 1999). Chimerism, defined as the presence of >1% donor cells in peripheral blood lineages, was periodically assessed by measuring expression of donor versus recipient-specific CD45 allelic markers, which are expressed on all nucleated blood cells. While unconditioned mice did not become chimeric, all 4Ab-conditioned mice developed multi-lineage “mixed” chimerism, in that both donor and host blood cells co-existed (Fig S1a). Importantly, chimerism was also observed in the HSC compartment (Fig. S1b), indicating that peripheral donor cells were the product of active hematopoiesis rather than long-lived mature cells circulating after transplantation.
We tested permutations of the 4Ab regimen and found no single antibody alone permitted engraftment (Supplemental Table 1) while 4Ab conditioning induced chimerism in all recipient mice transplanted with haploidentical WBM (Fig. S1c). Anti-KIT and anti-CD47 combined with either anti-CD40L or anti-CD122 alone induced chimerism in a fraction of mice (Fig. S1c), with anti-CD40L proving more effective at increasing chimerism. Since attaining high doses of donor cells can be challenging in the clinical setting (Negrin et al., 2000, Müller et al., 2012), we attempted a 10-fold lower dose of WBM. Since NK cell ablation is critical in low-dose HCT (Westerhuis et al., 2005), we attempted engrafting 3×106 WBM cells with and without anti-CD122 conditioning (Supplemental Table 1). When anti-CD122 was excluded, the percentage of mice that became chimeric significantly decreased at the lower cell dose (Fig. 1c).
A major limitation of HCT is graft-versus-host disease (GvHD), which is mediated by transplanted donor T cells attacking the host, resulting in potentially lethal organ damage as well as immune deficiency carried out by lymphoid organ homing donor T cells (Gallatin et al 1986, Krensky et al., 1990, Weissman, 2000, Tsao et al., 2009, Müller et al., 2012). GvHD can be avoided by transplanting purified HSCs which lack mature T cells (Shizuru et al., 1996, Uchida et al., 1998). However, in MHC-mismatched transplants, purified HSCs are particularly susceptible to host rejection (Shizuru et al., 1996, Gandy and Weissman, 1998). Therefore, after establishing proof-of-concept in WBM we attempted transplantation of haploidentical Lineage− Sca1+ KIT+ (LSK) cells, which are highly enriched for HSC and multipotent progenitors (Morrison et al., 1994, Kiel et al., 2005). We transplanted 9×103 LSK cells, which is the number of LSK cells present within 3×106 WBM cells. However, LSK cells failed to engraft 4Ab-conditioned mice (Fig 1d).
WBM populations engraft more robustly than purified HSCs, as they contain cell populations that may induce immune suppression by actively attacking host immune cells and/or by secreting immunosuppressive factors (Gandy et al., 1999, Grimes et al., 2004, Nilsson et al., 2007). We have previously shown that transplanting purified HSCs can be achieved by additional immune suppression to eliminate T cells (Shizuru et al., 1996; Chhabra et al., 2016); therefore, we added anti-CD4 and anti-CD8 depleting antibodies to the 4Ab regimen. The usage of this six antibody cocktail (anti-KIT, anti-CD47, anti-CD40L, anti-CD122, anti-CD4, and anti-CD8; referred to as 6Ab conditioning hereafter (Fig. 1a)) induced multi-lineage chimerism in recipients transplanted with 9×103 haploidentical LSK cells (Fig. 1e). Withholding any single antibody from the 6Ab cocktail resulted in fewer chimeric animals and lower chimerism; removal of both anti-CD40L anti-CD122 antibodies generally prevented any engraftment (Fig. S1d). Both 4Ab and 6Ab regimens specifically depleted desired cell-types, while generally sparing B cells and tissue-resident myeloid cells (Fig. S2a-d), highlighting the targeted nature of these monoclonal antibodies.
To assess whether antibody conditioning could enable fully MHC-mismatched transplants we used DBA/1J (H2q) mice as donors and CB6F1 (H2b/d) as hosts (Fig. 1f). 6Ab conditioning enabled efficient LSK transplantation in all mice while only 40% of 4Ab-conditioned mice receiving WBM achieved chimerism (Fig. 1g). These findings were compared to outcomes in irradiated mice that also received fully MHC-mismatched transplants. Although all irradiated CB6F1 mice transplanted with WBM were chimeric by week 3, 80% died by 9 weeks following transplantation (Fig. S2e). Given that the LSK transplanted radiation-conditioned mice did not succumb and that the WBM transplanted radiation-conditioned mice were initially engrafted, this lethality is possibly due to GvHD in this strain combination. In summary, 6Ab conditioning enabled engraftment of low doses of both haploidentical and fully-MHC mismatched HSCs without recourse to chemotherapy or radiation.
In chimeric mice, the co-existence of donor and recipient immune systems implied the induction of immune tolerance. However, it was unclear if this was due to central tolerance, where thymic negative selection prevents the formation of donor-reactive host T cells that can induce rejection (Kappler et al., 1987, Guidos et al., 1990). To gauge central tolerance in the transplanted mice, we measured the presence of the Vβ6 chain of the T cell receptor (TCR), which is reactive to the Mtv-7 provirus-encoding super-antigen expressed by the AKR background (Kanagawa et al., 1989; Guidos et al, 1990, Shizuru et al., 2000). The coexistence of AKR x C57BL/6 (AB6F1) cells in CB6F1 mice would likely result from thymic deletion of Vβ6 + T cells by donor HSC-derived thymic medullary dendritic cells and possibly host medullary epithelial cells (Rouse et al., 1979, 1985). Deletion of Vβ6 + T cells was indeed observed in all chimeric animals receiving antibody conditioning for haploidentical WBM or LSK transplants (Fig. 2a-b). Thus, both 4Ab and 6Ab antibody conditioning followed by immune-system replacement can instill central immunological tolerance to the donor mouse strain.
Figure 2. Antibody conditioned animals have intact immune systems and display tolerance to matched organ grafts.
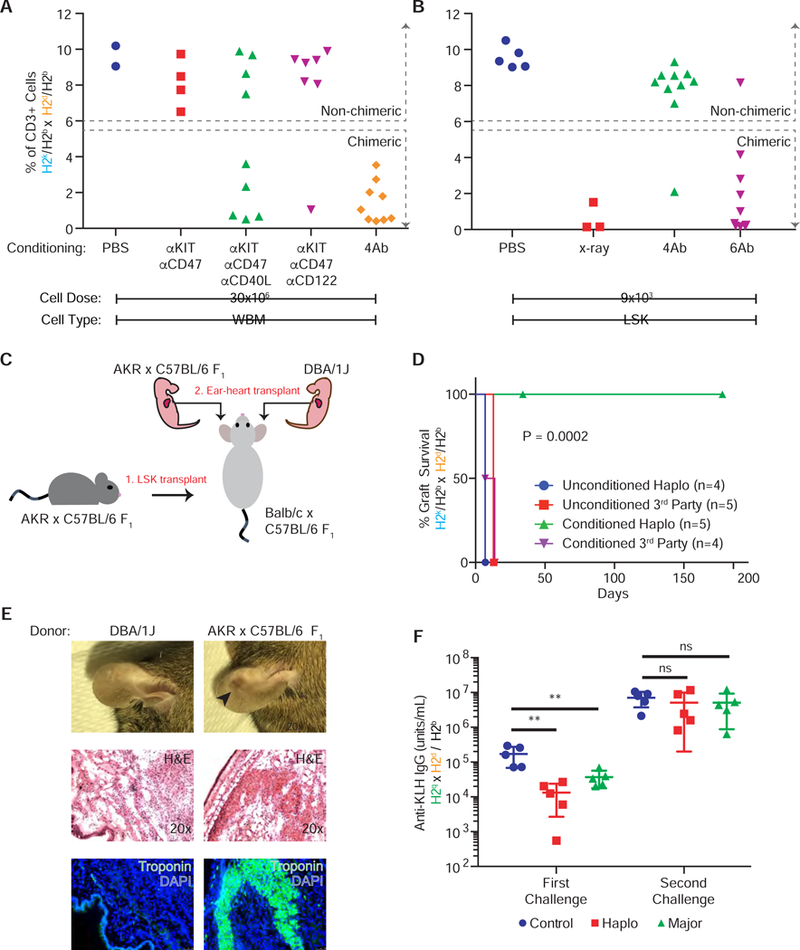
(a-b) Abundance of host Vβ6 + T cells in peripheral blood following (a) WBM and (b) LSK haploidentical transplantation (pooled data from two replicate experiments, pooled, n=2–10). (c) Fetal heart into ear transplantation schematic. (d) Kaplan-Meier curve showing donor heart survival in haploidentical chimeras (n=5). (e) Gross examination, H&E, and IF of representative HSC-donor and 3rd party heart tissue in ears at 34 days following tissue transplant in haploidentical chimeras. (f) Anti-KLH IgG production following KLH immunization six (first challenge) and eight (second challenge) weeks after fully-MHC mismatched HSC transplantation (n=5). Data in (d) was subjected to a log-rank (Mantel-Cox) test and yielded P = 0.0002. Data and error bars in (f) represent means ± SD and one way ANOVA was performed where ****P≤0.0001.
To test whether this central tolerance through antibody conditioning would allow engraftment of donor-matched solid organs, we transplanted neonatal heart grafts (Gandy and Weissman, 1998) from HSC-donor (AB6F1, which are H2k/b) or third-party (DBA/1J strain, which are homozygous for H2q) newborn mice pups into the ear pinna of both naïve and 6Ab-conditioned haploidentical transplant chimeras (Fig. 2c). Graft survival was measured by visualization of the beating heart graft. In naïve, unconditioned and untransplanted mice, both AB6F1 and DBA/1J hearts failed to beat at 14 days (Fig. 2d). In 6Ab-conditioned chimeric mice, AB6F1 hearts began beating at 14 days and persisted for at least 181 days while DBA/1J hearts were rejected. At 34 days, AB6F1 hearts were still visible in the pinna while DBA/1J hearts were no longer visible (Fig. 2e). H&E and immunofluorescence (IF) analysis indicated troponin+ cardiac tissue lacking immune cell infiltrates in the AB6F1-engrafted pinna; troponin+ tissue within the pinna containing DBA/1J hearts was not detectable (Fig. 2e). These results indicate that following antibody-conditioning and haploidentical HSC engraftment, tolerance to matched heart grafts is feasible while transplantation immunity against completely foreign tissues is preserved.
Given that 6Ab-conditioned chimeras either share half or no MHC molecules and adaptive immunity is mediated through MHC recognition, it is important to understand whether T cells of donor origin were re-educated to respond to antigens presented by host MHC. To test functional immunity, which requires interactions between T cells, B cells, and antigen presenting cells following immunization, we challenged mice following a fully-MHC mismatched HSC transplant with keyhole limpet hemocyanin (KLH). Animals with intact T cell dependent antibody responses will generate IgG against KLH. Six weeks after transplant, antibody conditioned mice were able to generate anti-KLH antibodies (Fig. 2f); however, their response lagged behind that of unconditioned, untransplanted mice. Importantly, upon second challenge two weeks later, antibody conditioned mice were able to generate a secondary IgG antibody responses comparable to control mice (Fig. 2f).
DISCUSSION
In sum, here we have developed a method to transplant half (haploidentical) and fully-MHC mismatched purified HSCs into immune-competent animals without the use of chemotherapy and/or radiation. Importantly, the use of purified HSCs dramatically reduces the risk of GvHD (Weissman, 2000). If translatable to human patients, this antibody-based conditioning strategy to overcome immunological barriers could both expand the HSC donor pool to enable most recipients to find a HCT match and decrease the often prohibitive risks of conditioning that prevent widespread use of HCT.
While we have demonstrated the efficacy of antibody conditioning in mice, realizing the potential of such conditioning in humans will require further exploration. Despite the cell type specificity of monoclonal antibodies, as compared to the pleiotropic action of radiation or chemotherapy, the former is not without toxicity. For instance, an anti-CD40L antibody was found to induce thromboembolic events (Kawai et al., 2000, Robles-Carrillo et al., 2010) in humans by cross-linking platelets. However, this effect was found to be Fc-mediated and newer anti-CD40L therapies that lack Fc-domains or contain an Fc-dead domain (Shock et al., 2015, Xie et al., 2014) have proven to be immunosuppressive without inducing thrombosis. Therefore, further studies to better understand the human expression of the proteins targeted here are needed for clinical translation.
Importantly, the ability to induce immunological tolerance to foreign organs could increase opportunities for all patients requiring lifesaving organ transplants. Today the donor of an organ, tissue or HSC transplant is a living or recently deceased person. The ultimate goal of regenerative medicine will be to differentiate a pluripotent (embryonic or induced pluripotent) stem cell line into HSCs and other needed tissue stem cells (such as neural (Uchida et al., 2000), bone and cartilage (Chan et al., 2015), or liver− (Wang et al., 2015)), either in vitro (Loh et al., 2014, Loh et al., 2016) or in vivo within a large-animal host, such as a pig (Rashid et al., 2014, Yamaguchi et al., 2017). Our approach may enable the use of these methods to result in more robust, rapid, and gentler transplant schemes reducing the burden on an organ donation system that is currently dramatically underserving the patients most in need.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Irving L. Weissman (irv@stanford.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
For HSC transplantation experiments, CB6F1/J (Jax: 100007) and DBA/1J (Jax: 670) mice were purchased from Jackson laboratories, while AKR/J x C57Bl/6 F1 (AB6F1) mice were bred at Stanford University. At the time of transplant, all donors and recipients were 8–12 weeks in age and female unless otherwise noted on Supplemental Table 1. For ear-heart transplantation, untimed pregnant DBA/1J mice were purchased from Taconic Biosciences. Mice for all experiments were immunocompetent and group housed; littermates of the same sex were randomly assigned to experimental groups. All experiments were performed according to guidelines established by the Stanford University Administrative Panel on Laboratory Animal Care.
METHOD DETAILS
Pre-Transplantation Conditioning
For antibody conditioning, all antibodies were suspended in phosphate buffered saline (PBS) and injected intraperitoneally unless otherwise noted. When antibodies were withheld or animals were not conditioned, equivalent volumes of PBS were given intraperitoneally instead. Day 0 corresponds to the day of transplantation. For anti-CD47 (clone mIAP410), mice received 100μg on Day −8 and 500μg daily from Day −6 through Day −2. For anti-KIT (clone ACK2), which is injected retro-orbitally, 500μg was given on Day −6. Thirty minutes prior to anti-KIT injections, mice received 400μg of diphenhydramine intraperitoneally. Both anti-CD4 (clone GK1.5) and anti-CD8 (clone YTS169.4) were given as 100μg injections daily from Day −2 through Day 0 (Chabbra et al., 2016). For anti-CD122 (clone Tm-β1), 250μg was given on Day −2 (Tanaka et al., 1993, Seung et al., 2003). For anti-CD40L (clone MR-1), 500μg was given on Day 0 (Wekerle et al., 2000). Irradiation control mice were exposed to two doses of 6.5Gy x-ray radiation on the day of transplantation.
Graft Preparation and Transplantation
Whole bone marrow was extracted from tibias, femurs, hips, and spines of donor mice. Bones were crushed with a mortar and pestle, filtered, and subsequently underwent red blood cell (RBC) lysis. For LSK cell transplants, RBC lysed whole bone marrow was bound to the Miltenyi Lineage Cell Depletion kit cocktail as per the manufacturer’s instructions. Flow-through from the magnetic separation columns was collected and stained in PBS with 2% fetal bovine serum (FBS) and the following antibodies: CD3 PE (clone 17A2; 0.66μg/mL final concentration), CD4 PE (clone GK1.5; 0.66μg/mL final concentration), CD5 PE (clone 53–7.3; 0.66μg/mL final concentration), CD8a PE (clone 53–6.7; 0.66μg/mL final concentration), B220 PE (clone RA3–6B2; 0.66μg/mL final concentration), Gr-1 PE (clone RB6–8C5; 0.5μg/mL final concentration), Mac-1 PE (clone M1/70; 0.5μg/mL final concentration), Ter119 PE (clone TER119; 0.66μg/mL final concentration), SCA1 Pe-Cy7 (clone D7; 1.0μg/mL final concentration), and CD117 APC (clone 2B8; 1.0μg/mL final concentration). Propidium iodide was added as a viability stain just prior to sorting on a BD Aria. All cells for transplant were re-suspended at the desired concentration in PBS with 2% FBS. All mice were anesthetized using isoflurane and then transplanted with 100uL of cell suspension via retro-orbital injection.
Peripheral Blood Analysis
Mice were periodically bled retro-orbitally into EDTA coated tubes. Blood was then incubated in 1% dextran with 5mM EDTA at 37C for 1 hour. The supernatant from each tube was extracted, lysed, and then stained with the following antibodies for peripheral blood chimerism: CD3 APC (clone 17A2; 2.0μg/mL final concentration), CD19 PE-Cy7 (clone ebio103; 2.0μg/mL final concentration), Gr-1 eFluor-450 (clone RB6–8C5; 1.0μg/mL final concentration), Mac-1 APC-Cy7 (clone M1/70; 1.0μg/mL final concentration), CD45.1 FITC (clone A20; 2.0μg/mL final concentration), and CD45.2 PE (104; 2.0μg/mL final concentration). To assess Vβ6 TCR expression, peripheral blood was processed as above and stained with Vβ6 APC (RR4–7; 2.0μg/mL final concentration). Propidium iodide was added as a viability stain just prior to analysis. Samples were analyzed on a BD Fortessa. Donor versus host chimerism was distinguished based on CD45 allelic differences. For complete blood counts, 20μl of peripheral blood was analyzed on a Heska HemaTrue Veterinary Hematology Analyzer.
Spleen and Bone Marrow Population Analysis
Spleens were harvested from conditioned mice and directly mashed in a 70-μm filter. Hips, femurs and tibia were crushed as described above for bone marrow analysis. Single cells from spleen and bones were lysed, filtered, and stained with the following antibodies for immune cell analysis: CD4 APC (clone GK1.5; 2.0μg/mL final concentration), CD8 FITC (clone 53–6.7; 5.0μg/mL final concentration), CD19 PE (clone 1D3; 2.0μg/mL final concentration), NK1.1 PE-Cy7 (clone PK136; 2.0μg/mL final concentration), Mac-1 APC-Cy7 (clone M1/70; 0.5μg/mL final concentration), and Gr1 eFluor-450 (clone RB6–8C5; 1.0μg/mL final concentration). For long-term HSC chimerism, cells were stained with the following antibodies: CD3 PE (clone 17A2; 0.66μg/mL final concentration), CD4 PE (clone GK1.5; 0.66μg/mL final concentration), CD5 PE (clone 53–7.3; 0.66μg/mL final concentration), CD8a PE (clone 53–6.7; 0.66μg/mL final concentration), B220 PE (clone RA3–6B2; 0.66μg/mL final concentration), Gr-1 PE (clone RB6–8C5; 0.5μg/mL final concentration), Mac-1 PE (clone M1/70; 0.5μg/mL final concentration), Ter119 PE (clone TER119; 0.66μg/mL final concentration), SCA1 Pe-Cy7 (clone D7; 1.0μg/mL final concentration), CD117 APC (clone 2B8; 1.0μg/mL final concentration), CD150 Pacific Blue (clone TC15–12F12.2; 5.0μg/mL final concentration), CD34 FITC (clone RAM34; 5.0μg/mL final concentration), CD45.1 FITC (clone A20; 2.0μg/mL final concentration), and CD45.2 PE (clone 104; 2.0μg/mL final concentration). Propidium iodide was added as a viability stain just prior to analysis. Samples were analyzed on a BD Fortessa.
Ear-Heart Graft
Neonatal mice were euthanized 1–2 days after birth and their hearts were harvested and placed in ice cold PBS. Recipient mice were prepared by making a small incision on the dorsal side of their ear near the skull. Afterward, using a trocar, a pouch was created by tunneling from the incision site to the tip of the pinna. Neonatal hearts were delivered at the distal end of the pouch with the trocar. The tunnel was closed by gently pushing the lifted skin back to the dermis. Heart viability was monitored for beating by visualizing the graft through a dissecting microscope. At the noted time points, ears were dissected from representative animals and embedded in optimal cutting temperature (O.C.T.) compound. Tissue was sectioned and stained with hematoxylin and eosin, as well as cardiac troponin I. For troponin staining, sections were blocked with PBS containing 10% FBS for 1 hour, and then stained overnight at 4 degrees Celsius in 0.5% bovine serum albumin (BSA) with 10μg/mL of anti-cardiac troponin I antibody. Secondary staining was performed with 10μg/mL of goat anti-rabbit IgG Alexa Fluor 488 for 30 minutes, and then subsequently stained with DAPI. Images were collected on a Leica DMI6000B epifluorescence-equipped inverted microscope.
KLH immunization and monitoring of KLH antibody production in serum
KLH (Sigma-Aldrich) were mixed with an equal volume of the complete Freund adjuvant (Sigma-Aldrich) for primary immunization or incomplete Freund adjuvant (Sigma-Aldrich) for secondary immunization to form an emulsion by vortexing in 15 mL tube. KLH emulsion (100 µg KLH in 100 μl emulsion/mouse) was intraperitoneally injected into transplanted mice 4 weeks post-transplantation for primary immunization. Two (2) weeks after primary immunization, secondary immunization was performed. Serum was collected by retro-orbital bleeding of KLH-immunized mice 2 weeks after secondary immunization. Anti-KLH antibody titer in the serum was determined using KLH IgG mouse ELISA kit (Abnova) and a SpectraMax i3x plate reader (Molecular Device) according to the manufacturer’s instructions.
QUANTIFICATION AND STATISTICAL ANALYSIS
Analyses were performed on GraphPad Prism. One- and two-way ANOVA, log-rank test, and unpaired and multiple t-tests were used where appropriate; n signifies the number of animals used.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
| Antibodies | ||
| Mouse monoclonal anti-CD47 | BioXCell | RRID: AB_2687806 |
| Rat monoclonal anti-CD117 | BioXCell | RRID: AB_2687818 |
| Rat monoclonal anti-CD122 | BioXCell | RRID: AB_2687820 |
| Armenian hamster monoclonal anti-CD40L | BioXCell | RRID: AB_1107601 |
| Rat monoclonal anti-CD4 | BioXCell | RRID: AB_1107636 |
| Rat monoclonal anti-CD8 | BioXCell | RRID: AB_10950145 |
| Rat monoclonal anti-CD3 PE | BioLegend | RRID: AB_312662 |
| Rat monoclonal ant-CD4 PE | BioLegend | RRID: AB_11152678 |
| Rat monoclonal anti-CD5 PE | Thermo Fisher Scientific | RRID: AB_2539168 |
| Rat monoclonal anti-CD8 PE | Thermo Fisher Scientific | RRID: AB_465529 |
| Rat monoclonal anti-B220 PE | Thermo Fisher Scientific | RRID: AB_10371899 |
| Rat monoclonal anti-Gr1 PE | Thermo Fisher Scientific | RRID: AB_10376319 |
| Rat monoclonal anti-Mac1 PE | Thermo Fisher Scientific | RRID: AB_11154207 |
| Rat monoclonal anti-TER199 PE | Thermo Fisher Scientific | RRID: AB_2535273 |
| Rat monoclonal anti-Sca1 PE-Cy7 | Thermo Fisher Scientific | RRID: AB_469669 |
| Rat monoclonal anti-CD117 APC | Thermo Fisher Scientific | RRID: AB_469429 |
| Rat monoclonal anti-CD3 APC | Thermo Fisher Scientific | RRID: AB_2536039 |
| Mouse monoclonal anti-CD19 PE-Cy7 | BD Biosciences | RRID: AB_394495 |
| Rat monoclonal anti-Gr1 Pacific Blue | Thermo Fisher Scientific | RRID: AB_10376182 |
| Rat monoclonal anti-Mac1 APC-Cy7 | BD Biosciences | RRID: AB_396772 |
| Mouse monoclonal anti-CD45.1 FITC | Thermo Fisher Scientific | RRID: AB_2534248 |
| Mouse monoclonal anti-CD45.2 PE | Thermo Fisher Scientific | RRID: AB_2534922 |
| Rat monoclonal anti-Vb6 | BioLegend | RRID: AB_2564056 |
| Rat monoclonal anti-CD4 APC | Thermo Fisher Scientific | RRID: AB_11152647 |
| Rat monoclonal anti-CD8 FITC | Thermo Fisher Scientific | RRID: AB_11153636 |
| Mouse monoclonal anti-CD19 PE | Thermo Fisher Scientific | RRID: AB_465579 |
| Mouse monoclonal anti-NK1.1 PE-Cy7 | Thermo Fisher Scientific | RRID: AB_469665 |
| Rat monoclonal anti-Mac1 APC-Cy7 | Thermo Fisher Scientific | RRID: AB_2534404 |
| Rat monoclonal anti-Gr1 eFluor-450 | Thermo Fisher Scientific | RRID: AB_1548788 |
| Rat monoclonal anti-CD150 Pacific Blue | BioLegend | RRID: AB_2187962 |
| Rat monoclonal anti-CD34 FITC | Thermo Fisher Scientific | RRID: AB_465021 |
| Rabbit polyclonal anti-Cardiac Troponin I | Abcam | RRID: AB_869982 |
| Goat polyclonal anti-Rabbit IgG Alexa Fluor 488 | Thermo Fisher Scientific | RRID: AB_143165 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| KLH | Sigma-Aldrich | Cat# H7017 |
| Complete Freund’s Adjuvant | Sigma-Aldrich | Cat# F5881 |
| Incomplete Freund’s Adjuvant | Sigma-Aldrich | Cat# F5506 |
| Diphenhydramine | APP Pharmaceuticals | Cat# 63323066401 |
| Critical Commercial Assays | ||
| KLH IgG (Mouse) ELISA Kit | Abnova | Cat# KA2460 |
| Experimental Models: Organisms/Strains | ||
| CB6F1 | Jackson Laboratories | Cat# 100007 |
| DBA/1J | Jackson Laboratories | Cat# 670 |
| DBA/1J Untimed Pregnant Mice | Taconic Biosciences | N/A |
| AKR x C57Bl/6 F1 (AB6F1) | Bred in the Weissman Laboratory, Stanford University | N/A |
| Other | ||
| Lineage Cell Depletion Kit, mouse | Miltenyi Biotec | Cat# 130–110-470 |
Highlights.
Six antibodies suppress a mouse’s HSCs, T cells and NK cells in 8 days
Antibody treatment enables HSC transplants without radiation/chemotherapy
Transplants can be performed with fully MHC mismatched donors
Following HSC transplants, animals are tolerant to solid organs from the same donor
ACKNOWLEDGEMENTS
We thank Agnieszka Czechowicz, Deepta Bhattacharya, and Daniel Kraft for helping to initiate this field while in the laboratory, and for discussion of the subject matter that resulted in the paper. We thank Kenneth I. Weinberg, Ryan A. Flynn, and Adam J. Rubin for editorial comments. We thank Aaron McCarty, Theresa Storm, Teja Naik, and Steve Jungers for technical assistance. We thank Pauline Chu for providing histological services. We thank Patty Lovelace and Stephen Weber for flow cytometry assistance. This study was supported by the California Institute for Regenerative Medicine (RT3–07683, to I.L.W. and J.A.S.), the Ludwig Cancer Foundation and an NIH/NCI Outstanding Investigator Award (R35CA220434) (to I.L.W), NIH Director’s Early Independence Award DP5OD024558, Stanford-UC Berkeley Siebel Stem Cell Institute, Stanford Beckman Center, Anonymous and DiGenova Families, and the Baxter Foundation Faculty Scholar Award (to K.M.L.), and PHS Grant Number CA09302, awarded by the National Cancer Institute, DHHS (to B.M.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATIONS OF INTEREST
A.Chh., J.A.S., B.M.G. and I.L.W. are inventors on patents describing antibody-mediated HSC clearance and engraftment (US20180214524A1, US62/041,989, US20100226927A1). I.L.W. is a cofounder, director, and stockholder of Forty Seven, Inc., the company that licensed the above stated patents. B.M.G., J.A.S., K.S.K. and A.Chh. are stockholders of and/or were paid consultants for Forty Seven, Inc. J.A.S. is cofounder and stockholder of Jasper, Inc, which licensed an above stated patent or patents.
REFERENCES
- Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. (1992). Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA 89(7):2804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty PG, Clift RA, Mickelson EM, Nisperos BB, Flournoy N, Martin PJ, Sanders JE, Stewart P, Buckner CD, Storb R, et al. (1985). Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med 313(13):765–71. [DOI] [PubMed] [Google Scholar]
- Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. (1991). Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature 349(6307):329–31. [DOI] [PubMed] [Google Scholar]
- Bolaños-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, Brodsky RA. (2012). HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood 120(22):4285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, et al. (2015). Identification and specification of the mouse skeletal stem cell. Cell 160(1–2):285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra A, Ring AM, Weiskopf K, Schnorr PJ, Gordon S, Le AC, Kwon HS, Ring NG2, Volkmer J, Ho PY, et al. (2016). Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Sci Transl Med 8(351):351ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold SP, Martin G, Qin S, Waldmann H. (1986). Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature 323(6084):164–6. [DOI] [PubMed] [Google Scholar]
- Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. (2007). Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 318(5854):1296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham MM, Bingaman AW, Adams AB, Ha J, Waitze SY, Pearson TC, Larsen CP. (2000). Cutting edge: administration of anti-CD40 ligand and donor bone marrow leads to hemopoietic chimerism and donor-specific tolerance without cytoreductive conditioning. J Immunol 165(1):1–4. [DOI] [PubMed] [Google Scholar]
- Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, et al. (2011). Spectrum of cancer risk among US solid organ transplant recipients. JAMA 306(17):1891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, Weissman IL. (2011). Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood 118(20):5439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin M, St John TP, Siegelman M, Reichert R, Butcher EC, Weissman IL. (1986). Lymphocyte homing receptors. Cell 44(5):673–80. [DOI] [PubMed] [Google Scholar]
- Gandy KL, Domen J, Aguila H, Weissman IL. (1999). CD8+TCR+ and CD8+TCR− cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity 11(5):579–90. [DOI] [PubMed] [Google Scholar]
- Gandy KL, Weissman IL. (1998). Tolerance of allogeneic heart grafts in mice simultaneously reconstituted with purified allogeneic hematopoietic stem cells. Transplantation 65(3):295–304. [DOI] [PubMed] [Google Scholar]
- Grimes HL, Schanie CL, Huang Y, Cramer D, Rezzoug F, Fugier-Vivier I, Ildstad ST. (2004). Graft facilitating cells are derived from hematopoietic stem cells and functionally require CD3, but are distinct from T lymphocytes. Exp Hematol 32(10):946–54. [DOI] [PubMed] [Google Scholar]
- Guidos CJ, Danska JS, Fathman CG, Weissman IL. (1990). T cell receptor-mediated negative selection of autoreactive T lymphocyte precursors occurs after commitment to the CD4 or CD8 lineages. J Exp Med 172(3):835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AR, Williams SF, Dillon JJ. (1998). Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transplant 22(5):439–43. [DOI] [PubMed] [Google Scholar]
- Hoogerbrugge PM, Brouwer OF, Bordigoni P, Ringden O, Kapaun P, Ortega JJ, O’Meara A, Cornu G, Souillet G, Frappaz D, et al. (1995). Allogeneic bone marrow transplantation for lysosomal storage diseases. The European Group for Bone Marrow Transplantation. Lancet 345(8962):1398–402. [DOI] [PubMed] [Google Scholar]
- Kanagawa O, Palmer E, Bill J. (1989). The T cell receptor V beta 6 domain imparts reactivity to the Mls-1a antigen. Cell Immunol 119(2):412–26. [DOI] [PubMed] [Google Scholar]
- Kappler JW, Roehm N, Marrack P. (1987). T cell tolerance by clonal elimination in the thymus. Cell 49(2):273–80. [DOI] [PubMed] [Google Scholar]
- Kärre K, Ljunggren HG, Piontek G, Kiessling R. (1986). Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319(6055):675–8. [DOI] [PubMed] [Google Scholar]
- Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. (2000). Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med 6(2):114. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. (2005). SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121(7):1109–21. [DOI] [PubMed] [Google Scholar]
- Krensky AM, Weiss A, Crabtree G, Davis MM, Parham P. (1990). T-lymphocyte-antigen interactions in transplant rejection. N Engl J Med 322(8):510–7. [DOI] [PubMed] [Google Scholar]
- Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. (1992). Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help). J Exp Med 175(4):1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl KF, Wilson DB. (1977). Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. J Exp Med 145(3):508–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KM, Ang LT, Zhang J, Kumar V, Ang J, Auyeong JQ, Lee KL, Choo SH, Lim CY, Nichane M, et al. (2014). Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell 14(2):237–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KM, Chen A, Koh PW, Deng TZ, Sinha R, Tsai JM, Barkal AA, Shen KY, Jain R, Morganti RM, et al. (2016). Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types. Cell 166(2):451–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli G, Galimberti M, Polchi P, Angelucci E, Baronciani D, Giardini C, Politi P, Durazzi SM, Muretto P, Albertini F. (1990). Bone marrow transplantation in patients with thalassemia. N Engl J Med 322(7):417–21. [DOI] [PubMed] [Google Scholar]
- Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, et al. (2008). HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 14(6):641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv W, Fan Z, Huang F, Xu N, Xuan L, Guopan Yu, a Q, Zhou H, Lin R, Zhang X, et al. (2017). Autoimmune hematological diseases following haploidentical donor hematopoietic stem cell transplant compared with matched sibling and unrelated donor. Oncotarget 8(16):26505–26514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R, Park CY, Weissman IL. (2007). Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 1(6):635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markees TG, Phillips NE, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA. (1997). Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation 64(2):329–35. [DOI] [PubMed] [Google Scholar]
- Michel G, Socié G, Gebhard F, Bernaudin F, Thuret I, Vannier JP, Demeocq F, Leverger G, Pico JL, Rubie H, et al. (1997). Late effects of allogeneic bone marrow transplantation for children with acute myeloblastic leukemia in first complete remission: the impact of conditioning regimen without total-body irradiation--a report from the Société Française de Greffe de Moelle. J Clin Oncol 15(6):2238–46. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL. (1994). The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1(8):661–73. [DOI] [PubMed] [Google Scholar]
- Müller AM, Shashidhar S, Küpper NJ, Kohrt HE, Florek M, Negrin RS, Brown JM, Shizuru JA. (2012). Co-transplantation of pure blood stem cells with antigen-specific but not bulk T cells augments functional immunity. Proc Natl Acad Sci USA 109(15):5820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller AM, Kohrt HE, Cha S, Laport G, Klein J, Guardino AE, Johnston LJ, Stockerl-Goldstein KE, Hanania E, Juttner C, et al. (2012). Long-term outcome of patients with metastatic breast cancer treated with high-dose chemotherapy and transplantation of purified autologous hematopoietic stem cells. Biol Blood Marrow Transplant 18(1):125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrin RS, Atkinson K, Leemhuis T, Hanania E, Juttner C, Tierney K, Hu WW, Johnston LJ, Shizurn JA, Stockerl-Goldstein KE, et al. (2000). Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with metastatic breast cancer. Biol Blood Marrow Transplant 6(3):262–71. [DOI] [PubMed] [Google Scholar]
- Neven B, Leroy S, Decaluwe H, Le Deist F, Picard C, Moshous D, Mahlaoui N, Debré M, Casanova JL, Dal Cortivo L, et al. (2009). Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood 113(17):4114–24. [DOI] [PubMed] [Google Scholar]
- Nikolic B, Zhao G, Swenson K, Sykes M. (2000). A novel application of cyclosporine A in nonmyeloablative pretransplant host conditioning for allogeneic BMT. Blood 96(3):1166–72. [PubMed] [Google Scholar]
- Nilsson SK, Dooner MS, Tiarks CY, Weier HU, Quesenberry PJ. (1997). Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood 89(11):4013–20. [PubMed] [Google Scholar]
- Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. (1992). A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA 89(14):6550–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, Gennery A, Kröger N, Kuball J, Lanza F, et al. (2017). Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant 52(6):811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine JJ, Wang M, Zhang M, Zeng D. (2014). Induction of mixed chimerism depletes pre-existing and de novo-developed autoreactive B cells in autoimmune NOD mice. Diabetes 63(6):2051–62. [DOI] [PubMed] [Google Scholar]
- Rashid T, Kobayashi T, Nakauchi H. (2014). Revisiting the flight of Icarus: making human organs from PSCs with large animal chimeras. Cell Stem Cell 15(4):406–409. [DOI] [PubMed] [Google Scholar]
- Robles-Carrillo L, Meyer T, Hatfield M, Desai H, Dávila M, Langer F, Amaya M, Garber E, Francis JL, Hsu YM, et al. (2010). Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol 185(3):1577–83. [DOI] [PubMed] [Google Scholar]
- Rouse RV, Van ewijk W, Jones PP, Weissman IL. (1979). Expression of MHC antigens by mouse thymic dendritic cells. J Immunol 122(6):2508–15. [PubMed] [Google Scholar]
- Rouse RV, Ezine S, Weissman IL. (1985). Expression of major histocompatibility complex antigens in the thymuses of chimeric mice. Transplantation 40(4):422–6. [DOI] [PubMed] [Google Scholar]
- Sachs DH, Kawai T, Sykes M. (2014). Induction of tolerance through mixed chimerism. Cold Spring Harb Perspect Med 4(1):a015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung E, Mordes JP, Rossini AA, Greiner DL. (2003). Hematopoietic chimerism and central tolerance created by peripheral-tolerance induction without myeloablative conditioning. J Clin Invest 112(5):795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabi Y, Sachs DH. (1989). Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med 169(2):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuru JA, Jerabek L, Edwards CT, Weissman IL. (1996). Transplantation of purified hematopoietic stem cells: requirements for overcoming the barriers of allogeneic engraftment. Biol Blood Marrow Transplant 2(1):3–14. [PubMed] [Google Scholar]
- Shizuru JA, Weissman IL, Kernoff R, Masek M, Scheffold YC. (2000). Purified hematopoietic stem cell grafts induce tolerance to alloantigens and can mediate positive and negative T cell selection. Proc Natl Acad Sci USA 97(17):9555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shock A, Burkly L, Wakefield I, Peters C, Garber E, Ferrant J, Taylor FR, Su L, Hsu YM, Hutto D, et al. (2015). CDP7657, an anti-CD40L antibody lacking an Fc domain, inhibits CD40L-dependent immune responses without thrombotic complications: an in vivo study. Arthritis Res Ther 17:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. (1988). Purification and characterization of mouse hematopoietic stem cells. Science 241(4861):58–62. [DOI] [PubMed] [Google Scholar]
- Spitzer TR, McAfee SL, Dey BR, Colby C, Hope J, Grossberg H, Preffer F, Shaffer J, Alexander SI, Sachs DH, et al. (2003). Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation 75(10):1748–51. [DOI] [PubMed] [Google Scholar]
- Straathof KC, Rao K, Eyrich M, Hale G, Bird P, Berrie E, Brown L, Adams S, Schlegel PG, Goulden N, et al. (2009). Haemopoietic stem-cell transplantation with antibody-based minimal-intensity conditioning: a phase ½ study. Lancet 374(9693):912–20. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kitamura F, Nagasaka Y, Kuida K, Suwa H, Miyasaka M. (1993). Selective long-term elimination of natural killer cells in vivo by an anti-interleukin 2 receptor beta chain monoclonal antibody in mice. J Exp Med 178(3):1103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ED, Buckner CD, Clift RA, Fefer A, Johnson FL, Neiman PE, Sale GE, Sanders JE, Singer JW, et al. (1979). Marrow transplantation for acute nonlymphoblastic leukemia in first remission. N Engl J Med 301(11):597–9. [DOI] [PubMed] [Google Scholar]
- Tsao GJ, Allen JA, Logronio KA, Lazzeroni LC, Shizuru JA. (2009). Purified hematopoietic stem cell allografts reconstitute immunity superior to bone marrow. Proc Natl Acad Sci USA 106(9):3288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. (2000). Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA 97(26):14720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Tsukamoto A, He D, Friera AM, Scollay R, Weissman IL. (1998). High doses of purified stem cells cause early hematopoietic recovery in syngeneic and allogeneic hosts. J Clin Invest 101(5):961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhao L, Fish M, Logan CY, Nusse R. (2015). Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 524(7564):180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL. (1966). Studies on the mechanism of split tolerance in mice. Transplantation 4(5):565–71. [DOI] [PubMed] [Google Scholar]
- Weissman IL. (1973). Transfer of tolerance. Transplantation 15(3):265–9. [DOI] [PubMed] [Google Scholar]
- Weissman IL. (2000). Stem cells: units of development, units of regeneration, and units in evolution. Cell 100(1):157–68. [DOI] [PubMed] [Google Scholar]
- Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, Shaffer J, Sayegh MH, Sykes M. (2000). Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med 6(4):464–9. [DOI] [PubMed] [Google Scholar]
- Westerhuis G, Maas WG, Willemze R, Toes RE, Fibbe WE. (2005). Long-term mixed chimerism after immunologic conditioning and MHC-mismatched stem-cell transplantation is dependent on NK-cell tolerance. Blood 106(6):2215–20. [DOI] [PubMed] [Google Scholar]
- Worth AJ, Nikolajeva O, Chiesa R, Rao K, Veys P, Amrolia PJ. (2013). Successful stem cell transplant with antibody-based conditioning for XIAP deficiency with refractory hemophagocytic lymphohistiocytosis. Blood 121(24):4966–8. [DOI] [PubMed] [Google Scholar]
- Xie JH, Yamniuk AP, Borowski V, Kuhn R, Susulic V, Rex-Rabe S, Yang X, Zhou X, Zhang Y, Gillooly K, et al. (2014). Engineering of a novel anti-CD40L domain antibody for treatment of autoimmune diseases. J Immunol 192(9):4083–92. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sato H, Kato-Itoh M, Goto T, Hara H, Sanbo M, Mizuno N, Kobayashi T, Yanagida A, Umino A, et al. (2017). Interspecies organogenesis generates autologous functional islets. Nature 542(7640):191–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


