Summary/Abstract
Precision cancer medicine requires effective genotyping of every patient’s tumor to optimally design treatment plans. Despite its imperfect sensitivity, the rapidity and convenience of cell-free DNA (cfDNA) sequencing makes it an essential complement to tumor genotyping which, when used appropriately, can aide the pursuit of effective genotyping for all patients.
In this issue of Clinical Cancer Research, Leighl and colleagues describe a prospective study demonstrating the clinical utility of cell-free DNA (cfDNA) analysis for front-line genotyping of metastatic non-small cell lung cancer (NSCLC) as compared to tissue genotyping (1). Their data raise important and timely questions about the best approaches to achieve effective cancer genotyping in patients with metastatic NSCLC.
Our evolving diagnostic capabilities are moving us towards an inflection point in precision cancer medicine. Since the inception of medical oncology, we have attempted to characterize tumors by their various subsets – beginning with tissue type and morphology, progressing to include key genetic changes (driver mutations, resistance mutations) and broad genomic features (tumor mutational burden, other signatures), and now capturing immunologic factors (tumor microenvironment, expression of immune checkpoint receptors/ligands). Our collective understanding of the molecular makeup of NSCLC and other solid tumors across these categories at the time of initial diagnosis, progression, and therapy resistance is growing rapidly. We have more molecular targets than ever before, and we also have more pharmaceuticals available than we could have dreamed just a few decades ago.
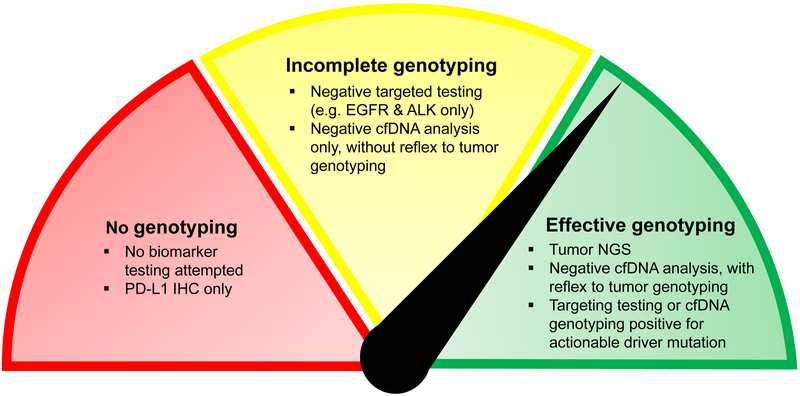
This explosion in development of targeted therapies has been accompanied by a similarly exponential growth in the availability of sophisticated diagnostic tools for genotyping. Nonetheless, more than a decade after the discovery that NSCLCs harboring EGFR driver mutations are responsive to tyrosine kinase inhibitors (TKIs), EGFR genotyping is still not performed for every newly diagnosed NSCLC. Indeed, Leighl and colleagues reference multiple studies in which up to 20–30% of patients with newly diagnosed NSCLC did not have EGFR or ALK genotyping results prior to initiating therapy. The reasons for this lack of genotyping are myriad, and can include inadequate tumor tissue, testing fatigue, the increasing complexity of care, and perhaps diagnostic nihilism. In some cases, clinicians send PD-L1 IHC to guide immunotherapy treatment without any genetic sequencing for known drivers – this is still clearly inadequate, as the response rate to pembrolizumab in PD-L1-high (>50%) NSCLC was only 39% in the recent KEYNOTE-042 study (2). Genotyping must inform this decision point, as outcomes on immunotherapy are reliably poor in patients with targetable genotypes and are reliably better in those with a high tumor mutational burden (3). Finally, in addition to these cases in which no genotyping is done, another common scenario is ‘under-genotyping,’ or incomplete genotyping (Figure 1). This can also happen for a variety of reasons, but often results from single-gene testing which can exhaust tissue and preclude more comprehensive NGS.
Figure. Achieving effective cancer genotyping:
The availability of convenient plasma NGS assays with a high positive predictive value (PPV) means that, today, no patients with advanced NSCLC should receive no molecular testing or PD-L1 testing only (red). However, the limitations of our genotyping assays must be recognized if we wish to avoid incomplete genotyping (yellow). Targeted assays for common driver mutations (e.g. EGFR, ALK) can take time and exhaust tissue, making it harder to then test for rarer driver mutations if negative, while cfDNA analysis has imperfect sensitivity such that targetable driver mutations can be missed. These more limited assays can be useful if positive, but further testing must be pursued if they are negative. To offer our patients effective genotyping (green), the most reliable approaches either involve a panel-based assay tested on a high-quality tissue biopsy, or cfDNA analysis first, followed by reflex to tumor genotyping if the “liquid biopsy” is negative for a targetable driver mutation.
In this study, Leighl and colleagues attempt to address the problem of ‘under-genotyping’ by studying the potential for cfDNA sequencing to provide a breadth of genotyping information with a shorter turnaround time (TAT) than tumor tissue genotyping. In their study, 282 patients underwent cfDNA sequencing with Guardant360, an NGS platform assessing for alterations (point mutations, indels, fusions, and amplifications) in 73 genes of interest. They find that cfDNA analysis via the Guardant360 NGS assay detected clinically-relevant NSCLC-associated biomarkers at a similar rate as standard of care (SOC) testing, noting that during this study the SOC was not tumor NGS for all patients. They further demonstrate that the combination of tissue-based genotyping and cfDNA analysis resulted in a meaningfully higher frequency of identification of NSCLC driver mutations than either method alone. Tissue-based genotyping identified 67% (60/89) of the guideline-recommended biomarkers in first pass, with reflex cfDNA testing identifying the final 33%. In cases where cfDNA genotyping was utilized first, 87% (77/89) of the biomarkers were identified initially, with reflex tumor genotyping identifying the remaining 13%. Notably, the median TAT for cfDNA analysis was significantly lower than that for SOC tumor genotyping (9 days vs 15 days), with progressive shortening of TAT over the course of the trial as technologies improved.
We share the authors’ enthusiasm for cfDNA sequencing as a convenient option for routine NSCLC genotyping. There is now a body of data demonstrating high positive predictive value (PPV) of cfDNA analysis in solid tumor genotyping (4) – a necessary performance characteristic of this diagnostic platform, given the unacceptability of false positives. However, we and others have shown that sensitivity of cfDNA genotyping can be low in patients with lower metastatic burden, likely due to reduced shed of tumor DNA into the plasma (4). This insensitivity of cfDNA sequencing must be acknowledged as a significant barrier to its application. While it does not preclude the use of cfDNA sequencing in the appropriate clinical situation, it does require us to be thoughtful about how we apply this technology and the results we conclude from the information gained. Ultimately, negative cfDNA sequencing may be better than no genotyping at all, but it is not sufficient to rule out the presence of targetable driver mutations given the impaired sensitivity of these assays and unknown rate of tumor shed in any given patient. Without careful attention to the sensitivity and specificity of our diagnostic tools, we risk being fooled into thinking we have more information than we actually do.
We must be cautious in thinking, as Leighl et al suggest, that sequencing of cfDNA alone offers “guideline-complete genotyping” – the recent ASCO-CAP joint review highlights the need for “reflex to tumor biopsy testing in patients with negative liquid biopsy results” (5). We echo the authors’ suggestion for a role of cfDNA analysis as complementary to tumor analysis, but not a replacement. One could hope that cfDNA sequencing, with its potential for faster TAT and readily available sample in a simple blood draw, could neatly fill some of the gaps that result in incomplete genotyping (Figure 1). Like our other available diagnostics, it is but one useful addition to our toolkit, and one that we must learn to use well.
In sum, cancer genotyping is not binary. For any given clinical scenario, there are multiple ways to obtain the necessary information to make treatment decisions. Our task as a community of medical oncologists is to first discern what is the necessary and sufficient genotyping information to fully inform treatment decisions. Second, we must acknowledge and overcome the logistical barriers that prevent our ability to achieve this for every patient. Thanks to a rapidly progressing field in both diagnostics and therapeutics, this will be an iterative process for decades to come. If we can continue to leverage our collective understanding of the biology of solid tumors, the targeted therapies available for clinical use, and the healthcare delivery systems we work within, we have the ability to make meaningful advances in the field of precision cancer medicine. Through establishing criteria for what qualifies as effective cancer genotyping, we maximize the chance of it being implemented universally for our patients with NSCLC.
Acknowledgements
GRO is the Damon Runyon-Gordon Family Clinical Investigator supported by the Damon Runyon Cancer Research Foundation (CI-86–16). GRO is also supported by the Pamela Elizabeth Cooper Research Fund and the National Institutes of Health (R35CA220497; P.I.: Janne).
Footnotes
Conflicts of interest:
GRO: Consulting fees from Astra-Zeneca, DropWorks, Inivata, Janssen, GRAIL, Sysmex, and Honoraria from Foundation Medicine, Guardant
CBM: No conflicts to report
References
- 1.Leighl NB, Page RD, Raymond VM, Daniel DB, Divers S, Reckamp K, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res 2019; [published online ahead of print, April 15]. [DOI] [PubMed] [Google Scholar]
- 2.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Tuma HZ et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomized, open-label, controlled, phase 3 trial. Lancet 2019; [published online ahead of print, April 4]. [DOI] [PubMed] [Google Scholar]
- 3.Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L et al. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin Cancer Res 2019; [published online ahead of print, March 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacher AG, Komatsubara KM, and Oxnard GR. Application of Plasma Genotyping Technologies in Non-Small Cell Lung Cancer: A Practical Review. J Thorac Oncol 2017;12:1344–1356. [DOI] [PubMed] [Google Scholar]
- 5.Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol 2018;36:1631–1641. [DOI] [PubMed] [Google Scholar]