Abstract
Objective:
Artificial pancreas (AP) systems have been shown to improve glycemic control throughout the day and night in adults, adolescents, and children. However, AP testing remains limited during intense and prolonged exercise in adolescents and children. We present the performance of the Tandem Control-IQ AP system in adolescents and children during a winter ski camp study, where high altitude, low temperature, prolonged intense activity, and stress challenged glycemic control.
Methods:
In a randomized controlled trial, 24 adolescents (ages 13–18 years) and 24 school-aged children (6–12 years) with Type 1 diabetes (T1D) participated in a 48 hours ski camp (~5 hours skiing/day) at three sites: Wintergreen, VA; Kirkwood, and Breckenridge, CO. Study participants were randomized 1:1 at each site. The control group used remote monitored sensor-augmented pump (RM-SAP), and the experimental group used the t: slim X2 with Control-IQ Technology AP system. All subjects were remotely monitored 24 hours per day by study staff.
Results:
The Control-IQ system improved percent time within range (70–180 mg/dL) over the entire camp duration: 66.4 ± 16.4 vs 53.9 ± 24.8%; P = .01 in both children and adolescents. The AP system was associated with a significantly lower average glucose based on continuous glucose monitor data: 161 ± 29.9 vs 176.8 ± 36.5 mg/dL; P = .023. There were no differences between groups for hypoglycemia exposure or carbohydrate interventions. There were no adverse events.
Conclusions:
The use of the Control-IQ AP improved glycemic control and safely reduced exposure to hyperglycemia relative to RM-SAP in pediatric patients with T1D during prolonged intensive winter sport activities.
Keywords: artificial pancreas, closed loop, pediatrics, type 1 diabetes
1 |. INTRODUCTION
The landmark diabetes trial (DCCT) established the association between poor glycemic control and long-term complications including retinopathy, nephropathy, neuropathy, and how intensive insulin therapy will reduce these complications.1 Despite the reduction of micro-vascular complications, improvement in glycemic control in the DCCT resulted in a significant increase in severe hypoglycemic episodes especially in children with type 1 diabetes (T1D).2 These concerns continue to this day with an average hemoglobin A1C (HbA1c) >8% in children 8 and above, reaching 9% by 16 years of age.3 Less than 20% of youth achieve HbA1c levels in target range per current American Diabetes Association guidelines (HbA1c ≤7.5%) .4,5 In addition, about 10% suffer from one or more episodes of diabetes ketoacidosis (DKA) per year and 6% have one or more severe hypoglycemic events per year.6
Automated glucose control is a promising approach to regulate glucose and decrease the burden of diabetes care for the pediatric patients and their parents. An artificial pancreas (AP) or closed loop system includes a continuous subcutaneous insulin infusion pump, a continuous glucose monitor (CGM) and a control algorithm that adjusts insulin infusion frequently (ie, every 5 minutes) based on the data from CGM.7 These systems have been in development for about two decades, with one commercial system currently available in the United States (Medtronic Minimed 670G), and shown to significantly improve glycemic control in patients with T1D.8–10
Both inpatient and outpatient trials of AP systems in children have showed efficacy in glucose control.11–13 In AP studies, despite the increase in time in range (TIR) and the reduction in hypoglycemia, optimal management following meals and exercise remains challenging. The impact of exercise on glucose level response varies by age, fitness level, duration, and the intensity of exercise.14,15 Prolonged and intense exercise is often associated with an increased risk of hyperglycemia and immediate or delayed hypoglycemia.16 The insulin dosing adjustment (eg, reduction in basal rate) and carbohydrate treatment prior to the exercise in free-living condition might not lead to optimal glycemic control because of the slow absorption rate of current insulin analogs or blunted counter regulatory hormone responses.17 Several studies have shown the efficacy and safety of the of AP use in in the pediatric and adolescent population.18–20 There are multiple approaches to the specific management of physical activity by these systems that will assist the insulin dosing modulation; including bi-hormonal (insulin and glucagon) closed loop systems, or exercise-informed control algorithms by using heart rate monitor, accelerometer and exercise announcement.21–27
Several of the distinctive features of winter sport activities lead to especially challenging glycemic control including an increase in glucose uptake, depletion in muscle glycogen, variable oxygen consumption, stress, fear, elevated altitude, and cold temperature. The AP system developed at the University of Virginia was challenged previously in a series of ski and summer camps where physical activity and meal sizes are greater than usual, and the controller was shown to improve the glycemic control and reduced exposure to hypoglycemia in adolescent participants.20,28,29 In these previous studies, the AP system consisted in an Android smartphone (DiAs platform, UVA) running the algorithm, linked to a G4 505 CGM (Dexcom, San Diego) and either a Combo Spirit (Roche, Mannheim, Germany) or t: slim (Tandem, San Diego) insulin pump. 24 hours/day remote monitoring was available through the DiAs Web Monitoring cloud system.
The present randomized control study is the first pediatric study of the UVA/TypeZero algorithm on-boarded onto the Tandem T:slim X2 pump integrated with the Dexcom G6 CGM. The trial was conducted in three winter/ski camp environments (the first in Wintergreen, VA, at an elevation of 1071 m, the second in Breckenridge, CO, at an altitude of 2960 m, and the third in Kirkwood, CA, at an elevation of 2400 m). This study aimed to show the superiority of closed-loop control using the t:slim X2 with Control-IQ Technology, (Control-IQ) and assess usability in a supervised setting in a controlled environment compared to current state-of-the-art Sensor-Augmented Pump (SAP) therapy for the treatment of T1D in adolescents (13–18 years old) and school-aged children (6–12 years old) and to challenge the different components (algorithm, pump, CGM) of the system. Both the Control-IQ group and the SAP group were remotely monitored by study staff using the Dexcom Share app.
1.1 |. Research design and methodss
We completed a multicenter, randomized controlled clinical trial (clincialtrial.gov ) designed to assess if t:slim X2 with Control-IQ Technology can safely improve time spent in target range during prolonged outdoor winter sports (48-hours ski camp). The research protocol was approved by the Food & Drugs Administration (IDE G170267), UVA, the University of Colorado and Stanford University institutional review boards. Study participants and their parent(s) signed consent/assent prior to enrollment.
1.1.1 |. Devices and systems
As described earlier, the experimental AP Group used the Tandem t: slim X2 with Control-IQ Technology (Tandem Diabetes Care, San Diego, California) which was integrated with the Dexcom G6 CGM (Dexcom, San Diego, Callifornia).28 The control SAP group used their home insulin pumps; pumps used included the Tandem t:slim (N = 9), the Insulet Omnipod (N = 4), a variety of Medtronic pumps (N = 7, 670G, 530G, revel, Paradigm, 751), and the Animas Ping (N = 4); automated insulin modes (AP, predictive low glucose suspend, and threshold low glucose suspend) were deactivated during the study. All participants were fitted with a Dexcom CGM G5 continuous glucose monitor with share capability with minimum calibration before breakfast and dinner (7:00 am and 7:00 pm) using a study-provided blood glucose meter (BGM) (ContourNext Link; Ascencia Diabetes Care, Parsippany, New Jersey); therefore, AP group participants were asked to wear two different CGMs. Participants also wore physical activity (PA) trackers (Fitbit Charge HR; Fitbit, San Francisco, CA); PA data were collected after the study completion and not used by the Control-IQ system.
The study was split into two sequential phases (Figure 1). All participants were between 13 and 18 years old in phase 1 and between 6 and 12 years old in phase 2, and had a clinical diagnosis of T1D, were insulin-treated for at least 1 year, and were on insulin pump treatment for at least 3 months. Exclusion criteria included a recent history of severe hypoglycemia or diabetes ketoacidosis (within the last 6 months), requiring long-acting or any noninsulin anti-diabetic medications, pregnancy, active renal or cardiac illness, and history of altitude sickness.
FIGURE 1.
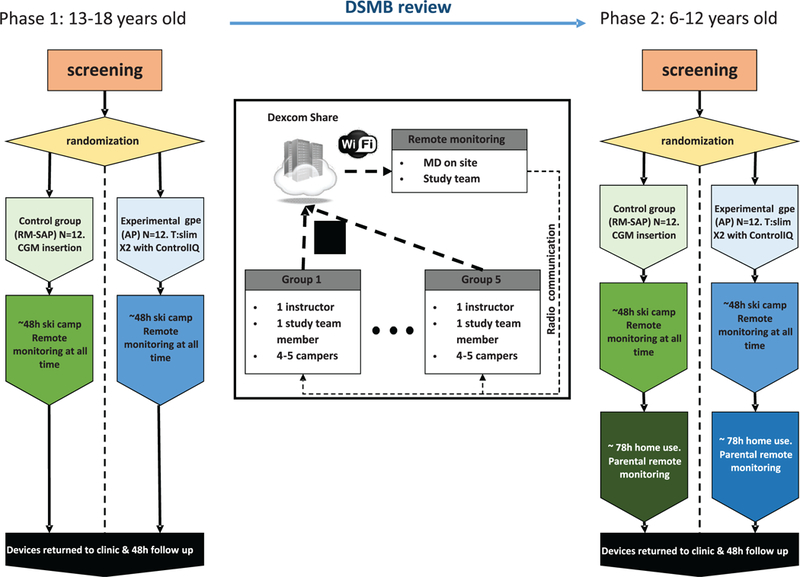
Study design. There was remote monitoring by a physician 24 hours per day. Each skiing group of four to five campers was led by one riding on insulin instructor and followed by a study team member
All of the insulin pump parameters were reviewed by the camp medical team and adjustments were made to address the increase in physical activity that occurred during the study. There was an initial 10% to 20% reduction in insulin dosing upon arriving at camp for participants in both groups. If significant hypoglycemia was observed, further reduction of total insulin was made on the following day. Meal boluses for both groups were computed using personal settings and were supervised by the study staff. Insulin pump infusion sets were systematically changed at camp onset, and locations on body varied based on the subject’s preference.
Study participants were divided into two equal groups at each site, half using SAP, and half Control-IQ. Using a randomized block design, the eligible participants were assessed and put in blocks of two according to age and HbA1c, if an identical match was not possible the closest in age and HbA1c value were paired and then randomized. The members of each block were then randomly assigned, one to each of the two treatment groups.
Closed loop control mode was initiated on the Tandem pump once a CGM value was available, and a confirmatory blood glucose measurement (BGM) was between 80 and 300 mg/dL.
All the camp’s recreational activities were managed by “Riding on Insulin” with study staff supervision. “Riding on Insulin” (www.ridingoninsulin.org) is a non-profit organization specialized in engaging the pediatric T1D population in skiing and snowboarding.
1.1.2 |. Remote monitoring and safety protocols
During system use (both phase 1 and 2), participants in both groups were remotely monitored in real time by the study staff including a study physician using the Dexcom G5 share/follow system. They had immediate access to medical and technical personnel. Notifications were set for CGM readings below 80 mg/dL during the day and 70 mg/dL at night or above 300 mg/dL to alert study staff of the need for treatment or BGM confirmation. During Phase 2, considering the younger age of the participants, the upper threshold alert was reduced to 250 mg/dL.
Participants performed confirmatory BGM before each meal, snack and 30 minutes prior to the activity. A minimum of 100 mg/dL prior to skiing was required, and non-insulinized carbohydrates were consumed if this condition was not met. The management of hyperand hypoglycemia was based on standardized protocol glycemic treatment guidelines (see protocol in Appendix S1).
During skiing, one or two study staff were assigned to each group with access to cellphones to monitor up to five campers on the mountain. Concurrent monitoring was conducted at a central location inside of the resort where study stuff was monitoring all the campers using the transmission from each participant’s Dexcom G5 CGM.
Following the completion of the ski camp, the participants of the Stanford University and Barbara Davis Center sites continued the study at home for 72 hours using either the Control-IQ or SAP therapy. The results are reported in a separate manuscript.30
1.1.3 |. Outcomes and statistical analysis
All glycemic outcomes were calculated based on the Dexcom G5 CGM readings. The primary outcome was the percent time spent between 70 and 180 mg/dL. The secondary outcomes were derived from published guidelines31: percent time spent <70, <60, and < 54 mg/dL, percent time spent >180, and > 250 mg/dL, percent time spent between 70 and 150 mg/dL overnight (11 pm–7 am), percent time spent between 70 and 180 mg/dL during the day (7 am–11 pm), as well as total amount of CHO treatments. Outcomes were reported in segments of the day: daytime (7:00 am to 11:00 pm), overnight (11:00 pm to 7:00 am), and ski (9:00 am to 12:00 pm and 1:00 pm to 4:00 pm).
Primary statistical analysis between treatment groups was performed using univariate analysis of variance (ANOVA) with the treatment mode and age group as fixed factors. Years since diagnosis, weight, and activity (measured as average heart rate) were included as covariates to the analysis. Outcomes are reported as mean ± SD. For outcomes too skewed for ANOVA analysis (eg, percent time below 60 mg/dL) we compared median between groups and reported median (quartiles). P- value <0.05 was considered significant. The statistical analysis was performed in SPSS 23 (IBM), and data formatting and preparation were conducted in Matlab 2018a (Mathwork).
1.2 |. Results
1.2.1 |. Subjects
In total, 54 subjects were enrolled across three sites, and 48 participated and completed the study in three sites (24 in UVA, 12 in Colorado and 12 at Stanford). One subject signed consent but did not meet eligibility criteria (no history of pump use), and five subjects left the study after a change in the Stanford camp dates due to blizzard cancellation. Table 1 lists baseline data for the 48 participants who completed the study. The average age was 12.3 ± 3.2 years with a range of 7 to 17 years old that included two groups of school-aged children (6–12 years) and adolescents (13–18 years). The baseline HbA1C was 7.8% ± 1.1%. There was no significant difference between any of these variables between Control-IQ and SAP groups in each site.
TABLE 1.
Demographics characteristics of the participants
| Site |
CA |
CO |
VA |
Overall cohort |
||||
|---|---|---|---|---|---|---|---|---|
| Study arm | RM-SAP | Control-IQ | RM-SAP | Control-IQ | RM-SAP | Control-IQ | RM-SAP | Control-IQ |
| Number | N = 6 | N = 6 | N = 6 | N = 6 | N = 12 | N = 12 | 24 | 24 |
| Gender (% female) | 50 | 50 | 50 | 50 | 54 | 46 | 54 | 46 |
| Age (years) | 9.7 ± 1.4 | 10.8 ± 2.4 | 8.8 ± 0.4 | 9.2 ± 1.6 | 14.8 ± 1.8 | 15.0 ± 1.4 | 12.0 ± 3.2 | 12.5 ± 3.1 |
| Height (cm) | 138.4 ± 13 | 4 143.9 ± 12 | 136.5 ± 2.4 | 137.9 ± 8.9 | 168.9 ± 8.7 | 166.6 ± 10.2 | 153.2 ± 18.8 | 153.8 ± 16.6 |
| Weight (kg) | 36.7 ± 4.4 | 41.2 ± 9.2 | 31 ± 3.1 | 36.5 ± 10.7 | 73.6 ± 16.8 | 60.1 ± 10.9 | 53.7 ± 24.2 | 49.5 ± 14.2 |
| BMI (kg/m2) | 18.9 ± 0.8 | 19.6 ± 1.5 | 16.6 ± 1.6 | 18.9 ± 3.8 | 25.7 ± 4.7 | 21.5 ± 2.4 | 21.7 ± 5.7 | 20.4 ± 2.8 |
| Diabetes duration (years) | 4.0 ± 0.7 | 4.7 ± 0.2 | 4.8 ± 1.6 | 4.7 ± 2.8 | 5.6 ± 2.4 | 6.7 ± 3.2 | 5.0 ± 2.0 | 5.7 ± 2.9 |
| Pump use (years) | 3.3 ± 0.7 | 4.2 ± 1.7 | 3.7 ± 2.0 | 4 ± 2.3 | 4.9 ± 2.1 | 6.5 ± 3.5 | 4.2 ± 2.0 | 5.3 ± 3.0 |
| Daily insulin dose (U/kg) | 0.7 ± 0.0 | 0.8 ± 0.1 | 0.7 ± 0.2 | 0.7 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| Glycated hemoglobin (%) | 7.2 ± 0.3 | 7.4 ± 0.7 | 7.6 ± 0.7 | 7.3 ± 0.8 | 8.0 ± 1.1 | 8.3 ± 1.5 | 7.7 ± 0.9 | 7.8 ± 1.3 |
The Control-IQ group spent 94% of time in closed loop (22 hours and 34 minutes/day) with minimal connectivity issues mainly due to offline CGM (3.8% or 54 minutes/day); all other causes (eg, CGM warmup, manual disconnect, cold alerts, maximum delivery condition, etc.) accounted for the remaining 2.2%, or 32 minutes/day. For Ap group participants, the G5 and G6 sensors did disagree significantly (Precision Absolute Relative Deviation, PARD, of 11.4%, or an average absolute deviation of 16.0 mg/dL), especially the first 24 hours (PARD = 14%, equivalent to an average absolute deviation of 20.2 mg/dL).
1.2.2 |. Adverse events
Data for three participants were excluded. For one subject, a software error resulted in prolonged AP system downtime. The error was identified during the data analysis of phase 1 and was resolved by a software update prior to phase 2; no glycemic adverse events, % < 70 = 1%, TIR = 34.5%. For a second subject, repeated pump occlusions resulted in prolonged system downtime; no glycemic adverse events, % < 70 = 0%, TIR = 43.1%. For the third subject, the Tandem pump was initialized with a sibling’s pump settings resulting in strongly biased results; no glycemic adverse events, % < 70 = 7.05%, TIR = 88.4%. There were four participants in phase 2 whose pumps were found to have an error in the software causing the initial total insulin dose estimation to be 50% of the value entered by the user. The error was found following the completion of the study. The data for these participants were included in the data analysis, as the impact on system function was judged to be minimal, and any impact would bias the results to the null hypothesis. There were no episodes of severe hypoglycemia in any participants.
1.2.3 |. Glycemic outcomes
Use of the Control-IQ system was associated with a significant increase (12%, or ~3 hours) in percent time-in-range (70–180 mg/dL) over the entire camp duration (66.4 ± 16.4 vs 53.9 ± 24.8% [Control-IQ vs RM-SAP], P = .010), as depicted in Figure 2. Of note 5 out of 12 participants in RM-SAP group had no hypoglycemia with sub-optimal TIR. The difference between the two groups was more pro-found (28%) during the night 78.6 ± 20.3 vs 50.9 ± 34.2%, P = .001. The Control-IQ system was associated with a significantly lower average glucose based on CGM data: 161 ± 30 vs 177 ± 37 mg/dL, P = .023. The reduction in average overnight glucose was even more significant (143 ± 36 vs 175 ± 53 mg/dL, P = .005, Figures 2 and 3).
FIGURE 2.
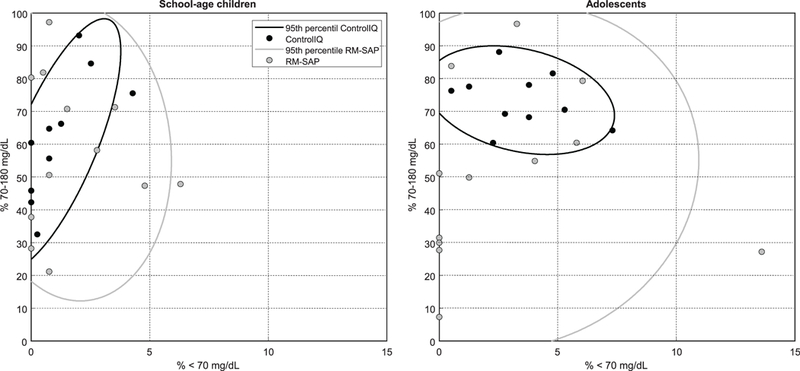
Plots showing the glycemic control outcomes (percent time < 70 mg/dL and time in target range) and the confidence intervals in two arms (Control-IQ and RM-SAP), each participant identified as circles
FIGURE 3.

Glycemic control as represented by mean (plain and dotted line) and quartiles (gray envelopes) of CGM values during the day for both RM-SAP (light gray and dotted line) and Control-IQ (dark gray and plain line). The glucose traces are aligned with the average hourly increase in heart rate (compared to resting heart rate, right axis), without group contrast
There was no difference between groups for the average time in hypoglycemia (<70 mg/dL) 2% [0.5–3.8] vs 0.8% [0–3.7], not significant (ns). The total amount of carbohydrate treatments for hypoglycemia (Control-IQ: 45.5 ± 27.8 vs RM-SAP: 57.7 ± 57.8 g) and the number of treatments were similar in both groups (Control-IQ: 2.8 ± 1.5 vs RM-SAP: 3.2 ± 2.4). The Control-IQ system was also associated with a 15% reduction in time spent with hyperglycemia (>180 mg/dL) 31.4 ± 17.6% vs 43 ± 24.5%, P = .015. This reduction was more than doubled overnight 18.2 ± 21.4% v.s. 44.5 ± 36.9%, P = .001. The exposure to significant hyperglycemia (>250 mg/dL) was reduced in the Control-IQ group by 5.6%: 10.4 ± 11.4% vs 16 ± 13.6% P = .059 with no significant overnight difference: 5.3 ± 13.5% vs 13.2 ± 19.0%. The mean total daily amount of insulin was not different between groups.
1.2.4 |. The interaction between age group and glycemic control
Glycemic control was similar in the two age groups of the participants (6–12 years old vs 13–18 years old). The glycemic outcomes during the experiment, contrasting the two age groups are presented in Table 2. In summary, the increase in overall percent TIR (70–180 mg/dL) using Control-IQ was significantly greater in adolescents: 73.5 ± 8.4% vs 50 .0 ± 26.8%, than in younger children: 59.9 ± 19.5% vs 57.7 ± 23.1%, P = .024. This difference was mainly driven by daytime control 68.9 ± 11.1% vs 50.0 ± 27.3%; younger children: 56.4 ± 22.7% vs 59.6 ± 22.3%, P = .042, and potentially, but not significantly, overnight: adolescents: 87.3 ± 10.8% vs 49.9 ± 35.6%; younger children: 70.6 ± 24% vs 52 ± 34.3%, P = .065. Exposure to hyperglycemia >180 mg/dL was decreased more significantly in adolescents 22.9 ± 8.2% vs 45.5 ± 26.7%, than in younger children 39.0 ± 20.5% vs 40.5 ± 23.1%, P = .025. This was similar for percent time > 250 mg/dL and > 300 mg/dL; P = .017, and P = .034, respectively. The reduction in the average CGM between the Control-IQ and SAP groups was also greater for the adolescents (– 35 mg/dL) than for the younger children (– 5 mg/dL), P = .016. There was a similar pattern for day vs night time (Figure 3).
TABLE 2.
Glycemic outcomes measured by continuous glucose monitor during the day, night, and skiinga
| Control-IQ |
RM-SAP |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | School-age | Teens | All | School-age | Teens | Tx | Age group | Tx* Age gpe | |
|
Time between 70 and 180 mg/dL [%] |
66.4 ± 16.4 | 59.9 ± 19.5 | 73.5 ± 8.4 | 53.9 ± 24.8 | 57.7 ± 23.1 | 50 ± 26.8 | 0.010 | 0.089 | 0.024 |
| Time below 50 mg/dL [%] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0.1] | 0 [0–0] | ns | ns | ns |
| Time below 54 mg/dL [%] | 0 [0–0] | 0 [0–0] | 0 [0–0.2] | 0 [0–0.1] | 0 [0–0.5] | 0 [0–0] | ns | ns | ns |
| Time below 60 mg/dL [%] | 0 [0–0.8] | 0 [0–0.3] | 0.5 [0–1.6] | 0 [0–0.6] | 0 [0–0.9] | 0 [0–0.5] | ns | ns | ns |
| Time below 70 mg/dL | 2 [0.5–3.8] | 0.8 [0–1.6] | 3.3 [2.3–4.5] | 0.8 [0–3.7] | 0.8 [0.4–3] | 0.9 [0–4.5] | ns | 0.062 | ns |
| Time above 180 mg/dL [%] | 31.4 ± 17.6 | 39 ± 20.5 | 22.9 ± 8.2 | 43 ± 24.5 | 40.5 ± 23.1 | 45.5 ± 26.7 | 0.015 | 0.199 | 0.025 |
| Time above 250 mg/dL [%] | 10.4 ± 11.4 | 14.8 ± 14 | 5.6 ± 4.9 | 16 ± 13.6 | 13.2 ± 12.5 | 18.8 ± 14.5 | 0.059 | 0.064 | 0.017 |
| Time above 300 mg/dL [%] | 3.9 ± 5.9 | 6.5 ± 7.2 | 1 ± 1.3 | 6.9 ± 6.7 | 6.6 ± 6.6 | 7.3 ± 7 | 0.034 | 0.074 | 0.034 |
| Mean glucose [mg/dL] | 161 ±29.9 | 175.9 ± 33.9 | 144.5 ± 11.7 | 176.8 ± 36.5 | 174.5 ± 30.7 | 179 ± 42.7 | 0.023 | ns | 0.016 |
| Glucose variability [coefficient of variation %] |
34.2 ± 6.1 | 32.4 ± 5.3 | 36.2 ± 6.6 | 33.9 ± 8.4 | 33.2 ± 8 | 34.6 ± 9 | ns | ns | ns |
| Insulin [U/kg] | 40.5 ± 16.7 | 29.5 ± 10.9 | 52.7 ± 13.1 | 43.9 ± 28.4 | 21.4 ± 8.4 | 66.3 ± 22.8 | ns | 0.081 | ns |
| CHO treatment [g] | 45.5 ± 27.8 | 42.1 ± 27.3 | 49.3 ± 29.2 | 57.7 ± 57.8 | 55.8 ± 36.1 | 59.6 ± 75.4 | ns | ns | ns |
| Daytime [7 am – 11 pm] | |||||||||
| Time below 50 mg/dL [%] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | ns | ns | ns |
| Time below 54 mg/dL [%] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0.1] | 0 [0–0] | ns | ns | ns |
| Time below 60 mg/dL [%] | 0 [0–0.3] | 0 [0–0] | 0 [0–0.8] | 0 [0–0.1] | 0 [0–0.8] | 0 [0–0] | ns | ns | ns |
| Ttime below 70mgdl | 1 [0–3.3] | 0.7 [0–1.7] | 2.5 [0.4–4.7] | 0.8 [0–2] | 1 [0.5–2.7] | 0.7 [0–1.8] | ns | ns | ns |
| Time between 70 and 180 mg/dL [%] |
62.4 ± 18.8 | 56.4 ± 22.7 | 68.9 ± 11.1 | 54.8 ± 24.9 | 59.6 ± 22.3 | 50 ± 27.3 | 0.095 | 0.104 | 0.042 |
| Time above 180 mg/dL [%] | 35.7 ± 19.8 | 42.6 ± 23.7 | 28.1 ± 11.3 | 42.5 ± 24.9 | 38.4 ± 22.4 | 46.5 ± 27.6 | 0.124 | 0.187 | 0.040 |
| Time above 250 mg/dL [%] | 12.1 ± 13.6 | 17.1 ± 16.3 | 6.6 ± 6.9 | 16.9 ± 14.4 | 13.3 ± 12 | 20.4 ± 16.3 | 0.108 | 0.053 | 0.010 |
| Time above 300 mg/dL [%] | 4.6 ± 7.2 | 7.6 ± 9 | 1.3 ± 1.7 | 7.3 ± 7.5 | 6.5 ± 6.4 | 8.1 ± 8.7 | 0.080 | 0.038 | 0.016 |
| Mean glucose [mg/dL] | 166.9 ± 34 | 180.3 ± 40.6 | 152 ± 16.4 | 177.2 ± 37.6 | 170.4 ± 30.1 | 183.9 ± 44.1 | 0.111 | 0.130 | 0.017 |
| Glucose variability [coefficient of variation %] |
33.5 ± 4.8 | 32.2 ± 3.3 | 34.9 ± 5.9 | 34.2 ± 8.2 | 35.3 ± 8.1 | 33.2 ± 8.5 | ns | ns | ns |
| Insulin [U/kg] | 30.9 ± 13.1 | 22.1 ± 8 | 40.7 ± 10.5 | 32.7 ± 20.4 | 16.3 ± 6.2 | 49 ± 15.8 | ns | 0.044 | ns |
| CHO treatment [g] | 38.5 ± 25.2 | 35.1 ± 23.6 | 42.3 ± 27.6 | 47.5 ± 50.2 | 46.8 ± 29.1 | 48.2 ± 66.5 | ns | ns | ns |
| Overnight [11 pm – 7 am] | |||||||||
| Time below 50 mg/dL [%] | 0[0–0] | 0[0–0] | 0[0–0] | 0 [0–0] | 0[0–0] | 0 [0–0] | ns | ns | ns |
| Time below 54 mg/dL [%] | 0[0–0] | 0[0–0] | 0[0–0] | 0 [0–0] | 0[0–0] | 0 [0–0] | ns | ns | ns |
| Time below 60 mg/dL [%] | 0 [0–1] | 0[0–0] | 0.5 [0–1.8] | 0 [0–0] | 0[0–0] | 0 [0–0.5] | ns | 0.141 | 0.149 |
| Time below 70mgdl [%] | 0 [0–8.2] | 0[0–0] | 6.1 [0.3–9.7] | 0 [0–6.4] | 0[0–0] | 0 [0–8.2] | ns | 0.094 | ns |
| Time between 70 and 180 mg/dL [%] |
78.6 ± 20.3 | 70.6 ± 24 | 87.3 ± 10.8 | 50.9 ± 34.2 | 52 ± 34.3 | 49.9 ± 35.6 | <0.001 | ns | 0.065 |
| Time between 70 and 150 mg/dL [%] |
60.8 ± 26.5 | 45.5 ± 27.7 | 77.6 ± 10.4 | 32.1 ± 33.2 | 24.7 ± 28.5 | 39.5 ± 37.1 | <0.001 | ns | 0.033 |
| Time above 180 mg/dL (%) | 18.2 ± 21.4 | 28.2 ± 23.3 | 7.1 ± 12.5 | 44.5 ± 37 | 46.7 ± 34.1 | 42.3 ± 41.1 | 0.001 | ns | 0.111 |
| Time above 250 mg/dL (%) | 5.3 ± 13.5 | 7.7 ± 17 | 2.7 ± 8.4 | 13.2 ± 19 | 12.7 ± 20.2 | 13.7 ± 18.6 | 0.118 | ns | ns |
| Time above 300 mg/dL (%) | 1.8 ± 7.8 | 3.2 ± 10.8 | 0.2 ± 0.6 | 5.8 ± 9.5 | 6.7 ± 11.7 | 4.8 ± 7 | 0.116 | ns | ns |
| Mean glucose (mg/dL) | 142.9 ± 36 | 162.4 ± 37 | 121.6 ± 19.7 | 175.4 ± 52.7 | 187.1 ± 39.9 | 163.8 ± 62.6 | 0.005 | ns | 0.193 |
| Glucose variability (coefficient of variation %) |
24.5 ± 9.9 | 22.6 ± 8.9 | 26.5 ± 10.9 | 24.3 ± 11.6 | 21 ± 13.3 | 27.6 ± 9 | ns | 0.106 | ns |
| Insulin (U/kg) | 7.5 ± 3.1 | 6.1 ± 3 | 9 ± 2.6 | 9.4 ± 10 | 4.1 ± 2.5 | 14.8 ± 11.9 | ns | ns | 0.157 |
| CHO treatment (g) | 2.8 ± 8.3 | 3.4 ± 10 | 2.1 ± 6.5 | 5.5 ± 12.4 | 4 ± 9.7 | 7 ± 14.9 | ns | ns | ns |
| Skiing [9:30 am – noon and 1:30 pm–4 pm] | |||||||||
| Time below 50 mg/dL [%] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | ns | ns | ns |
| Time below 54 mg/dL [%] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | ns | ns | ns |
| Time below 60 mg/dL [%] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | ns | ns | ns |
| Time below 70 mg/dL | 0 [0–0.8] | 0 [0–0.8] | 0 [0–0.8] | 0 [0–0.4] | 0 [0–1.9] | 0 [0–0] | ns | ns | ns |
| Time between 70 and 180 mg/dL [%] |
57.8 ± 27.3 | 50 ± 30.7 | 66.4 ± 21.1 | 55.9 ± 31.1 | 65.2 ± 24 | 46.6 ± 35.5 | ns | ns | 0.063 |
| Time above 180 mg/dL [%] | 41.4 ± 27.8 | 49.3 ± 31.4 | 32.8 ± 21.5 | 41.5 ± 30.3 | 33.4 ± 23.4 | 49.5 ± 35 | ns | ns | 0.073 |
| Time above 250 mg/dL [%] | 14.4 ± 17.3 | 19.8 ± 20.7 | 8.5 ± 10.6 | 18.2 ± 20.4 | 14.4 ± 16.4 | 22 ± 23.8 | ns | ns | 0.064 |
| Time above 300 mg/dL [%] | 5.9 ± 11.1 | 9.2 ± 14.6 | 2.3 ± 3.2 | 7.5 ± 10.9 | 8.1 ± 10.5 | 6.9 ± 11.7 | ns | ns | ns |
| Mean glucose (mg/dL) | 173.2 ± 44.7 | 185.3 ± 53.8 | 159.8 ± 29 | 178 ± 47.4 | 167.5 ± 33.3 | 188.5 ± 57.8 | ns | ns | 0.104 |
| Glucose variability [coefficient of variation %] |
30.2 ± 7.7 | 30 ± 9.3 | 30.3 ± 6 | 29.9 ± 10.5 | 35.2 ± 10.2 | 24.5 ± 7.9 | ns | ns | 0.198 |
| Insulin (U/kg) | 6.8 ± 2.6 | 6.6 ± 2.4 | 6.9 ± 2.9 | 6.6 ± 5.8 | 5 ±3.1 | 8.1 ± 7.4 | 0.105 | 0.001 | ns |
| CHO treatment (g) | 13.4 ± 14.8 | 10.1 ± 9 | 17.1 ± 19.3 | 14.6 ± 19.3 | 14.3 ± 14.7 | 14.9 ± 23.7 | ns | ns | ns |
Mean and SD are reported. For not normally distributed data median and IQRs are reported. Significance levels <0.05 are presented in bold font, levels above 0.2 are presented as ns (not significant).
Abbreviations: CHO, carbohydrate; RM-SAP, remote monitored sensor-augmented pump.
Statistical significances are presented for the treatment effect (Tx: AP vs SAP), the age group effect (Teens vs school age), and their interaction (Tx*Agegpe).
2 |. DISCUSSION
In this multicenter randomized controlled trial, t:slim X2 with Control-IQ Technology safely improved glycemic control specifically time in range (70–180 mg) during day and night in both adolescents and younger children and after controlling for a participant’s age group. The system decreased the average CGM and exposure time to hyperglycemia more significantly in adolescents compared to younger children. Overnight improvements in glycemic control based on time in range and average CGM was significant for both age groups.
These findings occurred despite prolonged exercise, cold temperatures, and high altitude. There was a high level of remote monitoring in the SAP group, which was necessary for patient safety, and the insulin pump settings were reduced by 20% for exercise in younger children. This was similar in both Control-IQ and SAP groups and may have biased the results towards better control for the SAP group. Carbohydrate treatments for hypoglycemia are likely to have been more frequent and prompt in both Control-IQ and SAP groups due to remote monitoring. We intentionally choose a control group which would be most challenging for the Control-IQ system to demonstrate an advantage, that is, a control group using sensor augmented pump therapy32 when only about 11% of patients with T1D are using CGM and insulin pumps to manage their diabetes,33 and few of these experience the degree of constant surveillance that occurred in our clinical trial.
The Control-IQ system functioned similarly to SAP in the reduction of time hypoglycemic and the amount and the number of carbohydrate treatments when the participants were monitored. Patients with T1D are at risk of nocturnal hypoglycemia following afternoon exercise due to increased metabolic demands and blunted counter-regulatory hormones.10 Prolonged exercise in an environment with cold temperature and high altitude make glycemic control even more challenging. Tandem t:slim X2 with Control-IQ Technology improved time in range by approximately 4 hours over the entire ski camp duration. The system has been proven to be safe and efficacious in improving time in range without a significant increase in hypoglycemia exposure during intense continuous activity. The only other study that tested a closed loop system during winter sports was published by this group and showed that the CLC system (DiAs system connected to t-slim or Roche insulin pumps) decreased time with hypoglycemia during the daytime, and overnight exposure to hypoglycemia was most decreased with beginner skiers compared to advanced skiers. In that study, the University of Virginia (UVA) AP system increased time in range by about 1.5 hours during camp duration.28 The current trial is unique in testing an artificial pancreas system in the younger children (younger than 12 years old) during winter sports.
Although not in a ski camp setting, there have been other closed-loop studies in prepubertal children and adolescents in supervised outpatient settings which provided physical activity. For 30, 5 to 9 year old patients used the DiAs system in a camp setting, there was a significant reduction in exposure to hypoglycemia (both overall and overnight) at the expense of a reduction in time-in-target, and increase in average CGM.34
In another randomized, crossover trial, the AP system resulted in increased time in range (73% vs 47%) with no significant change in time with hypoglycemia in 12 children with T1D age 5 to 8 years using the DiAs system.35 In a 48 hour randomized cross-over study in 24 children 5 to 9 years old using either the DiAs system (CL) or a low glucose suspend system (LGS), there was a significant improvement in both overall mean CGM (160.2 in CL vs 180 mg/dL in LGS) and time in range (63.8 in CL vs 48.5% in LGS) with no significant reduction in hypoglycemia.36 In another camp study by Ly et al testing the DiAs system in 10 to 20 year old participants time in range improved during closed loop at 92% vs 80% during SAP (P = .022).20 Using the same system in adolescents increased the time in range from 65.4% to 78.6% and decreased in percent time < 70 mg/dL from 4.2% to 1.8%.29
In randomized, crossover 5-day camp studies, both younger children (aged 6–11 years) and adolescents used a bi-hormonal closed loop system (insulin and glucagon) that was associated with a lower mean CGM glucose in both groups.19,37
Our study had some limitations: We included the data for four subjects in the analysis whose pumps initial estimated total daily dose was not correct because of an error in the software. However, the significant improvement in the primary outcomes was shown using the CLC system despite these four systems potentially under-delivering insulin in these four participants. Our ski camp study confirmed the results of two of the previous studies testing the DiAs system in pre-pubertal children.35,36 The only study testing a CLC system during ski camp by this group resulted in significant improvement in both time in range and hypoglycemia in an adolescent cohort.28 Both groups were supervised remotely through CGM by a physician. The remote monitoring might have improved the glycemia compared to real world control.
In conclusion, during a winter camp and intensive outdoor activities using DiAs algorithm in a Tandem t: slim X2 with Control-IQ Technology in prepubertal children and adolescents improved time in range and decreased average glucose. Future studies using CLC technology during challenging activities without remote monitoring in patients with T1D are necessary to move this field forward.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the volunteers, their families and staff of Riding On. Insulin, the research staff of the UVA Center for Diabetes Technology, Stanford University and the Barbara Davis Center for Childhood Diabetes. This study was funded by the University of Virginia Strategic Investment in Type 1 Diabetes (PrIMeD) Project and by Tandem Diabetes Care.
Funding information
Tandem Diabetes Care; The University of Virginia Strategic Investment in Type 1 Diabetes (PrIMeD) Project; Davis Center; Stanford University
Footnotes
CONFLICT OF INTEREST
L.E. and M.D.D. have no disclosures. L.H.M is a contracted trainer for Medtronic, and consults for Tandem Diabetes, Capillary Biomedical, and Clinical Sensors. R.PW. is an advisory board member for Eli Lilly & Co and has received honorarium from Dexcom. B.A.B. is on advisory boards for Novo Nordisk, Convatec, and Profusa; has received honorarium from Insulet and Tandem, and consulting fees from Medtronic. DMM reports consulting for Abbott, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly and Insulet. BK reports consulting/advisory board for Sanofi and Tandem, speaker bureau for Dexcom, and patent royalties handled by the University of Virginia from J&J, Sanofi, and Dexcom. DRC is a part-time employee of Dexcom (formerly TypeZero Inc.). MDB reports consulting/advisory board for Tandem and Ascensia, speaker bureau for Dexcom and Ascensia, and patent royalties handled by the University of Virginia from Sanofi, and Dexcom.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Diabetes Control and Complications Trial Resesarch Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329 (14):977–986. [DOI] [PubMed] [Google Scholar]
- 2.Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care. 1995;18(11): 1415–1427. [DOI] [PubMed] [Google Scholar]
- 3.Clements MA, Foster NC, Maahs DM, et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes. 2016;17(5): 327–336. [DOI] [PubMed] [Google Scholar]
- 4.Wood J BC, Quinn M, Wong J, et al. Impact of target HbA1c change in pediatric participants in the T1D exchange Clinic Registry. Orlando: American Diabetes Association Scientific Session; 2018. [Google Scholar]
- 5.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cengiz E, Xing D, Wong JC, et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D exchange clinic registry. Pediatr Diabetes. 2013;14(6):447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forlenza GP, Buckingham B, Maahs DM. Progress in diabetes technology: developments in insulin pumps, continuous glucose monitors, and Progress towards the artificial pancreas. J Pediatr. 2016;169: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5: 501–512. [DOI] [PubMed] [Google Scholar]
- 9.Dai X, Luo ZC, Zhai L, Zhao WP, Huang F. Artificial pancreas as an effective and safe alternative in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2018;9(3): 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375(9716):743–751. [DOI] [PubMed] [Google Scholar]
- 12.Elleri D, Allen JM, Nodale M, et al. Automated overnight closed-loop glucose control in young children with type 1 diabetes. Diabetes Technol Ther. 2011;13(4):419–424. [DOI] [PubMed] [Google Scholar]
- 13.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–939. [DOI] [PubMed] [Google Scholar]
- 14.Turksoy K, Paulino TM, Zaharieva DP, et al. Classification of physical activity: information to artificial pancreas control Systems in Real Time. J Diabetes Sci Technol. 2015;9(6):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddell MC, Zaharieva DP, Yavelberg L, Cinar A, Jamnik VK. Exercise and the development of the artificial pancreas: one of the more difficult series of hurdles. J Diabetes Sci Technol. 2015;9(6):1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben Brahim N, Place J, Renard E, Breton MD. Identification of Main factors explaining glucose dynamics during and immediately after moderate exercise in patients with type 1 diabetes. J Diabetes Sci Technol. 2015;9(6):1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel NS, Van Name MA, Cengiz E, et al. Mitigating reductions in glucose during exercise on closed-loop insulin delivery: the ex-snacks study. Diabetes Technol Ther. 2016;18(12):794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson SM, Raghinaru D, Pinsker JE, et al. Multinational home use of closed-loop control is safe and effective. Diabetes Care. 2016;39: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371(4): 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ly TT, Breton MD, Keith-Hynes P, et al. Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care. 2014;37(8): 2310–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs PG, El Youssef J, Reddy R, et al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab. 2016;18(11):1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBoer MD, Chernavvsky DR, Topchyan K, Kovatchev BP, Francis GL, Breton MD. Heart rate informed artificial pancreas system enhances glycemic control during exercise in adolescents with T1D. Pediatr Diabetes. 2017;18(7):540–546. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs PG, Resalat N, El Youssef J, et al. Incorporating an exercise detection, grading, and hormone dosing algorithm into the artificial pancreas using Accelerometry and heart rate. J Diabetes Sci Technol. 2015;9(6):1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasanayake IS, Bevier WC, Castorino K, et al. Early detection of physical activity for people with type 1 diabetes mellitus. J Diabetes Sci Technol. 2015;9(6):1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dadlani V, Levine JA, McCrady-Spitzer SK, Dassau E, Kudva YC. Physical activity capture technology with potential for incorporation into closed-loop control for type 1 diabetes. J Diabetes Sci Technol. 2015;9(6):1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenerson M, Cameron F, Wilson DM, et al. The impact of accelerometer and heart rate data on hypoglycemia mitigation in type 1 diabetes. J Diabetes Sci Technol. 2014;8(1):64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breton MD, Brown SA, Karvetski CH, et al. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther. 2014;16(8):506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breton MD, Chernavvsky DR, Forlenza GP, et al. Closed loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40: 1644–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ly TT, Buckingham BA, DeSalvo DJ, et al. Day-and-night closed-loop control using the unified safety system in adolescents with type 1 diabetes at camp. Diabetes Care. 2016;39:e106–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forlenza GP, Ekhlaspour L, Breton M, et al. Successful At-Home Use of the Tandem Control-IQ Artificial Pancreas System in Young Children During a Randomized Controlled Trial. Diabetes technology& therapeutics. 2019;21(4). 10.1089/dia.2019.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maahs DM, Buckingham BA, Castle JR, et al. Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care. 2016;39(7):1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell SJ, Beck RW. Design considerations for artificial pancreas pivotal studies. Diabetes Care. 2016;39(7):1161–1167. [DOI] [PubMed] [Google Scholar]
- 33.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38(6):971–978. [DOI] [PubMed] [Google Scholar]
- 34.Del Favero S, Boscari F, Messori M, et al. Randomized summer camp crossover trial in 5- to 9-year-old children: outpatient wearable artificial pancreas is feasible and safe. Diabetes Care. 2016;39(7):1180–1185. [DOI] [PubMed] [Google Scholar]
- 35.DeBoer MD, Breton MD, Wakeman C, et al. Performance of an artificial pancreas system for young children with type 1 diabetes. Diabetes Technol Ther. 2017;19(5):293–298. [DOI] [PubMed] [Google Scholar]
- 36.Renard E, Tubiana-Rufi N, Bonnemaison-Gilbert E, et al. Closed-loop driven by control-to-range algorithm outperforms threshold-low-glucose-suspend insulin delivery on glucose control albeit not on nocturnal hypoglycaemia in prepubertal patients with type 1 diabetes in a supervised hotel setting. Diabetes Obes Metab.2019;21(1):183–187. [DOI] [PubMed] [Google Scholar]
- 37.Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4(3):233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


