Abstract
Cytokine-induced memory-like (CIML) NK cells are endowed with the capacity to mediate enhanced effector functions upon cytokine or activating receptor re-stimulation for several weeks following short-term pre-activation with IL-12, IL-15 and IL-18. Promising results from a first-in-human clinical trial highlighted the clinical potential of CIML NK cells as adoptive immunotherapy for patients with hematologic malignancies. However, the mechanisms underlying CIML NK cell differentiation and increased functionality remain incompletely understood. Semaphorin 7A (SEMA7A) is a potent immunomodulator expressed in activated lymphocytes and myeloid cells. In this study, we show that SEMA7A is substantially upregulated on NK cells stimulated with cytokines, and specifically marks activated NK cells with a strong potential to release IFN-γ. In particular, pre-activation of NK cells with IL-12+IL-15+IL-18 resulted in greater than 10-fold upregulation of SEMA7A and enhanced expression of the ligand for SEMA7A, integrin-β1, on CIML NK cells. Strikingly, pre-activation in the presence of antibodies targeting SEMA7A lead to significantly decreased IFN-γ production following re-stimulation. These results imply a novel mechanism by which cytokine-enhanced SEMA7A/Integrin-β1 interaction promotes CIML NK cell differentiation and maintenance of increased functionality. Our data suggest that targeting SEMA7A/Integrin-β1 signaling might provide a novel immunotherapeutic approach to potentiate antitumor activity of CIML NK cells.
Keywords: NK cells, cytokine-induced memory-like NK cells, innate memory, Semaphorin 7A, integrin-β1
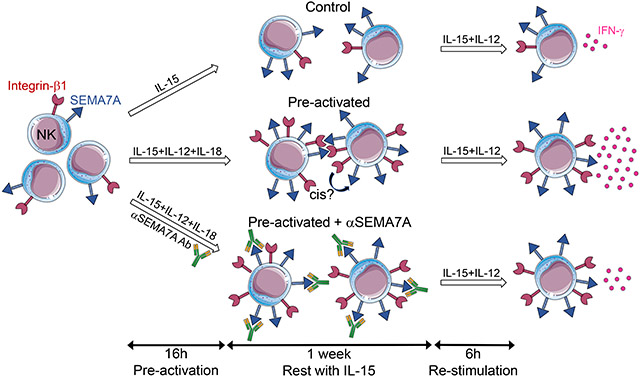
Graphical Abstract
The immunomodulatory molecule SEMA7A and its ligand integrin-β1 are potently upregulated on CIML NK cells. SEMA7A blockade during generation of CIML NK cells results in decreased IFN-y release following cytokine re-stimulation. These findings reveal that cytokine-enhanced SEMA7A/Integrin-β1 interaction promotes CIML NK cell differentiation and maintenance of increased functionality.
INTRODUCTION
Classically, natural killer (NK) cells are viewed as nonspecific effector cells of the innate immune system that play critical role(s) in defense against viral infections and nascent neoplasms. NK cell function is regulated by the integration of inhibitory and activating signals generated by an arsenal of cell surface receptors, with effector functions taking place when activating signals overcome inhibitory ones [1]. Human NK cells are defined as CD3negCD56pos lymphocytes, representing approximately 15% of peripheral blood lymphocytes, and can be subdivided into functionally distinct subpopulations based on expression levels of CD56 and CD16 [2]. CD56brightCD16neg NK cells have a high proliferation potential and the ability to secrete a large amount of cytokines, notably IFN-γ in response to IL-12, with limited cytotoxic functions [3], while CD56dimCD16pos NK cells display strong cytolytic activity as well as a significant capacity to secrete cytokines upon triggering of activating receptors [4, 5]. In addition, a subset of CD56negCD16pos NK cells appears to be expanded in chronic viral infections and might represent an exhausted/anergic subset of NK cells [6-8].
Unexpectedly, a vast amount of independent studies has demonstrated that subsets of murine and primate NK cells are capable of antigen-dependent expansion and long-lived immunological memory [9]. In particular, human NK cells do exhibit memory-like properties, including the capacity to mediate enhanced effector functions after a short pre-activation with a combination of IL-12, IL-15 and IL-18 followed by a prolonged rest period [10], similar to earlier observations in mice [11-13]. Re-stimulation of pre-activated human NK cells using leukemia target cells, cytokines or FcγRIIIa ligation is associated with increased responsiveness that can be retained for several weeks following their initial differentiation into cytokine-induced memory-like (CIML) NK cells [10, 14-18].
The relevance of human CIML NK cells in vivo has been emphasized by the demonstration that exposure to vaccines, and notably inactivated or live attenuated influenza viruses, induces CIML NK cells, thereby potentially promoting enhanced responsiveness to immunization [19-22]. Moreover, the long-lived properties of CIML NK cells have tremendous potential to be exploited for cancer immunotherapy, and preliminary results from a first-in-human phase 1 clinical trial have shown that NK cells pre-activated with IL-12, IL-15 and IL-18 exert robust responses against leukemia targets, leading to remission in a subset of acute myeloid leukemia (AML) patients [15]. A better understanding of CIML NK cell responses may lead to novel strategies to further enhance their antitumor function.
CD56bright and CD56dim NK cells both have the potential to differentiate into CIML NK cells endowed with increased cytotoxicity and IFN-γ production as well as enhanced proliferative capacity [10, 14, 15, 18]. Thus far, potent effector functions of CIML NK cells have been linked to expression of the high-affinity IL-2 receptor αβγ (IL-2Rαβγ), demethylation of the conserved upstream noncoding enhancer region of the IFN-γ gene, recruitment of anergic unlicensed NK cells, enhanced antibody-mediated functions and release from KIR-mediated inhibition [14, 16, 17, 23]. However, mechanisms underlying CIML NK cell differentiation and maintenance of superior functionality of CIML NK cells upon combined pre-activation with IL-12, IL-15 and IL-18 remain unclear.
Semaphorin 7A (SEMA7A), also known as CD108, is a member of the large semaphorin family of transmembrane and secreted proteins of which 20 are expressed in humans [24]. SEMA7A is the only member of the family that is anchored to the cell membrane via glycosylphosphatidylinositol (GPI) [25] and can also be found as a cleaved soluble form [26, 27]. SEMA7A has been implicated in both axon guidance [27] and regulation of immune cell activation [28-35]. In the immune system, SEMA7A is expressed on activated lymphocytes, including NK cells, and in myeloid cells [30, 36-38]. Immunomodulatory functions mediated by SEMA7A mainly rely on its interaction with β1 integrins [27, 28], although plexin C1 was also identified as a binding partner [39-41]. There is evidence that SEMA7A substantially contributes to inflammation and progression of immunopathology in several disorders such as rheumatoid arthritis, multiple sclerosis, pulmonary fibrosis and liver fibrogenesis and therefore this molecule is considered a promising therapeutic target to treat those conditions [33, 34, 42, 43].
SEMA7A has been reported to affect the function of immune cells, including dendritic cells (DCs) [44], monocytes [30, 33], eosinophils [32] and T cells [28, 31]. In activated DCs and monocytes, SEMA7A acts as a potent stimulator of cytokines production and chemotaxis [30, 44]. In mouse models of contact hypersensitivity, SEMA7A and β1 integrin are components of the immunological synapse between antigen-specific T cells and macrophages and their interaction is required to initiate T cell-mediated inflammation [28]. Murine SEMA7A has also been proposed to have T cell-intrinsic inhibitory activity, with the capacity to protect against autoimmunity by limiting antigen-specific T cell responses [31]. However, how SEMA7A expression affects human NK cell function, particularly memory-like responses mediated by NK cells, remains largely unexplored. In the present study, we investigated the expression of SEMA7A on human NK cells and its regulation by cytokines and provide evidence for a role played by SEMA7A and its ligand Integrin-β1 in modulating CIML NK cell function.
RESULTS
SEMA7A is predominantly expressed on CD56bright NK cells
SEMA7A upregulation on NK cells activated with mitogens has been reported [37], however, its expression on NK cells has not been investigated in detail. In a set of experiments not directly related to this project, we noticed expression of SEMA7A on a subset of in vitro expanded individual NK cells bearing distinct functional features. Since the medium used to expand NK cells contained several cytokines, including those known to promote differentiation of NK cells into CIML NK cells [10, 11], we sought to determine which specific subsets of primary NK cells express SEMA7A and how SEMA7A expression relates to NK cell function, particularly in response to cytokines.
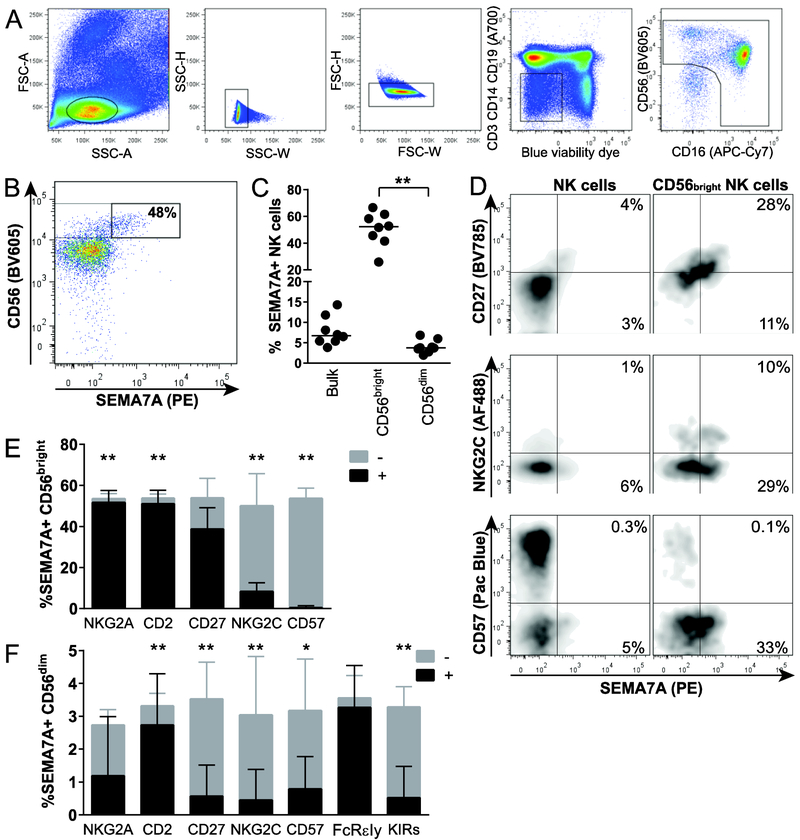
Using multiparameter flow cytometry, we first analyzed the expression of SEMA7A on NK cells in 8 healthy subjects and found that among unstimulated PBMCs, approximately 8% of peripheral blood NK cells were SEMA7Apos. Expression of SEMA7A was primarily restricted to the CD56bright NK cell subset; on average, 50% of the CD56bright NK cells expressed this surface receptor, with significant interindividual variation ranging from 26% to 67% (Fig.1A-C).
Figure 1. Differential expression of SEMA7A on distinct human NK cell subsets.
(A) Representative flow cytometry gating strategy to analyze NK cells. PBMC were first gated on forward angle light scatter (FSC-A) and side light scatter (SSC-A) to define lymphocytes and then on SSC-height and SSC-W gating followed by FSC-height and FSC-W to eliminate doublets. Dead cells were excluded by using LIVE/DEAD™ Fixable Blue Dead Cell Stain kit, and CD3, CD14 and CD19 staining were used to exclude T cells, monocytes and B cells, respectively. CD56 and CD16 were used to identify NK cells within the CD14−CD19−CD3− population. (B) Representative flow cytometry plot depicting SEMA7A expression on unstimulated NK cells from one healthy donor, with gating on CD56bright NK cell subset. (C) Each data point represents proportions of SEMA7Apos NK cells in bulk, CD56bright and CD56dim peripheral blood NK cells for each of 8 healthy donors. Bar indicates the median. Asterisks indicate significant differences between NK cell subpopulations. ** p<0.01. (D) Representative flow cytometry pots depicting co-expression of SEMA7A and CD27 (top), NKG2C (middle) and CD57 (bottom) on bulk (left) and CD56bright (right) NK cells. (E) and (F) Stacked bar charts represent mean + SEM percentages of all SEMA7Apos CD56bright (E) and SEMA7Apos CD56dim (F) NK cells expressing (black portion of the bar) or not expressing (grey portion of the bar) the indicated markers. Asterisks represents significant differences in proportions of SEMA7A+ NK cells expressing or not indicated markers. * p<0.05; ** p<0.01. Data are shown for n=8 donors and pooled from 2 independent experiments. Significance determined by Wilcoxon matched-pairs signed rank test.
While the exact hematopoietic origin of CD56bright NK cells as well as their relationship with other NK cell subpopulations remain a matter of debate [45], peripheral blood CD56bright NK cells probably represent a mixture of both immature NK cells that are direct precursors of CD56dim NK cells [46, 47], and mature NK cells, including CD56dim NK cells that have upregulated CD56 and lost CD16 upon activation in peripheral tissues [48]. To further characterize the subpopulation of CD56bright NK cells that is associated with SEMA7A expression, we assessed co-expression of SEMA7A and several markers classically used to define conventional, mature and/or adaptive NK cells. As expected, SEMA7A was co-expressed with receptors that are found on virtually all CD56bright NK cells such as NKG2A and CD2 [49] (Fig. 1D and E). In addition, more than half of SEMA7A+ CD56bright NK cells also expressed CD27, a marker found on up to 50% of the CD56bright NK cell subset, defining a subpopulation with enhanced ability to secrete IFN-γ, and which is absent on mature cytotoxic NK cells [50]. In both CD56bright and CD56dim NK cell subsets, SEMA7A was typically not or infrequently co-expressed with markers of terminally differentiated or adaptive NK cells such as CD57 or NKG2C, respectively [51-53] (Fig. 1D-F), and almost never expressed in the recently described memory-like NK cell subset lacking the FcR intracellular gamma signaling chain and endowed with potent and broad antibody-dependent properties [54-56]. Altogether, these data suggest that in the absence of ex vivo stimulation, SEMA7Apos NK cells do not bear phenotypic features typical of terminally differentiated or adaptive NK cells.
SEMA7A expression on NK cells is markedly upregulated by cytokines
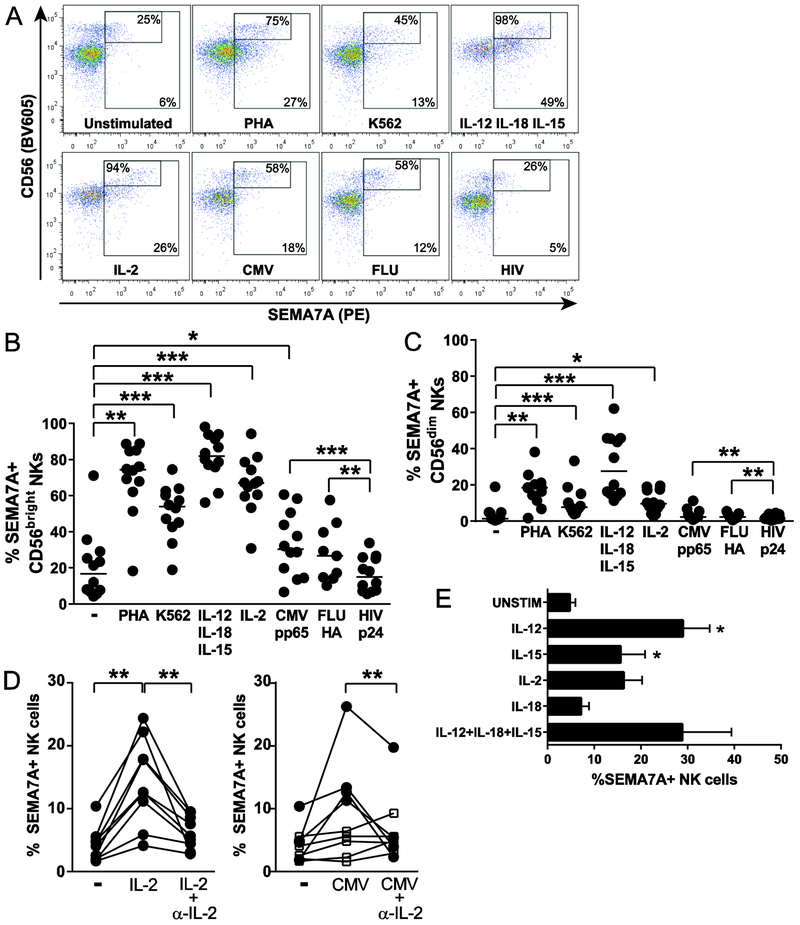
To further delineate the role played by SEMA7A on NK cells, we assessed the expression of SEMA7A on NK cells upon culture of PBMCs with a range of various stimuli. Co-culture was performed in the presence of IL-2 alone, which can potently activate NK cells [57], of combined IL-12+IL-18+IL-15, which can induce memory-like NK cells [10, 11], and of viral proteins, HLA-deficient K562 cells, or PHA. Except HIV p24, each of those stimuli induced upregulation of SEMA7A on NK cells (Fig. 2A-C). Proportions of both CD56bright and CD56dim NK cells expressing SEMA7A were markedly increased by stimulation with IL-12+IL-18+IL-15, and, to a lesser extent by co-culture with PHA, as previously reported [37], IL-2 and K562 cells. While proliferation of SEMA7Apos NK cells upon cytokine stimulation probably contributes to enhancing the frequency of SEMA7Apos NK cells, cell surface density of SEMA7A, as measured by mean fluorescent intensity (MFI), was also increased on activated NK cells, suggesting upregulation of SEMA7A expression by SEMA7Aneg/low NK cells (Supporting information Fig. 1).
Figure 2. Up-regulation of SEMA7A on activated NK cells.
(A) Representative flow cytometry plots depicting SEMA7A expression on NK cells left unstimulated or co-cultured overnight with the indicated stimuli, with gating on all NK cells and on the CD56bright NK cell subset. Stimuli tested included 2 μg/mL viral antigens, 100 U/mL IL-2, 20 ng/mL IL-12 + 10 ng/mL IL-18 + 1 ng/mL IL-15, 5 μg/mL PHA and HLA-deficient K562 cells at 10:1 E:T ratio. (B) and (C) Each data point represents proportions of SEMA7Apos CD56bright (B) or SEMA7Apos CD56dim (C) NK cells from 12 healthy donors co-cultured with indicated stimuli as described above. Bar indicates the median. Data are shown for n=12 donors and pooled from 4 independent experiments. (D) Proportions of SEMA7Apos bulk NK cells following co-culture with 20 U/mL IL-2 or 2 μg/mL CMV pp65 with or without 10 μg/mL anti-IL-2 blocking Ab. Filled circles represent subjects with increased SEMA7A expression on NK cells upon stimulation with IL-2 or CMV. Open squares represent subjects with no SEMA7A response to CMV. Data are shown for n=9 donors and pooled from 3 independent experiments. Statistics were applied on values represented by filled circles. (E) Bar graphs represent mean + SEM percentages of SEMA7Apos NK cells following overnight incubation with individual cytokines including 20 ng/mL IL-12, 1 ng/mL IL-15, 100 U/mL IL-2, 10 ng/mL IL-18 or a combination of 20 ng/mL IL-12 + 10 ng/mL IL-18 + 1 ng/mL IL-15 for 6 healthy donors except for IL-2 (n=4) and for IL-12+IL-18+IL-15 (n=2). Asterisks indicate significant differences in SEMA7A expression. Data are shown for n=6 donors and pooled from 2 independent experiments. * p<0.05, ** p<0.01, and *** p<0.001 determined by Wilcoxon matched-pairs signed rank test.
Interestingly, SEMA7A expression on NK cells was significantly increased in response to CMV and influenza antigens compared to stimulation with HIV p24 (Fig. 2A-C, Supporting information Fig. 1). It is likely that at least 50% of our HIV-negative healthy donors have been exposed to CMV and/or influenza viruses, suggesting an IL-2/antigen-specific CD4pos T cell help-dependent SEMA7A upregulation on NK cells [58-65]. Accordingly, the strongest effect was observed in CD56bright NK cells that constitutively express the high-affinity IL-2 receptor and can use T cell-derived IL-2 even under limiting concentrations. Corroborating this hypothesis, in healthy subjects displaying enhanced SEMA7A expression on NK cells following co-culture with CMV pp65 antigen, IL-2 blockade robustly impaired SEMA7A upregulation on NK cells (Fig. 2D).
Compared to other immune cell types, only resting B cells expressed higher levels of SEMA7A than unstimulated CD56bright NK cells, with over 60% of B cells being constitutively SEMA7Apos (Supporting information Fig. 2A-E). However, PHA stimulation led to a robust increase in SEMA7Apos CD4pos and CD8pos T cells, as previously shown [37], whereas SEMA7A expression on monocytes increased equally in response to PHA and IL-12+IL-18+IL-15. Proportions of SEMA7Apos DCs were enhanced mostly in response to tested cytokines. We also examined expression of integrin-β1, a functional ligand for SEMA7A, on NK cells and other immune cells. All cell types studied expressed integrin-β1 when cultured in medium alone, although to various levels, and expression was not or only slightly affected by stimulation in our 16h assay (Supporting information Fig. 3A-F). The proportions of activated NK cells, particularly cytokine-activated NK cells, expressing integrin-β1 were enhanced but did not statistically differ from those of unstimulated cells.
Finally, we tested the ability of individual cytokines to trigger SEMA7A expression on NK cells and could show that after 16h of stimulation, IL-12 was the most potent of tested cytokines to induce SEMA7A on NK cells, with IL-18 having almost no effect when used alone (Fig. 2E). Altogether, those data show that cytokines that are relevant for NK cell function induce a robust expression of SEMA7A on NK cells, and notably on CD56bright NK cells.
SEMA7A expression marks cytokine-responsive IFN-γ-producing NK cells
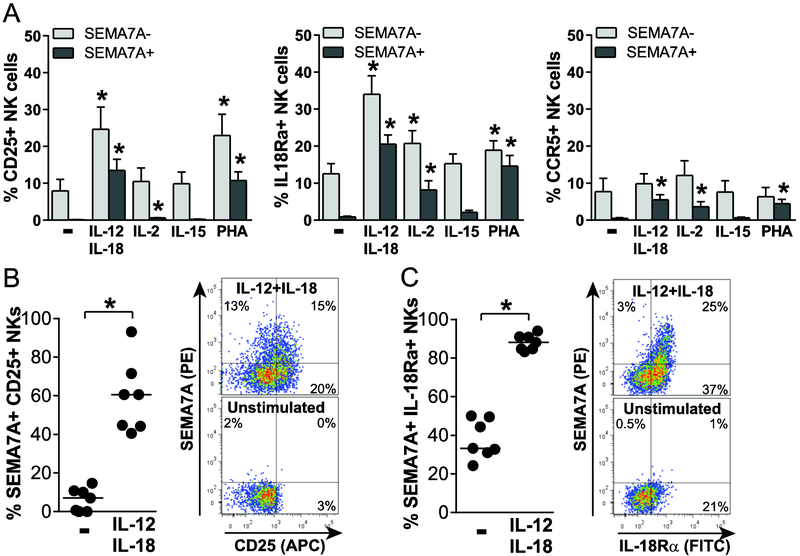
We next sought to determine the functional relevance of SEMA7A expression on activated NK cells and started by investigating expression of cytokine and chemokine receptors on activated SEMA7Apos NK cells. Upon stimulation with IL-2, IL-12+IL-18 or PHA, a significant proportion of CD25pos, IL-18Rαpos and CCR5pos NK cells co-expressed SEMA7A (Fig. 3A). The highest upregulation of CD25, IL-18Rα and SEMA7A was observed in response to IL-12+IL-18, leading to approximately 60% and 90% SEMA7Apos NK cells expressing CD25 (Fig. 3B) or IL-18Rα (Fig. 3C), respectively, with a positive correlation between proportions of SEMA7Apos NK cells and percentages of both CD25pos (Spearman r=0.8214, p=0.03) and IL-18Rαpos (Spearman r=0.8929, p=0.01) NK cells. Altogether, these observations strongly suggest that cytokine-activated SEMA7Apos NK cells can adequately respond to IL-2 and IL-18 and migrate to sites of inflammation.
Figure 3. SEMA7Apos NK cells express innate cytokine and chemokine receptors.
(A) Percentages of CD25pos (left), IL-18Rαpos (middle) or CCR5pos (right) NK cells that do not (light grey bars) or do (dark grey bars) express SEMA7A after overnight culture in the absence or presence of indicated stimuli, including 50 ng/mL IL-12 + 100 ng/mL IL-18, 100 U/mL IL-2, 1 ng/mL IL-15 and 5 μg/mL PHA, were determined by flow cytometry for 7 healthy donors. Bar graphs represent mean + SEM. Asterisks indicate significant differences in proportions of SEMA7Aneg or SEMA7Apos NK cells expressing each marker after stimulation with indicated stimuli compared to unstimulated. (B) and (C) Dot plots (left panel) and representative flow cytometry plot (right panel) depicting proportions of SEMA7Apos CD25pos (B) or SEMA7Apos IL-18Rαpos (C) NK cells after overnight culture in medium alone or supplemented with 50 ng/mL IL-12 + 100 ng/mL IL-18. Data are shown for n=7 donors and pooled from 2 independent experiments. * p<0.05.
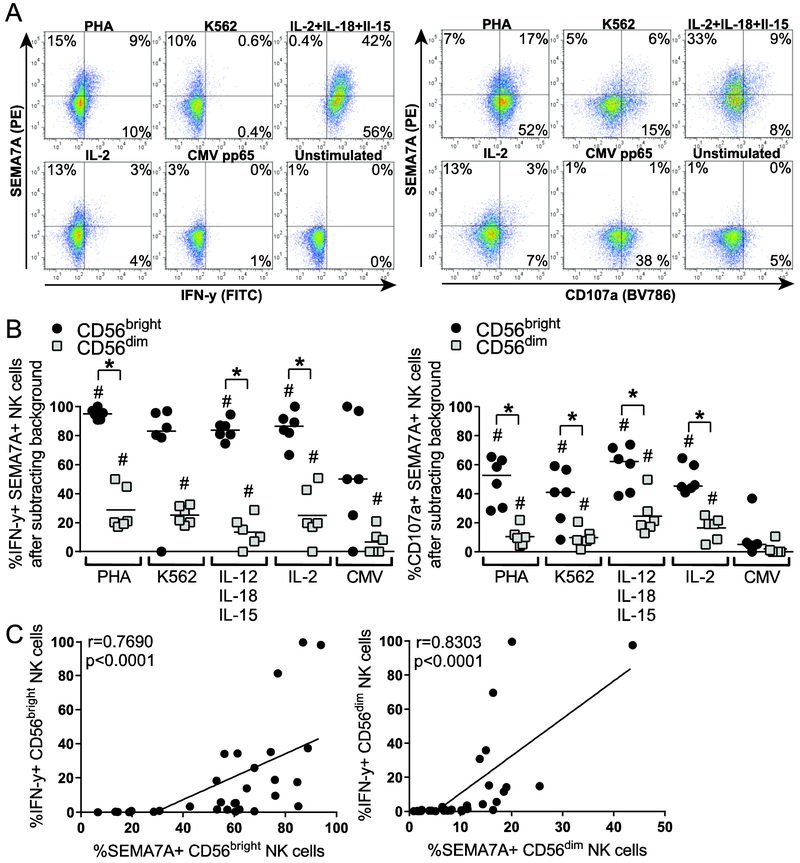
Pro-inflammatory cytokines such as IL-12 and IL-18 potently elicit NK cell cytotoxicity, expansion, and production of immunomodulatory cytokines such as IFN-γ. As IL-12+IL-18 also potently elicit SEMA7A upregulation on NK cells, we next assessed SEMA7A expression on NK cells producing IFN-γ and upregulating CD107a in response to various stimuli, including cytokines. Overall, approximately half of IFN-γpos NK cells expressed SEMA7A, with the vast majority of IFN-ypos CD56bright NK cells co-expressing SEMA7A (Fig. 4A and B). Indeed, over 80% of IFN-γpos CD56bright NK cells express SEMA7A in response to PHA, K562 cells or cytokines, while on average less than 30% IFNγpos CD56dim are SEMA7Apos. These observations are in line with the propensity of CD56bright NK cells to efficiently respond to cytokines and produce IFN-γ. Nevertheless, SEMA7A expression on both CD56bright and CD56dim NK cells significantly correlated with their respective production of IFN-γ upon stimulation (Fig. 4C). Accordingly, a higher proportion of IFN-γpos NK cells was found among SEMA7Apos NK cells than among SEMA7Aneg NK cells for activated CD56bright and CD56dim NK cell subsets and unstimulated CD56dim NK cells (Supporting information Fig. 4 and data not shown). Similarly, higher proportions of CD56bright than CD56dim activated CD107apos NK cells expressed SEMA7A (Fig. 4A and B), yet SEMA7A expression did not correlate with upregulation of the CD107a degranulation marker (data not shown). Altogether, these data support the fact that SEMA7A marks activated NK cells with a high potential to release IFN-γ, such as cytokine-activated CD56bright NK cells.
Figure 4. SEMA7A expression is associated with IFN-γ production by NK cells.
(A) Representative flow cytometry plots depicting co-expression of SEMA7A and IFN-γ (left) or CD107a (right) on bulk NK cells after overnight culture of PBMCs in medium alone or in the presence of 5 μg/mL PHA, K562 cells at 10:1 E:T ratio, 20 ng/mL IL-12 + 10 ng/mL IL18 + 1 ng/mL IL15, 100 U/mL IL-2, or 2.8 μg/mL CMV pp65 native protein. (B) Each data point represents proportions of IFN-γpos (left panel) or CD107apos (right panel) SEMA7Apos CD56bright (black circles) and CD56dim (grey squares) NK cells following overnight incubation with indicated stimuli after subtracting background (unstimulated). Bar indicates the median. Asterisks indicate significant differences in proportions of IFN-γpos NK cells that are positive for SEMA7A between CD56bright and CD56dim NK cells. * p<0.05. Pound signs indicate differences in IFN-γ production upon stimulation compared to unstimulated. ## p<0.01. Significance determined by Wilcoxon matched-pairs signed rank test. Data are shown for n=6 donors and pooled from 2 independent experiments. (C) Spearman correlation of frequencies of SEMA7Apos and IFN-γpos CD56bright (left panel) or CD56dim (right panel) activated NK cells.
SEMA7A blockade impairs memory-like responses mediated by cytokine-pre-activated NK cells
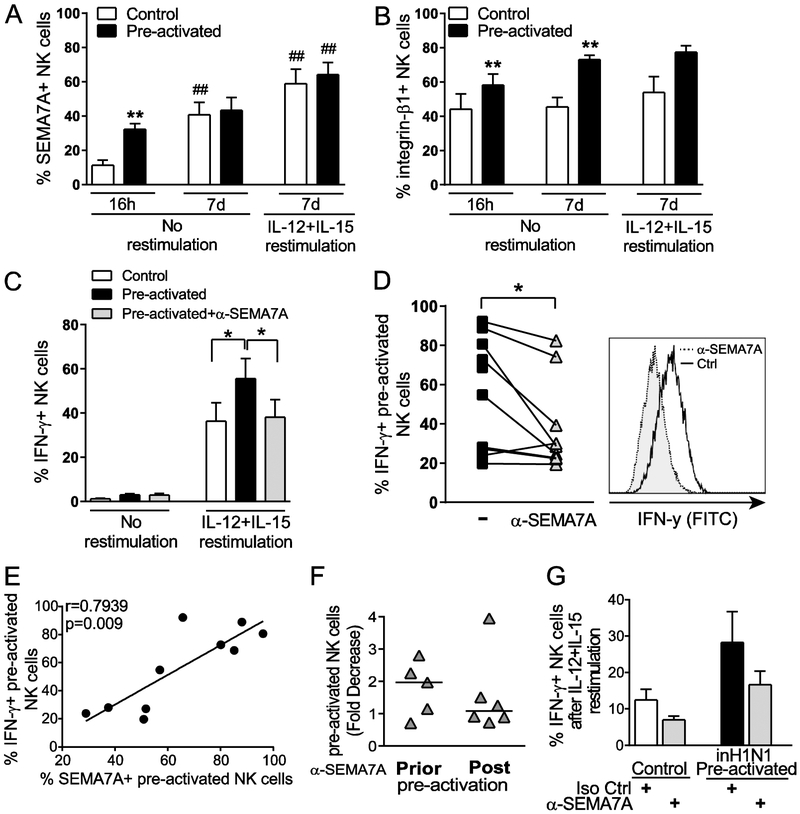
Short NK cell pre-activation with various combinations of IL-12, IL-18 and IL-15 has been associated with differentiation into memory-like NK cells [10] and linked to rapid and prolonged expression of CD25, resulting in a high affinity IL-2 receptor that confers responsiveness to picomolar concentrations of IL-2 [14]. As overnight stimulation with those cytokines potently upregulates SEMA7A on NK cells, with approximately 60% of SEMA7Apos NK cells co-expressing CD25, we next sought to determine the role played by SEMA7A signaling in cytokine-induced memory-like responses by NK cells. To do so, we pre-activated NK cells with IL-15, IL-12 and IL-18 for 16h followed by a week in culture with low concentrations of IL-15 to let the cells rest, as previously described [10]. After 7 days, NK cells were re-stimulated with IL-12 and IL-15 to assess expression of SEMA7A and integrin-β1, the functional ligand for SEMA7A, on control and pre-activated NK cells (Fig. 5A and B). SEMA7A expression was significantly higher on pre-activated NK cells after 16 hours compared to control NK cells. However, after 7 days in culture, we observed similar proportions of SEMA7Apos pre-activated and control NK cells, suggesting that long-term culture in the presence of low-dose IL-15 triggers SEMA7A upregulation. Re-stimulation further enhanced SEMA7A expression on both control and pre-activated NK cells, resulting in comparable levels, and therefore changes in SEMA7A expression itself are not likely to contribute directly to the higher functionality previously reported for pre-activated NK cells. In contrast, a significant increase in proportions of NK cells expressing the ligand for SEMA7A, integrin-β1, could only be observed in pre-activated NK cells even after a week of rest in low-dose IL-15 (Fig. 5B).
Figure 5. SEMA7A blockade impairs cytokine-induced memory-like NK cell responses.
Purified NK cells were incubated for 16 hours with 1 ng/mL IL-15 (Control) or 10 ng/mL IL-12 + 50 ng/mL IL-18 + 1 ng/mL IL-15 (Pre-activated). All NK cells were then washed and cultured with 1 ng/mL IL-15 for 7 days. After a week, NK cells were re-stimulated for 6 hours with 10 ng/mL IL-12 + 100 ng/mL IL-15 to evaluate SEMA7A (A) and Integrin-β1 (B) expression as well as IFN-γ production (C and D). Bar graphs represent mean + SEM proportions of SEMA7Apos (A) and Integrin-β1pos (B) bulk NK cells, comparing control (white bars) and pre-activated (black bars) NK cells at 16 hours and 7 days without re-stimulation and at day 7 after re-stimulation. Asterisks indicate significant differences in SEMA7A or Integrin-β1 expression between control and pre-activated NK cells at each time point. ** p<0.01. Pound signs indicate differences in SEMA7A or Integrin-β1 expression at day 7 compared to 16 hours for either control or pre-activated NK cells. ## p<0.01. (C) Bar graphs represent mean + SEM proportions of IFN-γpos control bulk NK cells (white bars) and of IFN-γpos bulk NK cells that were pre-activated in the presence of isotype control antibodies (black bars) or anti-SEMA7A antibodies (light grey bars) at day 7. (D) Proportions of re-stimulated IFN-γpos NK cells that were pre-activated in the presence of isotype control Ab or anti-SEMA7A Ab for each tested donor. A representative flow cytometry histogram overlay is displayed on the right to illustrate decreased production of IFN-γ production in the presence of anti-SEMA7A antibodies. (C and D) Asterisks indicate significant differences in IFN-γ production determined by Wilcoxon matched-pairs signed rank test. * p<0.05. Data are shown for n=9 donors and pooled from 4 independent experiments. (E) Spearman correlation of frequencies of SEMA7Apos and IFN-γpos CIML NK cells upon re-stimulation with IL-12+IL-15. Data are shown for n=10 donors and pooled from 4 independent experiments. (F) Each data point represents fold decrease in IFN-γ production by CIML NK cells achieved by pre-activating NK cells (Prior) or maintaining NK cells for a week following pre-activation (Post) in the presence of anti-SEMA7A antibodies. Fold decrease in IFN-γ production upon re-stimulation with IL-12 + IL-15 was calculated by dividing proportions of IFN-γpos control CIML NK cells (pre-activated with cytokines and cultured with isotype control antibodies) by proportions of IFN-γpos NK cells pre-activated with cytokines and exposed to anti-SEMA7A antibodies. Data are shown for n=5 (Prior) and n=6 (Post) donors and pooled from 2 independent experiments. No significant differences as determined by Wilcoxon matched-pairs signed rank test. (G) PBMCs were incubated for 16 hours with 1 ng/mL IL-15 (Control) or inactivated H1N1 influenza + 1 ng/mL IL-15 (Pre-activated) in the presence of isotype control or anti-SEMA7A antibodies as indicated. All cells were then washed and cultured with 1 ng/mL IL-15 for 7 days. After a week, cells were re-stimulated for 6 hours with 10 ng/mL IL-12 + 100 ng/mL IL-15 to evaluate IFN-γ production by NK cells. Bar graphs represent mean ± SEM proportions of IFN-γpos control NK cells cultured in the presence of isotype control (white bar) or anti-SEMA7A antibodies (light grey bars) and of IFN-γpos NK cells that were pre-activated in the presence of isotype control (black bars) or anti-SEMA7A antibodies (light grey bars) at day 7. Data are shown for n=3 donors and represent 1 experiment. No significant differences as determined by Wilcoxon matched-pairs signed rank test.
To further evaluate the role played by engagement of SEMA7A by integrin-β1 in cytokine-induced memory-like NK cell responses, we pre-activated NK cells in the presence of antibodies against SEMA7A or integrin-β1 and compared the ability of these pre-activated NK cells with that of NK cells pre-activated with isotype control antibodies to release IFN-γ following re-stimulation (Fig. 5C and D). Strikingly, NK cells pre-activated in the presence of antibodies against SEMA7A did not display enhanced memory-like responses upon restimulation with IL-12 + IL-15, with IFN-γ production being similar to that of non-pre-activated control NK cells. These observations could be recapitulated using purified NK cells from a subset of donors and integrin-β1 blockade, yet NK cell viability was significantly affected by long-term culture in the presence of anti-integrin-β1 antibodies (data not shown). On average, 83% of IFN-γpos NK cells expressed SEMA7A among both control and pre-activated NK cells, with approximately 50% of SEMA7Apos control NK cells participating in IFN-γ production, whereas 70% of SEMA7Apos pre-activated NK cells released IFN-γ upon cytokine re-stimulation (data not shown). Accordingly, SEMA7A expression on CIML NK cells strongly correlated with IFN-γ production upon re-stimulation (Fig. 5E), further supporting a role for SEMA7A in promoting enhanced responses by CIML NK cells.
In these blockade experiments, anti-SEMA7A antibodies were present not only during cytokine pre-activation but also during the following week of culture in medium containing only low dose IL-15. To investigate whether SEMA7A expression is crucial for differentiation into CIML NK cells and/or maintenance of CIML NK cells, we repeated these assays comparing IFN-γ responses by NK cells co-cultured with anti-SEMA7A antibodies exclusively during or after the overnight cytokine pre-activation (Fig. 5F). SEMA7A blockade during pre-activation led to impaired CIML NK cell responses upon re-stimulation, whereas addition of the SEMA7A antibody after pre-activation marginally affected CIML NK cell responses, suggesting that SEMA7A plays an important role early in the generation of CIML NK cells.
Differentiation into CIML NK cells is an intrinsic property of NK cells and can occur upon cytokine pre-activation of purified NK cell cultures [10]. CIML NK cell responses have also been described following exposure to influenza in vivo and in vitro, and in this context, generation of cytokine-induced memory involved support from accessory immune cells [19, 20]. To test if SEMA7A blockade also influences CIML NK cell generation elicited by influenza in the presence of other immune cells, we exposed PBMCs to inactivated influenza A/PR8/8/34 (H1N1) virus with isotype control or anti-SEMA7A antibodies for 16h. PBMCs were subsequently cultured in low dose IL-15 for 6 days prior to analyzing IFN-γ production by NK cells upon IL-12+IL-15 re-stimulation. We could show that SEMA7A blockade reduced CIML NK cell responses, suggesting that the function played by SEMA7A/integrin-β1 interaction in establishing and maintaining CIML NK cells is maintained in the presence of other immune cells, which may also contribute to the generation of CIML NK cells via expression of either receptor (Fig. 5G). Altogether, these results strongly suggest that cytokine-enhanced SEMA7A/Integrin-β1 interaction promotes CIML NK cell differentiation and/or maintenance of increased functionality.
SEMA7A or integrin-β1 blockade impairs the formation of conjugates between pre-activated NK cells
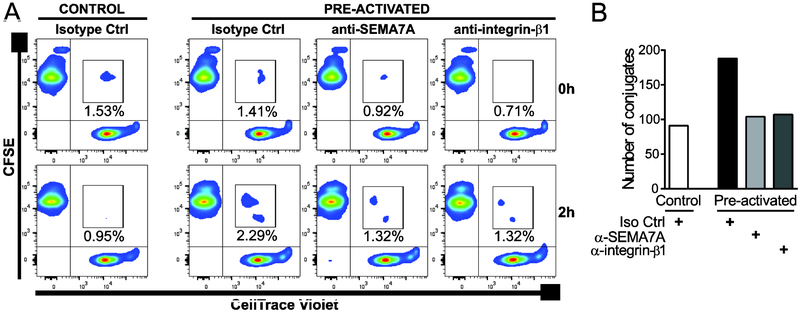
Homotypic interactions between receptors and ligands that are both expressed on NK cells have been shown to regulate NK cell effector functions [66-68], including accelerated proliferation kinetics and maximal effector activity via enhanced cytokine responsiveness [69]. It is tempting to speculate that enhanced expression of SEMA7A and integrin-β1 on cytokine pre-activated NK cells potentiates the formation of conjugates among NK cells and delivery of activating signals that stimulate differentiation into cytokine-induced memory-like NK cells and/or enhanced IFN-γ release by cytokine-induced memory-like NK cells. We therefore examined the impact of SEMA7A and integrin-β1 blockade on NK cell conjugates formation during IL-12+IL-18+IL-15 pre-activation. To this end, we employed a flow cytometry-based assay that enabled to determine the frequency of stable conjugates formed between cytokine-activated CFSE-labelled and CellTrace Violet-labelled NK cells from the same healthy donor. Conjugates were defined as double fluorescent events after co-incubating differently labelled NK cells for 2 hours, at a time when SEMA7A upregulation on NK cells in response to combined cytokines starts to be noticeable (data not shown). As illustrated in Figure 6, after this incubation time, expression of SEMA7A on cytokine-activated NK cells conferred these cells an enhanced ability to form conjugates among themselves. Notably, cytokine stimulation in the presence of anti-SEMA7A or anti-integrin-β1 antibodies led to a reduction in conjugate formation. These data support a role for trans associations between these surface molecules among NK cells.
Figure 6. SEMA7A blockade impairs conjugate formation between cytokine pre-activated NK cells.
Control or cytokine (50 ng/mL IL-12+ 10 ng/mL IL-18+ 1 ng/mL IL-15) pre-activated CFSE-labeled and CellTrace Violet-stained autologous NK cells were co-cultured at 1:1 ratio in the presence of isotype control, anti-SEMA7A or anti-integrin-β1 antibodies and the formation of conjugates analyzed after 0 or 2 hours. Conjugates were identified as double positive cells by flow cytometry. (A) Flow cytometry plots of one representative experiment out of 2 with the percentage of conjugates indicated for each condition. (B) Bar graphs representing the numbers of conjugates formed at 2 hours for control and pre-activated NK cells in the presence of indicated antibodies.
DISCUSSION
Multiple lines of evidence suggest human CIML NK cells represent potent antitumor effector cells for leukemia immunotherapies [13, 15-18], however there are many gaps in knowledge regarding the cellular and molecular processes regulating CIML NK cell responses. This study unveils new mechanistic insights underlying the generation and maintenance of enhanced functionality of human CIML NK cells. Herein, we provide the first characterization of SEMA7A expression on NK cells, demonstrating that SEMA7A serves as a marker for activated NK cells which is significantly upregulated on IL-12/18/15-exposed NK cells, and provide evidence that signaling through SEMA7A and its ligand integrin-β1 contributes to the robust effector functions of CIML NK cells.
One of the most striking findings in this study was that combined cytokine pre-activation of NK cells in the presence of antibodies targeting SEMA7A results in NK cells with similar IFN-γ responses to non-pre-activated NK cells following re-stimulation. Of note, while we did observe impaired CIML NK cell responses in a subset of donors when blocking integrin-β1, maintaining purified NK cells for a week in culture with anti-integrin-β1 antibodies led to extensive cell death, which might act as confounding factor in our interpretation of the results. Cell death deriving from long-term blockade of integrin-β1 is unsurprising as binding of integrins to extracellular matrix ligands is known to result in cell signals that have effects on cell proliferation and survival, with integrin-mediated signals being necessary in normal cells to block apoptosis and to stimulate cell cycle progression [70].
In our initial set of experiments, antibodies were present during the overnight pre-activation step as well as during the week of rest in culture, yet they were not added at the time of re-stimulation, suggesting that blockade of the SEMA7A/integrin-β1 axis abrogates differentiation into CIML NK cells rather than prevents IFN-γ production upon re-stimulation. By adding blocking antibodies either prior to or after overnight pre-activation, we could show that blockade of SEMA7A/integrin-β1 interaction specifically during cytokine stimulation is associated with the most remarkable impact on CIML NK cell responses, suggesting these molecules play a major role early in the differentiation process towards CIML NK cells (Fig. 5F). Furthermore, assays involving NK cell incubation with anti-SEMA7A antibodies alone or with IL-12+IL-15 did not reveal any impact of SEMA7A blockade on NK cell functionality, precluding a major role played by SEMA7A on conventional NK cell responses (data not shown). However, in experiments to investigate CIML NK cells, non-pre-activated control cells kept in culture for a week with SEMA7A antibodies displayed slightly decreased responses to IL-12+IL-15 stimulation compared to control NK cells cultured with isotype control antibodies. As long-term exposure to low dose IL-15 elicits a significant SEMA7A upregulation on control NK cells (Fig. 5A), it is not excluded that cis or trans interactions between SEMA7A and integrin-β1 contribute to the overall functionality of those cells. Further studies will be required to dissect the role played by SEMA7A and integrin-β1 in the establishment of cytokine-induced NK cell memory as well as in regulating conventional and memory NK cell responses to malignant cells or viral infections.
SEMA7A expression on antigen-specific T cells is required to initiate inflammation through activation of macrophages, which relies on clustering and engagement of SEMA7A with α1β1 integrins at the immunological synapse [28]. Human NK cells express fibronectin receptors α4β1 and α5β1 [71] and previous studies have suggested that integrin-β1 may function as an activating NK cell receptor. Indeed, engagement of integrin-β1 on NK cells elicits IFN-γ production via activation of the Ras/ERK signaling pathway [72] and while integrin-β1 cross-linking does not trigger cytotoxic functions, it co-stimulates cytotoxicity [73]. It is tempting to speculate that robust upregulation of both SEMA7A and integrin-β1 on CIML NK cells potentiates the formation of immunological synapses between NK cells (or between NK cells and other immune cells) and delivery of activating signals that stimulate IFN-γ release. This hypothesis is strongly supported by the increased formation of conjugates observed 2 hours after initiating cytokine pre-activation of purified NK cells, which can be partly abrogated by addition of antibodies against SEMA7A or integrin-β1 (Fig. 6). Interactions between NK cell receptors and their ligands on the surface of the same NK cell have also been demonstrated, and such cis interactions can for instance limit the recruitment of the ligand at the immunological synapse or enhance NK cell inhibitory signals. While we can’t exclude a role for binding in cis between SEMA7A and integrin-β1 on NK cells, our results illustrate enhanced SEMA7A/integrin-β1 trans interactions among cytokine-activated NK cells and suggest that potential cis associations, if they occur, do not prevent SEMA7A from binding to its ligand on other NK cells.
Overall, our data strongly suggest that SEMA7A expression on NK cells primarily modulates their ability to secrete IFN-γ. Whether cytotoxicity, and particularly that against leukemia target cells, is also affected by SEMA7A expression was not formally addressed in this study and warrants further investigations, including studies in appropriate in vivo models. Along those lines, it would be of interest to correlate SEMA7A and integrin-β1 expression with clinical outcomes and ex vivo NK cell ability to respond to malignant cells in patients receiving memory-like NK cell adoptive immunotherapy. In addition, based on the observed variability in expression of SEMA7A on unstimulated or activated NK cells between donors, baseline SEMA7A expression levels on NK cells and/or concentration of soluble SEMA7A in the plasma might be potential biomarkers for the outcome of therapeutic interventions involving cytokine-stimulated NK cells.
Collectively, these results imply a novel mechanism by which cytokine-enhanced SEMA7A/Integrin-β1 interaction promotes CIML NK cell differentiation and maintenance of increased functionality. Our data suggest that targeting the SEMA7A/Integrin-β1 axis might provide a novel immunotherapeutic approach to potentiate antitumor activity of CIML NK cells.
MATERIALS AND METHODS
Primary cells and cell lines
Whole blood from healthy anonymous donors who provided written informed consent was purchased from Research Blood Components. Ficoll centrifugation was used to isolate peripheral blood mononuclear cells (PBMC) from blood samples collected in ACD-containing tubes. PBMCs were either cryopreserved or used immediately in functional assays. The BIDMC institutional review board approved the research involving human participants reported in this study. 721.221 and K562 cells were obtained from ATCC and maintained in culture as per ATCC instructions.
NK cell analysis by multiparameter flow cytometry
To assess expression of SEMA7A ex vivo on unstimulated NK cells, cryopreserved PBMCs from healthy subjects were thawed and stained for cell surface markers using the following anti-human monoclonal antibodies from BD Biosciences: CD3 (SP34-2), CD14 (M5E2), CD19 (HIB19), CD56 (B159), CD16 (3G8), and SEMA7A (KS-2). Samples were fixed with Perm A (BD Biosciences) and analyzed on a BD LSRII.
To quantify SEMA7A on activated NK cells, CD4pos and CD8pos T cells, B cells, monocytes and DCs and identify surface receptors co-expressed with SEMA7A on NK cells, thawed PBMCs were incubated at 1*106/mL in RPMI-1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 μg/mL streptomycin and 100 U/mL penicillin for 16 hours without stimulus or in the presence of 2 μg/mL native Cytomegalovirus (CMV) (Strain AD169) protein (MyBiosource), 2 μg/mL influenza H1N1 (A/Brisbane/59/2007) Hemagglutinin (HA) protein (Sino Biological Inc), 2 μg/mL HIV-1 p24 protein (group M, subtype B, strain 92418) (Sino Biological Inc), 100 U/mL rhIL-2 (R&D), 20 ng/mL rhIL-12 (PreproTech) + 10 ng/mL rhIL-18 (MBL) + 1 ng/mL rhIL-15 (R&D), 50 ng/mL rhIL-12 (PreproTech) + 100 ng/mL rhIL-18 (MBL), 5 μg/mL PHA (Sigma-Aldrich), or K562 cells at 10:1 E:T ratio. Following stimulation, cells were subjected to surface staining using the following anti-human monoclonal antibodies from BD Biosciences: CD3 (SP34-2), CD14 (M5E2), CD19 (HIB19), CD56 (B159), CD16 (3G8), CD11c (B-ly6), CD8 (RPA-T8), CD4 (L200), HLA-DR (G46-6), SEMA7A (KS-2), CD29 (MAR4), CD2 (RPA2.10), CD27 (M-T271), CCR5 (3A9); R&D: NKG2C (134591), NKG2A (131411); BioLegend: CD57 (HCD57), IL18Rα (H44) or eBiosciences: CD25 (BC96). Samples fixed with Perm A (BD Biosciences) were analyzed on a BD LSRII. To evaluate IL-2-dependent increase in SEMA7Apos NK cells upon exposure to viral proteins, thawed PBMCs were co-cultured with medium alone, 20 U/mL rhIL-2, or 2 μg/mL CMV pp65 in the presence of purified rat IgG2a, κ, isotype control (R35-95) or purified rat anti-human IL-2 antibody (MQ1-17H12) (BD Biosciences). In assays comparing functionality of SEMA7Aneg and SEMA7Apos NK cells, fluorophore-conjugated CD107a antibodies were added for the 16 hours incubation period, and an IFN-γ secretion assay (Miltenyi) was performed according to manufacturer’s instructions prior to surface staining.
For all assays, dead cells were excluded using LIVE/DEAD® Fixable Blue Dead Cell Stain Kit (Invitrogen). NK cells were identified as CD3negCD14negCD19negCD56posCD16pos/neg lymphocytes and divided into CD56bright and CD56dim subsets based on CD56 and FcγRIIIa (CD16) expression. The phenotype and function of NK cells were defined using FlowJo™ version 7.6.5 (Treestar). Flow cytometry analysis adhered to the “Guidelines for the use of flow cytometry and cell sorting in immunological studies” [74].
Flow cytometry-based memory-like NK cell functional assays
Control and memory-like NK cells were generated as previously described [10]. Briefly, untouched NK cells isolated using RosetteSep NK cell enrichment cocktail (STEMCELL Technologies) were either cultured in the presence of rhIL-15 (1 ng/mL) alone and 2 μg/mL isotype control antibody (control) or pre-activated for 16 hours using rhIL-12 (10 ng/mL) + rhIL-18 (50 ng/mL) + rhIL-15 (1 ng/mL) (memory-like) in the presence of 2 μg/mL of either isotype control antibody (MOPC-21; BD and 20116; R&D), anti-SEMA7A Ab (310829; R&D) or anti-CD29 antibody (P5D2; BioLegend). For pre-activation of NK cells with influenza, 4*106 PBMCs were cultured in 1 ng/mL rhIL-15 (control) or in 1 ng/mL rhIL-15 combined with 8 μg inactivated A/PR/8/34 (H1N1) virus (Charles River Laboratories) for 16h in the presence of isotype control or blocking antibodies as described above. All NK cells were washed and allowed to differentiate in complete RPMI 1640 medium containing 10% human AB serum (Sigma-Aldrich) supplemented with rhIL-15 (1 ng/mL) and control or blocking antibodies for a week, with 50% of the medium being replaced after 2-3 days with fresh cytokines and antibodies. Control and memory-like NK cells were then stimulated with 10 ng/mL rhIL-12 + 100 ng/mL rhIL-15 for 6 hours prior to analyzing NK cell function using an IFN-γ secretion assay (Miltenyi) and surface staining with CD3 (SP34-2), CD56 (B159), CD16 (3G8), SEMA7A (KS-2), and CD29 (MAR4) antibodies (all BD). Dead cells were excluded using LIVE/DEAD® Fixable Blue Dead Cell Stain Kit (Invitrogen). Samples fixed with Perm A (BD Biosciences) were analyzed on a BD LSRII. The phenotype and function of NK cells were defined using FlowJo™ version 7.6.5 (Treestar).
Conjugate formation assay
Untouched primary NK cells isolated from a healthy donor were split equally to stain half with 5 μM CellTrace™ CFSE and the other half with 5 μM CellTrace™ Violet for 15 min at 37°C according to the manufacturer’s instructions (Invitrogen). After labelling, cells were washed extensively. Then, 7.5×104 CFSE-stained NK cells and 7.5×104 Violet-stained NK cells were co-cultured at 37°C for 0 or 2 hours in a final volume of 150 μL with or without 10 ng/mL rhIL-12 + 50 ng/mL rhIL-18 + 1 ng/mL rhIL-15. Co-cultures with the combined cytokines were performed in the presence of 10 μg/mL of either isotype control antibody (MOPC-21; BD and 20116; R&D), anti-SEMA7A Ab (310829; R&D) or anti-CD29 antibody (P5D2; BioLegend). Isotype control antibodies were also added to the co-culture in medium alone. Reactions were stopped by adding 50 μL of ice-cold PBS. Conjugates were detected by flow cytometry as double positive events.
Statistics
Statistical analysis was performed using GraphPad Prism version 6.05 (GraphPad). Differences in functional responses between conditions or time points were compared using Wilcoxon matched-pairs signed-rank test. P values inferior to 0.05 were considered significant. Significance levels are assigned as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 for all tests.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health R01 AI116363 (S.J.) and R01 AI120828 (R.K.R), and the Harvard University Center for AIDS Research grant P30 AI060354 (R.K.R.). We are grateful to Dr. Marcus Altfeld for helpful discussions, and we thank the CVVR flow cytometry core and Michelle Lifton for expert technical assistance. The graphical abstract was produced using Servier Medical Art.
Footnotes
CONFLICT OF INTEREST
The authors declare no commercial or financial conflict of interest.
REFERENCES
- 1.Lanier LL, NK cell recognition. Annu Rev Immunol 2005. 23: 225–274. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL, Le AM, Civin CI, Loken MR and Phillips JH, The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J. Immunol 1986. 136: 4480–4486. [PubMed] [Google Scholar]
- 3.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE et al. , Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 2001. 97: 3146–3151. [DOI] [PubMed] [Google Scholar]
- 4.Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA and Ritz J, Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J. Exp. Med 1990. 171: 1509–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper MA, Fehniger TA and Caligiuri MA, The biology of human natural killer-cell subsets. Trends Immunol 2001. 22: 633–640. [DOI] [PubMed] [Google Scholar]
- 6.Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, Streeck H et al. , Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 2005. 106: 3366–3369. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkstrom NK, Ljunggren HG and Sandberg JK, CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol 2010. 31: 401–406. [DOI] [PubMed] [Google Scholar]
- 8.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, Marcenaro E et al. , Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc. Natl. Acad. Sci. U. S. A 2003. 100: 15011–15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paust S, Blish CA and Reeves RK, Redefining Memory: Building the Case for Adaptive NK Cells. J. Virol 2017. 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA et al. , Cytokine activation induces human memory-like NK cells. Blood 2012. 120: 4751–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA and Yokoyama WM, Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. U. S. A 2009. 106: 1915–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keppel MP, Yang L and Cooper MA, Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J. Immunol 2013. 190: 4754–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni J, Miller M, Stojanovic A, Garbi N and Cerwenka A, Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J. Exp. Med 2012. 209: 2351–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA and Fehniger TA, Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol. Blood Marrow Transplant 2014. 20: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW et al. , Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med 2016. 8: 357ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner JA, Berrien-Elliott MM, Rosario M, Leong JW, Jewell BA, Schappe T, Abdel-Latif S et al. , Cytokine-Induced Memory-Like Differentiation Enhances Unlicensed Natural Killer Cell Antileukemia and FcgammaRIIIa-Triggered Responses. Biol. Blood Marrow Transplant 2017. 23: 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewen EM, Pahl JHW, Miller M, Watzl C and Cerwenka A, KIR downregulation by IL-12/15/18 unleashes human NK cells from KIR/HLA-I inhibition and enhances killing of tumor cells. Eur. J. Immunol 2018. 48: 355–365. [DOI] [PubMed] [Google Scholar]
- 18.Terren I, Mikelez I, Odriozola I, Gredilla A, Gonzalez J, Orrantia A, Vitalle J et al. , Implication of Interleukin-12/15/18 and Ruxolitinib in the Phenotype, Proliferation, and Polyfunctionality of Human Cytokine-Preactivated Natural Killer Cells. Front Immunol 2018. 9: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darboe A, Danso E, Clarke E, Umesi A, Touray E, Wegmuller R, Moore SE et al. , Enhancement of cytokine-driven NK cell IFN-gamma production after vaccination of HCMV infected Africans. Eur. J. Immunol 2017. 47: 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodier MR, Rodriguez-Galan A, Lusa C, Nielsen CM, Darboe A, Moldoveanu AL, White MJ et al. , Influenza Vaccination Generates Cytokine-Induced Memory-like NK Cells: Impact of Human Cytomegalovirus Infection. J. Immunol 2016. 197: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquardt N, Ivarsson MA, Blom K, Gonzalez VD, Braun M, Falconer K, Gustafsson R et al. , The Human NK Cell Response to Yellow Fever Virus 17D Is Primarily Governed by NK Cell Differentiation Independently of NK Cell Education. J. Immunol 2015. 195: 3262–3272. [DOI] [PubMed] [Google Scholar]
- 22.Suliman S, Geldenhuys H, Johnson JL, Hughes JE, Smit E, Murphy M, Toefy A et al. , Bacillus Calmette-Guerin (BCG) Revaccination of Adults with Latent Mycobacterium tuberculosis Infection Induces Long-Lived BCG-Reactive NK Cell Responses. J. Immunol 2016. 197: 1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luetke-Eversloh M, Hammer Q, Durek P, Nordstrom K, Gasparoni G, Pink M, Hamann A et al. , Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog 2014. 10: e1004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell 1999. 97: 551–552. [DOI] [PubMed] [Google Scholar]
- 25.Kolodkin AL, Matthes DJ and Goodman CS, The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 1993. 75: 1389–1399. [DOI] [PubMed] [Google Scholar]
- 26.Kopp MA, Brommer B, Gatzemeier N, Schwab JM and Pruss H, Spinal cord injury induces differential expression of the profibrotic semaphorin 7A in the developing and mature glial scar. Glia 2010. 58: 1748–1756. [DOI] [PubMed] [Google Scholar]
- 27.Pasterkamp RJ, Peschon JJ, Spriggs MK and Kolodkin AL, Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 2003. 424: 398–405. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T et al. , Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature 2007. 446: 680–684. [DOI] [PubMed] [Google Scholar]
- 29.Kamata M, Tada Y, Uratsuji H, Kawashima T, Asano Y, Sugaya M, Kadono T et al. , Semaphorin 7A on keratinocytes induces interleukin-8 production by monocytes. J. Dermatol. Sci 2011. 62: 176–182. [DOI] [PubMed] [Google Scholar]
- 30.Holmes S, Downs AM, Fosberry A, Hayes PD, Michalovich D, Murdoch P, Moores K et al. , Sema7A is a potent monocyte stimulator. Scand. J. Immunol 2002. 56: 270–275. [DOI] [PubMed] [Google Scholar]
- 31.Czopik AK, Bynoe MS, Palm N, Raine CS and Medzhitov R, Semaphorin 7A is a negative regulator of T cell responses. Immunity 2006. 24: 591–600. [DOI] [PubMed] [Google Scholar]
- 32.Esnault S, Kelly EA, Johansson MW, Liu LY, Han ST, Akhtar M, Sandbo N et al. , Semaphorin 7A is expressed on airway eosinophils and upregulated by IL-5 family cytokines. Clin. Immunol 2014. 150: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie J and Wang H, Semaphorin 7A as a potential immune regulator and promising therapeutic target in rheumatoid arthritis. Arthritis Res Ther 2017. 19: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez-Franco A, Eixarch H, Costa C, Gil V, Castillo M, Calvo-Barreiro L, Montalban X et al. , Semaphorin 7A as a Potential Therapeutic Target for Multiple Sclerosis. Mol. Neurobiol 2017. 54: 4820–4831. [DOI] [PubMed] [Google Scholar]
- 35.Sultana H, Neelakanta G, Foellmer HG, Montgomery RR, Anderson JF, Koski RA, Medzhitov RM et al. , Semaphorin 7A contributes to West Nile virus pathogenesis through TGF-beta1/Smad6 signaling. J. Immunol 2012. 189: 3150–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada A, Kubo K, Takeshita T, Harashima N, Kawano K, Mine T, Sagawa K et al. , Molecular cloning of a glycosylphosphatidylinositol-anchored molecule CDw108. J. Immunol 1999. 162: 4094–4100. [PubMed] [Google Scholar]
- 37.Angelisova P, Drbal K, Cerny J, Hilgert I and Horejsi V, Characterization of the human leukocyte GPI-anchored glycoprotein CDw108 and its relation to other similar molecules. Immunobiol 1999. 200: 234–245. [DOI] [PubMed] [Google Scholar]
- 38.Mine T, Harada K, Matsumoto T, Yamana H, Shirouzu K, Itoh K and Yamada A, CDw108 expression during T-cell development. Tissue antigens 2000. 55: 429–436. [DOI] [PubMed] [Google Scholar]
- 39.Konig K, Marth L, Roissant J, Granja T, Jennewein C, Devanathan V, Schneider M et al. , The plexin C1 receptor promotes acute inflammation. Eur. J. Immunol 2014. 44: 2648–2658. [DOI] [PubMed] [Google Scholar]
- 40.Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A et al. , Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 1999. 99: 71–80. [DOI] [PubMed] [Google Scholar]
- 41.Comeau MR, Johnson R, DuBose RF, Petersen M, Gearing P, VandenBos T, Park L et al. , A poxvirus-encoded semaphorin induces cytokine production from monocytes and binds to a novel cellular semaphorin receptor, VESPR. Immunity 1998. 8: 473–482. [DOI] [PubMed] [Google Scholar]
- 42.Roth JM, Kohler D, Schneider M, Granja TF and Rosenberger P, Semaphorin 7A Aggravates Pulmonary Inflammation during Lung Injury. PloS one 2016. 11: e0146930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Minicis S, Rychlicki C, Agostinelli L, Saccomanno S, Trozzi L, Candelaresi C, Bataller R et al. , Semaphorin 7A contributes to TGF-beta-mediated liver fibrogenesis. Am. J. Pathol 2013. 183: 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rijn A, Paulis L, te Riet J, Vasaturo A, Reinieren-Beeren I, van der Schaaf A, Kuipers AJ et al. , Semaphorin 7A Promotes Chemokine-Driven Dendritic Cell Migration. J. Immunol 2016. 196: 459–468. [DOI] [PubMed] [Google Scholar]
- 45.Michel T, Poli A, Cuapio A, Briquemont B, Iserentant G, Ollert M and Zimmer J, Human CD56bright NK Cells: An Update. J. Immunol 2016. 196: 2923–2931. [DOI] [PubMed] [Google Scholar]
- 46.Freud AG, Yu J and Caligiuri MA, Human natural killer cell development in secondary lymphoid tissues. Semin. Immunol 2014. 26: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luetke-Eversloh M, Killig M and Romagnani C, Signatures of human NK cell development and terminal differentiation. Front Immunol 2013. 4: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robertson MJ, Role of chemokines in the biology of natural killer cells. J. Leukoc. Biol 2002. 71: 173–183. [PubMed] [Google Scholar]
- 49.Angelo LS, Banerjee PP, Monaco-Shawver L, Rosen JB, Makedonas G, Forbes LR, Mace EM et al. , Practical NK cell phenotyping and variability in healthy adults. Immunol. Res 2015. 62: 341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vossen MT, Matmati M, Hertoghs KM, Baars PA, Gent MR, Leclercq G, Hamann J et al. , CD27 defines phenotypically and functionally different human NK cell subsets. J. Immunol 2008. 180: 3739–3745. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ et al. , CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010. 116: 3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP et al. , Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. U. S. A 2011. 108: 14725–14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hendricks DW, Balfour HH Jr., Dunmire SK, Schmeling DO, Hogquist KA and Lanier LL, Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J. Immunol 2014. 192: 4492–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Scott JM et al. , Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015. 42: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H et al. , Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015. 42: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang T, Scott JM, Hwang I and Kim S, Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J. Immunol 2013. 190: 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granzin M, Wagner J, Kohl U, Cerwenka A, Huppert V and Ullrich E, Shaping of Natural Killer Cell Antitumor Activity by Ex Vivo Cultivation. Front Immunol 2017. 8: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He XS, Draghi M, Mahmood K, Holmes TH, Kemble GW, Dekker CL, Arvin AM et al. , T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest 2004. 114: 1812–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horowitz A, Behrens RH, Okell L, Fooks AR and Riley EM, NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J. Immunol 2010. 185: 2808–2818. [DOI] [PubMed] [Google Scholar]
- 60.Horowitz A, Hafalla JC, King E, Lusingu J, Dekker D, Leach A, Moris P et al. , Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. J. Immunol 2012. 188: 5054–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jost S, Tomezsko PJ, Rands K, Toth I, Lichterfeld M, Gandhi RT and Altfeld M, CD4+ T-cell help enhances NK cell function following therapeutic HIV-1 vaccination. J. Virol 2014. 88: 8349–8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Long BR, Michaelsson J, Loo CP, Ballan WM, Vu BA, Hecht FM, Lanier LL et al. , Elevated frequency of gamma interferon-producing NK cells in healthy adults vaccinated against influenza virus. Clin Vaccine Immunol 2008. 15: 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White MJ, Nielsen CM, McGregor RH, Riley EH and Goodier MR, Differential activation of CD57-defined natural killer cell subsets during recall responses to vaccine antigens. Immunology 2014. 142: 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evans JH, Horowitz A, Mehrabi M, Wise EL, Pease JE, Riley EM and Davis DM, A distinct subset of human NK cells expressing HLA-DR expand in response to IL-2 and can aid immune responses to BCG. Eur. J. Immunol 2011. 41: 1924–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M and Caligiuri MA, CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 2003. 101: 3052–3057. [DOI] [PubMed] [Google Scholar]
- 66.Lee KM, Forman JP, McNerney ME, Stepp S, Kuppireddi S, Guzior D, Latchman YE et al. , Requirement of homotypic NK-cell interactions through 2B4(CD244)/CD48 in the generation of NK effector functions. Blood 2006. 107: 3181–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Assarsson E, Kambayashi T, Schatzle JD, Cramer SO, von Bonin A, Jensen PE, Ljunggren HG et al. , NK cells stimulate proliferation of T and NK cells through 2B4/CD48 interactions. J Immunol 2004. 173: 174–180. [DOI] [PubMed] [Google Scholar]
- 68.Kim EO, Kim TJ, Kim N, Kim ST, Kumar V and Lee KM, Homotypic cell to cell cross-talk among human natural killer cells reveals differential and overlapping roles of 2B4 and CD2. J Biol Chem 2010. 285: 41755–41764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim TJ, Kim M, Kim HM, Lim SA, Kim EO, Kim K, Song KH et al. , Homotypic NK cell-to-cell communication controls cytokine responsiveness of innate immune NK cells. Sci Rep 2014. 4: 7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hynes RO, Integrins: bidirectional, allosteric signaling machines. Cell 2002. 110: 673–687. [DOI] [PubMed] [Google Scholar]
- 71.Gismondi A, Morrone S, Humphries MJ, Piccoli M, Frati L and Santoni A, Human natural killer cells express VLA-4 and VLA-5, which mediate their adhesion to fibronectin. J Immunol 1991. 146: 384–392. [PubMed] [Google Scholar]
- 72.Mainiero F, Gismondi A, Soriani A, Cippitelli M, Palmieri G, Jacobelli J, Piccoli M et al. , Integrin-mediated ras-extracellular regulated kinase (ERK) signaling regulates interferon gamma production in human natural killer cells. J. Exp. Med 1998. 188: 1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palmieri G, Serra A, De Maria R, Gismondi A, Milella M, Piccoli M, Frati L et al. , Cross-linking of alpha 4 beta 1 and alpha 5 beta 1 fibronectin receptors enhances natural killer cell cytotoxic activity. J Immunol 1995. 155: 5314–5322. [PubMed] [Google Scholar]
- 74.Cossarizza A, Chang HD, Radbruch A, Akdis M, Andra I, Annunziato F, Bacher P et al. , Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol 2017. 47: 1584–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.