Abstract
Background:
Anxiety disorders are highly prevalent and cause substantial suffering and impairment. Whereas the amygdala has well-established contributions to anxiety, evidence from rodent and non-human primate models suggests that the bed nucleus of the stria terminalis (BNST) may play a critical, and possibly distinct, role in human anxiety disorders. The BNST mediates hypervigilance and anticipatory anxiety in response to unpredictable or ambiguous threat, core symptoms of social anxiety, yet little is known about the BNST’s role in social anxiety.
Methods:
Functional magnetic resonance imaging was used to measure neural responses during a cued anticipation task with unpredictable, predictable threat, and predictable neutral cues followed by threat or neutral images. Social anxiety was examined using a dimensional approach (N = 44 adults).
Results:
For unpredictable cues, higher social anxiety was associated with lower BNST-amygdala connectivity. For unpredictable images, higher social anxiety was associated with greater connectivity between the BNST and both the ventromedial prefrontal cortex and the posterior cingulate cortex and lower connectivity between the BNST and post-central gyrus. Social anxiety moderated the BNST-amygdala dissociation for unpredictable images; higher social anxiety was associated with BNST > amygdala response to unpredictable threat relative to unpredictable neutral images.
Conclusions:
Social anxiety was associated with alterations in BNST responses to unpredictability, particularly in the BNST’s interactions with other brain regions including the amygdala and prefrontal cortex. To our knowledge, these findings provide the first evidence for the BNST’s role in social anxiety, which may be a potential new target for prevention and intervention.
Keywords: BST
Introduction
Anxiety disorders are common, chronic disorders that cause substantial suffering and impairment (Baxter, Scott, Vos, & Whiteford, 2013; Kessler, Chiu, Demler, & Walters, 2005; Stein & Kean, 2000). A decade of neuroimaging studies have dissected the fear neurocircuitry in humans and provided compelling evidence that anxiety disorders are associated with amygdala hyperactivity and amygdalo-frontal hypoconnectivity (Brühl, Delsignore, Komossa, & Weidt, 2014; Etkin & Wager, 2007). Despite well-established behavioral and pharmacological therapies that have been shown to modify particular neural circuits, treatment outcomes are poor. Approximately half of individuals with anxiety disorders either fail to respond to treatment, only partially respond, or relapse (Blanco et al., 2010; Craske et al., 2015; Ginsburg et al., 2014). This tremendous variability in treatment response raises the question of whether other neural circuits are involved in anxiety disorders.
Two decades of research in rodents and non-human primates indicates that the bed nucleus of the stria terminalis (BNST, also referred to as the BST) plays an important role in anxiety (for reviews see 9, 10). While Studies in animal models of anxiety suggested that lesions to the BNST reduced responses to contextual, distant, or diffuse cues; in contrast to lesions to the amygdala which reduced responses to explicit or direct threats (Davis, Falls, Campeau, & Kim, 1993; Davis et al., 2010; Lee & Davis, 1997; Sullivan et al., 2004; Waddell, Morris, & Bouton, 2006; Walker & Davis, 1997). Despite compelling evidence from animal models that the BNST is critical to anxiety, the human BNST has been remarkably understudied. A major limitation to studying the BNST has been the technological challenges associated with its small size. In humans, the BNST is about the size of a sunflower seed and is located at the end of the stria terminalis (Alheid & Heimer, 1988). Fortunately, advances in MRI field strength, image acquisition, and analytic methods have provided the technological foundation for studying the BNST in humans. These methods have been reliably used to map the structural (Avery et al., 2014) and functional (Avery et al., 2014; Mcmenamin, Langeslag, Sirbu, Padmala, & Pessoa, 2014; Tillman et al., 2018; Torrisi et al., 2015) connections of the BNST and differentiate functional connectivity of the BNST and amygdala (Gorka, Torrisi, Shackman, Grillon, & Ernst, 2017; Tillman et al., 2018). Multiple studies have shown increased BNST response during threat anticipation (Alvarez, Chen, Bodurka, Kaplan, & Grillon, 2011; Choi, Padmala, & Pessoa, 2012; Grupe, Oathes, & Nitschke, 2013; Herrmann et al., 2016; Klumpers et al., 2015; Klumpers, Kroes, Baas, & Fernández, 2017; Mcmenamin et al., 2014) and a meta-analysis of threat anticipation studies shows robust engagement of the BNST (Avery, Clauss, & Blackford, 2016). Consistent with the findings in rodents (Davis et al., 2010) several studies in humans also report distinct BNST and amygdala responses (Alvarez et al., 2011; Herrmann et al., 2016; Klumpers et al., 2015; Mcmenamin et al., 2014; Somerville, Whalen, & Kelley, 2010); of note, there is emerging evidence that the human BNST also responds to conditioned and explicit threat stimuli (Shackman & Fox, 2016), thus the role of the BNST is continuing to be refined. While there is growing evidence that the BNST is involved in threat anticipation in healthy adults, investigation of the BNST’s role in human anxiety is in a nascent stage.
The BNST’s functions of hypervigilance and response to diffuse, ambiguous, or unpredictable threat is highly relevant for anxiety disorders, which are thought to result from maladaptive responses to uncertainty (Grupe & Nitschke, 2013). Social anxiety is characterized by anticipatory anxiety and hypervigilance to social stimuli (Kessler et al., 2005; Rapee & Heimberg, 1997; Richards, Benson, Donnelly, & Hadwin, 2014). During social-evaluative situations, individuals with social anxiety disorder anticipate and worry about potential social judgment. This potential social judgment is an inherently ambiguous and unpredictable situation. Social anxiety disorder is associated with elevated intolerance of uncertainty and symptoms of social anxiety disorder correlate with degree of intolerance of uncertainty, suggesting that ambiguous or uncertain situations are critical to the disorder (Boelen & Reijntjes, 2009; Carleton, Collimore, & Asmundson, 2010). Landmark studies in non-human primates have shown that anxious temperament‐characterized by individual differences in responses to ambiguous or potential social threat, similar to social anxiety in humans (Fox & Kalin, 2014)‐is associated with heightened BNST activity (Fox et al., 2015) during prolonged exposure to potential social threat and stronger BNST-amygdala intrinsic connectivity (Fox et al., 2018). Yet, it remains unknown whether BNST function or connectivity is altered in human social anxiety. To investigate the BNST’s role in social anxiety, we examined responses to unpredictable and predictable cues and images in individuals across a range from low to high social anxiety. We hypothesized that social anxiety would moderate BNST activation and connectivity to an unpredictable threat. Based on findings of a BNST-amygdala dissociation during threat anticipation in both rodents and humans reviewed above, we performed a secondary analysis to test the hypothesis that there would be a dissociation between BNST and amygdala responses--characterized by a greater BNST response to unpredictable threat and a greater amygdala response to predictable threat—and that social anxiety would moderate the degree of dissociation.
Methods
Participants
Participants were recruited with a stratified approach (across social anxiety spectrum) using both advertisements and email lists. Individuals were not eligible for the study if they had any of the following: failure on MRI safety screen; current use of psychoactive medications; major medical illness; or history of brain trauma. Individuals were not eligible if they had lifetime psychiatric disorders or substance abuse—with the exception of anxiety disorders—based on the Structured Clinical Interview for DSM IV Axis I disorders (First, Spitzer, Gibbon, & Williams, 2002). Social anxiety symptoms were calculated using the Social Phobia and Anxiety Inventory (Turner, Beidel, Dancu, & Stanley, 1989). The sample reported here partially overlaps with a previously reported study (Clauss et al., 2014). This research was conducted in accordance with the Vanderbilt Human Research Protection Program and all participants provided written informed consent. Participants received financial compensation.
Study participants were 44 young adults (27 females), 18-25 years of age (M = 21.9, SD = 1.99), of various ethnicities (68% Caucasian-non-Hispanic, 2% Caucasian-Hispanic, 16% African-American, 11% Asian, 3% other). Scores on the dimensional measure of social anxiety ranged from 0 – 157 (maximum range = 0-186; M = 56, SD = 44). Nine participants met criteria for social anxiety disorder or another anxiety disorder (social anxiety disorder only, n = 3; specific phobia only, n = 3; social anxiety disorder and specific phobia, n = 1, social anxiety disorder and specific phobia and generalized anxiety disorder, n = 1; social anxiety disorder and specific phobia and obsessive compulsive disorder, n = 1).
fMRI Task, Data Collection, and Preprocessing
To study the effects of unpredictability, we used a cued anticipation task with unpredictable and predictable conditions. Participants were trained to associate one cue (e.g., blue square or pink diamond) with a fear face and another cue with a neutral face. Four predictable runs included 10 fear and 10 neutral cue/image combinations. The unpredictable condition was untrained and was presented last; participants saw a novel cue (yellow circle) that was randomly followed by either a fear face or a neutral face (20 unpredictable cues, 10 fear faces, 10 neutral faces). Details of the task, fMRI data acquisition and data processing are provided in the Supplemental Methods.
Statistical Analysis
Normality.
All data were tested for normality; all variables were normally distributed except for BNST response to the unpredictable cue. BNST response had a substantial outlier (z = 4.2). Data for that score was winsorized (to 1 SD above the highest value) and the resulting distribution was normal. Data analysis and results for the button push behavior are presented in Supplementary Information.
Overall Approach.
A hierarchical linear model approach was used to determine whether social anxiety moderated the BNST responses to unpredictable threat. Tests of BNST activation and connectivity were performed for cues and images separately, specifically: (1a) BNST activation during cues; (1b) BNST activation during images; (2a) BNST functional connectivity during cues; and (2b) BNST functional connectivity during images. Based on previous findings of a BNST-amygdala dissociation in fMRI studies, we also performed a set of secondary analysis to examine whether social anxiety moderated the difference between BNST and amygdala response to unpredictable threat cues or unpredictable threat images. Details for each analysis are provided in the Supplementary Methods.
Results
BNST Activation
Cues
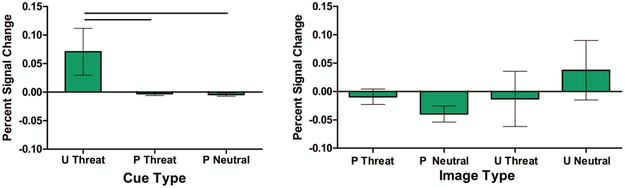
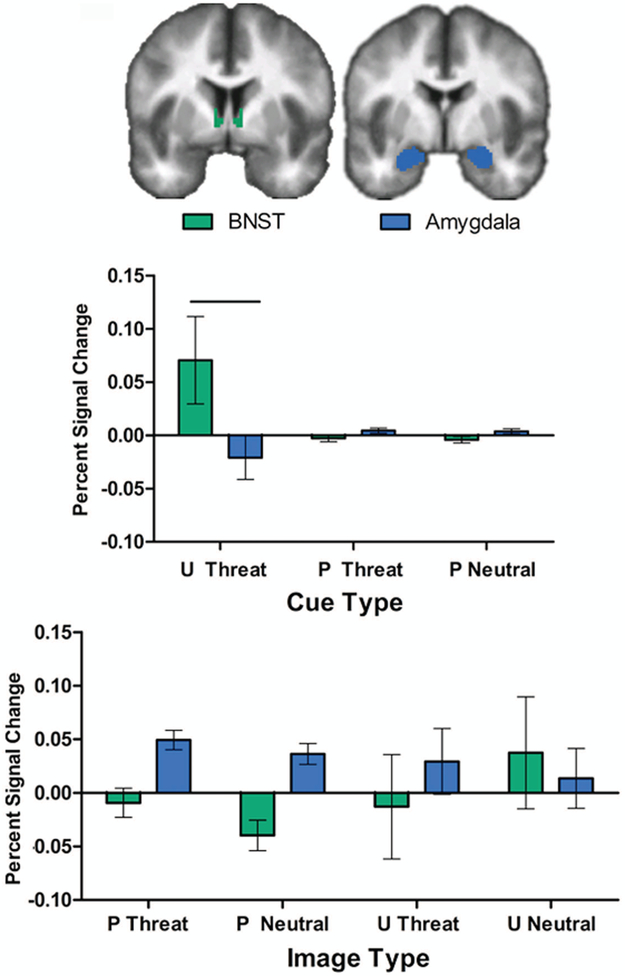
BNST activation differed by cue type (Figure 1; F(2,86) = 3.20, p = .05). Post-hoc pairwise comparisons revealed that the BNST had significantly greater response to unpredictable cues relative to either predictable threat cues (t(86) = 2.17 p = .04) or predictable neutral cues (t(86) = 2.21, p = .03). Social anxiety did not moderate BNST response to cues.
Figure 1.
BNST activation to cues and images. Left: The BNST shows significantly higher activation to unpredictable threat cues relative to predictable threat and predictable neutral cues (p < .05). Right: BNST response is consistently similar to baseline across all image conditions, with no significant effect of image type or valence. Horizontal lines indicate significant group differences.
Images
BNST response to images was not modulated by image type or image valence (Figure 1; predictable/unpredictable, p = .45; threat/neutral, p = .60; type × valence p = .07). Social anxiety did not moderate the effect of image type (p = .95), image valence (p = .37), nor the image × valence interaction (p = .10).
BNST Connectivity
Cues
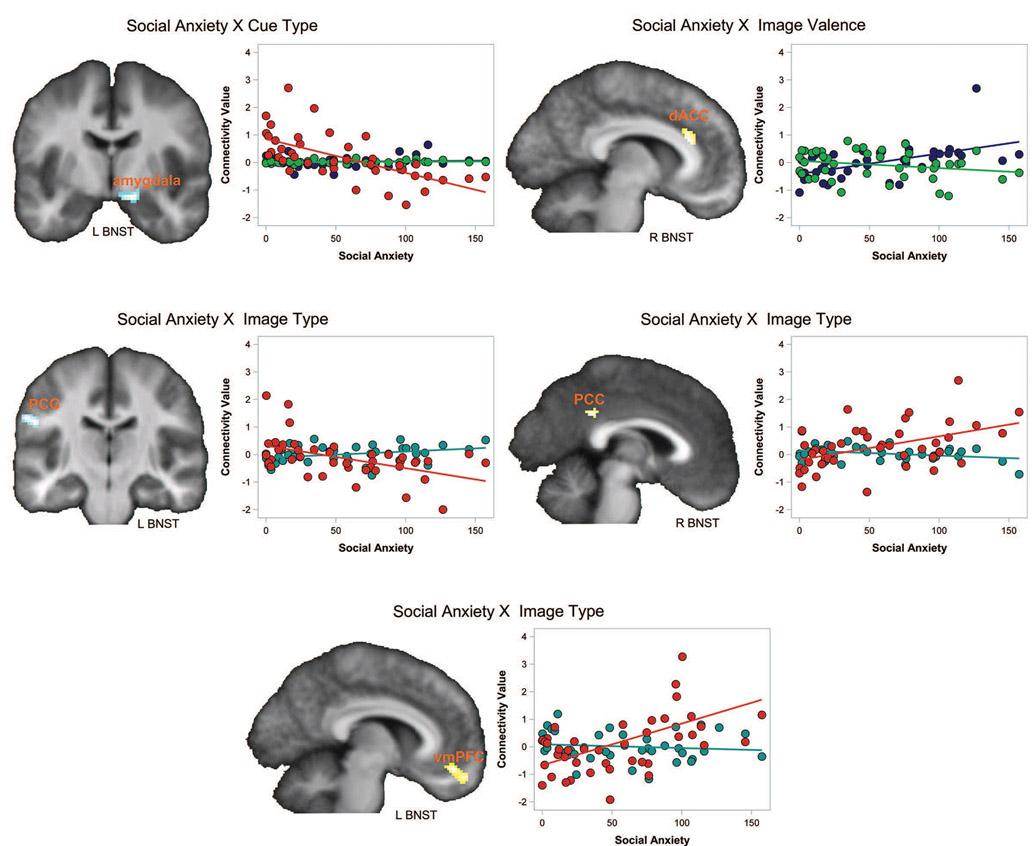
There were no significant main effects of cue type on left or right BNST connectivity. However, social anxiety moderated the main effect of cue on left BNST connectivity in a cluster in the dorsal right amygdala (Figure 2; Table 1). Post-hoc correlations between social anxiety and the connectivity values by cue type, revealed that social anxiety was associated with connectivity during the unpredictable cues (r = − .64, p < .001) but not during either of the predictable cue types (predictable threat: r = .08, p = .62; predictable neutral: r = .14, p = .38).
Figure 2. Social anxiety moderates BNST task-related connectivity to cues and images.
In the brain images, regions in blue represent negative correlations and regions in yellow represent positive correlations. The scatterplots illustrate the correlations with social anxiety by cue type, image valence, or image type; connectivity values were extracted from the significant clusters. Brain regions are labelled: dACC = dorsal anterior cingulate cortex; vmPFC = ventromedial prefrontal cortex; PCG = post-central gyrus; PCC = posterior cingulate cortex. The hemisphere of the seed region is indicated by text (L BNST or R BNST) under each image. For the scatterplots: Red = unpredictable, Blue = Threat, Green = Neutral, Teal = Predictable.
Table 1.
BNST Connectivity Results
| Hemisphere | MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region | Seed | Cluster | k | t | x | y | z | |
| Main Effects and Interactions | ||||||||
| Main Effect of Image Type | ||||||||
| Putamen | L | L | 100 | 4.20 | −24 | −2 | −2 | |
| Visual Cortex/BA 17 | R | R | 86 | 3.88 | 8 | −88 | 4 | |
| Interaction Image Type × Valence | ||||||||
| Fusiform Gyrus | L | R | 53 | 5.14 | 30 | −60 | −14 | |
| Interactions with Social Anxiety | ||||||||
| Hemisphere | MNI coordinates | |||||||
| Brain Region | Correlation | Seed | Cluster | k | t | x | y | z |
| Social Anxiety × Cue Type | ||||||||
| Amygdala | − | L | R | 67 | 4.64 | 12 | −6 | −16 |
| Social Anxiety × Image Type | ||||||||
| Ventromedial Prefrontal Cortex | + | L | L | 101 | 4.67 | −6 | 46 | −16 |
| Posterior Cingulate Cortex | + | R | L | 55 | 4.20 | −6 | −40 | 32 |
| Post-central Gyrus | − | L | L | 84 | 4.12 | −60 | −16 | 28 |
| Social Anxiety × Image Valence | ||||||||
| Dorsal Anterior Cingulate Cortex | + | L | L | 75 | 4.38 | −10 | 28 | 20 |
Images
During image viewing, there were several main effects and interactions for BNST connectivity (Table 1). First, there were main effects of image type (unpredictable > predictable), the BNST showed significantly lower connectivity with the putamen and greater connectivity with primary visual cortex. There was also an image type × valence interaction with BNST-fusiform gyrus connectivity.
Social anxiety moderated the effect of image type (unpredictable > predictable) on BNST connectivity in multiple regions (Figure 2; Table 1). First, higher social anxiety was associated with greater connectivity between the BNST and the ventromedial prefrontal cortex. Post-hoc correlations show this finding was driven by social anxiety correlations with the unpredictable images (r = .54, p = .0002) but not predictable images (r = −.12, p = .46). Second, higher social anxiety was associated with greater BNST connectivity with the posterior cingulate cortex; post-hoc correlations showed that social anxiety predicted connectivity for the unpredictable images (r = .47, p = .002) but not predictable images (r = −.29, p = .07). Third, higher social anxiety was associated with lower connectivity between the BNST and a cluster in the post-central gyrus; social anxiety was modestly correlated with connectivity for both unpredictable images (r = −.50, p = .0007) and predictable images (r = .33, p = .03). Finally, social anxiety moderated the effect of image valence (threat> neutral) between the left BNST and dorsal anterior cingulate cortex. Social anxiety was associated with BNST-dACC connectivity for the threat images (r = .54) but not neutral images (r = −.23, p = .14). Note: the one outlier is not influential, the correlations with the outlier removed are similar (threat images: r = .53, p = .0004); neutral images: r = −.28, p = .07). There were no significant effects of social anxiety on connectivity for the type × valence interaction.
BNST-Amygdala Comparison.
Cues
The direct comparison of BNST and amygdala responses to the cues revealed a significantly heightened and selective BNST response to unpredictable cues (Figure 3; region × cue interaction, (F(2,86)=4.56, p = .02). Post-hoc analyses by cue reveal a significant difference between regions for the unpredictable cue (F(1,86) = 11.585 p < .001), with a greater BNST relative to amygdala response, but no differences for the predictable threat cue (F(1,86) = .06, p = .81) or predictable neutral cue (F(1,210) = .07, p = .79). Social anxiety was not associated with BNST or amygdala responses to the cues.
Figure 3. BNST and amygdala responses to cues and image.
The BNST and amygdala show a significant dissociation by cue type (region × cue type interaction, p = .02), which represents a significant region effect for the unpredictable cue. In contrast, there are no significant regional differences by image type (predictable vs unpredictable) or valence (threat vs neutral).
Images
For the direct comparison of BNST and amygdala responses to images, there were no significant main effects of region (p = .16), image valence (p = .88), image type (p = .79), region × valence (p = .34), region × type (p = .24), type × valence (p = .13) or region × type × valence (p = .11) . However, as illustrated by the means in Figure 3, the amygdala generally showed activation to the images, whereas the BNST did not. Also of note, there was substantial variability in amygdala and BNST responses to the images that followed the unpredictable cues.
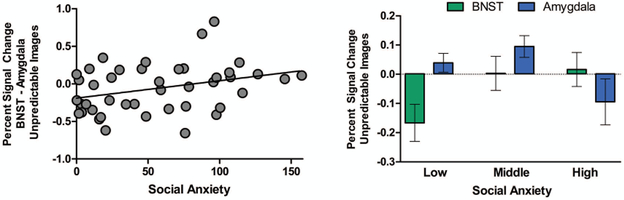
Social anxiety modulated the interaction of region × valence × type interaction (Figure 4; F(1,42) = 4.16, p = .05), with no significant main effects or other interactions with social anxiety. Post-hoc analyses by image type revealed that the effect of social anxiety on the region × valence interaction was significant for the unpredictable images (F(1,42)= 4.40 p = .05) and not the predictable images (F(1,42) = .10, p = .76). For the images following the unpredictable cues, individuals with the highest social anxiety had higher BNST relative to amygdala responses for threat vs neutral images, whereas those with low social anxiety had higher amygdala relative to BNST responses for threat vs neutral images.
Figure 4. Social anxiety moderates BNST vs amygdala responses to unpredictable threat images.
Left: Social anxiety scores are positively correlated with a difference between the BNST and amygdala responses to unpredictable (threat – neutral) images. Right: Social anxiety scores were binned into tertiles to illustrate the shift from amygdala > BNST to BNST > amygdala responses to unpredictable threat.
Discussion
The present findings provide new insights into the role of the BNST in social anxiety. The findings show that social anxiety moderates the relationship between the BNST and other brain regions in response to unpredictability. Although the hypothesis was that social anxiety would be associated with BNST activation in response to unpredictable cues and images, instead we discovered that the impact of social anxiety was largely on BNST connectivity and on the BNST-amygdala dissociation. To our knowledge, this is the first evidence of altered BNST function and connectivity in social anxiety. Difficulty tolerating uncertainty and unpredictability are central to anxiety disorders in general (Grupe & Nitschke, 2013) and social anxiety more specifically (Boelen & Reijntjes, 2009; Carleton et al., 2010); thus greater sensitivity to contexts of unpredictability may be a trait that contributes to heightened social anxiety and may also serve to maintain anxiety over time. These findings highlight the importance of the BNST in social anxiety-related differences in responses to unpredictability.
Social Anxiety and Unpredictable Cues.
Although cues signaling unpredictable threat robustly engaged the BNST--consistent with previous studies demonstrating heightened skin conductance responses to unpredictable threat cues (Alvarez et al., 2011) and with a previous meta-analysis of threat anticipation studies (Avery et al., 2016)—BNST response was not associated with social anxiety. Instead, social anxiety moderated BNST connectivity with the amygdala, specifically during unpredictable cues with lower BNST-amygdala connectivity in individuals with higher anxiety. Previous studies have shown BNST-amygdala resting state connectivity in humans (Avery et al., 2014; Gorka et al., 2017; Tillman et al., 2018; Torrisi et al., 2015), although much less is known about connectivity during tasks. At rest, the BNST and amygdala have overlapping connectivity with multiple brain regions involved in stimuli detection and processing (Gorka et al., 2017; Tillman et al., 2018), which may suggest an integrated system for detecting and evaluating stimuli. In this context, one hypothesis is that stronger task-related connectivity in those with lower social anxiety may reflect a coordinated system of BNST-amygdala cross-talk during detection and evaluation of unpredictable threat. The low social anxiety individuals also had greater amygdala relative to BNST responses to the unpredictable threat images which may reflect that BNST-amygdala connectivity, likely in concert with other brain regions, guided which region would process the images that followed. The lower BNST-amygdala connectivity to unpredictable cues in individuals with higher social anxiety might then reflect a failure of the coordinated cross-talk, resulting downstream in greater BNST response to unpredictable images. Future studies focused on network connectivity analyses and ideally dynamic network patterns will be critical for elucidating our understanding of the role of BNST-amygdala connectivity during unpredictable threat processing.
Social Anxiety and Unpredictable Images.
In contrast to the cue findings, unpredictable images did not engage the BNST and in fact, the BNST showed little response during image viewing. But there was substantial variability in BNST responses to the images and social anxiety accounted for a significant portion of that variance. For the effect of unpredictability, social anxiety was associated with stronger BNST connectivity with the ventromedial prefrontal cortex (vmPFC) and posterior cingulate cortex (PCC). The vmPFC and PCC are functionally connected with the BNST at rest (Avery et al., 2014; Tillman et al., 2018; Torrisi et al., 2015). During shock anticipation healthy individuals have decreased BNST connectivity with the vmPFC and PCC (Torrisi et al., 2017), consistent with the pattern observed for individuals with the lower social anxiety scores. In contrast, individuals with higher social anxiety had stronger connectivity with these two regions. The vmPFC is thought to promote BNST activity (Motzkin et al., 2015); in non-human primates, OFC lesions reduce both BNST activity and anxiety (Fox et al., 2010) and in humans vmPFC lesions are also associated with reduced BNST function at rest (Motzkin et al., 2015). Thus, the socially anxious individuals may have underlying alterations in BNST-vmPFC connectivity at rest, which may reflect tonic vmPFC hyperactivity and may drive BNST activation to unpredictable threat images. The PCC, perhaps best known for being a hub of the default mode network—is highly connected to many subcortical and cortical regions involved in emotion processing and also part of the dorsal attention network. The PCC has numerous functions that may be implicated in social anxiety including self-referential processing (Northoff et al., 2006), cognitive control (Leech, Kamourieh, Beckmann, & Sharp, 2011), and fear extinction recall (Ganella, Drummond, Ganella, Whittle, & Kim, 2018); however, future studies will be necessary to dissect the role of BNST-PCC connectivity in social anxiety.
Social anxiety and the BNST-Amygdala Dissociation.
Social anxiety was associated with a BNST vs amygdala difference in response to unpredictable images, but not unpredictable cues. Individuals with low and average social anxiety had a stronger amygdala response to threat images, consistent with a well-established function of the amygdala in responding to threatening stimuli (Zald, 2003). In comparison, the individuals with the highest social anxiety scores had a heightened BNST response to the unpredictable threat images. Our working hypothesis is that high social anxiety is associated with both a typical BNST response to unpredictable cues, seen in all individuals, combined with a sustained BNST response that persists through the presentation of the images, indicating sustained hypervigilance in the context of unpredictability. In contrast, in low social anxiety, the initial BNST response to the unpredictable cues is not sustained, and instead the amygdala shows the well-established activation to images. This hypothesis is consistent with previous studies demonstrating the BNST’s sustained activation during threat anticipation and role in hypervigilance (Somerville et al., 2010) and provides initial evidence for a BNST-amygdala dissociation in social anxiety.
BNST and Anxiety Disorders.
These study findings should be considered in the context of other anxiety studies. Previous investigations of BNST function in anxiety often studied normative levels of trait anxiety during threat anticipation and results have been mixed, including higher anxiety associated with increased or sustained BNST activation (Somerville et al., 2013, 2010) or altered BNST connectivity (Brinkmann et al., 2018; Mcmenamin et al., 2014), and other studies failing to find an association (Choi et al., 2012; Grupe et al., 2013; Pedersen, Balderston, et al., 2017) or finding a blunted BNST response (Pedersen, Tugan Muftuler, & Larson, 2017). Two studies showed BNST activation differences in spider phobia (Münsterkötter et al., 2015; Straube, Mentzel, & Miltner, 2007), but none in blood injection phobia (Brinkmann, Poller, Herrmann, Miltner, & Straube, 2017). Similarly, reports in generalized anxiety disorder have been mixed with both positive (Buff et al., 2017) and negative findings (Yassa, Hazlett, Stark, & Hoehn-Saric, 2012). The current study both extends early findings of a link between BNST activation and anxiety to social anxiety and also highlights the importance of studying BNST connectivity. The variable findings across studies likely reflect heterogeneity in tasks and samples, as well as replication challenges inherent in the modest sample sizes. Larger studies using the same task will be helpful in clarifying the BNST’s role across and within anxiety disorders.
Limitations and Conclusions.
Several study limitations should be noted. First, while advances in neuroimaging technology and methods have provided new opportunities to study small brain regions, such as the BNST, issues regarding limited resolution should still be considered. Second, we used a dimensional approach to test for associations with social anxiety. Dimensional approaches to understanding anxiety provide greater statistical power than categorical approaches and can provide evidence for linear dose-response relationships between behavioral traits and brain function. However, one limitation is that individuals who meet diagnostic criteria for a social anxiety disorder or those with the most severe functional impairment may have distinct brain responses. Third, the unpredictable threat cue was untrained and always presented in the last run, with the goal to maximized unpredictability. However, it is possible that the study findings are influenced by training or timing differences. It will be important for future studies to systematically investigate the effects of training and timing to determine the factors that contribute to BNST activation to cues. Fourth, emotional expressions of fear were used as the threat stimuli based on the salience of faces for social anxiety and the hypothesis about fear and anxiety circuitry; however, emotional expressions of anger are also salient for social anxiety and future studies should examine the impact of emotional valence on BNST responses in social anxiety. Finally, while the sample size was larger than many neuroimaging studies and provided statistical power to detect relatively large main effects, the sample was only powered to detect modest individual differences effects (Yarkoni, 2009). Two implications are that it is likely that social anxiety moderates responses to unpredictable threat in other brain regions not detected here and second, that it will be important to replicate these findings in future studies.
In summary, these findings provide novel evidence that the effect of social anxiety on neural responses to unpredictability is largely through altered BNST connectivity and BNST-amygdala dissociations, highlighting the importance of examining circuits in addition to single brain regions. This work adds to an emerging body of evidence that supports a distinct role for the BNST in unpredictability that may offer a novel target for therapeutic intervention in individuals with social anxiety disorder.
Supplementary Material
Acknowledgments
We are grateful for assistance by Ross VanDerKlok in data collection and processing.
This research was supported by the National Institutes of Health (NIMH: K01-MH083052 to JUB; F30-MH097344 to JAC), the Vanderbilt Institute for Clinical and Translational Research (NCRR; UL1-RR024975, TL1-RR024978), Developmental Psychopathology Training Grant (NIMH; T32-MH018921); and Vanderbilt Medical Scientist Training Program (NIGMS; T32-GM07347). Dr. Blackford’s effort was partially supported by a MERIT award (CX001226) and Dr. Clauss’ effort was also supported by NIMH R25-MH094612 (MGH/McLean Research Concentration Program).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
None of the authors have conflicts of interest to disclose.
References
- Alheid GF, & Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience, 27(1), 1–39. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, & Grillon C (2011). Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage, 55(1), 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, & Blackford JU (2016). The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology, 41(February), 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, & Blackford JU (2014). BNST neurocircuitry in humans. NeuroImage, 91, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AJ, Scott KM, Vos T, & Whiteford HA (2013). Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychological Medicine, 43(5), 897–910. [DOI] [PubMed] [Google Scholar]
- Blanco C, Heimberg RG, Schneier FR, Fresco DM, Chen H, Turk CL, … Liebowitz MR (2010). A Placebo-Controlled Trial of Phenelzine, Cognitive Behavioral Group Therapy, and Their Combination for Social Anxiety Disorder. Archives of General Psychiatry, 67(3), 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelen PA, & Reijntjes A (2009). Intolerance of uncertainty and social anxiety. Journal of Anxiety Disorders, 23(1), 130–135. [DOI] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Feldker K, Neumeister P, Heitmann CY, Hofmann D, … Straube T (2018). Inter-individual differences in trait anxiety shape the functional connectivity between the bed nucleus of the stria terminalis and the amygdala during brief threat processing. NeuroImage, 166(June 2017), 110–116. [DOI] [PubMed] [Google Scholar]
- Brinkmann L, Poller H, Herrmann MJ, Miltner W, & Straube T (2017). Initial and sustained brain responses to threat anticipation in blood-injection-injury phobia. NeuroImage: Clinical, 13(2017), 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, & Weidt S (2014). Neuroimaging in Social Anxiety Disorder–a meta-analytic review resulting in a new neurofunctional model. Neuroscience & Biobehavioral Reviews, 47, 260–280. [DOI] [PubMed] [Google Scholar]
- Buff C, Brinkmann L, Bruchmann M, Becker MPI, Tupak S, Herrmann MJ, & Straube T (2017). Activity alterations in the bed nucleus of the stria terminalis and amygdala during threat anticipation in generalized anxiety disorder. Social Cognitive and Affective Neuroscience, (January), 1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton RN, Collimore KC, & Asmundson GJG (2010). “It’s not just the judgements-It’s that I don’t know”: Intolerance of uncertainty as a predictor of social anxiety. Journal of Anxiety Disorders, 24(2), 189–195. [DOI] [PubMed] [Google Scholar]
- Choi JM, Padmala S, & Pessoa L (2012). Impact of state anxiety on the interaction between threat monitoring and cognition. NeuroImage, 59(2), 1912–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, Vanderklok RM, Rogers BP, Cowan RL, Benningfield MM, & Blackford JU (2014). Neurocircuitry underlying risk and resilience to social anxiety disorder. Depression and Anxiety, 31(10), 822–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Niles AN, Burklund LJ, Wolitzky-Taylor KB, Vilardaga-Jennifer CP, & Arch JJ (2015). Randomized controlled trial of cognitive behavioral therapy and acceptance and commitment therapy for social phobia: Outcomes and moderators. Journal of Consulting and Clinical Psychology Journal of Consulting Psychology, 82(6), No-Specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, & Kim M (1993). Fear-potentiated startle: A neural and pharmacological analysis. Behavioural Brain Research, 58(1–2), 175–198. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35(1), 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fox AS, & Kalin NH (2014). A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. The American Journal of Psychiatry, 171(11), 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Birn RM, Shackman AJ, Alexander AL, & Kalin NH (2018). Functional connectivity within the primate extended amygdala is heritable and associated with early-life anxious temperament. The Journal of Neuroscience, 0102–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, … Kalin NH (2015). Intergenerational neural mediators of early-life anxious temperament. Proceedings of the National Academy of Sciences of the United States of America, 201508593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, & Kalin NH (2010). Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. Journal of Neuroscience, 30(20), 7023–7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella DE, Drummond KD, Ganella EP, Whittle S, & Kim JH (2018). Extinction of conditioned fear in adolescents and adults: a human fMRI study. Frontiers in Human Neuroscience, 11(January), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, Becker EM, Keeton CP, Sakolsky D, Piacentini J, Albano AM, … Kendall PC (2014). Naturalistic follow-up of youths treated for pediatric anxiety disorders. JAMA Psychiatry, 21205, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka AX, Torrisi S, Shackman AJ, Grillon C, & Ernst M (2017). Intrinsic functional connectivity of the central nucleus of the amygdala and bed nucleus of the stria terminalis. NeuroImage, (September), 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe D, & Nitschke JB (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14(7), 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Oathes DJ, & Nitschke JB (2013). Dissecting the anticipation of aversion reveals dissociable neural networks. Cerebral Cortex, 23(8), 1874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Boehme S, Becker MPI, Tupak SV, Guhn A, Schmidt B, … Straube T (2016). Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Human Brain Mapping, 37(3), 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, & Walters EE (2005). Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Archives of General Psychiatry, 62(6), 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Kroes MC, Heitland I, Everaerd D, Akkermans SEA, Oosting RS, … Baas JMP (2015). Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biological Psychiatry, 78(8), 582–589. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Kroes MCW, Baas J, & Fernández G (2017). How human amygdala and bed nucleus of the stria terminalis may drive distinct defensive responses. The Journal of Neuroscience, 37(40), 3830–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, & Davis M (1997). Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. The Journal of Neuroscience, 17(16), 6434–6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, & Sharp DJ (2011). Fractionating the Default Mode Network: distinct dontributions of the ventral and dorsal posterior cingulate cortex to cognitive control. Journal of Neuroscience, 31(9), 3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcmenamin BW, Langeslag SJE, Sirbu M, Padmala S, & Pessoa XL (2014). Network organization unfolds over time during periods of anxious anticipation. Journal of Neuroscience, 34(34), 11261–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Oler JA, Kalin NH, Baskaya MK, & Koenigs M (2015). Ventromedial prefrontal cortex damage alters resting blood flow to the bed nucleus of stria terminalis. Cortex, 64, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münsterkötter AL, Notzon S, Redlich R, Grotegerd D, Dohm K, Arolt V, … Dannlowski U (2015). Spider or no spider? neural correlates of sustained and phasic fear in spider phobia. Depression and Anxiety, 32(9), 656–663. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, & Panksepp J (2006). Self-referential processing in our brain-A meta-analysis of imaging studies on the self. NeuroImage, 31(1), 440–457. [DOI] [PubMed] [Google Scholar]
- Pedersen WS, Balderston NL, Miskovich TA, Belleau EL, Helmstetter FJ, & Larson CL (2017). The effects of stimulus novelty and negativity on BOLD activity in the amygdala, hippocampus, and bed nucleus of the stria terminalis. Social Cognitive and Affective Neuroscience, 12(5), 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen WS, Tugan Muftuler L, & Larson CL (2017). Disentangling the effects of novelty, valence and trait anxiety in the bed nucleus of the stria terminalis, amygdala and hippocampus with high resolution 7T fMRI. NeuroImage, 156, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM, & Heimberg RG (1997). A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy, 35(8), 741–756. [DOI] [PubMed] [Google Scholar]
- Richards HJ, Benson V, Donnelly N, & Hadwin JA (2014). Exploring the function of selective attention and hypervigilance for threat in anxiety. Clinical Psychology Review, 34(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, & Fox AS (2016). Contributions of the Central Extended Amygdala to Fear and Anxiety. Journal of Neuroscience, 36(31), 8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, & Kelley WM (2013). Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex, 23(1), 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, & Kelley WM (2010). Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry, 68(5), 416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, & Kean YM (2000). Disability and quality of life in social phobia: epidemiologic findings. American Journal of Psychiatry, 157(10), 1606–1613. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, & Miltner WHR (2007). Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. NeuroImage, 37(4), 1427–1436. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, & Ledoux JE (2004). Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience, 128(1), 7–14. [DOI] [PubMed] [Google Scholar]
- Tillman RM, Stockbridge MD, Nacewicz BM, Torrisi S, Fox AS, Smith JF, & Shackman AJ (2018). Intrinsic functional connectivity of the central extended amygdala. Human Brain Mapping, 39(3), 1291–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, Gorka AX, Gonzalez-Castillo J, Balderston N, Grillon C, & Ernst M (2017). Extended amygdala connectivity changes during sustained shock anticipation. Translational Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, O’Connell K, Davis A, Reynolds R, Balderston N, Fudge JL, … Ernst M (2015). Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Human Brain Mapping, 36(10), 4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SM, Beidel DC, Dancu CV, & Stanley MA (1989). An empirically derived inventory to measure social fears and anxiety: The social phobia and anxiety inventory. Psychological Assessment, 1(1), 35–40. [Google Scholar]
- Waddell J, Morris R, & Bouton M (2006). Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: Aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behavioral Neuroscience, 120(2), 324–336. [DOI] [PubMed] [Google Scholar]
- Walker DL, & Davis M (1997). Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of Neuroscience, 17(23), 9375–9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T (2009). Big correlations in little studies. Perspect. Psychol. Stud, 4(3), 294–298. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Hazlett RL, Stark CEL, & Hoehn-Saric R (2012). Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. Journal of Psychiatric Research, 46(8), 1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH (2003). The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews, 41(1), 88–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.