Abstract
Background:
The relation between liver fibrosis scores and health outcomes in older people has been barely investigated. We aimed to evaluate the association of four liver fibrosis scores (fibrosis-4 -FIB-4-, NAFLD fibrosis score -NFS-, BARD and aspartate aminotransferase/alanine aminotransferase ratio -AST/ALT-) with mortality and incident disability at 6 years in an older population.
Methods:
We studied 962 individuals aged≥65 (mean age 74.4; female 55.5%) with a mean follow-up of 95.7 months, enrolled in the InCHIANTI study. The relationship between liver fibrosis scores and mortality and disability was assessed through Cox and log-binomial regressions.
Results:
NFS and FIB-4 were associated with higher overall (aHR ranging 1.38–1.78 for intermediate risk of fibrosis and 1.60–2.02 for high risk) and cardiovascular (aHR ranging 1.76–2.90 for intermediate and 2.22–2.42 for high risk) mortality. AST/ALT and BARD were only associated with overall mortality. Only NFS and FIB-4 high risk classes were associated with incident disability (aRR ranging 1.93–2.76). Despite poor sensitivity, all scores showed high specificity (ranging 0.88–0.95).
Conclusion:
Higher risk of liver fibrosis is associated with higher risk of poor health outcomes. Liver fibrosis scores may help to stratify the risk and, mainly, identify elderly patients with favorable prognosis.
Keywords: liver fibrosis scores, elderly, mortality, disability
Introduction
Chronic liver diseases (CLDs) have reached epidemic proportions in Western countries with relevant health and economic consequences.[1] While the burden of hepatitis C virus (HCV) is supposed to significantly decrease due to the availability of highly effective direct-acting antiviral drugs, alcoholic consumption still represents a common cause of CLD, and non-alcoholic fatty liver disease (NAFLD) has already become the most common liver disease in parallel with the relentless increase of prevalence of obesity, dyslipidemia and diabetes mellitus.[1]
The burden of CLD is expected to further increase as a result of population aging, because older age is associated with many risk factors for CLD or CLD progression to liver cirrhosis.[2] Indeed, the prevalence of CLD is high among older people, and NAFLD has its peak prevalence (30%) in the seventh decade of life, suggesting that CLD may be a relevant problem in this age group.[2–4] In turn, CLD is characterized by a low-grade chronic inflammation, that may underpin the association between CLD and other conditions. For example, CLD has been associated with cardiovascular disease independently of the shared metabolic risk factors, with risk increasing with the progression of liver disease in terms of fibrosis.[5,6] In older people, the same mechanism may be also associated with reduction of muscle mass and frailty.[7]
Independently of the etiology, liver fibrosis progression to cirrhosis can be considered as the most important prognostic indicator in CLD subjects[8,9]. Accordingly, adequately staging CLD represent a leading issue to stratify the risk of patients and tailor effective healthcare strategies. To this purpose, liver biopsy is the gold standard for the staging and, in selected case, for the diagnosis of CLD, but it is an invasive procedure that cannot be routinely performed. Alternative approaches are based on the use of non-invasive tests of liver fibrosis, i.e. imaging methods and combined scores of clinical and serum indicators. Among these scores, the fibrosis-4 (FIB-4) score[10] and the aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (AST/ALT ratio)[11] are made up of mainly “liver-specific” variables such as age, ALT, AST and platelets. Conversely, the NAFLD fibrosis score (NFS)[12] and the BARD[13] were specifically constructed in NAFLD patients and also include more “general” variables (such as BMI, impaired fasting glucose or diabetes mellitus and albumin), that are well-known to be per se associated with mortality and not necessarily via liver fibrosis. Beside their reasonable accuracy in detecting advanced liver fibrosis, these scores have been recently associated with overall, cardiovascular and liver-specific mortality in large population-based studies, both when restricted to NAFLD subjects[14–16], and when extended to the entire population[17], but no data are available on the elderly population, especially with respect to functional outcomes, such as disability, that are of particular interest in this age group.
Our hypothesis is that liver fibrosis scores may be associated with mortality and incident disability in the general population of older people, and that, compared to NFS and BARD, indices including “liver-specific” variables (FIB-4 and AST/ALT ratio) would show an at least comparable association when potential confounders are taken into account.
We used the InCHIANTI dataset to estimate the association between non-invasive liver fibrosis scores and mortality (both overall and cause-specific) and 6-years incident disability in a population of older subjects.
Methods
Data Source and Sample Selection
We used data from InCHIANTI study, a prospective population-based study of randomly selected 1453 subjects living in the Chianti area (Greve in Chianti and Bagno a Ripoli, Tuscany, Italy), aiming at investigating the factors contributing to the decline of mobility in older persons. The project was designed by the Laboratory of Clinical Epidemiology of the Italian National Research Council on Aging (INRCA, Florence, Italy) and the study protocol was ratified by INRCA Ethical Committee. The eligible participants were firstly interviewed at their homes on their health, physical and cognitive status. Then, physical examination and blood tests were performed at the study clinic. Comorbid diseases were ascertained examining clinical history, medical records and medication use. The first wave of study started in 1998 (baseline evaluation) and participants were followed up with evaluation every 3 years. A detailed description of the study design has been previously published[18].
From the original study population (N 1453), we selected participants aged more than 65 years (N 1155). Thereafter, we removed patients (N 193) with any missing data in variables necessary for the computation of the liver fibrosis scores (NFS, FIB-4, BARD and AST/ALT ratio; Supplementary Table 1). The final sample size was 962.
Variable measurement
Data on variables needed to calculate liver fibrosis scores and believed to affect mortality and incident disability were retrieved from the baseline evaluation. The Charlson Comorbidity Index was computed to represent the global burden of diseases.[19] Data on the following blood tests were also collected: ALT, AST, platelets, serum albumin, creatinine, total and low-density-lipoprotein (LDL) cholesterol, triglycerides, fibrinogen, interleukin-6 (IL-6), high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor (TNF)-alpha and oxidized LDL. Glomerular filtration rate was estimated through the CKD-EPI formula. Smoking habit (pack-years) and alcohol consumption (grams per day) were measured based on interview data. Physically active patients were considered those performing at least moderate exercise once or twice a week or light exercise more than 4 times per week.
Liver fibrosis scores were computed according to suggested formulas (Supplementary Table 1), that included age, AST, ALT and platelets for FIB-4; age, BMI, impaired fasting glycaemia or diabetes mellitus, AST to ALT ratio, platelets and albumin for NFS; BMI, AST to ALT ratio and diabetes mellitus for BARD; AST and ALT for AST/ALT ratio. The originally described cut-points were used for BARD (<2 and >3) and AST/ALT ratio (<0.8 and >1), while age-specific cut-points for subjects aged 65 or more were used for NFS (<0.12 and >0.676) and FIB-4 (<2 and >2.67), as suggested by McPherson et al[20]. Consequently, patients were categorized into 3 groups, i.e. those with low probability (score<lower cut-off), intermediate probability and high probability (score>upper cut-off) for advanced fibrosis.
Since data on ultrasonographic presence of liver steatosis were not available, the Fatty Liver Index (FLI), a validated algorithm to predict ultrasonographic liver steatosis based on BMI, waist circumference, triglycerides and GGT, was calculated for all subjects and was used to rule in hepatic steatosis with the cut-off ≥60.[21]
Frailty was assessed according to Fried and colleagues’ criteria and participants were considered frail if meeting 3 or more criteria and pre-frail if meeting 1 or 2 criteria.[22] Sarcopenia was defined, according to EWGSOP, as the presence of low muscle mass, plus low muscle strength or low physical performance; conversely, the presence of low muscle mass with normal muscle strength and normal physical performance was defined as pre-sarcopenia.[23] Muscle mass was measured through a right leg peripheral Quantitative Computed Tomography (pQCT), that evaluated the cross-sectional muscle and fat areas of the calf scanned at the 66% of the tibial length starting from the tibiotarsal joint. Low muscle mass was, then, assess with the lowest gender-specific tertile of the residuals of a linear regression model that predicted the dependent variable muscle mass area (in cm2) from height (in cm) and fat mass area (log value of cm2; independent variables).[24] Disability was defined as loss of one of the basic activities of daily living (ADL) at baseline and at 6 years’ follow-up: dressing, moving in and out of bed, using the toilet, washing, eating, and control urine and fecal continence.[25]
Vital status was available for all participants up to April 2010. Causes of death were registered through the International Classification of Disease (ICD)-9 code. Cause-specific mortality was considered for causes exceeding a 2.5% absolute mortality: cardiovascular (defined with ICD-9 codes from 390 to 459), cancer (ICD-9 codes from 140 to 239), respiratory (ICD-9 codes from 460 to 519).
Analytical approach
The main baseline characteristics of the study population were shown according to the 3 risk classes of the liver fibrosis scores. Comparison were carried out using ANOVA test or Kruskal-Wallis test for continuous variables, as appropriate, and by χ2 test for categorical variables. Then, to examine the longitudinal association between liver fibrosis scores the risk of disability at 6 years and overall and cause-specific mortality, we performed a log-binomial regression and Cox proportional hazard regressions, respectively. Multivariable models were adjusted for the following potential confounding factors: age, sex, BMI, arterial hypertension, diabetes mellitus, COPD, CHF, total cholesterol, Charlson comorbidity index, physical activity, alcohol consumption, smoking, IL-6 and TNF-alpha. Interaction terms were entered in the models to evaluate whether risk estimates may change across categories of predicted presence of ultrasonographic liver steatosis (as estimated by FLI ≥60) and alcohol consumption (below or above 30 g/day for males and 20 g/day for females). In addition, sensitivity analyses were run including only subjects with FLI≥60 and with low alcohol consumption. The proportional hazard assumption of Cox regressions was tested through the inspection of Schoenfeld residuals.
We also examined the discriminative capacities of liver scores in predicting mortality and disability, calculating the c-statistics of the Cox models and the area under the receiver operating characteristic curve (AUC) of the log-binomial models. Sensitivity and specificity were also calculated. All analyses were performed using R 3.3.3 software for Mac (R Foundation).
Results
Baseline characteristics
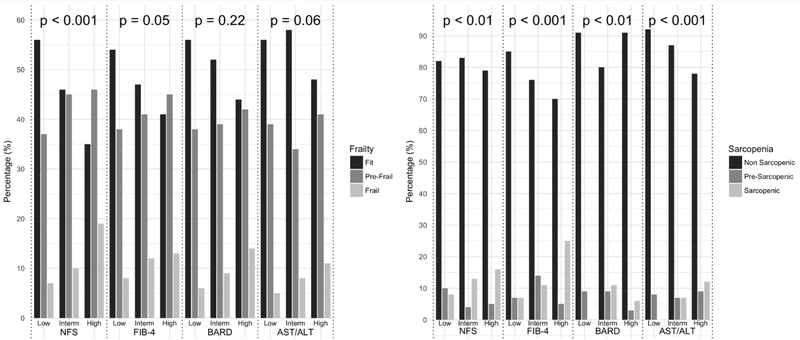
General characteristics of participants according to the liver scores risk classes at baseline are presented in Table 1 and Supplementary Table 2. The mean age was 74.4 years (SD: 6.9), and 55.5% were women. Prevalence of intermediate and high risk score varied according to specific liver score (20.0% and 8.5% for FIB-4, 11.6% and 14.4% for NFS, 80.9% and 11.7% for BARD, 25.8% and 64% for AST/ALT ratio). Relationships with demographic, anthropometric and clinical characteristics were variable depending on the liver score taken into account and no variable was found to be uniformly different in high risk patients compared to other risk categories. In general, persons in higher risk classes had more adverse metabolic characteristics with a greater burden of comorbidities and a more pronounced inflammatory profile (Table 1). Apparently, there were some exceptions, that could be explained by the consideration of potential confounders. For instance, the observed lower BMI values in higher FIB-4 classes are basically mediated by the confounding effect of age, as older subjects tend to have lower BMI and are more represented in the high risk group (data not shown). As reported in Figure 1, the prevalence of frailty and sarcopenia increased from low to high risk classes for NFS (from 7% to 19%, p<0.001, and from 8% to 16%, p<0.01, respectively), FIB4 (from 8% to 13%, p<0.05, and from 7% to 25%, p<0.001, respectively). Sarcopenia, but not frailty, was associated with BARD (from 0% in low risk to 6% in high risk class, p<0.01) and AST/ALT ratio risk classes (from 0% in low risk to 12% in high risk class, p<0.001).
Table 1.
Characteristics of study participants, according to FIB-4, NFS, BARD and AST/ALT ratio risk classes.
| NFS | ||||
|---|---|---|---|---|
| Low risk | Interm risk | High risk | p | |
| N (%) | 711 (73.9%) | 112 (11.6%) | 139 (14.5) | - |
| Age (years), mean(SD) | 73.2 (6.5) | 76.3 (6.8) | 78.4 (7) | < 0.001 |
| Sex (Female), n(%) | 403 (56.7%) | 72 (64.3%) | 59 (42.4%) | 0.001 |
| BMI (Kg/m2), mean(SD) | 26.8 (3.7) | 28.6 (4.4) | 29.9 (4.5) | < 0.001 |
| Hypertension, n(%) | 460 (64.7%) | 80 (71.4%) | 96 (69.1%) | 0.274 |
| Diabetes Mellitus, n(%) | 53 (7.5%) | 29 (25.9%) | 51 (36.7%) | < 0.001 |
| eGFR (mL/min), mean(SD) | 72.2 (13.5) | 68.8 (14.1) | 67.4 (15.1) | < 0.001 |
| Smoke (Pack-year), mean(SD) | 12.4 (20.6) | 10.8 (18.5) | 14.2 (22.3) | 0.643 |
| Alcohol consumption (g/day), median(IQR) | 12 (20.4) | 12 (16.8) | 12 (31.2) | 0.331 |
| Physically Active, n(%) | 291 (41.1%) | 38 (33.9%) | 38 (27.5%) | 0.007 |
| Sarcopenia, n(%) | 51 (7.8%) | 14 (13.5%) | 19 (15.7%) | 0.009 |
| Frailty, n(%) | 53 (7.5%) | 11 (9.8%) | 26 (18.7%) | < 0.001 |
| Disability at baseline, n(%) | 29 (4.1%) | 5 (4.5%) | 15 (10.8%) | 0.004 |
| Disability at 6 years follow-up, n(%) | 55 (9.8%) | 9 (11.7%) | 20 (22%) | 0.003 |
| Follow-up (months), mean(SD) | 98.7 (25.8) | 89 (34.7) | 86.1 (32.7) | < 0.001 |
| Deaths, n(%) | 203 (28.6%) | 49 (43.8%) | 78 (56.1%) | < 0.001 |
| CV-deaths, n(%) | 76 (11%) | 24 (22.2%) | 39 (29.5%) | < 0.001 |
| Cancer deaths, n(%) | 48 (6.9%) | 12 (11.1%) | 14 (10.6%) | 0.155 |
| Respiratory deaths, n(%) | 18 (2.6%) | 2 (1.9%) | 5 (3.8%) | 0.631 |
| FIB-4 | ||||
| Low risk | Interm risk | High risk | p | |
| N (%) | 687 (71.4%) | 193 (20.0%) | 82 (8.6%) | - |
| Age (years), mean(SD) | 73.1 (6.3) | 76.4 (6.9) | 80 (7.2) | < 0.001 |
| Sex (Female), n(%) | 408 (59.4%) | 93 (48.2%) | 33 (40.2%) | < 0.001 |
| BMI (Kg/m2), mean(SD) | 27.7 (4.1) | 26.9 (4.1) | 26.3 (3.9) | 0.001 |
| Hypertension, n(%) | 457 (66.5%) | 128 (66.3%) | 51 (62.2%) | 0.735 |
| Diabetes Mellitus, n(%) | 101 (14.7%) | 24 (12.4%) | 8 (9.8%) | 0.388 |
| eGFR (mL/min), mean(SD) | 72.2 (13.8) | 69.2 (13.5) | 65.8 (14.1) | < 0.001 |
| Smoke (Pack-year), mean(SD) | 12.3 (20.2) | 11.5 (20.4) | 16.3 (23.6) | 0.126 |
| Alcohol consumption (g/day), median(IQR) | 12 (19.2) | 12 (25.2) | 12 (32.7) | 0.275 |
| Physically Active, n(%) | 260 (38%) | 81 (42.2%) | 26 (32.1%) | 0.275 |
| Sarcopenia, n(%) | 47 (7.5%) | 19 (10.7%) | 18 (24.7%) | < 0.001 |
| Frailty, n(%) | 55 (8%) | 24 (12.4%) | 11 (13.4%) | 0.073 |
| Disability at baseline, n(%) | 27 (3.9%) | 15 (7.8%) | 7 (8.5%) | 0.033 |
| Disability at 6 years follow-up, n(%) | 50 (9.2%) | 18 (13%) | 16 (32.7%) | < 0.001 |
| Follow-up (months), mean(SD) | 98.1 (26.9) | 93 (30) | 82 (33.2) | < 0.001 |
| Deaths, n(%) | 196 (28.5%) | 77 (39.9%) | 57 (69.5%) | < 0.001 |
| CV-deaths, n(%) | 76 (11.4%) | 38 (20.5%) | 25 (32.1%) | < 0.001 |
| Cancer deaths, n(%) | 46 (6.9%) | 17 (9.2%) | 11 (14.1%) | 0.064 |
| Respiratory deaths, n(%) | 19 (2.8%) | 1 (0.5%) | 5 (6.4%) | 0.024 |
| BARD | ||||
| Low risk | Interm risk | High risk | p | |
| N (%) | 71 (7.4%) | 778 (80.8%) | 113 (11.8%) | - |
| Age (years), mean(SD) | 71 (5.1) | 74.5 (6.9) | 75.4 (6.8) | < 0.001 |
| Sex (Female), n(%) | 32 (45.1%) | 436 (56%) | 66 (58.4%) | 0.165 |
| BMI (Kg/m2), mean(SD) | 28.5 (3.8) | 26.7 (3.7) | 31.9 (3.3) | < 0.001 |
| Hypertension, n(%) | 41 (57.7%) | 508 (65.3%) | 87 (77%) | 0.015 |
| Diabetes Mellitus, n(%) | 14 (19.7%) | 69 (8.9%) | 50 (44.2%) | < 0.001 |
| eGFR (mL/min), mean(SD) | 74.2 (18.9) | 63.4 (19) | 71.3 (19.8) | < 0.001 |
| Smoke (Pack-year), mean(SD) | 18.2 (26.3) | 12.1 (20) | 11.6 (20.4) | 0.062 |
| Alcohol consumption (g/day), median(IQR) | 16.8 (25.2) | 12 (20.1) | 12 (26.4) | 0.053 |
| Physically Active, n(%) | 34 (48.6%) | 304 (39.2%) | 29 (25.7%) | 0.004 |
| Sarcopenia, n(%) | 0 (0%) | 78 (11%) | 6 (5.9%) | 0.006 |
| Frailty, n(%) | 4 (5.6%) | 70 (9%) | 16 (14.2%) | 0.113 |
| Disability at baseline, n(%) | 4 (5.6%) | 39 (5%) | 6 (5.3%) | 0.968 |
| Disability at 6 years follow-up, n(%) | 4 (6.6%) | 70 (11.9%) | 10 (12.2%) | 0.45 |
| Follow-up (months), mean(SD) | 104.6 (22.3) | 95.6 (28.3) | 90.8 (31.8) | < 0.001 |
| Deaths, n(%) | 15 (21.1%) | 271 (34.8%) | 44 (38.9%) | 0.036 |
| CV-deaths, n(%) | 5 (7.4%) | 111 (14.7%) | 23 (20.7%) | 0.049 |
| Cancer deaths, n(%) | 4 (5.9%) | 60 (8%) | 10 (9%) | 0.753 |
| Respiratory deaths, n(%) | 1 (1.5%) | 20 (2.7%) | 4 (3.6%) | 0.689 |
| AST/ALT ratio | ||||
| Low risk | Interm risk | High risk | p | |
| N (%) | 98 (10.2%) | 248 (25.8%) | 616 (64.0%) | - |
| Age (years), mean(SD) | 70.7 (5) | 72.2 (5.9) | 75.8 (7.1) | < 0.001 |
| Sex (Female), n(%) | 47 (48%) | 122 (49.2%) | 365 (59.3%) | 0.008 |
| BMI (Kg/m2), mean(SD) | 29.5 (3.9) | 28.5 (3.9) | 26.7 (4) | < 0.001 |
| Hypertension, n(%) | 64 (65.3%) | 168 (67.7%) | 404 (65.6%) | 0.819 |
| Diabetes Mellitus, n(%) | 29 (29.6%) | 42 (16.9%) | 62 (10.1%) | < 0.001 |
| eGFR (mL/min), mean(SD) | 76.3 (18.9) | 72 (19.3) | 60.6 (18) | < 0.001 |
| Smoke (Pack-year), mean(SD) | 17.3 (25.7) | 13.7 (21.2) | 11.2 (19.3) | 0.048 |
| Alcohol consumption (g/day), median(IQR) | 16.8 (26.4) | 12 (26.4) | 12 (19.2) | 0.044 |
| Physically Active, n(%) | 43 (44.3%) | 111 (44.9%) | 213 (34.7%) | 0.009 |
| Sarcopenia, n(%) | 0 (0%) | 16 (7%) | 68 (12.2%) | < 0.001 |
| Frailty, n(%) | 5 (5.1%) | 20 (8.1%) | 65 (10.6%) | 0.164 |
| Disability at baseline, n(%) | 6 (6.1%) | 9 (3.6%) | 34 (5.5%) | 0.462 |
| Disability at 6 years follow-up, n(%) | 7 (8.2%) | 13 (6.6%) | 64 (14.3%) | 0.011 |
| Follow-up (months), mean(SD) | 105 (20.5) | 97.3 (28.4) | 93.6 (29.3) | < 0.001 |
| Deaths, n(%) | 20 (20.4%) | 69 (27.8%) | 241 (39.1%) | < 0.001 |
| CV-deaths, n(%) | 6 (6.4%) | 22 (9.1%) | 111 (18.6%) | < 0.001 |
| Cancer deaths, n(%) | 6 (6.4%) | 22 (9.1%) | 46 (7.7%) | 0.674 |
| Respiratory deaths, n(%) | 1 (1.1%) | 8 (3.3%) | 16 (2.7%) | 0.521 |
Groups compared by Kruskal-Wallis test for continuous variables and by χ2 test for categorical variables. BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate by CKD-EPI formula; CHF, congestive heart failure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDL, low density lipoprotein; hs-CRP, high sensitivity C reactive protein; TNF-alpha, tumor necrosis factor alpha; SD, standard deviation; IQR, interquartile range.
Figure 1. Distribution of liver fibrosis scores’ risk classes across categories of frailty and sarcopenia.
(p values are for chi-squared test; post-hoc chi-squared comparison with Bonferroni correction was performed to verify the statistical significance of the reduced prevalence of sarcopenia in BARD high compared to intermediate risk class: p=0.48)
Liver fibrosis scores and mortality
Over a mean follow-up of 95.7 months, 330 subjects (34.4%) died, 139 (14.9%) for cardiovascular disease, 74 (7.9%) for neoplasms and 25 (2.7%) for respiratory disease.
Mortality risk increased in people with intermediate (HR 1.51, 95%CI 1.16–1.96, for overall mortality and HR 1.96, 95%CI 1.33–2.9, for CV mortality) and high risk (HR 2.86, 95%CI 2.12–3.85, for overall mortality and HR 3.57, 95%CI 2.27–5.62, for CV mortality) of fibrosis according to FIB-4 compared to those at low risk, even after correction for potential confounders (for overall mortality, aHR in intermediate risk class 1.34, 95%CI 1.02–1.77 and aHR in high risk class 2.02, 95%CI 1.47–2.77; for CV mortality, aHR in intermediate risk class 1.76, 95%CI 1.17–2.64, and aHR in high risk class 2.22, 95%CI 1.36–3.6).
The same relationship was also observed for NFS intermediate (for overall mortality: HR 1.64, 95%CI 1.2–2.24 and aHR 1.78, 95%CI 1.29–2.47; for CV mortality: HR 2.32, 95%CI 1.47–3.67 and aHR 2.9, 95%CI 1.80–4.66) and high risk classes (for overall mortality: HR 2.14, 95%CI 1.64–2.78, and aHR 1.6, 95%CI 1.21–2.1; for CV mortality: HR 3.24, 95%CI 2.2–4.77 and aHR 2.42, 95%CI 1.61–3.64).
Also AST/ALT ratio risk classes were associated with overall mortality with HR 1.8 (95%CI 1.07–3.04) in intermediate risk class, and 2.61 (95%CI 1.61–4.22) in high risk class; the corresponding aHR were 2.02 (95%CI 1.19–3.44) and 1.74 (95%CI 1.06–2.86), respectively. No association was present with CV mortality. Conversely, only BARD high risk class showed an increased overall and CV mortality (HR 2.90, 95%CI 1.55–5.42, and 3.29, 95%CI 1.25–8.66 for overall and CV mortality, respectively), that was confirmed in adjusted models for overall mortality (aHR 1.81, 95%CI 1.10–2.93, for BARD), but not for CV mortality. No association was found with regard to mortality for neoplasms and respiratory diseases (Supplementary Table 3). No interaction was found between mortality and FLI≥60 or excessive alcohol consumption. Sensitivity analyses after excluding subjects with excessive alcohol consumption (>30 g/day for male and >20 g/day for females) or selecting only subjects with high probability of liver steatosis (FLI≥60) did not change the strength of observed associations with mortality (Supplementary Tables 4–5).
Liver fibrosis scores and incident disability
Seventy-one (11.1%) subjects became disabled at 6 years’ follow-up. FIB-4 high risk class for liver fibrosis showed a significant association with incident disability (RR 3.69, 95%CI 1.95–6.57), even after correction for potential confounders (aRR 2.76, 95%CI 1.38–5.19).
In the same way, participants with high risk of fibrosis according to NFS, BARD and AST/ALT ratio had an increased incidence of disability at 6 years (RR 2.04, 95%CI 1.11–3.57, for NFS; RR 5.80, 95%CI 1.06–107.6, for BARD; RR 3.41, 95%CI 1.26–13.98, for AST/ALT ratio). These associations were confirmed in adjusted models only for NFS (aRR 1.93, 95%CI 1.13–2.93). In line with the results on mortality, we found no interaction between FLI≥60 and excessive alcohol consumption with incident disability. Sensitivity analyses after excluding subjects with excessive alcohol consumption or selecting only subjects with high probability of liver steatosis (FLI≥60) did not change the strength of observed associations with incident disability (Supplementary Tables 4–5).
Predictive properties of liver fibrosis scores
Table 3 shows discriminative capacities for the adverse outcomes of interest at 6 years’ follow-up. NFS, FIB-4 and BARD high risk classes evidenced high specificity (0.88–0.94 for overall and CV mortality and 0.89–0.95 for disability), while poor sensitivity (0.14–0.27 for overall and CV mortality and 0.11–0.21 for disability) and c-statistics/AUCs (0.53–0.62 for overall and CV mortality and 0.53–0.59 for disability).
Table 3.
Sensitivity, Specificity and c-statistics/AUC for Occurrence of Adverse Outcomes at 6 years According to Liver Score risk classes, Frailty, Sarcopenia and disability at baseline.
| Sensitivity | Specificity | c-statistics (95%CI) | |
|---|---|---|---|
| Death | |||
| NFS | 0.24 | 0.88 | 0.6(0.57–0.63) |
| FIB-4 | 0.17 | 0.93 | 0.6(0.57–0.63) |
| BARD | 0.15 | 0.89 | 0.53(0.5–0.56) |
| AST/ALT ratio | 0.74 | 0.38 | 0.57(0.54–0.6) |
| FRAILTY | 0.27 | 0.95 | 0.59(0.57–0.62) |
| SARCOPENIA | 0.26 | 0.94 | 0.59(0.56–0.61) |
| BASELINE DISABILITY | 0.17 | 0.98 | 0.56(0.54–0.58) |
| CV-death | Sensitivity | Specificity | c-statistics (95%CI) |
| NFS | 0.27 | 0.88 | 0.62(0.57–0.66) |
| FIB-4 | 0.16 | 0.94 | 0.61(0.56–0.65) |
| BARD | 0.14 | 0.89 | 0.54(0.51–0.58) |
| AST/ALT ratio | 0.83 | 0.38 | 0.6(0.56–0.63) |
| FRAILTY | 0.37 | 0.95 | 0.63(0.59–0.67) |
| SARCOPENIA | 0.34 | 0.94 | 0.6(0.55–0.64) |
| BASELINE DISABILITY | 0.24 | 0.98 | 0.59(0.55–0.62) |
| Incident Disability | Sensitivity | Specificity | AUC (95%CI) |
| NFS | 0.21 | 0.89 | 0.57(0.51–0.62) |
| FIB-4 | 0.2 | 0.95 | 0.59(0.53–0.66) |
| BARD | 0.11 | 0.89 | 0.53(0.5–0.56) |
| AST/ALT ratio | 0.79 | 0.4 | 0.57(0.54–0.6) |
| FRAILTY | 0.18 | 0.97 | 0.58(0.53–0.62) |
| SARCOPENIA | 0.22 | 0.96 | 0.59(0.54–0.64) |
Overall, discriminative performances were substantially comparable to those shown for frailty (c-statistics of 0.59 and 0.63 for overall and CV mortality and AUC of 0.58 for disability), sarcopenia (c-statistics of 0.59 and 0.60 for overall and CV mortality and AUC of 0.59 for disability) and baseline disability (c-statistics of 0.56 for mortality and 0.59 for CV mortality).
Discussion
The present study indicates that older persons classified by non-invasive scores as having higher risk of liver fibrosis are also at increased risk for mortality and incident disability. The strength of the association was similar using “liver-specific” scores (FIB-4 and AST/ALT ratio) and other scores including more “general” risk factors. While FIB-4 and NFS were associated with both mortality and disability, BARD and AST/ALT ratio were associated with mortality, but not with incident disability. To note, observed associations were independent of coexisting diseases and other known risk factors. Specificity towards all the outcomes was high, while we found poor sensitivity and c-statistics/AUCs.
Hitherto, liver fibrosis scores have been suggested as surrogate prognostic tools in epidemiological studies, showing strong association with liver and non-liver related mortality.[14–17] However, the proportion of older subjects included in previous studies was negligible. Thus, our study contributes to the literature by extending to an older population the data on the prognostic role of liver fibrosis scores.
The FIB-4, i.e. the score including mainly liver related variables, retained consistent associations with all the outcomes, also after correction for potential confounders. This finding is also strengthened by the concomitant observed association of AST/ALT ratio with mortality, that is in keeping with recent studies pointing at the prognostic role of liver specific tests, such as ALT, in the aging population.[26]
NFS confirmed the same relationships of FIB-4, while BARD was associated with mortality, but not with CV mortality and disability in multivariable models. In any case, the observation that scores (FIB-4 and AST/ALT ratio) made up of purely liver-specific variables showed similar associations with scores (i.e. NFS and BARD) also including general variables possibly associated with mortality, supports the concept that liver-related factors may play, together with other shared risk factors, an important and independent role in the pathophysiological underpinnings mediating the occurrence of the studied adverse outcomes. Surprisingly and apparently in contrast with this hypothesis, the associations of liver fibrosis scores were also independent of IL-6 and TNF-alpha, probably indicating chronic low-grade liver inflammation may impact on health status through different pathways, not involving the classical mediators.
In line with observations in the younger population, older patients at “intermediate risk” for fibrosis show increased mortality, particularly with scores allowing the correction with age-specific thresholds for discrimination of low from intermediate class (NFS and FIB-4).
With respect to cause-specific mortality, no association was found with respiratory and cancer mortality, as also reported by other similar studies.[15,16]
To our knowledge, this is the first study showing the association between liver fibrosis scores and disability. Many liver-related factors may interact with aging promoting a vicious circle leading to physical impairment and ultimately disability. Indeed, insulin resistance, which is the pathophysiological background of NAFLD, but also the consequence of all CLDs[27,28], is responsible for the increased inflammatory response that characterizes obesity and diabetes mellitus. Inflammatory cytokines, such as interleukin-6 and TNFα, activate muscle breakdown to generate amino-acids for energy, but also favor a progressive decrease in muscle mass, i.e. sarcopenia.[29] Among younger NAFLD patients, low muscle mass was independently associated with NASH and significant fibrosis,[30] which in turn may contribute to the overactive, insufficiently regulated and persistent inflammatory response that is typical of aging.[7] Moreover, sarcopenia is a key factor for the development of frailty and both have been linked to falls, functional decline, disability and mortality in the elderly.[31] Our data support these observations, showing that people in high risk classes of liver fibrosis scores have higher concentration of inflammatory markers and more comorbidities. Furthermore, the observation of an increased prevalence of frailty and sarcopenia in more advanced liver fibrosis risk classes probably reinforces the concept that liver fibrosis may increase the risk of disability through reduced muscle mass and physical function.
The sensitivity of the scores was low, and therefore liver fibrosis score cannot be used to identify people who will actually experience the outcomes. On the other hand, the specificity was high, allowing identifying with confidence elderly people who are less likely to experience the outcomes. These predictive capacities were, actually, in line with those observed for frailty, sarcopenia and disability and confirmed in many other studies[32–34], suggesting high risk classes of liver fibrosis scores along with classical elderly-specific factors could not be useful for screening purposes, rather than to rule out elderly subjects with more adverse prognosis.
Some potential limitations must be considered in the interpretation of our results. First, none of the deaths was attributed to liver disease and we could not analyze the association of liver scores with this outcome. Second, even though factors related to the specific etiology of liver disease (i.e. NAFLD, alcoholic or viral) may influence the observed study associations with mortality and disability, we could only partially take them into account, since limited data were available. For instance, the median alcohol intake of our population was relatively low, but around one third of subjects showed an elevated daily consumption. Therefore, we adjusted all our analyses for alcohol use and a sensitivity analyses excluding excessive alcohol users did not show significant change in the observed risk estimates. We did not have any information on markers of viral hepatitis. However, the prevalence of viral hepatitis among rural elderly populations in Central and Northern Italy has been reported to be around 3%,[35] and it is likely to have had a limited impact on our results. Moreover, no information about prevalence of actual liver steatosis, detected by ultrasonography, was available. To address this limitation, we used as surrogate the FLI, finding no effect modification when this variable was taken into account. Sensitivity analyses was also carried out only selecting subjects at high risk of liver steatosis (FLI≥60). As assumed also in a similar study[17], considering the limited impact of other etiologies, it is plausible that the background liver disease would be NAFLD in the majority of the subject.
In conclusion, our study showed that elderly subjects at higher risk of fibrosis are also at increased risk of overall and cardiovascular mortality, and incident disability, independently of comorbidities and other potential confounders. The observed associations were similar in scores including “liver-specific” variables (FIB-4 and AST/ALT ratio) compared to those including more “general” risk factors (NFS and BARD). Despite poor sensitivity, liver fibrosis scores showed clinically relevant specificity. Therefore, liver scores might be used along with established risk factors (sarcopenia and/or frailty) as an aid to stratify the risk of dying or becoming disabled and to identify those subject with more favorable prognosis.
Supplementary Material
Table 2.
Longitudinal associations between liver scores’ risk classes at baseline and incidence of overall and cause-specific mortality and disability at 6 years’ follow-up.
| NFS | FIB-4 | BARD | AST/ALT ratio | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | aHR (95%CI) | HR (95%CI) | aHR (95%CI) | HR (95%CI) | aHR (95%CI) | HR (95%CI) | aHR (95%CI) | |
| Overall M | ||||||||
| Low risk | ref | ref | ref | ref | ref | ref | ref | ref |
| Interm risk | 1.64 (1.2–2.24) | 1.78 (1.29–2.47) | 1.51 (1.16–1.96) | 1.34 (1.02–1.77) | 2.43 (1.38–4.27) | 1.49 (0.81–2.72) | 1.8 (1.07–3.04) | 2.02 (1.19–3.44) |
| High risk | 2.14 (1.64–2.78) | 1.6 (1.21–2.1) | 2.86 (2.12–3.85) | 2.02 (1.47–2.77) | 2.9 (1.55–5.42) | 1.81 (1.10–2.93) | 2.61 (1.61–4.22) | 1.74 (1.06–2.86) |
| CV mortality | ||||||||
| Low risk | ref | ref | ref | ref | ref | ref | ref | ref |
| Interm risk | 2.32 (1.47–3.67) | 2.9 (1.8–4.66) | 1.96 (1.33–2.9) | 1.76 (1.17–2.64) | 2.21 (0.9–5.41) | 0.97 (0.38–2.46) | 1.53 (0.62–3.77) | 1.15 (0.46–2.86) |
| High risk | 3.24 (2.2–4.77) | 2.42 (1.61–3.64) | 3.57 (2.27–5.62) | 2.22 (1.36–3.6) | 3.29 (1.25–8.66) | 1.23 (0.45–3.38) | 3.34 (1.47–7.59) | 1.27 (0.54–2.96) |
| RR (95%CI) | aRR (95%CI) | RR (95%CI) | aRR (95%CI) | RR (95%CI) | aRR (95%CI) | RR (95%CI) | aRR (95%CI) | |
| Disability at 6 y* | ||||||||
| Low risk | ref | ref | ref | ref | ref | ref | ref | ref |
| Interm risk | 1.39 (0.64–2.7) | 1.37 (0.62–2.71) | 1.3 (0.69–2.32) | 1.3 (0.68–2.35) | 6.25 (1.38–110.5) | 2.80 (0.60–49.9) | 1.64 (0.52–7.2) | 1.31 (0.42–5.79) |
| High risk | 2.04 (1.11–3.57) | 1.93 (1.13–2.93) | 3.69 (1.95–6.57) | 2.76 (1.38–5.19) | 5.8 (1.06–107.6) | 2.33 (0.40–44.2) | 3.41 (1.26–13.98) | 1.49 (0.53–6.23) |
Adjusted(a-) models corrected for age, sex, body mass index, arterial hypertension, diabetes mellitus, chronic obstructive pulmonary disease, congestive heart failure, total cholesterol, Charlson comorbidity index, physical activity, alcohol consumption, smoke, IL-6 and TNF-alpha. Age was not considered in models with NFS and FIB4 and diabetes mellitus and body mass index were not considered in models with NFS and BARD, because already included in the liver scores, themselves.
Disable patients (N 49) at baseline were excluded.
Acknowledgments
Financial support
Authors deny any financial support for the conduction of the present study.
The InCHIANTI study was supported as a targeted project (ICS-110.1/RS97.71) by the Italian Ministry of Health and in part by the US National Institute on Aging (contracts N01-AG-919413 and N01-AG-821336; grants R01 AG027012 and R01 AG029148).
Footnotes
Conflict of Interest
All authors deny any conflict of interest with the present work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol 2018;69:718–35. doi: 10.1016/j.jhep.2018.05.011. [DOI] [PubMed] [Google Scholar]
- [2].Kim H, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol 2015;31:184–91. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bertolotti M, Lonardo A, Mussi C, Baldelli E, Pellegrini E, Ballestri S, et al. Nonalcoholic fatty liver disease and aging: Epidemiology to management. World J Gastroenterol WJG 2014;20:14185–204. doi: 10.3748/wjg.v20.i39.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Koehler EM, Schouten JNL, Hansen BE, van Rooij FJA, Hofman A, Stricker BH, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: results from the Rotterdam study. J Hepatol 2012;57:1305–11. doi: 10.1016/j.jhep.2012.07.028. [DOI] [PubMed] [Google Scholar]
- [5].Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- [6].Petta S, Maida M, Macaluso FS, Barbara M, Licata A, Craxì A, et al. Hepatitis C Virus Infection Is Associated With Increased Cardiovascular Mortality: A Meta-Analysis of Observational Studies. Gastroenterology 2016;150:145–55.e4; quiz e15–6. doi: 10.1053/j.gastro.2015.09.007. [DOI] [PubMed] [Google Scholar]
- [7].Fulop T, McElhaney J, Pawelec G, Cohen AA, Morais JA, Dupuis G, et al. Frailty, Inflammation and Immunosenescence. Interdiscip Top Gerontol Geriatr 2015;41:26–40. doi: 10.1159/000381134. [DOI] [PubMed] [Google Scholar]
- [8].Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149:389–97.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xu F, Moorman AC, Tong X, Gordon SC, Rupp LB, Lu M, et al. All-Cause Mortality and Progression Risks to Hepatic Decompensation and Hepatocellular Carcinoma in Patients Infected With Hepatitis C Virus. Clin Infect Dis 2016;62:289–97. doi: 10.1093/cid/civ860. [DOI] [PubMed] [Google Scholar]
- [10].Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatol Baltim Md 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- [11].Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 1988;95:734–9. [DOI] [PubMed] [Google Scholar]
- [12].Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatol Baltim Md 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- [13].Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008;57:1441–7. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- [14].Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013;145:782–9.e4. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Treeprasertsuk S, Björnsson E, Enders F, Suwanwalaikorn S, Lindor KD. NAFLD fibrosis score: A prognostic predictor for mortality and liver complications among NAFLD patients. World J Gastroenterol WJG 2013;19:1219–29. doi: 10.3748/wjg.v19.i8.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatol Baltim Md 2013;57:1357–65. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Unalp-Arida A, Ruhl CE. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatol Baltim Md 2017;66:84–95. doi: 10.1002/hep.29113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 2000;48:1618–25. [DOI] [PubMed] [Google Scholar]
- [19].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- [20].McPherson S, Hardy T, Dufour J-F, Petta S, Romero-Gomez M, Allison M, et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol 2017;112:740–51. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- [23].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602–9. [DOI] [PubMed] [Google Scholar]
- [25].Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. The Gerontologist 1970;10:20–30. [DOI] [PubMed] [Google Scholar]
- [26].Vespasiani-Gentilucci U, De Vincentis A, Ferrucci L, Bandinelli S, Antonelli Incalzi R, Picardi A. Low Alanine Aminotransferase Levels in the Elderly: Frailty, Disability, Sarcopenia and Reduced Survival. J Gerontol A Biol Sci Med Sci 2017. doi: 10.1093/gerona/glx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kawaguchi T, Taniguchi E, Itou M, Sakata M, Sumie S, Sata M. Insulin resistance and chronic liver disease. World J Hepatol 2011;3:99–107. doi: 10.4254/wjh.v3.i5.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vespasiani-Gentilucci U, Gallo P, De Vincentis A, Galati G, Picardi A. Hepatitis C virus and metabolic disorder interactions towards liver damage and atherosclerosis. World J Gastroenterol 2014;20:2825–38. doi: 10.3748/wjg.v20.i11.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol 2016;229:R67–81. doi: 10.1530/JOE-15-0533. [DOI] [PubMed] [Google Scholar]
- [30].Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123–31. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- [31].Hirani V, Blyth F, Naganathan V, Le Couteur DG, Seibel MJ, Waite LM, et al. Sarcopenia Is Associated With Incident Disability, Institutionalization, and Mortality in Community-Dwelling Older Men: The Concord Health and Ageing in Men Project. J Am Med Dir Assoc 2015;16:607–13. doi: 10.1016/j.jamda.2015.02.006. [DOI] [PubMed] [Google Scholar]
- [32].Pedone C, Costanzo L, Cesari M, Bandinelli S, Ferrucci L, Antonelli Incalzi R. Are Performance Measures Necessary to Predict Loss of Independence in Elderly People? J Gerontol A Biol Sci Med Sci 2016;71:84–9. doi: 10.1093/gerona/glv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].García-García FJ, Carcaillon L, Fernandez-Tresguerres J, Alfaro A, Larrion JL, Castillo C, et al. A new operational definition of frailty: the Frailty Trait Scale. J Am Med Dir Assoc 2014;15:371.e7–371.e13. doi: 10.1016/j.jamda.2014.01.004. [DOI] [PubMed] [Google Scholar]
- [34].Pijpers E, Ferreira I, Stehouwer CDA, Nieuwenhuijzen Kruseman AC. The frailty dilemma. Review of the predictive accuracy of major frailty scores. Eur J Intern Med 2012;23:118–23. doi: 10.1016/j.ejim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- [35].Vespasiani-Gentilucci U, Galati G, Gallo P, De Vincentis A, Riva E, Picardi A. Hepatitis C treatment in the elderly: New possibilities and controversies towards interferon-free regimens. World J Gastroenterol 2015;21:7412–26. doi: 10.3748/wjg.v21.i24.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.