Abstract
Background:
Cannabis is categorized as an illicit drug in most US states, but legalization for medical indications is increasing. Policies and guidance regarding cannabis use in transplant patients remain controversial.
Methods:
We examined a database linking national kidney transplant records (n=52,689) with Medicare claims to identify diagnoses of cannabis dependence or abuse (CDOA) and associations (adjusted hazard ratio, 95% LCL aHR95% UCL) with graft, patient and other clinical outcomes.
Results:
CDOA was diagnosed in only 0.5% (n=254) and 0.3% (n=163) of kidney transplant recipients in the years before and after transplant, respectively. Patients with pretransplant CDOA were more likely to be aged 19-30 and black race, and less likely to be obese, college-educated, and employed. After multivariate and propensity adjustment, CDOA in the year before transplant was not associated with death or graft failure in the year after transplant, but was associated with posttransplant psychosocial problems such as alcohol abuse, other drug abuse, noncompliance, schizophrenia and depression. Further, CDOA in the first year posttransplant was associated with an approximately 2-fold increased risk of death-censored graft failure (aHR 1.592.293.32), all-cause graft loss (aHR 1.502.092.91), and death (aHR 1.061.793.04) in the subsequent 2 years. Posttransplant CDOA was also associated with cardiovascular, pulmonary, and psychosocial problems, and with events such as accidents and fractures.
Conclusions:
While associations likely, in part, reflect associated conditions or behaviors, clinical diagnosis of CDOA in the year after transplant appears to have prognostic implications for allograft and patient outcomes. Recipients with posttransplant CDOA warrant focused monitoring and support.
INTRODUCTION
Candidates for organ transplant undergo evaluation of comorbid conditions and overall fitness for surgery, and assessment of psychosocial status and risk factors for non-compliance after transplant 1,2. The psychosocial assessment includes a review of substance use and abuse habits. Recognition of a national epidemic of opioid abuse and emerging evidence of associations with adverse posttransplant outcomes have focused recent attention on screening for opioid use in potential recipients 3-7. However, less information is available regarding outcomes associated with other substance use. Cannabis (also known as marijuana) is currently the most commonly used illicit drug in the United States 8, and prevalence of use doubled in 2012-2013 versus 2002-2003 9. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, identified cannabis use disorder in 2.9% of respondents in a national data survey 10. The implications of cannabis use are complicated by expanded legalization for medical indications. Twenty-nine states and the District of Columbia have legalized cannabis for medical use 11, and eight of these states have legalized cannabis for recreational use 12. Cannabis use has been legal in many European countries, and recently was legalized in Canada. In contrast, cannabis is currently categorized as a Schedule 1 substance by the U.S. Controlled Substances Act (ie, having no accepted medical uses, high potential for abuse, and lack of an acceptable safety profile) 13.
With uncertain benefits and limited data, cannabis has been used for medical conditions such as glaucoma 14, chronic nausea 15, chronic neuropathic pain 16, multiple sclerosis 17, and epilepsy 18. Patients with kidney failure may use medical or recreational cannabis to manage symptoms of chronic pain, nausea, vomiting, anorexia, cachexia, and pruritus 19. Cannabis use has also been associated with adverse psychosocial, cognitive, and respiratory complications 20-22. Wolff et al. described the possible precipitation of stroke by cannabis use, even in young people without cardiovascular risk factors, due to multifocal intracranial arterial vasoconstriction 23.
Few studies have examined cannabis use among kidney transplant recipients. A retrospective chart review of kidney transplant patients in a single center, comparing 56 cannabis users with 1,169 non-users, did not identify associations of cannabis use with patient death or graft failure 24. This study was limited by a small sample size, short follow-up, single-center design, and cross-sectional approach. Other adverse outcomes associated with cannabis, use such as respiratory, cardiovascular, and psychosocial complications, have not been studied in kidney transplant recipients. Given the paucity of information on the effects of cannabis use on patient and graft outcomes after kidney transplant, policies and guidance regarding cannabis use in kidney transplant candidates and recipients remain controversial.
Currently, the national US transplant registry does not collect measures of substance use before or after transplant. However, linking the transplant registry with other data sources can combine the value of transplant recipient status, baseline clinical characteristics, and patient and graft survival records with novel exposure information. Administrative billings offer non-obtrusive measures of clinical diagnoses that may serve as surrogate measures of clinical conditions, including substance use behaviors, and overcome some of the disadvantages of self-reporting 5,25-29. To help advance understanding of the outcomes associated with cannabis use in the kidney transplant population, we identified diagnoses of cannabis dependence or abuse (CDOA) before and after transplant reported in Medicare claims data, and examined associations with patient survival, graft survival, and clinical complications.
METHODS
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR system includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight of the activities of the OPTN and SRTR contractors.
Medicare billing claims data include diagnostic and procedure codes for patients with Medicare fee-for-service primary or secondary insurance (service information is submitted to and tracked by Medicare, even if Medicare is not the primary payer). After regulatory approvals, beneficiary identifier numbers from Medicare’s electronic databases were linked using Social Security Number, sex, and birthdate to unique, anonymous registry identification numbers. Because of the large sample size, the anonymity of the patients studied, and the nonintrusive nature of the research, a waiver of informed consent was granted per the Department of Health and Human Services Code of Federal Regulations (Title 45, Part 46, Paragraph 46.116). Analyses were performed using Health Information Portability and Accountability Act (HIPAA)-compliant, limited datasets from which all direct identifiers were removed. This study was approved by the Institutional Review Board of Saint Louis University.
Sampling and Exposure Definitions
We selected kidney-only transplant recipients from 2007 through 2015. Eligible recipients had Medicare coverage 1 year prior to transplant (for assessment of pretransplant CDOA), and 1 year posttransplant (for assessment of posttransplant CDOA). Recipient clinical and demographic factors, and characteristics of the donated organ and other transplant factors, were defined by the OPTN Transplant Candidate Registration and Transplant Recipient Registration forms (Table 1).
Table 1.
Distributions of clinical traits in the study samples of Medicare-insured transplant recipients according to CDOA.
| 1-Year Pretransplant Assessment | 1-Year Posttransplant Assessment | |||||
|---|---|---|---|---|---|---|
| No CDOA (n=52,435) |
CDOA (n=254) |
Adjusted odds ratio | No CDOA (n=50,498) |
CDOA (n=163) |
Adjusted odds ratio | |
| Age (years) | ‡ | ‡ | ||||
| ≤18 | 2.1 | 1.2 | 0.26 (0.07-0.95) * | 4.2 | 8.0 | 0.68 (0.25-1.84) |
| 19-30 | 6.4 | 20.9 | Reference | 7.2 | 23.9 | Reference |
| 31-44 | 18.2 | 33.9 | 0.63 (0.44-0.90) * | 17.9 | 26.4 | 0.45 (0.28-0.71) † |
| 45-59 | 35.3 | 38.6 | 0.37 (0.26-0.54) ‡ | 36.7 | 34.4 | 0.30 (0.19-0.49) ‡ |
| ≥60 | 37.9 | 5.5 | 0.05 (0.03-0.09) ‡ | 34.0 | 7.4 | 0.08 (0.04-0.16) ‡ |
| Male | 61.2 | 76.4‡ | 2.23 (1.64-3.03) ‡ | 60.7 | 77.3‡ | 2.33 (1.58-3.42) ‡ |
| Race | ‡ | ‡ | ||||
| White | 47.2 | 36.6 | Reference | 52.3 | 31.3 | Reference |
| Black | 31.6 | 48.8 | 1.38 (1.02-1.85) * | 27.9 | 52.8 | 2.07 (1.40-3.06) † |
| Hispanic | 14.6 | 13.4 | 0.75 (0.50-1.12) | 13.6 | 12.3 | 0.85 (0.50-1.44) |
| Other | 6.7 | 1.2 | 0.17 (0.05-0.54) * | 6.3 | 3.7 | 0.76 (0.32-1.81) |
| BMI (kg/m2) | ‡ | † | ||||
| <18.5 | 2.8 | 2.4 | 0.61 (0.26-1.42) | 3.6 | 8.6 | 1.68 (0.87-3.24) |
| 18.5-24.9 | 27.3 | 41.7 | Reference | 27.2 | 36.8 | Reference |
| 25-30 | 32.2 | 32.7 | 0.76 (0.57-1.02) | 31.9 | 28.8 | 0.82 (0.55-1.21) |
| >30 | 34.9 | 22.4 | 0.45 (0.32-0.63) ‡ | 34.3 | 23.9 | 0.61 (0.40-0.93) * |
| Unknown | 2.9 | 0.8 | 0.20 (0.05-0.81) * | 3.1 | 1.8 | 0.52 (0.16-1.68) |
| Cause of ESRD | ‡ | * | ||||
| Diabetes | 27.1 | 16.5 | 1.02 (0.54-1.91) | 25.6 | 18.4 | 3.69 (1.11-12.29) * |
| Glomerulonephritis | 20.8 | 28.7 | 1.14 (0.81-1.62) | 21.4 | 27.6 | 1.13 (0.72-1.77) |
| Hypertension | 29.4 | 34.7 | Reference | 27.5 | 31.9 | Reference |
| Polycystic kidney disease | 7.6 | 3.5 | 0.62 (0.30-1.26) | 9.7 | 4.9 | 0.99 (0.45-2.16) |
| Other | 15.2 | 16.5 | 1.03 (0.68-1.58) | 15.8 | 17.2 | 1.00 (0.57-1.75) |
| Dialysis duration (months) | * | ‡ | ||||
| None | 6.7 | 3.5 | 1.59 (0.76-3.34) | 14.5 | 5.5 | 0.78 (0.37-1.63) |
| >0-24 | 21.4 | 14.6 | Reference | 30.5 | 24.5 | Reference |
| 25-60 | 41.5 | 41.3 | 1.20 (0.82-1.75) | 34.5 | 30.7 | 0.99 (0.63-1.57) |
| >60 | 29.7 | 39.8 | 1.31 (0.88-1.94) | 19.7 | 39.3 | 1.82 (1.13-2.93) * |
| Missing | 0.7 | 0.8 | 2.06 (0.49-8.69) | 0.9 | 0.0 | NA |
| Comorbid conditions | ||||||
| Diabetes | 36.8 | 22.1‡ | 0.79 (0.45-1.39) | 34.1 | 20.25† | 0.29 (0.09-0.92) * |
| Angina | 7.3 | 6.7 | 1.41 (0.84-2.36) | 7.0 | 4.3 | 0.84 (0.38-1.83) |
| COPD | 1.4 | 1.2 | 1.30 (0.41-4.14) | 1.2 | 2.5 | 3.50 (1.26-9.73) * |
| Hypertension | 81.0 | 83.1 | 1.08 (0.76-1.55) | 80.1 | 81.0 | 1.11 (0.72-1.72) |
| Cerebral vascular disease | 2.9 | 2.0 | 0.81 (0.33-2.00) | 2.7 | 1.2 | 0.57 (0.14-2.36) |
| Peripheral vascular disease | 4.3 | 2.4 | 0.77 (0.33-1.77) | 3.9 | 2.5 | 0.92 (0.33-2.57) |
| Education level | ‡ | ‡ | ||||
| College & higher | 43.5 | 31.1 | Reference | 44.1 | 20.9 | Reference |
| Grade/High schools | 46.9 | 61.0 | 1.51 (1.14-1.99) * | 45.1 | 67.5 | 2.38 (1.60-3.55) ‡ |
| Unknown | 9.6 | 7.9 | 1.11 (0.67-1.84) | 10.9 | 11.7 | 1.85 (1.03-3.31) * |
| Employment | * | † | ||||
| Working | 19.3 | 11.8 | 0.46 (0.31-0.67) ‡ | 26.9 | 13.5 | 0.47 (0.29-0.75) * |
| Not working | 70.3 | 80.7 | Reference | 60.5 | 73.0 | Reference |
| Unknown | 10.4 | 7.5 | 0.57 (0.34-0.95) * | 12.5 | 13.5 | 0.45 (0.22-0.90) * |
| Physical capacity | ||||||
| No limit | 65.1 | 67.7 | Reference | 64.8 | 63.2 | Reference |
| Limited | 8.1 | 7.9 | 1.06 (0.66-1.71) | 7.5 | 4.9 | 0.70 (0.34-1.47) |
| Unknown | 26.8 | 24.4 | 0.94 (0.70-1.27) | 27.7 | 31.9 | 1.13 (0.78-1.63) |
| Previous transplant | 14.3 | 11.4 | 0.45 (0.29-0.72) † | 2.6 | 0.6 | 0.31 (0.04-2.33) |
| Most Recent PRA | ||||||
| 0-9 | 68.3 | 70.1 | Reference | 77.5 | 79.8 | Reference |
| 10-79 | 19.0 | 19.3 | 1.16 (0.83-1.63) | 15.8 | 13.5 | 0.98 (0.62-1.56) |
| ≥80 | 7.7 | 7.9 | 1.31 (0.76-2.23) | 4.0 | 2.5 | 0.89 (0.31-2.52) |
| Missing | 5.0 | 2.8 | 0.66 (0.31-1.43) | 2.7 | 4.3 | 2.13 (0.97-4.67) |
| HLA mismatch | ||||||
| Zero A, B, DR | - | - | - | 7.1 | 4.9 | 0.98 (0.47-2.05) |
| Zero DR | - | - | - | 10.7 | 10.4 | 1.02 (0.61-1.70) |
| Other | - | - | - | 82.2 | 84.7 | Reference |
| Donor type | * | |||||
| Living | - | - | - | 35.0 | 25.8 | 1.00 (0.60-1.65) |
| SCD | - | - | - | 44.4 | 52.8 | Reference |
| ECD | - | - | - | 11.2 | 6.8 | 0.95 (0.49-1.82) |
| DCD | - | - | - | 9.4 | 14.7 | 1.37 (0.86-2.18) |
| Year of transplant | ||||||
| 2007-2010 | 33.7 | 31.9 | Reference | 61.0 | 57.1 | Reference |
| 2011-2015 | 66.3 | 68.1 | 1.14 (0.87-1.50) | - | - | - |
| 2011-2013 | - | - | - | 39.0 | 42.9 | 1.09 (0.79-1.50) |
Abbreviations: BMI, body mass index; CDOA, cannabis dependence or abuse; COPD, chronic obstructive pulmonary disease; DCD, donation after circulatory death; ECD, extended criteria donor; ESRD, end-stage renal disease; HLA, human leukocyte antigen; NA, not applicable; PRA, panel reactive antibody; SCD, standard criteria donor.
P values:
P < 0.05–0.002;
P = 0.001–0.0001;
P < 0.0001, for differences of distributions of clinical traits among patients with versus without CDOA.
“Other race” includes Asian, Native American, Pacific Islander and multiracial.
Cannabis use disorder was based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes for CDOA (304.3x or 305.2x). Cannabis use alone does not qualify for CDOA coding in the ICD system; rather, coders are instructed to reserve CDOA coding for habitual consumption of a substance, taken by his or her own initiative, even though the substance is known to be a detriment to one’s health or is not taken for therapeutic purposes 30. The physician’s documentation should include evidence that the physician addressed CDOA with the patient and/or it is directly related to a service or procedure provided during the encounter 30. ICD criteria for CDOA resonate with the Diagnostic and Statistical Manual of Mental Disorders, fifth revision (DSM-5) criteria for Cannabis Use Disorder (CUD), defined as continued use of cannabis despite clinically significant impairment, ranging from mild to severe 31. General population data support strong sensitivity and predictive value of these diagnostic codes for the identification of CDOA,32,33 and administrative billing claims data have been used to characterize use of CDOA and other drugs of abuse in observational research.34 Pretransplant CDOA was defined as evidence of a diagnostic code within 1 year prior to transplant. Posttransplant CDOA was defined as evidence of a diagnostic code within the first year posttransplant. Death-censored graft failure (DCGF) was defined as return to maintenance dialysis or re-transplant, censoring for death. All-cause graft failure (ACGF) was defined as return to maintenance dialysis, re-transplant, or death.
Other outcomes studied, including cardiovascular (hypotension, acute myocardial infarction [MI], stroke), pulmonary (hypercapnia, fungal pneumonia, aspiration pneumonia, other pneumonia), psychosocial (alcohol abuse, other drug abuse, noncompliance, mental status changes, delusions, psychosis, schizophrenia, depression), and other (accidents, fractures), were identified using ICD-9-CM diagnosis codes as previously defined 6,35(Table S1).
Statistical Analyses
Data management and analyses were performed with SAS for Windows software, version 9.4 (SAS Institute Inc., Cary, NC). Distributions of clinical and demographics traits among recipients with pretransplant and posttransplant CDOA were compared with distributions of traits among those without CDOA by the Chi-square test.
For analysis of outcomes in relation to pretransplant CDOA, at-risk time was considered to the first posttransplant anniversary. Outcomes in relation to first-year posttransplant CDOA were examined from >1 to 3 years posttransplant, because Medicare coverage expires at the third transplant anniversary in the absence of age >65 years or disability. At-risk time for all models was censored at the end of the assessment period, loss to follow-up, end of Medicare enrollment, or end of study (September 2017). Incidence of each posttransplant event was estimated by the Kaplan-Meier method, with use of the log-rank test to assess the statistical significance of unadjusted differences. Propensity scores for the likelihood of CDOA were estimated by logistic regression. Adjusted associations of posttransplant CDOA and new posttransplant CDOA with transplant outcomes and clinical complications were quantified by multivariate Cox regression (adjusted hazard ratio with 95% upper and lower confidence limits, LCLaHRUCL), including adjustment for recipient, donor, and transplant clinical factors listed in Table 1, and propensity for CDOA. In secondary analyses, we also adjusted primary models examining associations of CDOA before and after transplant with subsequent graft loss or death for diagnoses of alcohol abuse, other drug abuse and noncompliance in the same period.
RESULTS
Sample Characteristics
In the study period, 52,689 US kidney-only transplant recipients had linked transplant registry and Medicare claims in the year before transplant; only 0.5% (n=254) had CDOA in the year before transplant. Among recipients with first-year Medicare eligibility, 0.3% (n=163) had CDOA in the first year posttransplant. Compared with recipients without CDOA, those with CDOA in pre- and posttransplant periods were more likely to be younger (aged 19-30 years), male, and black (Table 1). Cannabis users were less likely to be obese (body mass index >30 kg/m2) or diabetic, or to have a college or higher degree, and less likely to be employed. Compared with recipients without CDOA posttransplant, those with posttransplant CDOA were more likely to have a chronic obstructive pulmonary disease (COPD), kidney failure due to diabetes, and longer pretransplant dialysis duration (>60 months) (Table 1).
Incidence of Clinical Complications and Associations with Pretransplant CDOA
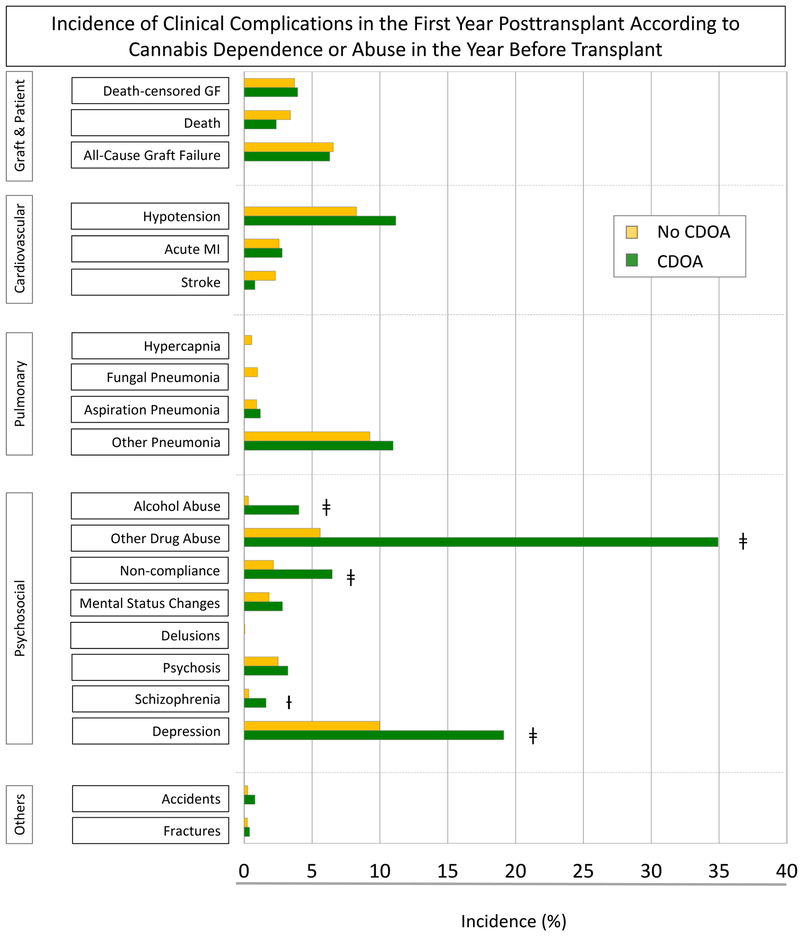
At 1 year posttransplant, rates of DCGF (3.9% vs. 3.7%), death (2.4% vs. 3.4%), and ACGF (6.3% vs. 6.6%) did not differ significantly for recipients with pretransplant CDOA compared with those without (Figure 1A). Pretransplant CDOA was associated with increased rates of psychosocial conditions such as alcohol abuse (4.0% vs. 0.3%), other drug abuse (34.9% vs. 5.6%), noncompliance (6.5 % vs. 2.2 %), schizophrenia (1.6% vs. 0.3%), and depression (19.1% vs. 9.9%) over the first posttransplant year.
Figure 1.
A. Incidence of clinical complications in the first year posttransplant according to cannabis dependence or abuse in the year before transplant. B. Adjusted association of cannabis dependence or abuse in the year before transplant with clinical complications in the first year posttransplant.
Abbreviations: ACGF, all-cause graft failure; CDOA, cannabis dependence or abuse; DCGF, death-censored graft failure.
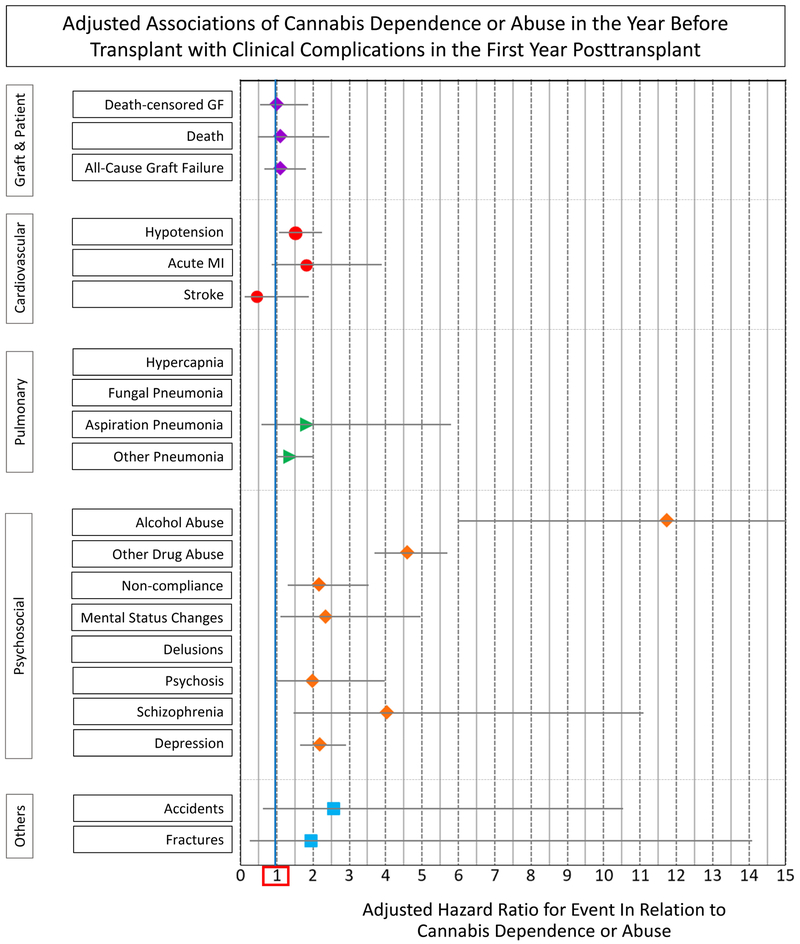
After multivariate adjustment for recipient, donor, and transplant factors, as well as propensity for the likelihood of pretransplant CDOA, pretransplant CDOA was not associated with increased risk of DCGF (aHR 0.541.001.87), death (aHR 0.491.092.44), or ACGF (aHR 0.671.101.80) (Figure 1B, Table S2). Pretransplant CDOA was not associated with adjusted risk of cardiovascular, pulmonary, or other complications in the first year posttransplant. However, CDOA was significantly associated with adjusted risk of alcohol abuse (aHR 5.9911.7322.95), other drug abuse (aHR 3.694.585.69), noncompliance (aHR 1.302.153.54), mental status changes (aHR 1.102.334.95), schizophrenia (aHR 1.474.0311.10), and depression (aHR 1.642.182.90) over the first posttransplant year.
Among patients with pretransplant CDOA, only 1% had pretransplant alcohol abuse, 2% had other drug abuse, and 12% had non-compliance diagnoses. Adding these other diagnoses (alcohol abuse, other drug abuse, and non-compliance) to the models of first-year outcomes in relation to pretransplant CDOA did not impact inferences, and pretransplant CDOA remained a non-significant correlate of patient death or graft failure in the first-year post-transplant (Table S3).
Incidence of Clinical Complications and Associations with Posttransplant CDOA
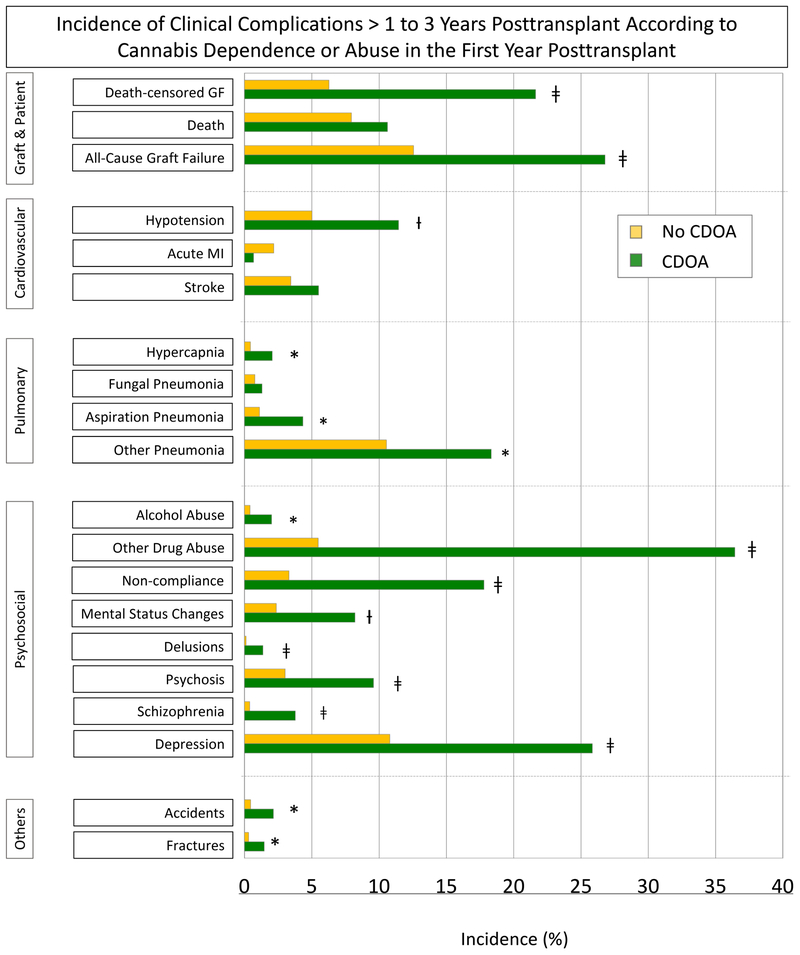
Rates of DCGF (21.6% vs. 6.3%), ACGF (26.8% vs. 12.6%), and death (10.6 % vs. 7.9%) >1 to 3 years posttransplant were significantly higher for recipients with CDOA in the first year posttransplant than for those without (Figure 2A). Posttransplant CDOA was associated with increased rates of cardiovascular conditions such as hypotension (11.5% vs. 5.0%); pulmonary conditions such as hypercapnia (0.7% vs. 0.5%), aspiration pneumonia (4.3% vs. 1.1%), and other pneumonia (18.3% vs. 10.6%); psychosocial problems such as alcohol abuse (2.0% vs. 0.4%), other drug abuse (36.4% vs. 5.5%), noncompliance (17.8% vs. 3.3%), mental status changes (8.2% vs. 2.4%), delusions (1.4% vs. 0.1%), psychosis (9.6% vs. 3.0%), schizophrenia (3.8% vs. 0.4%), and depression (25.9% vs. 10.8%); and other conditions such as accidents (2.2% vs. 0.5%) and fractures (1.5% vs. 0.3%) over the next 2 years.
Figure 2.
A. Incidence of clinical complications >1 to 3 years posttransplant according to cannabis dependence or abuse in the year before transplant. B. Adjusted association of cannabis dependence or abuse in the year after transplant with clinical complications > 1 to 3 years posttransplant.
Abbreviations: ACGF, all-cause graft failure; CDOA, cannabis dependence or abuse; DCGF, death-censored graft failure. Referent, no CDOA.
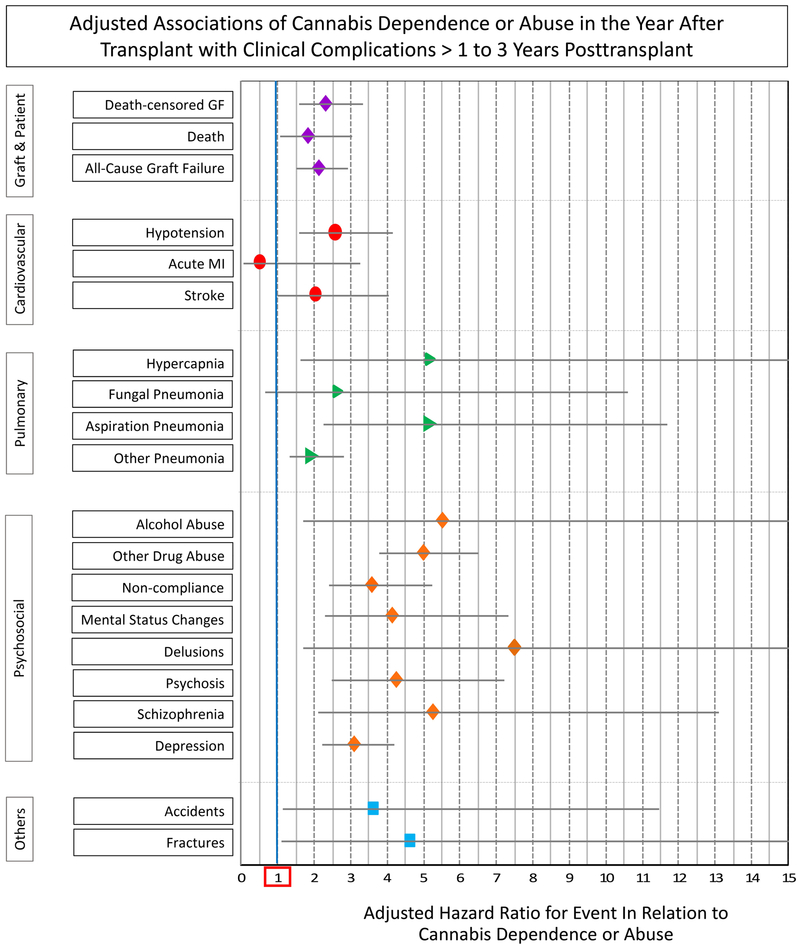
After multivariate adjustment for recipient, donor, and transplant factors, as well as propensity for the likelihood of posttransplant CDOA, posttransplant CDOA was independently associated with increased risk of DCGF (aHR 1.592.293.32), ACGF (aHR 1.502.092.91), and death (aHR 1.061.793.04) in years >1 to 3 posttransplant (Figure 2, Table S2). CDOA in the first year posttransplant was also associated with significantly increased adjusted risk of hypotension (aHR 1.582.554.14), hypercapnia (aHR 1.625.1616.46), aspiration pneumonia (aHR 2.275.1511.68), other pneumonia (aHR 1.311.922.80), alcohol abuse (aHR 1.715.5017.66), other drug abuse (aHR 3.794.976.52), noncompliance (aHR 2.423.555.23), mental status changes (aHR 2.314.117.32), delusions (aHR 1.717.4932.92), psychosis (aHR 2.484.247.23), schizophrenia (aHR 2.105.2513.11), depression (aHR 2.233.064.21), accidents (aHR 1.123.5811.45), and fractures (aHR 1.114.6019.05) over the subsequent 2 years of follow-up.
Among recipients with posttransplant CDOA, 2.5% had posttransplant alcohol abuse, 9% had other drug abuse, and 21% had non-compliance diagnoses. After adjusting for alcohol abuse, other drug abuse, and non-compliance, posttransplant CDOA remained a significant predictor of DCGF (aHR 1.251.812.63) and ACGF (aHR 1.151.612.24) in the period >1 to 3 years posttransplant. The association of CDOA with death remained significant when adjusted alcohol abuse (aHR 1.051.783.01), but not was not significant (aHR 0.761.292.20) after adjustment for other drug abuse and non-compliance (Table S4).
Among recipients with CDOA in the first year posttransplant, 56% had new onset CDOA and 44% showed indication of CDOA in the year before transplant. Outcomes >1 to 3 years posttransplant did not differ significantly (P > 0.05) according to whether CDOA in the first year was continued or newly reported.
DISCUSSION
We examined associations of CDOA diagnoses among Medicare-insured kidney transplant recipients with posttransplant patient survival, graft outcomes, and a variety of clinical complications. CDOA diagnoses were found in 0.5% and 0.3% in the years before and after transplant, respectively. CDOA in the year before transplant was not associated with death or graft survival in the year after transplant, but CDOA in the first year posttransplant was associated with 2-fold increased risk of DCGF, ACGF, and death in the subsequent 2 years (>1 to 3 years posttransplant). CDOA in the years before and after transplant was associated with psychosocial complications. Further, posttransplant CDOA was also associated with cardiovascular, pulmonary, and other complications such as accidents and fractures.
Available guidance on implications of cannabis use for kidney transplant candidacy are limited. In 2001, an American Society of Transplantation (AST) clinical practice guideline recommended that because of under-reporting by individuals anxious to be approved for transplant, independent sources of information should probably be used to discover whether there may be substance use problems, and that every effort should be made to ensure that substance use problems are adequately treated prior to transplant.36 The work group also concluded that is it reasonable to insist that candidates with a history of substance dependency undergo counseling and treatment, and advocated for documentation by caregivers of a drug-free period and periodic screening for continued abstinence, as relapse may occur. We believe that multiple sources of information are needed to understand cannabis use in the transplant population, and that administrative data can complement other information sources. In accord with the AST guidance, we also explored CDOA in more than one time period.
Kidney transplant patients with CDOA appear to have increased frequency of certain sociodemographic characteristics. Recipients with CDOA were more likely to be young, male, and black, and to have lower education and likelihood of employment. While unadjusted confounding by sociodemographic profile may in part explain the outcomes associated with posttransplant CDOA, these patterns highlight groups appropriate for tailored education regarding the potential impact of cannabis use on allograft function and other clinical complications. The higher tendency of CDOA in young black men should not be used to create stereotype or to decrease access to kidney transplant in this already disadvantaged group, but rather to direct education and support services.
Notably, pretransplant CDOA was not associated with increased risk of graft loss, patient death, or other clinical complications. Similar to our observation, a single-center study reported that pretransplant cannabis use was not associated with these clinical outcomes at 1 year posttransplant 24. While not all transplant programs require abstinence among cannabis users, selection for transplant means that in candidates with recognized substance abuse histories, the behavior has been deemed resolved or sufficiently controlled to not preclude transplant. Importantly, our study identifies clinical diagnoses of CDOA (ie, clinical-determined substance use disorder), not all forms of cannabis use. In July 2015, California enacted the Medical Cannabis Organ Transplant Act (Assembly Bill 258) to prohibit discrimination against medical cannabis patients in the organ transplant process, unless a doctor has determined that medical cannabis use is clinically detrimental to transplant 37. Subsequently, six additional states joined the organ transplant protection for medical cannabis users, including Arizona, Delaware, Illinois, Minnesota, New Hampshire, and Washington. An online survey of 360 heart transplant providers reported that three-quarters of providers who recommended denial of listing on the basis of cannabis use were in potential conflict with laws in their states 38. Therefore, the need for more evidence to provide a foundation for clinical practice guidelines is urgent.
In contrast to the generally neutral implications of pretransplant CDOA in this recipient sample, we observed strong associations of posttransplant CDOA with subsequent outcomes in the >1- to 3-year period, including the risk of graft loss. While vasoconstrictive properties of cannabis suggest putative mechanisms for nephrotoxicity, data evaluating associations of cannabis use with renal physiology and outcomes are limited. Two different cannabinoid receptors, type 1 (CB1) and type 2 (CB2), are predominantly expressed in the central nervous system, but low-level expression is also found in the glomerulus. The receptors seem to play opposing roles in the kidney. In rodent models of obesity and diabetes mellitus, endogenous cannabinoids generated in various renal cells activate CB1 receptors and contribute to the development of oxidative stress, inflammation, and renal fibrosis 39,40. In contrast, experimental models of murine diabetic nephropathy support that the CB2 receptor is expressed by the glomerular podocytes, and its activation reduces proteinuria 41. A recent study found modest cross-sectional association between higher cannabis exposure and lower kidney function among young adults, but no longitudinal association with glomerular filtration rate decline or albuminuria 42. However, in this study, the mean age of the study population was only 35 years, and patients did not have baseline kidney dysfunction. In preliminary analysis of hospitalized adults in the ASSESS-AKI cohort, cannabis use was not linked to incident chronic kidney disease or differences in estimated glomerular filtration rate (eGFR) slope over time, but notably, eGFR declined more rapidly among cannabis users with a baseline eGFR <60 mL/min/1.73 m2 compared with non-users (−3.2 vs. −1.4 mL/min/1.73 m2 per year). Cannabis users with a baseline eGFR <60 mL/min/1.73 m2 also had a higher risk for chronic kidney disease progression (aHR = 2.7; 95% CI, 0.83-8.5).43 Case reports and case series also suggest associations of cannabis use with acute kidney injury, membranous nephropathy, and hypertension 44-47. In the current study, recipients with CDOA were two to three times more likely to be diagnosed with “non-compliance.” Non-compliance is a major risk factor for under-immunosuppression, which may lead to rejection and potentially graft failure 48. In our analysis, associations of CDOA with graft loss persisted after adjustment for non-compliance diagnosis in the same period.
Available data examining associations of cannabis use with mortality are scant and controversial 49-52. We examined associations of CDOA in kidney transplant recipients with multiple complications previously examined in relation to cannabis use in the general population. Cannabis has been associated with bacterial and fungal pneumonia 53,54. Remote case reports linked contaminated cannabis with Aspergillum infections in transplant patients 55,56. A more recent case report described an exogenous lipid pneumonia in patients with a history of smoking cannabis oil for 10 years 57. In addition to the risk of infection, some evidence suggests that short-term cannabis use leads to bronchitis, pharyngitis, and worsening of asthma symptoms, and long-term use is linked to COPD 58. Consistent with the literature, posttransplant CDOA was associated with a 5-times greater risk of hypercapnia and aspiration pneumonia, and almost twice the risk of “other” pneumonia. There was also a trend toward increased risk of fungal pneumonia >1 to 3 years posttransplant, but the condition was uncommon and associations were not significant. Clinical literature suggests potential associations of cannabis use with acute MI 59, stroke 60, and postural hypotension 61. In our analysis, posttransplant CDOA was associated with a 2.6-fold increased risk of hypotension, but no significant difference in MI or stroke. Cannabis use has also been associated with accidents, including injuries related to motor vehicle crashes 62 and fractures 63; both were found to be associated with posttransplant CDOA in our analysis (3.6- and 4.6-fold increased risk, respectively).
In addition to the associations with renal, pulmonary, and cardiovascular disease, cannabis use has been strongly linked with neurocognitive, psychosocial, and psychiatric issues. Cannabis is known to affect neurocognitive function, short-term memory, judgment, and motor coordination 21,22,64, and to be associated with psychiatric conditions such as schizophrenia 64, other psychosis 65, and depression 66. Our study identified a strong association between pretransplant and posttransplant CDOA with increased risk of mental status changes, delusions, psychosis, schizophrenia, and depression. Our study also highlights the association between CDOA and a higher risk of alcohol and other drug abuse compared with no CDOA. The association with other substances was reported previously in non-transplant recipients 67,68. In secondary analysis in our study, posttransplant CDOA was associated with graft loss after adjustment for alcohol use, other drug use and noncompliance in the same period, but the mortality association was not significant after adjustment for other drug use or non-compliance, highlighting the complex interplay between multiple forms or substance use and patient compliance.
Our study has limitations. The frequency of CDOA in this sample of kidney transplant recipients was substantially lower than the frequency of any cannabis use reported in the general population 10. This can be attributed in part to design and sampling; the sample was limited to patients selected to undergo transplant, meaning that those with substance abuse behaviors that would preclude transplant were not included. In addition, clinical diagnoses of CDOA (as designated “disorder or abuse”) likely represent cases of significant use that raised the attention of the healthcare providers; our study was not designed to identify occasional “social” or legally prescribed medical cannabis use, as use without detrimental consequences does not qualify as CDOA 30. Coding practices may vary and are not subject to the rigorous data quality assessments that are typically conducted within clinical trials or prospective research. Additionally, the available data do not include frequency, duration, dose, or type (recreational vs. medical) of cannabis use. The retrospective design can identify associations but not prove causation. Our sample was limited to Medicare beneficiaries, which has relevance as Medicare is the largest single payer for transplant services, but may underestimate CDOA compared with younger, privately insured recipients. Despite these limitations, to our knowledge, this is the first study to examine the prognostic importance of CDOA for posttransplant outcomes in a large national sample. Although a relatively small number of CDOA cases were identified, we detected clinically significant associations of CDOA with important clinical outcomes.
In summary, diagnoses of CDOA are uncommon among Medicare-insured kidney transplant recipients, and likely reflect a select subgroup of cannabis users who raise the clinical attention of healthcare providers. While associations likely in part reflect associated conditions or behaviors, clinical diagnosis of CDOA in the year after transplant appears to have prognostic implications for subsequent graft and patient survival and other medical complications. Given these findings, we believe that focused assessment of substance use habits should continue beyond the transplant evaluation as part of routine posttransplant care. We advocate for support and education of kidney patients diagnosed with CDOA, and for ongoing work to determine how to achieve abstinence in those with CDOA, assess whether abstinence mitigates risk, and define the outcome implications of medical compared with illicit cannabis use.
Supplementary Material
ACKNOWLEDGMENTS
This work was conducted under the auspices of the Minneapolis Medical Research Foundation (MMRF), contractor for the Scientific Registry of Transplant Recipients (SRTR), as a deliverable under contract no. HHSH250201000018C (U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a U.S. Government-sponsored work, there are no restrictions on its use. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government. This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01-DK096008. NNL was supported by a KRESCENT New Investigator Award. The opinions, results, and conclusions reported in this article are those of the authors and are independent of the funding sources. The authors thank SRTR colleague Nan Booth, MSW, MPH, ELS, for manuscript editing. Portions of this work were presented as an oral abstract at the American Transplant Congress, June 4, 2018, in Seattle, WA.
ABBREVIATIONS
- ACGF
all-cause graft failure
- aHR
adjusted hazard ratio
- CB1 and CB2
cannabinoid receptors types 1 and 2
- CDOA
cannabis dependence or abuse
- COPD
chronic obstructive pulmonary disease
- DCGF
death-censored graft failure
- ESRD
end-stage renal disease
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- LCL
lower confidence limit
- MI
myocardial infarction
- OPTN
Organ Procurement and Transplantation Network
- SAS
Statistical Analysis Software
- SRTR
Scientific Registry of Transplant Recipients
- THC
tetrahydrocannabinol
- UCL
upper confidence limit
Footnotes
- TA, FMK, NNL, SK, ASN, HR, RO, RD, and BLK participated in study design, interpretation, and writing of the paper.
- MAS and KLL participated in study design, acquisition of data and regulatory approvals, data analysis, and writing of the paper. Support provided to the authors’ institution by the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- HX Participated in data analysis and manuscript preparation. Support provided to the author’s institution by the NIH/NIDDK.
- DAA, VRD, DCB, and DLS participated in study design, interpretation, and writing of the paper. Support provided to the authors’ institutions by the NIH/NIDDK.
DISCLOSURES: The authors declare no conflicts of interest.
REFERENCES
- 1.Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858–873. [DOI] [PubMed] [Google Scholar]
- 2.Denhaerynck K, Steiger J, Bock A, et al. Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant 2007;7(1):108–116. [DOI] [PubMed] [Google Scholar]
- 3.Minelli E, Bryan AL. Transplant Candidates and Substance Use: Adopting Rational Health Policy for Resource Allocation. Univ Mich J Law Reform. 2011;44(3). [Google Scholar]
- 4.European Renal Best Practice Transplantation Guideline Development Group. ERBP Guideline on the Management and Evaluation of the Kidney Donor and Recipient. Nephrol Dial Transplant 2013;28 Suppl 2:ii1–71. [DOI] [PubMed] [Google Scholar]
- 5.Lentine KL, Yuan H, Tuttle-Newhall JE, et al. Quantifying prognostic impact of prescription opioid use before kidney transplantation through linked registry and pharmaceutical claims data. Transplantation. 2015;99(1):187–196. [DOI] [PubMed] [Google Scholar]
- 6.Lentine KL, Lam NN, Xiao H, et al. Associations of pre-transplant prescription narcotic use with clinical complications after kidney transplantation. Am J Nephrol 2015;41(2):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lentine KL, Lam NN, Naik AS, et al. Prescription opioid use before and after kidney transplant: Implications for posttransplant outcomes. Am J Transplant 2018;18(12):2987–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Most Commonly Used Addictive Drugs. National Institute on Drug Abuse. https://www.drugabuse.gov/publications/media-guide/most-commonly-used-addictive-drugs. Published 2018. Accessed April 18, 2018.
- 9.Prevalence of Marijuana Use Among U.S. Adults Doubles Over Past Decade. National Institute on Alcohol Abuse; https://www.niaaa.nih.gov/news-events/news-releases/prevalence-marijuana-use-among-us-adults-doubles-over-past-decade. Published October 21, 2015. Accessed April 30, 2018. [Google Scholar]
- 10.Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of Marijuana Use Disorders in the United States Between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72(12):1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.33 Legal Medical Marijuana States and DC. ProCon.org. https://medicalmarijuana.procon.org/view.resource.php?resourceID=000881. Published 2017. Updated December 4, 2018. Accessed April 18, 2018.
- 12.State Marijuana Laws in 2018 Map. Governing. http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html. Published 2018. Accessed April 18, 2018.
- 13.Drug Fact Sheet: Marijuana. US Drug Enforcement Administration; https://www.dea.gov/druginfo/drug_data_sheets/Marijuana.pdf. Published 2018. Accessed April 18, 2018. [Google Scholar]
- 14.Merritt JC, Crawford WJ, Alexander PC, Anduze AL, Gelbart SS. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology. 1980;87(3):222–228. [DOI] [PubMed] [Google Scholar]
- 15.Sallan SE, Zinberg NE, Frei E 3rd. Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N Engl J Med 1975;293(16):795–797. [DOI] [PubMed] [Google Scholar]
- 16.Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9(6):506–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collin C, Davies P, Mutiboko IK, Ratcliffe S; Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol 2007;14(3):290–296. [DOI] [PubMed] [Google Scholar]
- 18.Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav 2013;29(3):574–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rein JL, Wyatt CM. Marijuana and Cannabinoids in ESRD and Earlier Stages of CKD. Am J Kidney Dis 2018;71(2):267–274. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med 2014;370(23):2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383–1391. [DOI] [PubMed] [Google Scholar]
- 22.Auer R, Vittinghoff E, Yaffe K, et al. Association Between Lifetime Marijuana Use and Cognitive Function in Middle Age: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Intern Med 2016;176(3):352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolff V, Zinchenko I, Quenardelle V, Rouyer O, Geny B. Characteristics and Prognosis of Ischemic Stroke in Young Cannabis Users Compared With Non-Cannabis Users. J Am Coll Cardiol 2015;66(18):2052–2053. [DOI] [PubMed] [Google Scholar]
- 24.Greenan G, Ahmad SB, Anders MG, Leeser A, Bromberg JS, Niederhaus SV. Recreational marijuana use is not associated with worse outcomes after renal transplantation. Clin Transplant 2016;30(10):1340–1346. [DOI] [PubMed] [Google Scholar]
- 25.Lentine KL, Naik AS, Ouseph R, et al. Antidepressant medication use before and after kidney transplant: implications for outcomes - a retrospective study. Transpl Int 2018;31(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lentine KL, Schnitzler MA, Xiao H, et al. Depression diagnoses after living kidney donation: linking U.S. Registry data and administrative claims. Transplantation. 2012;94(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam NN, Garg AX, Segev DL, et al. Gout after living kidney donation: correlations with demographic traits and renal complications. Am J Nephrol 2015;41(3):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lentine KL, Lam NN, Schnitzler MA, et al. Predonation Prescription Opioid Use: A Novel Risk Factor for Readmission After Living Kidney Donation. Am J Transplant 2017;17(3):744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randall HB, Alhamad T, Schnitzler MA, et al. Survival implications of opioid use before and after liver transplantation. Liver Transpl 2017;23(3):305–314. [DOI] [PubMed] [Google Scholar]
- 30.Safian SC. JustCoding News: Documentation for marijuana use and abuse. HCPro. http://blr.hcpro.com/content.cfm?content_id=318764. Published July 22, 2015. Accessed Decebmer 1, 2018.
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: 10.1176/appi.books.9780890425596. American Psychiatric Association; 2013. [DOI] [Google Scholar]
- 32.DeYoung K, Chen Y, Beum R, Askenazi M, Zimmerman C, Davidson AJ. Validation of a Syndromic Case Definition for Detecting Emergency Department Visits Potentially Related to Marijuana. Public Health Rep 2017;132(4):471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall KE, Monte AA, Chang T, et al. Mental Health-related Emergency Department Visits Associated With Cannabis in Colorado. Acad Emerg Med 2018;25(5):526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas CP, Hodgkin D, Levit K, Mark TL. Growth in spending on substance use disorder treatment services for the privately insured population. Drug Alcohol Depend 2016;160:143–150. [DOI] [PubMed] [Google Scholar]
- 35.Alhamad T, Brennan DC, Brifkani Z, et al. Pretransplant Midodrine Use: A Newly Identified Risk Marker for Complications After Kidney Transplantation. Transplantation. 2016;100(5):1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasiske BL, Cangro CB, Hariharan S, et al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant 2001;1 Suppl 2:3–95. [PubMed] [Google Scholar]
- 37.AB-258 Organ transplants: medical marijuana: qualified patients. California Legislative Information. http://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=201520160AB258&search_keywords=. Published July 6, 2015. Accessed December 28, 2017.
- 38.Neyer J, Uberoi A, Hamilton M, Kobashigawa JA. Marijuana and Listing for Heart Transplant: A Survey of Transplant Providers. Circ Heart Fail. 2016;9(7). pii: e002851. [DOI] [PubMed] [Google Scholar]
- 39.Lecru L, Desterke C, Grassin-Delyle S, et al. Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int 2015;88(1):72–84. [DOI] [PubMed] [Google Scholar]
- 40.Tam J The emerging role of the endocannabinoid system in the pathogenesis and treatment of kidney diseases. J Basic Clin Physiol Pharmacol 2016;27(3):267–276. [DOI] [PubMed] [Google Scholar]
- 41.Barutta F, Piscitelli F, Pinach S, et al. Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes. 2011;60(9):2386–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishida JH, Auer R, Vittinghoff E, et al. Marijuana Use and Estimated Glomerular Filtration Rate in Young Adults. Clin J Am Soc Nephrol 2017;12(10):1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rein JL, Coca SG, Texter L, et al. Marijuana Use and Kidney Outcomes in the ASSESS-AKI Cohort. J Am Soc Nephrol 2018;(Programs & Abstracts) Abstract FR-PO233. [Google Scholar]
- 44.Bohatyrewicz M, Urasinska E, Rozanski J, Ciechanowski K. Membranous glomerulonephritis may be associated with heavy marijuana abuse. Transplant Proc 2007;39(10):3054–3056. [DOI] [PubMed] [Google Scholar]
- 45.Bhanushali GK, Jain G, Fatima H, Leisch LJ, Thornley-Brown D. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol 2013;8(4):523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alshaarawy O, Elbaz HA. Cannabis use and blood pressure levels: United States National Health and Nutrition Examination Survey, 2005-2012. J Hypertens. 2016;34(8):1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yankey BA, Rothenberg R, Strasser S, Ramsey-White K, Okosun IS. Effect of marijuana use on cardiovascular and cerebrovascular mortality: A study using the National Health and Nutrition Examination Survey linked mortality file. Eur J Prev Cardiol 2017;24(17):1833–1840. [DOI] [PubMed] [Google Scholar]
- 48.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant 2009;9(11):2597–2606. [DOI] [PubMed] [Google Scholar]
- 49.Andreasson S, Allebeck P. Cannabis and mortality among young men: a longitudinal study of Swedish conscripts. Scand J Soc Med 1990;18(1):9–15. [DOI] [PubMed] [Google Scholar]
- 50.Manrique-Garcia E, Ponce de Leon A, Dalman C, Andreasson S, Allebeck P. Cannabis, Psychosis, and Mortality: A Cohort Study of 50,373 Swedish Men. Am J Psychiatry. 2016;173(8):790–798. [DOI] [PubMed] [Google Scholar]
- 51.Muhuri PK, Gfroerer JC. Mortality associated with illegal drug use among adults in the United States. Am J Drug Alcohol Abuse. 2011;37(3):155–164. [DOI] [PubMed] [Google Scholar]
- 52.Sidney S, Beck JE, Tekawa IS, Quesenberry CP, Friedman GD. Marijuana use and mortality. Am J Public Health. 1997;87(4):585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ungerleider JT, Andrysiak T, Tashkin DP, Gale RP. Contamination of marihuana cigarettes with pathogenic bacteria--possible source of infection in cancer patients. Cancer Treat Rep 1982;66(3):589–591. [PubMed] [Google Scholar]
- 54.Kagen SL, Kurup VP, Sohnle PG, Fink JN. Marijuana smoking and fungal sensitization. J Allergy Clin Immunol 1983;71(4):389–393. [DOI] [PubMed] [Google Scholar]
- 55.Hamadeh R, Ardehali A, Locksley RM, York MK. Fatal aspergillosis associated with smoking contaminated marijuana, in a marrow transplant recipient. Chest. 1988;94(2):432–433. [DOI] [PubMed] [Google Scholar]
- 56.Marks WH, Florence L, Lieberman J, et al. Successfully treated invasive pulmonary aspergillosis associated with smoking marijuana in a renal transplant recipient. Transplantation. 1996;61(12):1771–1774. [DOI] [PubMed] [Google Scholar]
- 57.Vethanayagam D, Pugsley S, Dunn E, Russell D, Kay JM, Allen C. Exogenous lipid pneumonia related to smoking weed oil following cadaveric renal transplantation. Can Respir J. 2000;7(4):338–342. [DOI] [PubMed] [Google Scholar]
- 58.Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med 2007;167(3):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001;103(23):2805–2809. [DOI] [PubMed] [Google Scholar]
- 60.Rumalla K, Reddy AY, Mittal MK. Recreational marijuana use and acute ischemic stroke: A population-based analysis of hospitalized patients in the United States. J Neurol Sci 2016;364:191–196. [DOI] [PubMed] [Google Scholar]
- 61.Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol 2002;42(S1):58S–63S. [DOI] [PubMed] [Google Scholar]
- 62.Rogeberg O, Elvik R. The effects of cannabis intoxication on motor vehicle collision revisited and revised. Addiction. 2016;111(8):1348–1359. [DOI] [PubMed] [Google Scholar]
- 63.Sophocleous A, Robertson R, Ferreira NB, McKenzie J, Fraser WD, Ralston SH. Heavy Cannabis Use Is Associated With Low Bone Mineral Density and an Increased Risk of Fractures. Am J Med 2017;130(2):214–221. [DOI] [PubMed] [Google Scholar]
- 64.Belbasis L, Kohler CA, Stefanis N, et al. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr Scand. 2018;137(2):88–97. [DOI] [PubMed] [Google Scholar]
- 65.Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr Bull. 2016;42(5):1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lev-Ran S, Le Foll B, McKenzie K, George TP, Rehm J. Bipolar disorder and co-occurring cannabis use disorders: characteristics, co-morbidities and clinical correlates. Psychiatry Res 2013;209(3):459–465. [DOI] [PubMed] [Google Scholar]
- 67.Buu A, Dabrowska A, Mygrants M, Puttler LI, Jester JM, Zucker RA. Gender differences in the developmental risk of onset of alcohol, nicotine, and marijuana use and the effects of nicotine and marijuana use on alcohol outcomes. J Stud Alcohol Drugs. 2014;75(5):850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blanco C, Hasin DS, Wall MM, et al. Cannabis Use and Risk of Psychiatric Disorders: Prospective Evidence From a US National Longitudinal Study. JAMA Psychiatry. 2016;73(4):388–395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.