Abstract
Introduction:
Despite advances in drug eluting technologies, neointimal hyperplasia (NIH) and restenosis still plagues endovascular therapy in atherosclerotic diseases. By appreciating atherosclerosis and NIH as complex inflammatory processes, specialized pro-resolving mediators (SPMs) are a superfamily of endogenous unsaturated fatty-acid derived lipids with the potential for inflammatory resolution.
Areas covered:
Inquiry into SPMs in this context is a novel approach and is the focus of this review, with emphasis on our understanding with NIH. Prior mechanistic understandings of SPM deficiency with atherosclerosis has offered insight, as well as the complexity and diversity of the SPM superfamily. Therapeutic investigation using SPMs to combat NIH is also evaluated here.
Expert commentary:
Endogenous deficiency of SPMs synthesis by 12/15-lipoxygenase underlies resolution deficits in atherosclerosis and NIH. Upstream PDGF inhibition by SPMs, most notably RvD1 and LXA4, confers a multifactorial attenuation of NIH that involves interconnected anti-inflammatory efforts, most notably switch pro-resolving smooth muscle cells (vSMCs) and macrophages. The ALX/ FPR2 is one receptor system identified on vSMCs that interacts with these SPMs to promote NIH resolution. Therapeutically, while shown to be promising with less stent burden or cytotoxicity, SPMs must be balanced by necessary mechanistic, pharmacokinetic and anatomical considerations.
Keywords: Atherosclerosis, Inflammation, Neointimal hyperplasia, Pro-resolving mediators, Resolvins, Lipoxins, Protectins
1. Introduction
Atherosclerosis is a chronic inflammatory process that is most susceptible to occurrence in the intimal layer of arteries, particularly at bifurcation points of the blood vessel. The earliest events of atherosclerosis include activation of the endothelium by certain risk factors such as hypercholesterolemia.1 In general, in atherosclerotic arteries, the clinical symptoms develop primarily due to two events: (a) The prototypical plaque formation that ensues within the inflammatory milieu results from oxidized lipids creating foam cells, a macrophage derivative.3 Plaque instability and rupture are attributed to a weakening of its fibrous cap due to matrix degradation by further macrophage secretions (cytokines, chemokines, growth-factors, and disintegrins).3–4 Subsequent vessel thrombosis can promote symptomatic stenosis or occlusion underlying the ischemic events seen in stroke of the brain, reduced coronary perfusion of the heart in patients with coronary artery disease (CAD), and limb claudication seen in peripheral arterial disease (PAD).1, and (b) Following mechanical injury to the endothelium that commonly occur during interventional procedures, including balloon angioplasty and intravascular stenting, circulating leukocytes, monocytes, and T-lymphocytes attach and infiltrate the intima and release mediators to promote smooth muscle cell (SMC) migration towards lumen with fibrous cap formation, resulting in the development of neointimal hyperplasia and restenosis.1–2
2. Current therapeutic options to prevent neointimal hyperplasia
In the realm of atherosclerotic sequelae across various vascular beds, endovascular revascularization remains a viable option with minimally invasive options amongst medical management. The earliest forms of such intervention started in the 1970s and included angioplasty, where obligatory vessel injury is utilized to obliterate lesions and improve vessel patency with endothelial denudation.5–6 However, employment of angioplasty alone can promote acute closure of vessels due to elastic recoil and post-injury endothelial denudation and sub-endothelial matrix exposure leading to platelet aggregation and infiltration of circulating cells into the intima.7–8 Additionally, further closure is mediated by neointimal hyperplasia (NIH) secondary to balloon insult, an additional inflammatory event that lead to excess scar tissue growth and restenosis secondary to proliferation of vascular smooth muscle cells (Figure 1)9. In coronary intervention, issues with both acute recoil and dissection issues with angioplasty alone have been moderately alleviated with the development and improvement of stent devices. Drug-eluting stents are coated with paclitaxel, which disrupts microtubule organization, or sirolimus, which inhibits mammalian target of rapamycin (mTOR) for cell cycle arrest, with the release of these drugs controlling cell proliferation, intimal hyperplasia, and restenosis at the site of stent deployment.6,10 However, delayed NIH remains an issue despite these advances in anti-proliferative and anti-inflammatory effects. In peripheral revascularization, the durability of stenting is even shorter and complicated by in-stent restenosis, with mixed evidence regarding the equivalency of femoropopliteal stenting to bypass in long-term patency outcomes.11,12 In addition, peripheral revascularization is complicated by mechanical stress and torsion of the arteries in the lower limbs, illuminating the particular necessity of improved balloon intervention with drug-coated technology.13 Nevertheless, paclitaxel-based drug coated balloons (DCBs) have revolutionized femoropopliteal revascularization in peripheral arterial disease (PAD) with long-term patency sustained at three years post-intervention, highlighting the yield of an anti-proliferative target.14 Lastly, in-stent restenosis and NIH also plague those with cerebrovascular atherosclerotic disease undergoing carotid artery angioplasty with stenting, despite long-term follow-up at ten years for ischemic outcomes being equivalent to carotid endarterectomy.15,16 Likewise, the advent and application of drug eluting techniques to limit restenosis in the carotid space are the least developed.
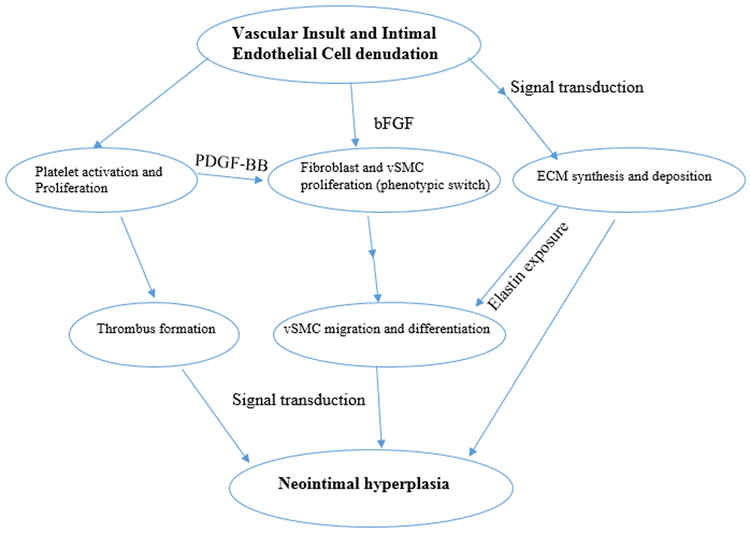
Figure 1: Event sequence in the pathophysiology of neointimal hyperplasia (NIH):
Therapeutic insult creates obligatory vessel injury and inflammation, which activates platelet. Recruited platelets induce thrombus formation and release PDGF-BB, which promotes fibroblast proliferation in addition to a downstream switch in vSMC to the dedifferentiated proliferating type (potentiated by bFGF release from subendothelial injury). Additionally, endothelial injury of the vessel promotes ECM remodeling with an imbalance of matrix metalloproteinases potentiating vSMC migration via exposure of elastin components.
EC endothelial cell, ECM – extracellular matrix, PDGF-BB – platelet derived growth factor-BB; bFGF- basic fibroblast growth factor
3. Pro-resolving lipid mediators: biosynthesis and subclasses
Given both the disease burden that atherosclerosis can ensue and the challenges of endovascular intervention with NIH, promotion of endogenous anti-inflammatory mediators is of therapeutic interest. By appreciating the link between an inflammatory state with both atherosclerosis and NIH, inquiry into the inflammation resolution program has identified a series of molecular and cellular effectors. One superfamily of interest are unsaturated fatty-acid derived lipid mediators referred to as specialized pro-resolving mediators (SPMs).17 With earlier evidence of the localization of SPMs within human arterial cells, endogenous biosynthesis is regulated by lipoxygenases and cyclooxygenases that utilize polyunsaturated fatty acid precursors derived from omega-6 and omega-3 fatty acids.18 These SPMs are further subdivided into four major classes of activators of potentially G-protein coupled receptors which include the lipoxins (LX), resolvins (Rv), protectins (P), and maresins (Mar), each of which attenuates inflammation and facilitates tissue repair.17, 19,20 While the role of these SPMs with both atherosclerosis and NIH is not fully defined, exogenous delivery of these mediators and inquiry into endogenous mechanisms have uncovered few clues to their therapeutic potential. This review summarizes and critically evaluates the preclinical and interventional findings in attempts to elucidate the role of SPMs and their targeted mechanisms in atherosclerotic disease with a particular focus on NIH.
4. SPMs and atherosclerosis
4.1. SPM deficiency and the resolution deficit
The earliest evidence for the role of SPMs with atherosclerosis was found in CAD patients undergoing percutaneous transluminal angioplasty, which showed intracoronary release of peptidoleukotrienes and lipoxin A4 (LXA4) distal to the plaque site and after plaque rupture .21 Additionally, it was found that patients undergoing aspirin (ASA) therapy had an increase in the appearance of these compounds. However, aspirin-induction of lipoxin levels has been shown to be reduced in CAD and PAD patients, highlighting a resolution deficit in counter-regulating platelet-derived growth factor (PDGF)-stimulated chemotaxis of SMCs.22,23 The hypothesis that atherosclerosis results from an inflammatory resolution failure is further supported in animal models that emphasize a pertinent deficiency in local 12/15-lipoxygenase (12/15-LOX) pathways to upregulate LXA4, resolving D1 (RvD1), and protectin D1 (PD1) via oxygenation of free-form and complex fatty acid assemblies.24,25 Conversely, it was shown prior to this that overexpression of 15-LOX and LXA4 dampened neutrophil-mediated inflammation with respect to bone and tissue loss in periodontitis and arthritis.26 Importantly, as indicated by Sansbury et al. 27, mere supplementation of omega-3 polyunsaturated fatty acids (a substrate of SPMs) has failed to show improvement in cardiovascular end points, indicating the possibility of altered downstreat mechanisms (e.g. SPM upregulation).
4.2. Dichotomy of the 12/15-LOX pathway and association with 5-LOX
Unlike the predominantly established pro-atherogenic mechanisms of the 5-LOX pathway, evolution of these findings within the 12/15-LOX pathway have led to the postulation that its activation can confer protective vascular effects via PPARγ with increased nitric oxide (NO) production and reduction in IL-12p40.25, 28 However, deletion of 12/15-LOX has also been shown to promote atherosclerosis, with specific evidence that increased 15-LOX-2 (an isoform of 15-LOX) expression is seen in human monocyte to macrophage differentiation.29 Nevertheless, with an array of metabolites exist for 12/15-LOX pathway, catalytic action may be attributed to be tissue- and species-specific.30 Additionally varying cell types offer a different intracellular redox state that would preferentially result in a pro-atherogenic state or an anti-inflammatory state. For example, evidence exists for 15-LOX-2 products in macrophages within human carotid plaques with increased hypoxia.31 In coronary atherosclerosis, heterozygote carriers with a null T560M allele in 15-LOX-1 had an increased risk, highlighting a genetic polymorphic component as well.32 Furthermore, a sequential action of LOX isoforms has been identified, such as 15-LOX with 12-LOX or 5-LOX, and may allow for bypass mechanisms that should be elucidated and targeted to explain atherogenic or anti-inflammatory effects towards atherosclerosis.30, 33–34 The common substrate for lipoxygenases is arachidonic acid (AA), where 5-LOX alone can create leukotriene A4 (LTA4) from the transformation of AA.35 Inquiry into the mechanism of macrophages promoting inflammatory plaque progression has shown that nuclear 5-LOX may upregulate a bypass via LTA4 hydrolase.36.37 Contrary to this and utilizing the same AA substrate, the sequential action of 5-LOX in association with 12/15-LOX creates anti-inflammatory lipoxins, a type of SPM. To this effort, non-nuclear 5-LOX is seen to localize near 12/15-LOX, facilitating the conversion of arachidonic acid to SPMs potentially via an efferocytosis receptor, MerTK.38,39
4.3. Plaque stability: central role of LOX-produced SPMs in atherosclerosis
The specific importance of SPMs produced by the LOX pathways primarily centers around plaque stability and underlies the therapeutic potential of SPMs in atherosclerosis. In addition to SPM deficiency conferring a resolution deficit that promotes plaque formation, the vulnerability to subsequent plaque rupture was identified in human carotid plaques where the ratio of RvD1 to LTB4 was significantly decreased.40 Similarly, in aortic lipid mediator profiling of ApoE −/− mice, increased leukotriene B4 (LTB4) and prostaglandin E2 (PGE2) and decreased SPM levels (RvD2 and Mar1) were associated with increasing vulnerability of the plaque rupture.41 To appreciate this to be secondary to the resolution deficit, RvD2 and Mar1 were shown to shift the macrophage profile to secondarily stimulate collagen synthesis in SMCs and inhibit expansion of the necrotic core in the plaque. Interestingly in this same study, therapeutic delivery of the two SPM types was successful in halting advanced atherosclerosis. Considering the atherogenic potential of nuclear 5-LOX alone, further inquiry into advanced plaque prevention showed that RvD1 is reciprocally capable of preventing nuclear localization of 5-LOX to promote SPM synthesis in macrophages and inhibit LTB4.38 Likewise, RvD1 administration in fat-fed Ldlr −/− mice improved the RvD1:LTB4 ratio to that of less advanced plaques, with greater stability.40 The lipoxin subset of the SPM family has also been shown to have importance with plaque stability. Aspirin-triggered LXA4 (ATL) is seen to provide athero-protection via stimulation of formyl peptide receptor 2 (FPR2) receptor, reducing macrophage and apoptotic cell infiltration to halt advanced plaque necrosis.42
Taken together, the importance of SPMs in a resolution deficit with atherosclerotic disease is well-supported in both animal and human studies. While the synthesis of SPMs via LOX pathways is critical, the diversity and sequence activation of LOX isoforms with plaque stability or atherogenic process must be elucidated individually. Additionally, changes in the microenvironment and genetic implications may play a role. Nevertheless, the 12/15-LOX pathway with nuclear exclusion of 5-LOX is proven to be important. Lastly, future therapeutic application of SPMs therapeutically in atherosclerosis may be two-fold, revolving around addressing both the formation and stability of plaques. However, considering both aspects, the mechanisms of consumption or degradation of SPMs in those that are deficient must be further considered and may emphasize targets specific to a LOX isoform. The consideration of plaque regression with SPMs is also an area of interest.
5. SPMs and neointimal hyperplasia (NIH)
5.1. Pathophysiology of NIH
The consequences of an unhindered atherosclerotic plaque instability can lead to acute thrombotic events seen in various vascular pathologies. As discussed above, an endovascular approach with vessel injury is often attempted for revascularization but is currently complicated in the long-term due to the development of NIH. NIH involves fibroblast and SMC proliferation in the intima layer of arteries and veins with deposition of extracellular matrix (ECM).43 The thickened intimal layer reduces the luminal area of the vessel. A phenotypic switch in SMC type from a differentiated contractile type to a dedifferentiated high proliferation type is believed to underlie the expansion of NIH.44 The phenotypic switch is associated with increased migration activity, proteolytic activity, and decreased levels of contractile and cytoskeletal proteins. However, the pathways and factors involved in this switch are less well known, with platelet derived growth factor-BB (PDGF-BB) and basic fibroblast growth factor (bFGF) believed to play a multifactorial role.45 The progression of migration and differentiation of these dedifferentiated SMC in NIH is perpetuated by downstream signal transduction, involving the Ras-MAPK and PI3K-Akt pathways.46 Downstream activation of NF-kB by MAPK signaling has previously been implicated in NIH with recent evidence of attenuated NF-kB activity and decreased medial cell proliferation upon exogenous delivery of another SPM subclass, protectins (specifically PD1).47–49 However, these signaling cascades also promote ECM synthesis, the second component of the vascular remodeling that occurs with NIH. ECM remodeling, as seen in the MMP/TIMP system, involves a harmony between degradation and synthesis, the former of which involves matrix metalloproteinases (MMPs) and the latter of which involves MMP tissue inhibitors.50 These components of NIH physiology are summarized in a simplified theoretical sequence (Figure 1). However, while the pathophysiology of NIH continues to be elucidated, therapeutic considerations of combating NIH might not involve a single downstream target, a belief indicated earlier by Collins et al. with the failure of the PREVENT trials.43 Secondary induction of NIH may only be bypassed by upstream targets, such as prevention of phenotypic switching.
5.2. ALX/FPR2-mediated signaling with SPMs
The developing role of SPMs with atherosclerosis previewed its therapeutic potential lying upstream in the pathophysiology of the closely related process of NIH after vascular insult. The earliest inquiry into this topic stemmed from the detection of the A type LX (ALX) receptor/ FPR2 as a receptor not only for LXA4 and ChemR23 as a receptor for RvE1 has been identified in human vascular SMCs (vSMCs).22 Within these findings in human vSMCs related to peripheral arterial atherosclerosis, the catalytic action of LXA4 and RvE1 attenuated PDGF-stimulated vSMC migration and PDGF receptor phosphorylation. However, it was uncertain whether differential expression of LXA4 and RvE1 receptors mediates this process. The first inquiry of these findings with NIH involved a rabbit model with in vivo arterial angioplasty insult.51 Upregulation of D-series resolvins (RvD1 and RvD2) exhibited a dose-dependent inhibition of vSMCs proliferation and migration, the culminating events of NIH. Additionally, increased expression levels of these SPMs after vessel injury highlighted endogenous biosynthetic pathways with its receptor presence known but poorly localized. Furthermore, successful attenuation of NIH with the exogenous delivery of RvD2 provided the earliest evidence for SPM based therapy. Subsequent to this, and after atheroprotective findings of ATLs via FPR stimulation were found, ATL signaling through the ALX/FPR2 receptor in vSMCs was explored after carotid artery ligation in a mouse model.52 It was seen that vSMCs had a significantly higher rate of proliferation in ALX/FPR2 knockout (KO) compared to wild type (WT) mice. Additionally, in vitro and in vivo administration of ATL to the ALX/FPR2 KO vSMCs did not alleviate this, emphasizing the role of the ALX/FPR2 receptor for ATL inhibition of PDGF-induced migration. Both of these initial studies with SPMs in NIH provides strong evidence for the upstream involvement of the ALX/FPR2 signaling in vSMCs but is best evidenced with RvD1 and LXA4.
5.3. SPM-induced phenotypic switch in macrophages and vSMC
Further inquiry into other SPM subtypes showed success with the exogenous delivery of maresin as an additional therapeutic agent in attenuating NIH.53 In an in vivo mouse model with carotid artery ligation, omega-3 polyunsaturated fatty acid-derived SPMs (Mar1 and RvD2) significantly inhibited NIH compared to vehicle via decreased cell proliferation, decreased neutrophil and macrophage recruitment, and increased polarization of M2 macrophages. The latter component augments the resolution phase of the inflammation with NIH by switching the dominant phenotype of macrophages from a pro-inflammatory state (M1) to a pro-resolving state (M2), a process that requires decreased PMN infiltration.17 Likewise, the ability for Mar1 and RvD2 to enable this phenotypic switch in macrophages was the first evidence of its kind in vascular injury and NIH resolution. Evidence for a SPM-induced macrophage phenotype switching to M2 is provided in a most recent study by Liu et al., where exogenous and endogenous RvE1substantially reduced NIH after femoral artery injury in mice.54 Similarly, the phenotypic switch occurred in the background of diminished vascular PMN infiltration and vSMC migration. Interestingly, Liu et al.54 also found that RvE1 attenuated T-cell trafficking by reducing chemokine [C-C motif] ligand 5 (CCL5) secretion from vSMC, thereby reducing leukocyte recruitment. In the in vitro part of the study by Akagi et al. 53, Mar1 and RvD2 treatment inhibited SMC migration secondary to a PDGF gradient and reduced responsiveness to TNF-α, facilitating both beneficial alterations in leukocyte-vessel wall interactions and the phenotypic switch to vSMC with reduced migratory capability. Nevertheless, unlike the elucidation of the RvD1 and LXA4 receptor (ALX/FPR2), ChemR23 for RvE1, and SRV2/GPR18 for RvD2,55 the receptor type is unclear for Mar, although a GPCR may be speculated to be the type. Furthermore, linking these elucidated receptors with the attenuation of PDGF signaling requires further evaluation of these downstream mechanisms.
5.4. Pharmacokinetics of potential SPM therapeutics in NIH
With some success with the exogenous delivery of SPMs to attenuate NIH in animal models, consideration is also given to the pharmacokinetic challenge of drug elution kinetics that has also complicated current treatment options in vascular disease. In addition to further investigating the role of RvD1 with NIH, Wu et al. 56 were the first to evaluate the safety and efficacy of perivascular delivery of SPMs (RvD1) through thin biodegradable three-layered poly (lactic-co-glycolic-acid) (PLGA) wraps or 25% Pluronic F127 gels in a rat model with carotid angioplasty. The therapeutic rationale of these delivery mediums is the ability for biodegradability in the case of PLGA, or the novelty of the Pluronic gels from proof-of-concept studies.57–58 Consistent with a previous study discussed here, exogenous RvD1 was shown to attenuate NIH processes of proliferation, migration, and ECM deposition via PDGF inhibition after carotid vessel insult without any hazardous cytotoxic evidence. However, reduced leukocyte recruitment was not seen as noted in the previous studies that attributed macrophage phenotype switching to it. Likewise, the delivery apparatus is believed to have had a role in this. A similar reduction in NIH was seen with both the gel and the PLGA wrap with no thrombotic or infectious events noted, or death. This safety profile demonstrates a useful property of SPM therapeutics along with having no cytotoxicity. Despite equivalent evidence, it is speculated that the PLGA wrap may be more suitable for translation to NIH attenuation in larger animals or humans due to prolonged kinetics and is less sensitive to temperature and positional sensitivity similar to Pluronic gels. Furthermore, previous evidence with a paclitaxel-loaded PLGA device was troubled by high infection rates, an issue Wu et al. did not see with their RvD1 apparatus.59 Finally, a therapeutic option that can be delivered via a biodegradable device like the PLGA wrap would also address the issue of in-stent restenosis that currently plagues stenting in vascular disease, in addition to stent fracture.
6. Expert commentary
The pathophysiology of NIH is complex, involving multiple signaling pathways, phenotypic switching of vSMCs, and matrix deposition. However, upstream PDGF inhibition was consistently demonstrated in elucidating a multifactorial therapeutic potential of SPMs in the preliminary animal studies discussed here. Furthermore, the endogenous or exogenous involvement of SPMs in the resolution of NIH through PDGF inhibition, is mostly mediated by GPCRs in vSMCs. The best understood one to date is the ALX/FPR signaling via RvD1 and LXA4, which facilitated decreased leukocyte and macrophage recruitment, decreased proliferation, and a switch in macrophages and vSMCs to a proresolving phenotype. Additionally, further anti-inflammatory efforts identified in these studies included diminished TNF-α sensitivity, NF-kB activity, and CCL5 secretion, which all may be SPM amplifiers of NIH resolution. A proposed early mechanism that incorporates these parts is provided (Figure 2). While other SPMs showed evidence for these effects in NIH, such as other resolvin types, maresins and protectins, their direct interactions with PDGF are more poorly understood.
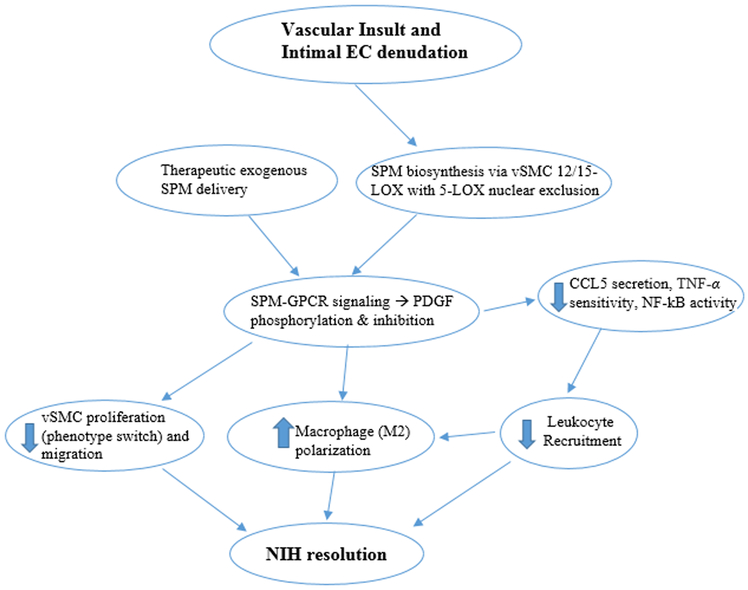
Figure 2: Proposed mechanism of neointimal hyperplasia (NIH) resolution with SPMs:
Therapeutic insult creates obligatory endothelial injury and inflammation, which promotes SPM biosynthesis. Subsequently, synthesized SPMs bind to GPCRs on vSMCs to inhibit PDGF, creating direct and amplifying anti-inflammatory effects, in addition to promoting NIH resolution phenotypes within macrophages and vSMCs (differentiated).
7. Five-year view
It will be pertinent to further characterize SPM receptor types to optimize pharmacokinetics with exogenous delivery of SPMs. Likewise, understanding differential expression for SPM receptors, such as ALX/FPR2 receptor along the arterial wall will be a pertinent therapeutic consideration in deciding exogenous delivery mechanisms with excipients. Mechanistically, understanding the mode of signaling (ex. paracrine or autocrine) would facilitate understanding of how an endogenous SPM balance is kept. These practical considerations to the therapeutic application of SPMs warrants even further investigation given the benefits described. The first study of its kind discussed here is promising for delivery mechanisms that have less vessel burden and cytotoxicity. However, long term patency remains the primary goal with any SPMs or combination of SPMs, thus requiring considerations in addition to a delivery vehicle such as PLGA. Finally, our understanding of the importance of SPMs in a resolution deficit with atherosclerosis offers additional diagnostic and therapeutic routes in the context of endogenous SPM biosynthesis through the 12/15-LOX pathway with nuclear exclusion of 5-LOX. Evidence of the sequence activation described presents an area of additional inquiry into their biosynthesis with dual properties that are both anti-inflammatory and atherogenic. Diagnostically, the RvD1:LTB4 ratio may serve to show an underlying deficit in biosynthesis and SPM resolution. However, the feasibility of assessing such a biomarker is not well-understood. It should be stressed from the varying success of vascular intervention in relation to the type of vascular bed (e.g. peripheral vs. coronary), that macro- and microenvironments and vessel bed differences are important considerations as well with further inquiry of SPMs. The interaction of even the same subclass of SPMs within different vessel bed microenvironments may confer a differing response that could also have a genomic determinant underlying the varied response, as appreciated in atherosclerotic processes.60
Thus, the elucidation of the endogenous role of SPMs in atherosclerotic disease has offered valuable insight into NIH, an intimately related process stemming from its treatment. The therapeutic investigation of SPMs is a worthy endeavor in NIH, but their biosynthetic and resolution events require further evaluation. Likewise, the exogenous delivery of SPM treatment may offer an efficacious but also safe way to address atherosclerotic vascular disease.
8. Key issues
Atherosclerosis is a chronic inflammatory process that is most susceptible to occur in the intimal layer of arteries, particularly at the bifurcation points of the blood vessel.
In the realm of atherosclerotic sequelae across various vascular beds, endovascular revascularization is complicated by the development of NIH and restenosis.
One superfamily of endogenous mediators for resolution of inflammation is unsaturated fatty-acid derived lipid mediators referred to as specialized pro-resolving mediators, or SPMs.
Atherosclerosis results from inflammatory resolution failure from deficiency in local 12/15-lipoxygenase (12/15-LOX) pathways that upregulate SPMs.
Non-nuclear 5-LOX is seen to localize near 12/15-LOX, facilitating the conversion of arachidonic acid to SPMs. Other LOX isoforms may exist.
Importance of SPMs produced by the LOX pathways centers around plaque stability in atherosclerosis.
NIH involves fibroblast and smooth muscle cell (SMC) proliferation in the intimal layer of arteries and veins with deposition of extracellular matrix (ECM). The thickened intimal layer reduces the luminal area of the vessel.
A phenotypic switch in SMC type from a differentiated contractile type to a dedifferentiated high proliferation type is believed to underlie the expansion of NIH.
Several signal transduction pathways may be implicated in NIH and a sole target may not be sufficient, but PDGF signaling is well described.
Detection of the ALX/FPR2 as a receptor not only for LXA4, but also for RvD1 in human vSMCs stemmed inquiry into SPM involvement with NIH.
Each of these SPM receptor types (potentially all GPCRs) may be modulated by PDGF phosphorylation but are not fully investigated like ALX/FPR2.
SPMs inhibits NIH via decreased cell proliferation, decreased leukocyte, and increased polarization to M2 macrophages.
Therapeutic delivery mechanisms of SPMs may offer less vessel burden (vs. intravascular stenting) and less cytotoxicity. However, long-term vessel patency with SPMs is not understood.
Acknowledgement
The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
The research work of D K Agrawal is supported by research grants R01HL112597, R01HL116042, R01HL120659, and R01HL144125 from the National Institutes of Health, USA.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has disclosed they are an inventor on patents related to this area held by UCSF and Partners Healthcare/Brigham and Women’s Hospital, and they are a co-founder of VasaRx, a biotechnology start up focused in this area. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- [1].Immunity Frostegård J., atherosclerosis and cardiovascular disease. BMC Medicine. 2013;11(1). doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Frostegård J, Ulfgren A-K, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145(1):33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- [3].Koenig W, Khuseyinova N. Biomarkers of Atherosclerotic Plaque Instability and Rupture. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(1):15–26. doi: 10.1161/01.atv.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- [4].Shah PK. Mechanisms of plaque vulnerability and rupture. Journal of the American College of Cardiology. 2003;41(4). doi: 10.1016/s0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- [5].Grüntzig A Transluminal Dilatation of Coronary Artery Stenosis — Experimental Report. Percutaneous Vascular Recanalization. 1978:57–65. doi: 10.1007/978-3-642-46381-5_9. [DOI] [Google Scholar]
- [6].Iqbal J, Gunn J, Serruys PW. Coronary stents: historical development, current status and future directions. British Medical Bulletin. 2013;106(1):193–211. doi: 10.1093/bmb/ldt009. [DOI] [PubMed] [Google Scholar]
- [7].Bauters C, Meurice T, Hamon M, Mcfadden E, Lablanche J- M, Bertrand ME. Mechanisms and prevention of restenosis: from experimental models to clinical practice. Cardiovascular Research. 1996;31(6):835–846. doi: 10.1016/s0008-6363(96)00038-7. [DOI] [PubMed] [Google Scholar]
- [8].Chandrasekar B, Tanguay J- F. Platelets and restenosis. Journal of the American College of Cardiology. 2000;35(3):555–562. doi: 10.1016/s0735-1097(99)00596-3. [DOI] [PubMed] [Google Scholar]
- [9].Yoshida Y, Mitsumata M, Ling G, Jiang J, Shu Q. Migration of Medial Smooth Muscle Cells to the Intima after Balloon Injury. Annals of the New York Academy of Sciences. 1997;811(1 Atherosclerosis:459–470. doi: 10.1111/j.1749-6632.1997.tb52027.x. [DOI] [PubMed] [Google Scholar]
- [10].Yamaji K, Räber L, Zanchin T, et al. Ten-year clinical outcomes of first-generation drug-eluting stents: the Sirolimus-Eluting vs. Paclitaxel-Eluting Stents for Coronary Revascularization (SIRTAX) VERY LATE trial. European Heart Journal. 2016;37(45):3386–3395. doi: 10.1093/eurheartj/ehw343. [DOI] [PubMed] [Google Scholar]
- [11].Zaag EVD, Legemate D, Prins M, Reekers J, Jacobs M. Angioplasty or Bypass for Superficial Femoral Artery Disease? A Randomised Controlled Trial. European Journal of Vascular and Endovascular Surgery. 2004;28(2):132–137. doi: 10.1016/j.ejvs.2004.04.003. [DOI] [PubMed] [Google Scholar]
- [12].Islam J Comparison between superficial femoral artery stenting and bypass surgery in severe lower-limb ischaemia : a retrospective study : cardiovascular topic. Cardiovascular Journal of Africa. 2015;26(1):34–37. doi: 10.5830/cvja-2014-074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Adlakha S, Sheikh M, Wu J, et al. Stent Fracture in the Coronary and Peripheral Arteries. Journal of Interventional Cardiology. 2010;23(4):411–419. doi: 10.1111/j.1540-8183.2010.00567.x. [DOI] [PubMed] [Google Scholar]
- [14].Schneider PA, Laird JR, Tepe G, et al. Treatment Effect of Drug-Coated Balloons Is Durable to 3 Years in the Femoropopliteal Arteries. Circulation: Cardiovascular Interventions. 2018;11(1). doi: 10.1161/circinterventions.117.005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wholey MH, Wholey M, Mathias K, et al. Global experience in cervical carotid artery stent placement. Catheterization Cardiovascular Interventions. 2000, 50:160–167. [DOI] [PubMed] [Google Scholar]
- [16].Brott T, Howard G, Roubin G. Long-Term Results of Stenting Versus Endarterectomy for Carotid-Artery Stenosis. Journal of Vascular Surgery. 2016;64(2):535–536. doi: 10.1016/j.jvs.2016.06.056. [DOI] [Google Scholar]
- [17].Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chatterjee A, Komshian S, Sansbury BE, et al. Biosynthesis of proresolving lipid mediators by vascular cells and tissues. The FASEB Journal. 2017;31(8):3393–3402. doi: 10.1096/fj.201700082r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Buckley CD, Gilroy DW, Serhan CN. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity. 2014;40(3):315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thorp EB. Proresolving Lipid Mediators Restore Balance to the Vulnerable Plaque. Circulation Research. 2016;119(9):972–974. doi: 10.1161/circresaha.116.309794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brezinski DA, Nesto RW, Serhan CN. Angioplasty triggers intracoronary leukotrienes and lipoxin A4. Impact of aspirin therapy. Circulation. 1992;86(1):56–63. doi: 10.1161/01.cir.86.1.56. [DOI] [PubMed] [Google Scholar]
- [22].Ho KJ, Spite M, Owens CD, et al. : Aspirin-Triggered Lipoxin and Resolvin E1 Modulate Vascular Smooth Muscle Phenotype and Correlate with Peripheral Atherosclerosis. The American Journal of Pathology. 2010;177(4):2116–2123. doi: 10.2353/ajpath.2010.091082.*This article provides a good description on SPM deficiency in PAD – resolution deficits
- [23].Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. The FASEB Journal. 2016;30(8):2792–2801. doi: 10.1096/fj.201500155R.**This article discusses SPM deficiency in CAD – resolution deficits
- [24].Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. The FASEB Journal. 2008;22(10):3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kühn H, O’Donnell VB. Inflammation and immune regulation by 12/15 lipoxygenases. Progress in Lipid Research. 2006;45(4):334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [26].Serhan CN, Jain A, Marleau S, et al. Reduced Inflammation and Tissue Damage in Transgenic Rabbits Overexpressing 15-Lipoxygenase and Endogenous Anti-inflammatory Lipid Mediators. The Journal of Immunology. 2003;171(12):6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- [27].Sansbury BE, Spite M. Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology. Circulation Research. 2016;119(1):113–130. doi: 10.1161/circresaha.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovascular Research. 2010;86(2):243–253. doi: 10.1093/cvr/cvq016. [DOI] [PubMed] [Google Scholar]
- [29].Wuest SJ, Crucet M, Gemperle C, Loretz C, Hersberger M. Expression and regulation of 12/15-lipoxygenases in human primary macrophages. Atherosclerosis. 2012;225(1):121–127. doi: 10.1016/j.atherosclerosis.2012.07.022. [DOI] [PubMed] [Google Scholar]
- [30].Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Progress in Lipid Research. 2011;50(1):115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hulten LM, Olson FJ, Aberg H, et al. 15-Lipoxygenase-2 is expressed in macrophages in human carotid plaques and regulated by hypoxia-inducible factor-1alpha. European Journal of Clinical Investigation. 2010;40(1):11–17. doi: 10.1111/j.1365-2362.2009.02223.x. [DOI] [PubMed] [Google Scholar]
- [32].Assimes TL, Knowles JW, Priest JR, et al. A near null variant of 12/15-LOX encoded by a novel SNP in ALOX15 and the risk of coronary artery disease. Atherosclerosis. 2008;198(1):136–144. doi: 10.1016/j.atherosclerosis.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: Lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;73(3–4):163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- [34].Poeckel D, Berry KAZ, Murphy RC, Funk CD. Dual 12/15- and 5-Lipoxygenase Deficiency in Macrophages Alters Arachidonic Acid Metabolism and Attenuates Peritonitis and Atherosclerosis in ApoE Knock-out Mice. Journal of Biological Chemistry. 2009;284(31):21077–21089. doi: 10.1074/jbc.m109.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kobe M, Neau D, Mitchell C, Bartlett S, Newcomer M. The structure of human 15-lipoxygenase-2 with a substrate mimic. Journal of Biological Chemistry. 2014; 289(12): 8562–8469; doi: 10.1074/jbc.M113.543777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brock TG, Maydanski E, Mcnish RW, Peters-Golden M. Co-localization of Leukotriene A4Hydrolase with 5-Lipoxygenase in Nuclei of Alveolar Macrophages and Rat Basophilic Leukemia Cells but Not Neutrophils. Journal of Biological Chemistry. 2001;276(37):35071–35077. doi: 10.1074/jbc.m105676200. [DOI] [PubMed] [Google Scholar]
- [37].Luo M, Jones SM, Peters-Golden M, Brock TG. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proceedings of the National Academy of Sciences. 2003;100(21):12165–12170. doi: 10.1073/pnas.2133253100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fredman G, Ozcan L, Spolitu S, et al. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proceedings of the National Academy of Sciences. 2014;111(40):14530–14535. doi: 10.1073/pnas.1410851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cai B, Thorp EB, Doran AC, et al. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proceedings of the National Academy of Sciences. 2016;113(23):6526–6531. doi: 10.1073/pnas.1524292113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nature Communications. 2016;7:12859. doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Viola J, Lemnitzer P, Jansen Y, et al. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circulation Research. 2016. doi: 10.1161/circresaha.116.309492.** The major conclusion of the article is that nuclear exclusion of 5-LOX promotes 12/15 LOX biosynthesis of SPMs.
- [42].Petri MH, Laguna-Fernandez A, Arnardottir H, et al. Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E−/− mice. British Journal of Pharmacology. 2017;174(22):4043–4054. doi: 10.1111/bph.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Collins MJ, Li X, Lv W, et al. Therapeutic strategies to combat neointimal hyperplasia in vascular grafts. Expert Review of Cardiovascular Therapy. 2012;10(5):635–647. doi: 10.1586/erc.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Muto A, Fitzgerald TN, Pimiento JM, et al. Smooth Muscle Cell Signal Transduction: Implications of vascular biology for vascular surgeons. Journal of Vascular Surgery. 2007;45(6S):15–24. doi: 10.1016/j.jvs.2007.02.06.** This article elegantly shows that vSMC phenotypic switch underlies NIH expansion
- [45].Hao H Arterial Smooth Muscle Cell Heterogeneity: Implications for Atherosclerosis and Restenosis Development. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(9):1510–1520. doi: 10.1161/01.atv.0000090130.85752.ed. [DOI] [PubMed] [Google Scholar]
- [46].Ross R The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- [47].Monaco C Nuclear factor κB: a potential therapeutic target in atherosclerosis and thrombosis. Cardiovascular Research. 2004;61(4):671–682. doi: 10.1016/j.cardiores.2003.11.038. [DOI] [PubMed] [Google Scholar]
- [48].Breuss J Activation of Nuclear Factor-kappaB Significantly Contributes to Lumen Loss in a Rabbit Iliac Artery Balloon Angioplasty Model. Circulation. 2002;105(5):633–638. doi: 10.1161/hc0502.102966. [DOI] [PubMed] [Google Scholar]
- [49].Makino Y, Miyahara T, Nitta J, et al. Proresolving lipid mediators resolvin D1 and protectin D1 isomer attenuate neointimal hyperplasia in the rat carotid artery balloon injury model. Journal of Surgical Research. 2019;233:104–110. doi: 10.1016/j.jss.2018.07.049. [DOI] [PubMed] [Google Scholar]
- [50].Wang X, Khalil RA. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Advances in Pharmacology Vascular Pharmacology: Cytoskeleton and Extracellular Matrix. 2018:241–330. doi: 10.1016/bs.apha.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Miyahara T, Runge S, Chatterjee A, et al. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. The FASEB Journal. 2013;27(6):2220–2232. doi: 10.1096/fj.12-225615.** SPM (LXA4 and RvD1) mechanism in NIH works through ALX/FPR2 receptor – best known receptor signaling pathway of SPMs with NIH to date, may explain others.
- [52].Petri MH, Laguna-Fernandez A, Tseng C- N, Hedin U, Perretti M, Bäck M. Aspirin-triggered 15-epi-lipoxin A4 signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation. International Journal of Cardiology. 2015;179:370–372. doi: 10.1016/j.ijcard.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. The FASEB Journal. 2015;29(6):2504–2513. doi: 10.1096/fj.14-265363.**Macrophage (M1 to M2) phenotypic switching is another pertinent component of NIH resolution, and may require reduction in PMN recruitment.
- [54].Liu G, Gong Y, Zhang R, et al. Resolvin E1 attenuates injury-induced vascular neointimal formation by inhibition of inflammatory responses and vascular smooth muscle cell migration. The FASEB Journal. March 2018. doi: 10.1096/fj.201800173r. [DOI] [PubMed] [Google Scholar]
- [55].Chiang N, Rosa XDL, Libreros S, Serhan CN. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. The Journal of Immunology. 2016;198(2):842–851. doi: 10.4049/jimmunol.1601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wu B, Mottola G, Chatterjee A, et al. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. Journal of Vascular Surgery. 2017;65(1). doi: 10.1016/j.jvs.2016.01.030.** This article represents the first inquiry into SPM pharmacokinetics with NIH – evidence for potentially better safety profile and without device or implant burden.
- [57].Kerimoglu O, Alarcin E. Poly(Lactic-Co-Glycolic Acid) Based Drug Delivery Devices For Tissue Engineering And Regenerative Medicine. ANKEM Dergisi. 2012;26(2):86–98. doi: 10.5222/ankem.2012.086. [DOI] [Google Scholar]
- [58].Wang GJ. Regulation of Vein Graft Hyperplasia by Survivin, an Inhibitor of Apoptosis Protein. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(10):2081–2087. doi: 10.1161/01.atv.0000183885.66153.8a. [DOI] [PubMed] [Google Scholar]
- [59].Ostrovsky G Angiotech suspends Vascular Wrap trial enrollment. Medgadget 2008 [Google Scholar]
- [60].Steenman M, Espitia O, Maurel B, et al. Identification of genomic differences among peripheral arterial beds in atherosclerotic and healthy arteries. Scientific Reports. 2018;8(1). doi: 10.1038/s41598-018-22292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]