Abstract
Prader–Willi syndrome (PWS) is a rare genetic disorder associated with distinct abnormal behaviors including hyperphagia, profound social deficits, and obsessive-compulsive tendencies. PWS males showed reduced oxytocin receptor (OTR) gene expression and density in the hypothalamic paraventricular nucleus that may play a role in PWS psychopathology. Oxytocin is an anorexigenic neuropeptide similar to vasopressin that is associated with social cognition and obsessive-compulsive behavior. To evaluate oxytocin biology in PWS, we examined overnight fasting plasma oxytocin levels in 23 children with PWS (mean SD age: 8.2 ± 2.0 year) having genetic confirmation and 18 age matched healthy unrelated siblings without PWS (mean ± SD age: 8.2 ± 2.3 year) and a similar gender ratio under the same clinical assessments, specimen processing and laboratory conditions. Multiplex immune assays were carried out using the Milliplex Human Neuropeptide Magnetic panel and the Luminex system. Natural log-transformed oxytocin levels were analyzed using general linear model adjusting for diagnosis, gender, age and body mass index (BMI). Oxytocin plasma levels were significantly elevated in children with PWS (168 ± 121 pg/ml) compared with unrelated and unaffected siblings without the diagnosis of PWS (64.8 ± 83.8 pg/ml, F = 8.8, P < 0.01) and the diagnosis of PWS predicted oxytocin level (F = 9.5, P < 0.003) in controlled regression analysis with an overall model fit R2 = 0.33 (P < 0.01). The symptoms of hyperphagia, anxiety and repetitive behaviors classically seen in PWS may be related to the disruption of oxytocin responsivity or feedback in the hypothalamic paraventricular nucleus possibly influencing vasopressin signaling. Further study is needed to characterize oxytocin function in PWS.
Keywords: oxytocin, neuropeptide, children with Prader–Willi syndrome, healthy unrelated siblings, abnormal eating and social behavior
INTRODUCTION
Prader–Willi syndrome (PWS) is a rare neurodevelopmental disorder due to errors in genomic imprinting caused by absent expression of paternally active genes on chromosome 15q11.2–q13 region. Diagnostic criteria for PWS in children include a history of hypotonia with a poor suck and feeding difficulties, global developmental delay, hypogonadism/hypogenitalism, growth hormone deficiency with short stature and small hands/feet and excessive eating with central obesity, if uncontrolled during early childhood. Additional clinical features include mental deficiency, hypothalamic hypogonadism, and behavioral problems including, but not limited to self-injury, temper tantrums, poor social skills, and obsessive-compulsive behaviors [Butler, 1990, 2011; Holm et al., 1993; Gunay-Aygun et al., 2001; Butler et al., 2006; Cassidy et al., 2011].
PWS affects approximately 1 in 20,000 individuals and is commonly caused by a de novo deletion of the proximal long arm of the paternal chromosome 15 in 70% of the cases or maternal disomy 15 in 25% with both chromosome 15s from the mother [Nicholls et al., 1989; Butler, 1990, Bittel and Butler, 2005, Butler et al., 2006, Cassidy et al., 2011]. The remaining individuals with PWS are caused by defects (i.e., epimutations or microdeletions) in the genomic imprinting center [Ohta et al., 1999] and other chromosome 15 rearrangements or translocations [Butler, 1990; Butler and Thompson, 2000; Bittel and Butler, 2005]. Individuals with PWS caused by paternal chromosome 15q11–q13 deletions often have more cognitive and behavioral problems and a lower incidence of autism spectrum disorder (ASD) compared to individuals with PWS with maternal disomy 15 [Roof et al., 2000; Butler et al., 2004, 2006; Bittel et al., 2006; Ogata et al., 2014].
While infants with PWS display characteristic clinical features of hypotonia, decreased feeding activity, and failure to thrive [Butler, 1990; Butler et al., 2006; Lu et al., 2014], young children with PWS exhibit hyperphagia, which can lead to childhood obesity, if not properly managed. Hypothalamic dysfunction, which is the most likely cause of hyperphagia, results in decreased growth and thyroid-stimulating hormone deficiencies in affected individuals [Butler et al., 2006; Elena et al., 2012]. In addition to an insatiable appetite, hypogonadism and short stature among patients with PWS further suggest abnormalities of the hypothalamic-pituitary axis. Early morbid obesity, short stature, and small hands and feet are associated with growth hormone (GH) deficiency. When individuals with PWS are given GH treatment, improvements are seen with increased muscle size and decreased fat mass [Carrel et al., 2004; Butler et al., 2006; Sode-Carlsen et al., 2010; Cadoudal et al., 2014]. In addition to their characteristic physical features, individuals with PWS also have dysfunctional behavioral patterns. Their inability to control emotion is linked to stereotypic behavior and temper tantrums. These behaviors inhibit normal socialization and affected individuals often suffer from poor relationships with their peers [Tauber et al., 2011]. It is theorized that the neuropeptide oxytocin plays a significant role in modulating behavior, and therefore may be an important factor in treatment of PWS.
Neuropeptides are chains of linked amino acids produced in the brain, formed from the cleavage of larger polypeptides [Swaab, 2004]. Oxytocin is a nine amino acid peptide produced by the hypothalamus and stored and secreted by the posterior pituitary gland. The biologically active form is an oxidized octapeptide (oxytocin disulfide) but it may also exist in a reduced dithiol nonapeptide form called oxytoceine [duVigneaud, 1960]. In addition to its better-known reproductive effects on the uterus and the breast, oxytocin acts within the central nervous system as an anorexigenic signal to decrease food intake. It regulates reward-driven energy intake and hypothalamic-pituitary-adrenal axis activity in humans [Ott et al., 2013]. Central administration of oxytocin can decrease food intake and meal duration in animal models and limit the intake of more palatable food by inhibiting the reward pathway [Sabatier et al., 2013]. Oxytocin is also thought to act on neurons in the nucleus tractus solitarius to modulate the neurotensin response to peripheral satiety signals [Parker and Bloom, 2012]. High plasma neurotensin levels have recently been reported in PWS children in comparison with healthy unrelated children [Butler et al., 2015].
Long-term administration of oxytocin reduces body weight and food intake in diet-induced obesity and in genetic obese animal models [Blevins and Ho, 2013]. Increased oxytocin levels have also been positively associated with improved social cognition, facial recognition, and communication [Donaldson and Young, 2008]. Increased oxytocin levels have also been reported in behavioral disorders and correlated with severity measures [Leckman et al., 1994; Taurines et al., 2014]. A deficit in the expression of oxytocin receptor (OXTR) gene was reported using a whole genome micro-array expression analysis of RNA isolated from lymphoblasts in PWS males [Bittel et al., 2007]. In addition, individuals with PWS were also found to have significantly decreased numbers of oxytocin (OXT) expressing neurons representing an associated decrease in the total volume of the paraventricular nucleus containing the OXT-expressing neurons [Swaab et al., 1995]. The full implication of these findings on individuals with PWS has yet to be understood, but may explain select traits and behaviors seen in this syndrome.
Understanding the significance of oxytocin in individuals with PWS may be an important step in developing new treatments for the significant health and behavioral complications associated with this rare obesity related genetic disorder. In order to further appreciate the role this neuropeptide may play in the hypothalamic dysfunction seen in PWS, we examined plasma oxytocin levels in 23 children with PWS between the ages of 5 and 11 years and compared with 18 healthy unrelated siblings matched for age with a similar gender ratio.
MATERIALS AND METHODS
Subjects
All forty-one subjects in this study were recruited using signed consent forms approved by the local human subjects committee from a large, ongoing multi-site rare disease consortium on PWS in the USA carried out with oversight from the Institutional Review Board of the University at Kansas Medical Center and the University of Florida School of Medicine. Morning fasting peripheral blood samples were collected in EDTA vacutainer tubes and plasma separated immediately then frozen at −80° until use. Blood was collected from ten females (mean age ± SD = 8.46 years ± 1.94 years; age range = 5–11 years) and 13 males (mean age ± SD = 7.70 years ± 1.91 years; age range 5–11 years) with PWS. The children with PWS were diagnosed clinically and confirmed using genetic testing protocols of DNA methylation, chromosomal microarray, methylation specific-multiplex ligation probe amplification (MS-MLPA), genotyping of informative chromosome 15 DNA markers, and/or chromosome analyses with fluorescence in situ hybridization (FISH). Fifteen of the children with PWS had the 15q11–q13 deletion and eight had maternal disomy 15. Control children were selected from a pool of unaffected siblings of families where at least one child has PWS but data from that affected child was not included in the current sample. Unaffected siblings absent the PWS 15q11–q13 methylation abnormality should be representative of the general population. The control group consisted of eight healthy unrelated female siblings (mean age ± SD = 8.19 years ± 2.07 years; age range 5–11 years) and ten healthy unrelated male siblings (mean age ± SD = 8.27 years ± 2.52 years; age range = 5–11 years). All children were Causcasian Americans and those with PWS were prescribed growth hormone. All PWS study participants received dietary intervention with caloric restriction and participated in daily exercise programs (e.g., 30 min walking per day) for weight control and caloric maintenance. No child received sex steroids or treatment for adrenal insufficiency; four of the children with PWS were insulin resistant and three were being treated for hypothyroidism. Height (cm) and weight (kg) were obtained for each individual using standing stadiometers and calibrated electronic weight balances in the clinical setting; body mass index (BMI) was calculated from growth data. Body composition and body-fat percentages were determined using dual-energy X-ray absorptiometry (DXA) and the Lunar DXA Scanner (General Electric, Atlanta, GA).
Oxytocin Assay and Analysis
Plasma levels of oxytocin were determined using the multiplex sandwich immunoassays with the Milliplex Human Neuropeptide Kit (Millipore; Billlerica, MA) and the Luminex 200TM magnetic-based instrument (Luminex Molecular Diagnostic; Toronto, ON) following established protocols. Twenty-five micro liter of plasma, concentration standard, or Milliplex quality control standard were combined with assay buffer and 25 μl of pre-mixed antibody-coupled magnetic beads for overnight incubation at 4°C. The plate was then foil-wrapped and incubated with agitation on a plate shaker for 2 hr at room temperature (20–25°C). The next day, the plates were washed, detection antibodies added, and then incubated at room temperature for 1 hr. Streptavidin–Phycoerythrin was added and the plate covered, incubated and agitated on a plate shaker for 30 min. After incubation, the plates were washed again and 100 μl of Sheath Fluid added to each sample well. The plate was resuspended on a plate shaker for 5 min at room temperature and then read on the Luminex®200™with xPONENT software based on magnetic-bead technology and level of magnetic field to separate the beads. Median fluorescent intensity data were analyzed using a weight 5-parameter curve-fitting method for calculating analyte concentrations in the sample wells. The minimum detectable concentration level for oxytocin was 9.35 pg/ml. Plasma oxytocin levels were calculated using a standard curve derived from reference oxytocin concentration standards provided by the manufacturer. The inter-assay coefficient of variation for oxytocin levels ranged from 0% to 20% with an intra-assay coefficient of variation ranging from 0% to 10%. Plasma samples were then analyzed and blinded to gender and control versus PWS during each assay run.
Statistical Analysis
Data were presented as mean and/or median ± standard deviation of raw and/or natural log-transformed oxytocin levels by diagnosis (PWS or unrelated siblings). Natural log-transformed oxytocin levels were utilized to meet normality criteria required for analysis using simple analysis of variance (ANOVA) and general linear model adjusting for diagnosis, gender, age and body mass index (BMI). Data falling below detection limits of the Milliplex assay were replaced with one half of the minimum detectable level of oxytocin (4.675 pg/ml) as reported in previous studies using this methodology [Ashwood et al., 2010; Manzardo et al., 2012]. Final transformed data met statistical criteria for the assumption of normality with equal variance and near linear residual plots. The frequency of oxytocin levels below detection limits were statistically analyzed by PWS diagnosis and gender using Fisher Exact Test. Statistical analyses and descriptive statistics were generated with SAS statistical analysis software version 0.4 (SAS Inc., Cary, NC) with P-values <0.05 were considered significant.
RESULTS
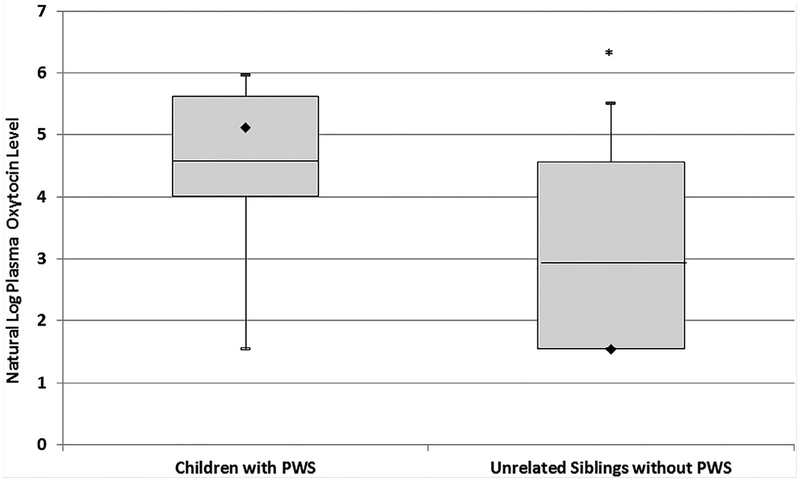
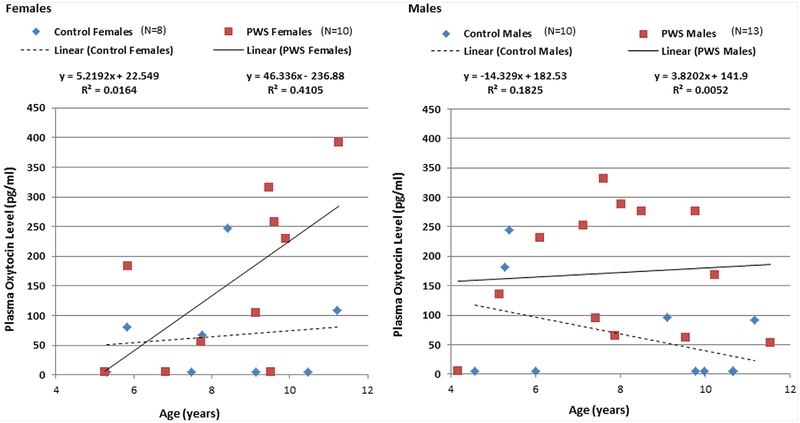
A significant elevation in plasma oxytocin levels was found between the cohort of 23 children with PWS (167 ± 121; range 4.68–391 pg/ml) when compared to the 18 healthy unrelated siblings of children with PWS (64.8 ± 83.8; range 4.68–247 pg/ml, F = 8.8, P < 0.01, see Table I, Fig. 1). PWS individuals also had significantly higher total body-fat than control subjects but did not differ in age, BMI or BMI z-scores (Table I). The PWS diagnosis predicted the oxytocin level (F = 9.5, P < 0.003) in controlled regression analyses with an overall model fit of R2= 0.33 (P < 0.01). Age (F = 2.1, P < 0.16), gender (F = 3.6, P < 0.07), BMI (F = 3.9, P < 0.06) and the interaction between age and gender (F = 3.3, P < 0.08) failed to meet significance criteria as predictors in these analyses (see Table II). A statistically significant correlation was observed between oxytocin level and age in PWS females (r 0.68, P < 0.03) which was not observed for control females, PWS or control males (see Fig. 2). No other correlations were noted between oxytocin levels and body-fat content, BMI or PWS genetic subtype. Healthy unrelated siblings had a statistically significant probability of having oxytocin levels at or below the minimum level of detection for the assay (N = 10) compared to PWS subjects (N = 4) (P < 0.02, odds ratio= 5.9, 95%CI: 1.4–24.6). This relationship did not differ by gender.
TABLE I.
Baseline Characteristics and Plasma Oxytocin Levels in Prader–Willi Syndrome and Healthy Unrelated Control Subjects
| Prader-Willi syndrome (N = 23) | Control subjects (N = 18) | |||
|---|---|---|---|---|
| Characteristic | [mean±SD (range)] | [mean ± SD (range)] | F-value | P-value |
| Age | 8.2 ± 2.0 year (5–11 year) | 8.2 ± 2.3 year (5–11 year) | 0.07 | 0.79 |
| BMI | 20.7 ± 5.0 (l4–28) | 18.2 ± 3.3 (15–25) | 2.2 | 0.15 |
| BMI z-score | 0.96 ± 1.4 (13–32) | 0.52 ± 1.35 (12–26) | 1.1 | 0.31 |
| Percent body fat | 33 ± 13% (13–54%) | 24 ± 10% (10–47%) | 5.5 | 0.02* |
| Oxytocin levels [mean pg/ml ± SD (range, N)] | ||||
| All subjects | 167 ± 121 (4.68–391, N = 23) | 64.8 ± 83.8 (4.68–247, N = 18) | 8.8 | 0.01* |
| Female subjects (N = 18) | 155 ± 141 (4.68–391, N = 10) | 65.3 ± 84.4 (4.68–247, N = 8) | 1.4 | 0.26 |
| Male subjects (N = 23) | 172 ± 109 (4.68–332, N = 13) | 64.3 ± 87.9 (4.68–245, N = 10) | 9.3 | 0.01* |
Indicated statistical significance.
FIG. 1.
Distribution of plasma oxytocin levels for individuals with Prader–Willi syndrome (PWS) and healthy unrelated sibling control children. Box plots represent mean (line), median (diamond), and interquartile ranges (25% and 75%). Error bars indicate maximum and minimum values form natural log-transformed data for each subject group. *P-value <0.05.
TABLE II.
Linear Regression Model of Natural Log Plasma Oxytocin Level
| F-value | P-value | |
|---|---|---|
| Diagnosis | 10.5 (df = 1) | 0.003* |
| Gender | 3.6 (df = 1) | 0.07 |
| Age | 2.1 (df = 1) | 0.16 |
| BMI | 3.9 (df = 1) | 0.06 |
| Age* gender | 3.3 (df = 1) | 0.08 |
| Overall model fit (R2 = 0.33) | 3.4 (df = 5,35) | 0.01* |
Indicated statistical significance.
FIG. 2.
Correlation between oxytocin level and age by gender in Prader–Willi syndrome (PWS) and healthy unrelated sibling control children. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmga].
DISCUSSION
Previous research has highlighted the importance of the neuropeptide oxytocin and its numerous effects in biology, pharmacology, and social behavior but a paucity of data exists in PWS particularly in relationship to plasma levels and correlations with genetic subtype, age, gender, and body composition measures. Reported age and sex effects in oxytocin biology including the presence and direction of sex differences in oxytocin plasma levels are inconsistent and remain inconclusive. Miller et al. [2013] reported higher plasma oxytocin concentrations in adolescent girls compared to boys, but the majority of studies found no sex difference in oxytocin levels in adults. This is similar to our observations between male and female control children. The posterior pituitary gland releases oxytocin in response to stimulation by birthing, nursing, stress, eating, and sex leading to a great deal of individual variability in oxytocin levels. Low levels of oxytocin are also produced by peripheral tissues such as muscle and fat which may also vary by age [Swaab, 2004; Harony and Wagner, 2010].
In this study, we measured morning fasting plasma levels of oxytocin in a cohort of 23 children with PWS and compared with 18 healthy unrelated siblings matched for age with similar gender ratio and no significant differences in BMI. The children with PWS were found to have significantly increased levels of plasma oxytocin (167.71 pg/ml ± 121.36) as compared to unrelated siblings (64.78 pg/ml ± 83.84). This finding is consistent with a study conducted by Martin et al. [1998] who reported elevated CSF oxytocin levels in PWS. Plasma oxytocin levels are significantly and positively correlated with CSF oxytocin levels, suggesting that plasma oxytocin levels may act as a surrogate for central nervous system measures of oxytocin activity [Carson et al., 2014]. We also observed an age-related increase in plasma oxytocin levels among females with PWS. The basis for the association is unclear but was not related to body-fat content or BMI. Changes in hormone status or peripheral oxytocin synthesis at the tissue level due to increased muscle or bone mass or possibly secondary to growth hormone exposure may have contributed to this change, but further studies are needed to ascertain the relevance of this observation.
Increased oxytocin levels have been reported in individuals with obsessive-compulsive disorder (OCD), a disorder that shares many common behavioral traits with individuals with PWS. Furthermore, Leckman et al. [1994] reported a positive correlation between cerebral spinal fluid oxytocin levels and OCD severity. Individuals with PWS show similar levels of symptom severity and number of compulsions as seen in non-PWS individuals with normal intelligence and OCD [Dykens et al., 1996]. A recent study measuring plasma oxytocin levels in patients with autism also found increased levels when compared to normally developing boys and in boys with ADHD [Taurines et al., 2014]. Furthermore, Greaves et al. [2006] reported that children with PWS and children with autism spectrum disorder displayed similar levels of repetitive and ritualistic behavior, extending beyond behavior limited to food. Behavioral problems, similar to those found in pervasive developmental disorder, are often seen in children with PWS [Greaves et al., 2006].
The increased oxytocin levels found in our children with PWS are in contrast to an oxytocin’s known role as an anorexigenic peptide such as following exogenous oxytocin administration in animal models, the volume of food consumption and time spent eating were both reduced [Arletti et al., 1989]. Oxytocin has also been positively associated with enhanced social behavior; consequently our finding of elevated levels in children with PWS would be unexpected in the presence of normal hypothalamic function and oxytocin receptor expression, which appears not to be the case in PWS. High levels of oxytocin have also been associated with increased facial processing and human interpersonal communication, a marked deficit in individuals with PWS. Furthermore, intranasal oxytocin administration leads to an enhanced ability to interpret facial cues and to determine the affective state of other individuals [Domes et al., 2007]. A separate randomized, double-blind trial involving the administration of intranasal oxytocin into adults with autism spectrum disorder showed significant improvements in social cognition and quality of life after 6 weeks duration [Anagnostou et al., 2012]. Given the pronounced communication and social deficits found in individuals with PWS, the high levels of oxytocin found in this study may be failing to achieve such normal physiological effect.
Despite elevated levels of oxytocin in our children with PWS, a significant deficit in oxytocin receptors and reduction in the volume of hypothalamic paraventricular nuclei-containing oxytocin-expressing neurons in PWS have been reported [Swaab et al., 1995], as well as whole genome microarray expression analysis of lymphoblasts from males with PWS showing significantly reduced expression of the oxytocin receptor (OXTR) gene [Bittel et al., 2007]. Gregory et al. [2009] further showed that the OXTR gene was hypermethylated in individuals with autism when compared with normal control subjects resulting in decreased levels of OXTR expression in the temporal cortex. Classical autism or ASD are reported components of a subset of children with PWS [Butler et al., 2006].
A reduced number of oxytocin receptors may lead to increased oxytocin secretion by the posterior pituitary due to loss of negative feedback. Human oxytocin (OXT) and arginine vasopressin (AVP) genes are both linked on chromosome 20p13, separated by 12 kilobases of DNA. Though they each have specific receptors, their close evolutionary relationship allows for interacting molecular systems and binding of OXT to the AVP receptor, and vice-versa [Francis et al., 2014]. Upregulation of oxytocin is necessary in order to maintain percent saturation and physiological oxytocin binding results. At high levels, as seen with our patient cohort, oxytocin may stimulate AVP receptors and produce the behavioral symptoms associated with vasopressin [Cho et al., 1999]. Stimulation of the AVP receptor in the paraventricular nucleus of the hypothalamus has been associated with increased levels of anxiety and arousal, in contrast to oxytocin, which typically induces relaxation and decreased anxiety [Domes et al., 2007, Harony and Wagner, 2010]. Increased AVP levels have also been linked to restrictive and repetitive behaviors in female children with autism spectrum disorder [Miller et al., 2013]. Interestingly, the same study also found a positive correlation between oxytocin levels and anxiety in these children. Due to OXTR deficits reported in PWS and elevated levels of oxytocin found in our PWS cohort, the expected effects of this peptide (i.e., decreased feeding and increased social functionality) may not be present. This reduced activity of oxytocin neurons in the paraventircular nucleus may account for hyperphagia and lack of satiety, common in PWS [Swaab, 2004]. If a significant reduction in oxytocin receptors leads to deficient oxytocin activity, then high plasma levels of oxytocin found in our study may not be as counter-intuitive as previously imagined. The results of this study support the hypothesis that the dysregulation of the oxytocin and AVP system in humans leads in marked changes in behavior including hyperphagia, social deficits, and increased anxiety, common in PWS.
The strengths of our study included that the laboratory conditions and data analysis were the same for all subjects and the PWS and control children cohorts had a similar age and gender ratio with no significant differences in BMI. All children with PWS included in this study were genetically confirmed. The findings of this study must be evaluated in the context of methodological limitations and inability to repeat the oxytocin measures with a second assay such as ELISA due to the small sample quantity of plasma available for experimentation which is often the case in the study of children with rare diseases. The multiplex method shows high sensitivity and good selectivity for oxytocin with minimal to no cross-reactivity to similar neuropeptides such as orexin A (0% cross-reactivity) and vasopressin (<1% cross-reactivity). Radioimmunoassay (RIA) methods combined with high pressure liquid chromatography or mass spectroscopy separation have been shown to discriminate the active oxidized (disulfide) form of oxytocin from the inactive oxytoceine nonapeptide more effectively than enzyme immunoassay methods [Szeto et al., 2011; McCullough et al., 2013]. The Luminex assay employs a capture antibody technique combined with biotinylated detection antibodies and has not been directly compared to results obtained by standard RIA.
Furthermore, most of our PWS participants were undergoing growth hormone treatment at the time of sampling which is standard therapy to improve stature, increase lean body mass and improve outcomes in PWS. Sparse and inconsistent data are available regarding possible cross-reactivity between oxytocin and growth hormone biology. In vitro growth hormone dose dependently stimulated oxytocin release in cultured bovine granulosa [Sirotkin and Nitray, 1994] while oxytocin dose dependently inhibited growth hormone release in cell cultures of rat anterior pituitary [Hulting et al., 1996]. A more recent study of humans found no relationship between exogenous oxytocin with ghrelin administration on growth hormone levels in normal males [Coiro et al., 2011]. More research is needed to fully characterize the obviously complex relationship between these neuropeptide and hormone levels. Additional weaknesses in our study include the small subject sample size, high variability in measured levels and lack of obsessive-compulsive, anxiety, cognitive and hyperphagia symptom quantification data per individual to correlate with the oxytocin levels. It is not possible to draw definitive connections between increased neuropeptide levels and expected behavior patterns due to limited access to our PWS subject cohort over time which would require long-term follow-up research studies.
An increased number of well-characterized subjects are needed for study to replicate our observations and for a longer period of time with analysis and quantification of hyperphagia, anxiety and other related symptomatology data that may relate to abnormal oxytocin levels. If behaviors in PWS are explained by incomplete oxytocin receptor activation or overstimulation of arginine vasopressin, then additional research should be performed to better understand the complex dysregulation and physiological compensation taking place leading to treatment modalities.
ACKNOWLEDGMENTS
We acknowledge the financial support of Prader–Willi Syndrome Association (USA) and the Angelman, Rett and Prader–Willi Syndromes Consortium (U54 HD06122) which is part of the National Institute of Health (NIH) and Rare Disease Clinical Research Network (RDCRN) supported through collaboration between the NIH Office of Rare Disease Research (ORDR) at the National Center of Advancing Translational Science (NCATS) and the National Institute of Child Health and Human Development (NICHD).
Grant sponsor: National Institute of Health.
Footnotes
Conflict of interest: None.
REFERENCES
- Anagnostou EL, Soorya W, Chaplin J, Bartz D, Halpern S, Wasserman AT, Wang L, Pepa N, Tanel A, Kushki A, Hollander E. 2012. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: A randomized controlled trial. Mol Autism 3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A. 1989. Influence of oxytocin on feeding behavior in the rat. Peptides 10:89–93. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Nguyen DV, Hessl D, Hagerman RJ, Tassone F. 2010. Plasma cytokine profiles in Fragile X subjects: Is there a role for cytokines in the pathogenesis. Brain Behav Immuno 24:898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Butler MG. 2005. Prader–Willi syndrome: Clinical genetics, cytogenetics, and molecular biology. Expert Rev Mol Med 7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Butler MG. 2006. Expression of four genes between chromosome 15 breakpoints 1 and 2 and behavioral outcomes in Prader–Willi syndrome. Pediatrics 118:e1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Sell SM, Strong TV, Butler MG. 2007. Whole genome microarray analysis of gene expression in Prader–Willi syndrome. Am J Med Genet Part A 143A:430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Ho JM. 2013. Role of oxytocin signaling in the regulation of body weight. Rev Endocr Metab Disord 14:311–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. 1990. Prader–Willi syndrome: Current understanding of cause and diagnosis. Am J Med Genet 35:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Thompson T. 2000. Prader–Willi syndrome: Clinical and genetic findings. The Endocrinol 10:3S–16S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Bittel DC, Kibiryeva N, Talebizadeh Z, Thompson T. 2004. Behavioral differences among subjects with Prader–Willi syndrome and type I or type II deletion and maternal disomy. Pediatrics 113:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Lee PDK, Whitman BY, eds. 2006. Management of Prader–Willi syndrome 3rd edition. New York: Springer. [Google Scholar]
- Butler MG. 2011. Prader–Willi syndrome: Obesity due to genomic imprinting. Curr Genomics 12:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Nelson TA, Driscoll DJ, Manzardo AM. 2015. High plasma neurotensin levels in children with Prader–Willi syndrome. Am J Med Genet Part A 167A:1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoudal T, Buléon M, Sengenès C, Diene G, Desneulin F, Molinas C, Eddiry S, Conte-Auriol F, Daviaud D, Martin PG, Bouloumié A, Salles JP, Tauber M, Valet P. 2014. Impairment of adipose tissue in Prader–Willi syndrome rescued by growth hormone treatment. Int J Obes (Lond) 38:1234–1240. [DOI] [PubMed] [Google Scholar]
- Carrel AL, Moerchen V, Myers SE, Bekx MT, Whitman BY, Allen DB. 2004. Growth hormone improves mobility and body composition in infants and toddlers with Prader–Willi syndrome. J Pediatr 145:744–749. [DOI] [PubMed] [Google Scholar]
- Carson DS, Berquist SW, Trujillo TH, Garner JP, Hannah SL, Hyde SA, Sumiyoshi RD, Jackson LP, Moss JK, Strehlow MC, Cheshier SH, Partap S, Hardan AY, Parker KJ. 2014. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol Psychiatry 20:1085–1090. [DOI] [PubMed] [Google Scholar]
- Cassidy SB, Schwartz S, Miller JL, Driscoll SJ. 2011. Prader–Willi syndrome. Genet Med 14:10–26. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. 1999. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav Neurosci 113:1071–1079. [DOI] [PubMed] [Google Scholar]
- Coiro V, Volpi R, Stella A, Cataldo S, Chiodera P. 2011. Oxytocin does not modify GH, ACTH, cortisol, and prolactin responses to ghrelin in normal men. Neuropeptides 45:139–142. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. 2007. Oxytocin improves “mind-reading” in humans. Biol Psychiatry 61:731–733. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. 2008. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322:900–904. [DOI] [PubMed] [Google Scholar]
- duVigneaud V 1960. Experiences in the polypeptide field: Insulin to oxytocin. Ann N Y Acad Sci 88:537–548. [Google Scholar]
- Dykens EM, Leckman JF, Cassidy SB. 1996. Obsessions and compulsions in Prader–Willi syndrome. J Child Psychol Psychiatry 37:995–1002. [DOI] [PubMed] [Google Scholar]
- Elena G,Bruna C,Benedetta M,Stefania DC,Giuseppe C.2012Prader–Willi syndrome: Clinical aspects. J Obes 2012:473941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SM, Sagar A, Levin-Decanini T, Liu W, Carter CS, Jacob S. 2014. Oxytocin and vasopressin systems in genetic syndromes and neuro-developmental disorders. Brain Res 1580:199–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves N, Prince E, Evans DW, Charman T. 2006. Repetitive and ritualistic behaviour in children with Prader–Willi syndrome and children with autism. J Intellect Disabil Res 50:92–100. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, Langford CF, Worley G, Delong GR, Murphy SK, Cuccaro ML, Persico A, Pericak-Vance MA. 2009. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med 7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunay-Aygun M, Schwartz S, Heeger S, O’Riordan MA, Cassidy SB. 2001. The changing purpose of Prader–Willi syndrome clinical diagnostic criteria and proposed revised criteria. Pediatrics 108:E92. [DOI] [PubMed] [Google Scholar]
- Harony H, Wagner S. 2010. The contribution of oxytocin and vasopressin to mammalian social behavior: Potential role in autism spectrum disorder. Neurosignals 18:82–97. [DOI] [PubMed] [Google Scholar]
- Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F. 1993. Prader–Willi syndrome: Consensus diagnostic criteria. Pediatrics. 91:398–402. [PMC free article] [PubMed] [Google Scholar]
- Hulting AL, Grenbäck E, Pineda J, Coya R, Hökfelt T, Meister B, Uvnäs-Moberg K. 1996. Effect of oxytocin on growth hormone release in vitro. Regul Pept. 67:69–73. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, Anderson GM, Riddle MA, McSwiggan-Hardin M, McDougle CJ, Barr LC, Cohen DJ. 1994. Elevated cerebrospinal fluid levels of oxytocin in obsessive-compulsive disorder. Comparison with Tourette’s syndrome and healthy controls. Arch Gen Psychiatry 51:782–792. [DOI] [PubMed] [Google Scholar]
- Lu W, Qi Y, Cui B, Chen XL, Wu BB, Chen C, Cao Y, Zhou WH, Xu H, Luo FH. 2014. Clinical and genetic features of Prader–Willi syndrome in China. Eur J Pediatr 173:81–86. [DOI] [PubMed] [Google Scholar]
- Manzardo AM, Henkhaus R, Dhillon S, Butler MG. 2012. Plasma cytokine levels in children with autistic disorder and unrelated siblings. Int J Dev Neurosci 30:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, State M, Anderson GM, Kaye WM, Hanchett JM, McConaha CW, North WG, Leckman JF. 1998. Cerebrospinal fluid levels of oxytocin in Prader–Willi syndrome: A preliminary report. Biological Psychiatry 44:1349–1352. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Churchland PS, Mendez AJ. 2013. Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev 37:1485–1492. [DOI] [PubMed] [Google Scholar]
- Miller M, Bales KL, Taylor SL, Yoon J, Hostetler CM, Carter CS, Solomon M. 2013. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: Sex differences and associations with symptoms. Autism Res 6:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls RD, Knoll JH, Butler MG, Karam S, Lalande M. 1989. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader–Willi syndrome. Nature 342:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H, Ihara H, Murakami N, Gito M, Kido Y, Nagai T. 2014. Autism spectrum disorders and hyperactive/impulsive behaviors in Japanese patients with Prader–Willi syndrome: A comparison between maternal uniparental disomy and deletion cases. Am J Med Genet Part A 164A:2180–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Gray TA, Rogan PK, Buiting K, Gabriel JM, Saitoh S, Muralidhar B, Bilienska B, Krajewska-Walasek M, Driscoll DJ, Horsthemke B, Butler MG, Nicholls RD. 1999. Imprinting-mutation mechanisms in Prader–Willi syndrome. Am J Hum Genet 64:397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, Hallschmid M. 2013. Oxytocin reduces reward-driven food intake in humans. Diabetes 62:3418–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JA, Bloom SR. 2012. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 63:18–30. [DOI] [PubMed] [Google Scholar]
- Roof E, Stone W, MacLean W, Feurer ID, Thompson T, Butler MG. 2000. Intellectual characteristics of Prader–Willi syndrome: Comparison of genetic subtypes. J Intellect Disabil Res 44:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Leng G, Menzies J. 2013. Oxytocin, feeding, and satiety. Front Endocrinol (Lausanne) 4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin AV, Nitray J. 1994. Growth hormone and prolactin affect oxytocin, vasopressin, progesterone, and cyclic nucleotide secretion by bovine granulosa cells in vitro. J Endocrinol 143:417–422. [DOI] [PubMed] [Google Scholar]
- Sode-Carlsen R, Farholt S, Rabben KF, Bollerslev J, Schreiner T, Jurik AG, Christiansen JS, Höybye C. 2010. One year of growth hormone treatment in adults with Prader–Willi syndrome improves body composition: Results from a randomized, placebo-controlled study. J Clin Endocrinol Metab 95:4943–4950. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Purba JS, Hofman MA. 1995. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader–Willi syndrome: A study of five cases. J Clin Endocrinol Metab 80:573–579. [DOI] [PubMed] [Google Scholar]
- Swaab DF. 2004. Neuropeptides in hypothalamic neuronal disorders. Int Rev Cytol 240:305–375. [DOI] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, Mendez AJ. 2011. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med 73:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber M, Mantoulan C, Copet P, Jauregui J, Demeer G, Diene G, Roge B, Laurier V,Ehlinger V,Arnaud C, Molinas C, Thuilleaux D. 2011. Oxytocin may be useful to increase trust in others and decrease disruptive behaviours in patients with Prader–Willi syndrome:A randomised placebo-controlled trial in 24 patients. Orphanet J Rare Dis 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurines R, Schwenck C, Lyttwin B, Schecklmann M, Jans T, Reefschlager L, Geissler J, Gerlach M, Romanos M. 2014. Oxytocin plasma concentrations in children and adolescents with autism spectrum disorder: Correlation with autistic symptomatology. Atten Defic Hyperact Disord 6:231–239. [DOI] [PubMed] [Google Scholar]