Abstract
Syndecans (SDCs) are a family of heparan sulfate proteoglycans (HSPGs) glycoproteins ubiquitously expressed on the cell surfaces and extracellular matrix of all mammalian tissues. There are four mammalian syndecans, SDC-1 thorough 4, which play a critical role in cell adhesion, migration, proliferation, differentiation, and angiogenesis through independent and growth factor mediated signaling. An altered expression of SDCs is often observed in autoimmune disorders, cancer, HIV infection, and many other pathological conditions. SDCs modulate disease progression by interacting with a diverse array of ligands, receptors, and other proteins, including extracellular matrix, glycoproteins, integrins, morphogens, and various growth factors and chemokines, along with their receptors and kinases. Specifically, SDCs present on cell surface can bind directly to chemokines to enhance their binding to receptors, downstream signaling, and migration. Alternatively, SDCs can be cleaved and shed to mediate negative regulation of chemokine and growth factor signaling pathways and ligand sequestration. Importantly, SDC shedding may be a biomarker of inflammation, especially in chronic inflammatory diseases. While the current therapies for cancer and several autoimmune disorders have revolutionized treatment outcomes, understanding the pathophysiological role of SDCs and the use of HSPG mimetic or antagonists on cytokine signaling networks may uncover potentially novel targeted therapeutic approaches. This review mainly summarizes the current findings on the role of individual SDCs in disease processes, mechanisms through which SDCs mediate their biological functions, and the possibility of targeting SDCs as future potential therapeutic approaches.
Keywords: autoimmune diseases, cancer, heparan sulfate proteoglycans, signaling pathways, syndecans, therapeutics
1 |. INTRODUCTION
Inflammation is an inherent response of the body’s immune system to an injury, exposure to viruses, bacteria, or xenobiotics. Under physiological conditions, inflammatory processes are tightly regulated by the immune system through a cascade of cellular networks to resolve acute inflammation and avoid tissue damage. However, chronic inflammation causes tissue damage that cannot be resolved by the immune system (Koch, 2005). Chronic inflammation is a characteristic feature of cancer and many other autoimmune disorders in which proinflammatory cytokines and chemokines play a vital role. In response to proinflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), the released chemokines employ their receptors to recruit leukocytes at the site of inflammation (Iwamoto, Okamoto, Toyama, & Momohara, 2008). Chemokines are small, secreted proteins classified into 4 subfamilies (C, CC, CXC, and CX3C) that differ in number of spacing of cysteine motif that direct cell migration in the development of immunity, inflammation, and cancer (Koch, 2005; Kufareva, 2016). They function by engaging G-protein coupled receptors (GPCR) on the surface of migrating cells thereby recruiting and developing gradients of monocytes, T lymphocytes, and many other leukocytes (Kufareva, 2016). Substantial evidence in recent years indicate that syndecans (SDCs), a family of transmembrane heparan sulfate proteoglycans (HSPGs), play a critical role in the pathological processes by interacting with a diverse array of ligands, including extracellular matrix (ECM) components, cytokines, chemokines, growth factors, and growth factor receptors (Kufareva, 2016). SDCs regulate cell adhesion, migration, proliferation, and differentiation through independent signaling mechanism as well. SDCs are processed by enzymes in the Golgi apparatus where the heparan sulfate (HS) chains are attached to the extracellular, N-terminal core protein by a number of serine residues and further modified by nucleotide sugars exported from the cytoplasm. SDCs constitutively modulate cell matrix adhesion by binding to heparan-binding motifs present in the ECM. Chemokines have a different mode of binding to HS than to GPCR. They oligomerize and fold in HS pockets due to strong electrostatic interaction and a unique HS sequence (Kufareva, 2016). In this regard, SDCs help build chemokine gradients on the luminal surfaces of endothelium while keeping them protected from proteolysis and facilitating an active transport of leukocytes toward inflammatory sites.
Furthermore, during inflammation, the expression of HSPGs is upregulated after activation of leukocytes by various cytokines. For example, human monocytes exposed to IL-1β rapidly express SDC-2, whereas unstimulated monocytes express little or no SDC-2 (Parish, 2006). Hence, the pro-inflammatory cytokines, chemokines, and SDCs functionally support each other to maintain an inflammatory microenvironment. This review mainly focuses on the importance of SDCs in inflammatory disorder and their potential as a therapeutic target.
1.1 |. Structural organization and function of SDCs
SDCs belong to a family of type I transmembrane HSPGs found in vertebrates. Almost all mammalian heparan bearing molecules that are expressed on cell surfaces, ECM, and basement membrane, are encoded by 13 genes (Iozzo, 2005). SDC-1 is the major SDC expressed at the basolateral surface of epithelial cells and in plasma cells, while SDC-2 is predominantly found in cells of mesenchymal origin. SDC-3 is primarily expressed by neuronal tissue, while SDC-4 is ubiquitously found in most tissues.
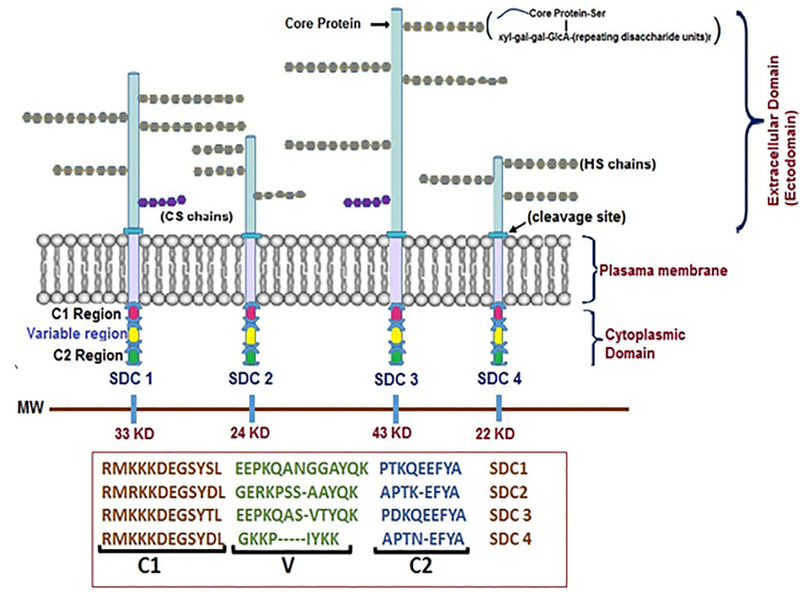
SDC core protein is comprised of extracellular, N-terminal domain, transmembrane domain, and cytoplasmic tail with two conserved, C-terminal domains (C1 and C2) with a flanking variable domain (V-domain) specific to each member of the SDC family (Afratis et al., 2017). The C1 region, located just beneath the membrane, is thought to interact with the cell cytoskeleton and c-Src proteins, while the C2 region, which contains a post-synaptic density-95/disc large protein/zonula occludens-1 or 2 (PDZ1 or PDZ2) domain, helps some SDCs with adaptor proteins to regulate downstream signaling and interaction with C-terminal EFYA sequence to mediate vesicular trafficking and exosome biogenesis (Baietti et al., 2012; Gao, Li, Chen, & Simons, 2000) (Figure 1). The V-region that is variable in all four known SDCs determines their role in signaling (Afratis et al., 2017). The transmembrane domain with GXXXG motif allows for a strong, SDS-resistant homodimerization of SDCs (Choi et al., 2005). The extracellular, N-terminal part of the core protein contains a variable number of serine residues to which all SDCs contain a common stem tetrasaccharide of xylose (xyl), two galactose units (gal), and a glucuronic acid (GlcA) residue followed by repeating disaccharide of HS glycosaminoglycans (GAGs) chains, which are N-acetyl-glucosamine (GlcNAc) and GlcA units. These side chains undergo several modifications in terms of sulfation and GlcA epimerization to L-iduronic acid (IdoA). The glucosamine units attached to SDCs can undergo N-, 6-O, 3-O sulfation, but IdoA is usually 2-O sulfated (Iozzo, 2005). In addition to HS chains on SDC core protein, chondroitin sulfate (CS) chains can also attach to SDC-1 and SDC-3 closer to the transmembrane domain through covalent bonds. Through these GAG chains and core protein, SDCs interact with a range of ECM molecules, growth factors, and chemokines, linking extracellular events to intracellular signaling.
FIGURE 1.
Structural organization of Syndecans. A schematic view of syndecan core protein and glycosaminoglycan chains. SDC-1 and SDC-3 core proteins are larger than SDC-2 and SDC-4 and in addition to heparan sulfate chains they also bear CS. The GAG chains are substituted on core protein serine residues and have common tetrasaccharide units attached to two units of galactose (gal) and GlcA residue with alternating units of GlcNAc and uronic acids. The HS chains undergo modification by sulfonation at 6-O or 3-O (rarely), CS 6-O, or 4-O while uronic acids undergo epimerization to IdoA. The cytoplasmic domain contains highly conserved regions (C1 and C2) with interceding variable (V) regions
Some of the proteins that can directly interact with SDC core proteins include transforming growth factor (TGF)-β, TGFβR, with betaglycan complex to facilitate TGF-β signaling (Mytilinaiou et al., 2013), and integrins and insulin-like growth factor-1 receptor to enhance adhesion (Beauvais & Rapraeger, 2010). Others require binding to HS chains such as paracrine fibroblast growth factors (FGFs), a subfamily that contain HS binding site, and chemokines. FGF and FGFR interaction is expedited by interaction of ligand and receptor with HS and has been shown structurally to form 2:2:2 symmetric dimers between heparin, FGF, and FGFR (Schlessinger et al., 2000).
Chemokines exhibit a complex interaction with HS GAG chains. In vitro binding studies indicate the inhibition of HS-bound chemokine binding to their cognate chemokine receptors (Monneau, Arenzana-Seisdedos, & Lortat-Jacob, 2016). However, targeted in vivo study using chemokine mutants with reduced HS-GAG binding activity showed a decline in chemotaxis, suggesting that chemokine-HS interaction plays an important role for its function (Weber et al., 2013). Further studies utilizing trapped monomer and trapped dimer mutants of CXCL1 in vivo indicated that the ability of wild-type CXCL1 to both monomerize and dimerize is critical for the most optimal neutrophil recruitment (Sawant et al., 2016) and can even promote tissue specificity in the case of CXCL8 (Gangavarapu et al., 2012). This disconnect between in vitro binding studies and in vivo trafficking studies involving chemokine and GAG binding was bridged when it was discovered that tyrosine sulfation of chemokine receptors at the N-terminal domain enhanced chemokine-chemokine receptor binding by assisting chemokines to dissociate from GAGs (Millard et al., 2014). Altogether, chemokine-HS interaction is important for establishing chemokine gradient especially on endothelial surfaces, while the tyrosine sulfated chemokine receptors on leukocytes release chemokines from GAGs to enhance chemokine-chemokine receptor binding and signaling for optimal chemotaxis.
1.2 |. Shedding of SDCs and their role in cell signaling
SDCs exist in membrane-bound or soluble ectodomain forms after proteolytic cleavage. A group of membrane-bound enzymes called sheddases, such as matrix metalloproteinase-2 (MMP-2), MMP-7, MMP-9, and a disintegrin and metalloproteinase domain-containing protein-17 (ADAM-17), cleave SDCs at the juxtamembrane site and produce soluble ectodomains, a process that is usually accelerated during diseased conditions (Manon-Jensen, Itoh, & Couchman, 2010). In addition, ADAM with thrombospondin motifs-1 (ADAMTS-1) and ADAMTS-4 cleave SDC-4 near the N-terminal tip of the first HS GAG chain attachment site (Gao et al., 2004; Rodriguez-Manzaneque et al., 2009). Both of these different cleaved fragments contain intact HS chains that retain similar biological activity as their parent molecule, thus possessing the ability to downregulate signal transduction by competing with the membrane-bound SDCs for extracellular ligand binding and sequestering the HS binding factors in ECM (Hayashida, Stahl, & Park, 2008). Furthermore, heparanase, an endo-β-D-glucuronidase, also plays a role in the shedding of SDCs. While the enzymatic activity of mammalian heparanase cleaves HSPGs at regions with less sulfation along the GAG chain that yield HS fragments of 10–20 sugar residues (Pikas, Li, Vlodavsky, & Lindahl, 1998), the enhanced shedding of SDCs by activated heparanase is due to upregulation of MMP-9 through ERK signaling (Purushothaman, Chen, Yang, & Sanderson, 2008). The remaining C-terminal, intramembrane fragments of SDCs are likely degraded by proteolysis as has been demonstrated with SDC-3 by membrane γ-secretase complex (Schulz et al., 2003). However, the intracellular mechanisms that regulate SDC-1 shedding are largely unknown except that Rab5 seems to be involved in SDC-1 shedding, suggesting that intracellular trafficking is required (Hayashida et al., 2008).
The shedding of SDCs (such as SDC-1) modulates the molecular and cellular processes central to the pathogenesis of inflammatory diseases and tumor growth and dissemination. For instance, genetically engineered ARH-77 B lymphoid cells that produce soluble forms of SDC-1 through transfection were shown to be more invasive than tumors that interact with the cell surface SDC-1 (Yang et al., 2002). Soluble ectodomains of SDCs do not only function as competitive inhibitors but can also work as agonists after modification. For example, SDC-1 ectodomain binds more competitively to FGF-2 than their cell surface counterparts and inhibits its mitogenicity. However, upon degradation of the soluble SDC-1 ectodomain HS chains by platelet-derived heparanase present in the wound fluids, FGF-2 is activated to enhance wound repair (Kato et al., 1998; Mahtouk et al., 2007; Yang et al., 2002). Hence, SDCs have diverse functions both as membrane bound and soluble forms. Further details of different functions of SDCs in various pathologies are discussed below.
1.3 |. SDCs in rheumatoid arthritis and psoriatic arthritis
Rheumatoid arthritis (RA) is an autoimmune arthritis characterized by symmetrical inflammation of joints, infiltration of leukocytes, and synovial hyperproliferation. Some of the RA patients fail to achieve remission partly due to activated fibroblast-like synoviocytes (SFs) that propagate joint inflammation and tissue destruction. Recent studies have identified the importance of SDC-4 and receptor-type PTP protein tyrosine phosphatase sigma (RPTPσ), a transmembrane tyrosine phosphatase, in the pathogenesis of RA (Doody et al., 2015; Korb-Pap et al., 2012). Earlier study with human TNF transgenic mouse model of RA showed that RASF’s attachment to damaged cartilage required SDC-4 (Korb-Pap et al., 2012). It was subsequently discovered that RPTPσ employs SDC-4 for arthritis progression using a mechanism called proteoglycan switch, a mechanism in which the binding of RPTPσ with CS is switched to HS-containing SDC-4 on the surface of RASFs and this, in turn, activates tyrosine phosphatase to facilitate the invasiveness of RASFs (Doody et al., 2015). Binding of RPTPσ decoy proteins or exogenous CS disrupted the interaction between RPTPσ and SDC-4 inhibiting cartilage attachment by RASFs. Therefore, it implicates that SDC-4 regulates RPTPσ mediated bone and cartilage destruction in RASFs (Doody et al., 2015; Korb-Pap et al., 2012).
In RA, disease progression is also characterized by angiogenesis (Carmeliet, 2003). In this process, the interaction of VEGF with ECM assists endothelial cells to become migratory and highly proliferative to form new blood vessels. In both human and rodent models of angiogenesis, SDC-2 expressing mesenchymal cells shed their extracellular core protein in the vasculature, thus inhibiting endothelial cell migration and inflammation (De Rossi et al., 2014). Mesenchymal cells shed SDCs like many other cell types through the action of several MMPs and heparanases influenced by a variety of inflammatory responses, although the precise molecular mechanism of SDC (Table 1) shedding is not yet fully understood. These findings demonstrate an unexplored pathway for the regulation of new blood vessel formation by SDC-2 and as a potential therapeutic target for angiogenesis in RA (Manon-Jensen et al., 2010).
TABLE 1.
Role of SDCs in rheumatic diseases
| SDC | Pathologies | Function | References |
|---|---|---|---|
| SDC-1 | OA | SDC-1 mRNA levels are downregulated at damaged cartilage sites, but upregulated during early stages of OA, maybe due to repair mechanism of affected joints. | Barre et al. (2000), De Rossi et al. (2014) |
| SDC-2 | RA/PsA | SDC-2 is expressed in higher levels on endothelial cells in RA and PsA patients. SDC-2, shed from mesenchymal cells into the vasculature, inhibited endothelial cell migration and inflammation. | De Rossi et al. (2014) |
| OA | SDC-2 may compensate for loss of SDC-4 during bone repair. | Bertrand et al. (2013) | |
| SDC-3 | Antigen-induced arthritis | SDC-3 establishes CXCL1 gradient on the synovial endothelium and promote neutrophil infiltration. | Kehoe et al. (2014), Rapraeger, (2013) |
| RA/PsA | CXCL8 binds to SDC-3 that’s expressed on sublining macrophages in the synovium. | Patterson et al. (2005) | |
| OA | SDC-3 positive cells are increased in the hypertrophic zones of OA cartilage along with an increase in annexin VI. | Pfander et al. (2001) | |
| SDC-4 | RA | In human TNF transgenic mouse model of RA, RASFs required SDC-4 to attach to cartilage. Binding of RPTPo to SDC-4 activates tyrosine phosphatase that facilitates RASFs invasion and bone and cartilage destruction. | Doody et al., (2015), Korb-Pap et al. (2012) |
| OA | SDC-4 mRNA levels are upregulated at damaged cartilage sites of OA patients. In surgically-induced model of OA, SDC-4 knock mice develop less severe OA-like cartilage degradation along with decreased levels of ADAMTS-5 that is involved in increased aggrecanolysis and thus cartilage degradation. SDC-4 has also been shown to be involved in chondrocyte differentiation, proliferation, and bone repair, including upregulation of TNF-α during bone repair. TNF-α is needed for chondrocyte differentiation and bone resorption. MiR-140 has been shown to downregulate SDC-4. | Barre et al. (2000), Bertrand et al. (2013), Echtermeyer et al. (2009), Gerstenfeld et al. (2003), Karlsen et al., (2016), Nugent, (2016), Stewart et al. (2006), Vasheghani et al. (2013) |
Administration of CXCL1 in the knee joints of wild-type and SDC-3 knockout mice resulted in reduced neutrophil infiltration into the synovium and CXCL1 accumulation on the synovial endothelium in the knockout mice compared to the wild type mice (Kehoe et al., 2014). Comparison of antigen-induced arthritis between these mice also showed reduced joint swelling and disease severity in the SDC-3 knockout mice. These results suggested that SDC-3 is crucial for the establishment of CXCL1 gradient on the synovial endothelium and thus neutrophil mobilization (Kehoe et al., 2014). CXCL8 also binds to SDC-3 in RA synovium (Patterson et al., 2005). This suggests that SDC-3 elicits pro-inflammatory function by facilitating the chemokine gradients and leukocyte recruitment to exert joint inflammation and cartilage damage.
Psoriasis is another autoimmune condition that occurs when skin cells develop scaly patches over the skin too quickly and creates lesions that cause flare-ups and further aggravate autoimmunity (Mok, Xie, Sham, Lin, & Cheng, 2013). This inflammation can further attack healthy joints causing inflammation and joint damage thus developing into psoriatic arthritis (PsA). A differential expression and distribution of SDCs and glypicans in an inflamed synovium was noted in synovia obtained from RA and PsA patients where SDC-2 was present mainly in blood vessels of endothelial cells, SDC-3 in sublining macrophages, and glypican 4 in both the endothelial lining and blood vessels (Patterson et al., 2005).
In general, endothelial cells, as well as macrophages in RA synovium express SDC-3, which is critically required for the development of CXCL1 and CXCL8 gradients. On the other hand, SDC-4 on SFs and SDC-2 on endothelial cells were identified to promote arthritis progression through the regulation of RPTPσ and angiogenesis, respectively. As chronic inflammation is the underlying mechanism in RA and PsA, the expression pattern and role of SDCs in normal and inflamed synovia might be similar even though SDCs role in PsA is still rudimentary.
1.4 |. SDCs in osteoarthritis
Unlike RA, which is an autoimmune disease, osteoarthritis (OA) is a common degenerative joint disease in the elderly cohort (typically 65+ years old) that initiates without a specific cause. OA is characterized by wear and tear of the protective cartilage around articular joints, which is further exacerbated by IL-1β-induced collagenases, and bone demineralization in chondrocytes (Scanzello & Goldring, 2012). One of the early studies that examined the alterations of SDC expressions in OA patients discovered that SDC-1 mRNA levels were down-regulated while SDC-4 mRNA expression was upregulated at the damaged cartilage sites when compared to the intact sites (Barre, Redini, Boumediene, Vielpeau, & Pujol, 2000). However, when chondrocytes from damaged and intact cartilage were isolated and cultured in monolayer, SDC-1 expression of chondrocytes isolated from damaged cartilage was higher than the chondrocytes isolated from intact sites, while SDC-4 expression levels were similar. These differences in expression, which amplified directly from cartilage versus cultured chondrocytes, may partly be due to SDC-mediated signals originating from the damaged cells that are mobilizing a number of ECM proteins, and/or partly due to a selective responsive mechanism of chondrocytes to different forms of HSPGs. Combined with the known role of SDC-1 in skeletal development and wound healing (Kim, Goldberger, Gallo, & Bernfield, 1994), up-regulation of SDC-1 in early stages of OA seems to be involved in the repair mechanism of the affected joints. However, down-regulation of chondrocyte mediated SDC-1 expression in cartilage tissues requires further validation.
In a related study using a surgically-induced model of OA, it was shown that intraarticular injection of a SDC-4 specific antibody resulted in a significant decrease in ADAMTS-5 that profoundly prevented articular damage (Echtermeyer et al., 2009). This finding was further supported by a SDC-4 deficient mouse model where less severe OA-like cartilage degradation was present in parallel with a marked decrease in ADAMTS-5. In addition, IHC analysis of OA murine joints showed an abundance of hyaluronan with poor aggrecan deposition due to increased aggrecanolysis mediated by ADAMTS-5, thus leading to cartilage degradation (Stewart et al., 2006). Furthermore, murine strains of null SDC-1 exhibited normal aggrecanase-mediated cleavages in chondrocytes. This clearly suggests that SDC-4 but not SDC-1 supports ADAMTS-5 activation and affects the process of articular damage in OA.
SDC-4 has a role not just in OA but also in bone development and repair. For example, detection of SDC-4 promoter activity in SDC-4 lacZ knock-in mice indicated SDC-4-mediated chondrocyte differentiation, whereas in its absence there was barely any detectable chondrocyte proliferation (Bertrand et al., 2013). Furthermore, immunohistochemical (IHC) analysis of embryos indicated that SDC-4 was elevated during the development of bones especially in long bones and ribs, and after post-fracture in adult mice. Even though endochondral bone formation in development and during bone fracture are near identical, surprisingly the absence of SDC-4 enhanced the fracture phenotype. It was further observed that the loss of SDC-4 was accompanied by approximately an eightfold increase in SDC-2 mRNA and threefold up-regulation of SDC-2 protein expression, suggesting a possible compensatory mechanism. In general, these findings suggest that both SDC-2 and SDC-4 are involved in osteoclast adhesion, and survival (Bertrand et al., 2013). In a related study, SDC-4 expression induced pro-inflammatory cytokines such as TNF-α and supported the repair of bone fracture in mice. In parallel to this finding, the loss of TNF-α receptor impaired chondrocyte differentiation and bone resorption during fracture healing (Gerstenfeld et al., 2003).
Peroxisome proliferator-activated receptor gamma (PPARγ), which plays a critical role in carbohydrate and lipid metabolism, are also implicated in OA where PPARγ knockout mice exhibited osteoarthritis phenotype. In human OA cartilage, it was identified that PPARγ was expressed at a very low level (Vasheghani et al., 2013). Further study has demonstrated that PPARγ deficient cartilage exhibited higher expression of ADAMTS-5, MMP-13, SDC-4, and several other inflammatory mediators as compared with controls indicating its involvement in cartilage degradation (Vasheghani et al., 2013). It is thus imperative that PPARγ agonists can be used as a potential therapeutic target for OA.
IHC analysis had shown the involvement of SDC-3 in the hypertrophic cartilage of OA in which the number of immunopositive SDC-3 cells had increased from 20% (in normal human articular cartilage) to 80% (in the severely affected human osteoarthritic cartilage) in hypertrophic zones along with a dramatic increase in annexin VI (a protein that predominantly gets expressed in proliferating chondrocytes) (Pfander, Swoboda, & Kirsch, 2001). Thus, SDC-3 seems to mediate ADAMTS-5 regulated aggrecanolysis in the pathological processes related to chondrocyte function. However, more evidence is required to validate these findings.
In recent years, microRNAs (miRNAs) have emerged as an attractive future therapeutic option for several inflammatory disorders, including OA. It has been shown that miRNAs are dysregulated in OA cartilage (Nugent, 2016). Using an in vitro model of IL-1β-induced OA and knockout studies, human articular chondrocytes expressing miR-140 were shown to inhibit cartilage ECM degradation and inflammation, thus protecting from OA (Karlsen, de Souza, Odegaard, Engebretsen, & Brinchmann, 2016). Furthermore, study showed that miR-140 plays a dual role in maintaining cartilage integrity by upregulating proteins like SOX9 and downregulating SDC-4. Although a number of different functions have been ascribed to SDCs in OA, further investigation is necessary to understand the molecular mechanism(s) governing each SDC during the cartilage damage of OA patients.
Expression of all four SDCs has been correlated in OA during chondrocyte differentiation and death. Importantly, the expressions of SDC-1 and −4 have been shown to be reciprocally regulated in that SDC-1-mRNA levels were downregulated as SDC-4 levels were up-regulated at mRNA level during osteoarthritic cartilage degradative processes. Furthermore, IHC analysis of chondrocytes from OA joints indicated increased aggrecanolysis mediated by ADAMTS5 and SDC-4 and regulated by PPARγ. It is also becoming apparent that miRNAs such as miR-140 down-regulated genes involved in SDC expression and activity in OA pathogenesis. Therefore, regulation of SDCs by modulating miRNA expression may serve as a future target for therapeutic intervention.
1.5 |. SDCs in diabetes
The two most common types of diabetes are type 1 and type 2. Type 1 diabetes mellitus (T1DM) is an autoimmune disease in which a faulty immune system mistakenly attacks and permanently destroys the β-cells of the pancreas that produce insulin. Type 2 diabetes mellitus (T2DM) arises due to insulin resistance in which the pancreas can no longer keep up with the insulin demand and fails to provide insulin efficiently. Even though the role of SDCs in T1DM and the proteases involved in SDC-1 shedding are not yet well characterized, SDC-1 expression and the sheddases are implicated in glomerular damage in T1DM (Kolseth et al., 2017). More studies have examined the role of SDCs in T2DM specifically or diabetic complications common in both T1DM and T2DM that are discussed below.
The health risk of obesity associated with diabetes is one of the critical and best studied hypothalamic circuits comprised of the melanocortinergic, orexigenic, and anorexigenic neurons in regulating body weight (Reizes et al., 2003). Orexigenic stimulus is induced by agouti-related protein (AgRP), while α-melanocyte stimulating hormone (α-MSH), an anorexigenic stimuli, inhibits feeding by binding to melanocortin 3 and 4 receptors (MC3-R and MC4-R). Mutations in the agouti gene caused an over-expression of agouti peptide which antagonized the effects of α-MSH in the brain and caused obese phenotypes (Mizuno, Makimura, & Mobbs, 2003). Studies have shown that amino-terminal domain of AgRP binds to SDC-3 in the brain, thereby enhancing the binding of carboxyl-terminal AgRP to MC4-R and increasing the feeding behavior and body weight (Reizes, Benoit, & Clegg, 2008). Thus, AgRP may regulate the body weight by competitively binding to MC4-R with the assistance of SDC-3, leading to differential sensitivity to orexigenic and anorexigenic ligands (Reizes et al., 2008).
So far, diabetes has been shown to cause HSPGs to undergo several modifications in various tissues and organs as a component of pathogenesis (Gowd, Gurukar, & Chilkunda, 2016). Interestingly, it has been found that insulin promotes shedding of SDC ectodomains (Reizes et al., 2006). This serves as a link between insulin signaling and SDCs mediated cellular and pathological processes apart from facilitating adhesion of integrins for normal growth and development. Recent studies on protein-losing enteropathy indicated the interaction between SDC-1 and heparanase in the maintenance of intestinal epithelial barrier (Bode et al., 2008). Studies on developed diabetic mice/cell models also showed dramatic SDC-1 shedding with a tremendous surge in heparanase activity correlated with abnormalities of intestinal permeability and the activation of p38 MAPK signaling pathway (Qing et al., 2015). Furthermore, soluble heparin has been shown to reverse the intestinal damage and effectively improved the impaired barrier function caused by SDC-1 and heparanase.
Furthermore, patients with diabetic nephropathy (DN), a complication in diabetes that causes thickened glomerular basement membrane due to increased ECM and thus leading to impaired glomerular filtration and albuminuria, showed an elevated serum level of SDC-1 as compared with patients without microalbuminuria, suggesting a role of soluble SDC-1 in the pathogenesis of T2 DN (Wang et al., 2012). Studies on rat model of peritoneal dialysis utilizing sulodexide (GAG), a treatment typically used for DN patients, demonstrated that sulodexide and heparin-derived drugs are effective in preventing TGF-β induced epithelial mesenchymal transition (EMT) and angiogenesis (Pletinck et al., 2012). Sulodexide inhibited heparanase-1 activity and prevented the decrease of SDC-1 expression and migration in FGF-2-treated HK2 human kidney cell line (Masola, Onisto, Zaza, Lupo, & Gambaro, 2012).
A study examining the possible correlation between SDC-1 and lipid profiles in the serum of T2DM patients showed a significant increase in soluble SDC-1 levels while showing a decrease in high-density lipoprotein cholesterol (HDL) (Wang et al., 2013). Considering that apoA1 is a major protein of HDL, there was a negative association between SDC-1 and apoA1 as compared with non-diabetic healthy subjects, but the mechanism of interaction between SDC-1 and apoA1 has not yet been clarified (Wang et al., 2013). Examination of SDC-1 in plasma membrane of cells in T2DM patients showed that the percentage of positive SDC-1 cells on neutrophils were significantly higher in subjects with diabetes than that of the controls, suggesting increased SDC-1 levels on neutrophils as a predictor of T2DM (Wang et al., 2012). These preliminary studies indicate that the role of SDCs in T2DM may be multifactorial. SDC-3 plays a role in eating behavior, while SDC-1 has multiple functions in metabolic changes, immune activation, scarring in the kidneys, and leaky gut. Prevention of SDC-1 shedding may be a potential therapeutic target for both diabetic enteropathy and nephropathy (Bode et al., 2008; Masola et al., 2012).
1.6 |. SDCs in human immunodeficiency virus
It is an established fact that human immunodeficiency virus (HIV) attachment to receptors is modulated by CD4, chemokine receptors, LFA-1 (lymphocyte function-associated protein-1), DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin), and HSPGs (Bobardt et al., 2003). T cells expressing low levels of CC chemokine receptor 5 (CCR5) typically lack the machinery to promote viral entry and replication to the host cell. However, T cells expressing DC-SIGN, despite lacking CCR5, facilitated the entry and replication of R5 viruses (Lee et al., 2001). Several lines of evidence further suggest that SDCs act in synergy with DC-SIGN, thus promoting HIV attachment to target cells (Mondor, Moulard et al., 1998). On the other hand, the removal of cell surface HS chains of proteoglycans using heparitinase diminishes both HIV attachment to and infectivity of CD4-positive HeLa cells and macrophages (Mondor, Ugolini, & Sattentau, 1998). A chemokine RANTES/CCL5 (regulated on activation normal T cell expressed and secreted/chemokine ligand 5) also induces cellular activation and enhances HIV type 1 (HIV-1) infectivity through the activation of ERK1/2 and protein tyrosine kinase (PTK) signaling pathways with a broad range of affinities for HS (Roscic-Mrkic et al., 2003). All of these findings suggest the role of SDCs for HIV entry into primary target cells (Bobardt et al., 2003; Roscic-Mrkic et al., 2003).
HIV-1 invades the human brain and preferentially attacks the nervous system by damaging astrocyte and myelin sheath migrating through brain microvascular endothelial cells (BMECs) (Edinger et al., 1997). As BMECs lack the entry receptor CD4, HIV-1 easily passes the brain-blood barrier (BBB) and gains access to neuronal cells by employing HSPGs (Edinger et al., 1997). This was discovered utilizing an artificial BBB transmigration assay in which both HS and CS proteoglycans were abundantly expressed on primary BMECs and promoted HIV-1 attachment and entry through electrostatic interaction between gp120 and the negative charges on the sulfate groups of proteoglycans (Edinger et al., 1997). In parallel to these findings, an association of stromal cell derived factor-1 (SDF-1), a natural ligand for CXCR4, with HSPGs determined the fusogenic activity of HIV-1 × 4 virus with CXCR4, thereby blocking the activation of gp41 necessary for the attachment and entry (Valenzuela-Fernandez et al., 2001). This clearly indicated that HSPGs are necessary for SDF-1 activity in downregulating HIV co-receptors. Further study into SDF-1 and HSPGs showed that SDF-1 alone does not interfere with the initial binding of HIV gp120 subunit to CD4 but rather occupy a site on CXCR4 (Valenzuela-Fernandez et al., 2001). Furthermore, attachment of SDF-1 to HSPGs promoted increased local concentration on the membrane and sustained occupancy between SDF-1 and CXCR4, thus preventing HIV-1 × 4 viral entry. However, other evidences suggested that HSPGs can participate directly in HIV-1 attachment to target cells. Unlike CD4+ T cells, the removal of cell surface polyanionic chains of proteoglycans using heparitinase totally abrogated both HIV-1 infection and attachment to CD4-positive HeLa cells. Thus, HSPGs may also serve as alternative binding sites for HIV-1 (Mondor, Ugolini et al. 1998).
It is uncommon to find individuals infected with HIV-1, who without any drug intervention, are able to maintain undetectable viremia for several years while being asymptomatic. This was recently discovered to be due to monocyte-derived dendritic cells (MDDC) that express high levels of SDC-3, DC-SIGN, and HIV controllers (HIC), leading to enhanced cytolytic CD8+ T cell responses in these patients. Thus, MDDCs stabilize the capture of HIV-1 through gp120 interaction with SDC-3 and prevent HIV-1 infected individuals from the deleterious effect of HIV-1 infection (de Witte et al., 2007). The same study indicated that silencing SDC-3 from immature DCs by siRNA, partially inhibited HIV-1 transmission, whereas neutralizing both SDC-3 and DC-SIGN completely abrogated HIV-1 capture and subsequent transmission. Thus, it seems that HIV-1 infection is inhibited by both SDC-3 and DC-SIGN (de Witte et al., 2007). SDC-1 is also commonly upregulated in AIDS-related B lymphoid malignancies in which HIV-1 trans-activating factor Tat engages SDC-1, chemokine receptors, and integrins in the pathogenesis of AIDS-related lymphomas (Urbinati et al., 2016). Therefore, modulating SDC-1 and SDC-3 may help in identifying new therapeutic targets for the treatment of AIDS-associated neoplasia and infection.
1.7 |. SDCs in cancer
Over the years, the association between SDCs and cancer have been established where SDCs are known to regulate tumor development and serve as prognostic markers for cancer progression as well as patient survival (Afratis et al., 2017; Barbouri et al., 2014). Among the four known members of mammalian HSPG family, low or high expression of SDC-1 was highly implicated in a number of cancers. High levels of SDC-1 detected in solid tumors such as head and neck, ovarian, breast, liver, and colorectal carcinomas serve as potential prognostic markers for cancer initiation and progression (Teng, Aquino, & Park, 2012; Wei, Guo, Dong, & Chen, 2015). For instance, SDC-1 overexpression was noted in early stages of prostate cancer by IHC staining (Shimada et al., 2009, 2013). Furthermore, IHC studies on triple-negative breast carcinoma also indicated an overexpression of SDC-1 to be associated with tumor aggressiveness and poor survival (Baba et al., 2006; Nguyen et al., 2013).
Considering that both SDC-1 and SDC-4 can bind to “heparin-binding” growth factors such as FGFs, hepatocyte growth factor, and epidermal growth factor to enhance ligand/receptor binding and thus cell survival and proliferation (Afratis et al., 2017), it is not surprising that these solid tumors have increased expression of SDC-1. SDC-1 expression is not only important on the tumor itself but also in the tumor microenvironment, as SDC-1 in stromal fibroblasts assisted in proliferation and angiogenesis of breast carcinoma (Maeda, Alexander, & Friedl, 2004; Maeda, Desouky,& Friedl, 2006). SDC-1 is also integral in various signaling pathways. Breast cancer stem cells from triple negative breast cancer require SDC-1 for IL-6/STAT3, Notch, and EGFR signaling pathways (Ibrahim et al., 2017). Moreover, it has also been shown that SDC-1 is a critical component of Wnt-1-induced mammary tumor formation (Alexander et al., 2000), although the role of SDC-1 may be further downstream of Wnt pathway to enhance the β-catenin/T-cell factor (TCF) transcriptional complex formation in mammary epithelial cells (Liu, Kim, Leatherberry, Cowin, & Alexander, 2003).
In this regard, even though there is limited evidence about the subcellular distribution and localization of SDCs, several studies have implicated SDCs, apart from their extracellular/transmembrane role in cell proliferation, and cytoskeletal organization, in the intracellular role in cancer and metastasis. A study using confocal laser microscopy has shown that during mitotic division, SDC-1 translocates to the nucleus and may modulate transcription factors, and nuclear proteins in myeloma tumor cells (Cheng, Petersson, Arroyo-Yanguas, & West-ergren-Thorsson, 2001). SDC-1 colocalizes with α-tubulin in the mitotic spindle, which suggests its nuclear localization and potential to regulate cell division, and additionally may serve as a vehicle for transporting growth factors and regulatory proteins. This emphasizes its potential role as a transcription factor, thereby, influencing gene regulation and cancer pathogenesis (Brockstedt, Dobra, Nurminen, & Hjerpe, 2002; Nilsson, Johnsson, Fransson, Ellervik, & Mani, 2010).
However contradictory to the above, low SDC-1 levels have also been associated with poor prognosis in many cancer types. Studies have shown that SDC-1 facilitated cancer cell adhesion to ECM thereby slowing invasion and metastasis. For example, an increased SDC-1 expression slowed down the progression of mesothelioma with high metastatic potential and increased survival in the patients with lung carcinoma after surgery (Anttonen, Heikkila, Kajanti, Jalkanen, & Joensuu, 2001; Kumar-Singh et al., 1998). Inversely, SDC-1 silencing improved adhesion and migration along with IL-6 induced activation of FAK in triple negative breast cancer (Hassan et al., 2013). Low levels of SDC-1 expression along with high expression of integrin β3 was also associated with metastasis of gastric carcinoma (Chu, Ye, Tao, Wang, & Zhao, 2008).
Low surface levels of SDC-1 could be due to shedding, and it is becoming apparent that endogenous endoglucuronidases that specifically cleave chains of GAGs increase their expression and activity in an aggressive tumor type, suggesting the emerging role of heparanase, and other sheddases in the up-regulation of cancer progression (Mikami et al., 2001; Ramani et al., 2013). Heparanase-induced shedding of SDC-1 has been shown to promote aggressive myeloma phenotype (Purushothaman et al., 2008). Similarly, heparanase overexpression in gallbladder carcinoma cell line decreased SDC-1 expression and increased invasion and migration (Jin, Zhou, Yang, & Cao, 2017).
In addition to sheddases, microRNAs have been shown to be overexpressed in breast cancer such as microRNA10b (miR-10b) and downregulated SDC-1 expression (Ibrahim et al., 2012). In a pre-invasive breast cancer study, a decreased expression of SDC-1 was noted with ectopic increase in the pro-metastatic miR-10b that significantly increased breast cancer invasiveness (Ibrahim et al., 2012). In parallel to this finding, significant downregulation of SDC-1 mRNA expression by miR-10b was observed both in MDA-MB-231 and in MCF-7 breast cancer cells. However, SDC-1 siRNA treatment caused an increase of β1-integrin and focal adhesion kinase-mediated cellular adhesion and migration (Hannafon, Sebastiani, de las Morenas, Lu, & Rosenberg, 2011; Ibrahim et al., 2012). These data seem to suggest that while SDC-1 may be needed for initial growth of solid tumors, at least surface levels of SDC-1 must be reduced for optimal invasion and metastasis during epithelial mesenchymal transition.
Taken together, SDC-1 has been shown to have a role in both tumor development and cancer metastasis, though much research remains to be done to investigate whether SDC-1 inhibitors, antagonists, or agonists may inhibit the disease progression. Currently, there are few studies showing the role of SDC-2 in cancer and tumor progression. Silencing SDC-2 in breast carcinoma cell lines significantly reduced breast cancer metastasis (Lim, Multhaupt, & Couchman, 2015). SDC-4 is ubiquitously expressed by most cell types, and little is known about its role in malignancy except in breast cancer where it was initially associated with estrogen receptor negative breast carcinoma (Baba et al., 2006). However, subsequent study suggest that SDC-4 may be associated with estrogen and progesterone receptor positive breast carcinoma (Lendorf, Manon-Jensen, Kronqvist, Multhaupt, & Couchman, 2011) and may mediate breast cancer cell adhesion and spreading (Beauvais & Rapraeger, 2003). SDC-3 has not currently been implicated in cancer, metastasis, or tumor progression.
1.8 |. Current therapies targeting SDCs
The clinical management of several inflammatory and autoimmune diseases is still a challenge, and multiple agents interfering with expression of HSPGs are under investigation. Currently, the heparanase/HSPG axis has been employed as a potential therapeutic target for several malignant tumor types. An increased expression level of heparanase in various cancers is correlated with an increased metastasis and poor prognosis, and therefore, sulodexide, which inhibits heparanase-1, has been used to prevent EMT as described above. Furthermore, targeting heparanase itself by silencing heparanase with shRNA has been shown to reduce the invasiveness and migratory capabilities of human osteosarcoma with decreased hypoxia-inducible factor −1 alpha (HIF-1α) (Fan, Wu, Xing, Liu, & Shao, 2011). In addition to investigating the potential therapeutic value of inhibiting heparanase and its enzyme activity, therapeutic regimens are emerging that target HSPGs with antibodies or short peptide inhibitors like synstatin (SSTN). SSTN is a selective inhibitor of SDC-1 that blocks αvβ3 or αvβ5 integrin and IGF-1R kinase from binding to SDC-1 and thus prevent tumor survival and invasion (Beauvais, Jung, Yang, Sanderson, & Rapraeger, 2016; Rapraeger, 2013). Zoledronate, a class of bisphosphonate drugs that inhibits bone resorption, hypercalcemia and metastasis, down-regulated SDC-1 by significantly reducing the levels of integrins (ανβ3, ανβ5, α5β1), and ECM interacting molecules, though the underlying mechanisms are still under intense investigation (Dedes et al., 2012). Moreover, SDC neutralizing antibodies that interfere with SDC-growth factor binding have been shown to inhibit SDC-mediated cell proliferation. In both in vivo and in vitro models, investigators have shown that chondrocytes growing in the presence of FGF-2 or Indian hedgehog (Ihh) failed to proliferate when SDC-3 neutralizing antibodies were added (Kirsch, Koyama, Liu, Golub, & Pacifici, 2002; Shimo et al., 2004). This suggests that both FGF-2 and Ihh activity in chondrocyte proliferation are largely influenced by SDC-3. Additionally, anti-SDC-4 antibody has been used to treat murine models of both allergic asthma and osteoarthritis (Echtermeyer et al., 2009; Polte et al., 2015). In light of these observations, the therapeutic regulation of autoimmune diseases and cancer progression through SDC chemical inhibitors, antibodies, and mimetic is a promising clinical strategy.
2 |. CONCLUSION
SDCs are an interesting family of transmembrane proteins that mediate a variety of cellular responses by coupling sugar and protein biochemistry to the classical GPCR. By virtue of their structural organization, it is now evident that they influence the biological activity of chemokines, several growth factors, growth factor receptors, and adhesion molecules while mediating normal and pathological processes. Nevertheless, much still remains unknown. For example, studies on SDC-1 have shown a conflicting role of SDC-1 in promoting, as well as suppressing tumor progression on the same tumor type (Table 2). Furthermore, the possible involvement of SDC-2 and SDC-4 in tumor metastasis, angiogenesis, and inflammation is yet rudimentary. So far, observations on the role of SDC-1 in cancer have failed to indicate its use as a prognostic marker for CRC and other cancer types (Wei et al., 2015).
TABLE 2.
Role of SDCs in other diseases
| SDC | Pathologies | Function | References |
|---|---|---|---|
| SDC-1 | T1DM/ T2DM DN | In diabetic patients with DN, serum levels of SDC-1 as well as surface expression of neutrophils was higher (only in T2DM). | Kolseth et al. (2017), Pletinck et al. (2012), Wang et al. (2012) |
| Diabetic enteropathy | Interaction between SDC-1 and heparanase is important for intestinal epithelial barrier. Shedding of SDC-1 and high heparanase activity correlates with increased intestinal permeability along with activation of p38 MAPK pathway. | Bode et al. (2008), Qing et al. (2015) | |
| HIV | Tat engages SDC-1, chemokine receptors, and intergrins to promote pathogenesis of AID-related lymphomas. | Urbinati et al. (2016) | |
| Cancer | High SDC-1 expression in many solid cancers including head and neck, ovarian, breast, prostate, and colon carcinomas is associated with cancer initiation, proliferation, and survival, since SDC-1 can bind to many growth factors and works as a cofactor to enhance downstream pathways including STAT3, Notch, EGFR, and Wnt-1 signaling pathways. SDC-1 can also function inside the cells by co-localizing with mitotic spindle during mitosis and modulating transcription factors and other nuclear protein to regulate cell division. However, lower levels have also been associated with poor prognosis in many cancer including mesothelioma, lung carcinoma, breast cancer, gastric carcinoma, and myeloma. In these cases, higher SDC-1 level facilitated adhesion to ECM and inhibited invasion and metastasis. Increased shedding of SDC-1 by heparanase and other sheddases promoted more aggressive tumor phenotype as observed in myeloma and gallbladder carcinoma. In addition to sheddases, miR-10b that downregulates SDC-1 expression has been shown to be overexpressed in breast cancer and overexpression of miR-10b increased invasiveness. | Alexander et al. (2000), Anttonen et al. (2001), Baba et al. (2006), Brockstedt et al. (2002), Cheng et al. (2001), Chu et al. (2008), Hannafon et al. (2011), Hassan et al. (2013), Ibrahim et al. (2012, 2017), Jin et al. (2017), Kumar-Singh et al. (1998), Liu et al. (2003), Maeda et al. (2004), Maeda et al. (2006), Mikami et al. (2001), Nguyen et al. (2013), Nilsson et al. (2010), Ramani et al. (2013), Shimada et al. (2009, 2013), Teng et al. (2012), Wei et al. (2015) | |
| SDC-2 | T2DM | In the brain, SDC-3 binds to N-terminal domain of AgRP, and this interaction promotes C-terminal domain of AgRP to competitively bind to MC4-R and thus enhance orexigenic stimulus. | Reizes et al. (2003, 2008) |
| Cancer | Silencing SDC-2 in breast cancer significantly reduced metastasis. | Lim et al. (2015) | |
| SDC-3 | HIV | MDCCs capture and present HIV antigens to cytolytic CD8+ T cells through gpl20 and SDC-3 interaction. | de Witte et al. (2007) |
| SDC-4 | Cancer | In breast cancer, SDC-4 expression is associated with estrogen and progesterone receptor positive cells and may function in cell adhesion and spreading. | Baba et al. (2006), Beauvais and Rapraeger, (2003) |
On the other hand, the role of heparanase and MMPs in creating shed ectodomains of soluble SDCs to facilitate the activation of innate immune cells, inflammatory processes, cellular signaling, angiogenesis, tumor metastasis, and regulate effector genes like VEGF and MMP-9 is still underdeveloped, especially in the context of diseases. In inflammatory disorders like RA, the roles of SDCs are beginning to emerge. SDC-3 has been shown to selectively promote inflammation by endothelial chemokine mobilization and leukocyte recruitment and contribute to antigen-induced arthritis that causes articular joint damage (Patterson et al., 2005). Despite these findings, the roles of SDCs in inflammatory processes need further investigation. Major areas of future investigation need to include the role of core protein cytoplasmic domain, how SDCs modulate chemokine or cytokine mediated signal transduction and its numerous interacting partners, and whether they affect transcriptional and epigenetic regulation during various diseases.
ACKNOWLEDGMENTS
SA was supported by the NIH grants AR063104 and AR068517. SAA was supported by the NIH supplemental grant AR063104S. No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Funding information
National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant numbers: AR063104 and AR068517, AR063104S
Footnotes
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest related to this work.
REFERENCES
- Afratis NA, Nikitovic D, Multhaupt HA, Theocharis AD, Couchman JR, & Karamanos NK (2017). Syndecans−key regulators of cell signaling and biological functions. FEBS Journal, 284(1), 27–41. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, & Bernfield M (2000). Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nature Genetics, 25(3), 329–332. [DOI] [PubMed] [Google Scholar]
- Anttonen A, Heikkila P, Kajanti M, Jalkanen M, & Joensuu H (2001). High syndecan-1 expression is associated with favourable outcome in squamous cell lung carcinoma treated with radical surgery. Lung Cancer, 32(3), 297–305. [DOI] [PubMed] [Google Scholar]
- Baba F, Swartz K, van Buren R, Eickhoff J, Zhang Y, Wolberg W, & Friedl A (2006). Syndecan-1 and syndecan-4 are overexpressed in an estrogen receptor-negative, highly proliferative breast carcinoma subtype. Breast Cancer Research and Treatment, 98(1), 91–98. [DOI] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, … David G (2012). Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nature Cell Biology, 14(7), 677–685. [DOI] [PubMed] [Google Scholar]
- Barbouri D, Afratis N, Gialeli C, Vynios DH, Theocharis AD, & Karamanos NK (2014). Syndecans as modulators and potential pharmacological targets in cancer progression. Frontiers in Oncology, 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre PE, Redini F, Boumediene K, Vielpeau C, & Pujol JP (2000). Semiquantitative reverse transcription-polymerase chain reaction analysis of syndecan-1 and −4 messages in cartilage and cultured chondrocytes from osteoarthritic joints. Osteoarthritis and Cartilage, 8(1), 34–43. [DOI] [PubMed] [Google Scholar]
- Beauvais DM, Jung OS, Yang Y, Sanderson RD, & Rapraeger AC (2016). Syndecan-1 (CD138) suppresses apoptosis in multiple myeloma by activating IGF1 receptor: Prevention by Synstatin(IGF1R) inhibits tumor growth. Cancer Research, 76(17), 4981–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais DM, & Rapraeger AC (2003). Syndecan-1-mediated cell spreading requires signaling by alphavbeta3 integrins in human breast carcinoma cells. Experimental Cell Research, 286(2), 219–232. [DOI] [PubMed] [Google Scholar]
- Beauvais DM, & Rapraeger AC (2010). Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation. Journal of Cell Science, 123(Pt 21), 3796–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J, Stange R, Hidding H, Echtermeyer F, Nalesso G, Godmann L, … Dreier R (2013). Syndecan 4 supports bone fracture repair, but not fetal skeletal development, in mice. Arthritis and Rheumatism, 65(3), 743–752. [DOI] [PubMed] [Google Scholar]
- Bobardt MD, Saphire AC, Hung HC, Yu X, Van der Schueren B, Zhang Z, … Gallay PA (2003). Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity, 18(1), 27–39. [DOI] [PubMed] [Google Scholar]
- Bode L, Salvestrini C, Park PW, Li JP, Esko JD, Yamaguchi Y, … Freeze HH (2008). Heparan sulfate and syndecan-1 are essential in maintaining murine and human intestinal epithelial barrier function. Journal of Clinical Investigation, 118(1), 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockstedt U, Dobra K, Nurminen M, & Hjerpe A (2002). Immunoreactivity to cell surface syndecans in cytoplasm and nucleus: Tubulin-dependent rearrangements. Experimental Cell Research, 274(2), 235–245. [DOI] [PubMed] [Google Scholar]
- Carmeliet P (2003). Angiogenesis in health and disease. Nature Medicine, 9(6), 653–660. [DOI] [PubMed] [Google Scholar]
- Cheng F, Petersson P, Arroyo-Yanguas Y, & Westergren-Thorsson G (2001). Differences in the uptake and nuclear localization of anti-proliferative heparan sulfate between human lung fibroblasts and human lung carcinoma cells. Journal of Cellular Biochemistry, 83(4), 597–606. [DOI] [PubMed] [Google Scholar]
- Choi S, Lee E, Kwon S, Park H, Yi JY, Kim S, … Oh ES (2005). Transmembrane domain-induced oligomerization is crucial for the functions of syndecan-2 and syndecan-4. Journal of Biological Chemistry, 280(52), 42573–42579. [DOI] [PubMed] [Google Scholar]
- Chu YQ, Ye ZY, Tao HQ, Wang YY, & Zhao ZS (2008). Relationship between cell adhesion molecules expression and the biological behavior of gastric carcinoma. World Journal of Gastroenterology, 14(13), 1990–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rossi G, Evans AR, Kay E, Woodfin A, McKay TR, Nourshargh S, & Whiteford JR (2014). Shed syndecan-2 inhibits angiogenesis. Journal of Cell Science, 127(Pt 21), 4788–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TB, & Gallay P (2007). Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proceedings of the National Academy of Sciences of the United States of America, 104(49), 19464–19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedes PG, Gialeli C, Tsonis AI, Kanakis I, Theocharis AD, Kletsas D, … Karamanos NK (2012). Expression of matrix macromolecules and functional properties of breast cancer cells are modulated by the bisphosphonate zoledronic acid. Biochimica et Biophysica Acta, 1820(12), 1926–1939. [DOI] [PubMed] [Google Scholar]
- Doody KM, Stanford SM, Sacchetti C, Svensson MN, Coles CH, Mitakidis N, … Bottini N (2015). Targeting phosphatase-dependent proteoglycan switch for rheumatoid arthritis therapy. Science Translational Medicine, 7(288), 288ra 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, … Pap T (2009). Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nature Medicine, 15(9), 1072–1076. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Mankowski JL, Doranz BJ, Margulies BJ, Lee B, Rucker J, … Doms RW (1997). CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proceedings of the National Academy of Sciences of the United States of America, 94(26), 14742–14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Wu Q, Xing X, Liu Y, & Shao Z (2011). Targeted silencing of heparanase gene by small interfering RNA inhibits invasiveness and metastasis of osteosarcoma cells. Journal of Huazhong University of Science and Technology Medical Sciences, 31(3), 348–352. [DOI] [PubMed] [Google Scholar]
- Gangavarapu P, Rajagopalan L, Kolli D, Guerrero-Plata A, Garofalo RP, & Rajarathnam K (2012). The monomer-dimer equilibrium and glycosaminoglycan interactions of chemokine CXCL8 regulate tissue-specific neutrophil recruitment. Journal of Leukocyte Biology, 91(2), 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Plaas A, Thompson VP, Jin S, Zuo F, & Sandy JD (2004). ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. Journal of Biological Chemistry, 279(11), 10042–10051. [DOI] [PubMed] [Google Scholar]
- Gao Y, Li M, Chen W, & Simons M (2000). Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration. Journal of Cellular Physiology, 184(3), 373–379. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, … Einhorn TA (2003). Impaired fracture healing in the absence of TNF-alpha signaling: The role of TNF-alpha in endochondral cartilage resorption. Journal of Bone and Mineral Research, 18(9), 1584–1592. [DOI] [PubMed] [Google Scholar]
- Gowd V, Gurukar A, & Chilkunda ND (2016). Glycosaminoglycan remodeling during diabetes and the role of dietary factors in their modulation. World Journal of Diabetes, 7(4), 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannafon BN, Sebastiani P, de las Morenas A, Lu J, & Rosenberg CL (2011). Expression of microRNA and their gene targets are dysregulated in preinvasive breast cancer. Breast Cancer Research, 13(2), R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H, Greve B, Pavao MS, Kiesel L, Ibrahim SA, & Gotte M (2013). Syndecan-1 modulates beta-integrin-dependent and interleukin-6-dependent functions in breast cancer cell adhesion, migration, and resistance to irradiation. FEBS Journal, 280(10), 2216–2227. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Stahl PD, & Park PW (2008). Syndecan-1 ectodomain shedding is regulated by the small GTPase Rab5. Journal of Biological Chemistry, 283(51), 35435–35444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SA, Gadalla R, El-Ghonaimy EA, Samir O, Mohamed HT, Hassan H, … Gotte M (2017). Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Molecular Cancer, 16(1), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SA, Yip GW, Stock C, Pan JW, Neubauer C, Poeter M, … Gotte M (2012). Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPase- and E-cadherin-dependent mechanism. International Journal of Cancer, 131(6), E884–E896. [DOI] [PubMed] [Google Scholar]
- Iozzo RV (2005). Basement membrane proteoglycans: From cellar to ceiling. Nature Reviews Molecular Cell Biology, 6(8), 646–656. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Okamoto H, Toyama Y, & Momohara S (2008). Molecular aspects of rheumatoid arthritis: Chemokines in the joints of patients. FEBS Journal, 275(18), 4448–4455. [DOI] [PubMed] [Google Scholar]
- Jin H, Zhou S, Yang S, & Cao HM (2017). Heparanase overexpression down-regulates syndecan-1 expression in a gallbladder carcinoma cell line. Journal of International Medical Research, 45(2), 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen TA, de Souza GA, Odegaard B, Engebretsen L, & Brinchmann JE (2016). MicroRNA-140 inhibits inflammation and stimulates chondrogenesis in a model of interleukin 1beta-induced osteoarthritis. Molecular Therapy–Nucleic Acids, 5(10), e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Wang H, Kainulainen V, Fitzgerald ML, Ledbetter S, Ornitz DM, & Bernfield M (1998). Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nature Medicine, 4(6), 691–697. [DOI] [PubMed] [Google Scholar]
- Kehoe O, Kalia N, King S, Eustace A, Boyes C, Reizes O, … Middleton J (2014). Syndecan-3 is selectively pro-inflammatory in the joint and contributes to antigen-induced arthritis in mice. Arthritis Research and Therapy, 16(4), R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CW, Goldberger OA, Gallo RL, & Bernfield M (1994). Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Molecular Biology of the Cell, 5(7), 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T, Koyama E, Liu M, Golub EE, & Pacifici M (2002). Syndecan-3 is a selective regulator of chondrocyte proliferation. J Biol Chem, 277(44), 42171–42177. [DOI] [PubMed] [Google Scholar]
- Koch AE (2005). Chemokines and their receptors in rheumatoid arthritis:Future targets? Arthritis Rheum, 52(3), 710–721. [DOI] [PubMed] [Google Scholar]
- Kolseth IB, Reine TM, Parker K, Sudworth A, Witczak BJ, Jenssen TG, & Kolset SO (2017). Increased levels of inflammatory mediators and proinflammatory monocytes in patients with type I diabetes mellitus and nephropathy. Journal of Diabetes and Its Complications, 31(1), 245–252. [DOI] [PubMed] [Google Scholar]
- Korb-Pap A, Stratis A, Muhlenberg K, Niederreiter B, Hayer S, Echtermeyer F, … Redlich K (2012). Early structural changes in cartilage and bone are required for the attachment and invasion of inflamed synovial tissue during destructive inflammatory arthritis. Annals of the Rheumatic Diseases, 71(6), 1004–1011. [DOI] [PubMed] [Google Scholar]
- Kufareva I (2016). Chemokines and their receptors: Insights from molecular modeling and crystallography. Current Opinion in Pharmacology, 30, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, Jacobs W, Dhaene K, Weyn B, Bogers J, Weyler J, & Van Marck E (1998). Syndecan-1 expression in malignant mesothelioma: Correlation with cell differentiation, WT1 expression, and clinical outcome. The Journal of Pathology, 186(3), 300–305. [DOI] [PubMed] [Google Scholar]
- Lee B, Leslie G, Soilleux E, O’Doherty U, Baik S, Levroney E, … Doms RW (2001). Cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. Journal of Virology, 75(24), 12028–12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendorf ME, Manon-Jensen T, Kronqvist P, Multhaupt HA, & Couchman JR (2011). Syndecan-1 and syndecan-4 are independent indicators in breast carcinoma. J Histochem Cytochem, 59(6), 615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HC, Multhaupt HA, & Couchman JR (2015). Cell surface heparan sulfate proteoglycans control adhesion and invasion of breast carcinoma cells. Molecular Cancer, 14, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BY, Kim YC, Leatherberry V, Cowin P, & Alexander CM (2003). Mammary gland development requires syndecan-1 to create a beta-catenin/TCF-responsive mammary epithelial subpopulation. Oncogene, 22(58), 9243–9253. [DOI] [PubMed] [Google Scholar]
- Maeda T, Alexander CM, & Friedl A (2004). Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Research, 64(2), 612–621. [DOI] [PubMed] [Google Scholar]
- Maeda T, Desouky J, & Friedl A (2006). Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene, 25(9), 1408–1412. [DOI] [PubMed] [Google Scholar]
- Mahtouk K, Hose D, Raynaud P, Hundemer M, Jourdan M, Jourdan E, … Klein B (2007). Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood, 109(11), 4914–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manon-Jensen T, Itoh Y, & Couchman JR (2010). Proteoglycans in health and disease: The multiple roles of syndecan shedding. FEBS Journal, 277(19), 3876–3889. [DOI] [PubMed] [Google Scholar]
- Masola V, Onisto M, Zaza G, Lupo A, & Gambaro G (2012). A new mechanism of action of sulodexide in diabetic nephropathy: Inhibits heparanase-1 and prevents FGF-2-induced renal epithelial-mesenchymal transition. Journal of Translational Medicine, 10, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami S, Ohashi K, Usui Y, Nemoto T, Katsube K, Yanagishita M, … Koike M (2001). Loss of syndecan-1 and increased expression of heparanase in invasive esophageal carcinomas. Japanese Journal of Cancer Research, 92(10), 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard CJ, Ludeman JP, Canals M, Bridgford JL, Hinds MG, Clayton DJ, … Stone MJ (2014). Structural basis of receptor sulfotyrosine recognition by a CC chemokine: The N-terminal region of CCR3 bound to CCL11/eotaxin-1. Structure, 22(11), 1571–1581. [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Makimura H, & Mobbs CV (2003). The physiological function of the agouti-related peptide gene: The control of weight and metabolic rate. Annals of Medicine, 35(6), 425–433. [DOI] [PubMed] [Google Scholar]
- Mok CF, Xie CM, Sham KW, Lin ZX, & Cheng CH (2013). 1,4-dihydroxy-2-naphthoic acid induces apoptosis in human keratinocyte: Potential application for psoriasis treatment. Evidence-Based Complementary and Alternative Medicine, 2013, 792840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondor I, Moulard M, Ugolini S, Klasse PJ, Hoxie J, Amara A, … Sattentau QJ (1998a). Interactions among HIV gp120, CD4, and CXCR4: dependence on CD4 expression level, gp120 viral origin, conservation of the gp120 COOH- and NH2-termini and V1/V2 and V3 loops, and sensitivity to neutralizing antibodies. Virology, 248(2), 394–405. [DOI] [PubMed] [Google Scholar]
- Mondor I, Ugolini S, & Sattentau QJ (1998b). Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. Journal of Virology, 72(5), 3623–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneau Y, Arenzana-Seisdedos F, & Lortat-Jacob H (2016). The sweet spot: How GAGs help chemokines guide migrating cells. Journal of Leukocyte Biology, 99(6), 935–953. [DOI] [PubMed] [Google Scholar]
- Mytilinaiou M, Bano A, Nikitovic D, Berdiaki A, Voudouri K, Kalogeraki A, … Tzanakakis GN (2013). Syndecan-2 is a key regulator of transforming growth factor beta 2/Smad2-mediated adhesion in fibrosarcoma cells. IUBMB Life, 65(2), 134–143. [DOI] [PubMed] [Google Scholar]
- Nguyen TL, Grizzle WE, Zhang K, Hameed O, Siegal GP, & Wei S (2013). Syndecan-1 overexpression is associated with nonluminal subtypes and poor prognosis in advanced breast cancer. American Journal of Clinical Pathology, 140(4), 468–474. [DOI] [PubMed] [Google Scholar]
- Nilsson U, Johnsson R, Fransson LA, Ellervik U, & Mani K (2010). Attenuation of tumor growth by formation of antiproliferative glycosaminoglycans correlates with low acetylation of histone H3. Cancer Research, 70(9), 3771–3779. [DOI] [PubMed] [Google Scholar]
- Nugent M (2016). MicroRNAs: Exploring new horizons in osteoarthritis. Osteoarthritis and Cartilage, 24(4), 573–580. [DOI] [PubMed] [Google Scholar]
- Parish CR (2006). The role of heparan sulphate in inflammation. Nature Reviews Immunology, 6(9), 633–643. [DOI] [PubMed] [Google Scholar]
- Patterson AM, Gardner L, Shaw J, David G, Loreau E, Aguilar L, … Middleton J (2005). Induction of a CXCL8 binding site on endothelial syndecan-3 in rheumatoid synovium. Arthritis and Rheumatism, 52(8), 2331–2342. [DOI] [PubMed] [Google Scholar]
- Pfander D, Swoboda B, & Kirsch T (2001). Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. The American Journal of Pathology, 159(5), 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikas DS, Li JP, Vlodavsky I, & Lindahl U (1998). Substrate specificity of heparanases from human hepatoma and platelets. Journal of Biological Chemistry, 273(30), 18770–18777. [DOI] [PubMed] [Google Scholar]
- Pletinck A, Van Landschoot M, Steppan S, Laukens D, Passlick-Deetjen J, Vanholder R, & Van Biesen W (2012). Oral supplementation with sulodexide inhibits neo-angiogenesis in a rat model of peritoneal perfusion. Nephrology, Dialysis, Transplantation, 27(2), 548–556. [DOI] [PubMed] [Google Scholar]
- Polte T, Petzold S, Bertrand J, Schutze N, Hinz D, Simon JC, … Averbeck M (2015). Critical role for syndecan-4 in dendritic cell migration during development of allergic airway inflammation. Nature Communications, 6, 7554. [DOI] [PubMed] [Google Scholar]
- Purushothaman A, Chen L, Yang Y, & Sanderson RD (2008). Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. Journal of Biological Chemistry, 283(47), 32628–32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Q, Zhang S, Chen Y, Li R, Mao H, & Chen Q (2015). High glucose-induced intestinal epithelial barrier damage is aggravated by syndecan-1 destruction and heparanase overexpression. Journal of Cellular and Molecular Medicine, 19(6), 1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani VC, Purushothaman A, Stewart MD, Thompson CA, Vlodavsky I, Au JL, & Sanderson RD (2013). The heparanase/syndecan-1 axis in cancer: Mechanisms and therapies. FEBS Journal, 280(10), 2294–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger AC (2013). Synstatin: A selective inhibitor of the syndecan-1-coupled IGF1R-alphavbeta3 integrin complex in tumorigenesis and angiogenesis. FEBS Journal, 280(10), 2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizes O, Benoit SC, & Clegg DJ (2008). The role of syndecans in the regulation of body weight and synaptic plasticity. The International Journal of Biochemistry and Cell Biology, 40(1), 28–45. [DOI] [PubMed] [Google Scholar]
- Reizes O, Benoit SC, Strader AD, Clegg DJ, Akunuru S, & Seeley RJ (2003). Syndecan-3 modulates food intake by interacting with the melanocortin/AgRP pathway. Annals of the New York Academy of Sciences, 994, 66–73. [DOI] [PubMed] [Google Scholar]
- Reizes O, Goldberger O, Smith AC, Xu Z, Bernfield M, & Bickel PE (2006). Insulin promotes shedding of syndecan ectodomains from 3T3–L1 adipocytes: A proposed mechanism for stabilization of extracellular lipoprotein lipase. Biochemistry, 45(18), 5703–5711. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Carpizo D, Plaza-Calonge Mdel C, Torres-Collado AX, Thai SN, Simons M, … Iruela-Arispe ML (2009). Cleavage of syndecan-4 by ADAMTS1 provokes defects in adhesion. The International Journal of Biochemistry and Cell Biology, 41(4), 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscic-Mrkic B, Fischer M, Leemann C, Manrique A, Gordon CJ, Moore JP, … Trkola A (2003). RANTES (CCL5) uses the proteoglycan CD44 as an auxiliary receptor to mediate cellular activation signals and HIV-1 enhancement. Blood, 102(4), 1169–1177. [DOI] [PubMed] [Google Scholar]
- Sawant KV, Poluri KM, Dutta AK, Sepuru KM, Troshkina A, Garofalo RP, & Rajarathnam K (2016). Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Scientific reports, 6, 33123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello CR, & Goldring SR (2012). The role of synovitis in osteoarthritis pathogenesis. Bone, 51(2), 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, … Mohammadi M (2000). Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Molecular Cell, 6(3), 743–750. [DOI] [PubMed] [Google Scholar]
- Schulz JG, Annaert W, Vandekerckhove J, Zimmermann P, De Strooper B, & David G (2003). Syndecan 3 intramembrane proteolysis is presenilin/gamma-secretase-dependent and modulates cytosolic signaling. Journal of Biological Chemistry, 278(49), 48651–48657. [DOI] [PubMed] [Google Scholar]
- Shimada K, Anai S, Fujii T, Tanaka N, Fujimoto K, & Konishi N (2013). Syndecan-1(CD138)contributestoprostatecancerprogressionbystabilizing tumour-initiating cells. The Journal of Pathology, 231(4), 495–504. [DOI] [PubMed] [Google Scholar]
- Shimada K, Nakamura M, De Velasco MA, Tanaka M, Ouji Y, & Konishi N (2009). Syndecan-1, a new target molecule involved in progression of androgen-independent prostate cancer. Cancer Science, 100(7), 1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimo T, Gentili C, Iwamoto M, Wu C, Koyama E, & Pacifici M (2004). Indian hedgehog and syndecans-3 coregulate chondrocyte proliferation and function during chick limb skeletogenesis. Developmental Dynamics, 229(3), 607–617. [DOI] [PubMed] [Google Scholar]
- Stewart MC, Fosang AJ, Bai Y, Osborn B, Plaas A, & Sandy JD (2006). ADAMTS5-mediated aggrecanolysis in murine epiphyseal chondrocyte cultures. Osteoarthritis and Cartilage, 14(4), 392–402. [DOI] [PubMed] [Google Scholar]
- Teng YH, Aquino RS, & Park PW (2012). Molecular functions of syndecan-1 in disease. Matrix Biology, 31(1), 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbinati C, Grillo E, Chiodelli P, Tobia C, Caccuri F, Fiorentini S, … Rusnati M (2016). Syndecan-1 increases B-lymphoid cell extravasation in response to HIV-1 Tat via alphavbeta3/pp60src/pp125FAK pathway. Oncogene, 36(18), 2618–2609. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Fernandez A, Palanche T, Amara A, Magerus A, Altmeyer R, Delaunay T, … Arenzana-Seisdedos F (2001). Optimal inhibition of X4 HIV isolates by the CXC chemokine stromal cell-derived factor 1 alpha requires interaction with cell surface heparan sulfate proteoglycans. Journal of Biological Chemistry, 276(28), 26550–26558. [DOI] [PubMed] [Google Scholar]
- Vasheghani F, Monemdjou R, Fahmi H, Zhang Y, Perez G, Blati M, … Kapoor M (2013). Adult cartilage-specific peroxisome proliferator-activated receptor gamma knockout mice exhibit the spontaneous osteoarthritis phenotype. The American Journal of Pathology, 182(4), 1099–1106. [DOI] [PubMed] [Google Scholar]
- Wang JB, Zhang YJ, Guan J, Zhou L, Sheng Y, Zhang Y, & Si YF (2012). Enhanced syndecan-1 expression on neutrophils in patients with type 2 diabetes mellitus. Acta Diabetologica, 49(1), 41–46. [DOI] [PubMed] [Google Scholar]
- Wang JB, Zhang YJ, Zhang Y, Guan J, Chen LY, Fu CH, … Zhang Y (2013). Negative correlation between serum syndecan-1 and apolipoprotein A1 in patients with type 2 diabetes mellitus. Acta Diabetologica, 50(2), 111–115. [DOI] [PubMed] [Google Scholar]
- Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, … Sixt M (2013). Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science, 339(6117), 328–332. [DOI] [PubMed] [Google Scholar]
- Wei HT, Guo EN, Dong BG, & Chen LS (2015). Prognostic and clinical significance of syndecan-1 in colorectal cancer: A meta-analysis. BMC Gastroenterology, 15, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yaccoby S, Liu W, Langford JK, Pumphrey CY, Theus A, … Sanderson RD (2002). Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood, 100(2), 610–617. [DOI] [PubMed] [Google Scholar]