Summary:
The carbon-fluorine bond engenders distinctive physicochemical properties and significant changes to general reactivity, which have impacted molecular design in material science, agrochemicals, and pharmaceutical research. These unique features are generally derived from fluorine having the highest electronegativity coupled to an enhanced bond strength of the C‒F bond compared to a C‒H bond. As a result, C‒F bonds are often leveraged as replacements for C‒H bonds when the latter presents a liability at a saturated (sp3) carbon centre especially for benzylic C‒H bonds prone to metabolic oxidation. In this context, the development of catalytic, enantioselective methods to set stereocenters containing a benzylic C‒F bond has been an important and rapidly evolving goal in synthetic chemistry. There have been notable advances enabling the construction of secondary stereocenters containing both a C‒F and a C‒H bond on the same carbon. In contrast, there are significantly fewer synthetic strategies defined for accessing stereocenters that incorporate a tertiary C‒F bond, especially those remote from pre-existing activating groups. Herein, we report a general method that establishes C‒F tertiary, benzylic stereocenters by forging a C‒C bond via a Pd-catalyzed enantioselective Heck reaction of acyclic alkenyl fluorides with arylboronic acids. This method provides a platform to rapidly incorporate significant functionality about the benzylic tertiary fluoride by virtue of the diversity of both reaction partners as well as the ability to install the stereocenters remotely from pre-existing functional groups.
The catalytic, enantioselective formation of tertiary C‒F bonds has traditionally been accomplished through bond formation adjacent (defined as α) to a pre-existing functional group (Fig. 1A).1–4 This is highlighted by: 1) the functionalization of carbonyl derivatives via the reaction of an enolate or an enolate equivalent with either an electrophilic fluorinating reagent5–13 or an arylating/alkylating reagent,14–18 leading to a plethora of α-functionalized carbonyl products or 2) the reaction of mainly cyclic alkene substrates with an electrophilic fluorinating source followed by elimination to form an allylic fluoride product.19–20 Trapping of the reactive intermediate in this process with a nucleophile to yield the formal alkene difunctionalization product is also possible.21–25 A recently reported strategy by Hartwig and co-workers provides an alternative approach to acyclic allylic fluorides involving nucleophilic attack onto a π-allyl-iridium species derived from a styrenyl fluoride electrophile.26 While these methods constitute excellent entries to produce the desired tertiary C‒F stereocenters, the specific synthetic positional limitation reflects the requirement of a functional group adjacent to the site at which the fluoride is placed. In order to access products that do not place the fluoride directly adjacent to either a carbonyl or alkenyl group (Fig. 1B), we envisioned a strategy by which an enantioselective Pd-catalyzed Heck reaction of alkenyl fluoride substrates with arylboronic acids could be developed.27,28 By performing such a reaction, a tertiary C‒F benzylic stereocenter could be constructed at modular locations from carbonyl functional groups. Successful implementation of this strategy would enable direct synthetic access to previously unavailable building blocks as both procedures to replace either the tertiary benzylic C‒H or C‒OH bonds with fluoride have yet to be reported at such sites.
Figure 1 |. Constructing chiral, non-racemic tertiary fluorides.
OTs, tosylate; M.S., molecular sieves; dba, dibenzylideneacetone; A, Conventional enantioselective, catalytic approaches. B, Strategy to use alkenyl fluorides as substrates in enantioselective C‒C forming arylation reactions to form remote F-bearing benzylic, tertiary stereocenters. C, preliminary results and optimization of reaction conditions.
Significant issues were anticipated in exploring this reaction sequence due to the general reactivity of alkenyl fluorides and accessibility of the substrate itself. Alkenyl fluorides are not traditional alkenes as exemplified by their unique 13C chemical shifts observed with a downfield shift of ~ 160 ppm, similar to that of an amide, for the C‒F bond (Figure 1B). Comparatively, the other carbon has a chemical shift ~105 and the C‒H bond attached has a rather upfield shift of 4.8 suggesting a highly electronically biased alkene. Thus, alkenyl fluorides have been reported as substrates for Heck-type reactions with insertion occurring at CB but with various caveats.29–35 The first is that tri-substituted alkenyl fluorides of type 1 have not been described as substrates in Heck reactions and presumably would be poorly reactive due to the high electronegativity of the F substituent and the congested nature of the alkene.32 Consistent with this, our lab has reported that alkenes bearing only carbon substituents with smaller groups (e.g., disubstituted vs. trisubstituted alkenes) as well as substrates that are more electron rich (e.g., alkyl vs. aryl) react faster in Heck-type reactions.27,28,36 The second issue is that if migratory insertion is effective, the resulting Pd-alkyl intermediate A (Fig. 1B) may have the propensity to undergo β-fluoride elimination according to reports involving alkenyl fluorides as Heck coupling partners.29,30,34 This would ultimately remove the F from the desired product and likely inhibit the necessary chain-walking process enabled by a cationic Pd catalyst as fluoride would likely form neutral Pd-intermediates.37 Moreover, a significant obstruction to progress in this area has been access to the desired substrates (e.g., 1a, Fig. 1B), which traditionally requires a lengthy synthetic sequence to prepare.38,39 Recently, a simple method has been reported by the Toste team, which overcomes previous limitations by enabling a modular preparation of geometrically pure allylic alkenyl fluoride derivatives in a two-step procedure.40
Using this route, substrate 1a was prepared and submitted to various previously reported conditions for Heck-type reactions of alkenyl alcohols reported by our lab (Fig 1C).27,28 The use of an allylic alcohol as a starting point for reaction optimization was anticipated to slow β-fluoride elimination through accelerating β-hydride elimination towards the alcohol and terminating the chain-walking process through irreversible formation of the aldehyde.27,28 Unfortunately, poor reactivity was observed under these conditions with the best preliminary result of 24% yield using DMF as solvent, a Cu salt, and PyrOx ligand (entry 1). An extensive evaluation of reaction conditions revealed three significant changes that were required to obtain a robust reaction: 1) methanol was identified as a uniquely effective solvent nearly doubling the yield compared to the use of any other common solvent (entry 2, see SI for complete screening details), 2) the addition of dba (dibenzylideneacetone) was essential (entry 3), which may rescue any long-lived Pd(0) intermediates that undergo slow oxidation and 3) in contrast to related oxidative Heck reactions, Cu salts are detrimental to the reaction outcome (entry 5).27,28 The inspiration for the use of dba was found through the observation that Pd(dba)2 gave modestly improved yields when used instead of a Pd(II) complex as the precatalyst (see SI for details). In all, the reaction yield was improved to 90% for the model substrate. Of particular note, by exploiting the simple chiral PyrOx ligand, which is readily prepared in two steps and commercially available, an exceptionally high enantiomeric ratio (er) of 99:1 was observed. Using the other alkene stereoisomer (entry 4) leads to conservation of high enantioselectivity (98:2 er) with the other enantiomer of product formed in reduced yield. Due to the ease in accessing the Z-alkene isomer and the better performance in terms of yield, this alkene isomer was utilized throughout the remainder of the study.
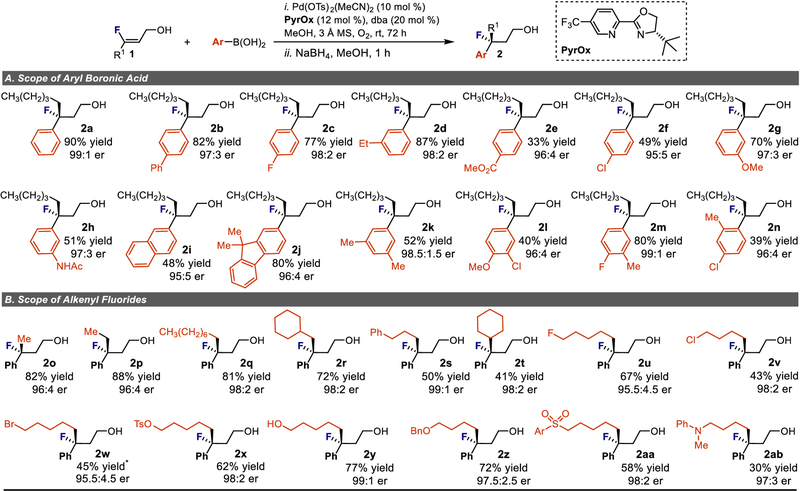
Applying the optimized conditions, the scope of the reaction was first evaluated by exploring the nature of the arylboronic acid coupling partner. In general, the use of more electron rich aryl rings gave enhanced yields compared to more electron poor examples (e.g., 2a-2d vs 2e, 2f). This is presumably due to a faster relative rate of migratory insertion with more nucleophilic arenes.27 In all cases though, the enantioselectivity remained high in an er range of 95:5 to 99:1. Both m-methoxy (2g) and m-acetamide (2h) groups were well-tolerated under the reaction conditions. Multi-substituted arenes including halogen substituted arylboronic acids were also effective coupling partners (2i-2n) highlighted by 2l and 2m. Finally, ortho-substitution on the arene was allowed as demonstrated by the success of 2n, which was produced in reduced yield but high enantioselectivity. Unfortunately, a number of heteroaromatic boronic acids were tested for this reaction with no product detected in most cases (for a complete list, see the SI).
The substrate scope of the alkenyl fluoride reaction partner was explored broadly, which was enabled by the simple synthetic procedure to access them (Fig. 2B).40 Different hydrocarbon substituents generally performed well (2o-2t). Surprisingly, a large cyclohexyl group (2t) attached to the alkene was allowed, leading to a modest product yield in high enantioselectivity (98:2 er). Incorporation of an array of functional groups on the alkene was also well-tolerated. This includes various halides on the alkyl chain (F, Cl, Br, 2u-2w) and a tosylate group (2×), which are substrates that can be reactive under Pd-catalysis.41 These illustrative examples suggest that the current catalyst system is precisely tuned to engage with the alkenyl fluoride in the presence of reactive functionality. Additionally, an alcohol (2y), an ether (2z), a sulfone (2aa) and an aniline (2ab) are all competent substrates in this reaction. Simple basic amines were not compatible with the reaction, likely due to displacement of the chiral ligand, and styrenylfluorides did not undergo the reaction presumably as a consequence of their mitigated nucleophilicity (vide infra). Of particular note, the enantioselectivity remained consistently high in all cases.
Figure 2 |. Enantioselective construction of remote tertiary fluorine-containing stereocenters.
Condition adjustments for 2w: 20 mol % Pd(CH3CN)2(OTs)2, 24 mol % ligand, 40 mol % dba. OTs, tosylate; Bn, benzyl; Ar, tolyl; A, Exploration of scope using various arylboronic acids. B, Evaluation of various alkene substituents and attached functional groups on reaction efficiency.
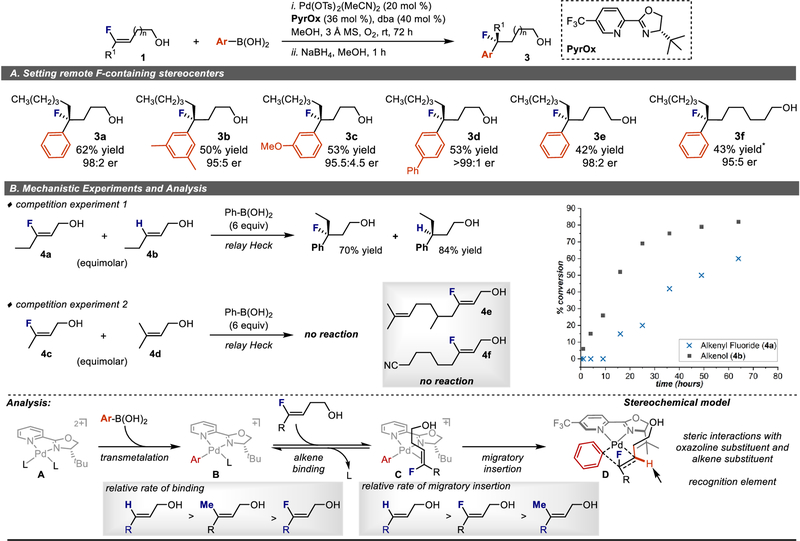
Further evaluation of substrate scope was aimed at highlighting the unique strategic advantage of using a Heck-type reaction of alkenyl fluorides to set stereocenters remote from a pre-existing functional group. Specifically, the scope evaluation in Figure 2 focused on alkenyl fluorides containing an allylic alcohol, which by virtue of the reaction forms an aldehyde product (the aldehyde was reduced in-situ with NaBH4 to avoid decomposition issues associated with isolation of the β-fluoro aldehyde). By adding methylene units between the alcohol and the alkenyl fluoride, a successful reaction would offer a direct route to a remotely located fluorinated benzylic stereocenter (Fig. 3A). In the event, a homoallylic alcohol underwent the desired reaction in reasonable chemical yields and consistently high enantioselectivity (3a-3d). This process provides products with a benzylic fluoride stereocenter three carbons from the resulting alcohol functional group. Additional methylene units were incorporated from the pre-existing functional group as highlighted by 3e and 3f, which have four and six carbons between the installed stereocenter and the oxygen containing functional group. While poorer yields were observed for these cases, the enantioselectivity remained excellent.
Figure 3 |. Evaluation of alkene substrates to establish remote stereocenters and mechanistic studies.
Condition adjustments for 3f: 30 mol % Pd(CH3CN)2(OTs)2, 36 mol % ligand, 60 mol % dba. A, Effect of chain length between the alcohol and the alkenyl fluoride on reaction. B, Mechanistic experiments and analysis.
As described in the introduction, a key mechanistic question is: how does the reactivity of alkenyl fluorides compare to their non-fluorinated derivatives in terms of catalyst binding and migratory insertion aptitude? To probe this question, several relative rate experiments were performed (Fig 3B, upper panel). The first experiment used equimolar amounts of alkenyl fluoride 4a and disubstituted alkenol 4b with excess boronic acid under the optimal conditions identified for alkenyl fluorides. The reaction progress showcases that disubstitued alkenol 4b reacted significantly faster than alkenyl fluoride 4a. Of particular interest, 4b was nearly consumed prior to reaction of the alkenyl fluoride suggesting that migratory insertion is faster for the more electron rich alkene. Perhaps of greater interest is a second experiment evaluating the relative rates of a simple alkenyl fluoride (4c) versus the trisubstituted alkene (4d) in which, surprisingly, no Heck products were detected. It should be noted that trisubstituted alkene 4d, while a competent substrate under previously reported reaction conditions (88% yield),27 does not react under the current conditions even in the absence of the alkenyl fluoride. Taken together, these results suggest that 4d, the trisubstituted alkene, is a considerably better ligand for the Pd catalyst thus inhibiting the reaction of the alkenyl fluoride. Consistent with this hypothesis, substrates containing competitive binding groups such as a trisubstituted alkene (4e) and a nitrile (4f), do not react under our current conditions although other factors may contribute to their poor reactivity.
This information suggests that alkene binding to form intermediate C is reversible after forming B via boronic acid transmetallation (Fig 3B, bottom panel). This hypothesis was developed on the basis of previous mechanistic studies42 and the observation of poorer yields with less nucleophilic boronic acids. Thus, the faster reacting alkene undergoes turnover limiting migratory insertion in C, which is likely under Curtin-Hammett control. Enantioselectivity is determined in this step, wherein the stereochemical model depicted in D showcases that the recognition element of carbon versus hydrogen on the proximal alkene carbon, relative to the alcohol, defines the stereochemical outcome. In terms of general reactivity of alkenyl fluorides, the results detailed above are consistent with the alkenyl fluoride being significantly less efficient at binding the catalyst than either simple di or tri-substituted alkenes, with the latter acting as an inhibitor likely as a result of the enhanced electron density of these alkenes. In contrast, the migratory insertion step is less sensitive to alkene electronics; in fact, the alkenyl fluoride reacts significantly faster than the trisubstituted alkene under the reaction conditions. This observation suggests that the steric component of the alkene has the greatest impact on the consequential migratory insertion step.
In this work, we have described a synthetic strategy to access enantiomerically enriched tertiary fluorides, which would be difficult to form using conventional approaches. The scope of the reaction is broad with uniformly high enantioselective. Remote tertiary fluoride stereocenters can be readily prepared with our strategy providing chemists with a new platform to explore fluorine-containing molecular scaffolds. While halogens have often been leveraged as reactive functional groups in transition metal-catalysed carbon-carbon bond forming reactions, this report demonstrates that catalytic systems can be developed that retain the carbon-fluorine bond. More broadly, we envision that this work will inspire further investigation of the transition metal-catalysed carbon-carbon bond forming reaction previously developed for non-fluorinated alkenes with their fluorinated analogues as a means to forge carbon-fluorine containing stereocenters.
Supplementary Material
Acknowledgements
We thank the National Institutes of Health (NIGMS RO1 GM063540) for their financial support. J. L. would like to thank Shanghai institute of Organic Chemistry, Chinese Academy of Sciences (SIOC) for a postdoctoral fellowship. Q.Y. acknowledges Shanghai Jiao Tong University for a postdoctoral fellowship.
Footnotes
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Data Statement: The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files.
References
- 1.Brunet VA & O’Hagan D Catalytic asymmetric fluorination comes of age. Angew. Chem. Int. Ed 47, 1179–1182 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Ma J-A & Cahard D Update 1 of: asymmetric fluorination, trifluoromethylation, and perfluoroalkylation reactions. Chem. Rev 108, PR1–PR43 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Wu T, Phipps RJ & Toste FD Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev 115, 826–870 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y et al. Modern approaches for asymmetric construction of carbon−fluorine quaternary stereogenic centers: synthetic challenges and pharmaceutical needs. Chem. Rev 118, 3887–3964 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Differding E & Lang RW New fluorinating reagents-I. the first enantioselec-tive fluorination reaction. Tetrahedron Lett. 29, 6087–6090 (1988). [Google Scholar]
- 6.Shibata N, Suzuki E, Asahi T & Shiro M Enantioselective fluorination mediated by cinchona alkaloid derivatives/selectfluor combinations: reaction scope and structural information for N-fluorocinchona alkaloids. J. Am. Chem. Soc 123, 7001–7009 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Mohar B, Baudoux J, Plaquevent J-C & Cahard D Electrophilic fluorination mediated by cinchona alkaloids: highly enantioselective synthesis of α-fluoro-α-phenylglycine derivatives. Angew. Chem., Int. Ed 40, 4214–4216 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Marigo M et al. Enantioselective formation of stereogenic carbon−fluorine centers by a simple catalytic method. Angew. Chem., Int. Ed 44, 3703–3706 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Steiner DD, Mase N & Barbas CF Direct asymmetric α- fluorination of aldehydes. Angew. Chem., Int. Ed 44, 3706–3710 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Shibatomi K et al. Enantioselective fluorination of α-branched aldehydes and subsequent conversion to α-hydroxyacetals via stereospecific C−F bond cleavage. Chem. Sci 7, 1388–1392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You Y, Zhang L & Luo S Reagent-controlled enantioselectivity switch for the asymmetric fluorination of β-ketocarbonyls by chiral primary amine catalysis. Chem. Sci, 8, 621–626 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata N et al. Highly enantioselective catalytic fluorination and chlorination reactions of carbonyl compounds capable of two- point binding. Angew. Chem. Int. Ed 44, 4204–4207 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Reddy DS et al. Desymmetrization-like catalytic enantioselective fluorination of malonates and its application to pharmaceutically attractive molecules. Angew. Chem., Int. Ed 47, 164–168 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Jiao Z et al. Palladium-catalyzed enantioselective α-arylation of α-fluoroketones. J. Am. Chem. Soc 138, 15980–15986 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Bélanger É et al. Enantioselective Pd-catalyzed allylation reaction of fluorinated silyl enol ethers. J. Am. Chem. Soc 129, 1034–1035 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Liang Y & Fu GC Catalytic asymmetric synthesis of tertiary alkyl fluorides: Negishi cross-couplings of racemic α,α-dihaloketones. J. Am. Chem. Soc 136, 5520–5524 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han X et al. Asymmetric Mannich reaction of fluorinated ketoesters with a tryptophan-derived bifunctional thiourea Catalyst. Angew. Chem., Int.Ed 48, 7604–7607 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Xie C et al. Assembly of fluorinated quaternary stereogenic centers through catalytic enantioselective detrifluoroacetylative Aldol reactions. Angew. Chem., Int. Ed 54, 6019–6023 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Ishimaru T et al. Cinchona alkaloid catalyzed enantioselective fluorination of allyl silanes, silyl enol ethers, and oxindoles. Angew. Chem., Int. Ed 47, 4157–4161 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Wu J et al. A combination of directing groups and chiral anion phase-transfer catalysis for enantioselective fluorination of alkenes. Proc. Natl. Acad. Sci. U. S. A 110, 13729–13733 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozano O et al. Organocatalyzed enantioselective fluorocyclizations. Angew. Chem., Int. Ed 50, 8105–8109 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Wolstenhulme JR & Gouverneur V Asymmetric fluorocyclizations of alkenes. Acc. Chem. Res 47, 3560–3570 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Rauniyar V, Lackner AD, Hamilton GL & Toste FD Asymmetric electrophilic fluorination using an anionic chiral phase-transfer catalyst. Science 334, 1681–1684 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Shunatona HP, Fruü; N, Wang Y-M, Rauniyar V & Toste FD Enantioselective fluoroamination: 1,4-addition to conjugated dienes using anionic phase-transfer catalysis. Angew. Chem. Int. Ed 52, 7724–7727 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Egami H et al. Dianionic phase-transfer catalyst for asymmetric fluoro-cyclization. J. Am. Chem. Soc 140, 2785–2788 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Butcher TW & Hartwig JF Enantioselective synthesis of tertiary allylic fluorides by iridium-catalyzed allylic fluoroalkylation. Angew. Chem. Int. Ed 57, 13125–13129 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Mei T-S, Patle HH & Sigman MS Enantioselective construction of remote quaternary stereocentres. Nature 508, 340–344 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mei T-S, Werner EW, Burckle AJ & Sigman MS Enantioselective redox-relay oxidative Heck arylations of acyclic alkenyl alcohols using boronic acids J. Am. Chem. Soc 135, 6830–6833 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amii H & Uneyama K C–F bond activation in organic synthesis. Chem. Rev 109, 2119–2183 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Heitz W & Knebelkamp A Synthesis of fluorostyrenes via palladium-catalyzed reactions of aromatic halides with fluoroolefins. Makromol. Rapid Commun. 12, 69–75 (1991). [Google Scholar]
- 31.Patrick TB, Agboka TY & Gorrell K Heck reaction with 3-fluoro-3-buten-2-one. J. Fluor. Chem 129, 983–985 (2008). [Google Scholar]
- 32.Rousee K, Bouillon JP, Couve-Bonnaire S & Pannecoucke X Stereospecific synthesis of tri- and tetrasubstituted α-fluoroacrylates by Mizoroki-Heck reaction. Org. Lett 18, 540–543 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Hirotaki K & Hanamoto T Mizoroki-Heck reaction of (1-fluorovinyl)methyldi-phenylsilane with aryl iodides. J. Org. Chem 76, 8564–8568 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Thornbury R,T & Toste FD Palladium-catalyzed defluorinative coupling of 1-aryl-2,2- difluoroalkenes and boronic acids: stereoselective synthesis of monofluorostilbenes. Angew. Chem. Int. Ed 55, 11629–11632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Zhao H-W, He J & Zhang C-P Pd-catalyzed Mizoroki-Heck reactions using fluorine-containing agents as the cross-coupling partners. Catalysts 8, 23–57 (2018). [Google Scholar]
- 36.Werner EW, Mei T-S, Burckle AJ & Sigman MS Enantioselective heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science 338, 1455–1458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wada S & Jordan RF Olefin insertion into a Pd–F bond: catalyst reactivation following β-F elimination in ethylene/vinyl fluoride copolymerization. Angew. Chem. Int. Ed 56, 1820–1824 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Lee SH & Schwartz J Stereospecific synthesis of alkenyl fluorides (with retention) via organometallic intermediates. J. Am. Chem. Soc 108, 2445–2447 (1986). [DOI] [PubMed] [Google Scholar]
- 39.Furuya T & Ritter T Fluorination of boronic acids mediated by silver(I) triflate. Org. Lett 11, 2860–2863 (2009). [DOI] [PubMed] [Google Scholar]
- 40.O’Connor T,J & Toste FD Gold-catalyzed hydrofluorination of electron-deficient alkynes: stereoselective synthesis of β‐fluoro michael acceptors. ACS Catal. 8, 5947–5951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jana R, Pathak TP & Sigman MS Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl- organometallics as reaction partners. Chem. Rev 111, 1417–1492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilton MJ et al. Relative reactivity of alkenyl alcohols in the palladium-catalyzed redox-relay Heck reaction. Tetrahedron 71, 6513–6518 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.