Abstract
The Impella device is a catheter-based miniaturized ventricular assist device. Using a retrograde femoral artery access, it is placed in the left ventricle across the aortic valve. The device pumps blood from left ventricle into ascending aorta and helps to maintain a systemic circulation at an upper rate between 2.5 and 5.0 L/min. This results in almost immediate and sustained unloading of the left ventricle, while increasing overall systemic cardiac output. The most common indications for using the Impella device are in the treatment of acute myocardial infarction complicated by cardiogenic shock and to facilitate high risk coronary angioplasty. Other indications include the treatment of cardiomyopathy with acute decompensation, postcardiotomy shock, and off-pump coronary bypass surgery. A growing body of observational and registry data suggest a potentially valuable role for the Impella system in reducing the mortality associated with cardiogenic shock. However, there are, as of yet, no randomized controlled trial data supporting this observation.
Keywords: Impella device, mechanical circulatory support, ventricular assist device, cardiogenic shock, high risk coronary angioplasty, postcardiotomy shock, Archimedes' screw
In 1968, Dr. Adrian Kantrowitz and colleagues at Maimonides Medical Center in New York reported the first successful use of a novel mechanical circulatory support system, intra-aortic balloon pump counter pulsation (IABP), in the treatment of a patient with cardiogenic shock. 1 Subsequently, this technique was rapidly accepted as a valuable treatment in cardiogenic shock, as well as, later, in the treatment of refractory angina pectoris. 2 Indeed, by 1980, over 50,000 IABP procedures had been performed. 3 Bregman and Casarella at Columbia/Presbyterian Center in New York reported successful percutaneous placement of the IABP in 1980. 3 This simplified technical approach soon became the standard with consequent even greater use of the IABP. As coronary angioplasty (PCI) developed in the 1980s and 1990s, interventional cardiologists began performing this procedure in increasingly challenging patient subgroups (such as depressed left ventricular function and multivessel coronary artery disease). Awareness of the potential of IABP to reduce the ischemic burden during such high-risk PCI procedures led to its use to increase the efficacy and safety of high-risk PCI (so-called facilitated PCI). 4 5 6 Remarkably, 50 years after the initial report of the device by Kantrowitz et al 1 , the IABP remains the most commonly used method of mechanical circulatory support in the cardiac catheterization laboratory and in the intensive care units. 5 However, in recent years, large scale, randomized clinical trials have failed to demonstrate a clinically useful role for the IABP in the treatment of patients with acute myocardial infarction complicated by cardiogenic shock 7 and in patients undergoing high-risk PCI. 8 This has resulted in increased attention to other available forms of mechanical circulatory support systems: the Impella device (Abiomed, Danvers, Massachusetts), the Tandem Heart system (Cardiac Assist, Pittsburgh, PA), and ECMO (percutaneous extracorporeal cardiopulmonary support). 9
The Impella device is a catheter-based miniaturized ventricular assist device that pumps blood from left ventricle (LV) into ascending aorta and responsible for systemic circulation at an upper rate between 2.5 and 5.0 L/min. It is notable that, in his initial clinical paper on IABP, Kantrowitz et al 1 emphasized that, for widespread use of a mechanical circulatory support system, simplicity of initiation and maintenance are crucial requirements. 1 As will be explained later in this review, the Impella system admirably fulfills these requirements.
Development of the Impella Device: A Journey from Ancient Greece and Egypt to Modern-Day Medicine
Before further describing the mechanics and set-up of the Impella device, we feel it would be of interest to the readers to provide a brief outline of the remarkable history behind the development of this device. The mechanism by which the Impella device pumps blood directly from the LV to the aorta is based on a machine developed by the Greek mathematician Archimedes (282–212 BC) while he was living in Alexandria, Egypt. 10 This machine, known as Archimedes' screw which is a type of pump used for raising water up. The screw is a helical surface surrounding a central cylindrical shaft and is placed inside a hollow tube. In ancient times, the machine was powered by hand or by cattle and was used to empty water out of leaking ships. It was also used to water fields of crops by using the screw to pull water from lakes and rivers. To use the screw to lift water, the tube must sit on an angle with one end in a body of water, and the screw is turned with a hand crank. As the bottom of the screw turns, it will scoop up water and this water will be carried up to the top of the tube where it spills out. Irrigation systems based on the Archimedes' screw have persisted in some part of the world up to current times.
In 1976, Dr. Richard Wampler, a brilliant young American physician, was visiting Egypt on a medical mission. He became extremely interested in well irrigation pumps in that country. He rapidly realized that these pumps were based on the Archimedes' screw. 11 Returning to the United States, Dr. Wampler became closely involved in designing artificial hearts and left ventricular assist devices. Remembering his observations in Egypt, he set out to design a system, based on the Archimedes' screw, to pump blood from the LV to the aorta. In 1985, while working with Nimbus Corporation in Rancho Cordova, California, he achieved this goal with the invention of the Hemopump (Medtronic, Minneapolis, MN). This device, the forerunner of the Impella device, was catheter-based and introduced via the femoral artery. A rotating screw within a covered housing pulled blood from a port positioned in the LV and pumped it through the housing to an outlet port positioned in the proximal aorta. The screw was powered by an external rotating motor that connected to the screw via a long metal shaft running through the center of the catheter. It provided an additional cardiac output of 3 to 4 L/min. 12 Following a series of meticulous experimental studies by Dr. Wampler, 13 the Hemopump was finally implanted in a patient in 1988. The procedure, performed by Dr. O.H. Frazier at the Texas Heart Institute in Houston, was a spectacular success, even making front page news in the New York Times. 14 However, the Hemopump never became a commercial success and eventually was discontinued. Fortunately, the important concepts of this device were not lost to medicine. Starting in 1991, Siess and colleagues in Aachen, Germany began working on modifications of the Hemopump design to make a more effective assist device. 15 Major advances they devised included using a short rotating impeller rather than a long screw to pump blood and putting a miniature motor on the catheter itself. These changes led to the Impella class of devices. 15 Subsequently, Meyns and colleagues (2000 and 2003) at the University of Leuven in Belgium conducted experimental studies to evaluate the ability of this newly designed device to attenuate the effects of acute myocardial ischemia. Importantly, they found that implantation of the Impella device prior to induction of experimental ischemia reduced myocardial oxygen consumption during ischemia and reperfusion and led to a reduction in infarct size. 16 17 These experimental findings helped lead to the approval in 2005 for clinical use of the Impella system in Europe.
In the U.S., the first of the Impella models (the Impella 2.5) received Food and Drug Administration (FDA) approval in 2008 and the Impella Cardiac Power (CP) model (known as cVAD in Europe) received approval in 2012. Since its introduction, there has been enthusiastic adoption of the Impella with > 50,000 Impella devices having been to date implanted in the U.S. 18 Currently, Impella devices are implanted at > 1,000 sites in the U.S. 19 The cost of an Impella 2.5 device is $22,000 (vs. $800 to 1,000 for an IABP). 20
Setup and Hemodynamic Effects of the Impella
The current left sided Impella devices comprise the Impella 2.5, Impella CP, Impella 5.0, and Impella 5.0/LD (left direct). The Impella 2.5 and Impella CP models are generally inserted via a retrograde femoral arterial approach, similar to the technique used for placement of an IABP ( Fig. 1 ). The larger sized Impella 5.0 model requires an arteriotomy for placement. The Impella 5.0/LD can be placed directly into the proximal aorta via an end-to-end anastomotic conduit. All models are placed across the aortic valve using fluoroscopic or echocardiographic guidance. The pigtail shape of the catheter facilitates crossing of the aortic valve and promotes a stable position. Once in a satisfactory position within the LV, the Impella catheter is connected distally to a portable mobile console that displays invasive pressures with the actual revolutions per minute of the pump, thus guiding the correct positioning of the device. Once activated, the Impella continuously draws blood from the LV via the inlet port and then expels it into the ascending aorta via the outlet port ( Fig. 2 ). The Impella 2.5, Impella CP, and Impella 5.0 can provide antegrade flow up to 2.5 L/min, 4.0 L/min, and 5.0 L/min, respectively. In comparison, the ability of the IABP to augment cardiac output is very modest; no more than 0.5 L/min. By continuously drawing blood from the LV, the Impella unloads the LV, thereby decreasing LV work and myocardial oxygen demand. 9 21 In addition, by delivering large volumes of blood to the aorta, Impella operation results in an increase in mean arterial pressure and cardiac output, resulting, in turn, in improved systemic perfusion and increased coronary flow. Finally, Impella leads to a decrease in pulmonary wedge pressure and a secondary reduction in right ventricular afterload. 9 21
Fig. 1.
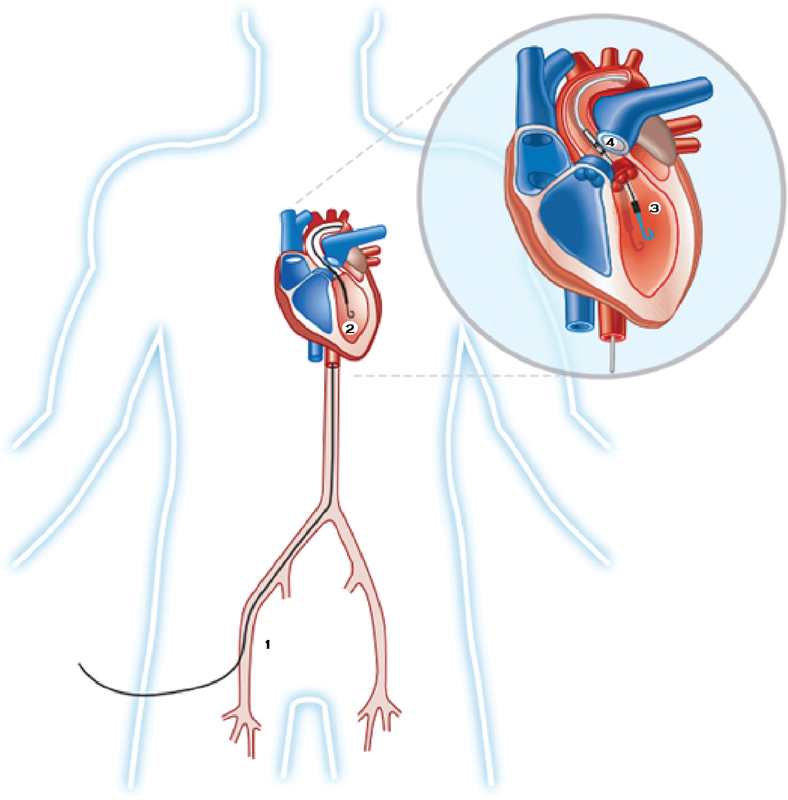
The Impella catheter is inserted percutaneously through the femoral artery (1) and advanced under fluoroscopic or echocardiographic monitoring into the left ventricular cavity (2). The inlet is within the left ventricle (3) and the outlet is in the ascending aorta (4).
Fig. 2.
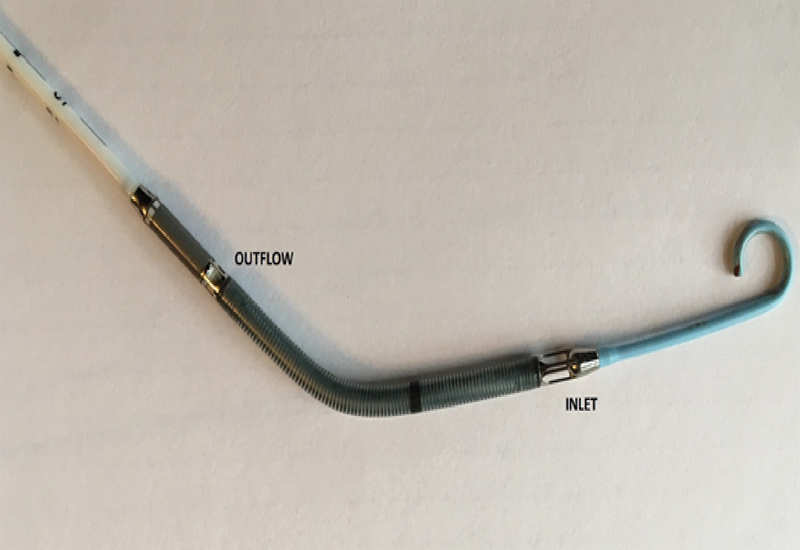
Details of Impella 2.5 catheter. Note the pigtail configuration of the catheter. Blood is drawn from the left ventricle cavity via the inlet and then expelled into the ascending aorta via the outflow.
Indications for Impella Use
The most common indications for using Impella are in the treatment of acute myocardial infarction complicated by cardiogenic shock (AMICS) and to facilitate high risk PCI. Other indications include treatment of cardiomyopathy with acute decompensation, postcardiotomy cardiogenic shock (PCCS), and off-pump coronary bypass surgery. 9 21
Cardiogenic Shock
For nearly 50 years, cardiologists and cardiac surgeons viewed the IABP as an often vital aid in the treatment of patients with cardiogenic shock. Accordingly, the publication in 2012 of the landmark randomized controlled IABP-SHOCK II trial left many of these physicians feeling quite bewildered. The results of this large scale, rigorously executed and meticulously analyzed study concluded that IABP did not reduce mortality in AMICS. 7 Indeed, such was the influence of this trial that use of IABP in AMICS fell from a class I to a class II-a indication in the United States and from a class I to a class II-b indication in Europe. Even when treated with an invasive approach (cardiac catheterization, PCI, or coronary bypass surgery) AMICS is associated with an in-hospital mortality approaching 40%. 22 23 Accordingly, in recent years investigators have directed their attention to other forms of mechanical circulatory support systems that may reduce mortality in this challenging condition. The superior hemodynamic effects of the Impella system, as well as its relative ease of insertion, have led to considerable focus on this device as a possible way of making some dent on the frustratingly high mortality rate associated with cardiogenic shock.
The USpella Registry (that included 38 high volume U.S. medical centers, Danvers, MA) reported on the outcome of 154 patients with AMICS, treated with combined PCI and Impella, between June 2009 and March 2012. 24 A particularly favorable outcome was noted for patients who received the Impella device before PCI was performed. Of these, 65% survived to hospital discharge. 24 Griffith et al performed a prospective multicenter study (the RECOVER I trial, a multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support) in 16 patients with PCCS. 25 The latter condition is associated with a 70% in-hospital mortality. 26 This study was conducted between October 2006 and May 2008. Very encouraging outcomes were reported. Hemodynamics improved immediately after initiation of mechanical support. Cardiac index increased from a mean of 1.65 to 2.7 L/min/m 2 ( p = 0.001) and mean arterial pressure increased from 71.4 to 82.1 mm Hg ( p = 0.01). The primary safety endpoints of death or stroke occurred in only two (17%) patients (one death; one stroke). Survival to 30 days, 3 months, and 1 year was 94, 81, and 75%, respectively. In 2016, on the basis of analysis of the findings of this study and the USpella Registry observations, the U.S. FDA granted approval for use of the Impella device for the treatment of AMICS. 27 Supporting the above encouraging observations were the findings of a single center retrospective study by Lemaire et al that described the outcome of 47 patients with cardiogenic shock who were treated with an Impella device. The authors reported successful removal of the device in 34 of 37 patients (72%) with only four patients requiring transition to long-term left ventricular assist device (LVAD) support. 28 The 30-day, 90-day, and 1-year survival was 72, 66, and 64%, respectively.
Despite the above impressive observational and registry data, it is important to note that there is no evidence based on randomized controlled trials (RCT) to support a role for the Impella device in improving survival in AMICS. 27 There have been three small RCT comparing mortality rates with the Impella and with IABP in cardiogenic shock. 29 30 31 No mortality difference was seen in any of these trials nor with meta-analysis (total 98 patients) of these trials. 32 However, all these trials were markedly underpowered to detect a mortality difference. Cheng et al 33 performed meta-analysis of trials of percutaneous LVADs (Tandem Heart and Impella) in the treatment of cardiogenic shock. In comparison to IABP, these devices, although providing superior hemodynamic support, did not improve early survival. The findings of the IABP-SHOCK II trial flew in the face of conventional thinking and thus serve as a reminder of the necessity of RCT in evaluating the efficacy of Impella in AMICS. While it is appreciated that RCT in the emergency setting of AMICS are exceptionally difficult to conduct, 32 such trials will be required before the Impella device can be confidently embraced or abandoned. Currently, of AMICS cases in the U.S., 42% are treated with an IABP. 34 In sharp contrast, only 6% were treated with an Impella device. 35
In recent years, there has been increasing evidence for the importance of early (i.e., prior to PCI) Impella implantation in improving survival in patients with AMICS. 36 Equally, routine performance of right and left heart catheterization (to allow calculation of measurements, such as cardiac power) has been advocated to optimize management of patients receiving Impella for AMICS. O'Neill et al designed a trial (the Detroit Cardiogenic Shock Initiative) in which both of these practices were routinely employed in AMICS patients. 35 Between July 2016 and April 2017, 41 patients with cardiogenic shock presenting to four metro Detroit sites were enrolled. Survival to explant for the entire cohort was 85%, a significant improvement from institutional historical controls (85 vs. 51%; p < 0.001). Moreover, survival to discharge was an impressive 76%. 37 These encouraging observations resulted in the launch of a national, multicenter, quality initiative entitled the National Cardiogenic Shock Initiative. This initiative will track metrics that have been associated with improved survival in AMICS. Included among these metrics are: (1) Impella use prior to PCI, (2) duration of shock to Impella support time of ≤90 minutes, (3) attainment of cardiac power output > 0.6 W after completion of therapy. It is hoped that with optimal Impella use, survival rates of ≥80% can eventually be achieved in AMICS.
High-Risk Nonemergent PCI
Patients with multivessel or left main coronary artery disease and severely depressed left ventricular function are generally considered for mechanical revascularization by coronary artery bypass graft surgery, particularly if disabling anginal symptoms are present. However, in some of these patients, adverse clinical and angiographic features, such as multiple comorbidities, advanced age, or poor distal targets make surgery an unattractive option. Such patients may be considered for high-risk PCI. 6 This option, of course, is also potentially hazardous as transient ischemia caused by coronary balloon or stent inflation may result in hemodynamic collapse or lethal dysrhythmias. Timely and effective mechanical circulatory support, initiated prior to intervention, may allow complex PCI without abrupt circulatory deterioration during coronary occlusion, thus allowing for more complete revascularization. 21 A large, contemporary, RCT examining the effects of IABP insertion prior to high-risk PCI, showed no reduction in the occurrence of major adverse cardiac events, such as death, stroke, or myocardial infarction. 8 As regards Impella supported PCI, feasibility and safety as well as registry studies 38 39 40 41 have demonstrated that the Impella 2.5 system is safe, easy to implant, and provides excellent hemodynamic support during high-risk PCI. 38 39 40 There has been one randomized controlled trial (the PROTECT II study) with regard to the potential benefits of Impella support in high-risk PCI. 42 The 30-day incidence of major adverse events (the primary end point of the study) was not different for patients with IABP or Impella 2.5 hemodynamic support. However, trends for improved outcomes were observed for Impella 2.5-supported patients at 90 days.
Impella Support for Other Indications
A growing indication for Impella use is to provide hemodynamic support during ablation of ventricular tachycardia (VT). It has been noted that 50 to 80% of patients with structural heart disease referred for VT ablation have unstable VT, thus making electrophysiological testing a considerable hemodynamic challenge. 43 In the PERMIT 1 study Miller et al evaluated the hemodynamic support provided by the Impella 2.5 device during scar-related VT ablation in 20 patients. 44 During fast simulated VT, the device provided a considerably more favorable hemodynamic profile compared with pharmacological agents alone. The authors concluded that Impella supported scar-VT ablation was safe and feasible.
Suradi and Breall have reported use of the Impella device as a bridge to permanent LVAD placement. 45 They described a patient who presented with cardiogenic shock secondary to giant cell myocarditis. The patient was supported hemodynamically with the Impella recover LP 2.5 device until a permanent LVAD could be surgically implanted. The Impella device has also been used to provide temporary mechanical circulatory support in patients with acute heart failure secondary to a variety of conditions, including postpartum cardiomyopathy, 46 Takotsubo cardiomyopathy, 47 and nonischemic cardiomyopathy. 48
Severe aortic stenosis has traditionally been viewed as a relative contraindication to use the Impella device. However, there have been a growing number of reports regarding successful and safe use of the device to support high risk aortic valvuloplasty and PCI. 49 50 51 52 In addition, emergency use of the Impella device to treat acute circulatory collapse following transaortic valve replacement has been described. 53
Contraindications to the Impella Placement
The presence of thrombus in the LV is an important contraindication to the placement of the device. Thrombus may be sucked up by the Impella screw, blocking it and causing it to stop working. In addition, as with any other catheter placed in the LV, the Impella catheter has the potential to dislodge thrombus, thus potentially causing systemic embolization. Accordingly, if time permits, prior echocardiographic visualization of the LV to exclude thrombus is advised in all patients being considered for Impella placement. Moderate to severe aortic valve regurgitation is another important contraindication. In such patients, Impella support will increase aortic pressure and thus worsen aortic regurgitation and LV dilation. Severe peripheral vascular disease will preclude attempting Impella placement via the femoral artery. In such cases, consideration should be given to an axillary artery approach. 54
Complications
The main complications associated with the Impella device are related to vascular access site issues. Percutaneous femoral arterial access for the Impella 2.5 device is obtained with a 14F sheath. This is considerably bigger than the sheath size used for an angioplasty guide catheter (6F) or for an IABP (8F). Vascular complications include hematoma formation, bleeding requiring transfusion, and vascular injury requiring surgical intervention. Meticulous attention should be paid to selection and management of the access site for Impella support to reduce the complications associated with large bore sheaths.
Future Directions
A key future development will be decreasing the size of the Impella catheters so that they can be inserted percutaneously through smaller sized arterial sheaths. This will likely lead to a reduction in vascular complications and make the procedure more widely acceptable to operators.
It is likely that there will soon be increasing adoption of a strategy of early Impella placement in the treatment of patients with cardiogenic shock, as proposed in the National Cardiogenic Shock Initiative. In turn, it is hoped that this strategy will result in improved survival.
Conclusions
A growing body of registry and observational data suggest an important role for the Impella system in the treatment of cardiogenic shock. Recently, it has been appreciated that a strategy of early use of Impella (i.e., prior to performance of PCI) in shock patients is associated with improved survival. Equally, routine performance of right heart catheterization during an Impella-supported PCI helps to guide the therapy and may improve outcomes. There has, however, been, to date, a lack of evidence based on RCT to support a clear role for Impella in the treatment of cardiogenic shock. Equally, while there is much evidence for a useful place for Impella in facilitating selected high-risk PCI procedures, there are no definitive RCT data to support this. Accordingly, RCT examining these issues are urgently needed.
Funding Statement
Funding None.
Conflicts of Interest Dr. A.K. serves as a proctor and speaker for Abiomed.
Authorship
All authors had access to the data and a role in writing the manuscript.
References
- 1.Kantrowitz A, Tjonneland S, Freed P S, Phillips S J, Butner A N, Sherman J L., Jr Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968;203(02):113–118. [PubMed] [Google Scholar]
- 2.Weintraub R M, Aroesty J M, Paulin S et al. Medically refractory unstable angina pectoris. I. Long-term follow-up of patients undergoing intraaortic balloon counterpulsation and operation. Am J Cardiol. 1979;43(05):877–882. doi: 10.1016/0002-9149(79)90348-5. [DOI] [PubMed] [Google Scholar]
- 3.Bregman D, Casarella W J. Percutaneous intraaortic balloon pumping: initial clinical experience. Ann Thorac Surg. 1980;29(02):153–155. doi: 10.1016/s0003-4975(10)61654-2. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson T M, Ohman E M, O'Neill W W, Rab T, Cigarroa J E; Interventional Scientific Council of the American College of Cardiology.A practical approach to mechanical circulatory support in patients undergoing percutaneous coronary intervention: an interventional perspective JACC Cardiovasc Interv 2016909871–883. [DOI] [PubMed] [Google Scholar]
- 5.Rihal C S, Naidu S S, Givertz M M et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015;65(19):e7–e26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Myat A, Patel N, Tehrani S, Banning A P, Redwood S R, Bhatt D L. Percutaneous circulatory assist devices for high-risk coronary intervention. JACC Cardiovasc Interv. 2015;8(02):229–244. doi: 10.1016/j.jcin.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Thiele H, Zeymer U, Neumann F J et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 8.Perera D, Stables R, Thomas M et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA. 2010;304(08):867–874. doi: 10.1001/jama.2010.1190. [DOI] [PubMed] [Google Scholar]
- 9.Burkhoff D, Moscucci M, Henriques J PS. Philadelphia, PA: Lippincott Williams &Wilkins; 2014. Percutaneous circulatory support: intra-aortic balloon counterpulsation, Impella, TandemHeart, and extracorporeal bypass; pp. 601–625. [Google Scholar]
- 10.Oleson J P. Dordrecht, Holland: D. Reidel Publishing Company; 1984. Greek and Roman Mechanical Water-Lifting Devices: The History of a Technology. [Google Scholar]
- 11.Phillips S.Project bionics pioneer interview Richard K. Wampler, MD: interviewed by Steven Phillips, MD 2011. Available from:https://www.asaio.com/wp-content/uploads/2015/04/Richard-Wampler-interview-2011-transcript-final.pdf. Accessed July 26, 2018
- 12.Sweeney M S. The Hemopump in 1997: a clinical, political, and marketing evolution. Ann Thorac Surg. 1999;68(02):761–763. doi: 10.1016/s0003-4975(99)00587-1. [DOI] [PubMed] [Google Scholar]
- 13.Wampler R K, Moise J C, Frazier O H, Olsen D B. In vivo evaluation of a peripheral vascular access axial flow blood pump. ASAIO Trans. 1988;34(03):450–454. [PubMed] [Google Scholar]
- 14.Altman L K.A tiny heart pump saves its first life, researchers reportNew York Times;1988. Available from:https://www.nytimes.com/1988/05/05/us/a-tiny-heart-pump-saves-its-first-life-researchers-report.html. Accessed November 16, 2018.
- 15.Siess T, Nix C, Menzler F. From a lab type to a product: a retrospective view on Impella's assist technology. Artif Organs. 2001;25(05):414–421. doi: 10.1046/j.1525-1594.2001.025005414.x. [DOI] [PubMed] [Google Scholar]
- 16.Meyns B, Sergeant P, Nishida T, Perek B, Zietkiewicz M, Flameng W. Micropumps to support the heart during CABG. Eur J Cardiothorac Surg. 2000;17(02):169–174. doi: 10.1016/s1010-7940(00)00317-1. [DOI] [PubMed] [Google Scholar]
- 17.Meyns B, Stolinski J, Leunens V, Verbeken E, Flameng W. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J Am Coll Cardiol. 2003;41(07):1087–1095. doi: 10.1016/s0735-1097(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 18.Abiomed surpasses 50,000 Impella patients treated in the United States. DAIC,2017. Available from:https://www.dicardiology.com/content/abiomed-surpasses-50000-impella-patients-treated-united-states. Accessed July 14, 2018
- 19.Abiomed Impella Quality (IQ) Database AbiomedDanvers, Massachusetts
- 20.TCT feature: Impella comes in under $100k threshold 2011. Available from:www.cardiovascularbusiness.com/topics/coronary-intervention-surgery/tct-feature-impella-comes-under-100k-threshold. Accessed April 14, 2018
- 21.Burzotta F, Trani C, Doshi S N et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. Int J Cardiol. 2015;201:684–691. doi: 10.1016/j.ijcard.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 22.Kolte D, Khera S, Aronow W S et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3(01):e000590. doi: 10.1161/JAHA.113.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bangalore S, Gupta N, Guo Y et al. Outcomes with invasive vs conservative management of cardiogenic shock complicating acute myocardial infarction. Am J Med. 2015;128(06):601–608. doi: 10.1016/j.amjmed.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill W W, Schreiber T, Wohns D H et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol. 2014;27(01):1–11. doi: 10.1111/joic.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith B P, Anderson M B, Samuels L E, Pae W E, Jr., Naka Y, Frazier O H. The RECOVER I: a multicenter prospective study of Impella 5.0/LD for postcardiotomy circulatory support. J Thorac Cardiovasc Surg. 2013;145(02):548–554. doi: 10.1016/j.jtcvs.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 26.Khorsandi M, Dougherty S, Bouamra O et al. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Surg. 2017;12(01):55. doi: 10.1186/s13019-017-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glazier J J, Kaki A. Improving survival in cardiogenic shock: is Impella the answer? Am J Med. 2018;131(10):e403–e404. doi: 10.1016/j.amjmed.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 28.Lemaire A, Anderson M B, Lee L Y et al. The Impella device for acute mechanical circulatory support in patients in cardiogenic shock. Ann Thorac Surg. 2014;97(01):133–138. doi: 10.1016/j.athoracsur.2013.07.053. [DOI] [PubMed] [Google Scholar]
- 29.Ouweneel D M, Engstrom A E, Sjauw K D et al. Experience from a randomized controlled trial with Impella 2.5 versus IABP in STEMI patients with cardiogenic pre-shock. Lessons learned from the IMPRESS in STEMI trial. Int J Cardiol. 2016;202:894–896. doi: 10.1016/j.ijcard.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 30.Seyfarth M, Sibbing D, Bauer I et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 31.Ouweneel D M, Eriksen E, Sjauw K D et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(03):278–287. doi: 10.1016/j.jacc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Ouweneel D M, Eriksen E, Seyfarth M, Henriques J P. Percutaneous mechanical circulatory support versus intra-aortic balloon pump for treating cardiogenic shock: meta-analysis. J Am Coll Cardiol. 2017;69(03):358–360. doi: 10.1016/j.jacc.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Cheng J M, den Uil C A, Hoeks S E et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30(17):2102–2108. doi: 10.1093/eurheartj/ehp292. [DOI] [PubMed] [Google Scholar]
- 34.Sandhu A, McCoy L A, Negi S I et al. Use of mechanical circulatory support in patients undergoing percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circulation. 2015;132(13):1243–1251. doi: 10.1161/CIRCULATIONAHA.114.014451. [DOI] [PubMed] [Google Scholar]
- 35.O'Neill W W, Grines C, Schreiber T et al. Analysis of outcomes for 15,259 U.S. patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. 2018;202:33–38. doi: 10.1016/j.ahj.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Flaherty M P, Khan A R, O'Neill W W. Early initiation of Impella in acute myocardial infarction complicated by cardiogenic shock improves survival: a meta-analysis. JACC Cardiovasc Interv. 2017;10(17):1805–1806. doi: 10.1016/j.jcin.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Basir M B, Schreiber T, Dixon S et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: the Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv. 2018;91(03):454–461. doi: 10.1002/ccd.27427. [DOI] [PubMed] [Google Scholar]
- 38.Dixon S R, Henriques J P, Mauri L et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (the PROTECT I trial): initial U.S. experience. JACC Cardiovasc Interv. 2009;2(02):91–96. doi: 10.1016/j.jcin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Henriques J P, Remmelink M, Baan J, Jr et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella recover LP 2.5. Am J Cardiol. 2006;97(07):990–992. doi: 10.1016/j.amjcard.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 40.Burzotta F, Paloscia L, Trani C et al. Feasibility and long-term safety of elective Impella-assisted high-risk percutaneous coronary intervention: a pilot two-centre study. J Cardiovasc Med (Hagerstown) 2008;9(10):1004–1010. doi: 10.2459/JCM.0b013e3282f9abe7. [DOI] [PubMed] [Google Scholar]
- 41.Sjauw K D, Konorza T, Erbel R et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol. 2009;54(25):2430–2434. doi: 10.1016/j.jacc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 42.O'Neill W W, Kleiman N S, Moses J et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126(14):1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 43.Reddy Y M, Chinitz L, Mansour M et al. Percutaneous left ventricular assist devices in ventricular tachycardia ablation: multicenter experience. Circ Arrhythm Electrophysiol. 2014;7(02):244–250. doi: 10.1161/CIRCEP.113.000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller M A, Dukkipati S R, Chinitz J S et al. Percutaneous hemodynamic support with Impella 2.5 during scar-related ventricular tachycardia ablation (PERMIT 1) Circ Arrhythm Electrophysiol. 2013;6(01):151–159. doi: 10.1161/CIRCEP.112.975888. [DOI] [PubMed] [Google Scholar]
- 45.Suradi H, Breall J A. Successful use of the Impella device in giant cell myocarditis as a bridge to permanent left ventricular mechanical support. Tex Heart Inst J. 2011;38(04):437–440. [PMC free article] [PubMed] [Google Scholar]
- 46.Schroeter M R, Unsöld B, Holke K, Schillinger W. Pro-thrombotic condition in a woman with peripartum cardiomyopathy treated with bromocriptine and an Impella LP 2.5 heart pump. Clin Res Cardiol. 2013;102(02):155–157. doi: 10.1007/s00392-012-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pineton de Chambrun M, Bréchot N, Combes A. Mechanical circulatory devices in acute heart failure. Curr Opin Crit Care. 2018;24(04):286–291. doi: 10.1097/MCC.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 48.Cena M, Karam F, Ramineni R, Khalife W, Barbagelata A. New Impella cardiac power device used in patient with cardiogenic shock due to nonischemic cardiomyopathy. Int J Angiol. 2016;25(04):258–262. doi: 10.1055/s-0034-1384822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez C A, Singh V, Londoño J C et al. Percutaneous retrograde left ventricular assist support for interventions in patients with aortic stenosis and left ventricular dysfunction. Catheter Cardiovasc Interv. 2012;80(07):1201–1209. doi: 10.1002/ccd.24303. [DOI] [PubMed] [Google Scholar]
- 50.Londoño J C, Martinez C A, Singh V, O'Neill W W. Hemodynamic support with impella 2.5 during balloon aortic valvuloplasty in a high-risk patient. J Interv Cardiol. 2011;24(02):193–197. doi: 10.1111/j.1540-8183.2010.00625.x. [DOI] [PubMed] [Google Scholar]
- 51.Ludeman D J, Schwartz B G, Burstein S. Impella-assisted balloon aortic valvuloplasty. J Invasive Cardiol. 2012;24(01):E19–E20. [PubMed] [Google Scholar]
- 52.Spiro J, Venugopal V, Raja Y, Ludman P F, Townend J N, Doshi S N. Feasibility and efficacy of the 2.5 L and 3.8 L impella percutaneous left ventricular support device during high-risk, percutaneous coronary intervention in patients with severe aortic stenosis. Catheter Cardiovasc Interv. 2015;85(06):981–989. doi: 10.1002/ccd.25355. [DOI] [PubMed] [Google Scholar]
- 53.Martinez C A, Singh V, Heldman A W, O'Neill W W. Emergent use of retrograde left ventricular support in patients after transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2013;82(02):E128–E132. doi: 10.1002/ccd.24583. [DOI] [PubMed] [Google Scholar]
- 54.Lotun K, Shetty R, Patel M, Arain S A. Percutaneous left axillary artery approach for Impella 2.5 liter circulatory support for patients with severe aortoiliac arterial disease undergoing high-risk percutaneous coronary intervention. J Interv Cardiol. 2012;25(02):210–213. doi: 10.1111/j.1540-8183.2011.00696.x. [DOI] [PubMed] [Google Scholar]


