Abstract
Introduction.
Potentially carcinogenic hazardous air pollutants (air toxics) have been inconsistently associated with breast cancer. Whether metabolic factors modify these associations is unknown. We studied 29 non-metallic air toxics classified as mammary gland carcinogens in animal studies in relation to breast cancer risk.
Methods.
Participants included 49,718 women from the Sister Study. Census tract air toxic concentration estimates from the 2005 National Air Toxics Assessment were linked to enrollment residential addresses. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for individual air toxics were estimated using Cox regression. Body mass index (BMI) was considered a potential modifier. Relevant mixtures were identified using classification trees.
Results.
Over follow-up (average=8.4 years), 2,975 women were newly diagnosed with breast cancer (invasive or ductal carcinoma in situ). Several air toxics, including methylene chloride, polycyclic organic matter, propylene dichloride, and styrene, were associated with increased risk. Of these, methylene chloride was most consistently associated with risk across multiple analyses. It was associated with overall (HRquintile 4vs1=1.21 (95%CI=1.07–1.38)) and estrogen receptor positive (ER+) invasive breast cancer (HRquintile 4vs1=1.28 (95%CI=1.08–1.52)) in individual pollutant models, although no dose-response was observed. Associations were stronger among overweight/obese (vs. non-overweight/obese) women (p<0.05) for six air toxics. The classification tree identified combinations of age, methylene chloride, BMI, and four other toxics (propylene dichloride, ethylene dibromide, ethylidene dichloride, styrene) related to overall breast cancer.
Conclusions.
Some non-metallic air toxics, particularly methylene chloride, were associated with the hazard for overall and ER+ breast cancer. Overweight/obese women may be particularly susceptible to air toxics.
Keywords: hazardous air pollutants, air toxics, breast cancer, obesity
1. INTRODUCTION
The United States (US) Environmental Protection Agency (EPA) Clean Air Act designates 187 chemicals as hazardous air pollutants, or air toxics, because they are known or suspected carcinogens or cause other serious health effects [1]. Although levels of air toxics have declined in the US over the past few decades, millions of tons are still emitted annually from a variety of sources such as gas stations, vehicular traffic, dry cleaners, and manufacturers [2].
A number of air toxics demonstrate estrogen-like activity [3–6], increase oxidative stress [7–16], or increase inflammation [8,9,17], which are hypothesized to play a role in breast carcinogenesis [18–25]. Breast cancer is the most common cancer among US women, with 266,120 diagnoses estimated in 2018 [26]. Due to the high burden of breast cancer and the ubiquitous nature of air toxics, a better understanding of the association between air toxics and breast cancer risk is of substantial public health interest. Three studies have examined the association between non-metallic air toxics and breast cancer risk, and results have been inconsistent as to whether specific air toxics are related to breast cancer risk [27–29]. Only one study has examined the non-metallic air toxic associations on a US-wide scale [28].
Previous studies of non-metallic air toxics have not comprehensively considered effect measure modification by physical activity or body mass index (BMI). Physical activity is inversely associated with breast cancer incidence [30–32], can lead to higher clearance efficiency of inhaled pollutants, and can reduce oxidative stress [33]. However, being more physically active could lead to greater exposure, increased ventilation, and a higher respiratory rate [34]. Obesity is associated with elevated postmenopausal breast cancer risk [35,36]. Higher BMI is also related to oxidative stress which may act in concert with that from air toxics [37].
We examined breast cancer incidence in relation to 29 non-metallic hazardous air pollutants that were previously found to be mammary gland carcinogens in animal models [38] and were estimated as part of the 2005 National Air Toxics Assessment (NATA). We further evaluated effect-measure modification by physical activity and BMI. Since individuals are not exposed to air toxics in isolation, but inhale air pollution as a complex mixture, we also used classification trees to explore whether there are combinations of air toxics that are particularly related to breast cancer risk.
2. MATERIALS AND METHODS
2.1. Study Design and Sister Study Population
We used data from the Sister Study, a prospective cohort of 50,884 women from across the US who were ages 35–74 at enrollment [39]. Participants were recruited from 2003–2009 using a national advertising campaign in English and Spanish. Women were eligible for the Sister Study if they had a sister who had been diagnosed with breast cancer, but no prior breast cancer themselves.
At baseline, women completed a computer-assisted telephone interview and written questionnaires to assess demographics, lifestyle factors, medical and family history, and residential history. Participants complete annual health updates and triennial follow-up questionnaires to assess changes in health and risk factor information. Response rates have remained above 91% over follow-up [40].
All women provided written informed consent. The Institutional Review Boards of the National Institute of Environmental Health Sciences and the Copernicus Group approved the study. The research presented here includes follow-up through September 15th, 2016 (data release 6.0).
2.2. Air Toxics Exposure Assessment
NATA is a database created by the US EPA of modeled air toxic concentrations at the census tract level [41]. The estimates from the 2005 version were chosen for this study because they represented the middle of the enrollment period (2003–2009) for the Sister Study. Further, 94% of women enrolled in 2005 or later, and thus the exposure assessment primarily predated enrollment for the majority of Sister Study participants. NATA methods have been described previously [42]. Briefly, emissions of air toxics from point (e.g. large factories/waste incinerators), non-point (e.g. prescribed burns/dry cleaners/small manufacturers), and on-road and non-road mobile (e.g. cars/buses/boats) sources are compiled into the National Emissions Inventory (NEI). These emissions are used as inputs to two air dispersion models, ASPEN (non-point sources) and HEM-3 (point and mobile sources). The dispersion models account for a number of parameters, such as meteorological factors, census data, and the rates, location, and height of release from the source, to estimate the ambient concentration of each air toxic in a census tract. NATA also accounts for background (long-range transport or persistence from past years) and secondary formation (reactions causing formation or decay of air toxics in the atmosphere). Sister Study participants reported their residential address at baseline, which was geocoded and linked to NATA by census tract number.
The 2005 NATA includes 177 EPA-targeted air toxics. A review identified 216 chemicals as mammary gland carcinogens based on animal studies [38]. Of these, 37 were air toxics and were available in the 2005 NATA. However, eight had extensive missing information (>75%) so the remaining 29 were examined.
2.3. Incident Breast Cancer
Women who reported a breast cancer diagnosis on the annual health updates or follow-up questionnaires were asked to provide additional diagnosis information, and to grant permission for the study to obtain medical records and pathology reports. Medical records were obtained for 81% of breast cancer diagnoses. Agreement between self-reported breast cancers and medical records was high (positive predictive value >99%) [39], so self-report was used when medical records were not available. Tumor characteristics (stage; histology; and estrogen receptor (ER) status) were abstracted from medical records, or self-reported.
2.4. Population Included in this Study
Of the 50,884 women in the Sister Study, we excluded 163 women whose breast cancer was diagnosed before their enrollment was complete or did not have follow-up information. Additionally, the baseline residential address for 1,003 (<2%) women could not be geocoded at the census tract level for linkage to NATA concentrations, so they were excluded from our study. Women with addresses that could not be geocoded include all Sister Study participants who were residents of Puerto Rico (n=882), as well as a small percentage of those who resided in West Virginia (n=19; 7.2% of participants from West Virginia), Missouri (n=16; 1.0% of participants from Missouri), and Oklahoma (n=11; 2.6% of participants from Oklahoma). Therefore, our study included 49,718 women.
For 1,4-dioxane, 2-chloroacetophenone, and acrylamide, air toxic concentrations were missing for 1,210 (2.4%), 369 (0.7%), and 4,096 (8.2%) women, respectively, because NATA modeling was not complete for their census tract; therefore, these women with missing air toxic estimates were not included in models that considered those three toxics or in the multipollutant analysis. Estimates for the remaining 26 air toxics were 100% complete for the 49,718 women in this study.
2.5. Covariates
Most covariates were assessed by self-report at the baseline interview. Confounders were determined using a directed acyclic graph (DAG) [43,44]. We reviewed the literature to determine risk factors for breast cancer that are also related to the exposure directly or through a relationship with another variable, for inclusion on our DAG (Supplemental Figure 1). These consisted of age, race, education, cigarette smoking, marital status, menopausal status, parity, hormone replacement therapy use, BMI, and residence type. The final DAG-determined minimally sufficient adjustment set used in our models was race (non-Hispanic white/non-Hispanic black/Hispanic/other), residence type (urban/suburban/small town/rural), highest level of education (<high school/high school/some college/≥4-year degree), and cigarette smoking (never/past/current).
BMI and physical activity were examined as potential modifiers of the air toxic-breast cancer associations. Height and weight were measured by trained examiners during a baseline home visit and used to calculate BMI. At the baseline interview, women were asked to report what sport/exercise activities they participated in during the past 12 months and the number of months, days per week and amount of time per day that they did each activity. This was used to calculate the average hours/week of exercise. To maximize power for the modification analyses, BMI was dichotomized as <25.0 kg/m2 and ≥25 kg/m2, and physical activity as ≤median (≤2 hours/week) and >median (>2 hours/week).
2.5. Statistical Analysis
2.5.1. Single Pollutant Analysis.
In single pollutant models, air toxics were categorized based on quintiles of each exposure among the study population. We considered models that examined the air toxic exposures continuously, but the assumption of linearity did not hold so we only reported the categorized results. We estimated hazard ratios and 95% confidence intervals (CIs) comparing index categories of exposure to exposures below the first quintile using Cox proportional hazards regression [45], with age as the time scale. Individuals were considered at-risk from study enrollment until the earliest of date of breast cancer diagnosis, date of study withdrawal, last follow-up, or death. We estimated p-values for trend by using a linear term for the quintile-category specific median of each exposure. The proportional hazards assumption was evaluated by conducting a Wald test for an interaction between the continuous form of the air toxic measure and time; violations were not observed. We estimated HRs for overall breast cancer (ductal carcinoma in situ (DCIS) and invasive combined) as well as for breast cancer stratified by invasive ER status (ER status was less frequently available for in situ tumors so the analysis by ER status was restricted to invasive tumors), and by pre-/post- menopause status. Women who transitioned from premenopausal to postmenopausal during follow-up were censored at the age of menopause for premenopausal risk time. Person-time occurring after menopause contributed to postmenopausal risk time.
Effect-measure modification by BMI and physical activity were each examined on the additive and multiplicative scales. On the additive scale, single referent hazard ratios were used to calculate the interaction contrast ratio (ICR) and 95% CIs [46–48]. On the multiplicative scale, an interaction term was used to estimate stratified HRs in each category of the modifier, and the ratio of the HRs (and corresponding 95% CIs). To further examine multiplicative modification, nested models (with and without an interaction term) were used to compute the likelihood ratio test (α=0.05) [49].
In sensitivity analyses, we examined whether the single-pollutant associations with overall breast cancer changed when: (a) restricted to invasive disease; (b) restricted to non-Hispanic whites (85% of the study population); (c) restricted to those who had lived at their baseline address >10 years (49% of the study population); (d) restricted to those who enrolled in 2005 or later (94% of the study population); (e) restricted to cases confirmed by medical record (81% of cases); and (f) additionally adjusted for US region (Northeast/Midwest/South/West) of residence. All analyses were completed in SAS 9.4 (Cary, NC.).
2.5.2. Multipollutant Analysis.
We used Classification and Regression Tree (CART) methods to explore whether there are combinations of pollutants that may be more or less harmful for breast cancer than would be expected based on exposure to only a single pollutant in isolation, while also addressing co-pollutant confounding [50,51]. CART is a forward-selection approach that selects, at each step, the variable with the strongest association with the outcome and performs a binary split according to that variable. Further partitions occur within the previous partitions, which forms “branches” that characterize the expected risk in strata of the variables on the branch. Risk is estimated for each individual by taking the average outcome among the participants in the end of each branch in which that participant is partitioned. We included the 29 air toxics, age at baseline, BMI, and physical activity in the classification trees. Age, BMI, and physical activity were included to parallel the single pollutant analyses. The most common splitting criterion for classification trees, and the one used in this analysis, is the Gini index [51]. For the stopping criteria, we specified that the tree grow to a maximum depth of five levels and that the minimum number of total observations in a node be 12 (leading to at least five cases per node). Cost-complexity pruning [50] was used to create a tree with a total of 11 terminal nodes. These specifications were used to control the size of the tree so that it wouldn’t be too large and lose interpretability, while still identifying meaningful groups. The final tree was chosen by balancing model fit and interpretability across combinations of maximum depth, node size, and number of terminal nodes. CART was conducted using SAS function PROC HPSPLIT.
3. RESULTS
3.1. Population Characteristics
After an average of 8.4 years of follow-up 2,975 (658 DCIS and 2,309 invasive) breast cancers were diagnosed. Characteristics of women in the Sister Study have been described previously [39]. Among the 49,718 women (98% of Sister Study participants) included in this study, 85.1% were non-Hispanic white, 51.0% had a college degree or higher, 48.9% had lived in their baseline address >10 years, 55.8% never smoked, and 61.7% were overweight/obese (Supplemental Table 1).
In our study population, the mean census tract estimated air toxic concentrations ranged from 2.30×10−7μg/m3 for benzidine to 2.21μg/m3 for toluene (Table 1). Quintile cutpoints for each air toxic are also provided in Table 1.
Table 1.
Mean concentrations and quintile cutpoints of the individual hazardous air pollutants (μg/m3) among the 49,718 Sister Study women in this study
| Air Toxic | Mean | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 |
|---|---|---|---|---|---|---|
| 1,2-Dibromo-3-chloropropane | 4.81×10−6 | 0–1.30×10−6 | >1.30×10−6–1.39×10−6 | >1.39×10−6–2.47×10−6 | >2.47×10−6–5.59×10−6 | >5.59×10−6 |
| 1,3-butadiene | 0.07 | 0–0.03 | >0.03–0.05 | >0.05–0.07 | >0.07–0.09 | >0.09 |
| 1,4-dioxanea | 1.17×10−4 | 0 | -- | >0–1.33×10−6 | >1.33×10−6–6.08×10−5 | >6.08×10−5 |
| 2,4-dinitrotoluene | 1.41×10−5 | 0–9.27×10−7 | >9.27×10−7–4.239×10−6 | >4.23×10−6–8.70×10−6 | >8.70×10−6–1.68×10−5 | >1.68×10−5 |
| 2,4-toluene diisocyanate | 1.45×10−4 | 0–8.79×10−6 | >8.79×10−6–2.21×10−5 | >2.21×10−5–4.11×10−5 | >4.11×10−5–8.29×10−5 | >8.29×10−5 |
| 2-chloroacetophenonea | 4.23×10−7 | 0 | -- | >0–9.94×10−8 | >9.94×10−8–3.07×10−7 | >3.07×10−7 |
| Acrylamidea | 6.17×10−6 | 0 | -- | >0–9.95×10−8 | >9.95×10−8–8.52×10−7 | >8.52×10−7 |
| Acrylonitrile | 0.01 | 0–1.58×10−3 | >1.58×10−3–2.92×10−3 | >2.92×10−3–5.33×10−3 | >5.33×10−3–9.43×10−3 | >9.43×10−3 |
| Benzene | 0.99 | 0–0.58 | >0.58–0.79 | >0.79–0.99 | >0.99–1.30 | >1.30 |
| Benzidinea | 2.30×10−7 | 9.90×10−9 | -- | >9.90×10−9–2.27×10−8 | >2.27×10−8–2.70×10−7 | >2.70×10−7 |
| Carbon tetrachloride | 0.61 | 0–0.610 | >0.610–0.610 | >0.610–0.610 | >0.610–0.610 | >0.610 |
| Chloroprene | 3.84×10−4 | 0–6.06×10−7 | >6.06×10−7–2.14×10−6 | >2.14×10−6–4.28×10−6 | >4.28×10−6–8.54×10−6 | >8.54×10−6 |
| Ethylbenzene | 0.20 | 0–0.05 | >0.05–0.11 | >0.11–0.18 | >0.18–0.31 | >0.31 |
| Ethylene dibromide | 4.79×10−4 | 0–2.18×10−4 | >2.18×10−4–3.21×10−4 | >3.21×10−4–4.59×10−4 | >4.59×10−4–6.51×10−4 | >6.51×10−4 |
| Ethylene dichloride | 3.69×10−3 | 0–2.27×10−3 | >2.27×10−3–2.68×10−3 | >2.68×10−3–3.15×10−3 | >3.15×10−3–4.06×10−3 | >4.06×10−3 |
| Ethylene oxide | 6.40×10−3 | 0–1.54×10−3 | 1.54×10−3–3.17×10−3 | >3.17×10−3–5.566×10−3 | >5.57×10−3–9.10×10−3 | >9.10×10−3 |
| Ethylidene dichloride | 6.49×10−4 | 0–1.31×10−5 | >1.31×10−5–4.66×10−5 | >4.66×10−5–1.13×10−4 | >1.13×10−4–4.28×10−4 | >4.28×10−4 |
| Hydrazine | 4.82×10−6 | 0–1.30×10−7 | >1.30×10−7–1.31×10−7 | >1.31×10−7–3.91×10−7 | >3.91×10−7–3.60×10−6 | >3.60×10−6 |
| Methylene chloride | 0.34 | 0–0.16 | >0.16–0.23 | >0.23–0.31 | >0.31–0.41 | >0.41 |
| Nitrobenzene | 1.25×10−5 | 0–1.04×10−7 | >1.04×10−7–5.701×10−7 | >5.70×10−7–1.27×10−6 | >1.27×10−6–2.75×10−6 | >2.75×10−6 |
| o-Toluidine | 2.78×10−6 | 0–6.51×10−9 | >6.51×10−9–3.07×10−8 | >3.07×10−8–1.07×10−7 | >1.07×10−7–3.85×10−7 | >3.85×10−7 |
| Polycyclic organic matter | 0.02 | 0–0.004 | >0.004–0.01 | >0.007–0.01 | >0.01–0.02 | >0.02 |
| Propylene dichloride | 1.59×10−3 | 0–5.15×10−4 | >5.15×10−4–5.95×10−4 | >5.95×10−4–7.92×10−4 | >7.92×10−4–1.93×10−3 | >1.93×10−3 |
| Propylene oxide | 4.32×10−4 | 0–2.86×10−5 | >2.86×10−5–9.50×10−5 | >9.50×10−5–2.03×10−4 | >2.03×10−4–4.09×10−4 | >4.09×10−4 |
| Styrene | 0.06 | 0–0.01 | >0.01–0.03 | >0.03–0.04 | >0.04–0.07 | >0.07 |
| Toluene | 2.21 | 0–1.02 | >1.02–1.60 | >1.60–2.22 | >2.22–3.11 | >3.11 |
| Vinyl chloride | 2.22×10−3 | 0–3.73×10−5 | >3.73×10−5–1.45×10−4 | >1.45×10−4–4.06×10−4 | >4.06×10−4–1.85×10−3 | >1.85×10−3 |
| Vinylidene chloride | 1.86×10−4 | 0–2.53×10−5 | >2.53×10−5–5.79×10−5 | >5.79×10−5–1.05×10−4 | >1.05×10−4–1.94×10−4 | >1.94×10−4 |
| Xylenes | 0.96 | 0–0.25 | >0.25–0.53 | >0.53–0.88 | >0.88–1.43 | >1.43 |
Air toxic categorized with a concentration of 0 (9.9×10−9 for benzidine) as the referent and remaining values split based on tertiles
3.2. Results from Single Pollutant Models
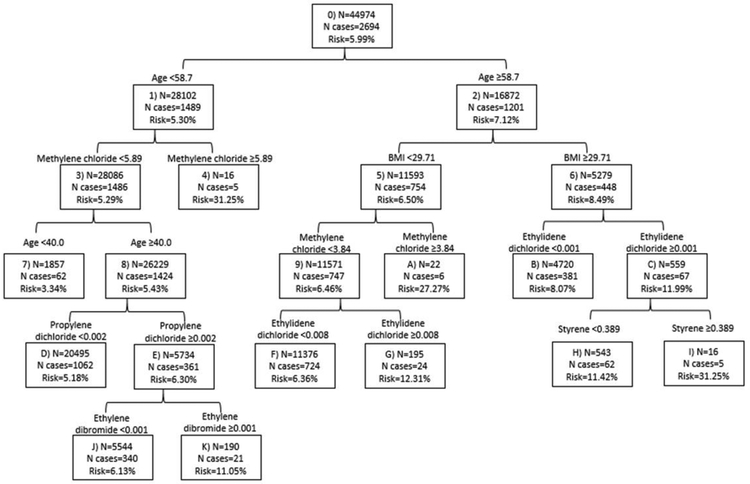
Methylene chloride was associated with an increased risk of overall (HRquintile 4vs1=1.21; 95% CI=1.07–1.38) (Table 2) and ER+ invasive breast cancer (HRquintile 4vs1=1.28; 95% CI=1.08–1.52) (Table 3). However, the associations were non-monotonic. For ER+ breast cancer, HRs were elevated for all quintile-based categories of exposure relative to the lowest category. The positive association with methylene chloride was also observed for postmenopausal breast cancer, and was maintained across sensitivity analyses when restricting, separately, to invasive cases, non-Hispanic whites, those who enrolled in 2005 or later, cases confirmed by medical record, and when adjusted for region (data not shown). Methylene chloride was also identified as a variable of importance in the classification tree (Figure 1). The combination of these results indicates that, among the 29 air toxics we considered, methylene chloride was most consistently associated with elevated breast cancer risk.
Table 2.
HRs (95% CIs) for the associations between individual hazardous air pollutants and overall breast cancer risk, the Sister Study
| Air Toxic | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | p-trend |
|---|---|---|---|---|---|---|
| 1,2-Dibromo-3-chloropropane | 1.00 (ref) | 0.99 (0.87, 1.14) | 1.05 (0.95, 1.17) | 1.08 (0.97, 1.19) | 1.01 (0.91, 1.12) | 1.0 |
| 1,3-butadiene | 1.00 (ref) | 0.99 (0.88, 1.11) | 1.05 (0.92, 1.19) | 1.05 (0.92, 1.20) | 1.06 (0.92, 1.22) | 0.3 |
| 1,4-dioxaneb | 1.00 (ref) | -- | 1.01 (0.91, 1.12) | 1.00 (0.89, 1.11) | 0.93 (0.83, 1.04) | 0.2 |
| 2,4-dinitrotoluene | 1.00 (ref) | 1.02 (0.91, 1.15) | 0.97 (0.87, 1.09) | 0.95 (0.85, 1.07) | 0.97 (0.86, 1.09) | 0.5 |
| 2,4-toluene diisocyanate | 1.00 (ref) | 1.00 (0.88, 1.13) | 1.02 (0.89, 1.16) | 1.11 (0.97, 1.27) | 1.03 (0.90, 1.18) | 0.8 |
| 2-chloroacetophenoneb | 1.00 (ref) | -- | 1.06 (0.96, 1.17) | 1.05 (0.95, 1.16) | 1.00 (0.90, 1.10) | 0.7 |
| Acrylamideb | 1.00 (ref) | -- | 0.98 (0.86, 1.11) | 0.99 (0.88, 1.12) | 1.08 (0.96, 1.22) | 0.2 |
| Acrylonitrile | 1.00 (ref) | 1.12 (1.00, 1.26) | 0.99 (0.89, 1.12) | 0.98 (0.87, 1.10) | 1.04 (0.93, 1.17) | 0.8 |
| Benzene | 1.00 (ref) | 1.05 (0.93, 1.18) | 1.04 (0.92, 1.19) | 0.97 (0.85, 1.12) | 1.05 (0.92, 1.21) | 0.8 |
| Benzidineb | 1.00 (ref) | -- | 1.03 (0.92, 1.14) | 1.11 (1.00, 1.23) | 1.04 (0.93, 1.16) | 0.7 |
| Carbon tetrachloride | 1.00 (ref) | 1.03 (0.92, 1.16) | 1.01 (0.90, 1.14) | 0.97 (0.86, 1.09) | 1.03 (0.91, 1.16) | 0.9 |
| Chloroprene | 1.00 (ref) | 1.07 (0.95, 1.20) | 1.02 (0.91, 1.15) | 0.96 (0.85, 1.08) | 1.00 (0.89, 1.13) | 0.6 |
| Ethylbenzene | 1.00 (ref) | 1.00 (0.88, 1.13) | 1.04 (0.91, 1.19) | 1.02 (0.89, 1.17) | 1.08 (0.94, 1.25) | 0.2 |
| Ethylene dibromide | 1.00 (ref) | 0.96 (0.86, 1.07) | 0.96 (0.85, 1.07) | 0.99 (0.88, 1.11) | 0.90 (0.80, 1.01) | 0.1 |
| Ethylene dichloride | 1.00 (ref) | 1.03 (0.92, 1.15) | 0.97 (0.87, 1.09) | 1.05 (0.94, 1.18) | 1.01 (0.90, 1.13) | 0.7 |
| Ethylene oxide | 1.00 (ref) | 0.99 (0.88, 1.11) | 1.02 (0.91, 1.14) | 1.03 (0.92, 1.15) | 1.00 (0.89, 1.13) | 0.9 |
| Ethylidene dichloride | 1.00 (ref) | 0.98 (0.87, 1.10) | 0.96 (0.86, 1.08) | 1.01 (0.90, 1.13) | 1.01 (0.90, 1.14) | 0.5 |
| Hydrazine | 1.00 (ref) | 0.98 (0.80, 1.21) | 1.02 (0.93, 1.13) | 0.90 (0.81, 1.00) | 1.01 (0.91, 1.11) | 0.7 |
| Methylene chloride | 1.00 (ref) | 1.10 (0.98, 1.24) | 1.03 (0.91, 1.17) | 1.21 (1.07, 1.38) | 1.05 (0.92, 1.21) | 0.6 |
| Nitrobenzene | 1.00 (ref) | 1.02 (0.91, 1.14) | 0.98 (0.87, 1.10) | 0.96 (0.85, 1.08) | 0.97 (0.86, 1.09) | 0.5 |
| o-Toluidine | 1.00 (ref) | 0.94 (0.84, 1.05) | 0.92 (0.82, 1.03) | 0.96 (0.85, 1.07) | 0.93 (0.83, 1.05) | 0.7 |
| Polycyclic organic matter | 1.00 (ref) | 1.01 (0.90, 1.14) | 1.03 (0.91, 1.16) | 1.07 (0.95, 1.21) | 1.07 (0.95, 1.21) | 0.3 |
| Propylene dichloride | 1.00 (ref) | 1.00 (0.89, 1.12) | 1.07 (0.95, 1.20) | 1.00 (0.89, 1.13) | 1.10 (0.98, 1.23) | 0.09 |
| Propylene oxide | 1.00 (ref) | 0.93 (0.83, 1.05) | 0.92 (0.82, 1.04) | 0.99 (0.88, 1.12) | 0.97 (0.86, 1.09) | 0.9 |
| Styrene | 1.00 (ref) | 1.07 (0.95, 1.21) | 1.08 (0.95, 1.22) | 1.02 (0.89, 1.16) | 1.08 (0.95, 1.23) | 0.5 |
| Toluene | 1.00 (ref) | 0.97 (0.86, 1.10) | 1.02 (0.90, 1.16) | 0.99 (0.86, 1.13) | 0.99 (0.86, 1.14) | 0.9 |
| Vinyl chloride | 1.00 (ref) | 0.95 (0.85, 1.07) | 1.04 (0.93, 1.17) | 0.92 (0.82, 1.04) | 1.00 (0.89, 1.13) | 0.8 |
| Vinylidene chloride | 1.00 (ref) | 0.96 (0.85, 1.08) | 0.92 (0.82, 1.04) | 0.94 (0.83, 1.06) | 0.97 (0.86, 1.10) | 1.0 |
| Xylenes | 1.00 (ref) | 0.98 (0.87, 1.11) | 1.04 (0.91, 1.18) | 1.06 (0.93, 1.22) | 1.04 (0.90, 1.20) | 0.5 |
Models adjusted for race, residence type, education, and smoking status
Air toxic categorized with a concentration of 0 (9.9×10−9 for benzidine) as the referent and remaining values split based on tertiles
Table 3.
HRs (95% CIs) for the associationsa between hazardous air pollutants and ER+ invasive breast cancerb, the Sister Study
| Air Toxic | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | p-trend |
|---|---|---|---|---|---|---|
| 1,2-Dibromo-3-chloropropane | 1.00 (ref) | 0.99 (0.83, 1.18) | 0.98 (0.85, 1.12) | 1.09 (0.96, 1.25) | 1.00 (0.87, 1.14) | 0.9 |
| 1,3-butadiene | 1.00 (ref) | 0.96 (0.82, 1.13) | 1.07 (0.90, 1.27) | 1.06 (0.89, 1.27) | 1.08 (0.90, 1.30) | 0.3 |
| 1,4-dioxanec | 1.00 (ref) | -- | 1.04 (0.91, 1.20) | 0.99 (0.86, 1.15) | 1.04 (0.90, 1.20) | 0.6 |
| 2,4-dinitrotoluene | 1.00 (ref) | 0.99 (0.86, 1.15) | 0.94 (0.81, 1.10) | 0.94 (0.81, 1.10) | 0.94 (0.81, 1.10) | 0.5 |
| 2,4-toluene diisocyanate | 1.00 (ref) | 1.08 (0.92, 1.27) | 1.28 (1.09, 1.51) | 1.20 (1.00, 1.42) | 1.11 (0.93, 1.33) | 0.9 |
| 2-chloroacetophenonec | 1.00 (ref) | -- | 1.00 (0.88, 1.14) | 1.04 (0.91, 1.18) | 0.99 (0.86, 1.13) | 0.9 |
| Acrylamidec | 1.00 (ref) | -- | 1.08 (0.92, 1.27) | 1.07 (0.91, 1.26) | 1.06 (0.90, 1.25) | 0.6 |
| Acrylonitrile | 1.00 (ref) | 1.22 (1.05, 1.41) | 1.06 (0.91, 1.23) | 0.97 (0.83, 1.13) | 1.08 (0.93, 1.27) | 0.7 |
| Benzene | 1.00 (ref) | 1.02 (0.87, 1.20) | 1.13 (0.96, 1.34) | 1.02 (0.85, 1.21) | 1.07 (0.89, 1.28) | 0.7 |
| Benzidinec | 1.00 (ref) | -- | 1.07 (0.93, 1.23) | 1.22 (1.07, 1.40) | 1.03 (0.89, 1.19) | 0.2 |
| Carbon tetrachloride | 1.00 (ref) | 1.01 (0.87, 1.17) | 1.01 (0.87, 1.18) | 0.95 (0.81, 1.11) | 1.00 (0.85, 1.17) | 0.8 |
| Chloroprene | 1.00 (ref) | 1.15 (0.99, 1.34) | 1.08 (0.92, 1.26) | 1.04 (0.89, 1.22) | 1.06 (0.90, 1.25) | 0.9 |
| Ethylbenzene | 1.00 (ref) | 1.02 (0.87, 1.20) | 1.08 (0.91, 1.28) | 1.10 (0.92, 1.32) | 1.10 (0.91, 1.33) | 0.3 |
| Ethylene dibromide | 1.00 (ref) | 0.94 (0.81, 1.09) | 0.95 (0.82, 1.10) | 1.13 (0.98, 1.31) | 0.85 (0.73, 0.99) | 0.2 |
| Ethylene dichloride | 1.00 (ref) | 0.95 (0.81, 1.10) | 0.93 (0.80, 1.08) | 1.17 (1.01, 1.35) | 0.99 (0.84, 1.15) | 0.3 |
| Ethylene oxide | 1.00 (ref) | 0.93 (0.80, 1.08) | 1.00 (0.86, 1.16) | 1.04 (0.90, 1.21) | 1.01 (0.87, 1.18) | 0.5 |
| Ethylidene dichloride | 1.00 (ref) | 0.91 (0.78, 1.05) | 0.96 (0.82, 1.12) | 1.00 (0.86, 1.17) | 0.99 (0.84, 1.15) | 0.7 |
| Hydrazine | 1.00 (ref) | 0.91 (0.68, 1.20) | 1.04 (0.91, 1.18) | 0.82 (0.71, 0.94) | 1.01 (0.89, 1.15) | 0.5 |
| Methylene chloride | 1.00 (ref) | 1.17 (1.00, 1.37) | 1.12 (0.94, 1.32) | 1.28 (1.08, 1.52) | 1.13 (0.95, 1.35) | 0.4 |
| Nitrobenzene | 1.00 (ref) | 0.96 (0.83, 1.12) | 0.96 (0.83, 1.12) | 0.95 (0.82, 1.11) | 0.95 (0.82, 1.11) | 0.7 |
| o-Toluidine | 1.00 (ref) | 0.91 (0.78, 1.05) | 0.97 (0.83, 1.12) | 0.96 (0.82, 1.12) | 0.93 (0.80, 1.08) | 0.6 |
| Polycyclic organic matter | 1.00 (ref) | 1.08 (0.92, 1.26) | 1.14 (0.97, 1.34) | 1.07 (0.90, 1.26) | 1.18 (1.00, 1.38) | 0.09 |
| Propylene dichloride | 1.00 (ref) | 0.99 (0.85, 1.15) | 1.05 (0.90, 1.22) | 0.99 (0.85, 1.15) | 1.19 (1.02, 1.38) | 0.01 |
| Propylene oxide | 1.00 (ref) | 0.92 (0.79, 1.07) | 0.96 (0.82, 1.11) | 1.06 (0.91, 1.23) | 0.95 (0.81, 1.12) | 1.0 |
| Styrene | 1.00 (ref) | 1.13 (0.96, 1.32) | 1.13 (0.96, 1.34) | 1.04 (0.87, 1.23) | 1.15 (0.97, 1.37) | 0.3 |
| Toluene | 1.00 (ref) | 0.96 (0.82, 1.12) | 1.03 (0.87, 1.22) | 1.02 (0.85, 1.22) | 0.97 (0.80, 1.16) | 0.8 |
| Vinyl chloride | 1.00 (ref) | 0.90 (0.78, 1.05) | 1.00 (0.86, 1.16) | 0.94 (0.81, 1.10) | 0.98 (0.84, 1.15) | 0.7 |
| Vinylidene chloride | 1.00 (ref) | 1.00 (0.86, 1.16) | 0.90 (0.76, 1.05) | 0.99 (0.84, 1.17) | 0.98 (0.83, 1.16) | 0.9 |
| Xylenes | 1.00 (ref) | 1.03 (0.88, 1.21) | 1.07 (0.90, 1.27) | 1.12 (0.93, 1.34) | 1.04 (0.86, 1.26) | 0.8 |
Models adjusted for race, residence type, education, and smoking status
There were 1,710 ER+ cases total, all case cell sample sizes were above 100 (majority above 300) with the exception of Q2 of 2-chloroacetophenone (n=95) and Q2 of hydrazine (n=52)
Air toxic categorized with a concentration of 0 (9.9×10−9 for benzidine) as the referent and remaining values split based on tertiles
Figure 1.
Classification tree of hazardous air pollutants and breast cancer, the Sister Study
Suggestive positive associations were also observed for overall breast cancer in the top category for acrylamide (HR=1.08; 95% CI: 0.96, 1.22), polycyclic organic matter (HR=1.07; 95% CI: 0.95, 1.21), propylene dichloride (HR=1.10; 95% CI: 0.98, 1.23), and styrene (HR=1.08; 95% CI: 0.95, 1.23) (Table 2). However, monotonic trends for air toxics with overall breast cancer were not observed.
Associations were also evident for ER+ breast cancer, particularly the top category of polycyclic organic matter (HR=1.18; 95% CI=1.00–1.38), styrene (HR=1.15; 95% CI=0.97, 1.37), and propylene dichloride (HR=1.19; 95% CI=1.02–1.38), which demonstrated evidence of a monotonic trend (p=0.01) (Table 3). 2,4-toluene diisocyanate, chloroprene, ethylbenzene, and styrene were associated with reduced ER- breast cancer risk, while acrylamide and benzidine were associated with increased ER- breast cancer risk, including a significant trend for acrylamide (p=0.01) (Table 4). Ethylbenzene demonstrated an inverse association with ER- breast cancer in all categories above the referent.
Table 4.
HRs (95% CIs) for the associationsa between hazardous air pollutants and ER- invasive breast cancerb, the Sister Study
| Air Toxic | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | p-trend |
|---|---|---|---|---|---|---|
| 1,2-Dibromo-3-chloropropane | 1.00 (ref) | 0.95 (0.61, 1.48) | 1.04 (0.75, 1.44) | 1.07 (0.77, 1.48) | 1.10 (0.79, 1.53) | 0.5 |
| 1,3-butadiene | 1.00 (ref) | 0.84 (0.59, 1.20) | 0.84 (0.57, 1.25) | 0.76 (0.50, 1.16) | 0.80 (0.52, 1.24) | 0.4 |
| 1,4-dioxanec | 1.00 (ref) | -- | 1.09 (0.78, 1.53) | 1.03 (0.73, 1.47) | 1.13 (0.80, 1.60) | 0.5 |
| 2,4-dinitrotoluene | 1.00 (ref) | 1.00 (0.71, 1.39) | 0.66 (0.45, 0.96) | 0.85 (0.59, 1.22) | 0.75 (0.51, 1.10) | 0.2 |
| 2,4-toluene diisocyanate | 1.00 (ref) | 0.72 (0.50, 1.03) | 0.52 (0.34, 0.79) | 0.88 (0.59, 1.29) | 0.65 (0.43, 0.98) | 0.3 |
| 2-chloroacetophenonec | 1.00 (ref) | -- | 0.97 (0.71, 1.33) | 1.14 (0.84, 1.54) | 0.85 (0.61, 1.19) | 0.4 |
| Acrylamidec | 1.00 (ref) | -- | 0.80 (0.51, 1.25) | 1.14 (0.77, 1.67) | 1.55 (1.11, 2.17) | 0.01 |
| Acrylonitrile | 1.00 (ref) | 0.92 (0.65, 1.30) | 0.70 (0.49, 1.02) | 0.86 (0.61, 1.23) | 0.90 (0.63, 1.29) | 0.9 |
| Benzene | 1.00 (ref) | 1.00 (0.71, 1.40) | 0.61 (0.40, 0.92) | 0.66 (0.43, 1.00) | 0.67 (0.43, 1.03) | 0.05 |
| Benzidinec | 1.00 (ref) | -- | 0.95 (0.66, 1.36) | 1.10 (0.78 1.54) | 1.33 (0.97, 1.82) | 0.1 |
| Carbon tetrachloride | 1.00 (ref) | 1.09 (0.78, 1.51) | 0.70 (0.48, 1.03) | 0.73 (0.50, 1.07) | 0.91 (0.63, 1.33) | 0.6 |
| Chloroprene | 1.00 (ref) | 0.79 (0.57, 1.10) | 0.57 (0.39, 0.83) | 0.70 (0.48, 1.00) | 0.65 (0.45, 0.95) | 0.1 |
| Ethylbenzene | 1.00 (ref) | 0.66 (0.46, 0.95) | 0.64 (0.43, 0.96) | 0.56 (0.36, 0.86) | 0.61 (0.39, 0.94) | 0.1 |
| Ethylene dibromide | 1.00 (ref) | 0.99 (0.69, 1.41) | 1.19 (0.84, 1.67) | 0.75 (0.51, 1.09) | 0.85 (0.59, 1.23) | 0.2 |
| Ethylene dichloride | 1.00 (ref) | 0.85 (0.60, 1.22) | 0.95 (0.67, 1.36) | 0.74 (0.51, 1.08) | 1.02 (0.71, 1.45) | 1.0 |
| Ethylene oxide | 1.00 (ref) | 0.99 (0.69, 1.42) | 0.98 (0.68, 1.40) | 1.02 (0.71, 1.46) | 0.94 (0.65, 1.37) | 0.8 |
| Ethylidene dichloride | 1.00 (ref) | 0.82 (0.58, 1.15) | 0.63 (0.43, 0.91) | 0.79 (0.56, 1.13) | 0.82 (0.57, 1.17) | 1.0 |
| Hydrazine | 1.00 (ref) | 1.18 (0.65, 2.14) | 0.79 (0.56, 1.10) | 0.89 (0.64, 1.22) | 1.05 (0.77, 1.42) | 0.4 |
| Methylene chloride | 1.00 (ref) | 0.85 (0.59, 1.21) | 0.60 (0.40, 0.91) | 0.87 (0.59, 1.30) | 0.88 (0.58, 1.32) | 1.0 |
| Nitrobenzene | 1.00 (ref) | 1.06 (0.76, 1.47) | 0.71 (0.49, 1.02) | 0.67 (0.46, 0.98) | 0.84 (0.58, 1.20) | 0.5 |
| o-Toluidine | 1.00 (ref) | 0.87 (0.63, 1.22) | 0.64 (0.44, 0.92) | 0.66 (0.45, 0.96) | 0.85 (0.60, 1.21) | 0.9 |
| Polycyclic organic matter | 1.00 (ref) | 0.87 (0.62, 1.22) | 0.54 (0.36, 0.80) | 0.72 (0.49, 1.05) | 0.74 (0.51, 1.07) | 0.4 |
| Propylene dichloride | 1.00 (ref) | 0.78 (0.54, 1.12) | 0.95 (0.67, 1.35) | 0.88 (0.61, 1.25) | 0.85 (0.59, 1.22) | 0.7 |
| Propylene oxide | 1.00 (ref) | 0.81 (0.58, 1.14) | 0.76 (0.53, 1.08) | 0.73 (0.50, 1.05) | 0.80 (0.55, 1.17) | 0.5 |
| Styrene | 1.00 (ref) | 0.78 (0.55, 1.12) | 0.75 (0.51, 1.09) | 0.73 (0.49, 1.08) | 0.67 (0.44, 1.01) | 0.1 |
| Toluene | 1.00 (ref) | 0.80 (0.56, 1.14) | 0.90 (0.61, 1.33) | 0.67 (0.43, 1.03) | 0.77 (0.49, 1.19) | 0.3 |
| Vinyl chloride | 1.00 (ref) | 0.79 (0.56, 1.11) | 0.76 (0.53, 1.08) | 0.76 (0.53, 1.09) | 0.76 (0.52, 1.10) | 0.5 |
| Vinylidene chloride | 1.00 (ref) | 0.80 (0.57, 1.13) | 0.79 (0.55, 1.13) | 0.51 (0.34, 0.77) | 0.80 (0.55, 1.18) | 0.5 |
| Xylenes | 1.00 (ref) | 0.69 (0.48, 1.00) | 0.69 (0.46, 1.04) | 0.69 (0.45, 1.06) | 0.66 (0.42, 1.03) | 0.3 |
Models adjusted for race, residence type, education, and smoking status
There were 297 ER- cases total, 77% of all case cell sample sizes were above 50, smallest cells were for Q2 of 2-chloroacetophenone (n=13) and Q2 of hydrazine (n=12)
Air toxic categorized with a concentration of 0 (9.9×10−9 for benzidine) as the referent and remaining values split based on tertiles
When we considered single pollutant associations stratified by menopausal status, the results for postmenopausal breast cancer were similar to those for overall breast cancer, while many, but not all, of those for premenopausal breast cancer were slightly attenuated and less precise (data not shown). Tests of heterogeneity revealed no differences by menopausal status.
There was evidence of synergistic effect-measure modification, on the additive and multiplicative scales, by BMI for six air toxics, including 2,4-toluene diisocynate, benzidene, ethylene dichloride, ethylene oxide, hydrazine, and propylene dichloride (Table 5). In the stratified analyses, the direction of the modification suggested a stronger association between the air toxics and breast cancer among those who were overweight/obese compared to those who were not. For example, on the multiplicative scale, ≥median vs. <median concentration of propylene dichloride was associated with a HR of 1.11 (95% CI: 1.01–1.22) among those who were overweight/obese, but a HR of 0.89 (95% CI: 0.79–1.01) among those who were not overweight/obese (ratio of HR=1.25; 95% CI: 1.07–1.45; LRT p=0.003). Physical activity did not modify the air toxic-breast cancer associations (Supplemental Table 2).
Table 5.
Additive and multiplicative effect measure modification by BMI for the associationsa between hazardous air pollutants and breast cancer risk, the Sister Study
| BMI (kg/m2) | Air toxic | Cases (N) | Single referent HRs (95% CIs) | Additive ICR (95% CI) | Stratified HRs (95% CIs) | Multiplicative RHR (95% CI) | p interaction |
|---|---|---|---|---|---|---|---|
| 1,2-Dibromo-3-chloropropane | |||||||
| <25 | < median | 571 | 1.00 | 1.00 | |||
| ≥ median | 527 | 0.94 (0.84, 1.06) | 0.94 (0.84, 1.06) | ||||
| ≥25 | < median | 915 | 1.05 (0.95, 1.17) | 1.00 | |||
| ≥ median | 958 | 1.13 (1.01, 1.25) | 0.13 (−0.02, 0.28) | 1.07 (0.98, 1.17) | 1.13 (0.98, 1.32) | 0.1 | |
| 1,3-butadiene | |||||||
| <25 | < median | 511 | 1.00 | 1.00 | |||
| ≥ median | 587 | 1.02 (0.90, 1.16) | 1.02 (0.90, 1.16) | ||||
| ≥25 | < median | 903 | 1.08 (0.97, 1.20) | 1.00 | |||
| ≥ median | 970 | 1.19 (1.06, 1.33) | 0.09 (−0.06, 0.24) | 1.10 (0.99, 1.22) | 1.08 (0.93, 1.25) | 0.3 | |
| 1,4-dioxane | |||||||
| <25 | < median | 619 | 1.00 | 1.00 | |||
| ≥ median | 444 | 0.92 (0.81, 1.04) | 0.92 (0.81, 1.04) | ||||
| ≥25 | < median | 1,087 | 1.08 (0.98, 1.20) | 1.00 | |||
| ≥ median | 748 | 1.10 (0.99, 1.23) | 0.10 (−0.05, 0.25) | 1.02 (0.93, 1.12) | 1.11 (0.95, 1.29) | 0.2 | |
| 2,4-dinitrotoluene | |||||||
| <25 | < median | 554 | 1.00 | 1.00 | |||
| ≥ median | 544 | 0.93 (0.83, 1.05) | 0.93 (0.83, 1.05) | ||||
| ≥25 | < median | 939 | 1.10 (0.99, 1.23) | 1.00 | |||
| ≥ median | 934 | 1.06 (0.95, 1.18) | 0.03 (−0.15, 0.20) | 0.96 (0.87, 1.06) | 1.03 (0.89, 1.20) | 0.7 | |
| 2,4-toluene diisocyanate | |||||||
| <25 | < median | 545 | 1.00 | 1.00 | |||
| ≥ median | 553 | 0.94 (0.83, 1.06) | 0.94 (0.83, 1.06) | ||||
| ≥25 | < median | 901 | 1.03 (0.93, 1.15) | 1.00 | |||
| ≥ median | 972 | 1.14 (1.02, 1.27) | 0.17 (0.02, 0.31) | 1.10 (1.00, 1.21) | 1.17 (1.01, 1.36) | 0.04 | |
| 2-chloroacetophenone | |||||||
| <25 | < median | 554 | 1.00 | 1.00 | |||
| ≥ median | 536 | 0.98 (0.87, 1.10) | 0.98 (0.87, 1.10) | ||||
| ≥25 | < median | 901 | 1.09 (0.98, 1.21) | 1.00 | |||
| ≥ median | 959 | 1.13 (1.02, 1.26) | 0.06 (−0.09, 0.22) | 1.04 (0.95, 1.14) | 1.06 (0.91, 1.23) | 0.4 | |
| Acrylamide | |||||||
| <25 | < median | 699 | 1.00 | 1.00 | |||
| ≥ median | 325 | 0.96 (0.84, 1.09) | 0.96 (0.84, 1.09) | ||||
| ≥25 | < median | 1,151 | 1.07 (0.97, 1.17) | 1.00 | |||
| ≥ median | 555 | 1.13 (1.01, 1.26) | 0.10 (−0.06, 0.27) | 1.06 (0.95, 1.17) | 1.11 (0.94, 1.31) | 0.2 | |
| Acrylonitrile | |||||||
| <25 | < median | 573 | 1.00 | 1.00 | |||
| ≥ median | 525 | 0.91 (0.81, 1.03) | 0.91 (0.81, 1.03) | ||||
| ≥25 | < median | 938 | 1.07 (0.96, 1.19) | 1.00 | |||
| ≥ median | 935 | 1.08 (0.97, 1.19) | 0.09 (−0.05, 0.24) | 1.00 (0.92, 1.10) | 1.10 (0.95, 1.28) | 0.2 | |
| Benzene | |||||||
| <25 | < median | 528 | 1.00 | 1.00 | |||
| ≥ median | 570 | 0.95 (0.84, 1.08) | 0.95 (0.84, 1.08) | ||||
| ≥25 | < median | 915 | 1.07 (0.96, 1.19) | 1.00 | |||
| ≥ median | 958 | 1.12 (1.00, 1.25) | 0.10 (−0.05, 0.24) | 1.04 (0.94, 1.16) | 1.10 (0.94, 1.27) | 0.2 | |
| Benzidine | |||||||
| <25 | < median | 666 | 1.00 | 1.00 | |||
| ≥ median | 432 | 0.96 (0.85, 1.08) | 0.96 (0.85, 1.08) | ||||
| ≥25 | < median | 1,070 | 1.05 (0.95, 1.16) | 1.00 | |||
| ≥ median | 803 | 1.18 (1.06, 1.31) | 0.17 (0.02, 0.32) | 1.12 (1.02, 1.23) | 1.17 (1.00, 1.36) | 0.03 | |
| Carbon tetrachloride | |||||||
| <25 | < median | 545 | 1.00 | 1.00 | |||
| ≥ median | 553 | 0.98 (0.87, 1.11) | 0.98 (0.87, 1.11) | ||||
| ≥25 | < median | 927 | /1.12 (1.00, 1.24) | 1.00 | |||
| ≥ median | 946 | 1.11 (0.99, 1.24) | 0.01 (−0.14, 0.16) | 0.99 (0.90, 1.09) | 1.01 (0.87, 1.17) | 0.9 | |
| Chloroprene | |||||||
| <25 | < median | 556 | 1.00 | 1.00 | |||
| ≥ median | 542 | 0.92 (0.82, 1.04) | 0.92 (0.82, 1.04) | ||||
| ≥25 | < median | 922 | 1.07 (0.96, 1.19) | 1.00 | |||
| ≥ median | 951 | 1.08 (0.97, 1.21) | 0.09 (−0.06, 0.23) | 1.01 (0.92, 1.11) | 1.09 (0.94, 1.27) | 0.2 | |
| Ethylbenzene | |||||||
| <25 | < median | 510 | 1.00 | 1.00 | |||
| ≥ median | 588 | 1.02 (0.90, 1.16) | 1.02 (0.90, 1.16) | ||||
| ≥25 | < median | 914 | 1.10 (0.99, 1.23) | 1.00 | |||
| ≥ median | 959 | 1.17 (1.04, 1.32) | 0.05 (−0.11, 0.20) | 1.06 (0.96, 1.18) | 1.04 (0.90, 1.21) | 0.6 | |
| Ethylene dibromide | |||||||
| <25 | < median | 599 | 1.00 | 1.00 | |||
| ≥ median | 499 | 0.95 (0.85, 1.07) | 0.95 (0.85, 1.07) | ||||
| ≥25 | < median | 917 | 1.10 (0.99, 1.22) | 1.00 | |||
| ≥ median | 956 | 1.10 (0.99, 1.22) | 0.05 (−0.11, 0.20) | 1.00 (0.91, 1.09) | 1.05 (0.90, 1.22) | 0.5 | |
| Ethylene dichloride | |||||||
| <25 | < median | 575 | 1.00 | 1.00 | |||
| ≥ median | 523 | 0.92 (0.82, 1.04) | 0.92 (0.82, 1.04) | ||||
| ≥25 | < median | 896 | 1.03 (0.92, 1.14) | 1.00 | |||
| ≥ median | 977 | 1.13 (1.02, 1.26) | 0.18 (0.04, 0.33) | 1.10 (1.01, 1.21) | 1.20 (1.03, 1.39) | 0.02 | |
| Ethylene oxide | |||||||
| <25 | < median | 583 | 1.00 | 1.00 | |||
| ≥ median | 515 | 0.90 (0.80, 1.01) | 0.90 (0.80, 1.01) | ||||
| ≥25 | < median | 916 | 1.04 (0.94, 1.16) | 1.00 | |||
| ≥ median | 957 | 1.09 (0.98, 1.21) | 0.15 (0.004, 0.29) | 1.04 (0.95, 1.14) | 1.16 (1.00, 1.35) | 0.05 | |
| Ethylidene dichloride | |||||||
| <25 | < median | 518 | 1.00 | 1.00 | |||
| ≥ median | 580 | 1.05 (0.94, 1.19) | 1.05 (0.94, 1.19) | ||||
| ≥25 | < median | 911 | 1.11 (1.00, 1.24) | 1.00 | |||
| ≥ median | 952 | 1.19 (1.07, 1.33) | 0.03 (−0.13, 0.18) | 1.07 (0.97, 1.18) | 1.02 (0.88, 1.18) | 0.8 | |
| Hydrazine | |||||||
| <25 | < median | 601 | 1.00 | 1.00 | |||
| ≥ median | 497 | 0.85 (0.75, 0.95) | 0.85 (0.75, 0.95) | ||||
| ≥25 | < median | 934 | 1.03 (0.93, 1.14) | 1.00 | |||
| ≥ median | 939 | y1.05 (0.94, 1.16) | 0.17 (0.04, 0.31) | 1.02 (0.93, 1.12) | 1.21 (1.04, 1.40) | 0.01 | |
| Methylene chloride | |||||||
| <25 | < median | 519 | 1.00 | 1.00 | |||
| ≥ median | 579 | i 0.99 (0.88, 1.13) | 0.99 (0.88, 1.13) | ||||
| ≥25 | < median | 902 | 1.06 (0.95, 1.19) | 1.00 | |||
| ≥ median | 971 | 1.17 (1.05, 1.31) | 0.12 (−0.04, 0.27) | 1.10 (1.00, 1.22) | 1.11 (0.96, 1.29) | 0.2 | |
| Nitrobenzene | |||||||
| <25 | < median | 554 | 1.00 | 1.00 | |||
| ≥ median | 544 | 0.93 (0.83, 1.05) | 0.93 (0.83, 1.05) | ||||
| ≥25 | < median | 930 | 1.09 (0.98, 1.21) | 1.00 | |||
| ≥ median | 943 | 1.08 (0.97, 1.20) | 0.05 (−0.10, 0.20) | 0.99 (0.90, 1.09) | 1.06 (0.91, 1.23) | 0.4 | |
| o-Toluidine | |||||||
| <25 | < median | 561 | 1.00 | 1.00 | |||
| ≥ median | 537 | 0.94 (0.84, 1.07) | 0.94 (0.84, 1.07) | ||||
| ≥25 | < median | 935 | 1.10 (0.99, 1.22) | 1.00 | |||
| ≥ median | 938 | 1.09 (0.98, 1.21) | 0.05 (−0.10, 0.20) | 0.99 (0.90, 1.09) | 1.05 (0.90, 1.22) | 0.5 | |
| Polycyclic organic matter | |||||||
| <25 | < median | 513 | 1.00 | 1.00 | |||
| ≥ median | 585 | 1.02 (0.90, 1.15) | 1.02 (0.90, 1.15) | ||||
| ≥25 | < median | 902 | 1.07 (0.96, 1.19) | 1.00 | |||
| ≥ median | 971 | 1.19 (1.07, 1.33) | 0.10 (−0.05, 0.26) | 1.11 (1.01, 1.22) | 1.09 (0.94, 1.27) | 0.2 | |
| Propylene dichloride | |||||||
| <25 | < median | 571 | 1.00 | 1.00 | |||
| ≥ median | 527 | 0.89 (0.79, 1.01) | 0.89 (0.79, 1.01) | ||||
| ≥25 | < median | 908 | 1.01 (0.90, 1.12) | 1.00 | |||
| ≥ median | 965 | 1.12 (1.01, 1.24) | 0.22 (0.08, 0.36) | *1.11 (1.02, 1.22) | 1.25 (1.07, 1.45) | 0.003 | |
| Propylene oxide | |||||||
| <25 | < median | 554 | 1.00 | 1.00 | |||
| ≥ median | 544 | 0.96 (0.85, 1.09) | 0.96 (0.85, 1.09) | ||||
| ≥25 | < median | 901 | 1.07 (0.96, 1.19) | 1.00 | |||
| ≥ median | 972 | 1.13 (1.01, 1.26) | 0.10 (−0.05, 0.25) | 1.06 (0.96, 1.17) | 1.10 (0.95, 1.28) | 0.2 | |
| Styrene | |||||||
| <25 | < median | 556 | 1.00 | 1.00 | |||
| ≥ median | 542 | 0.92 (0.81, 1.04) | 0.92 (0.81, 1.04) | ||||
| ≥25 | < median | 911 | 1.06 (0.95, 1.18) | 1.00 | |||
| ≥ median | 962 | 1.09 (0.98, 1.22) | 0.12 (−0.03, 0.26) | 1.03 (0.94, 1.14) | 1.12 (0.97, 1.30) | 0.1 | |
| Toluene | |||||||
| <25 | < median | 513 | 1.00 | 1.00 | |||
| ≥ median | 585 | 0.99 (0.88, 1.13) | 0.99 (0.88, 1.13) | ||||
| ≥25 | < median | 927 | 1.11 (0.99, 1.23) | 1.00 | |||
| ≥ median | 946 | 1.13 (1.00, 1.27) | 0.03 (−0.13, 0.18) | 1.02 (0.92, 1.13) | 1.03 (0.88, 1.19) | 0.7 | |
| Vinyl chloride | |||||||
| <25 | < median | 557 | 1.00 | 1.00 | |||
| ≥ median | 541 | 0.92 (0.82, 1.04) | 0.92 (0.82, 1.04) | ||||
| ≥25 | < median | 942 | 1.07 (0.97, 1.20) | 1.00 | |||
| ≥ median | 931 | 1.08 (0.97, 1.20) | 0.08 (−0.06, 0.23) | 1.00 (0.91, 1.10) | 1.09 (0.94, 1.26) | 0.3 | |
| Vinylidene chloride | |||||||
| <25 | < median | 563 | 1.00 | 1.00 | |||
| ≥ median | 535 | 0.90 (0.80, 1.02) | 0.90 (0.80, 1.02) | ||||
| ≥25 | < median | 920 | 1.06 (0.95, 1.18) | 1.00 | |||
| ≥ median | 953 | 1.08 (0.97, 1.20) | 0.12 (−0.03, 0.26) | 1.02 (0.93, 1.12) | 1.13 (0.97, 1.31) | 0.1 | |
| Xylenes | |||||||
| <25 | < median | 518 | 1.00 | 1.00 | |||
| ≥ median | 580 | 1.00 (0.88, 1.14) | 1.00 (0.88, 1.14) | ||||
| ≥25 | < median | 903 | 1.08 (0.96, 1.20) | 1.00 | |||
| ≥ median | 970 | 1.17 (1.04, 1.31) | 0.09 (−0.06, 0.24) | 1.09 (0.98, 1.20) | 1.08 (0.93, 1.26) | 0.3 | |
Abbreviations: BMI, body mass index; ICR, interaction contrast ratio; RHR, ratio of hazard ratios;
Models adjusted for race, residence type, education, and smoking status
In sensitivity analyses, results were similar to the results for all women when we restricted models to invasive breast cancer alone (Supplemental Table 3) or to non-Hispanic whites, those who enrolled in 2005 or later, or those who had lived at their baseline address for >10 years, and when additionally adjusted for region (data not shown). Results were slightly attenuated, but interpretation did not change, when restricted to the cases confirmed by medical record (data not shown).
Results of this study were interpreted with an emphasis on the magnitude of point estimates and precision of confidence intervals rather than statistical significance testing. Further, air toxics were selected for this study based on biological plausibility and a review of animal studies. Therefore, our results are focused on those not adjusted for multiple comparisons. However, due to the large number of air toxics and associations examined we explored a multiple comparisons adjustment using the false discovery rate method as a sensitivity analysis [52]. Associations for overall, ER+, ER- breast cancer, and modification by BMI observed in the primary analyses were not significant when adjusted for multiple comparisons.
3.3. Results from the Multipollutant Classification Tree
Classification tree methods were used in exploratory analyses to identify patterns and combinations of the 29 air toxics and other covariates of interest (age, BMI, physical activity). As shown in Figure 1, in addition to age, BMI, and methylene chloride, ethylidene dichloride, styrene, propylene dichloride, and ethylene dibromide were important in the formation of the groups. The terminal nodes exhibiting the highest risk of breast cancer consisted of those: (1) younger than 58.7, with higher (≥5.89μg/m3) methylene chloride; (2) older than 58.7, with a BMI <29.7kg/m2 and with higher (≥3.84μg/m3) methylene chloride; or (3) older than 58.7, with a BMI ≥29.7kg/m2 and with both higher (≥0.001μg/m3) ethylidene dichloride and higher (≥0.389μg/m3) styrene. The most common subgroup on the tree consisted of those between the ages of 40 and 58.7, with methylene chloride <5.89μg/m3 and propylene dichloride <0.002μg/m3. It is noteworthy, however, that when the classification tree determined the best splitting point for some air toxics, it was occasionally at a high concentration. As an example, the distribution of methylene chloride concentrations in this population (overall and among those < age 58.7) shown in Supplemental Figure 2 demonstrates that the cutpoints identified by the tree (≥5.89μg/m3 or ≥3.84μg/m3) would be well into the top quintile (>0.412μg/m3) that was used for single pollutant analyses.
4. DISCUSSION
In this large, US-wide cohort study, methylene chloride was consistently associated with breast cancer across different single and multipollutant models. Evidence of increased overall or ER+ breast cancer risk was also observed for multiple other air toxics, including acrylamide, polycyclic organic matter, propylene dichloride, and styrene. Results were inconsistent for ER- breast cancer; two air toxics demonstrated a positive association, while multiple others demonstrated an inverse association. BMI, but not physical activity, modified some air toxic-breast cancer associations in the direction of a stronger association among women who were overweight/obese.
The increased hazard of overall and ER+ breast cancer for multiple air toxics is biologically plausible. Some air toxics have been shown to have estrogenic activity [3–6], and increase oxidative stress [7–16] and inflammation [8,9,17], all processes implicated in breast cancer [18–25]. Specifically, methylene chloride, an industrial solvent in a variety of products, has been classified as Group 2A (probably carcinogenic to humans) by the International Agency for Research on Cancer (IARC) [53]. Methylene chloride is mutagenic, as it induces chromosomal instability and DNA damage [54] and is metabolized through two pathways, CYP2E1 and glutathione S-transferase (GST), that can lead to the formation of reactive intermediates. The importance of the GST pathway was primarily determined from lung and liver cancer models, but GSTT1 (a key enzyme in the pathway) activity has been detected in mammary tissue [54].
Styrene, propylene dichloride, and polycyclic organic matter had suggestive associations for overall and ER+ breast cancer. In a study using MCF-7 estrogen-sensitive human breast cancer cells, styrene oligomers increased cell proliferation, demonstrated estrogenicity, and had an affinity to estrogen receptor alpha [55]. Propylene dichloride has been classified by IARC as Group 1 (carcinogenic to humans) [53]. In mice it has been shown to increase oxidative stress through cytochrome P450 metabolism [56] and to induce mammary adenocarcinoma [57]. The primary components of polycyclic organic matter are polycyclic aromatic hydrocarbons, which have shown estrogenic and anti-estrogenic activity in vitro [58–62] and have been associated with breast cancer risk in previous studies [63–66].
Acrylamide and benzidine were associated with increased ER- breast cancer risk, but other air toxics, particularly 2,4-toluene diisocyanate, benzene, chloroprene, ethylbenzene, and styrene, were associated with reduced ER- breast cancer risk. These inverse associations were unexpected. However, a few studies on air pollution have also found inverse associations for some other pollutants with ER- breast cancer [28,67,68] so this area warrants further study, particularly in a population with a greater number of ER- cancers.
The finding of effect-measure modification by BMI for multiple air toxics is consistent with our a priori hypothesis that associations would be stronger among women who are overweight/obese. Obesity, which is associated with postmenopausal breast cancer [35,36], leads to increases in oxidative stress and inflammation [36,37,69]. Given that air toxics also increase oxidative stress, a synergy between the oxidative stress and inflammation from obesity and air toxics is plausible [37,70]. Additionally, obesity has been shown to impair the oxidant defense system which could make obese individuals more susceptible to the oxidative stress from air toxics [37,71]. Given that over 70% of US adults are overweight/obese [72] and the prevalence continues to increase [73], this is an important, potentially vulnerable subgroup that should be considered in the regulation of air toxics.
Our use of classification tree methods was useful in recognizing complex relations between air toxics and covariates in our population and also supported findings from the single pollutant analyses. Similar to the single pollutant analysis, methylene chloride appeared to be an air toxic of relevance for breast cancer; among women <58.7 years of age, breast cancer risk was higher among those with higher vs. lower methylene chloride. Further, among women >58.7 years of age and with a BMI <29.7kg/m2, breast cancer risk was elevated among those with higher vs. lower methylene chloride. However, the best cut-point identified by the classification tree was at a high concentration in the methylene chloride distribution. Therefore, although the 4th quintile was most strongly associated with breast cancer risk in the single pollutant models, the multipollutant models indicated that very high levels may also be of relevance (which could have been masked in the 5th quintile as a whole in the single pollutant models). Due to the ease with which CART handles non-linear and non-additive associations, CART methods were able to identify this grouping. While it was a strength that CART identified important cutpoints that would not have been found using traditional regression methods, the results based on these splits should be interpreted cautiously as the number of women impacted by them is small. Propylene dichloride and styrene were also identified as important in the formation of multipollutant groups in the classification tree and showed some evidence of association with breast cancer risk in the single pollutant models. The tree methodology was used as a tool for exploring complex relations between the air toxics that may reflect harmful co-exposures for breast cancer of interest for future evaluation. We considered different combinations of the number of total terminal nodes, minimum number of observations in a node, and maximum depth before settling on our final tree that was not too large to lose interpretability, but still identified potentially important groups. Generally, the nodes at the top of our tree were most robust to changes in stopping parameters, but the known instability of trees to changes in parameters is a limitation worth noting [74]. However, we emphasize that this analysis was exploratory as CART does not provide measures of statistical precision and the size of the tree is controlled by investigator-specified parameters.
Only one previous study has reported on associations between the non-metallic air toxics and breast cancer risk on a US-wide scale [28]. In the Nurses’ Health Study II (NHSII), 1,2-dibromo-3-chloropropane was the only air toxic that demonstrated a consistent increased risk of breast cancer (HRquartile 4vs1=1.12; 95% CI: 0.98–1.29; p-trend=0.004). Although in our study there was a suggestive association in the 3rd quintile of 1,2-dibromo-3-chlorpropane (HR=1.09; 95% CI: 0.97–1.19), there was no evidence of a monotonic trend and the magnitude was not as strong as for other air toxics. The NHSII found additional suggestively elevated risks for overall or ER+ breast cancer for 1,3-butadiene, 2,4-dinitrotoluene, 2,4-toluene diisocyante, benzene, carbon tetrachloride, hydrazine, nitrobenzene, propylene oxide, and vinylidene chloride. We similarly found an increased risk for 2,4-toluene diisocyanate (quintiles 2 and 3) and ER+ breast cancer, but not the others. Differences in approaches between the NHSII and our study reported here could explain the differing results. The NHSII utilized the 2002 NATA, whereas the 2005 version was used in our study. The 2005 version included multiple updates to improve upon the exposure assessment in earlier versions (including use of an up-to-date NEI with more recent information on industrial and other sources, use of HEM with AERMOD (a refined dispersion model) for more source types, and a new dataset for airport-related emissions with 5-times as many airports [42]). Additionally, the follow-up for the NHSII was conducted from 1989–2011, whereas Sister Study follow-up was from 2003–2009 through 2016. Therefore, changes in air toxics levels or distributions over those periods could partially explain the differences between the two studies.
A previous study that also used the Sister Study population reported that metal air toxics, particularly mercury, cadmium, and lead, were associated with an increased risk of postmenopausal breast cancer in individual pollutant models and as a mixture using a weighted quantile sum [68]. Together, the results from both of these Sister Study-based studies support an association between some air toxics, metallic or non-metallic, and breast cancer risk.
Limitations of our study relate to exposure misclassification from NATA. Concentrations at the census tract level do not fully account for variation in an individual’s daily activities that could lead to higher or lower exposure, and all women within a census tract are assigned the same concentration. Although NATA captures many ambient sources that are a major contributor to an individual’s exposure, exposure to some air toxics can also come from cigarette smoke, occupation, indoor air, and drinking water; sources not captured by NATA. Finally, the air toxics levels from NATA were linked to Sister Study women’s baseline residential addresses, so an assumption of our study is that these levels represent those from relevant etiologic exposure periods for breast cancer or that recent exposure is an important window. Given that NATA air toxic concentration estimates represent a one-year average [42] they may be indicative of a long-term or typical, rather than acute, exposure. The Sister Study population has been residentially stable; nearly half of the population had lived in their baseline address for at least 10 years and 80% have remained in that address throughout follow-up. This increases the likelihood that the residential levels at baseline reflect past residential levels and those until diagnosis.
The Sister Study is a fairly homogenous population composed mostly of women who are non-Hispanic White, well-educated, and postmenopausal. Further, these women have a family history of breast cancer. These aspects do not affect the internal validity of our study results, but may limit generalizability to other groups.
This study has a number of strengths. The Sister Study is a large, prospective cohort, which provided sufficient study power to examine the main air toxic-breast cancer associations as well as modification of these associations by obesity and physical activity. Additionally, these Sister Study participants came from all 50 US states which allowed us to examine nationwide associations and have a larger range of exposure upon which to estimate associations compared to focusing on a population limited to one state or geographic region. We used the 2005 NATA, which had undergone a number of modeling improvements to increase the scope and improve the modeling techniques compared to the 1996, 1999, and 2002 versions [42]. Further, given that NATA provides the only available nationwide data on air toxics, it is a valuable resource. Ours is the first study to comprehensively examine physical activity and BMI as effect modifiers for these air toxics with breast cancer risk and the first to explore multipollutant combinations that could be relevant for further examination. Further, we conducted a large number of sensitivity analyses and found that our primary results were robust to factors such as adjustment for residential region, restriction to non-Hispanic whites, and restriction to those that lived in their baseline address >10 years.
5. CONCLUSIONS
In a large, US-wide population, methylene chloride, along with several other hazardous air pollutants (including polycyclic organic matter, propylene dichloride, and styrene), showed some evidence of association with an increased risk of overall and ER+ breast cancer. We also found that the air toxic-breast cancer associations were stronger among overweight/obese women. Complex relations between air toxics involving methylene chloride, ethylidene dichloride, propylene dichloride, ethylene dibromide, and styrene were identified with exploratory classification tree methods and warrant further study.
Supplementary Material
Highlights.
Methylene chloride was positively associated with overall and ER+ breast cancer
Multiple other air toxics were also associated with increased risk
Body mass index (BMI) modified air toxic-breast cancer associations
Classification trees were used to explore complex relations between the air toxics
ACKNOWLEDGEMENTS
The authors appreciate the helpful comments of Jason Sacks at the Environmental Protection Agency, and Drs. Katie O’Brien and Emily Werder at the National Institute of Environmental Health Sciences.
Sources of Funding: This work was supported by a National Institute of Environmental Health Sciences training grant to the University of North Carolina (T32ES007018), by the UNC Lineberger Comprehensive Cancer Center Cancer Control Education Program (T32CA057726), and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES044005).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Environmental Protection Agency. What are hazardous air pollutants. https://www.epa.gov/haps/what-are-hazardous-air-pollutants.
- 2.Environmental Protection Agency. Report on the environment: Air toxics emissions. Environmental Protection Agency, 2015. [Google Scholar]
- 3.Mori Y, Taneda S, Hayashi H, Sakushima A, Kamata K, Suzuki AK, Yoshino S, Sakata M, Sagai M, Seki K. Estrogenic activities of chemicals in diesel exhaust particles. Biol Pharm Bull 2002;25(1):145–6. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi K, Toriba A, Chung SW, Kizu R, Hayakawa K. Identification of estrogenic/anti-estrogenic compounds in diesel exhaust particulate extract. Biomed Chromatogr 2007;21(11):1135–42. [DOI] [PubMed] [Google Scholar]
- 5.Oh SM, Ryu BT, Chung KH. Identification of estrogenic and antiestrogenic activities of respirable diesel exhaust particles by bioassay-directed fractionation. Arch Pharm Res 2008;31(1):75–82. [DOI] [PubMed] [Google Scholar]
- 6.Taneda S, Hayashi H, Sakushima A, Seki K, Suzuki AK, Kamata K, Sakata M, Yoshino S, Sagai M, Mori Y. Estrogenic and anti-estrogenic activities of two types of diesel exhaust particles. Toxicology 2002;170(1–2):153–61. [DOI] [PubMed] [Google Scholar]
- 7.Chang FK, Mao IF, Chen ML, Cheng SF. Urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to ethylbenzene. Ann Occup Hyg 2011;55(5):519–25. [DOI] [PubMed] [Google Scholar]
- 8.Jia R, Cao LP, Du JL, Wang JH, Liu YJ, Jeney G, Xu P, Yin GJ. Effects of carbon tetrachloride on oxidative stress, inflammatory response and hepatocyte apoptosis in common carp (Cyprinus carpio). Aquat Toxicol 2014;152:11–9. [DOI] [PubMed] [Google Scholar]
- 9.Ju R, Jia Q, Meng T, Wang C, Chen X, Niu Y, Meng X, Geng X, Ma Y, Jia Q, Miao P, Dai Y, Zheng Y, Shao H. Effect of occupational exposure to toluene diisocyanate on workers’ health. Chinese journal of industrial hygiene and occupational diseases 2016;34(1):23–6. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Moon JY, Park EY, Lee KH, Hong YC. Changes in oxidative stress biomarker and gene expression levels in workers exposed to volatile organic compounds. Ind Health 2011;49(1):8–14. [DOI] [PubMed] [Google Scholar]
- 11.Lai CH, Liou SH, Lin HC, Shih TS, Tsai PJ, Chen JS, Yang T, Jaakkola JJ, Strickland PT. Exposure to traffic exhausts and oxidative DNA damage. Occup Environ Med 2005;62(4):216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mo GW, Cai SX, Zhao HJ, Li WJ, Tong WC, Liu LY. Effect of toluene diisocyanate on reactive oxygen species production and permeability of human bronchial epithelial cells in vitro. Journal of Southern Medical University 2011;31(2):239–43. [PubMed] [Google Scholar]
- 13.Pu X, Kamendulis LM, Klaunig JE. Acrylonitrile-induced oxidative stress and oxidative DNA damage in male Sprague-Dawley rats. Toxicol Sci 2009;111(1):64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sati PC, Khaliq F, Vaney N, Ahmed T, Tripathi AK, Banerjee BD. Pulmonary function and oxidative stress in workers exposed to styrene in plastic factory: occupational hazards in styrene-exposed plastic factory workers. Hum Exp Toxicol 2011;30(11):1743–50. [DOI] [PubMed] [Google Scholar]
- 15.Sisto R, Botti T, Cerini L, Sanjust F, Tranfo G, Bonanni RC, Paci E, Pigini D, Moleti A. Oxidative stress biomarkers and otoacoustic emissions in humans exposed to styrene and noise. Int J Audiol 2016;55(9):523–31. [DOI] [PubMed] [Google Scholar]
- 16.Uzma N, Kumar BS, Hazari MA. Exposure to benzene induces oxidative stress, alters the immune response and expression of p53 in gasoline filling workers. Am J Ind Med 2010;53(12):1264–70. [DOI] [PubMed] [Google Scholar]
- 17.Strafella E, Bracci M, Staffolani S, Manzella N, Giantomasi D, Valentino M, Amati M, Tomasetti M, Santarelli L. Occupational styrene exposure induces stress-responsive genes involved in cytoprotective and cytotoxic activities. PLoS One 2013;8(9):e75401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Press DJ, Pharoah P. Risk factors for breast cancer: a reanalysis of two case-control studies from 1926 and 1931. Epidemiology 2010;21(4):566–72. [DOI] [PubMed] [Google Scholar]
- 19.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med 2006;354(3):270–82. [DOI] [PubMed] [Google Scholar]
- 20.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420(6917):860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res 2007;9(4):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jezierska-Drutel A, Rosenzweig SA, Neumann CA. Role of oxidative stress and the microenvironment in breast cancer development and progression. Adv Cancer Res 2013;119:107–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 2004;44:239–67. [DOI] [PubMed] [Google Scholar]
- 24.Rossner P Jr., Gammon MD, Terry MB, Agrawal M, Zhang FF, Teitelbaum SL, Eng SM, Gaudet MM, Neugut AI, Santella RM. Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2006;15(4):639–44. [DOI] [PubMed] [Google Scholar]
- 25.Tobias DK, Akinkuolie AO, Chandler PD, Lawler PR, Manson JE, Buring JE, Ridker PM, Wang L, Lee IM, Mora S. Markers of inflammation and incident breast cancer risk in the Women’s Health Study. Am J Epidemiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 27.Garcia E, Hurley S, Nelson DO, Hertz A, Reynolds P. Hazardous air pollutants and breast cancer risk in California teachers: a cohort study. Environ Health 2015;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart JE, Bertrand KA, DuPre N, James P, Vieira VM, VoPham T, Mittleman MR, Tamimi RM, Laden F. Exposure to hazardous air pollutants and risk of incident breast cancer in the nurses’ health study II. Environ Health 2018;17(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu R, Nelson DO, Hurley S, Hertz A, Reynolds P. Residential exposure to estrogen disrupting hazardous air pollutants and breast cancer risk: the California Teachers Study. Epidemiology 2015;26(3):365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Cancer Society. Cancer facts & figures 2018. Atlanta: American Cancer Society, 2018. [Google Scholar]
- 31.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res 2011;188:125–39. [DOI] [PubMed] [Google Scholar]
- 32.Niehoff NM, Nichols HB, Zhao S, White AJ, Sandler DP. Adult physical activity and breast cancer risk in women with a family history of breast cancer. Cancer Epidemiol Biomarkers Prev 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong CM, Ou CQ, Thach TQ, Chau YK, Chan KP, Ho SY, Chung RY, Lam TH, Hedley AJ. Does regular exercise protect against air pollution-associated mortality? Prev Med 2007;44(5):386–92. [DOI] [PubMed] [Google Scholar]
- 34.Giles LV, Koehle MS. The health effects of exercising in air pollution. Sports Med 2014;44(2):223–49. [DOI] [PubMed] [Google Scholar]
- 35.White AJ, Nichols HB, Bradshaw PT, Sandler DP. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer 2015;121(20):3700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fortner RT, Katzke V, Kuhn T, Kaaks R. Obesity and breast cancer. Recent Results Cancer Res 2016;208:43–65. [DOI] [PubMed] [Google Scholar]
- 37.Weichenthal S, Hoppin JA, Reeves F. Obesity and the cardiovascular health effects of fine particulate air pollution. Obesity (Silver Spring, Md.) 2014;22(7):1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudel RA, Attfield KR, Schifano JN, Brody JG. Chemicals causing mammary gland tumors in animals signal new directions for epidemiology, chemicals testing, and risk assessment for breast cancer prevention. Cancer 2007;109(12 Suppl):2635–66. [DOI] [PubMed] [Google Scholar]
- 39.Sandler DP, Hodgson ME, Deming-Halverson SL, Juras PS, D’Aloisio AA, Suarez LM, Kleeberger CA, Shore DL, DeRoo LA, Taylor JA, Weinberg CR. The Sister Study Cohort: Baseline Methods and Participant Characteristics. Environ Health Perspect 2017;125(12):127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Institute of Environmental Health Sciences. The Sister Study: Participation rates for annual and detailed follow-ups. https://sisterstudy.niehs.nih.gov/English/tables.htm, 2017.
- 41.Environmental Protection Agency. 2005 NATA: Assessment results. 2010.
- 42.Environmental Protection Agency. An overview of methods for EPA’s National-Scale Air Toxics Assessment. Durham, North Carolina: Office of Air Quality, Planning, and Standards, 2011. [Google Scholar]
- 43.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10(1):37–48. [PubMed] [Google Scholar]
- 44.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allison P Survival analysis using SAS: A practical guide. 2 ed. Cary, NC, 2010. [Google Scholar]
- 46.de Mutsert R, Jager KJ, Zoccali C, Dekker FW. The effect of joint exposures: examining the presence of interaction. Kidney Int 2009;75(7):677–81. [DOI] [PubMed] [Google Scholar]
- 47.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol 2007;17(3):227–36. [DOI] [PubMed] [Google Scholar]
- 48.VanderWeele TJ. Causal interactions in the proportional hazards model. Epidemiology (Cambridge, Mass.) 2011;22(5):713–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology: 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 50.Breiman L, Friedman J, Olshen R, Stone C. Classification and regression trees. 2nd ed. Pacific Grove, CA: Wadsworth, 1984. [Google Scholar]
- 51.Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med 2003;26(3):172–81. [DOI] [PubMed] [Google Scholar]
- 52.Benjamini Y, Hochberg Y. Controlling The False Discovery Rate - A Practical And Powerful Approach To Multiple Testing. Vol. 57, 1995. [Google Scholar]
- 53.International Agency for Research on Cancer. Agents classified by the IARC Mongraphs, Volumes 1–122. https://monographs.iarc.fr/list-of-classifications-volumes/.
- 54.Schlosser PM, Bale AS, Gibbons CF, Wilkins A, Cooper GS. Human health effects of dichloromethane: key findings and scientific issues. Environ Health Perspect 2015;123(2):114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohyama KI, Nagai F, Tsuchiya Y. Certain styrene oligomers have proliferative activity on MCF-7 human breast tumor cells and binding affinity for human estrogen receptor. Environ Health Perspect 2001;109(7):699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toyooka T, Yanagiba Y, Suda M, Ibuki Y, Wang RS. 1,2-Dichloropropane generates phosphorylated histone H2AX via cytochrome P450 2E1-mediated metabolism. Toxicol Lett 2017;272:60–67. [DOI] [PubMed] [Google Scholar]
- 57.International Agency for Research on Cancer. Some chemicals used as solvents in polymer manufacture. Vol. 110 Lyon, France: World Health Organization, 2017;141–176. [PubMed] [Google Scholar]
- 58.Zhang Y, Dong S, Wang H, Tao S, Kiyama R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ Pollut 2016;213:809–824. [DOI] [PubMed] [Google Scholar]
- 59.Santodonato J Review of the estrogenic and antiestrogenic activity of polycyclic aromatic hydrocarbons: relationship to carcinogenicity. Chemosphere 1997;34(4):835–48. [DOI] [PubMed] [Google Scholar]
- 60.Fertuck KC, Kumar S, Sikka HC, Matthews JB, Zacharewski TR. Interaction of PAH-related compounds with the alpha and beta isoforms of the estrogen receptor. Toxicol Lett 2001;121(3):167–77. [DOI] [PubMed] [Google Scholar]
- 61.Arcaro KF, O’Keefe PW, Yang Y, Clayton W, Gierthy JF. Antiestrogenicity of environmental polycyclic aromatic hydrocarbons in human breast cancer cells. Toxicology 1999;133(2–3):115–27. [DOI] [PubMed] [Google Scholar]
- 62.Kummer V, Maskova J, Zraly Z, Neca J, Simeckova P, Vondracek J, Machala M. Estrogenic activity of environmental polycyclic aromatic hydrocarbons in uterus of immature Wistar rats. Toxicol Lett 2008;180(3):212–21. [DOI] [PubMed] [Google Scholar]
- 63.White AJ, Bradshaw PT, Herring AH, Teitelbaum SL, Beyea J, Stellman SD, Steck SE, Mordukhovich I, Eng SM, Engel LS, Conway K, Hatch M, Neugut AI, Santella RM, Gammon MD. Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ Int 2016;89–90:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonner MR, Han D, Nie J, Rogerson P, Vena JE, Muti P, Trevisan M, Edge SB, Freudenheim JL. Breast cancer risk and exposure in early life to polycyclic aromatic hydrocarbons using total suspended particulates as a proxy measure. Cancer Epidemiol Biomarkers Prev 2005;14(1):53–60. [PubMed] [Google Scholar]
- 65.Gammon MD, Santella RM, Neugut AI, Eng SM, Teitelbaum SL, Paykin A, Levin B, Terry MB, Young TL, Wang LW, Wang Q, Britton JA, Wolff MS, Stellman SD, Hatch M, Kabat GC, Senie R, Garbowski G, Maffeo C, Montalvan P, Berkowitz G, Kemeny M, Citron M, Schnabel F, Schuss A, Hajdu S, Vinceguerra V. Environmental toxins and breast cancer on Long Island. I. Polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol Biomarkers Prev 2002;11(8):677–85. [PubMed] [Google Scholar]
- 66.Mordukhovich I, Beyea J, Herring AH, Hatch M, Stellman SD, Teitelbaum SL, Richardson DB, Millikan RC, Engel LS, Shantakumar S, Steck SE, Neugut AI, Rossner P, Santella RM, Gammon MD. Vehicular traffic-related polycyclic aromatic hydrocarbon exposure and breast cancer incidence: The Long Island Breast Cancer Study Project (LIBCSP). Environ Health Perspect 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amadou A, Praud D, Coudon T, Danjou AMN, Faure E, Leffondre K, Le Romancer M, Severi G, Salizzoni P, Mancini FR, Fervers B. Chronic long-term exposure to cadmium air pollution and breast cancer risk in the French E3N cohort. Int J Cancer 2019. [DOI] [PubMed] [Google Scholar]
- 68.White AJ, O’Brien KM, Niehoff NM, Carroll R, Sandler DP. Metallic air pollutants and breast cancer risk in a nationwide cohort study. Epidemiology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4(7):1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Eviron Health Perspect 2006;114(7):992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci 2013;14(5):10497–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.National Institute of Diabetes and Digestive and Kidney Diseases. Overweight & obesity statistics. https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity.
- 73.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. Jama 2018;319(16):1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods 2009;14(4):323–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.