Abstract
Previous studies have suggested increased risk of respiratory diseases and mortality following short-term exposures to ionizing radiation. However, the short-term respiratory effects of low-level environmental radiation associated with air pollution particles have not been previously considered. Although ambient particulate matter (PM) has been reproducibly linked to decreased lung function and to increased respiratory related morbidity, the properties of PM promoting its toxicity are uncertain. As such, we evaluated whether lung function was associated with exposures to radioactive components of ambient PM, referred to as particle radioactivity (PR). For this, we performed a repeated-measures analysis of 839 men to examine associations between PR exposure and lung function using mixed-effects regression models, adjusting for potential confounders. We examined whether PR-lung function associations changed after adjusting for PM2.5 (particulate matter≤2.5 μm) or black carbon, and vice versa. PR was measured by the USEPA’s radiation monitoring network. We found that higher PR exposure was associated with a lower forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1). An IQR increase in 28-day PR exposure was associated with a 2.4% lower FVC [95% confidence interval (CI): 1.4, 3.4% p<0.001] and a 2.4% lower FEV1 (95% CI: 1.3, 3.5%, p<0.001). The PR-lung function associations were partially attenuated with adjustment for PM2.5 and black carbon. This is the first study to demonstrate associations between PR and lung function, which were independent of and similar in magnitude to those of PM2.5 and black carbon. If confirmed, future research should account for PR exposure in estimating respiratory health effects of ambient particles. Because of widespread exposure to low levels of ionizing radiation, our findings may have important implications for research, and environmental health policies worldwide.
Keywords: Particle radioactivity, lung function, epidemiology, particulate matter, particle toxicity
1. INTRODUCTION
Previous studies have suggested increased risk of mortality, cancer- and non-cancer respiratory disease following short-term exposures to ionizing radiation from the atomic bomb (Furukawa et al., 2013; Kamiya et al., 2015; Pham et al., 2013; Preston et al., 2003), from radiotherapy to treat thoracic malignancies, or longer term occupational radiation exposures (Vrijkeid et al., 2007; Vacquier et al., 2008; Belyaeva et al., 2008). A number of epidemiological studies have provided evidence of an association between radon, a naturally occurring radioactive gas, and lung cancer risk and mortality (Krewski et al., 2005; WHO, 2009; Turner et al., 2011; Kim et al., 2016). However, there is little research on the association between radon and non-malignant respiratory disease (Turner et al., 2012; NRC, 1988). Results from animal studies have shown associations of pulmonary fibrosis and emphysema with exposure to either radon progeny alone or in combination with uranium ore dust (NRC, 1988; Archer et al., 1998). The short-term respiratory effects of low-level radiation associated with air pollution particles have not previously been considered.
Particulate matter (PM) air pollution has been linked to short- and long-term respiratory conditions that include exacerbation and development of asthma, chronic obstructive pulmonary disease (COPD), pulmonary infections, bronchitis and cystic fibrosis (Dominici et al., 2006; Kelly et al., 2011; Peng et al., 2008; Liu et al., 2017). Studies have reported associations between short-term air pollution exposure and decreased lung function, specifically lower forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) (Lepuele et al., 2014; Rice et al., 2013; Int Panis et al., 2017). However, there is little knowledge of the properties of PM that promote its toxicity.
While the radiometric composition of PM has been measured (Dueñas et al., 1999; Hernández et al., 2004), the association between particle radioactivity (PR) and lung function has not been investigated. Therefore, we investigate the effects of PR on respiratory health. We hypothesize that exposure to PR will decrease FVC and FEV1, independent of other air pollution exposures.
Radiation is produced through the decay of unstable atoms called radionuclides (UNSCEAR, 2008). Radionuclides may decay by one of several processes: emission of alpha particles or beta particles, or by isomeric transitions such as gamma or X-ray emissions. Radon is an ubiquitous radioactive gas, formed by the decay of uranium radionuclides naturally present in rocks in the earth’s crust. After formation, radon emanates from the soil and continues to decay into radon progeny. When radionuclide decay products are inhaled, they continue to decay and irradiate lung tissues (ICRP, 2007; US NAS, 2006; IARC, 2001; Kendall and Smith, 2002). Please see the Online Data Supplement for further details on the radioactive decay, radiation exposure, and dose.
The radiometric composition of airborne particulates has been quantified previously (Dueñas et al., 1999; Hernández et al., 2004). This includes analyzing particulate samples for gross alpha and gross beta activities, and gamma rays. Hernández et al. (2004) analyzed the radiometric compositions of airborne particulate samples over a four year period. For this, gross alpha and beta activities were measured, showing correlation with each other (R=0.72). In the United States, environmental radioactivity is measured by the US Environmental Protection Agency’s RadNet monitoring network (USEPA, 2017). In this study, we use gross beta activity as a surrogate for PR.
Nyhan et al., (2018) examined the associations between PR exposure and blood pressure. However, the impact of PR exposure from particulate matter on lung function have not been studied previously. In addressing a critical gap in the literature, we examine the associations of short- to medium-term average exposures (defined as from 7- to 28-days) to PR and lung function. Radionuclides attached to inhaled particulates may continue to emit radiation in the human respiratory tract, where they may promote inflammation and oxidative stress, and in turn affect respiratory function. We examined this in a longitudinal study in a cohort of men, with repeated clinical measurements taken approximately every four years. This study testing for independent PR-lung function associations complements previous research demonstrating decreased lung function with increased particle mass less than 2.5 μm (PM2.5) and black carbon (BC).
2. METHODS
1.1. Study population
Our study included 839 participants who were enrolled in the Normative Aging Study (NAS) cohort, a longitudinal investigation established in Boston in 1963 by the U.S. Veterans Administration (VA) and limited to healthy men (Bell et al., 1972). At the time of initial enrollment, participants were free of heart disease, hypertension, diabetes, cancer, recurrent asthma, or bronchitis. Subjects completed one to seven clinical examinations with intervals of 3–5 years (a total of 2,228 observations) during the period 1998 to 2013. Medical visits included on-site physical examinations and detailed questionnaires after smoking abstinence and an overnight fast. This study was approved by the Harvard School of Public Health and the VA Institutional Review Boards (IRBs). Subjects provided written informed consent to participate in this study. At each visit, information about medication use (corticosteroids, sympathomimetics, anticholinergics), pulmonary disorders, and smoking history were collected using the American Thoracic Society (ATS) questionnaire (Ferris et al., 1978).
Spirometric tests were performed following strict protocol in accordance with ATS guidelines, as previously reported (Sparrow et al., 1987). Spirometry was assessed in the standing position with a nose-clip using a 10-L water-filled survey-recoding spirometer and an Eagle II minicomputer (Warren E. Collins, Braintree, MA, USA). Values were adjusted for body temperature and pressure. A minimum of three acceptable spirograms were obtained, of which at least two were reproducible within 5% for both FVC and FEV1. Technicians underwent training before taking measurements for the study.
Methacholine challenge tests were conducted using procedures adapted from Chatham et al. (1982). Participants with ischemic heart disease or baseline FEV1 <60% of the predicted value were excluded, and some elected not to participate. Methacholine inhalations were administered at incremental doses corresponding to 0, 0.330, 1.98, 8.58, 16.8, and 49.8 µmol. Participants whose FEV1 declined by 20% in response to any of the doses at or before 8.58 µmol were classified as having airway hyperresponsiveness. Participants whose FEV1 did not decline by 20% in response to any of the administered doses, and participants who demonstrated a 20% decline in FEV1 at higher methacholine dosages (16.8 or 49.8 µmol) only were categorized as having no airway hyperresponsiveness.
1.2. Particle radioactivity and gross beta activity
Similar to Nyhan et al., (2018) and Li et al. (2018), gross beta activity was used as a surrogate for PR in this study. Gross beta activity is a measurement of all particle bound ambient beta activity, regardless of the specific radionuclide source (USEPA, 2017). A note on radioactive decay and the release of radiation is included in the Supplementary Materials. Gross beta activity data were acquired from the US EPA’s RadNet monitoring network (USEPA, 2017). The RadNet stationary sampling stations provide airborne particulate samples on synthetic fiber filters. Gross beta activity on the filters are measured following a 5–15 day period to permit decay of short-lived radon progenies (e.g., 214Pb, 214Bi) that may be attached to the particles. Despite the decay of most of the short-lived radionuclides, there is still residual radioactivity especially from the last one to two days of the sampled particles, which can be related to the relatively long-lived radon progenies (e.g., 210Pb and 210Bi). We use these data as an indicator of radiation activity of particles collected on the filter. The validity of this assumption has been demonstrated previously (Hernández et al., 2004) where a significant linear correlation (R=0.72) between gross beta and gross alpha activity was observed. To quantify the beta activity on filters, a background subtraction procedure is applied. As samples are collected over several days (typically from 5 to 7 days for each sampling period), we assigned all days within each sampling period with the same beta activity levels. Subsequently, we calculated moving averages based on these measures. Due to this multi-day sampling protocol, we calculated PR exposures for durations longer than 7 days.
Gross beta activity measurements were attained from monitoring sites in Boston MA, Worcester MA, Providence RI and Albany NY. The locations correspond to the geographical distribution of the residential locations of the NAS participants (please see Figure S2 in the Supplementary Materials). A regional mean gross beta activity was calculated based on the data collected and used as the PR exposure for the study. The data collected from each site did not cover the entire study period and different sites covered different periods. We therefore, used measurements from the Albany NY site, which covered the entire study period from 1998 to 2013, to predict levels in each of the other three sites. We did this using a multiple linear regression analysis. We then took an average of these three sites to represent the daily regional beta concentrations. The regression analyses used to model the missing PR exposures for the study population residing in the eastern MA area are described in Nyhan et al., (2018) and have been adopted by Li et al., (2018) also. We examined the effects of 7-, 14-, 21-, and 28-day moving average PR exposures.
1.3. Air pollution
Similar to a previous air pollution studies investigating the association between air pollution and respiratory-related outcomes (Lepuele et al., 2014), we explored short- and medium-term exposure windows. For short-term exposures, we focused on moving average air pollution concentrations computed from seven to 28 days prior to medical visits. From the year 1998 onwards, we measured ambient particle concentrations at the Harvard US EPA Supersite located in downtown Boston, MA and approximately 1 km from the medical center where the study participants were examined. The site (42°20´ north latitude, 71°06´ west longitude) is located on the roof of the Countway Library (a six-floor building) of the Harvard Medical School. This site is located within one block of a four-lane street with truck traffic and with two major highways nearby. Semi-continuous and integrated filter-based measurements of PM2.5 (particles with an aerodynamic diameter of ≤2.5 µm) and BC concentrations were conducted. Hourly PM2.5 mass concentrations were measured using a Tapered Element Oscillation Microbalance (Model 1400A, Rupprecht and Pastachnick, East Greenbush, NY). Ambient hourly concentrations of BC were measured using an Aethalometer (Magee Scientific Company, model AE-16, Berkeley, CA) on the basis of optical transmittance at a single wavelength (γ = 880 nm). The precision of this method was examined using two collocated Aethalometers. BC concentrations were determined based on the attenuation of light transmitted through a sampled quartz-fiber filter tape. The attenuation coefficient used was a fixed value of 16.6 m2/g provided by the manufacturer. We calculated 7-, 14-, 21-, and 28-day moving average exposure using these hourly data. A detailed description of the supersite has been previously published (Kang et al., 2010).
1.4. Statistical methods
In separate models, we examined whether 7-, 14-, 21- and 28-day moving average PR and air pollution exposures (PM2.5 and BC) were associated with lung function outcomes (FVC and FEV1). Our primary models included a single exposure variable (PR, PM2.5 and BC), while a second set of models included two exposure variables (PR and PM2.5; PR and BC). We analyzed associations using linear mixed effects models with a random intercept for each subject. We evaluated FVC and FEV1 as dependent variables. The models took the form:
where Yit is the log-transformed lung function measurement in subject at visit t, β0 was the intercept, βE was the effect of the exposure variable on lung function measurement, Eit is the mean exposure concentration for the subject i in the day-of and days prior to the visit t, the covariates for subject i at visit t are denoted by X1it to Xkit, εit and was the within-participant error. Here ui represents a subject-specific intercept, reflecting unexplained heterogeneity in subjects’ overall level of outcome and accounting for longitudinal correlation among measurements taken on the same subject. We assume that ui values are generated from a normal distribution with common variance, yielding the compound symmetry variance structure. The model accommodates unbalanced data (i.e., varying numbers of repeated measurements on each subject) under the assumption that any missing data is missing at random. We reported the effect estimates as the percent difference in FVC and FEV1 outcome per interquartile range increase (IQR) in exposure, adjusting for the other covariates in the model. Exposure IQRs were calculated separately for each exposure window studied (from seven to 28-days prior to the medical visit). The IQR reflects the distribution (25th-75th percentiles) in the observed data, while also enabling a comparison of the effects of different exposure types measured with different units such as radioactivity and mass concentration units.
Based on previous NAS studies, we have selected the following adjustment covariates and added a quadratic term whenever it was significant: age (linear and quadratic), ln(height) (linear and quadratic) and standardized weight (linear), race, education level, smoking status, cumulative smoking in pack-years, season of the medical examination (using sine and cosine of time), day of the week, visit number, temperature and relative humidity (matched on exposure windows), physician-diagnosed chronic lung conditions (asthma, emphysema, chronic bronchitis), methacholine responsiveness, and medication use. We visually assessed the linearity of the association of PR and air pollution exposures on both FVC and FEV1 by fitting penalized splines, using generalized additive mixed models. Because participants with chronic lung conditions are expected to be sicker than average, we explored potential modification of PR and air pollution effects by asthma, emphysema, chronic bronchitis, and chronic obstructive pulmonary disease [COPD; defined as GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage II (FEV1/FVC <70% and FEV1 <80% predicted) or higher] by incorporating these variables into our primary analysis.
Several sensitivity analyses were performed. In these tests, we included each of the air pollutants (PM2.5 and BC) in regression models with PR. In doing so, we examined whether the PR-lung function effect was changed by each of the air pollutant parameters. We also estimated the extent to which the air pollution-lung function effect estimates were modified by PR exposure. Furthermore, we examined potential interactions between PR and each of the air pollutants (PM2.5 and BC).
We furthermore conducted a number of sensitivity tests whereby we completely excluded participants with cardiovascular diseases (coronary heart diseases, stroke), diabetes, and hypertension. Following this, we excluded participants with physician-diagnosed asthma, emphysema, and chronic bronchitis. These sensitivity tests were included for the PR-lung function and the particulate air pollution-lung function models. Finally, to account for the fact that healthier individuals are more likely to participate in follow-up visits, we applied inverse probability weighting to calculate the probability of having a follow-up visit given their age, education level, BMI, smoking status, pack-years, hypertension, cholesterol, diabetes, FEV1, asthmas, emphysema, chronic bronchitis, methacholine responsiveness, and air pollution concentration at previous visits (Hernan et al., 2006). All analyses were performed using R software (http://cran-r-project.org). The R libraries and packages used are listed in the Supplementary Materials.
3. RESULTS
3.1. Descriptive results
Table 1 shows the longitudinal characteristics of the population. By study design, participants were all male, with a mean age of 75 years. At baseline, less than 10% were current smokers, but the majority (67%) were former smokers. Participants had a mean BMI of 28 kg/m2. Furthermore, 29.2% of participants had coronary heart disease, while 72.6% were hypertensive and 13.3% had diabetes during the study. The Spearman correlation between FVC and FEV1 was 0.90.
Table 1.
Descriptive statistics for the NAS cohort, including lung function-related outcomes and demographic characteristics reported on the first medical examination (baseline) (n=839 subjects) and over all clinical examinations (N=2,228). Visit characteristics are also reported.
| Study variable (unit) | All visits Mean (SD) |
|---|---|
| FVC (L) | 3.4 (0.7) |
| FEV1 (L) | 2.5 (0.6) |
| Age (years) | 75.1 (7.0) |
| Height (m) | 173.6 (7.0) |
| Weight (kg) | 84.4 (14.3) |
| BMI (kg/m2) | 27.95 (4.07) |
| Cumulative smoking (pack-yearsa) | 19.85 (24.25) |
| Years of education (individual) | 14.61 (2.91) |
| Categorical variables | N (%) |
| Race | |
| White | 818 (97.5) |
| Black | 14 (1.7) |
| Missing | 7 (1.0) |
| Smoking status | |
| Never | 236 (28.1) |
| Former | 564 (67.2) |
| Current | 39 (4.6) |
| Asthma | 49 (5.8) |
| Chronic bronchitis | 54 (6.4%) |
| Emphysema | 30 (3.6%) |
| Methacholine responsiveness | 87 (10.1) |
| Missing | 138 (16.0) |
| Corticosteroids | 54 (6.4) |
| Sympathomimetics (α, β) | 67 (8.0) |
| Anticholinergic | 15 (1.8) |
| Coronary heart disease | 245 (29.2) |
| Stroke | 53 (6.3) |
| Hypertension | 609 (72.6) |
| Diabetesb | 112 (13.3) |
| Visit Characteristics | N (%) |
| Season | |
| Spring (March-May) | 505 (22.7) |
| Summer (June-August) | 609 (27.3) |
| Fall (September-November) | 733 (32.9) |
| Winter (December-February) | 381 (17.1) |
Values for the continuous variables are reported as mean ± SD, while values for the categorical variables are no. (%).
Pack-year is defined as the number of packs of cigarettes smoked per day times the number of years the person has smoked.
Diabetic status was diagnosed by a physician.
Characteristics of the ambient PR and air pollution exposures during the study period and their correlations are presented in Table 2, Table 3 and Table E2. The IQR exposures used to scale the effect estimates are also described. The mean 28-day PR exposure was found to be 2.68 ×10−4 Bq/m3, with an IQR of 0.5 ×10−4 Bq/m3. Mean 28-day PM2.5 exposure was 9.66 µg/m3 with an IQR of 3.78 µg/m3. Mean 28-day BC exposure was 0.71 µg/m3 with an IQR of 0.25 µg/m3. The mean ambient temperature was 12.8 °C during all study visits.
Table 2.
Distributions of ambient measured PR (gross beta activity) and particulate air pollution variables (PM2.5 mass and BC), starting in the year 1998 and ending in year 2013.
| Exposure (unit) | PR (Bq/m3) | PM2.5 (µg/m3) | BC (µg/m3) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD ×10−4 | IQR ×10−4 | Mean ± SD | IQR | Mean ± SD ×10−1 | IQR ×10−1 | ||
| Exposure window (days) | 7 | 2.69 ± 0.73 | 0.90 | 9.61 ± 3.98 | 4.67 | 7.16 ± 2.23 | 3.13 |
| 14 | 2.67 ± 0.62 | 0.82 | 9.61 ± 3.35 | 4.16 | 7.15 ± 1.88 | 2.60 | |
| 21 | 2.68 ± 0.56 | 0.74 | 9.63 ± 3.12 | 3.90 | 7.13 ± 1.76 | 2.43 | |
| 28 | 2.68 ± 0.53 | 0.69 | 9.66 ± 3.01 | 3.78 | 7.14 ± 1.70 | 2.49 | |
Table 3.
Correlation matrix of 7-day moving average PR (gross beta activity) and particulate air pollution variables (PM2.5 mass and BC), for clinical examination days (N=2,228).
| R2 | |||
|---|---|---|---|
| Exposure | PR | PM2.5 | BC |
| PR | 1 | ||
| PM2.5 | 0.22 | 1 | |
| BC | 0.14 | 0.47 | 1 |
All p-values were <0.05.
3.2. Exposures and lung function
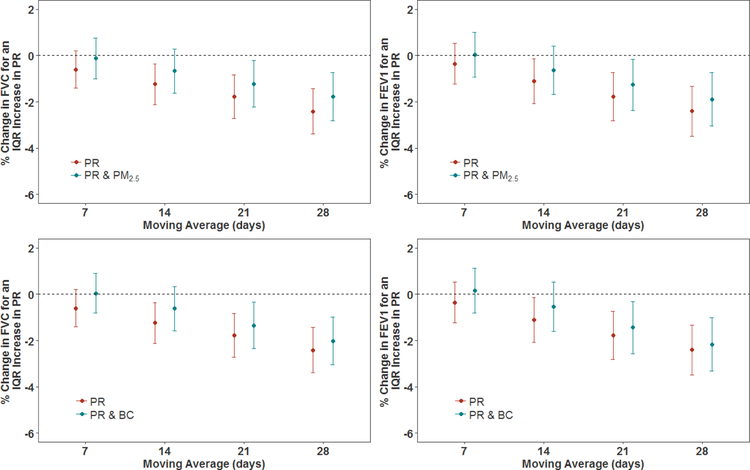
Higher PR exposures were significantly (p<0.05) associated with lower FVC and FEV1, after adjusting for confounders (see Figure 1 and Table 4). The magnitude of the estimated associations increased as the exposure window increased. An IQR difference in 14-day PR exposure was significantly (p<0.05) associated with differences of −1.24% (95% CI: −2.11, −0.36 %) and −1.10% (−2.07, −0.14%) in FVC and FEV1, respectively. An IQR increase in PR exposures in the previous 28-days was significantly (p<0.001) associated with a −2.41% (95% CI: −3.39, −1.43%) change in FVC and a −2.41% (95% CI: −3.49, −1.33%) change in FEV1.
Figure 1.
Associations of PR exposure with FVC and FEV1, and associations of PR with FVC and FEV1 where the models have also included particulate air pollution (PM2.5 and BC) exposures, in a cohort of men (n=839 subjects). Results were adjusted for age, race, height, weight, education level, smoking status, cumulative smoking, sine and cosine terms of the day of the year, day of the week, visit number, temperature, relative humidity, asthma, chronic bronchitis, emphysema, methacholine responsiveness, corticosteroids, sympathomimetics, anticholinergics. The y-axis represents the percent difference (and 95% CIs) in FVC or FEV1 per IQR increase in PR exposure. The IQRs for PR are shown in Table 2.
Table 4.
Differences in lung function (FEV1 and FVC) associated with an IQR increase in exposure to PR, as observed in the cohort of men, for 2,228 clinical visits (n=839 subjects). Outcomes are expressed as the percent difference in FEV1 or FVC per IQR increase in PR. IQRs for PR are shown in Table 2 in the main manuscript.
| Difference in FVC per IQR increase in exposure to PR (95 % CI) | ||||
|---|---|---|---|---|
| Exposures in model | PR | PR & PM2.5 | PR & BC | |
| Exposure window (days) | 7 | −0.60 (−1.40, 0.20) | −0.12 (−1.00, 0.76) | 0.05 (−0.81, 0.91) |
| 14 | −1.24 (−2.11, −0.36)** | −0.67 (−1.61, 0.28) | −0.62 (−1.57, 0.33) | |
| 21 | −1.77 (−2.72, −0.83)*** | −1.22 (−2.22, −0.21)** | −1.34 (−2.34, −0.35)** | |
| 28 | −2.41 (−3.39, −1.43)*** | −1.77 (−2.81, −0.73)*** | −2.02 (−3.04, −0.99)*** | |
| Difference in FEV1 per IQR increase in exposure to PR (95 % CI) | ||||
| Exposures in model | PR | PR & PM2.5 | PR & BC | |
| Exposure Window (days) | 7 | −0.35 (−1.24, 0.53) | 0.03 (−0.93, 1.00) | 0.16 (−0.81, 1.13) |
| 14 | −1.10 (−2.07, −0.14)** | −0.64 (−1.68, 0.41) | −0.52 (−1.59, 0.54) | |
| 21 | −1.77 (−2.81, −0.73)*** | −1.26 (−2.36, −0.16)** | −1.44 (−2.56, −0.31)** | |
| 28 | −2.41 (−3.49, −1.33)*** | −1.89 (−3.04, −0.74)*** | −2.17 (−3.33, −1.01)*** | |
p-value<0.1
p-value<0.05
p-value<0.001.
All models were adjusted for age, race, height, weight, education level, smoking status, cumulative smoking, sine and cosine terms of the day of the year, day of the week, visit number, temperature, relative humidity, asthma, chronic bronchitis, emphysema, methacholine responsiveness, corticosteroids, sympathomimetics, anticholinergics.
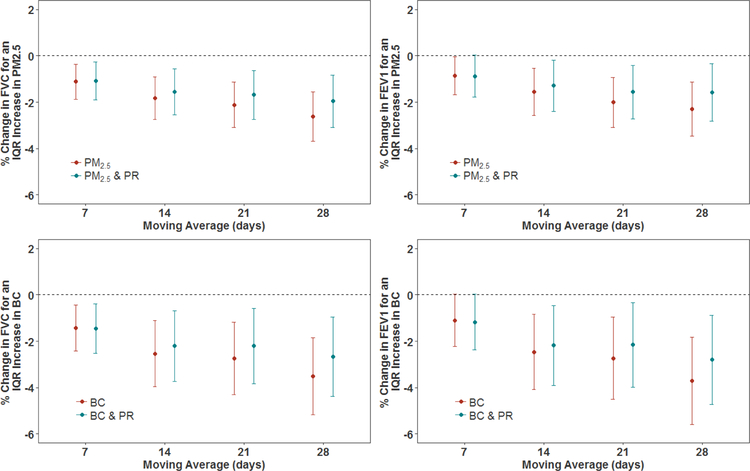
We evaluated the impact of additionally controlling for PM2.5 and BC levels on the PR-lung function relationship, results of which are shown in Figures 1 and 2, along with Tables 4 and 5. When models included both PR and PM2.5, associations between PR exposure with both FVC and FEV1 were attenuated across all exposure windows, but remained significant (p<0.05) for 21- and 28-day exposures for both FVC and FEV1. When PR and BC were included together in a model, the association of PR with FVC and FEV1 was also attenuated. Even though the PR effect estimates were reduced, they remained statistically significant for the 21- and 28-day exposure windows.
Figure 2.
Associations of particulate air pollution exposures (PM2.5 and BC) with FVC and FEV1, and associations of particulate air pollution exposure with FVC and FEV1 where the models have also included PR exposures, in a cohort of men (n=839 subjects). All models were adjusted for age, race, height, weight, education level, smoking status, cumulative smoking, sine and cosine terms of the day of the year, day of the week, visit number, temperature, relative humidity, asthma, chronic bronchitis, emphysema, methacholine responsiveness, corticosteroids, sympathomimetics, anticholinergics. The y-axis represents the percent difference (and 95% CIs) in FVC or FEV1 per IQR increase in PR exposure. The IQRs for PR are shown in Table 2. The IQRs for PM2.5 mass and BC are shown in Table 2.
Table 5.
Difference in lung function (FEV1 and FVC) associated with an IQR increase in exposure to particulate air pollution, as observed in the cohort of men, for 2,228 clinical visits (n=839 subjects). Outcomes are expressed as the difference in FEV1 or FVC per IQR increase in particulate air pollution (PM2.5 mass or BC). IQRs for PM2.5 mass and BC are shown in Table 2.
| Exposures in model | Difference in FVC per IQR increase in PM2.5 (95% CI) | Difference in FEV1 per IQR increase in PM2.5 (95% CI) | |||
|---|---|---|---|---|---|
| PM2.5 | PM2.5 & PR | PM2.5 | PM2.5 & PR | ||
| Exposure window (days) | 7 | −1.12 (−1.86, −0.37)** | −1.07 (−1.89, −0.25)** | −0.86 (−1.68, 0.04)** | −0.87 (−1.77, 0.03)* |
| 14 | −1.83 (−2.75, −0.90)*** | −1.56 (−2.56, −0.55)** | −1.55 (−2.56, −0.53)*** | −1.29 (−2.39, −0.18)** | |
| 21 | −2.11 (−3.10, −1.13)*** | −1.68 (−2.73, −0.63)** | −2.01 (−3.09, −0.92)*** | −1.56 (−2.71, −0.41)** | |
| 28 | −2.62 (−3.68, −1.56)*** | −1.95 (−3.08, −0.83)*** | −2.30 (−3.47, −1.13)*** | −1.59 (−2.83, −0.34)** | |
| Exposures in model | Difference in FVC per IQR increase in BC (95% CI) | Difference in FEV1 per IQR increase in BC (95% CI) | |||
| BC | BC & PR | BC | BC & PR | ||
| Exposure window (days) | 7 | −1.43 (−2.43, −0.43)** | −1.45 (−2.51, −0.39)** | −1.10 (−2.23, −0.03)* | −1.17 (−2.37, 0.03)* |
| 14 | −2.53 (−3.97, −1.10)*** | −2.21 (−3.73, −0.68)** | −2.46 (−4.09, −0.84)** | −2.18 (−3.91, −0.46)** | |
| 21 | −2.75 (−4.32, −1.18)*** | −2.21 (−3.82, −0.59)** | −2.73 (−4.50, −0.96)** | −2.15 (−3.97, −0.33)** | |
| 28 | −3.51 (−5.17, −1.85)*** | −2.66 (−4.37, −0.96)** | −3.71 (−5.59, −1.83)*** | −2.80 (−4.73, −0.87)** | |
p-value<0.1
p-value<0.05
p-value<0.001.
All models were adjusted for age, race, height, weight, education level, smoking status, cumulative smoking, sine and cosine terms of the day of the year, day of the week, visit number, temperature, relative humidity, asthma, chronic bronchitis, emphysema, methacholine responsiveness, corticosteroids, sympathomimetics, anticholinergics.
PM2.5 exposure was significantly (p<0.05 or p<0.001) associated with FVC and FEV1 for all exposure windows (see Figure 2 and Table 5). An IQR increase in 28-day PM2.5 exposure was associated with a difference of −2.62% (95% CI: −3.68, −1.56%) in FVC and −2.30% (95% CI: −3.47, −1.13%) in FEV1. When PR was simultaneously included in the models, PM2.5-lung function associations remained stable for 7-day PM2.5 exposures; however, they were attenuated for the 14-, 21- and 28-day exposures. BC exposure had a significant (p<0.001) association with both FVC and FEV1. For example, an IQR increase in 28-day BC exposure was associated with a −3.51% (95% CI: −5.17, −1.85%) difference in FVC. When the models were adjusted for PR exposure also, this did not affect the results of 7-day BC exposures. However, for 14-, 21-, and 28-day exposures, the BC-lung function associations were attenuated but remained significant (p<0.05).
We assessed the linearity of the association of PR and air pollution exposures on both FVC and FEV1 by fitting penalized splines for all exposure durations studies. For all the effects of 7-, 14-, 21-, and 28-day PR exposures on FVC and FEV1, the AIC for each was minimized with a curve of more than 1 degree of freedom; however, the overall trends were also approximately linear with no evidence of a threshold (data not shown). In the models that included two exposure variables (PR and PM2.5, and PR and BC), we tested interactions by also including a multiplicative interaction term between the two pollutants in the model, but this interaction term was never significant (p<0.05).
3.3. Sensitivity tests
By omitting participants with cardiovascular diseases including coronary heart disease (29.2% of participants) and stroke (6.3%), diabetes (13.3 %), and hypertension (72.6 %), the results did not change (see the Online Data Supplement, Table S5 and S6). When participants with physician diagnosed asthma, emphysema and chronic bronchitis were excluded from the analysis (n = 1,948 following the exclusions), model estimates were consistent with the main analysis, although p-values were larger (see the Supplementary Materials, Table S7 and S8). With further controlling for potential survival bias using inverse probability weighting, results only varied slightly (data not shown).
4. DISCUSSION
4.1. Significant findings
To our knowledge, this is the first study to investigate the effect of PR exposure on lung function in a cohort. We investigated the effect of PR exposure on FVC and FEV1, using longitudinal data with repeated measurements for each subject. We examined the independent PR health effects while controlling for PM2.5 and BC, and vice versa. This is the first study to apply PR exposure for a respiratory health effects study using data collected from a radiation monitoring network such as the USEPA’s RadNet.
In quantifying the impact of PR exposure on FVC and FEV1 in the cohort, we observed statistically significant negative associations. The size of PR-induced lung function effects were comparable to those produced by PM2.5 and BC exposures in this study. When PR models also included PM2.5 exposures, the PR-lung function associations were partially attenuated, but retained statistical significance for two exposure windows. Similar results were observed when PR-lung function models simultaneously included BC. This suggests that PR may have an independent effect on lung function. However, as beta activity is measured from and depends on TSP (up to 30 µm in diameter including ultrafine, fine, and coarse particles), the radioactivity that the beta activity represents may be associated with both fine and coarse particles. Despite this, even after accounting for regional particle concentrations, PR-lung function effects were observed.
The associations between increased particulate air pollution, namely PM2.5 and BC exposures, and decreased FVC and FEV1 were significant in the study. When models controlled for PR exposure, the adverse PM2.5- and BC-lung function associations were attenuated for 14-, 21-, and 28-day exposures. This suggests that PR could potentially explain some of the previously reported associations between lung function and air pollution. The 7-day PM- and BC-lung function associations were not affected and therefore, may be independent of PR exposures. The associations with longer moving averages (14-, 21-, and 28-day exposures) were partially attenuated, suggesting that some of that particle toxicity may depend on radioactivity. The longer moving averages of beta activity (14-, 21-, and 28-day exposures) may be a more stable measure of radioactivity than the shorter 7-day moving averages, which depend more on sampling protocol. The PR measurements do not capture short-lived radionuclides, which may be important for acute lung function effects.
4.2. Comparison to the literature
As this is the first study to examine how PR exposure affects FVC and FEV1, we are unable to directly compare our PR results to other studies. The associations between PM2.5 and BC with lung function that we identified are comparable in magnitude, although slightly larger, than the effects of air pollution exposures determined in a previous NAS study (Lepuele et al., 2014). Although we defined our air pollution exposures using the same Harvard supersite and adjusted for similar covariates in our models to Lepuele et al., (2014), we used many more clinical measurements for a longer study period (from 1998 to 2013) than Lepuele et al. (2014) (from 1999 to 2009).
4.3. Biological mechanisms
Research regarding mechanisms mediating PR’s negative effect on respiratory health is limited. However, the biological mechanisms for the health effects of high doses of ionizing radiation from radiotherapy have been reported in the literature. Pulmonary irradiation can produce reactive oxygen and nitrogen, which cause oxidative damage of DNA, lipids, and proteins. The resulting apoptosis of alveolar epithelial cells and vascular endothelial cells then induces inflammatory reactions and chemotaxis of monocytes, lymphocytes, and granulocytes in the lungs (Huang et al., 2016). Radiation-induced lung injury leads to radiation pneumonitis, an inflammatory response that involves alveolar cell destruction and inflammatory cell influx in the interstitial and alveolar space (Judge et al., 2015). When radiation-induced lung injury becomes chronic, it may lead to pulmonary fibrosis, which is an irreversible process characterized by fibroblast proliferation, collagen accumulation and destruction of normal lung structure (Huang et al., 2016; Christofidou-Solomidou et al., 2017). Our findings raise the question of whether ambient particle-bound ionizing radiation, at much lower levels than the dosage of pulmonary irradiation, may explain some of the toxic effects of particulate matter on the lung, which has also been found to cause intracellular oxidative stress and airway inflammation (Kelly and Fussell, 2011; Zhang et al., 2009; Rahman and MacNee, 2000; Stringer and Kobzik, 1998). Our results for PR suggest a symmetrical reduction in FVC and FEV1, especially for exposure durations of 21- and 28-days. This symmetrical reduction may be indicative of restrictive lung conditions including interstitial lung disease and pulmonary fibrosis. In response to irradiation, other studies have suggested surfactant impairment, which affects interactions between alveolar membrane and respiration (Christofidou-Solomidou et al., 2017).
4.4. Limitations
There is potential for confounding by unaccounted factors in our study. However, we adjusted for a comprehensive list of individual and meteorological confounders, and ran sensitivity analyses, including additional adjustment for chronic disease, and inverse probability weighting, and our findings remained consistent. There may be confounding by specific PM constituents such as metals. Our study population was homogeneous, consisting entirely of men, 97% of whom were Caucasian. Therefore, our results cannot be generalized to other populations without further research.
A single monitoring site was used to estimate regional daily levels of particle pollution, as has been used in previous studies on the same NAS cohort (Lepuele et al., 2014). For PR, we assigned the average level measured by the USEPA’s monitoring network, from multiple monitoring stations in the study area. We have included a correlation matrix of the PR measurements by study site in the Supplementary Materials however (see Table S3). We assumed the measurement error of the air pollutants and PR levels to be primarily Berkson measurement error, which is random and causes little or no bias in exposures. Previous research supports this assumption for air pollution exposures determined at a central monitoring site (Zeger et al., 2000), and the same would likely be true for PR. The PR measurements are likely due to radon concentrations, which does not exhibit spatial variation.
There are additional limitations of the PR exposure estimate. As per the USEPA’s protocol, integrated filter samples are collected over several days (from 5 to 7 days) (USEPA, 2017). As such, we examined the main effects of 7-, 14-, 21-, and 28-day averages of PR. As PM-bound gross beta activity is measured after the end of the multi-day sampling period, we were unable to assess any respiratory effects of short-lived radionuclides that had decayed prior to beta activity measurement. Also, we used PM-bound gross beta activity as a surrogate for PR, whereas particles also emit alpha and gamma radiation. Previous research has shown that gross alpha activity is correlated with beta activity (Hernández et al., 2004), while gamma rays may or may not be correlated with the beta activity.
5. CONCLUSION
In this study, we demonstrate that PR can impair lung function, with effect sizes similar to that of ambient particle pollution. The effects of radioactivity are attenuated after adjustment for particles. Our findings also suggest that a portion of the lung function effects of ambient particle exposure, averaged over 21 to 28 days, may be explained by the radioactivity level. We speculate that radionuclides associated with ambient particles, upon inhalation and deposition in the human respiratory tract, may subsequently lead to the activation of inflammatory pathways and oxidative stress, resulting in reduced lung function. Understanding these mechanisms of lung injury may inform future public health and air quality policy worldwide.
Supplementary Material
Highlights.
Particulate matter (PM) adversely affects lung function but factors promoting its toxicity are uncertain
The respiratory effects of low-level environmental radioactivity from PM are unknown
We evaluate how particle-bound radioactivity (PR) is associated with lung function
Independent of PM, we demonstrate that PR is associated with decreased FVC and FEV1
Our findings will have important implications for research and environmental health policy
ACKNOWLEDGEMENTS
This publication was made possible by USEPA grant (RD-835872–01) through the Harvard University USEPA sponsored Air, Climate & Environment (ACE) Centre. The contents of the study are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. This publication was also funded by the National Institute of Health (NIH) grants (P01-ES009825 and R01-ES019853) and the National Institute of Environmental Health Sciences (NIEHS) (P30-ES000002). The Veterans Administration (VA) Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center (ERIC) of the Department of Veteran Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC). This study was also supported by resources and the use of facilities at the VA Boston Healthcare System.
Footnotes
Declaration of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Furukawa K, Preston D, Lönn S, Funamoto S, Yonehara S, Matsuo T, Egawa H, Tokuoka S, Ozasa K, Kasagi F, Kodama K, Mabuchi K. 2013. Radiation and smoking effects on lung cancer incidence among atomic-bomb survivors. Radiation Research 174(1). doi: 10.1667/RR2083.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamiya K, Ozasa K, Akiba S, Niwa O, Kodama K, Takamura N, et al. 2015. From Hiroshima and Nagasaki to Fukushima 1: Long-term effects of radiation exposure on health. Lancet 386:469–78. doi: 10.1016/S0140-6736(15)61167-9. [DOI] [PubMed] [Google Scholar]
- 3.Pham T-M, Sakata R, Grant EJ, et al. Radiation exposure and the risk of mortality from noncancer respiratory diseases in the Life Span Study, 1950–2005. Radiat Res 2013; 180: 539–45. [DOI] [PubMed] [Google Scholar]
- 4.Preston DL, Shimizu Y, Pierce DA, et al. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 2003; 160:381–407. [DOI] [PubMed] [Google Scholar]
- 5.Vrijkeid M, Cardis E, Asmore P, Auvinen A, Bae JM, Engels H, et al. 2007. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int J Epidemiol 36:1126–1135. doi: 10.1093/ije/dym138 [DOI] [PubMed] [Google Scholar]
- 6.Vacquier B, Caer S, Rogel A, et al. Mortality risk in the French cohort of uranium miners: extended follow-up 1946–1999. Occup Environ Med 2008; 65: 597–604. [DOI] [PubMed] [Google Scholar]
- 7.Belyaeva ZD, Osovets SV, Scott BR, et al. Modeling of respiratory system dysfunction among nuclear workers: a preliminary study. Dose Response 2008; 6:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, Field RW, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16:137–45. [DOI] [PubMed] [Google Scholar]
- 9.WHO. 2009. WHO Handbook on Indoor Radon: A Public Health Perspective Geneva: World Health Organization. [PubMed] [Google Scholar]
- 10.Turner MC, Krewski D, Chen Y, Pope CA, Gapstur SM, Thun MJ. 2011. Radon and lung cancer in the American Cancer Society cohort. Cancer Epidemiol Biomarkers Prev 20(3):438–448. doi: 10.1158/1055-9965.EPI-10-1153. [DOI] [PubMed] [Google Scholar]
- 11.Kim S-H, Hwang WJ, Cho J-S, Kang DR, 2016. Attributable risk of lung cancer deaths due to indoor radon exposure. Annals of Occupational and Environmental Medicine 28:8 Doi: 10.1186/s-40557-016-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner MC, Krewski D, Chen Y, Pope CA, Gapstur SM, Thun MJ, 2012. Radon and COPD mortality in the American Cancer Society Cohort. European Respiratory Journal 39:1113–1119. Doi: 10.1183/09031936.00058211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Research Council (NRC). Health risks of radon and other internally deposited alpha-emitters. Beir IV Washington, National Academy Press, 1988. [PubMed] [Google Scholar]
- 14.Archer V, Renzetti A, Doggett R, et al. Chronic diffuse interstitial fibrosis of the lung of uranium miners. J Occup Environ Med 1998; 40:460–474. [DOI] [PubMed] [Google Scholar]
- 15.Dominici F, Peng RD, Bell ML et al. 2006. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 295: 1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly F and Fussell JC. 2011. Air pollution and airway disease. Clinical and Experimental Allergy 41, 1059–1071. Doi: 10.1111/j.1365-2222.2011.03776.x [DOI] [PubMed] [Google Scholar]
- 17.Peng RD, Chang HH, Bell ML, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. JAMA, 2008, 299(18): 2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q, Xu C, Ji G, Liu H, Shao W, Zhang C, Gu A, Zhao P. 2017. Effect of exposure to ambient PM2.5 pollution on the risk of respiratory tract diseases: a meta-analysis of cohort studies. J of Biomedical Research 31(2): 130–142. doi: 10.7555/JBR.31.20160071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepuele J, Bind M-AC, Baccarelli AA, Koutrakis P, Tarantini L, Litonjua A, Sparrow D, Vokonas P, Schwartz JD. 2014. Epigenic influences on associations between air pollutants and lung function in elderly men: the Normative Aging Study. Environmental Health Perspectives 122 (6): 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P, Washko GR, O’Connor GT, Mittleman MA. 2013. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med 188(11): 1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Int Panis L, Provost EB, Cox B, Louwies T, Laeremans M, Standaert A, Dons E, Holmstock L, Nawrot T, De Boever P. 2017. Short-term air pollution exposure decreases lung function: a repeated measures study in healthy adults. Environmental Health 16:60. doi: 10.1186/s12940-017-0271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dueñas C, Fernández MC, Liger E, Carretero J. 1999. Gross alpha, gross beta activities and 7Be concentrations in surface air: analysis of their variations and prediction model. Atmos Environ 33:3705–3715. doi: 10.1016/S1352-2310(99)00172-7. [DOI] [Google Scholar]
- 23.Hernández F, Hernández-Armas J, Catalán A, Fernández-Aldecoa JC, Karlsson L. 2004. Gross alpha, gross beta activities and gamma emitting radionuclides composition of airborne particulate samples in an oceanic island. Atmos Environ 39:4057–4066. doi: 10.1016/j.atmosenv.2005.03.035. [DOI] [Google Scholar]
- 24.UNSCEAR. 2008. Volume I: effects of ionizing radiation exposure. UNSCEAR Report 2006 New York: United Nations Scientific Committee on the Effects of Atomic Radiation. [Google Scholar]
- 25.US National Academy of Sciences, 2006. National Research Council Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Health risks from exposure to low levels of ionizing radiation. BEIR VII Phase 2 Washington, DC: National Academies Press; 13. [PubMed] [Google Scholar]
- 26.ICRP. 2007. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Annals of the ICRP 37: 1–332. [DOI] [PubMed] [Google Scholar]
- 27.IARC (International Agency for Research on Cancer). 2001. IARC monographs on the evaluation of carcinogenic risks to humans: Volume 78: Ionizing radiation, part 2: Some internally deposited radionuclides. IARC Working Group on the Evaluation of Carcinogen Risks to Humans, World Health Organization, IARC Press, Lyon, France. [Google Scholar]
- 28.Kendall and Smith. 2002. Doses to organs and tissues from radon and its decay products. J Radiol Prot 22:389–406. [DOI] [PubMed] [Google Scholar]
- 29.USEPA (United States Environmental Protection Agency). RadNet Monitoring network. 2017 Available online at www.epa.gov/radnet.
- 30.Nyhan MM, Coull BA, Blomberg AJ, Vieira CLZ, Garshick E, Aba A, Vokonas P, Gold DR, Schwartz J, Koutrakis P. Associations between ambient particle radioactivity and blood pressure; the Normative Aging Study (NAS). J Am Heart Assoc 2018;7:e008245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell B, Rose CL, Damon A. 1972. The normative aging study: an interdisciplinary and longitudinal study of health and aging. Agin Hum Dev 3:4–17. doi: 10.2190/GGVP-XLB5-PC3N-EF0G. [DOI] [Google Scholar]
- 32.Ferris BG. 1978. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 118(6 pt 2):1–20. [PubMed] [Google Scholar]
- 33.Sparrow D, O’Connor G, Colton T, Barry CL, Weiss ST, 1987. The relationship of nonspecific bronchial responsiveness to the occurrence of respiratory symptoms and decreased levels of pulmonary function. The Normative Aging Study. Am Rev Respir Dis 135: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 34.Chatham M, Bleecker ER, Norman P, Smith PL, Mason P. 1982. A screening test for airways reactivity. An abbreviated methacholine inhalation challenge. Chest 82:15–8. [DOI] [PubMed] [Google Scholar]
- 35.Kang CM, Koutrakis P, Suh HH. 2010. Hourly measurements of fine particulate sulfate and carbon aerosols at the Harvard-U.S. Environmental Protection Agency Supersite in Boston. J Air Waste Manag Assoc 60(11):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernan MA, Lanoy E, Costagliola D, Robins JM. 2006. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol 98: 237–242. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Zhang W, Yu F, Gao F, 2016. The cellular and molecular mechanism of radiation-induced lung injury. Medical Science Monitor 23:3446–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christofidou-Solomidou M, Pietrofesa RA, Arguiri E, Koumenis C, Segal R. 2017. Radiation mitigating properties of intranasally administered KL4 surfactant in a murine model of radiation-induced lung damage. Radiat Res 188(5):491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Judge JL, Owens KM, Pollock SJ, et al. Ionizing radiation induces myofibroblast differentiation via lactate dehydrogenase. American Journal of Physiology - Lung Cellular and Molecular Physiology 2015;309(8):L879–L887. doi: 10.1152/ajplung.00153.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang JJ, McCreanor JE, Cullinan P, Chung KF, Ohman-Strickland P, Han I-K, Jarup L, Nieuwenhuijsen MJ. Health effects of real-world exposure to diesel exhaust in persons with asthma. Res Rep Health Eff Inst 2009;(138):5–109, discussion 111–123. [PubMed] [Google Scholar]
- 41.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 2000; 16: 534–554. [DOI] [PubMed] [Google Scholar]
- 42.Stringer B, Kobzik L. Environmental particulate-mediated cytokine production in lung epithelial cells (A549): role of preexisting inflammation and lung oxidative stress. J Toxicol Environ Health A 1998; 55:31–44. [DOI] [PubMed] [Google Scholar]
- 43.Zeger SL, Thomas D, Dominici F, Samet Jm, Schwartz J, Dockery D, et al. 2000. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect 108:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Nyhan MM, Wilker EH, Vieira CLZ, Lin H, Schwartz JD, et al. , 2018. Recent exposure to particle radioactivity and biomarkers of oxidative stress and inflammation: The Framingham Heart Study. Environ International doi: 10.1016/j.envint.2018.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.