Abstract
Study Design
Prospective clinical study.
Purpose
We evaluated the challenges faced during diagnosis and management of patients with subacute pyogenic discitis and discussed various clues in clinical history, radiologic and hematologic parameters of these patients that helped in establishing their diagnosis.
Overview of Literature
Present literature available shows that in patients with subacute spondylodiscitis and infection with less virulent organisms, the clinical picture often is confusing and the initial radiologic and hematologic studies do not contribute much toward establishing the diagnosis.
Methods
Demographic pattern, predisposing factors, clinical presentation, comorbidities, microbiology, treatment, neurologic recovery, and complications of 11 patients were prospectively reviewed regarding their contribution toward the conformation of diagnosis of subacute pyogenic discitis.
Results
Mean age at presentation was 46.0 years with average preoperative Oswestry Disability Index and Visual Analog Scale scores of 83.4 and 7.18, respectively. Mean follow-up duration was 12.0 months. The most common site of infection was the lumbar spine, followed by the thoracic spine (n=1). Infective organisms were isolated in only 45% of cases. Staphylococcus aureus was the most common causative organism isolated.
Conclusions
Diagnosing subacute spondylodiscitis in a patient presenting with subacute low backache poses a diagnostic challenge. Clinical and radiologic picture are deceiving, and bacteriologic results often are negative, further complicating the picture. A detailed medical history along with clinical, radiologic, and biochemical parameters prevents missing the diagnosis. Serial serum Creactive protein and alkaline phosphatases were more reliable blood parameters in cases of subacute presentation.
Keywords: Discitis, Low back pain, Staphylococcus aureus, Alkaline phosphatase, Lumbar vertebrae, Staphylococcal infections
Introduction
Subacute pyogenic spondylodiscitis remains a diagnostic challenge, and affected patients are at risk for permanent neurologic deficits and chronic pain if the appropriate treatment is delayed [1-11]. Spondylodiscitis accounts for 0.15%–5% of all cases of osteomyelitis [1,3-5]. The incidence of nontuberculosis, nonpostoperative spondylodiscitis has been estimated previously to be 2.2–2.4 cases per 100,000 person-years [7,8,10-13] and is increasing mainly due to an increasing elderly population [7,8,10,13]. The onset of symptoms usually is insidious, with back or neck pain. Fever typically is not present and accounts for <20% of patients [9]. Other symptoms include nausea, vomiting, anorexia, weight loss, lethargy, and confusion. Predisposing factors include age, previous spine surgery, distant infectious focus, diabetes mellitus, advanced age, intravenous drug use, human immunodeficiency virus infection, immunosuppression, oncologic history, and renal failure [2,5,6,12,14]. On the basis of the duration of symptoms, infections can be classified as acute (<1 week), subacute (>2 weeks), and chronic. Staphylococcus aureus is the most frequent causative microorganism, accounting for approximately half of the cases of bacterial spondylodiscitis and also is predominant in iatrogenic spondylodiscitis. In approximately one-third of patients with pyogenic spondylodiscitis, the infectious agent never is identified, which may increase up to 44% in iatrogenic cases by direct inoculation [5,13,14]. Due to the nonjudicious use of antibiotics in the Indian subcontinent, the low grade pyogenic spine infections may present in subacute or chronic phase as a nonrelieving backache. In these cases, the clinical picture is very confusing with vague clinical signs and symptoms, modifying the picture of infectious spondylodiscitis. Diagnosis is based on clinical, radiologic, and microbiological grounds. Delayed diagnosis is common and can range from 2 to 12 weeks and occasionally after 3 months [9,15-18]. However, conflicting reports exist regarding the optimal methods for diagnosis and treatment. We discussed and evaluated various challenges faced during establishing the diagnosis in cases of spontaneous subacute pyogenic spondylodiscitis in this prospective study.
Materials and Methods
A total of 11 consecutive immunocompetent patients with the clinicoradiologic diagnosis of subacute pyogenic spondylodiscitis were prospectively included in the study between January 2016 and December 2017 at All India Institute of Medical Sciences, Rishikesh, a tertiary care teaching institute of northern india. A written informed consent was taken from all the patients included in the study and the approval from the institutional review board for this study was taken (IRB approval no., 79/ IEC/PGM/2016). Each patient was followed for 1 year after the diagnosis. Clinical data on each patient were recorded and consisted of demographic pattern, predisposing factors, clinical presentation, comorbidities, microbiology, treatment, neurologic recovery, complications, and various challenges encountered subsequently during the diagnosis and management of each case.
Inclusion criteria were subacute presentation >2 weeks, radiologic correlation of spondylodiscitis, culture positive/culture negative with clinicoradiologic diagnosis, and biopsy positive/GeneXpert [19,20] negative for mycobacterium. Exclusion criteria were acute presentation, nonpyogenic (e.g., tubercular, fungal, parasitic, and immunocompromized) patients, malignancy, trauma, and no radiologic finding correlating with discitis.
Spondylodiscitis was diagnosed on the basis of clinical, radiologic, and microbiologic grounds, involving a combination of clinical history, physical examination, radiologic assessment, and positive organism culture from blood culture or spinal biopsy. Additionally, spinal infection was diagnosed in cases with suggestive clinical features and appropriate radiologic changes, which remained culture negative, but had elevated inflammatory markers and responded favorably to antimicrobial therapy. In our series, blood markers considered were total leukocyte count (TLC), direct leukocyte count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and alkaline phosphatase. For diagnosis of subacute spondylodiscitis caused by bacteria, bone aspiration specimens obtained through needle biopsy under C-arm or computed tomography (CT) where previous methods failed in sterile conditions were transferred immediately to BACTEC 9050 culture bottles (a series of blood culture instruments designed for the rapid detection of microorganisms in clinical specimens). When bacterial growth occurred on automated systems, conventional methods were used for bacteria identification.
Partial recovery was labeled when the symptoms improved, but the patient failed to return to his activities of daily living. Persistence or aggravation of the disease was deemed when the symptoms continued unabated for more than a month, with increased ESR and CRP levels, after spondylodiscitis treatment was started. Neurologic deterioration or worsening of the radiologic picture indicated treatment failure or lack of response. These patients were given the option of open biopsy and instrumented fusion for confirmation of diagnosis and alleviation of symptoms. Functional status was defined as interrupted activities of daily activities, and it was calculated by using Oswestry Disability Index (ODI). Pain was accessed by the Visual Analog Scale (VAS) score. Neurology was documented by Frankel grading, which was recorded during the pretreatment period and at 1, 2, 3, and 6 months and 1 year (final follow-up) after treatment.
For medical management of spondylodiscitis patients, flowchart 1 was followed with some modifications as reported by Duarte and Vaccaro and illustrated in Fig. 1 [3]. The patient was started on intravenous antibiotics after all hemato-radiological investigations with the most sensitive two drugs available according to culture sensitivity results for 4 weeks, which was later converted to oral therapy for 4 more weeks. Empiric antibiotics were administered in case of a negative biopsy report. In case of nonresponders, previous culture negative patients were switched to the surgical option with open biopsy and fusion for confirmation of diagnosis along with decompression and stabilization of the spine. These patients were started postoperatively on empirical therapy and later switched on to an intravenous and oral antibiotics regime according to culture reports.
Fig. 1.
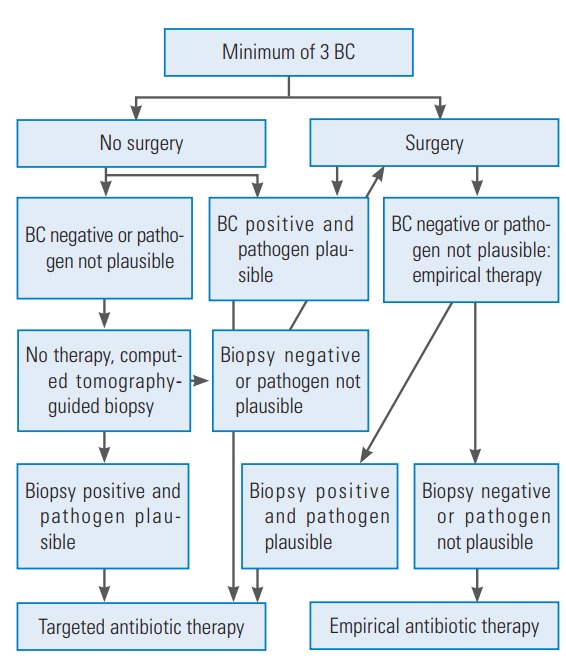
Algorithm for the diagnosis and treatment of spondylodiscitis. BC, blood cultures.
Results
Our study included 11 patients with a mean age of 46 years. S. aureus was the most common organism detected in five patients (one had methicillin-resistant S. aureus and one had salmonella).
We could not isolate organisms in the remaining six cases even after open biopsy. Among 11 patients, 10 had spondylodiscitis involving the lumbar spine and one the thoracic spine. On careful and elaborated history taking of recent illness, the probable infection source was identified in most cases (Table 1).
Table 1.
Demographic data and predisposing factors of patients
| Patient no. | Age (yr) | Sex | Predisposing factors |
|---|---|---|---|
| 1 | 17 | M | Recent history of typhoid fever |
| 2 | 19 | M | No significant history |
| 3 | 32 | F | Recent history of urinary tract infection |
| 4 | 35 | F | Recent history of lower respiratory tract infection |
| 5 | 51 | M | History of chest infection |
| 6 | 54 | F | History of septicaemia |
| 7 | 59 | F | Recent history of urinary tract infection |
| 8 | 56 | M | Diabetes mellitus |
| 9 | 57 | F | No significant history |
| 10 | 63 | M | History of 2 spinal injections |
| 11 | 65 | M | Diabetes mellitus |
M, male; F, female.
The most common clinical symptom was backache, which was mild-to-moderate, nagging, and localized in the lower back region without radiation causing discomfort in daily life activities. Fever was present in only five of 11 patients (45%), which was low grade or intermittent in nature with spikes. Preoperatively, mean ODI was 83.4 and VAS was 7.18. Patients had neurologic deficit of varying degree of involvement, such as Frankel grades A (n=1), B (n=1), C (n=2), and D (n=2). Among them, two patients presented with bladder and bowel involvement. TLC was not a very sensitive marker. In most cases the TLC was within the normal range.
ESR was mildly raised in most cases (mean±standard deviation [SD], 25.4±16.6; n<20) (Fig. 2), except for two patients with frank extradural abscess formation. Serial CRP proved to be a much more sensitive marker and was raised significantly in all of our patients (mean±SD, 37.2±19.36; n<10). Also during the follow-up period, serial CRP values proved to be sensitive markers of response to treatment, showing a decreasing trend of values in patients responding to treatment and returned to the baseline normal value of <10 in most patients. In our series, serial alkaline phosphatase was a sensitive marker of subacute spondylodiscitis. Alkaline phosphatase was raised in all patients significantly (average±SD, 239±80.3 international units) (Fig. 2) and higher in patients with longer symptom durations. During follow-up, serial alkaline phosphatase values showed a decreasing trend in correlation with the response to treatment and decreased to normal values in most patients at the 6-month follow-up.
Fig. 2.
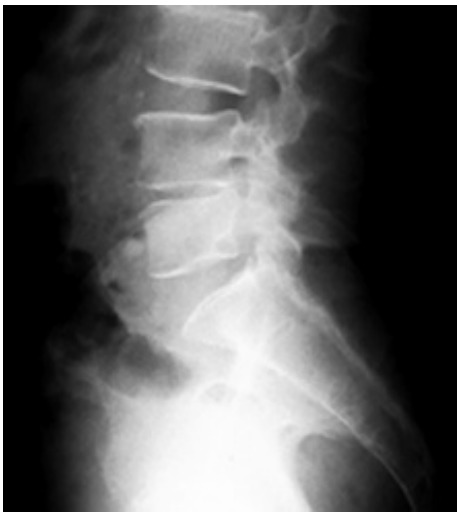
Lumbosacral spine X-ray of a 63-year-old male with severe back pain shows only decreased disc space height.
In microbiological workup for spondylodiscitis, blood culture yielded positive results in only one patient with suspected septicemia as a predisposing cause. Percutaneous C-arm/CT-guided discal biopsy for culture and histopathology was done for all patients before starting antimicrobial treatment and yielded positive results in only two of 11 patients (18%) in our series. In these two patients, frank pus was found on discal biopsy, which was positive for S. aureus on aerobic culture and sensitivity testing. All these discal biopsy specimens were subjected to testing for mycobacterium using polymerase chain reaction/Gene Xpert to rule out tubercular spondylodiscitis [19,20], which is the most common cause of subacute/chronic spondylodiscitis in our country. Mycobacterium was not detected in any of our patients, and 54% underwent surgical procedures as either they were not responding to empirical antimicrobial treatment or they were having progressive neurologic involvement. Only three of six open biopsies (50%) yielded positive culture results with S. aureus in two and salmonella in one culture result (Table 2). Overall, percutaneous and open biopsies yielded positive culture results only in five of 11 patients (45%). All patients received intravenous antibiotic therapy for 3 weeks and oral therapy for 3 weeks. Antibiotics were administered as per antibiotic sensitivity in six patients and empirically (injection cefuroxime) in the remainder. Mean VAS and ODI were 32.5 and 3, and 12.5 and 1, respectively, at 1 month and 1 year after treatment, respectively. Postoperative neurology improved at final follow-up in all patients with Frankel E in 10 and D in 1. There was no long-term morbidity or mortality. All patients except one with upper dorsal spine involvement recovered well with conservative and surgical treatment. All patients achieved bony fusion at the affected level on final follow-up of 1 year, which was decided on follow-up X-rays and CT.
Table 2.
Different operative procedures performed in patients
| Neurology (Frankel grade) | Level of spine | Operative intervention |
|---|---|---|
| A | T1–T2 | Posterior decompression and instrumentation with anterior column reconstruction with bone graft |
| B | L4–L5 | TLIF |
| C | L3–L4 | TLIF |
| C | L3–L4 | TLIF |
| D | L5–S1 | Disc debridement with TLIF |
| D | L4–L5 | Fenestration and discal biopsy |
TLIF, transforaminal lumbar interbody fusion.
Discussion
Subacute spinal infections pose a diagnostic and treatment challenge to clinicians because of their subtle presentation and vague course. Advanced imaging studies, laboratory tests, and a detailed clinical history and examination can alert spinal surgeons to the correct diagnosis in a timely fashion [2,6].
1. Clinical challenges
Subacute spondylodiscitis, a relatively uncommon clinical scenario, is becoming more common because of aging and higher incidence of bacteremia and sepsis episodes following use of invasive diagnostic and treatment methods [13,21]. The onset of symptoms usually is insidious, with persistent back or neck pain being the most common presenting complaint [6,7]. In our series mild-to-moderate intensity back pain was seen in 100% of cases. In the literature, age at presentation is in the 5th and 6th decades [13,14]. However, in our study, presentation was slightly earlier with a mean age of 45.6 years, possibly because most literature available is of acute cases. In the elderly, diagnosis of spondylodiscitis frequently is delayed. The reasons for this include a higher prevalence of comorbidities, such as degenerative back pain that may cloud diagnostic evaluation. Clinical assessment may be difficult because of preexisting cognitive impairment or delirium and atypical or subtle symptoms and biochemical marker abnormalities.
Relevant medical history can be one of the most important aids toward clinching the diagnosis in subacute cases presenting with persistent nonrelieving backache [16-18]. In our series, a thorough medical history revealed a recent history of medical illness in 63% of cases, for which the patient had undergone an incomplete course of antibiotics providing a clue to the bacterial origin of spondylodiscitis. The pathogenesis of spinal infection can be caused by direct inoculation (e.g., surgery, trauma, and contiguous spread from adjacent infected vertebrae) or hematogenous spread [4,22,23].
Abscess continues to be a rare (<1%) cause of acute back pain in the primary care setting [16]. Several case reports continue to highlight the importance of diligent pursuit of an etiology in cases of back pain that do not improve or resolve. Ramos et al. [16] presented a case of fusobacterium species as the causal agent in vertebral osteomyelitis. Their case was particularly remarkable because the affected patient had back pain for 3 months and a remarkable medical history. Additionally, other recent cases highlight the importance of considering an infectious etiology in back pain without other specific etiologies [17,18]. High-grade fever, one of the most common constitutional symptoms, was present in none of our subacute cases; however, fever of mild and intermittent nature was present in 45%. Similarly, such an insidious clinical course and a delayed onset of symptoms are seen in tubercular cases [15]. In our country, tuberculosis is endemic [20], which adds further to the confusion as the subacute type clinical picture without microbiological growth warrants the diagnosis of tubercular spondylodiscitis [15] and undue prolonged treatment with antitubercular drugs. Hence, the differential diagnosis of subacute pyogenic spondylodiscitis should be kept in mind even in tuberculosis endemic countries in cases of backache with subacute presentation.
2. Diagnostic challenges
1) Radiologic
All patients in our series underwent radiologic investigations for confirmation of the diagnosis. All patients with nonrelieving backache >2 weeks in duration were initially screened by X-rays, followed by magnetic resonance imaging (MRI) and noncontrast CT. In most subacute cases, X-rays were nonalarming except for a slight reduction of disk space (Fig. 2). Because many patients have degenerative changes on imaging studies, a high index of suspicion must be maintained in these cases [6,7]. MRI in all our cases revealed hyperintense signals of disk space and adjacent vertebral bodies on T2-weighted and short T1 inversion recovery images (Figs. 3, 4), which suggests local infective or inflammatory pathology [6,8,24-26]. However, because of abundance of spinal tuberculosis patients, these subacute pyogenic cases can be very easily confused with tuberculosis because of subtle symptoms.
Fig. 3.
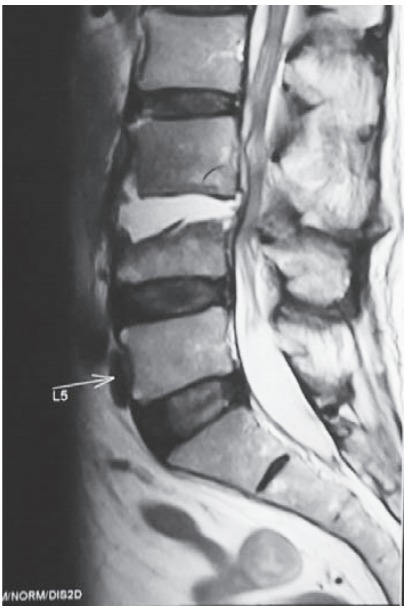
Contrast-enhanced magnetic resonance imaging of a 65-yearold male shows hyperintense shadow in T2-weighted images suggestive of fluid collection.
Fig. 4.
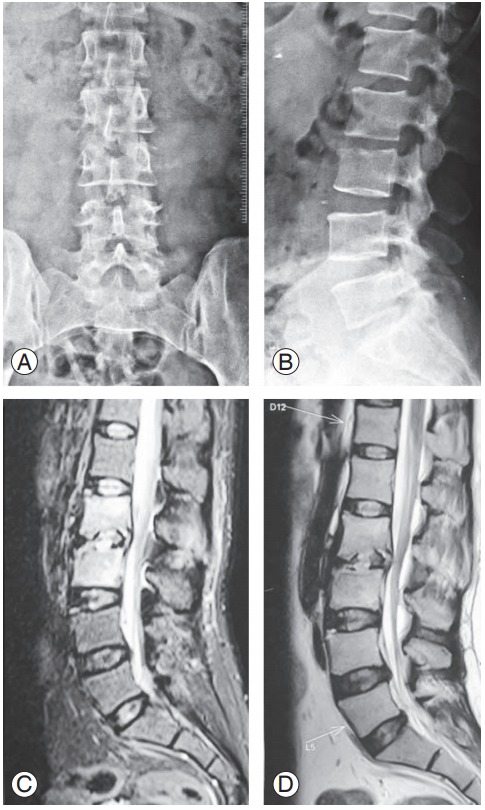
(A–D) A 35-year-old female with doubtful disc space narrowing at L2–L3 on X-rays with hyperintense lesion in T2-weighted and short T1 inversion recovery images.
Sensitivity, specificity, and accuracy of MRI are reported as 96%, 92%, and 94%, respectively [10]. The typical signal pattern of acute spinal infection on MRI is an increase in fluid signal because of marrow edema with signal decrease in T1-weighted sequences and signal increase in T2-weighted sequences with contrast enhancement (Figs. 4, 5). In most cases, the infection starts in the anterolateral vertebral body near the endplate [8,26]. Associated edema typically is pronounced and affects much of the vertebral body and intervertebral disc [26,27]. Spinal neoplasms also may present with similar T1- and T2-weighted findings on MRI. However, the presence of disk space involvement will serve as one way to distinguish infections from neoplasms. Although MRI cannot diagnose the specific causative organism, some features suggest certain infections. For example, tuberculous spondylitis is characterized by meningeal involvement, subligamentous spread of infection, and paravertebral and intraosseous abscesses, which can be well demonstrated with contrast MRI [27]. In postoperative patients, the interpretation of MRI images may be more difficult because there will be some increased T2-weighted signal and contrast uptake at the surgery site [24]. Though there can be overlap of less florid or early discitis with normal postoperative changes, if the adjacent vertebral body shows low signal on T1-weighted images and contrast enhancement, infection is more likely. Newer diagnostic facilities, such as fludeoxyglucose-positron emission tomography (FDG-PET), have shown promising results in conformation of infection etiology of spine infections [28]. Schmitz et al. [28] reported that all patients with spinal infection confirmed by histopathology had positive FDG-PET imaging. Stumpe et al. [29] reported the use of FDG-PET for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine that were detected on MRI. PET did not show FDG uptake in the intervertebral spaces of any patient with degenerative disease. In our series, we could not use this modality because of nonavailability at the time of study.
Fig. 5.
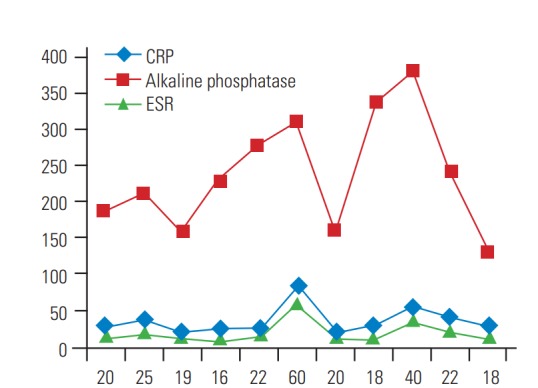
Line diagram shows distribution of initial values of ESR, CRP, and alkaline phosphatase among patients. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
2) Microbiological
Identifying the offending pathogen is critical for guiding appropriate antibiotic therapy. Timely relevant identification of the causative agents and specific antimicrobial treatment are essential for avoiding severe complications in infectious discitis [11]. When pathogen identification is lacking, there is no option other than to use empirically based (trial and error), broad-spectrum, and long-term antibiotic therapy. CT-guided and C-arm–guided biopsy has been recommended for isolating causative pathogens [24]; however, the aspirate often is inadequate and sometimes no organism has been cultured. In the absence of bacteremia, percutaneous disc aspiration or biopsy performed for pathogen identification by conventional microbiologic techniques fails to identify the causative agent in 30%–50% of patients [13]. In our series, we attempted C-arm–guided biopsy in all cases, which, if it resulted in a negative biopsy, was followed by CT-guided biopsy in all patients before starting antimicrobial therapy. However, conclusive results were obtained in only 18% patients, which were significantly less compared with the previous literature on acute spondylodiscitis (Table 3). The reported accuracy of spinal biopsy varies from 36% to 91% according to the organism isolated [24,25]. In a retrospective study of postoperative spondylodiscitis, Fouquet et al. [24] obtained positive bacteriologic results in nine (36%) of 25 patients who had biopsies. They concluded the features of septic or chemical postoperative discitis can be distinguished by ESR, CRP, and percutaneous discal biopsy. Due to a low detection rate of microbial organisms in percutaneous biopsies, a greater number of cases needed to be converted to open biopsy who did not respond to initial empirical medical treatment. In our case series, only 50% yielded positive results, which further increased the diagnostic dilemma (Table 3). So, overall in our series, only 45% were culture positive; thus, we could suggest that while suspecting a case of subacute discitis, a high index of suspicion should be maintained even in culture negative cases and not bluntly categorize them as tubercular infection even in endemic regions.
Table 3.
Distribution of involved vertebral regions by pathogen found in spondylodiscitis
| Involved vertebral region | Causative organism |
||
|---|---|---|---|
| Staphylococcus aureus | Salmonella typhi | Couldn’t be isolated | |
| Cervical | - | - | - |
| Thoracic | 1 | - | - |
| Lumbar | 3 | 1 | 6 |
3) Hematologic
Laboratory tests usually are inconclusive in diagnosing subacute and chronic spondylodiscitis. White blood cell (WBC) count is of limited diagnostic value, as it is commonly nonspecific, being elevated in less than half of patients [15]. ESR and CRP levels are more helpful. ESR is a sensitive laboratory test, being elevated in >90% of patients. CRP is more sensitive than ESR, with its levels also elevated in most cases [26]. In our series, we found that in subacute cases of spine infections WBC and ESR were inconsistent with the diagnosis and were not significantly raised (Fig. 5). Serial CRP and alkaline phosphatase proved to be more sensitive markers in our series, raised significantly in 100% of cases (Fig. 5), and helped in establishing their diagnosis and monitoring of healing during the post-treatment period [15,26]. However, those associations could not be statistically proven because of the small sample size. Nevertheless, these markers also are nonspecific, since they are unable to distinguish between a pyogenic, granulomatous, or other inflammatory process. Patients with acute disease may demonstrate a significant increase in these markers, whereas in chronic infections (as in tuberculosis infections), these values may be normal or only slightly elevated.
Conclusions
In patients with longstanding (>3 weeks) nonrelieving localized back pain without significant constitutional symptoms, with a significant positive medical history especially of urinary tract infection, the suspicion of subacute discitis should be raised even if there is no fever and no increase in white cell count, ESR rate, or CRP level, and radiography is normal. These patients should not be bluntly categorized as having tubercular discitis in view of radiologic findings with subtle clinical symptoms and negative discal biopsy results. In total, the clinical picture, including all the clinical, radiologic, and biochemical parameters, should be considered for establishing the proper diagnosis and treatment.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lee KY. Comparison of pyogenic spondylitis and tuberculous spondylitis. Asian Spine J. 2014;8:216–23. doi: 10.4184/asj.2014.8.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lecouvet F, Irenge L, Vandercam B, Nzeusseu A, Hamels S, Gala JL. The etiologic diagnosis of infectious discitis is improved by amplification-based DNA analysis. Arthritis Rheum. 2004;50:2985–94. doi: 10.1002/art.20462. [DOI] [PubMed] [Google Scholar]
- 3.Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. Eur Spine J. 2013;22:2787–99. doi: 10.1007/s00586-013-2850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meys E, Deprez X, Hautefeuille P, Flipo RM, Duquesnoy B, Delcambre B. Role of iatrogenic spondylodiscitis among pyogenic spondylodiscitis: 136 cases observed between 1980 and 1989. Rev Rhum Mal Osteoartic. 1991;58:839–46. [PubMed] [Google Scholar]
- 5.Cheung WY, Luk KD. Pyogenic spondylitis. Int Orthop. 2012;36:397–404. doi: 10.1007/s00264-011-1384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Euba G, Narvaez JA, Nolla JM, et al. Long-term clinical and radiological magnetic resonance imaging outcome of abscess-associated spontaneous pyogenic vertebral osteomyelitis under conservative management. Semin Arthritis Rheum. 2008;38:28–40. doi: 10.1016/j.semarthrit.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM. Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center. Spine (Phila Pa 1976) 2006;31:2695–700. doi: 10.1097/01.brs.0000244662.78725.37. [DOI] [PubMed] [Google Scholar]
- 8.Kaya S, Ercan S, Kaya S, et al. Spondylodiscitis: evaluation of patients in a tertiary hospital. J Infect Dev Ctries. 2014;8:1272–6. doi: 10.3855/jidc.4522. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh PC, Wienecke RJ, O’Shaughnessy BA, Koski TR, Ondra SL. Surgical strategies for vertebral osteomyelitis and epidural abscess. Neurosurg Focus. 2004;17:E4. doi: 10.3171/foc.2004.17.6.4. [DOI] [PubMed] [Google Scholar]
- 10.Luzzati R, Giacomazzi D, Danzi MC, Tacconi L, Concia E, Vento S. Diagnosis, management and outcome of clinically- suspected spinal infection. J Infect. 2009;58:259–65. doi: 10.1016/j.jinf.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Goteborg, Sweden: a retrospective study of patients during 1990-95. Scand J Infect Dis. 2001;33:527–32. doi: 10.1080/00365540110026566. [DOI] [PubMed] [Google Scholar]
- 12.Kehrer M, Pedersen C, Jensen TG, Lassen AT. Increasing incidence of pyogenic spondylodiscitis: a 14-year population-based study. J Infect. 2014;68:313–20. doi: 10.1016/j.jinf.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Martinez Hernandez PL, Amer Lopez M, Zamora Vargas F, et al. Spontaneous infectious spondylodiscitis in an internal medicine department: epidemiological and clinical study in 41 cases. Rev Clin Esp. 2008;208:347–52. doi: 10.1157/13124314. [DOI] [PubMed] [Google Scholar]
- 14.Mavrogenis AF, Megaloikonomos PD, Igoumenou VG, et al. Spondylodiscitis revisited. EFORT Open Rev. 2017;2:447–61. doi: 10.1302/2058-5241.2.160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy RL. Low back pain: an approach to diagnosis and management. Prim Care. 2010;37:729–41. doi: 10.1016/j.pop.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Ramos A, Berbari E, Huddleston P. Diagnosis and treatment of Fusobacterium nucleatum discitis and vertebral osteomyelitis: case report and review of the literature. Spine (Phila Pa 1976) 2013;38:E120–2. doi: 10.1097/BRS.0b013e31827b4d61. [DOI] [PubMed] [Google Scholar]
- 17.O’Keeffe AB, Terris J, Hormbrey P. A sinister cause of back pain in a young man. BMJ. 2012;344:e3015. doi: 10.1136/bmj.e3015. [DOI] [PubMed] [Google Scholar]
- 18. doi: 10.1136/bcr-2012-007398. Wangjirapan A, Kongthavonsakul K, Oberdorfer P. An 8-year-old boy with severe disseminated Staphylococcus aureus infection. BMJ Case Rep 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18:76–84. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Global Health Education . Horsham: Global Health Education; 2017. TB Statistics India: national, treatment outcome, state [Internet] [cited 2018 Jan 10]. Available from: https://www.tbfacts.org/tb-statisticsindia/ [Google Scholar]
- 21.Amadoru S, Lim K, Tacey M, Aboltins C. Spinal infections in older people: an analysis of demographics, presenting features, microbiology and outcomes. Intern Med J. 2017;47:182–8. doi: 10.1111/imj.13300. [DOI] [PubMed] [Google Scholar]
- 22.Wiley AM, Trueta J. The vascular anatomy of the spine and its relationship to pyogenic vertebral osteomyelitis. J Bone Joint Surg Br. 1959;41-B:796–809. doi: 10.1302/0301-620X.41B4.796. [DOI] [PubMed] [Google Scholar]
- 23.Chew FS, Kline MJ. Diagnostic yield of CTguided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;218:211–4. doi: 10.1148/radiology.218.1.r01ja06211. [DOI] [PubMed] [Google Scholar]
- 24.Fouquet B, Goupille P, Jattiot F, et al. Discitis after lumbar disc surgery: features of “aseptic” and “septic” forms. Spine (Phila Pa 1976) 1992;17:356–8. doi: 10.1097/00007632-199203000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157–66. doi: 10.1148/radiology.157.1.3875878. [DOI] [PubMed] [Google Scholar]
- 26.James SL, Davies AM. Imaging of infectious spinal disorders in children and adults. Eur J Radiol. 2006;58:27–40. doi: 10.1016/j.ejrad.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65 Suppl 3:iii11–24. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz A, Risse JH, Grunwald F, Gassel F, Biersack HJ, Schmitt O. Fluorine-18 fluorodeoxyglucose positron emission tomography findings in spondylodiscitis: preliminary results. Eur Spine J. 2001;10:534–9. doi: 10.1007/s005860100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stumpe KD, Zanetti M, Weishaupt D, Hodler J, Boos N, von Schulthess GK. FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. AJR Am J Roentgenol. 2002;179:1151–7. doi: 10.2214/ajr.179.5.1791151. [DOI] [PubMed] [Google Scholar]


