Abstract
Background
Lung cancers are classified into small-cell lung cancer (SCLC) and non-small-cell lung cancer due to their different treatment and prognosis. Although many studies have reported the specific survival of SCLC patients treated at cancer hospitals, survival from population-based data has rarely been reported.
Methods
We analyzed survival of SCLC cases diagnosed from 1993 through 2006 from a population-based cancer registry of six prefectures. To assess trends in SCLC survival, we defined three periods that mirrored developments in SCLC treatment: period 1, 1993–1998; period 2, 1999–2001; and period 3, 2002–2006. Assessments were based on relative survival (RS), excess hazard, and conditional survival.
Results
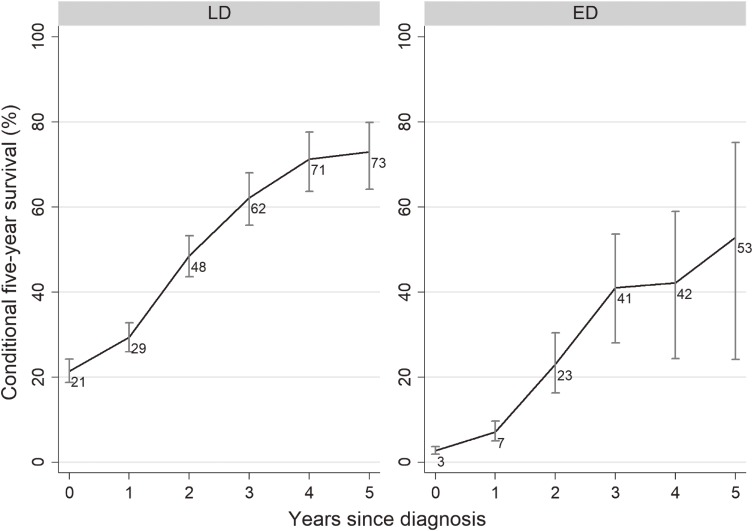
A total of 10,911 SCLC patients were analyzed. Five-year RS among limited disease SCLC (LD-SCLC) in periods 1 to 3 was 16.8%, 21.1%, and 21.4%, respectively. Five-year RS among extensive disease SCLC (ED-SCLC) in periods 1 to 3 was 2.3%, 2.8%, and 2.7%, respectively. Improvement in 5-year RS in periods 2 and 3 compared with period 1 was significant among both LD- and ED-SCLC patients (all P < 0.001). Conditional 5-year RS of LD-SCLC increased from 21% at year 0 to 73% at year 5, while that of ED-SCLC was 3% at year 0 and 53% at year 5.
Conclusions
The prognosis of SCLC patients improved from 1999–2001 but plateaued in 2002–2006, after which no further significant improvement was seen. Continuous survey based on population-based data is helpful in monitoring the impact of developments in treatment.
Key words: cancer registry, population-based, small cell lung cancer, survival
INTRODUCTION
Lung cancers are classified into two broad classes, small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC).1,2 These cancers differ biologically and, accordingly, also differ in their therapy and prognosis.3 National rates of survival of total lung cancer patients have been reported for countries all over the world,4 and while reporting of histologic subtype-specific survival of lung cancer patients treated in cancer hospitals is also common,5,6 reporting of this survival from population-based data is rare. With regard to SCLC status, however, this may be problematic for three reasons: cancer patients treated in cancer hospitals have a relatively better health status than those treated at general hospitals; survival reports from cancer hospitals are often restricted to patients who undergo surgery; and overall lung cancer survival from population-based data mainly reflects the survival of patients with NSCLC, given that NSCLC accounts for more than 80% of lung cancer cases.7 For these reasons, overall lung cancer survival data might not be applicable to patients with SCLC.
Prognosis of cancer patients is modified by disease stage and treatment.8 Treatment plans in patients with SCLC are commonly determined using a two-stage system originally introduced by the Veterans’ Affairs Lung Study Group, together with the TNM staging system.9,10 SCLC patients are classified into two stages, limited disease (LD) or extensive disease (ED), which are utilized for treatment selection. Tumor confined to the ipsilateral hemithorax and regional nodes is defined as LD, and tumor beyond the boundaries of LD is defined as ED. In general, patients with LD-SCLC are treated using multimodal treatment, while those with ED-SCLC receive systemic therapy.11
SCLC treatment has changed over time. Around 1999, several clinical studies supported the efficacy of concurrent chemoradiotherapy and hyperfractionated radiotherapy for LD-SCLC.12,13 The efficacy of new combination chemotherapy with cisplatin and irinotecan for Japanese patients with ED-SCLC was established in 2002.14 In addition, new drugs for ED-SCLC, amrubicin and topotecan, were approved in Japan in 2002 and 2003, respectively.15–17 Although these developments in SCLC treatment might have improved prognosis, scarce evidence for their impact is available based on population-based data.
Here, to determine specific survival of SCLC with consideration to disease stage and developments in treatment, we estimated recent trends in 10-year survival of patients with SCLC based on population-based data in Japan.
MATERIAL AND METHODS
Data source
This study was conducted using the framework of the Japanese Cancer Survival Information for Society (J-CANSIS) study. Details of the J-CANSIS study are provided elsewhere.18 In brief, the J-CANSIS study aimed to analyze recent trends in cancer survival and report long-term survival based on population-based cancer registry data of six prefectures (Yamagata, Miyagi, Fukui, Niigata, Osaka, and Nagasaki) in Japan. These six registries provided a total of 98,475 lung cancer cases diagnosed between 1993 and 2006. The population covered in our study represents 13.4% of the total Japanese population and includes both urban and rural areas. These prefectural cancer registries have high data quality (% of death certificate only = 3.1–24.6%) and have long been used to estimate national statistics for cancer survival in Japan.19 Morphologies of lung cancer were recorded using the morphology codes of the International Classification of Diseases for Oncology, Third Edition (ICD-O-3).20 Data from cancer patients followed for 5 years or more were used. Patients were linked to the prefecture death certificate database to confirm their vital status. The Yamagata, Fukui, Osaka, and Nagasaki registries additionally confirm the vital status of patients using linkage to the residential database. We excluded data that were registered using death certificate only cases from the analysis.
Grouping of morphology was defined according to Cancer Incidence in Five Continents, Volume IX.21 Morphology codes of 8041–8045 and 8246 were defined as SCLC, and all SCLC patients (n = 10,911) were included in the study. Lung cancer patients with other morphologies were excluded. Disease stage at diagnosis was categorized using a summary staging system.22 LD-SCLC and ED-SCLC were defined using the Veterans Administration Lung Cancer Study Group (VALSG) staging system.9 In short, SCLC confined to one hemithorax was defined as LD. Ipsilateral lymph node metastasis and contralateral hilar lymph node metastasis was defined as ED. LD-SCLC was defined as localized and regional stage on the summary staging system. ED-SCLC was defined as distant stage on the summary staging system. Localized and regional stages correspond to T1-2, N0-2, and M0 in the American Joint Committee of Cancer (AJCC) TNM staging system.
This study was approved by the ethics committee of Osaka Medical Center for Cancer and Cardiovascular Diseases (Osaka, Japan) in September 2013. Use of the data was approved by the six prefectural cancer registries.
Statistical analysis
We defined three periods (period 1, 1993–1998; period 2, 1999–2001; period 3, 2002–2006) to mirror the development of SCLC treatment. Elderly lung cancer patients were often defined as those who were aged 65, 70, or 75 years and older in clinical research. Therefore, age at diagnosis was classified into three groups: less than 65 years, between 65 and 74 years, and 75 years or older.
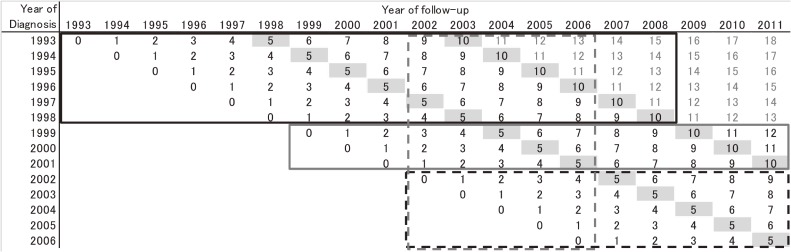
Trends in SCLC survival were assessed using relative survival (RS), because this is a standard method used to adjust for competing causes of death.23 RS is the ratio of the observed (overall) survival and expected survival. The background mortality of cancer patients was derived using the complete national population life tables by birth year, age, and sex.24 We estimated RS by applying the maximum likelihood method proposed by Esteve et al.25 We calculated the 1-, 3-, 5-, and 10-year RS for patients diagnosed in period 1 and period 2 using a conventional approach (cohort approach). Instead of 10-year RS, 1-, 3-, and 5-year RS for patients diagnosed in period 3 were calculated using the cohort approach, because 10-year survival data for these patients were not available (Figure 1, black dash frame). One-, 3-, 5-, and 10-year RS for patients diagnosed in period 3 were estimated using the period approach. Long-term RS could be estimated using the period approach from recently followed-up data. Ten-year RS for patients diagnosed in period 3 (2002–2006) was estimated using the survival data for patients diagnosed between 1993 and 2006 and followed-up between 2002 and 2006 (Figure 1, gray dashed frame).
Figure 1. Patient data used in the survival analysis. Black figures indicate the data from six prefectural cancer registries, and the numbers within the cells indicate years of follow-up. Data in the black and gray solid frames were used to calculate 10-year relative survival by the cohort approach for patients diagnosed in period 1 (1993–1998) and period 2 (1999–2001), respectively. Data in the black dashed frame were used to calculate 5-year relative survival by the cohort approach for patients diagnosed in period 3 (2002–2006). Data in the gray dashed frame were used to calculate 10-year relative survival using period analysis for patients diagnosed in period 3 (2002–2006).
RS was compared using the excess hazards model,26 a multivariate regression approach based on generalized linear models which adopts the Poisson assumption for the observed number of deaths. The excess hazards model is based on the idea that the total mortality hazard of cancer patients is decomposed into an excess hazard of death from cancer, and a hazard for other causes of death, derived from population life tables as background mortality of general populations. Period, sex, and age at diagnosis were included in the excess hazard model.
Using data of patients diagnosed in period 3, conditional 5-year survival was calculated. Conditional 5-year survival was 5-year survival with the pre-condition of having already survived a certain length of time (0 to 5 years in this report). Conditional 5-year survival for x-year survivors is calculated as follows: divide the (x+5)-year cumulative survival rate by the x-year cumulative survival, or calculate (x+5)-year cumulative survival, limited to the x-year survivors, in accordance with other studies.27–30
All analyses were conducted using STATA version 14.2 (StataCorp, College Station, TX, USA). The strel command in this software was used to calculate RS in both the cohort and period approaches.31
RESULTS
In total, 98,475 lung cancer patients, including 10,911 SCLC patients, were registered in the six prefectural cancer registries between 1993 and 2006. Proportions of SCLC in periods 1, 2 and 3 were 11.6%, 11.1%, and 10.7%, respectively. Characteristics of SCLC patients in the three periods are shown in Table 1. Proportions of female patients were approximately 18% throughout the periods. Proportions of elderly patients (aged ≥75 years) in periods 1, 2, and 3 were 25.2%, 29.1%, and 33.3%, respectively. The proportion of patients with ED-SCLC increased from 42.9% in period 1 to 50.0% in period 3.
Table 1. Characteristics of study subjects.
| 1993–1998 | 1999–2001 | 2002–2006 | Total | |||||
| Number | % | Number | % | Number | % | Number | % | |
| Sex | ||||||||
| Male | 2,817 | 82.2 | 2,501 | 81.2 | 3,639 | 82.6 | 8,957 | 82.1 |
| Female | 610 | 17.8 | 579 | 18.8 | 765 | 17.4 | 1,954 | 17.9 |
| Age, years | ||||||||
| ≤64 | 1,093 | 31.9 | 842 | 27.3 | 1,169 | 26.5 | 3,104 | 28.4 |
| 65–74 | 1,469 | 42.9 | 1,341 | 43.5 | 1,770 | 40.2 | 4,580 | 42.0 |
| ≥75 | 865 | 25.2 | 897 | 29.1 | 1,465 | 33.3 | 3,227 | 29.6 |
| Disease stage | ||||||||
| Limited disease (LD) | 1,482 | 43.2 | 1,369 | 44.4 | 1,756 | 39.9 | 4,607 | 42.2 |
| Extensive disease (ED) | 1,469 | 42.9 | 1,371 | 44.5 | 2,203 | 50.0 | 5,043 | 46.2 |
| Unknown | 476 | 13.9 | 340 | 11.0 | 445 | 10.1 | 1,261 | 11.6 |
| Total | 3,427 | 100.0 | 3,080 | 100.0 | 4,404 | 100.0 | 10,911 | 100.0 |
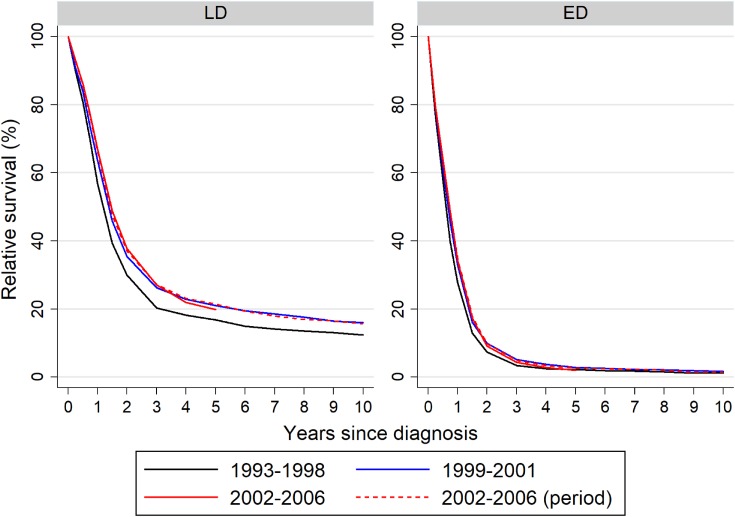
Ten-year RS curves of each period by disease stage are shown in Figure 2 and Table 2. Five-year RS curves of both LD- and ED-SCLC patients in period 3 calculated using the cohort approach were similar to those estimated by the period approach. One-, 3-, 5-, and 10-year RSs of LD-SCLC patients in period 2 were better than those in period 1. The 10-year RS curve of LD-SCLC patients in period 3 estimated using the period approach was similar to that in period 2 estimated using the cohort approach. One- and 3-year RSs of ED-SCLC patients in period 3 were better than those in period 1, whereas 5- and 10-year RSs of ED-SCLC in period 1 were similar to those in period 3 estimated using the period approach. Ten-year RS curves in each period were similar between male and female patients with SCLC. RS of SCLC patients aged 75 years and more was approximately half that of patients aged less than 65 years in each period (eTable 1).
Figure 2. Ten year relative survival of patients with small-cell lung cancer. Relative survival was stratified by disease stage. ED, extensive disease; LD, limited disease.
Table 2. 1-, 3-, 5-, and 10-year relative survival of patients with SCLC stratified by disease stage.
| Relative survival (%) | Years since diagnosis | |||||||
| 1 | 3 | 5 | 10 | |||||
| Survival | (95% CI) | Survival | (95% CI) | Survival | (95% CI) | Survival | (95% CI) | |
| Limited Disease (LD) | ||||||||
| Period 1 (1993–1998) | 56.8 | (54.3–59.1) | 20.3 | (18.3–22.3) | 16.8 | (14.9–18.7) | 12.4 | (10.6–14.4) |
| Period 2 (1999–2001) | 63.6 | (60.5–66.6) | 26.2 | (23.4–29.1) | 21.1 | (18.4–23.8) | 16.1 | (13.5–18.8) |
| Period 3 (2002–2006) | 66.9 | (64.5–69.2) | 27.0 | (24.8–29.3) | 19.9 | (17.8–22.0) | ||
| Period 3 (perioda) | 66.2 | (63.8–68.5) | 27.2 | (25.0–29.5) | 21.4 | (19.3–23.6) | 15.6 | (13.4–18.0) |
| Extensive Disease (ED) | ||||||||
| Period 1 (1993–1998) | 27.7 | (25.6–29.8) | 3.4 | (2.6–4.4) | 2.3 | (1.6–3.1) | 1.2 | (0.7–1.8) |
| Period 2 (1999–2001) | 33.0 | (30.1–35.9) | 5.2 | (3.9–6.7) | 2.8 | (1.9–4.0) | 1.7 | (1.0–2.9) |
| Period 3 (2002–2006) | 34.3 | (32.3–36.4) | 4.3 | (3.5–5.3) | 2.0 | (1.4–2.7) | ||
| Period 3 (perioda) | 34.8 | (32.8–36.9) | 5.0 | (4.0–6.0) | 2.7 | (2.0–3.6) | 1.4 | (0.8–2.3) |
CI, confidence interval; ED, extensive disease; LD, limited disease; SCLC, small-cell lung cancer.
aRelative survival and CIs were estimated using the period method. Survival data of patients followed between 2002 and 2006 were used.
We estimated the EHR of SCLC patients’ hazard of death from cancer within 5 years (Table 3). When stratified by disease stage, LD- and ED-SCLC showed similar trends. Excess mortality in periods 2 and 3 was significantly lower than that in period 1. Female LD-SCLC patients showed no statistically significant difference in mortality from male patients, whereas female ED-SCLC patients showed significantly better survival than male patients.
Table 3. Excess hazard ratio (EHR) of death by excess mortality model stratified by disease stage.
| Limited disease (LD) | Extensive disease (ED) | |||||
| EHR | 95% CI | P value | EHR | 95% CI | P value | |
| Period | ||||||
| 1993–1998 | 1 | Reference | 1 | Reference | ||
| 1999–2001 | 0.84 | 0.77–0.92 | <0.001 | 0.86 | 0.80–0.94 | <0.001 |
| 2002–2006 | 0.77 | 0.72–0.84 | <0.001 | 0.85 | 0.79–0.90 | <0.001 |
| Sex | ||||||
| Male | 1 | Reference | 1 | Reference | ||
| Female | 1.04 | 0.95–1.13 | 0.401 | 0.92 | 0.85–0.99 | 0.028 |
| Age at diagnosis, years | ||||||
| ≤64 | 1 | Reference | 1 | Reference | ||
| 65–74 | 1.29 | 1.19–1.40 | <0.001 | 1.23 | 1.15–1.31 | <0.001 |
| ≥75 | 1.92 | 1.76–2.10 | <0.001 | 1.71 | 1.58–1.85 | <0.001 |
CI, confidence interval; EHR, excess hazard ratio.
Period, sex and age were included in the model.
Conditional 5-year RS stratified by disease stage are shown in Figure 3. Conditional 5-year survival for patients with LD-SCLC increased from 21% at year 0 to 73% at year 5, while that in patients with ED-SCLC increased from 3% at year 0 to 53% at year 5. Because the 2-year RS of patients with ED-SCLC was 9.8%, confidence intervals for the conditional 5-year survival of ED-SCLC patients at years 3 to 5 are wide.
Figure 3. Conditional 5-year relative survival and 95% confidence intervals of SCLC patients stratified by disease stage. ED, extensive disease; LD, limited disease; SCLC, small-cell lung cancer.
DISCUSSION
In this study, we found that the RS of patients with SCLC slightly improved between 1993 and 2006, despite increases in the number of elderly patients and relative proportion of ED-SCLC. This improvement in RS was confirmed after adjustment for period, sex, and age at diagnosis. To the best of our knowledge, this is the first study to show the RS of patients with SCLC stratified by disease stage using population-based data.
Among results, we found that the RS of patients with LD-SCLC in periods 2 and 3 were better than that in period 1. This improvement in survival was consistent with the development of chemoradiotherapy.12 A new chemoradiotherapy method, concurrent radiotherapy and hyperfractionated radiotherapy, improved the RS of patients with LD-SCLC diagnosed after 1997. LD-SCLC patients had a similar survival in period 3 to that in period 2. This seems consistent with the fact that no significant new treatment for LD-SCLC was developed during this time.
The improvement in ED-SCLC survival in periods 2 and 3 compared with period 1 was inconsistent with the development of chemotherapy. A clinical study in Japan showed that patients with ED-SCLC treated with the new combination of cisplatin and irinotecan had longer survival than those treated using standard chemotherapy with cisplatin and etoposide.14 In a replication study, however, cisplatin and irinotecan showed no significant benefit compared with standard chemotherapy.32–34 In addition, the higher rate of nonhematologic toxicity with the cisplatin and irinotecan regimen might decrease feasibility, and the new regimen might, therefore, have lacked impact on survival using population-based data. The RS of ED-SCLC patients in period 2 was better than that in period 1, despite no obvious improvement in ED-SCLC treatment. One reason might be the development of supportive care and palliative care. Total usage of opioids, a proxy for supportive care,35 was 706 kg of morphine equivalent in Japan in 1995, rapidly increasing to 891 kg in 2000 and 2,696 kg in 2004.36 Given that supportive care impacts the prognosis of patients with lung cancer, this increase in supportive care might have improved the prognosis of patients.37
The RS curves in period 2 were better than those in period 1 for both LD-SCLC and ED-SCLC. The RS curves in period 2 were similar to those in period 3 for both LD-SCLC and ED-SCLC. This similarity should be carefully considered because the improvement might have been due to stage migration. Improvements in diagnostic methods allow the detection of very small metastatic tumors. Patients with small distant metastasis would have been classified as LD in period 1. With the detection of small metastases with improved imaging, however, the patient would be diagnosed as ED. Movement of such patients with small metastases from LD to ED would improve the prognosis of LD patients, because their prognosis would be poorer than that of those without metastasis. Similarly, the prognosis of ED patients would be improved via the addition of patients with small metastases. The increased proportion of ED patients may support this hypothesis.
Conditional 5-year survival shows the conditional probability of surviving a further 5 years for cancer survivors.38 It is a more informative way for survivors to see their evolving prognosis over time. Conditional 5-year survival was low in our patients with LD-SCLC compared with other malignancies.18 Even 5 years post-diagnosis, conditional 5-year survival was 73%. The low conditional 5-year survival was mainly due to the poor prognosis of SCLC. In addition, the low conditional survival might be partly explained by the high proportion of heavy smokers among patients with SCLC.39 Even SCLC patients with long survival may eventually die due to other cigarette-associated disease and comorbidities.
Lung cancer screening might be another potential factor to influence SCLC survival. Because of aggressive growth of SCLC, most SCLC cases were discovered as symptomatic cancers during the interval of annual lung cancer screening,40 which suggests that lung cancer screening is unlikely to improve survival in patients with SCLC.41 Even if SCLC could be screened effectively, it is less likely that screening affects stage-specific survival. Therefore, lung cancer screening programs were unlikely to affect the results of our study.
The strength of this study is its use of population-based cancer registry data. Because all SCLC incident cases in six prefectures were included, the study is unlikely to have suffered from the selection bias which confounds clinical trials and hospital-based cancer registries. A second strength was its large sample size. Most reports of SCLC survival have been derived from hospital-based studies.5,42 The largest Japanese hospital-based lung cancer registry, the Japanese Joint Committee for Lung Cancer Registration, reported histology in specific lung cancer survival.5 However, their study included only 243 SCLC cases versus 10,911 incident SCLC cases in our present study.
This study has a number of limitations. First, long-term survival was estimated using data from only six prefectural cancer registries. Second, data quality was not particularly high. The proportion of death certificate only cases among registries was 3.1% to 24.6%. The generalizability of the results should, therefore, be interpreted cautiously. Thanks to the enactment of the Cancer Registry Law in 2013, the quality of population-based cancer registry data will shortly improve.43,44 This will allow new estimations of cancer survival with greater timeliness, longer follow-up, and inclusion of many more prefectures in Japan. Considering the decreasing trend in the incidence of SCLC,45,46 analysis might require larger coverage to attain a stable estimation. Third, detailed information, such as treatment, comorbidity, and smoking status, was not available. These variables affect cancer survival, but the data are not fully collected in population-based cancer registries. Verification of the influence of these clinical factors on prognosis would require studies using detailed clinical data from hospital-based cancer registries.
In conclusion, we reported the 10-year RS and conditional survival of patients with LD- and ED-SCLC. RS after 1999 was better than that before 1998, although conditional survival was poor even among the patients with LD-SCLC. The forthcoming improvement in the quality and timeliness of cancer registry data in Japan will allow continuous survey using population-based data from many prefectures to estimate the progress of treatment.
ACKNOWLEDGEMENTS
We thank the Yamagata, Miyagi, Fukui, Niigata, Osaka and Nagasaki Cancer Registries for understanding our research concept and providing data, and all the medical institutes which cooperated by submitting data to population-based cancer registries.
Funding: This study was supported by the Ministry of Health, Labour and Welfare of Japan by a Grant-in-aid for a Health and Labour Science Research Grant for the Third Term Comprehensive Control Research for Cancer; Grant number: H25-008 and JSPS KAKENHI Grant number: 17K15841.
Conflicts of interest: None declared.
APPENDIX A. SUPPLEMENTARY DATA
The following is the supplementary data related to this article:
eTable 1. Relative survival of patients with SCLC stratified by sex and age
REFERENCES
- 1.Travis WD, Brambilla E, Nicholson AG, et al. . The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243–1260. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 2.Hirsch FR, Matthews MJ, Aisner S, et al. . Histopathologic classification of small cell lung cancer. Changing concepts and terminology. Cancer. 1988;62(5):973–977. [DOI] [PubMed] [Google Scholar]
- 3.Elias AD. Small cell lung cancer: state-of-the-art therapy in 1996. Chest. 1997;112(4)(Suppl):251S–258S. 10.1378/chest.112.4_Supplement.251S [DOI] [PubMed] [Google Scholar]
- 4.Allemani C, Weir HK, Carreira H, et al. . Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010. 10.1016/S0140-6736(14)62038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawabata N, Fujii Y, Asamura H, et al. . Lung cancer in Japan: analysis of lung cancer registry cases resected in 2004. Jpn J Lung Cancer. 2010;50(7):875–888. 10.2482/haigan.50.875 [DOI] [PubMed] [Google Scholar]
- 6.Chansky K, Detterbeck FC, Nicholson AG, et al. . The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2017;12(7):1109–1121. 10.1016/j.jtho.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 7.Govindan R, Page N, Morgensztern D, et al. . Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. 10.1200/JCO.2005.04.4859 [DOI] [PubMed] [Google Scholar]
- 8.van Meerbeeck JP, Janssens A. The seventh tumour-node-metastasis staging system for lung cancer: Sequel or prequel? EJC Suppl. 2013;11(2):150–158. 10.1016/j.ejcsup.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3. 1973;4(2):31–42. [PubMed] [Google Scholar]
- 10.Mountain CF. A new international staging system for lung cancer. Chest. 1986;89(4)(Suppl):225S–233S. 10.1378/chest.89.4_Supplement.225S [DOI] [PubMed] [Google Scholar]
- 11.Hanna NH, Einhorn LH. Small-cell lung cancer: state of the art. Clin Lung Cancer. 2002;4(2):87–94. 10.3816/CLC.2002.n.018 [DOI] [PubMed] [Google Scholar]
- 12.Takada M, Fukuoka M, Kawahara M, et al. . Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20(14):3054–3060. 10.1200/JCO.2002.12.071 [DOI] [PubMed] [Google Scholar]
- 13.Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: a randomized study. J Clin Oncol. 1997;15(3):893–900. 10.1200/JCO.1997.15.3.893 [DOI] [PubMed] [Google Scholar]
- 14.Noda K, Nishiwaki Y, Kawahara M, et al. . Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346(2):85–91. 10.1056/NEJMoa003034 [DOI] [PubMed] [Google Scholar]
- 15.von Pawel J, Schiller JH, Shepherd FA, et al. . Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17(2):658–667. 10.1200/JCO.1999.17.2.658 [DOI] [PubMed] [Google Scholar]
- 16.Jotte R, Conkling P, Reynolds C, et al. . Randomized phase II trial of single-agent amrubicin or topotecan as second-line treatment in patients with small-cell lung cancer sensitive to first-line platinum-based chemotherapy. J Clin Oncol. 2011;29(3):287–293. 10.1200/JCO.2010.29.8851 [DOI] [PubMed] [Google Scholar]
- 17.Inoue A, Sugawara S, Yamazaki K, et al. . Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J Clin Oncol. 2008;26(33):5401–5406. 10.1200/JCO.2008.18.1974 [DOI] [PubMed] [Google Scholar]
- 18.Ito Y, Miyashiro I, Ito H, et al. . Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci. 2014;105(11):1480–1486. 10.1111/cas.12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hori M, Matsuda T, Shibata A, et al. . Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45(9):884–891. 10.1093/jjco/hyv088 [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. International Classification of Diseases for Oncology (ICD-O). 3rd ed. WHO Press; 2013. [Google Scholar]
- 21.Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents. Vol 10. Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- 22.Young JJ, Roffers S, Ries L, Fritz A, Hurlbut A. SEER Summary Staging Manual - 2000: Codes and Coding Instructions. Bethesda, MD: NIH Pub; 2001. [Google Scholar]
- 23.Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260(2):103–117. 10.1111/j.1365-2796.2006.01677.x [DOI] [PubMed] [Google Scholar]
- 24.Ministry of Health, Labour and Welfare. A bridged Life Tables in Japan for 1962–2011. Tokyo, Japan: Center for Cancer Control and Information Services, National Cancer Center; 2011. [Google Scholar]
- 25.Estève J, Benhamou E, Croasdale M, Raymond L. Relative survival and the estimation of net survival: elements for further discussion. Stat Med. 1990;9(5):529–538. 10.1002/sim.4780090506 [DOI] [PubMed] [Google Scholar]
- 26.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23(1):51–64. 10.1002/sim.1597 [DOI] [PubMed] [Google Scholar]
- 27.Ito Y, Nakayama T, Miyashiro I, Ioka A, Tsukuma H. Conditional survival for longer-term survivors from 2000–2004 using population-based cancer registry data in Osaka, Japan. BMC Cancer. 2013;13:304. 10.1186/1471-2407-13-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu XQ, Baade PD, O’Connell DL. Conditional survival of cancer patients: an Australian perspective. BMC Cancer. 2012;12:460. 10.1186/1471-2407-12-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellison LF, Bryant H, Lockwood G, Shack L. Conditional survival analyses across cancer sites. Health Rep. 2011;22(2):21–25. [PubMed] [Google Scholar]
- 30.Merrill RM, Hunter BD. Conditional survival among cancer patients in the United States. Oncologist. 2010;15(8):873–882. 10.1634/theoncologist.2009-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cancer Research UK Cancer Survival Group, London School of Hygiene and Tropical Medicine. Strel computer program version 1.2.7 for cancer survival analysis. 2009; http://csg.lshtm.ac.uk/tools-analysis/strel-strel2/. Accessed 12 May 2018.
- 32.Zatloukal P, Cardenal F, Szczesna A, et al. . A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol. 2010;21(9):1810–1816. 10.1093/annonc/mdq036 [DOI] [PubMed] [Google Scholar]
- 33.Lara PN Jr, Natale R, Crowley J, et al. . Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27(15):2530–2535. 10.1200/JCO.2008.20.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanna N, Bunn PA Jr, Langer C, et al. . Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24(13):2038–2043. 10.1200/JCO.2005.04.8595 [DOI] [PubMed] [Google Scholar]
- 35.International Narcotics Control Board. Report of the International Narcotics Control Board on the Availability of Internationally Controlled Drugs: Ensuring Adequate Access for Medical and Scientific Purposes. New York: United Nations; 2011. [Google Scholar]
- 36.The Editorial Board of the Cancer Statistics in Japan. Cancer Statistics in Japan 2016. Tokyo: Foundation for Promotion of Cancer Research (FPCR); 2017. [Google Scholar]
- 37.Temel JS, Greer JA, Muzikansky A, et al. . Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- 38.Hieke S, Kleber M, König C, Engelhardt M, Schumacher M. Conditional survival: a useful concept to provide information on how prognosis evolves over time. Clin Cancer Res. 2015;21(7):1530–1536. 10.1158/1078-0432.CCR-14-2154 [DOI] [PubMed] [Google Scholar]
- 39.Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526–4527. 10.1200/JCO.2006.07.3841 [DOI] [PubMed] [Google Scholar]
- 40.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva M, Galeone C, Sverzellati N, et al. . Screening with low-dose computed tomography does not improve survival of small cell lung cancer. J Thorac Oncol. 2016;11(2):187–193. 10.1016/j.jtho.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 42.Nakamura K, Ukawa S, Okada E, et al. . Characteristics and prognosis of Japanese male and female lung cancer patients: the BioBank Japan Project. J Epidemiol. 2017;27(3S):S49–S57. 10.1016/j.je.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka H, Matsuda T. Arrival of a new era in Japan with the establishment of the Cancer Registration Promotion Act. Eur J Cancer Prev. 2015;24(6):542–543. 10.1097/CEJ.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 44.Naito M. Utilization and application of public health data in descriptive epidemiology. J Epidemiol. 2014;24(6):435–436. 10.2188/jea.JE20140182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinoshita FL, Ito Y, Nakayama T. Trends in lung cancer incidence rates by histological type in 1975–2008: a population-based study in Osaka, Japan. J Epidemiol. 2016;26(11):579–586. 10.2188/jea.JE20150257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito H, Matsuo K, Tanaka H, et al. . Nonfilter and filter cigarette consumption and the incidence of lung cancer by histological type in Japan and the United States: analysis of 30-year data from population-based cancer registries. Int J Cancer. 2011;128(8):1918–1928. 10.1002/ijc.25531 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.