Key Points
IL-36γ conferred MDMs M. tuberculosis resistance through activation of autophagy.
IL-36γ induced autophagy relying on WNT5A/COX-2/AKT/mTOR axis.
IL-36γ may be a potential candidate for immune therapy of tuberculosis.
Abstract
Mycobacterium tuberculosis, which primarily infects mononuclear phagocytes, remains the leading bacterial cause of enormous morbidity and mortality because of bacterial infections in humans throughout the world. The IL-1 family of cytokines is critical for host resistance to M. tuberculosis. As a newly discovered subgroup of the IL-1 family, although IL-36 cytokines have been proven to play roles in protection against M. tuberculosis infection, the antibacterial mechanisms are poorly understood. In this study, we demonstrated that IL-36γ conferred to human monocyte-derived macrophages bacterial resistance through activation of autophagy as well as induction of WNT5A, a reported downstream effector of IL-1 involved in several inflammatory diseases. Further studies showed that WNT5A could enhance autophagy of monocyte-derived macrophages by inducing cyclooxygenase-2 (COX-2) expression and in turn decrease phosphorylation of AKT/mTOR via noncanonical WNT signaling. Consistently, the underlying molecular mechanisms of IL-36γ function are also mediated by the COX-2/AKT/mTOR signaling axis. Altogether, our findings reveal a novel activity for IL-36γ as an inducer of autophagy, which represents a critical inflammatory cytokine that control the outcome of M. tuberculosis infection in human macrophages.
Introduction
Tuberculosis remains one of the leading causes of global morbidity and mortality among infectious diseases (1). Macrophages are the major cellular niche for bacterial replication during early infection, as well as critical defense components in the antituberculosis innate immune response (2). Infection with Mycobacterium tuberculosis results in significant alterations in the macrophage physiology, including rapid secretion of cytokines and antimicrobial peptides (APs), induction of autophagy, apoptosis, and other innate immune functions, and active M. tuberculosis resistance (3–6). Among cytokines secreted by infected macrophages, the IL-1 superfamily attracts great attention for its important roles in immunity against M. tuberculosis with its multiple members. For example, IL-1β controls infection by autocrine signaling from infected macrophages, and IL-1β−/− or IL-1R−/− mice showed increased susceptibility and succumbed rapidly to low-dose aerosol infection (7). IL-12 + IL-18 cosignaling can trigger AP expression and autophagy, resulting in inhibition of intracellular mycobacteria in macrophages and lung epithelial cells (8).
Recently, a new member of the IL-1 superfamily, the IL-36 family, has emerged as an important mediator of inflammatory diseases. The IL-36 subfamily comprises the proinflammatory molecules IL-36α, IL-36β, and IL-36γ and a natural inhibitor, IL-36R antagonist (IL-36Ra), which play important roles in stimulation of immune responses (9). IL-36γ can induce inflammatory cytokine expression in bone marrow–derived macrophages and keratinocytes (10) and regulate neutrophilic airway inflammation (11). IL-36β is constitutively present in bone marrow–derived dendritic cells, enhancing activation of IL-36R high helper T cells and connecting the innate and adaptive immune responses against bacillus Calmette–Guérin (BCG) infection in vivo (12). In human PBMCs, in contrast, only APCs but not T cells express IL-36R (13), suggesting the importance of IL-36γ in human innate immune regulation. Recently, it was reported that increased IL-36γ in macrophages upon M. tuberculosis infection induced several kinds of APs and restricted intracellular M. tuberculosis growth, whereas such effects were attenuated in IL-36R knockdown macrophages (14, 15), demonstrating the importance of IL-36γ in the control of M. tuberculosis. Therefore, induction of IL-36γ expression is important in the host resistance to M. tuberculosis, with unelucidated functional mechanisms.
The members of the IL-36 family were originally discovered in sequence databases because of their homology with IL-1α and IL-1β (9). Like all other IL-1 family members, IL-36γ and IL-36Ra exhibit the typical β-trefoil fold composed of 12 β-strands connected by 11 loops and share the coreceptor of IL-1R accessory protein with IL-1 (9, 16). For these reasons, the function of IL-36 is mainly explored based on the knowledge about IL-1. It was reported that IL-1β induced the expression of WNT5A and the coreceptor, receptor tyrosine kinase-like orphan receptor 2, in human mesenchymal stem cells during their osteoblastic differentiation and mineralization under inflammatory conditions mainly through the noncanonical WNT–receptor tyrosine kinase-like orphan receptor 2 pathway (17). In SKOV-3 cells, a significant increase of WNT5A stimulated by recombinant human (rh) IL-1β induced secretion of G-CSF, GM-CSF, IL-1α, IL-13, and monocyte chemoattractant protein 3 (18). However, whether IL-36 functions through WNT5A during M. tuberculosis infection is still unknown.
WNT5A belongs to the WNT family of glycoprotein signal transducers. Two main branches of classical WNT signaling cascades have been demonstrated: β-catenin–dependent (canonical) and β-catenin–independent (noncanonical) WNT signaling, the use of which depends on the context (19, 20). Many studies indicated that WNT5A exerts proinflammatory functions on various types of immune and nonimmune cells, thus playing key roles in tissue homeostasis and modulation of immune responses in pathology (21, 22). WNT5A not only contributes to maintain IFN levels in macrophages but also supports macrophage survival (20). Importantly, WNT5A has been shown to be involved in regulation of the human Th1 response to M. tuberculosis, indicating its antimicrobial role (23). However, whether WNT5A could regulate the anti–M. tuberculosis response of macrophages and its role in the effects of IL-36 are still unknown.
In this study, we screened out the most potent member of the IL-36 family functioning in macrophage anti–M. tuberculosis responses and explored the existence of the IL-36γ/WNT5A axis and its effects and mechanisms on bactericidal activity. Our results demonstrated the involvement of noncanonical WNT signaling and downstream autophagy. These results provide valuable insights into the molecular mechanism of IL-36γ–regulated immune responses, suggesting that IL-36γ may be a potential candidate for immune therapy of tuberculosis.
Materials and Methods
Cell culture
PBMCs were isolated from peripheral blood donated from healthy individuals or patients with active pulmonary tuberculosis (APT) (Guangzhou Municipal Hospital of Chest Medicine). Tuberculosis was determined as defined by the China health industry standard for the classification of tuberculosis (WS 196-2017). All blood donors provided informed consent to participate in the study. The studies were reviewed and approved by the Medical Ethics Board and the Biosafety Management Committee of Southern Medical University. Monocytes were isolated from PBMCs by CD14+ microbead separation (Miltenyi Biotec) and differentiated into macrophages by culturing them in complete medium [RPMI 1640 medium supplemented (Corning) with 10% FBS (Corning), 1% glutamine, 1% antibiotics, 1% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] with the addition of 50 ng/ml of GM-CSF (PeproTech) (named as GM-CSF medium) for 7 d.
Mycobacteria culture and infection
BCG and M. tuberculosis virulent strain H37Rv (American Type Culture Collection) were grown in Middlebrook 7H9 broth (Becton Dickinson), supplemented with 10% oleic acid, albumin, dextrose, and catalase at 37°C, to an early log phase at an OD600 of 0.2–0.6. Single-cell suspension of mycobacteria was prepared as follows: bacterial culture was centrifuged at 3000 rpm for 5 min and the supernatant was removed. The pellet was resuspended in 1640 complete medium and grinded to generate bacterium suspension. Then, bacterium suspension was centrifuged at 3100 rpm for 5 min to remove the agglomeration bacteria. The supernatant was the single-cell suspension used for infections, and the density of bacteria was measured at OD600. The suspensions were used for infection within 12 h to prevent bacterial clumping. All infections were performed at a multiplicity of infection of 2 unless stated otherwise. The cells were incubated at 37°C for different periods, and the details are specified in figure legends.
RNA extraction and quantitative real time PCR
RNA was isolated using TRIzol (Thermo Fisher Scientific) and quantified using a Nanodrop 2000c (Thermo Fisher Scientific). RNA was reverse-transcribed at equal concentrations using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech) and then used for quantitative real time PCR (qRT-PCR) analysis using TB Green Premix Ex Taq II (Clontech, TaKaRa, Cellartis) in a LightCycler 480 thermocycler (Roche). The two-step qRT-PCR was used with 3 μl of RNA per 10 μl reaction. Thermal cycling conditions were 95°C for 5 min; 40 cycles of 95°C for 10 s, and 60°C for 45 s. The primers complementary to the IL-36G, WNT5A, and GAPDH sequences were synthesized by Sangon Biotech and used for initial amplification (Table I). All data were reanalyzed using the 2−ΔΔCt (relative quantification) method, where “Ct” is the cycle threshold. GAPDH was used as an internal control.
Table I. Primer sequences used for qRT-PCR amplification of target genes.
| Target Genes | Sequences 5′→ 3′ |
|---|---|
| WNT5A-F | 5′-GAGAGTGCTCGCATCCTCATG-3′ |
| WNT5A-R | 5′-TCTTGTGTGGTGGGCGAGTT-3′ |
| IL-36G-F | 5′-TTGTATTGTGAGAAGGTTGGAGAACA-3′ |
| IL-36G-R | 5′-GTCAGAATGATGGGCTGGTCTCT-3′ |
| GAPDH-F | 5′-GGATATTGTTGCCATCAATGACC-3′ |
| GAPDH-R | 5′-AGCCTTCTCCATGGTGGTGAAGA-3′ |
Primer sequence information of target genes, including WNT5A, IL-36G, and GAPDH, used in the study can be provided in this table.
F, forward; R, reverse.
Western blot
Cells were lysed with 80% whole cell extract buffer (20 mM HEPES, 10 mM KCl, 1 mM EDTA, 400 mM NaCl, 0.05% Nonidet P-40, 20% glycerol) supplemented with protease inhibitor mixture (Roche). The protein concentration was determined by the Bradford method, subjected to separation and transfer of the proteins on PVDF membranes, and the membranes were exposed to the following Abs: IL-36γ (1:500), WNT5A (1:250) (R&D Systems), phospho-AKT, AKT (1:2000; Cell Signaling Technology), phospho-ERK 1/2, ERK, phospho-p38, p38, phospho-JNK 1/2, JNK1/2, phospho-p65 (Ser536) (93H1) Rabbit mAb no. 3033, p65, cyclooxygenase-2 (COX-2), LC3A/B, ATG5, SQSTM1/p62, phospho-mTOR, mTOR (1:1000; Cell Signaling Technology), and GAPDH (1:2000; Zhongshan Golden Bridge Biotechnology). After washing, the membranes were incubated with either secondary rabbit anti-rat or goat anti-rabbit/mouse HRP-conjugated Abs (Zhongshan Golden Bridge Biotechnology). Following a second wash, the separated protein bands were visualized using Immobilon Western Chemiluminescence HRP substrate (MilliporeSigma) and imaged and analyzed using the ChemiDoc imaging system (Protein Simple). Densitometric quantifications of relative protein expression were carried out by calculating the band volume intensity using Image J software (National Institutes of Health). Signal intensity for the levels of GAPDH was served as control and normalized to 1 or 0.1. The changes among experimental groups were presented with the reference value of control.
ELISA
Supernatants were harvested from infected or stimulated cells. Prior to assay, supernatants of infected cells were centrifuged at 12,000 × g for 10 min to discard the debris and stored at −80°C until use. Samples were assayed using ELISA kits for IL-36γ (R&D Systems), IL-1β, TNF-α, and IL-6 (ExCell Bio). All procedures were performed according to the manufacturer’s instructions.
Small interfering RNA transfection
Transient small interfering RNA (siRNA) transfection was performed with Lipofectamine 2000 (Thermo Fisher Scientific). For knockdown experiments, monocyte-derived macrophages (MDMs) were pretreated with siRNA according to the manufacturer’s instructions (RiboBio) for 24 h. The following siRNA oligonucleotides were used: IL-36γ siRNA, WNT5A siRNA (100 mM; Thermo Fisher Scientific), p65 siRNA, and COX-2 siRNA (100 mM; RiboBio).
Inhibitor treatment
For inhibitor treatment, cells in 24-well plates were washed with PBS and were further cultured in GM-CSF–free complete media overnight to reduce the basal expression of WNT5A. In all experiments, when needed, rhIL-36γ (100 ng/ml) or rhWNT5A (200 ng/ml) were used unless stated otherwise, as indicated in figure. To investigate whether IL-36γ regulated the expression of WNT5A, MDMs were pretreated with rhIL-36Ra (100 ng/ml; PeproTech), specific inhibitor JSH-23 for p65 (20 μM; Selleck), U0126 for ERK (20 μM; Selleck), SB203580 for p38 (10 μM; Selleck), SP600125 for JNK (20 μM; Selleck), LY294002 for AKT (20 μM; Selleck), and DMSO as solvent control for 1 h before the addition of rhIL-36γ (PeproTech), and infected with H37Rv in the presence of above inhibitors for 24 or 48 h at 37°C. To confirm that the biological functions of IL-36γ mediated the autophagy activity partly through the noncanonical WNT pathway, MDMs were incubated with WNT antagonist III, BOX5 (100 μM; Thermo Fisher Scientific), or XAV-939 (10 nM; MedChem Express) for 12 h. Then, cells were infected with H37Rv and stimulated with rhIL-36γ or rhWNT5A (R&D Systems) simultaneously in the presence of BOX5 or XAV-939 for 24 h at 37°C. To inhibit autophagy, cells were infected with H37Rv and treated with rhIL-36γ in 24-well plates with or without the specific PI3K class III inhibitor, 3-methyladenine (3-MA) (10 mM; Selleck), for 24 h. To inhibit COX-2 activity, cells were pretreated with NS-398 (10 μM; Selleck) and DMSO as solvent control for 1 h before infection with H37Rv and treatment with rhIL-36γ in 24-well plates with or without the NS-398 for 24 h. At the end of the experiments, cells were washed in PBS, and protein was extracted and stored at −80°C until Western blot analysis.
CFU assay
To assess uptake of H37Rv, macrophages (2 × 105 cells) were pretreated with rhIL-36γ for 48 h and then incubated with H37Rv at a multiplicity of infection of 10 for 2 h at 37°C in 5% CO2. To estimate H37Rv killing, an equal number of H37Rv-infected cells were extensively washed with PBS and treated with rhIL-36γ for estimating internalized bacteria at 72 h. The time of CFU assay calculation began after 2 h infection. Then, cells were washed with PBS to remove extracellular bacteria and lysed in ddH2O containing 0.01% Triton X-100. The diluted aliquots were then spread on 7H10 Middlebrook agar (Becton Dickinson), and CFU were counted after 21–28 d of culture.
Confocal fluorescence microscopy
For immunostaining, human macrophages were seeded on 24-well chamber slides (2 × 104 cells per well). The cells were infected with or without BCG and treated with rhIL-36γ or rhWNT5A for 24 h. Thereafter, cells were fixed with 4% paraformaldehyde in PBS at room temperature (25°C) for 10 min and permeabilized with 0.1% Triton X-100 (SigmaAldrich) and 0.1% sodium citrate in PBS for 5 min. Then, the cells were treated with 10% BSA (SigmaAldrich) for 1 h at room temperature and stained with rabbit anti-human LC3A/B (Cell Signaling Technology) overnight at 4°C. After washing, the cells were incubated for 1 h at room temperature with the fluorescently labeled secondary Ab, anti-rabbit IgG–Alexa Fluor 488 (Thermo Fischer Scientific), and then washed three times with PBS. Cells were loaded with DAPI mounting media (Sangon Biotech) for visualization of nuclei and imaged with an Olympus confocal microscope (Olympus FluoView FV1000 confocal microscope). Quantification of autophagy was performed based on the percentage of the cells with LC3-II punctate dots by Image J software (National Institutes of Health).
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5.0 software (GraphPad Software). Results are presented as means ± SEM or means ± SD. All experiments were repeated at least three times. All data were considered to be significant if the p value <0.05.
Results
Expression profiles of IL-36 family members in PBMCs of patients with pulmonary tuberculosis
To investigate the role of IL-36 family members in tuberculosis, their expression was first detected in PBMCs of APT patients. Significantly upregulated IL-36A, IL-36G, and IL-36R expression in patient PBMCs compared with healthy controls was observed (Fig. 1A, Table I). Considering the bifurcated activities of IL-36 family members, the ratios of proinflammatory IL-36A, IL-36B, and IL-36G versus anti-inflammatory IL-36RA were calculated and compared between patients and healthy controls. Interestingly, only the IL-36G/IL-36RA ratio was significantly higher in patients (Fig. 1B). The prominently enhanced production of IL-36γ in APT PBMCs suggested its immune regulation role in tuberculosis.
FIGURE 1.
Upregulated mRNA expression of IL-36 family members and IL-36R in M. tuberculosis. (A) The relative mRNA expression levels of IL-36A, IL-36G, and IL-36R in PBMCs of patients with APT and HC detected by qRT-PCR. (B) The ratio of IL-36A/IL-36RA, IL-36B/IL-36RA, and IL-36G/IL-36RA mRNA level in the PBMCs of patients with APT. Data are shown as means ± SEM. *p ≤ 0.05, **p ≤ 0.01. HC, healthy control.
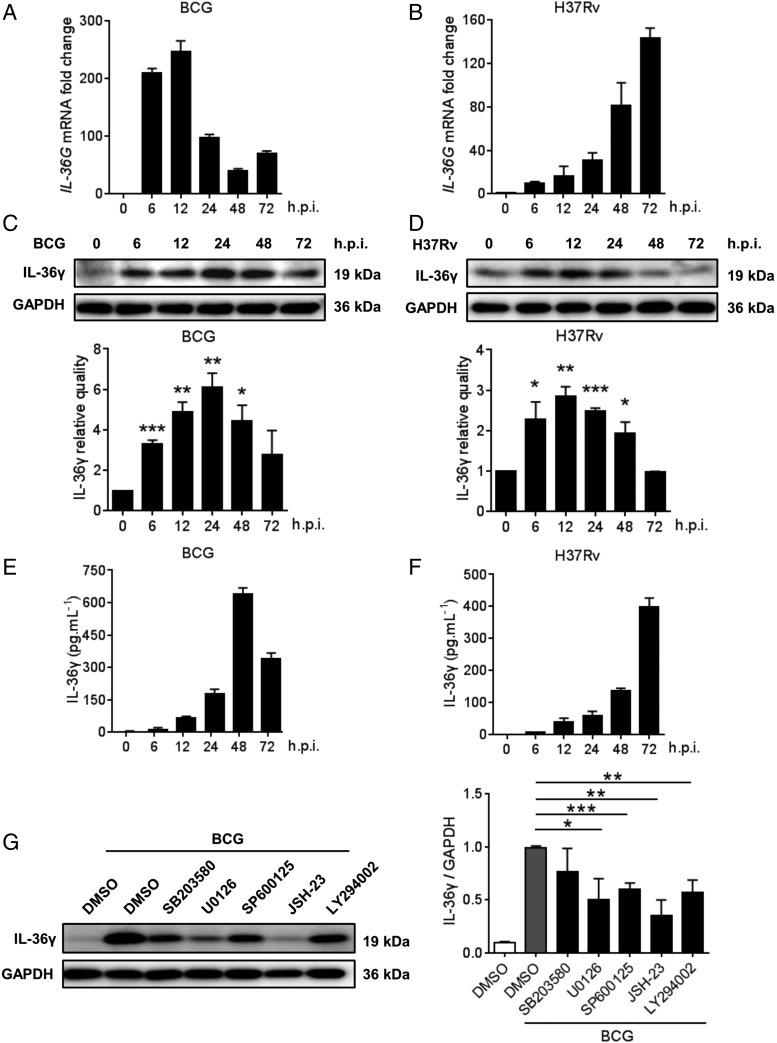
Upregulation of IL-36γ in M. tuberculosis–infected macrophages
Because macrophages play an important role in antituberculosis immunity, IL-36γ expression in human MDMs following M. tuberculosis infection was detected. As expected, both BCG and H37Rv significantly increased IL-36γ expression and secretion, with different peaking time points postinfection induced by different strains (Fig. 2A–F). Detection of the signaling pathways involved in IL-36γ production in response to M. tuberculosis infection according to previous studies (14) showed that the BCG-induced IL-36γ increase was abolished by inhibiting ERK, JNK, NF-κB, and AKT signaling but not by inhibiting p38 signaling (Fig. 2G, Supplemental Fig. 1A).
FIGURE 2.
M. tuberculosis induced expression of IL-36γ in human macrophages. (A–D) IL-36γ expression in MDMs postinfection with BCG or H37Rv was monitored by quantitative qRT-PCR (A and B) and Western blot (C and D). (E and F) IL-36γ expression in the supernatant of MDMs infected with BCG or H37Rv was measured by ELISA. (G) MDMs were pretreated with SB203580, U0126, SP600125, JSH-23, or LY294002, with DMSO as the solvent control for 1 h before infection with BCG for 24 h in the presence of above inhibitors, and IL-36γ protein expression was analyzed by Western blot. The densitometric scanning results of (C), (D), and (G) contain at least three independent experiments, and data are shown as means ± SEM. The results of (A), (B), (E), and (F) presented are from one of least three independent experiments with similar results, and data are shown as means ± SD. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. h.p.i., h postinfection.
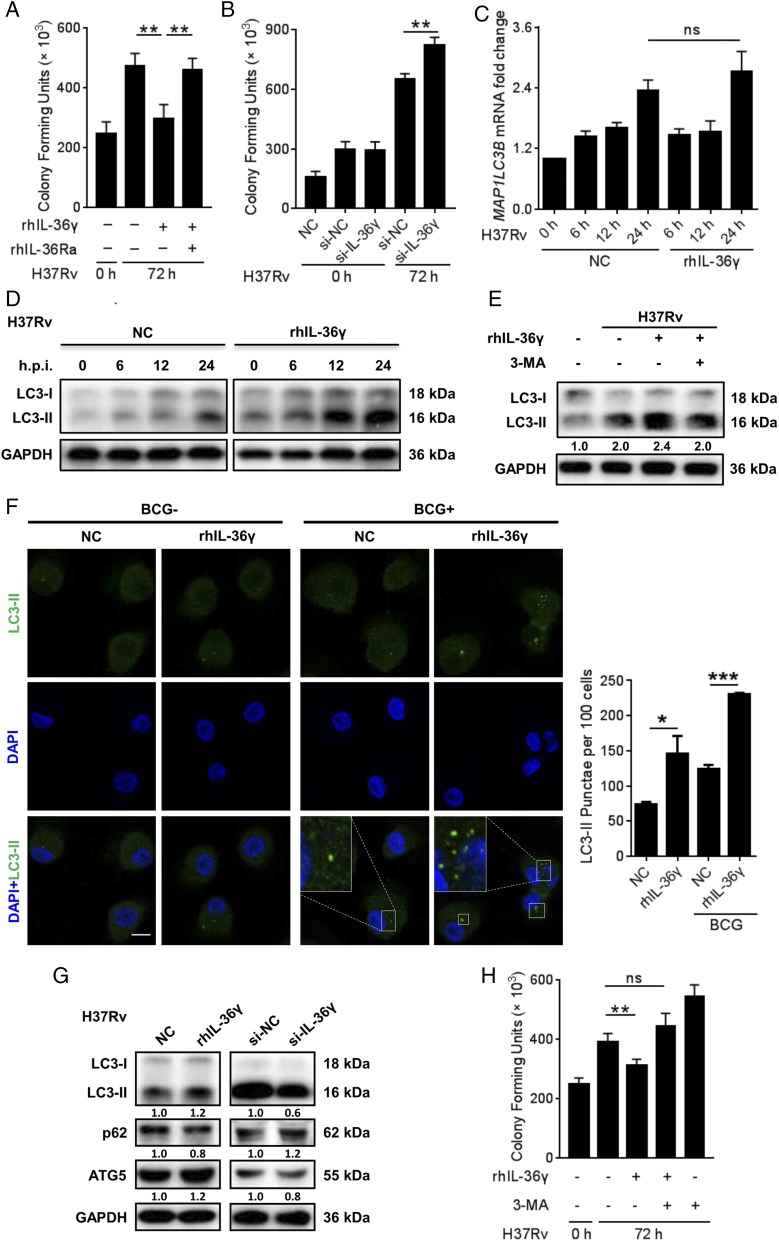
IL-36γ–induced autophagy contributed to defense against intracellular Mycobacteria
The M. tuberculosis–induced IL-36γ elevation inspired us to investigate its role in antituberculosis immunity of macrophages. Following exclusion of possible cell death influence (Supplemental Fig. 1B), CFU assays showed that rhIL-36γ treatment enhanced uptake of BCG but not H37Rv by macrophages (Supplemental Fig. 1C, 1D), but further inhibited intracellular survival of both strains (Fig. 3A, Supplemental Fig. 1E). Such effects depended on IL-36/IL-36R interactions, as rhIL-36Ra impaired IL-36γ–inhibited intracellular survival of H37Rv. Consistently, the intracellular survival of H37Rv increased following IL-36γ silence (Fig. 3B, Supplemental Fig. 1F, 1G). These data indicated the important role of IL-36γ in M. tuberculosis restriction by macrophages.
FIGURE 3.
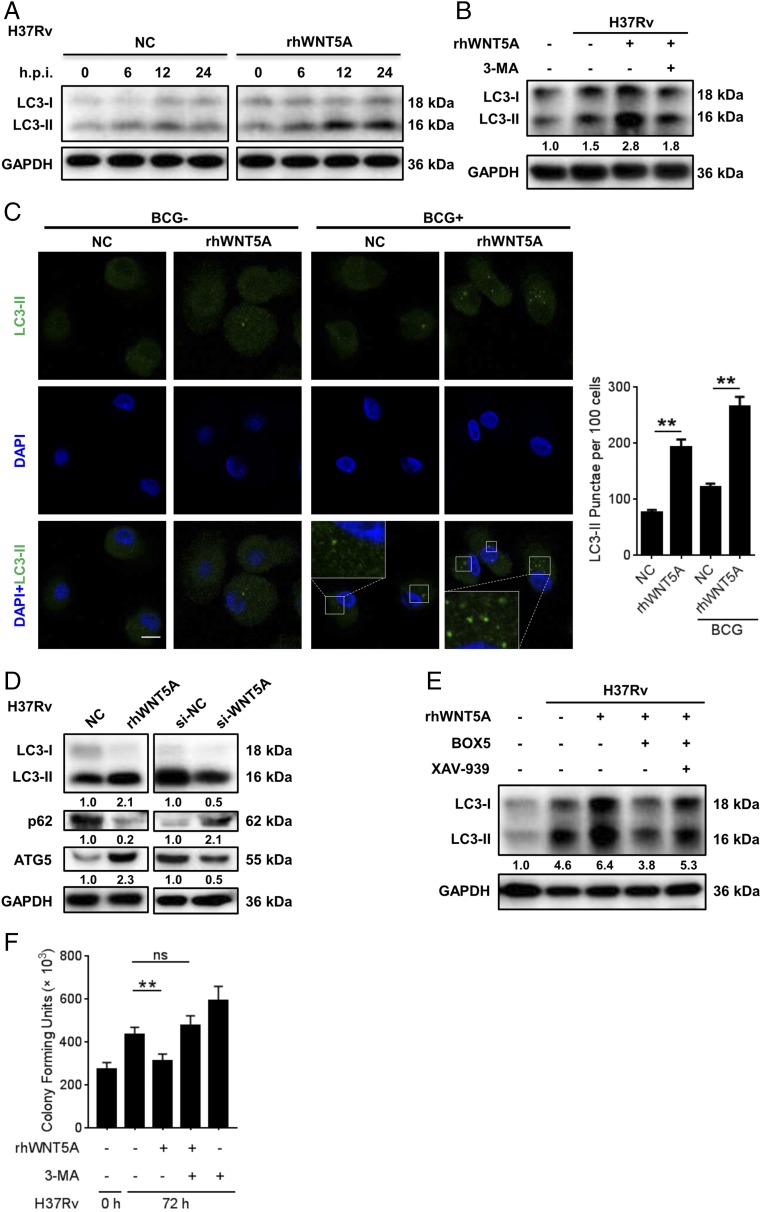
IL-36γ mediated autophagy to kill intracellular bacteria. (A and B) Intracellular H37Rv loads in MDMs treated with or without rhIL-36γ and rhIL-36Ra (A) or transfected with IL-36γ siRNA or control siRNA (B) were measured using CFU assays. (C) MAP1LC3B mRNA expression in H37Rv-infected MDMs and treatment with or without rhIL-36γ were analyzed using qRT-PCR. (D and E) Western blot analysis of endogenous LC3-I conversion to LC3-II in MDMs upon infection with H37Rv and treatment with rhIL-36γ at different time points, or treatment with combination of rhIL-36γ and 3-MA. (F) Percentages of MDMs with LC3+ puncta following infection with BCG and treatment with rhIL-36γ for 24 h were observed by confocal microscopy and measured from three representative experiments. Original magnification ×126. One of the representative results was shown. Scale bar, 10 μm. (G) Protein levels of LC3-II, p62, and ATG5 in MDMs infected with H37Rv and treated with rhIL-36γ (left), or transfected with IL-36γ siRNA (right), were measured by Western blot at 24 h postinfection. (H) H37Rv-infected MDMs treated with rhIL-36γ and/or 3-MA were plated for CFU assays. Data presented are from one of at least three independent experiments with similar results, and data are shown as means ± SD. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Subsequent exploration of the underlying mechanisms did not enhance proinflammatory mediator production by IL-36γ in response to H37Rv infection as described in human keratinocytes (24) or bone marrow–derived dendritic cells (10) (Supplemental Fig. 2A, 2B), but involvement of autophagy, the important bactericidal mechanism involved in restriction of intracellular M. tuberculosis (3, 25), was observed. We observed that rhIL-36γ did not result in significant induction of MAP1LC3B mRNA over control (Fig. 3C). By comparison, Western blot indicated that in the H37Rv-infected MDMs, rhIL-36γ promoted the conversion of LC3-I to LC3-II, the essential protein in autophagosome formation, which was attenuated by the specific autophagy inhibitor 3-MA (Fig. 3D, 3E, Supplemental Fig. 2C). Immunofluorescence staining showed a significant increase in the percentage of BCG-infected MDMs with LC3-positive puncta following rhIL-36γ (Fig. 3F), when treatment augmentation of autophagy was obviously diminished by IL-36γ siRNA employment (Supplemental Fig. 2D). Consistently, both ATG5 increase and p62 reduction indicated the enhancement of autophagy by rhIL-36γ (Fig. 3G). Such elevation of autophagy following H37Rv infection was abolished by IL-36γ silencing (Fig. 3G). The physiological relevance of IL-36γ–activated autophagy was elucidated by significantly increased H37Rv amounts in MDMs by 3-MA treatment (Fig. 3H).
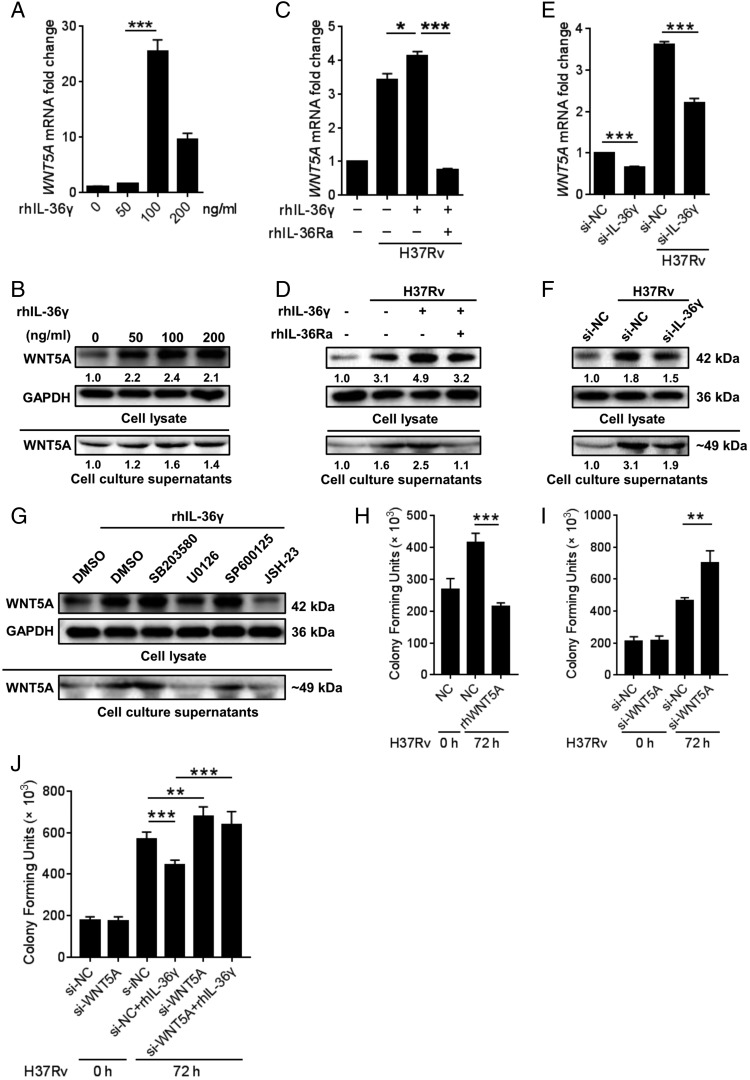
Induction of WNT5A by IL-36γ mediated the bactericidal activity
Studies have shown that IL-1β induces WNT5A expression to increase inflammatory cytokine secretion by SKOV-3 cells (18). Given the close relevance between IL-36γ and IL-1β, the association of WNT5A with IL-36γ in M. tuberculosis–infected macrophages was explored. Analysis of WNT5A expression of intracellular and supernatant was performed for Western blots because there was no appropriate human WNT5A ELISA kit for us. First, a significant increase of WNT5A induced by sequential concentrations of rhIL-36γ up to 100 ng/ml accompanied with or without H37Rv infection was observed (Fig. 4A–D, Supplemental Fig. 3A–D), which was abolished by IL-36γ silence (Fig. 4E, 4F). Such effects depended on IL-36/IL-36R interactions, as rhIL-36Ra impaired rhIL-36γ–stimulated WNT5A expression (Fig. 4C, 4D, Supplemental Fig. 3E, 3F). Subsequently, detection of the possible signal pathways regulating this process according to previous research (26) showed that only inhibiting ERK1/2 and p65, but not p38 and JNK signaling, significantly suppressed rhIL-36γ–induced WNT5A expression in M. tuberculosis–infected macrophages (Fig. 4G, Supplemental Fig. 3G).
FIGURE 4.
IL-36γ increased the expression of WNT5A in MDMs and inhibited H37Rv survival in MDMs. (A–F) qRT-PCR and Western blot analysis of WNT5A expression in MDMs treated with different levels of rhIL-36γ (A and B), with combination of rhIL-36γ and rhIL-36Ra and infected with H37Rv (C and D) or transfected with small interfering IL-36γ and then infected with H37Rv (E and F). (G) MDMs were pretreated with SB203580, U0126, SP600125, JSH-23, or LY294002, with DMSO as the solvent control for 1 h before infection with BCG in the presence of rhIL-36γ and above inhibitor for 48 h. WNT5A protein was analyzed by Western blot. (H–J) The numbers of viable intracellular bacteria in MDMs infected with H37Rv and treated with rhWNT5A (H), transfected with WNT5A siRNA (I), or combined with treatment of rhIL-36γ (J) were determined using CFU assays. Data presented are from one of least three independent experiments with similar results, and data are shown as means ± SD. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Potent antimycobacterial effects of IL-36R signaling (14, 15) and enhancement of bacterial clearance by IL-36γ (Fig. 3A, 3B) prompted us to explore if the accompanied WNT5A mediated the antimicrobial activity of IL-36γ. As shown in Fig. 4H, rhWNT5A significantly reduced the H37Rv load of MDMs, whereas WNT5A silence resulted in opposite results (Fig. 4I, Supplemental Fig. 3H) and, more importantly, also blocked the bacteriostatic effect of rhIL-36γ (Fig. 4J), suggesting the role of WNT5A as the effector of IL-36γ.
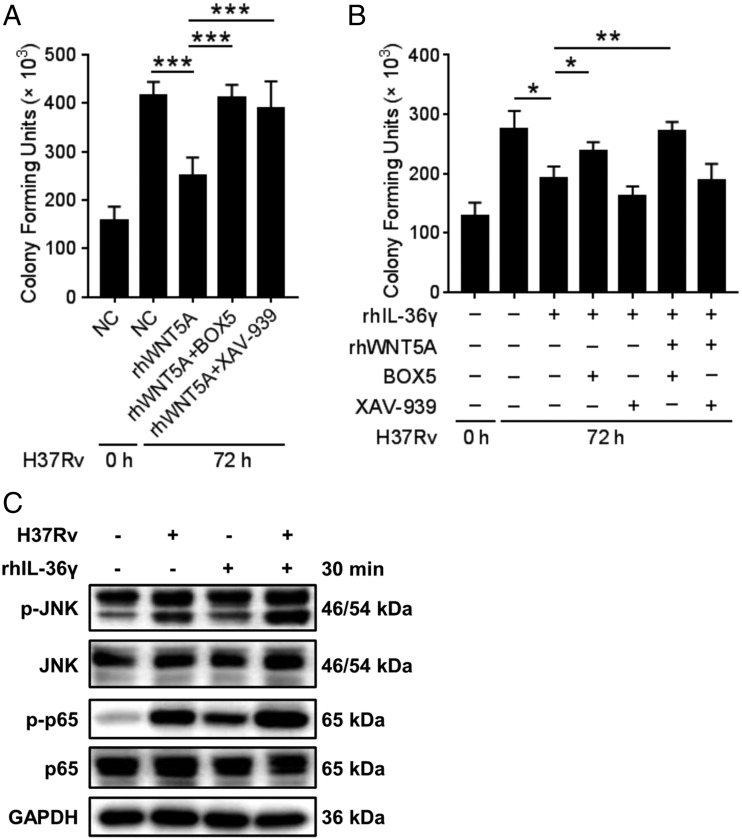
IL-36γ mediated the bactericidal activity partly through the WNT5A-noncanonical WNT pathway
To gain further insight into the mechanism, we investigated whether canonical and noncanonical WNT signaling pathways under the WNT5A are involved in inhibiting the bacterial growth effect of IL-36γ. CFU assays showed that rhWNT5A-induced killing of H37Rv was reduced significantly in the presence of both BOX5 (27), which inhibits the noncanonical WNT pathway, and XAV-939, the selective inhibitor of downstream β-catenin–mediated transcription of the canonical WNT pathway (28) (Fig. 5A), whereas treatment with XAV-939, but not BOX5, with or without rhWNT5A had no effect on rhIL-36γ–mediated bacterial killing (Fig. 5B). These results indicated the pivotal role of IL-36γ–upregulated WNT5A in macrophage defense against mycobacterial infection via the noncanonical WNT pathway. The results also discard the possibility that the presence of IL-36γ changes the signaling pathway mediated by WNT5A leading to bacterial killing. In addition, JNK and NF-κB are downstream pathways of noncanonical WNT signaling pathway (19, 29, 30). Ours results showed that both rhIL-36γ and rhWNT5A induced increased p-JNK and p-p65 (Fig. 5C, Supplemental Fig. 3I), which indicated that the bacteriostatic effect of IL-36γ on H37Rv-infected macrophages is mediated at least mainly through noncanonical WNT signaling.
FIGURE 5.
IL-36γ inhibited H37Rv survival in MDMs through the noncanonical WNT pathway of WNT5A. (A and B) CFU assays of H37Rv loads in MDMs treated with rhWNT5A combined with or without BOX5 or XAV-939 (A), or treated with rhIL-36γ combined with or without rhWNT5A, BOX5, or XAV-939 (B). (C) Phosphorylation of JNK and NF-κB in H37Rv-infected MDMs treated with or without rhIL-36γ was analyzed using Western blot. Data presented are from one of least three independent experiments with similar results, and data are shown as means ± SD. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
WNT5A induces autophagy via noncanonical WNT signaling to eliminate M. tuberculosis
Jati et al. (31) reported the WNT5A signaling-induced autophagic killing (xenophagy) of Pseudomonas aeruginosa and Streptococcus pneumoniae in RAW 264.7 cells. Meanwhile, autophagy is also the main bactericidal mechanism of IL-36γ (Fig. 3H). Thus, induction of autophagy in M. tuberculosis–infected MDMs by WNT5A was explored, and substantial promotion in the conversion of LC3-I to LC3-II by rhWNT5A in H37Rv-infected MDMs was observed (Fig. 6A, Supplemental Fig. 4A), which was markedly attenuated by 3-MA (Fig. 6B). Consistently, rhWNT5A treatment also reduced the p62 level and enhanced the ATG5 level (Fig. 6D), whereas such effects were reversed by WNT5A silencing (Fig. 6D). Furthermore, puncta of LC3-II were significantly increased after treatment with rhWNT5A, and the highest level appeared following the combinatory treatment of BCG and rhWNT5A (Fig. 6C), when the puncta of LC3-II was markedly enhanced by WNT5A siRNA (Supplemental Fig. 4B). The above effects depended on noncanonical WNT signaling, which could be suppressed by BOX5 but not XAV-939 (Fig. 6E). Furthermore, 3-MA significantly enhanced H37Rv survival in rhWNT5A-treated MDMs (Fig. 6F), indicating that WNT5A contributed to restriction of intracellular M. tuberculosis by inducing autophagy.
FIGURE 6.
WNT5A-induced autophagy in MDMs depended on the noncanonical WNT pathway. (A and B) Western blot analysis of endogenous LC3-I conversion to LC3-II in MDMs upon infection with H37Rv and treatment with rhWNT5A at different time points (A), or treatment with combination of rhWNT5A and 3-MA (B). (C) Percentages of MDMs with LC3+ puncta following infection with BCG and treatment with rhWNT5A for 24 h were observed by confocal microscopy and measured from three representative experiments. Original magnification ×126. One of the representative results was shown. Scale bar, 10 μm. (D) Protein levels of LC3-II, p62, and ATG5 in MDMs infected with H37Rv and treated with rhWNT5A (left), or transfected with WNT5A siRNA (right), were measured by Western blot. (E) LC3 conversion in H37Rv-MDMs treated with rhWNT5A combined with or without BOX5 or XAV-939 was analyzed by Western blot. (F) H37Rv-infected MDMs treated with rhWNT5A and/or 3-MA were plated for CFU assays. Data presented are from one of least three independent experiments with similar results, and data are shown as means ± SD. Data are shown as means ± SEM. **p ≤ 0.01.
WNT5A mediated activation of autophagy through the COX-2/AKT/mTOR axis
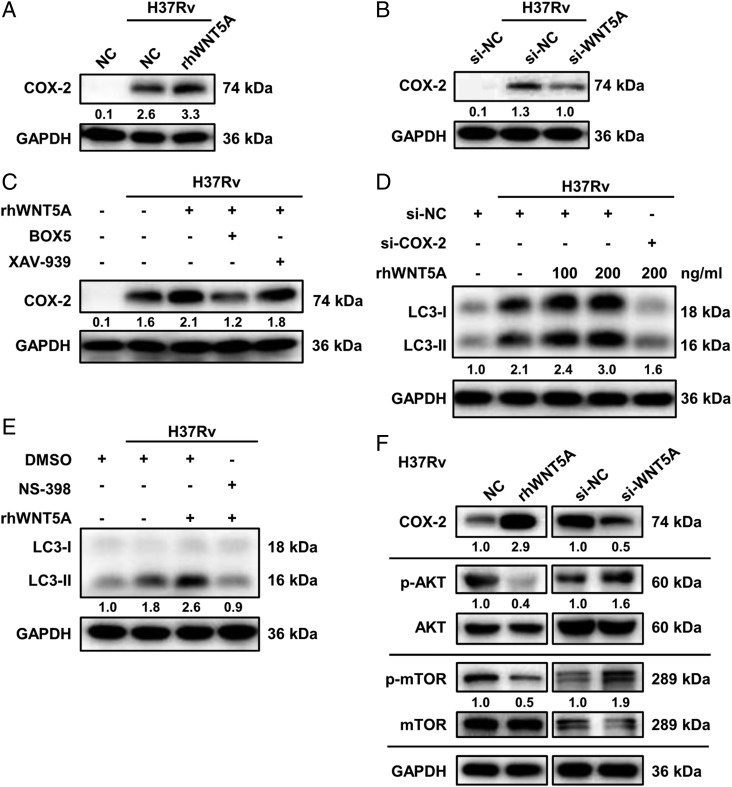
Next, the downstream factor responsible for WNT5A-mediated autophagy was explored. It was reported that expression of cyclooxygenase 2 (COX-2), a crucial catalyst in prostanoid synthesis, increased upon WNT5A exposure (32, 33). Consistently, our results indicated that 24 h of rhWNT5A stimulation increased COX-2 expression substantially (Fig. 7A), which was abrogated by WNT5A siRNA (Fig. 7B). Furthermore, WNT5A could activate NF-κB signaling (Supplemental Fig. 3I), whereas suppression of the p65/RelA subunit via specific siRNA (inhibitory effects were shown in Supplemental Fig. 4C, 4D) or JSH-23 attenuated the WNT5A-induced increase of COX-2 (Supplemental Fig. 4E, 4F), similar to that previously reported in endothelial cells (34) and consistent with the presence of NF-κB response elements within the COX-2 gene promoter (32, 35). This process depended mainly on β-catenin–independent signaling demonstrated by BOX5 but not XAV-939 use (Fig. 7C). COX-2 could be a downstream molecule involved in WNT5A function, because pretreatment of MDMs with NS-398, a COX-2 inhibitor, or COX-2 siRNA (inhibitory effects were shown in Supplemental Fig. 4G) resulted in remarkable suppression of WNT5A-mediated autophagy (Fig. 7D, 7E). These observations demonstrated that COX-2 participates in the regulation of autophagy induced by WNT5A following M. tuberculosis infection.
FIGURE 7.
WNT5A triggered autophagy via the COX-2/AKT/mTOR signaling pathway. (A and B) COX-2 expression in H37Rv-infected MDMs treated with or without rhWNT5A (A) or transfected with WNT5A siRNA or control siRNA (B) was detected by Western blot. (C) COX-2 expression in H37Rv-MDMs treated with rhWNT5A combined with or without BOX5 or XAV-939 was analyzed by Western blot. (D) LC3 conversion in MDMs transfected with COX-2 siRNA or control siRNA before infection with H37Rv and incubated rhWNT5A was analyzed by Western blot. (E) LC3 conversion in MDMs pretreated with NS-398, with DMSO as the solvent control before infection with H37Rv and incubated with combination of rhWNT5A and NS-398, was analyzed by Western blot. (F) Protein levels of COX-2, p-AKT/AKT, and p-mTOR/mTOR in MDMs infected with H37Rv and treated with rhWNT5A (left), or transfected with WNT5A siRNA (right), were measured by Western blot. All the samples of Western blot were collected and tested after 24 h of infection. Data presented are from one of least three independent experiments with similar results, and data are shown as means ± SD.
It has recently been reported that activation of autophagy by COX-2 in macrophages is through AKT/mTOR signaling to suppress mycobacterial survival in bone marrow–derived macrophages (36). Consistently, a decrease in activation of AKT and mTOR was observed in MDMs exposed to rhWNT5A, whereas small interfering WNT5A (si-WNT5A) upregulated their levels (Fig. 7F), indicating that WNT5A induced autophagy via upregulation of COX-2 and subsequent inactivation of AKT/mTOR signaling.
IL-36γ–mediated activation of autophagy depended on WNT5A
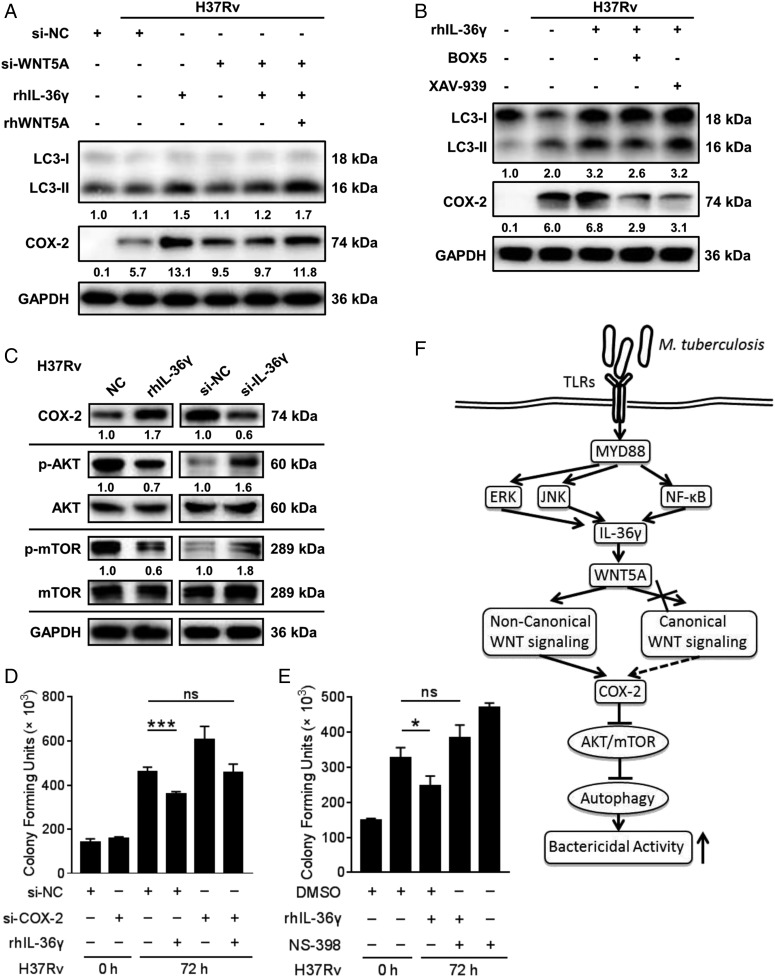
The induction of WNT5A by IL-36γ, and both of them exerting autophagy-mediated bactericidal activities, prompted us to explore if IL-36γ also activates autophagy through the WNT5A-induced COX-2/AKT/mTOR axis in H37Rv-infected MDMs. First, the role of WNT5A in IL-36γ–induced autophagy was measured in MDMs. As expected, si-WNT5A reduced rhIL-36γ–mediated LC3-II conversion (Fig. 8A). With WNT5A silencing, rhIL-36γ failed to convert LC3-I to LC3-II, which was restored with rhWNT5A and rhIL-36γ stimulation together, consistent with the expression of COX-2 (Fig. 8A). Furthermore, treating macrophages with BOX5 but not XAV-939 reduced rhIL-36γ–mediated LC3-II conversion (Fig. 8B). Unexpectedly, both BOX5 and XAV-939 reduced rhIL-36γ–mediated COX-2 expression (Fig. 8B), suggesting that this process depended on both WNT canonical and noncanonical pathways. rhIL-36γ increased the COX-2 level and reduced AKT and mTOR activation, which were reversed by silencing IL-36γ (Fig. 8C). Furthermore, both NS-398 and small interfering COX-2 significantly enhanced H37Rv survival in rhIL-36γ-treated MDMs (Fig. 8D, 8E), amply highlighting the protective role of IL-36γ through regulating autophagy via induction of COX-2 expression. Altogether, these results demonstrated that IL-36γ–mediated induction of autophagy relied on the release of the WNT5A ligand, which functions through the COX-2/AKT/mTOR axis.
FIGURE 8.
Requirement for WNT5A in IL-36γ mediated autophagic killing of H37Rv. (A and B) LC3-II and COX-2 levels in MDMs transfected with si-WNT5A or a control siRNA before infection with H37Rv and stimulated with rhIL-36γ and/or rhWNT5A (A), or incubated with BOX5 or XAV-939 before infection with H37Rv and stimulated with combination of rhIL-36γ and BOX5/XAV-939 (B), were measured by Western blot. (C) Protein levels of COX-2, p-AKT/AKT, and p-mTOR/mTOR in MDMs infected with H37Rv and treated with rhIL-36γ (left), or transfected with IL-36γ siRNA (right), were measured by Western blot. (D and E) The numbers of viable intracellular bacteria in MDMs infected with H37Rv and transfected with COX-2 siRNA or combined with rhIL-36γ treatment (D), or pretreated with NS-398 with DMSO as the solvent control before infection with H37Rv and then treated with combination of rhIL-36γ and NS-398 (E), were determined using CFU assays. (F) Illustration of model of IL-36γ promotes killing of M. tuberculosis by macrophages via the WNT5A-induced noncanonical WNT signaling pathway. Data presented are from one of least three independent experiments with similar results, and data are shown as means ± SD. *p ≤ 0.05, ***p ≤ 0.001.
Discussion
Although the protective role of IL-36γ against M. tuberculosis through regulation of oxysterol and AP expression has been reported (14, 15), the underlying mechanisms are not yet fully elucidated. In this study, we showed a novel, autophagy-involved bactericidal mechanism of IL-36γ in human macrophages. IL-36γ induced WNT5A to suppress intracellular M. tuberculosis growth by regulating the formation of autolysosomes through noncanonical WNT signaling and the COX-2/AKT/mTOR pathway. These results described the novel activity of IL-36γ in the induction of autophagy as its bactericidal mechanism in M. tuberculosis–infected MDMs.
The IL-36 family consists of potent proinflammatory IL-36α, IL-36β, and IL-36γ and the antagonist of IL-36R signaling, IL-36Ra. IL-36 cytokines contribute to the pathogenesis of human diseases owing to their unique imbalance between agonist and antagonist (37, 38). In this study, the substantial increase in IL-36A, IL-36G, and IL-36R and the highest ratio of IL-36G/IL-36RA compared with other members in the PBMCs of APT suggested the immune regulatory role of IL-36γ. Unexpectedly, although we have demonstrated that IL-36G mRNA is induced by both BCG and M. tuberculosis infection, the kinetics of induction are quite different, with induction starting to decrease from 24 h after BCG infection, in comparison with continuous elevation until 72 h after H37Rv infection, at the end of the observation. One possible explanation for this difference is the existence of the early secreted antigenic target protein 6, one of the key mediators for mycobacterial pathogenicity, also one of the most prominent Ags expressed by virulent M. tuberculosis but not by avirulent BCG (39). Besides induction of pathogenicity, early secreted antigenic target protein 6 and other virulent factors expressed by H37Rv may also induce the expressive differences in factors involved in immunologic defense. Consistently, both bacterial flagellin and the TLR 1/2 agonist, PAM3Cys, also induced IL-36G mRNA at 4 h, with mRNA levels returning to baseline by 18 h, similar with our observations after BCG infection (40). Furthermore, although the mechanism of transcription regulation of the IL-36G was not yet revealed, previous studies established that endotoxin triggers transient transcription and steady-state levels of IL-1B mRNA which accumulates for 4 h, followed by a rapid decrease due to synthesis of a transcriptional repressor and/or a decrease in mRNA t1/2 (41). Thus, another possible reason is that IL-36G cannot overcome an intrinsic inhibition to process precursor mRNA. The continuous induction by H37Rv might be a more sustained response over a longer period, which could be advantageous for maintaining the defenses of macrophages against infection. Thus, such difference in IL-36γ may be the host defensive reaction responding to M. tuberculosis infection. Intriguingly, we found the kinetics of intracellular expression and secretion of IL-36γ protein show discord in H37Rv-infected cells. The release of IL-36γ over time from macrophages continued to increase in the culture supernatant, whereas no increase was observed in the intracellular IL-36γ, which, instead significantly decreased within 24 h after the infection. These observations suggested that the secretion mechanism of IL-36 family members, which is poorly understood at present, is complex. In addition, IL-36γ significantly increased the phagocytosis of BCG but not H37Rv by MDMs, suggesting the existence of some escape strategy of virulent H37Rv. Further mechanistic studies will contribute to understanding of the interaction between the host and M. tuberculosis.
Previously, research has shown that WNT5A was observed in PBMCs and various immune cells (20, 23, 42), induced by inflammatory conditions or proinflammatory cytokines such as IL-1β and TNF-α (17, 18), and involved in macrophage differentiation (43), survival (20), cytokine production, and phagocytosis (44), suggesting its crucial role in the maintenance of macrophage immune function. Recently, Jati S. (31) reported that WNT5A induced autophagy in bone marrow–derived macrophages through Rac1-Disheveled, the well-known intermediates of WNT5A noncanonical signaling, enhancing clearance of Pseudomonas aeruginosa and S. pneumoniae infection. In this study, we confirmed that IL-36γ suppresses M. tuberculosis growth in macrophages by triggering WNT5A-induced noncanonical WNT signaling-mediated autophagy. Our study revealed that IL-36γ or WNT5A can activated the noncanonical WNT pathway by inducing JNK and p65 phosphorylation (19, 29, 30), although the specific downstream mechanisms are worthy to be studied. Furthermore, we have filled in the pathway of WNT5A-induced autophagy, verifying it was mediated by COX-2, a key rate-limiting enzyme in the arachidonic acid pathway, and subsequent inactivation of AKT/mTOR signaling. Concordant with regulation of autophagy, WNT5A increased COX-2 expression in MDMs depending on the noncanonical WNT pathway.
Our data indicated, by observation of reduction in IL-36γ–induced LC3-II following silencing of WNT5A, that IL-36γ activates autophagy through upstream regulation of WNT5A. COX-2 protein expression was also upregulated by IL-36γ; however, this was suppressed by both the inhibitors of the canonical pathway and the noncanonical pathway of WNT5A, suggesting the incomplete dependence of COX-2 induction by IL-36γ on the noncanonical WNT signaling. In any case, the detailed regulatory function of IL-36γ in macrophages during infection and anti–M. tuberculosis immunity deserves further study. Previous studies have shown that WNT5A regulates the production of inflammatory factors and acts as a critical determinant of the inflammatory response (45, 46). Therefore, it is interesting and meaningful to clarify whether WNT5A in turn regulates the expression or function of IL-36γ. As mentioned above, there are great diversity in the distribution of IL-36R between mice and human (13). In this case, we need a more appropriate approach to further study the association and antimicrobial effects of IL-36γ and WNT5A in an in vivo setting.
Collectively, IL-36γ provided protection upon M. tuberculosis infection via autophagy to enhance bacterial clearance in macrophages. A unique regulatory mechanism leading to WNT5A production, which later induces autophagy and restricts M. tuberculosis growth, was identified. Our data provided new insight into the IL-36γ–driven regulation of anti-infective function by macrophages, which might offer a novel therapeutic target for the treatment of tuberculosis or other infectious diseases.
Supplementary Material
Acknowledgments
We thank Prof. Xiao-Ping Zhong (Department of Immunology, Duke University Medical Center, Durham, NC), for his valuable instruction, and our colleagues, for their helpful advice.
This work was supported by National Science and Technology Major Project (2017ZX10201301-008), the National Natural Science Foundation of China (81772150 and 81571951), the Guangdong Natural Science Foundation (2016A030311001), the Science and Technology Project of Guangdong Province (2017A020212007), and the Science and Technology Project of Guangzhou (201707010215).
The online version of this article contains supplemental material.
- AP
- antimicrobial peptide
- APT
- active pulmonary tuberculosis
- BCG
- bacillus Calmette–Guérin
- COX-2
- cyclooxygenase 2
- IL-36Ra
- IL-36R antagonist
- 3-MA
- 3-methyladenine
- MDM
- monocyte-derived macrophage
- qRT-PCR
- quantitative real time PCR
- rh
- recombinant human
- siRNA
- small interfering RNA
- si-WNT5A
- small interfering WNT5A.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.World Health Organization 2018. Global Tuberculosis Report 2018. WHO, Geneva. [Google Scholar]
- 2.Danelishvili L., Everman J., Bermudez L. E. 2016. Mycobacterium tuberculosis PPE68 and Rv2626c genes contribute to the host cell necrosis and bacterial escape from macrophages. Virulence 7: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco L. H., Nair V. R., Scharn C. R., Xavier R. J., Torrealba J. R., Shiloh M. U., Levine B. 2017. The ubiquitin ligase Smurf1 functions in selective autophagy of Mycobacterium tuberculosis and anti-tuberculous host defense. [Published erratum appears in 2017 Cell Host Microbe 22: 421–423.] Cell Host Microbe 21: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNab F. W., Ewbank J., Howes A., Moreira-Teixeira L., Martirosyan A., Ghilardi N., Saraiva M., O’Garra A. 2014. Type I IFN induces IL-10 production in an IL-27-independent manner and blocks responsiveness to IFN-γ for production of IL-12 and bacterial killing in Mycobacterium tuberculosis-infected macrophages. J. Immunol. 193: 3600–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verway M., Bouttier M., Wang T. T., Carrier M., Calderon M., An B. S., Devemy E., McIntosh F., Divangahi M., Behr M. A., White J. H. 2013. Vitamin D induces interleukin-1β expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 9: e1003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kundu M., Pathak S. K., Kumawat K., Basu S., Chatterjee G., Pathak S., Noguchi T., Takeda K., Ichijo H., Thien C. B., et al. 2009. A TNF- and c-Cbl-dependent FLIP(S)-degradation pathway and its function in Mycobacterium tuberculosis-induced macrophage apoptosis. Nat. Immunol. 10: 918–926. [DOI] [PubMed] [Google Scholar]
- 7.Mayer-Barber K. D., Barber D. L., Shenderov K., White S. D., Wilson M. S., Cheever A., Kugler D., Hieny S., Caspar P., Núñez G., et al. 2010. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184: 3326–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang R., Yang E., Shen L., Modlin R. L., Shen H., Chen Z. W. 2018. IL-12+IL-18 cosignaling in human macrophages and lung epithelial cells activates cathelicidin and autophagy, inhibiting intracellular mycobacterial growth. J. Immunol. 200: 2405–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassoy E. Y., Towne J. E., Gabay C. 2018. Regulation and function of interleukin-36 cytokines. Immunol. Rev. 281: 169–178. [DOI] [PubMed] [Google Scholar]
- 10.Vigne S., Palmer G., Lamacchia C., Martin P., Talabot-Ayer D., Rodriguez E., Ronchi F., Sallusto F., Dinh H., Sims J. E., Gabay C. 2011. IL-36R ligands are potent regulators of dendritic and T cells. Blood 118: 5813–5823. [DOI] [PubMed] [Google Scholar]
- 11.Ramadas R. A., Ewart S. L., Medoff B. D., LeVine A. M. 2011. Interleukin-1 family member 9 stimulates chemokine production and neutrophil influx in mouse lungs. Am. J. Respir. Cell Mol. Biol. 44: 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vigne S., Palmer G., Martin P., Lamacchia C., Strebel D., Rodriguez E., Olleros M. L., Vesin D., Garcia I., Ronchi F., et al. 2012. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood 120: 3478–3487. [DOI] [PubMed] [Google Scholar]
- 13.Foster A. M., Baliwag J., Chen C. S., Guzman A. M., Stoll S. W., Gudjonsson J. E., Ward N. L., Johnston A. 2014. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J. Immunol. 192: 6053–6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahsan F., Moura-Alves P., Guhlich-Bornhof U., Klemm M., Kaufmann S. H., Maertzdorf J. 2016. Role of interleukin 36γ in host defense against tuberculosis. J. Infect. Dis. 214: 464–474. [DOI] [PubMed] [Google Scholar]
- 15.Ahsan F., Maertzdorf J., Guhlich-Bornhof U., Kaufmann S. H. E., Moura-Alves P. 2018. IL-36/LXR axis modulates cholesterol metabolism and immune defense to Mycobacterium tuberculosis. Sci. Rep. 8: 1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Günther S., Sundberg E. J. 2014. Molecular determinants of agonist and antagonist signaling through the IL-36 receptor. J. Immunol. 193: 921–930. [DOI] [PubMed] [Google Scholar]
- 17.Sonomoto K., Yamaoka K., Oshita K., Fukuyo S., Zhang X., Nakano K., Okada Y., Tanaka Y. 2012. Interleukin-1β induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2 pathway. Arthritis Rheum. 64: 3355–3363. [DOI] [PubMed] [Google Scholar]
- 18.Arabzadeh S., Hossein G., Zarnani A. H. 2016. Wnt5A exerts immunomodulatory activity in the human ovarian cancer cell line SKOV-3. Cell Biol. Int. 40: 177–187. [DOI] [PubMed] [Google Scholar]
- 19.Chae W. J., Bothwell A. L. M. 2018. Canonical and non-canonical wnt signaling in immune cells. Trends Immunol. 39: 830–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naskar D., Maiti G., Chakraborty A., Roy A., Chattopadhyay D., Sen M. 2014. Wnt5a-Rac1-NF-κB homeostatic circuitry sustains innate immune functions in macrophages. J. Immunol. 192: 4386–4397. [DOI] [PubMed] [Google Scholar]
- 21.Shao Y., Zheng Q., Wang W., Xin N., Song X., Zhao C. 2016. Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget 7: 67674–67684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandenburg J., Reiling N. 2016. The wnt blows: on the functional role of wnt signaling in Mycobacterium tuberculosis infection and beyond. Front. Immunol. 7: 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumenthal A., Ehlers S., Lauber J., Buer J., Lange C., Goldmann T., Heine H., Brandt E., Reiling N. 2006. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 108: 965–973. [DOI] [PubMed] [Google Scholar]
- 24.Carrier Y., Ma H. L., Ramon H. E., Napierata L., Small C., O’Toole M., Young D. A., Fouser L. A., Nickerson-Nutter C., Collins M., et al. 2011. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J. Invest. Dermatol. 131: 2428–2437. [DOI] [PubMed] [Google Scholar]
- 25.Ouimet M., Koster S., Sakowski E., Ramkhelawon B., van Solingen C., Oldebeken S., Karunakaran D., Portal-Celhay C., Sheedy F. J., Ray T. D., et al. 2016. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol. 17: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopetuso L. R., Chowdhry S., Pizarro T. T. 2013. Opposing functions of classic and novel IL-1 family members in gut Health and disease. Front. Immunol. 4: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenei V., Sherwood V., Howlin J., Linnskog R., Säfholm A., Axelsson L., Andersson T. 2009. A t-butyloxycarbonyl-modified Wnt5a-derived hexapeptide functions as a potent antagonist of Wnt5a-dependent melanoma cell invasion. Proc. Natl. Acad. Sci. USA 106: 19473–19478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crespo M., Vilar E., Tsai S. Y., Chang K., Amin S., Srinivasan T., Zhang T., Pipalia N. H., Chen H. J., Witherspoon M., et al. 2017. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. [Published erratum appears in 2018 Nat. Med. 24: 526.] Nat. Med. 23: 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villaseñor T., Madrid-Paulino E., Maldonado-Bravo R., Urbán-Aragón A., Pérez-Martínez L., Pedraza-Alva G. 2017. Activation of the wnt pathway by Mycobacterium tuberculosis: a wnt-wnt situation. Front. Immunol. 8: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouchi N., Higuchi A., Ohashi K., Oshima Y., Gokce N., Shibata R., Akasaki Y., Shimono A., Walsh K. 2010. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329: 454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jati S., Kundu S., Chakraborty A., Mahata S. K., Nizet V., Sen M. 2018. Wnt5A signaling promotes defense against bacterial pathogens by activating a host autophagy circuit. Front. Immunol. 9: 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao C., Bu X., Wang W., Ma T., Ma H. 2014. GEC-derived SFRP5 inhibits Wnt5a-induced macrophage chemotaxis and activation. PLoS One 9: e85058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halleskog C., Schulte G. 2013. WNT-3A and WNT-5A counteract lipopolysaccharide-induced pro-inflammatory changes in mouse primary microglia. J. Neurochem. 125: 803–808. [DOI] [PubMed] [Google Scholar]
- 34.Kim J., Kim J., Kim D. W., Ha Y., Ihm M. H., Kim H., Song K., Lee I. 2010. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J. Immunol. 185: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 35.Kang Y. J., Mbonye U. R., DeLong C. J., Wada M., Smith W. L. 2007. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog. Lipid Res. 46: 108–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong W., Wen Q., Du X., Wang J., He W., Wang R., Hu S., Zhou X., Yang J., Gao Y., Ma L. 2018. Novel function of cyclooxygenase-2: suppressing mycobacteria by promoting autophagy via the protein kinase B/mammalian target of rapamycin pathway. J. Infect. Dis. 217: 1267–1279. [DOI] [PubMed] [Google Scholar]
- 37.Tortola L., Rosenwald E., Abel B., Blumberg H., Schäfer M., Coyle A. J., Renauld J. C., Werner S., Kisielow J., Kopf M. 2012. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J. Clin. Invest. 122: 3965–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumberg H., Dinh H., Trueblood E. S., Pretorius J., Kugler D., Weng N., Kanaly S. T., Towne J. E., Willis C. R., Kuechle M. K., et al. 2007. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J. Exp. Med. 204: 2603–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandey H., Tripathi S., Srivastava K., Tripathi D. K., Srivastava M., Kant S., Srivastava K. K., Arora A. 2017. Characterization of culture filtrate proteins Rv1197 and Rv1198 of ESAT-6 family from Mycobacterium tuberculosis H37Rv. Biochim. Biophys. Acta, Gen. Subj. 1861: 396–408. [DOI] [PubMed] [Google Scholar]
- 40.Kovach M. A., Singer B. H., Newstead M. W., Zeng X., Moore T. A., White E. S., Kunkel S. L., Peters-Golden M., Standiford T. J. 2016. IL-36γ is secreted in microparticles and exosomes by lung macrophages in response to bacteria and bacterial components. J. Leukoc. Biol. 100: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinarello C. A. 1996. Biologic basis for interleukin-1 in disease. Blood 87: 2095–2147. [PubMed] [Google Scholar]
- 42.Chakraborty A., Kurati S. P., Mahata S. K., Sundar S., Roy S., Sen M. 2017. Wnt5a signaling promotes host defense against Leishmania donovani infection. J. Immunol. 199: 992–1002. [DOI] [PubMed] [Google Scholar]
- 43.Sessa R., Yuen D., Wan S., Rosner M., Padmanaban P., Ge S., Smith A., Fletcher R., Baudhuin-Kessel A., Yamaguchi T. P., et al. 2016. Monocyte-derived Wnt5a regulates inflammatory lymphangiogenesis. Cell Res. 26: 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maiti G., Naskar D., Sen M. 2012. The Wingless homolog Wnt5a stimulates phagocytosis but not bacterial killing. Proc. Natl. Acad. Sci. USA 109: 16600–16605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rauner M., Stein N., Winzer M., Goettsch C., Zwerina J., Schett G., Distler J. H., Albers J., Schulze J., Schinke T., et al. 2012. WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J. Bone Miner. Res. 27: 575–585. [DOI] [PubMed] [Google Scholar]
- 46.Li B., Shi Y., Shu J., Gao J., Wu P., Tang S. J. 2013. Wingless-type mammary tumor virus integration site family, member 5A (Wnt5a) regulates human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120)-induced expression of pro-inflammatory cytokines via the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) signaling pathways. J. Biol. Chem. 288: 13610–13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.