Abstract
Objectives
During initial assessment of trauma patients, vital signs do not identify all patients with life‐threatening hemorrhage. We hypothesized that a novel vital sign, muscle oxygen saturation (SmO2), could provide independent diagnostic information beyond routine vital signs for identification of hemorrhaging patients who require packed red blood cell (RBC) transfusion.
Methods
This was an observational study of adult trauma patients treated at a Level I trauma center. Study staff placed the CareGuide 1100 tissue oximeter (Reflectance Medical Inc., Westborough, MA), and we analyzed average values of SmO2, systolic blood pressure (sBP), pulse pressure (PP), and heart rate (HR) during 10 minutes of early emergency department evaluation. We excluded subjects without a full set of vital signs during the observation interval. The study outcome was hemorrhagic injury and RBC transfusion ≥ 3 units in 24 hours (24‐hr RBC ≥ 3). To test the hypothesis that SmO2 added independent information beyond routine vital signs, we developed one logistic regression model with HR, sBP, and PP and one with SmO2 in addition to HR, sBP, and PP and compared their areas under receiver operating characteristic curves (ROC AUCs) using DeLong's test.
Results
We enrolled 487 subjects; 23 received 24‐hr RBC ≥ 3. Compared to the model without SmO2, the regression model with SmO2 had a significantly increased ROC AUC for the prediction of ≥ 3 units of 24‐hr RBC volume, 0.85 (95% confidence interval [CI], 0.75–0.91) versus 0.77 (95% CI, 0.66–0.86; p < 0.05 per DeLong's test). Results were similar for ROC AUCs predicting patients (n = 11) receiving 24‐hr RBC ≥ 9.
Conclusions
SmO2 significantly improved the diagnostic association between initial vital signs and hemorrhagic injury with blood transfusion. This parameter may enhance the early identification of patients who require blood products for life‐threatening hemorrhage.
To help clinicians determine which trauma patients have life‐threatening hemorrhage, several clinical scores have been developed for predicting the need for massive blood transfusion.1 However, these scores cannot be computed immediately upon emergency department (ED) arrival because they require either blood testing or imaging results. An alternative methodology that could be used upon initial evaluation (or even during prehospital transport) for detecting significant risk of exsanguination could be useful. Near‐infrared spectrometry (NIRS), which has shown to correlate with high acuity and poor outcomes in trauma patients,2, 3, 4 has been studied specifically as a triage tool in one study, where it was found to predict the need for blood transfusion in combat casualties who lacked early hypotension.5 To further investigate whether NIRS offers potential value as a triage tool in the preliminary assessment of trauma patients upon ED arrival (i.e., prior to the availability of any diagnostic testing except for routine vital signs), we undertook an investigation, testing the hypothesis that NIRS tissue oxygen monitoring improves the early identification of patients with major hemorrhage compared with initial vital signs alone.
Materials and methods
Study Setting and Population
We received protocol approval from the institutional review board (IRB), including a waiver of informed consent as per 45 CFR § 46.116(d). We studied a convenience sample of trauma patients ≥ 18 years of age evaluated in the ED of a Level I trauma center. A priori exclusion criteria were: 1) transfer from another hospital if prior workup already ruled out hemorrhagic injury; 2) no suitable NIRS sensor placement site overlying the deltoid or thigh due to either tattoos, visible skin injury, gross blood, visible rash, clothing, request of treating clinician, or evident hirsutism (when patients had visible body hair, we did not attempt to place the oximeter because subsequent removal of the adhesive from hairy skin was expected to be painful, and the subjects had not provided consent for any painful procedure); 3) per manufacturer's recommendation, estimated body mass index < 19 or > 40 kg/m2; 4) minor trauma, e.g., fall from standing to flat ground; and 5) failure to record muscle oxygen saturation (SmO2), heart rate (HR), and blood pressure (BP) within a matching 10‐minute interval during the patient's initial evaluation.
Measurements
SmO2 was measured using the CareGuide 1100 tissue oximeter (Reflectance Medical, Inc., Westborough, MA) placed by dedicated study staff on skin overlying the deltoid or thigh. The sensor remained in place for a minimum of 3 minutes. The CareGuide sensor measures SmO2 using principles that are similar to other NIRS oximeters, while incorporating proprietary technology that is designed to eliminate spectral inference from skin pigmentation and fat.6
Vital signs were measured as per clinical routine using Solar patient monitors (General Electric, Milwaukee, WI). In most ED bays, data were electronically archived using BedMasterEx software (Excel Medical, Jupiter, FL). Unreliable vital sign data were identified and excluded using validated software algorithms.7 For ED bays that lacked the BedMasterEx system, we relied on the vital signs documented by ED nurses and corroborated by vital signs simultaneously documented by dedicated study staff.
Outcome
We studied the prediction of patients with hemorrhagic injuries and the receipt of ≥3 units of packed red blood cells (RBCs) in the first 24 hours. (Hemorrhagic injury was defined as any of the following: laceration or fracture of a solid organ; documented hematoma within the thorax, peritoneum, retroperitoneum, or pelvis; vascular injury that required operative repair or angioembolization; or limb amputation.) The secondary outcome was receipt of ≥9 units of RBCs in patients with hemorrhagic injury. Injuries, injury severity score, Glasgow coma scale, and operative interventions were obtained from the medical record (clinical documentation, radiology reports, and operative reports) and the trauma registry. Data were archived electronically using REDCap.8 We determined whether or not the patient had documented hemorrhagic injury by automated text search, searching for injuries that met the aforementioned criteria (all records were also jointly reviewed by two investigators to confirm that the automated text search had not omitted any applicable hemorrhagic injuries, nor included nonhemorrhagic injuries).
Patients who received RBCs but lacked a documented hemorrhagic injury were excluded from analysis, because of unresolvable uncertainty about whether the RBC transfusion was clinically indicated in the absence of explicitly hemorrhagic injuries.
Data Analysis
We computed the mean values of SmO2, HR, systolic BP (sBP), pulse pressure (PP = sBP – diastolic BP), and the shock index (SI = HR/sBP) measurements from a 10‐minute window starting upon the first simultaneous occurrence of a full set of HR, BP, and SmO2 values.
We applied DeLong's test to the areas under receiver operating characteristic curves (ROC AUCs) from two logistic regression models, the first using only routine vital signs (HR, sBP, PP) and the second adding the investigational metric (HR, sBP, PP, SmO2). The null hypothesis was that SmO2 did not provide additional diagnostic information compared with using routine vital signs alone.
Results
Between June 2012 and October 2014, we enrolled 487 subjects. Figure 1 shows the enrollment flowchart. Subjects were predominantly male (68%), mechanism of injury was predominantly blunt (90%), and median age was 47 years (interquartile range [IQR] = 31–64). Median injury severity score was 16 (IQR = 9–25) and mortality was 3%. There were 23 patients who received ≥3 units of RBCs within 24 hours and 11 who received ≥9 units.
Figure 1.
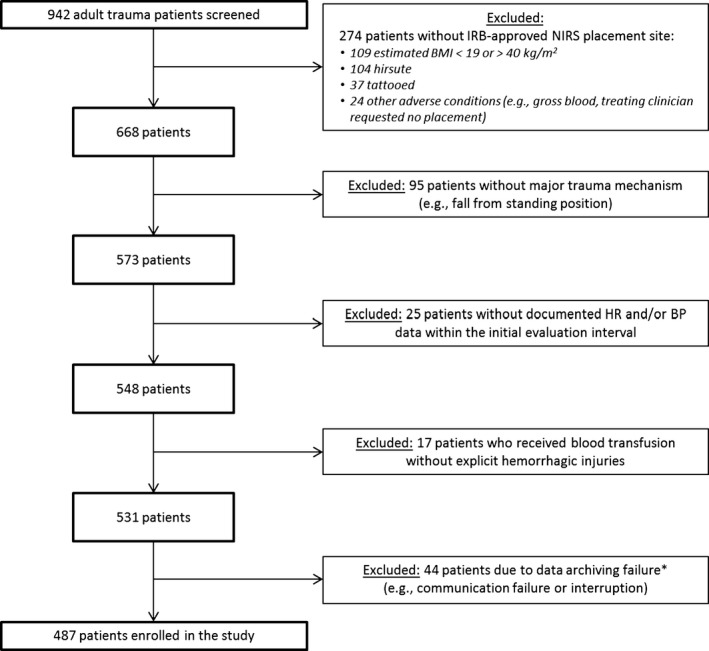
Flowchart of subject enrollment. *Data archiving failures involved the archiving system (ruggedized GoBook personal computer connected to the CareGuide SmO2 sensor) that we assembled for this investigation. For the final 16 months of the investigation, we reinforced the electronic and mechanical connectivity, and had only one additional subject with data archiving failure in that time interval. BMI = body mass index; BP = blood pressure; HR = heart rate; IRB = institutional review board; NIRS = near‐infrared spectrometry.
SmO2, BP, and HR were collected early in the subjects' clinical courses: the median time elapsed between the ED admission time and the onset of the initial evaluation time window (i.e., the 10‐minute window used for analysis) was 3.65 minutes (IQR = 1.85–6.22 minutes). Median values for vital signs and SmO2 are presented in Table 1.
Table 1.
Association of Initial Vital Signs and SmO2 With 24‐Hour RBC Transfusion Volume ≥ 3 Units
| Median (IQR) for Patients With 24‐Hour RBC Volume < 3 Units (n = 464) | Median (IQR) for Patients With 24‐Hour RBC Volume ≥ 3 Units (n = 23) | ROC AUCs (95% CIs) | |
|---|---|---|---|
| HR (beats/min) | 83 (70–96) | 99 (82–119) | 0.70 (0.56–0.81) |
| sBP (mm Hg) | 141 (127–158) | 136 (96–147) | 0.62 (0.47–0.75) |
| PP (mm Hg) | 61 (50–73) | 48 (33–63) | 0.68 (0.54–0.80) |
| SI ([beats/min]/[mm Hg]) | 0.59 (0.49–0.70) | 0.80 (0.63–0.94) | 0.75 (0.61–0.85) |
| SmO2 (%) | 67 (62–72) | 61 (50–64) | 0.76 (0.65–0.84) |
HR = heart rate; IQR = interquartile range; PP = pulse pressure (= sBP – diastolic blood pressure); RBC = packed red blood cells; ROC AUC = area under the receiver operating characteristic curve; sBP = systolic blood pressure; SI = shock index (= HR/sBP); SmO2 = muscle oxygen saturation.
In terms of diagnostic association between hemorrhagic injury with 24‐hour RBC transfusion volume ≥ 3 units:
The multivariate regression model using HR, sBP, and PP alone yielded a ROC AUC of 0.77 (95% confidence interval [CI], 0.66–0.86), which was similar to the ROC AUC for SI (Table 1).
The multivariate regression model using HR, sBP, and PP plus SmO 2 yielded a ROC AUC of 0.85 (95% CI, 0.75–0.91).
Per DeLong's test, these ROC AUCs were significantly different (p < 0.05).
Repeating the same analysis for the alternative RBC cutoff, i.e., ≥9 units of RBCs within 24 hours, we found similar results: the regression model that included SmO2 in addition to HR, sBP, and PP yielded an increased ROC AUC (0.89; 95% CI, 0.76–0.95) that was significantly greater (p < 0.05) than the ROC AUC (0.77; 95% CI, 0.61–0.87) of the regression model with HR, sBP, and PP alone.
Discussion
In this investigation, we found that SmO2 added significant discriminatory information beyond the initial values of HR and BP. The significantly higher ROC AUC implies that using SmO2 offered a higher combination of sensitivity and specificity than could be achieved using only routine vital signs.
Why would low SmO2 indicate a patient with life‐threatening hemorrhage, if routine vital signs are not patently abnormal? Presumably, such a patient would be physiologically compensating for blood loss with marked peripheral vasoconstriction, maintaining BP during blood loss, but at the expense of peripheral tissue hypoperfusion (and a resultant low SmO2). We note that some hemorrhagic patients with low SmO2 also had simultaneous hypotension, while others did not.
Our findings suggest that SmO2 can enhance initial vital signs, but cannot replace them: some hemorrhagic patients had hypotension but without low SmO2. We speculate that this reflects individual variability in vasoconstriction during blood loss:9 Patients with maximum vasoconstriction can maintain BP at the expense of peripheral perfusion/SmO2, while those patients with minimal vasoconstriction can maintain peripheral perfusion/SmO2 while experiencing earlier hypotension. (As well, there were hemorrhagic patients with neither hypotension nor reduced SmO2; possibly these patients had not yet suffered significant blood loss or had baseline hypertension and/or bradycardia that masked their progression into hypovolemic physiology.)
The current investigation provides evidence that NIRS oximetry can provide additional information for early detection of hemorrhage than initial routine vital signs alone. These findings are consistent with a prior report5 evaluating NIRS oximetry as a triage tool, which likewise found that it could predict the need for blood transfusion in patients who lacked early hypotension. The NIRS sensor is relatively easy to place, attaching to the patient's skin via an adhesive sleeve. A simple measure that can improve the early identification of patients with major hemorrhage may be particularly useful when caregivers are novice, distracted, or fatigued.
Limitations
A substantial number of subjects were excluded for lacking a suitable IRB‐approved NIRS placement site, and our outcome, blood transfusion, involved subjective clinical decision‐making; both factors limit the generalizability of the findings. This investigation only analyzed the initial ED assessment, and our findings do not address whether or not better information than initial vital signs alone translates into better clinical judgments and patient outcomes. We did not evaluate whether there is value of NIRS oximetry in later phases of trauma care when additional sources of diagnostic data are available, i.e., vital sign trends through time, lab results, such as lactate and base deficit, and imaging. Sources of diagnostic error for the CareGuide sensor, whether it is more reliable than other NIRS oximeters and how to interpret temporal trends in SmO2, were not investigated. We note that the State of Minnesota has added tissue spectroscopy to their official guidelines for “Tier‐One Trauma Team Activation Criteria.”10 Our findings corroborate the usefulness of this metric for early identification of major hemorrhage, but also highlight remaining questions about relying on this technology for clinical decision‐making.
Conclusions
We compared muscle oxygen saturation to heart rate, sBP, and pulse pressure alone in the early ED evaluation of trauma patients and found that use of muscle oxygen saturation significantly improved the diagnostic association between vital signs and hemorrhagic injury requiring blood transfusion. The results offered prima facie evidence that near‐infrared spectrometry might provide a tool for the early ED identification of patients with life‐threatening hemorrhage.
Academic Emergency Medicine 2016;23:353–357 © 2016 by the Society for Academic Emergency Medicine26743804
Presented at the 19th Annual New England Society for Academic Emergency Medicine Regional Conference (NERDS), Newton, MA, April 1, 2015; and the Society of Academic Emergency Medicine Annual Meeting, San Diego, CA, May 12–15, 2015.
This work was supported by the Combat Casualty Care Research Area Directorate of the U.S. Army Medical Research and Materiel Command, Fort Detrick, MD. The study sponsors did not have any role in the study design, data collection, analysis and interpretation of data, report writing, or decision to submit the article for publication. The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army or of the U.S. Department of Defense. This paper has been approved for public release with unlimited distribution.
The authors have no relevant financial information or potential conflicts to disclose.
References
- 1. Brockamp T, Nienaber U, Mutschler M, et al. Predicting on‐going hemorrhage and transfusion requirement after severe trauma: a validation of six scoring systems and algorithms on the TraumaRegister DGU. Crit Care 2012;16:R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohn SM, Nathens AB, Moore FA, et al. Tissue oxygen saturation predicts the development of organ dysfunction during traumatic shock resuscitation. J Trauma 2007;62:44–54. [DOI] [PubMed] [Google Scholar]
- 3. Moore FA, Nelson T, McKinley BA, et al. Massive transfusion in trauma patients: tissue hemoglobin oxygen saturation predicts poor outcome. J Trauma 2008;64:1010–23. [DOI] [PubMed] [Google Scholar]
- 4. Sagraves SG, Newell MA, Bard MR, et al. Tissue oxygenation monitoring in the field: a new EMS vital sign. J Trauma 2009;67:441–3. [DOI] [PubMed] [Google Scholar]
- 5. Beekley AC, Martin MJ, Nelson T, et al. Continuous noninvasive tissue oximetry in the early evaluation of the combat casualty: a prospective study. J Trauma 2010;69(Suppl 1):S14–25. [DOI] [PubMed] [Google Scholar]
- 6. Yang Y, Soyemi O, Scott PJ, et al. Quantitative measurement of muscle oxygen saturation without influence from skin and fat using continuous‐wave near infrared spectroscopy. Opt Express 2007;15:13715–30. [DOI] [PubMed] [Google Scholar]
- 7. Reisner AT, Chen L, McKenna TM, Reifman J. Automatically‐computed prehospital severity scores are equivalent to scores based on medic documentation. J Trauma 2008;65:915–23. [DOI] [PubMed] [Google Scholar]
- 8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Convertino VA, Rickards CA, Ryan KL. Autonomic mechanisms associated with heart rate and vasoconstrictor reserves. Clin Auton Res 2012;22:123–30. [DOI] [PubMed] [Google Scholar]
- 10. Minnesota Department of Health . Level 3 Trauma Hospital Criteria. Available at: http://www.health.state.mn.us/traumasystem/hospresources/criteria_level3.pdf. Accessed Oct 6, 2015.


