Abstract
We identify a novel endothelial membrane behaviour in transgenic zebrafish. Cerebral blood vessels extrude large transient spherical structures that persist for an average of 23 min before regressing into the parent vessel. We term these structures “kugeln”, after the German for sphere. Kugeln are only observed arising from the cerebral vessels and are present as late as 28 days post fertilization. Kugeln do not communicate with the vessel lumen and can form in the absence of blood flow. They contain little or no cytoplasm, but the majority are highly positive for nitric oxide reactivity. Kugeln do not interact with brain lymphatic endothelial cells (BLECs) and can form in their absence, nor do they perform a scavenging role or interact with macrophages. Inhibition of actin polymerization, Myosin II, or Notch signalling reduces kugel formation, while inhibition of VEGF or Wnt dysregulation (either inhibition or activation) increases kugel formation. Kugeln represent a novel Notch‐dependent NO‐containing endothelial organelle restricted to the cerebral vessels, of currently unknown function.
Keywords: angiogenesis, endothelial cell, Notch, VEGF, Wnt, zebrafish
Subject Categories: Development & Differentiation, Vascular Biology & Angiogenesis
Introduction
Formation of mature blood vessels requires a wide range of endothelial behaviours. These include proliferation, migration, anastomosis, lumen formation, remodelling and pruning, alongside recruitment of non‐endothelial cell types such as pericytes and vascular smooth muscle cells 1, 2, 3, 4. Many of these processes can be studied in vitro, but in vivo models of vascular development allow observation of endothelial behaviour within a multicellular and complex physiological environment.
Because visualizing real‐time embryonic vascular development in mammals is technically challenging, the zebrafish has become a widely applied model of vertebrate vascular development. Their translucency enables detailed observation of cellular behaviour without invasive instrumentation in vivo 5, 6. An increasing array of transgenic reporter lines that drive fluorescent gene expression in vascular cells is available. Coupling these with state‐of‐the‐art imaging techniques, such as light sheet fluorescence microscopy (LSFM), enables detailed cellular and subcellular imaging for hours or even days during embryonic development 7, 8. This ability to observe vascular development in more detail and for longer durations provides new insights into blood vessel formation.
Endothelial and other cells are known to form a variety of membranous vesicles 9. These include apoptotic bodies (1–4 μm diameter), microvesicles (0.15–1 μm diameter) and exosomes (40–150 nm diameter) 10. There is increasing evidence that such vesicles play important roles in intracellular signalling 11 and in vascular diseases such as atherosclerosis 12. Understanding the roles of such vesicles is therefore of both biological and clinical significance.
Here, we report a novel type of endothelial cell vesicle formation with characteristics that are entirely distinct from any previously described membrane behaviour. We show that endothelial cells (ECs) of the zebrafish cerebral vasculature (but not other vessels) extrude spherical structures far larger than any previously described microvesicle 10, and unlike previously described vesicles never detach from the parent endothelial cell.
Due to their unknown nature and function, we termed these structures kugeln (German for spheres) and we here characterize their morphology, dynamics, and sites of occurrence. We find that kugeln are membranous protrusions that do not contain either nuclei or cytoplasm. Although very different in size and behaviour to other vesicular structures, inhibition of the cytoskeletal components filamentous actin (F‐actin) formation or Myosin II reduced kugeln formation, as previously shown for cellular blebs 13. Kugeln do not interact with brain lymphatic endothelial cells (BLECs) 14, 15 or macrophages, nor do they serve as scavengers. Kugeln formation is not influenced by either increased membrane rigidity, nor osmotic pressure, which are known to impact platelet ballooning and cellular blebbing 13, 16.
Kugel formation can proceed in the absence of blood flow. Furthermore, we show that central orchestrators of vascular development such as Notch, VEGF and Wnt signalling 17, 18, 19, 20 all influence kugel formation.
Together, our data suggest that kugeln represent a previously undescribed EC membrane behaviour restricted to the cerebral vessels. Their function remains unknown, but their existence emphasizes that our understanding of the intricate processes during vascular development is far from complete.
Results
Endothelial cells of zebrafish cerebral vessels frequently develop large extruding spherical structures
We initially observed that the endothelial cells of the cerebral vessels of transgenic Tg(kdrl:HRAS‐mCherry) s916 zebrafish expressing an endothelial membrane‐tagged reporter protein 21 displayed rounded protrusions (Fig 1A). Although the appearance of these on single z‐slices was similar to cross‐sections of lumenized vessels, three‐dimensional reconstruction showed them to be spherical abluminal protrusions (Movie EV1). Due to their shape and unknown nature, we termed these structures kugeln, after the German for sphere (singular kugel). The mean diameter of kugeln was 10.1 ± 0.5 μm (s.e.m.) at 3 dpf (Fig 1B) exceeding the size of previously described membrane‐derived vesicles 10.
Figure 1. Endothelial cells of the zebrafish embryonic cerebral vessels develop “kugeln”; large spherical membrane protrusions.
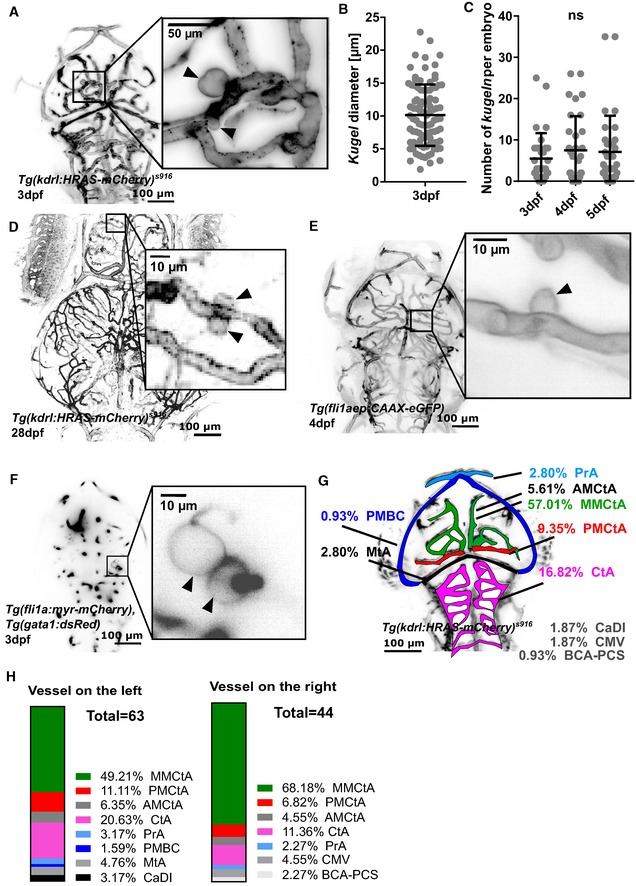
- MIP of cerebral vessels of 3 dpf Tg(kdrl:HRAS‐mCherry) s916 embryo (grey LUT; inverted). Higher magnification panel showing two kugeln (arrowheads) arising from the middle mesencephalic central artery (MMCtA).
- Diameter of kugeln at 3 dpf (mean ± s.e.m. 10.13 ± 0.49; n = 93 kugeln from 32 3 dpf embryos; three experimental repeats).
- Number of kugeln per embryo in 3, 4 and 5 dpf animals was not statistically significantly different (mean ± s.e.m. 3 dpf 5.47 ± 1.09, 4 dpf 7.47 ± 1.46, 5 dpf 7.09 ± 1.55; P = 0.8571; 3 dpf: 175 kugeln from 32 embryos; 4 dpf: 239 kugeln from 32 embryos, 5 dpf: 227 kugeln from 32 embryos; four experimental repeats; Kruskal–Wallis test).
- Kugeln (arrowheads) could be observed in 28 dpf animals.
- MIP of cerebral vessels of 4 dpf Tg(fli1aep:CAAX‐eGFP) embryo (grey LUT; inverted). Higher magnification panel shows a kugel (arrowhead) protruding from the posterior mesencephalic central artery (PMCtA).
- Single Z‐plane micrograph of cerebral vessels of a 3 dpf Tg(gata1:dsRed), injected with a Tol2‐fli1a:myr‐Cherry construct showing two kugeln (arrowheads) in the higher magnification panel protruding from the MMCtA.
- Locations and proportion of kugeln on cerebral vessels (colour coded by vessel; total n = 107 kugeln from 34 4 dpf embryos; three experimental repeats). AMCtA—anterior mesencephalic central artery, BCA—basal communicating artery, CaDI—caudal division of internal carotid artery, CMV—communicating vessel, CtA—central arteries, MMCtA—middle mesencephalic central artery, MtA—metencephalic artery, PCS—posterior communicating segment, PMBC—posterior midbrain channel, PMCtA—posterior mesencephalic central artery, PrA—prosencephalic artery.
- Location of kugeln by vessel and laterality (107 kugeln from 34 4 dpf embryos; three experimental repeats comparison of left vs right P = 0.3592; Mann–Whitney U‐test).
Mean number of kugeln per embryo was 5.55 ± 1.12 (s.e.m.) at 3 dpf and was not significantly different between 3 and 5 dpf (P = 0.8571; Fig 1C). We were able to observe kugeln in 28 dpf animals (Fig 1D), confirming that kugeln are present beyond embryonic stages.
To exclude the possibility that kugeln arise as a strain‐specific feature of the transgenic line Tg(kdrl:HRAS‐mCherry) s916, we imaged a different transgenic Tg(fli1aep:CAAX‐eGFP) 22, which utilizes the pan‐endothelial promotor fli1a 8 and prenylation to target eGFP to the endothelial membrane. Again, kugeln were observed to arise from cerebral vessels (Fig 1E). We furthermore induced transient expression of the plasmid pTol2‐fli1a:myr‐mCherry 23, 24, which uses the same fli1a promotor, and utilizes myristoylation to label the EC membrane. This was performed in Tg(gata1:dsRed) transgenics to enable vessel localization. Again, kugeln were observed in these embryos (Fig 1F), demonstrating that kugel formation was independent of the transgenic construct used, the promotor driving its expression and the method of membrane tagging. Therefore, we concluded that kugeln represent a physiological behaviour of the EC membrane of the cranial vasculature.
We mapped the location of kugeln on parent vessels to determine their distribution. > 90% of kugeln arose from the central vessels of the cerebral vasculature (Fig 1G) and none from the trunk vasculature (aorta, intersegmental vessels, dorsal longitudinal anastomotic vessel or caudal vein) at the examined time‐points. The specific vessels giving rise to kugeln are shown in Fig 1H.
Although individual kugeln were often unilateral (present left‐side but not right‐sided vessels, or vice versa), overall left/right distribution was not significantly different (Fig 1H).
Kugeln are highly dynamic transient structures
We next observed kugel behaviour over time. Time‐lapse imaging revealed kugeln were both transient and highly dynamic (Fig 2A). Although we expected kugeln to form new vessels, interact with other ECs to anastomose or detach from parent vessels, kugeln always either regressed back into the parent vessel or persisted to the end of imaging without separation or anastomosis.
Figure 2. Endothelial kugeln are transient and dynamically alter shape and size.
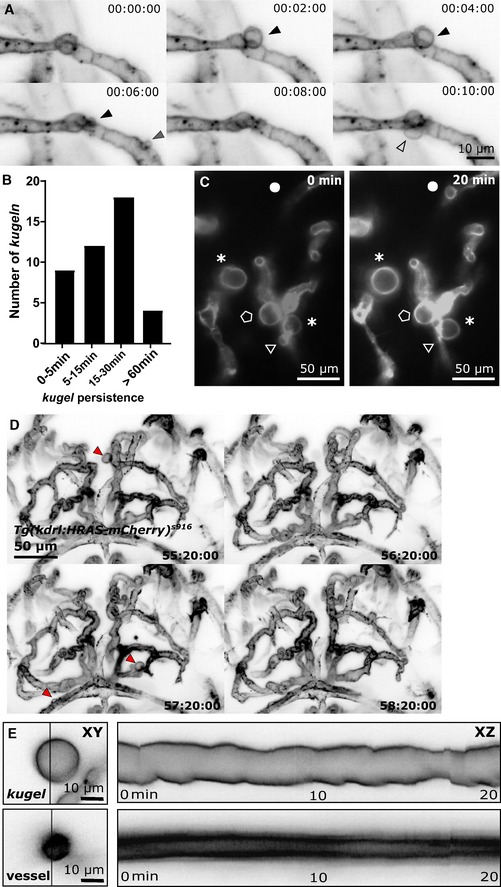
- MIPs of a time‐series light sheet acquisition shows three different kugeln (kugel 1—black arrowhead; kugel 2—grey arrowhead; kugel 3—unfilled arrowhead) protruding and retracting from parent vessels (2 min intervals; inverted LUT).
- Kugeln persisted for variable durations before regression into the parent vessel (43 kugeln from 9 4 dpf embryos; four experimental repeats).
- MIPs taken 20 min apart showing examples of different kugel behaviour including shape changes (asterisk), expansion (circle), retraction (triangle) or little change (pentagon).
- MIPs taken 1 h apart show that kugeln may be observed at one time‐point (red arrowhead, 55:20:00) but not 1 h later in the same animal (56:20:00) and to develop on other vessels 1 h later (57:20:00) which again have regressed by 58:20:00.
- Kymographs generated by line‐scanning across the diameter of a typical kugel shows that kugel diameter oscillated with a periodicity of minutes, while no such oscillations were observed in adjacent similarly sized cerebral vessels (all images grey LUT; inverted).
Quantification of kugel lifespan showed that while some regressed after minutes, others persisted for hours (Fig 2B). Further examination showed that kugeln displayed dynamic alteration in shape and size, including shape changes, enlargement and retraction (Fig 2C; Movie EV2). This dynamic behaviour was observed not only in Tg(kdrl:HRAS‐mCherry) s916 animals but also in Tg(fli1ep:CAAX‐eGFP) (Fig EV1A and B) and Tg(fli1a:myr‐mCherry), Tg(gata1:dsRed) (Fig EV1C).
Figure EV1. Kugeln were observed in the transgenic Tg(fli1aep:eGFP‐CAAX) .
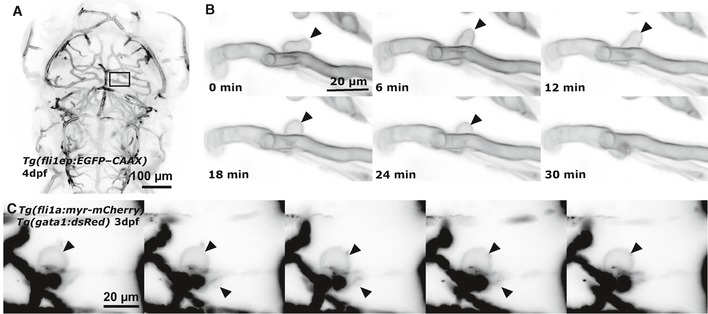
- MIP of cerebral vessels of 4 dpf Tg(fli1aep:eGFP‐CAAX) embryo.
- Time‐lapse acquisitions showed that kugeln were dynamic (black arrowhead) as in Tg(kdrl:HRAS‐mCherry) s916, showing protrusion, shape changes, oscillation and retraction.
- Additional time‐lapse acquisitions in the Tg(fli1a:myr‐mCherry), Tg(gata1:dsRed) showed equally dynamics of kugeln (black arrowhead; images grey LUT; inverted).
Although some animals displayed no kugeln when observed at a single time‐point (Fig 1C), time‐lapse imaging showed kugeln could develop at subsequent time‐points (Fig 2D).
When we studied kugel shape change over time, this revealed that some kugeln displayed oscillatory diameter changes with a periodicity of minutes (Fig 2E). No such changes were observed in parent vessels, suggesting alterations of kugel size or shape are not directly related to blood pressure or flow but related to active membrane remodelling.
Kugeln are membranous structures whose formation is dependent on the cytoskeleton
We next investigated the composition and biogenesis of kugeln. Examining double‐transgenic embryos Tg(kdr:nls‐eGFP) zf109 , Tg(kdrl:HRAS‐mCherry) s916 that labelled endothelial nuclei and membrane, we never observed kugeln to contain a nucleus, nuclear defragmentation or observed nuclear mitosis nearby kugeln (Fig 3A). This suggests kugeln do not represent an atypical form of cell proliferation, apoptosis or angiogenic sprouting.
Figure 3. Endothelial kugeln are non‐nucleated with a filamentous actin‐enriched neck.
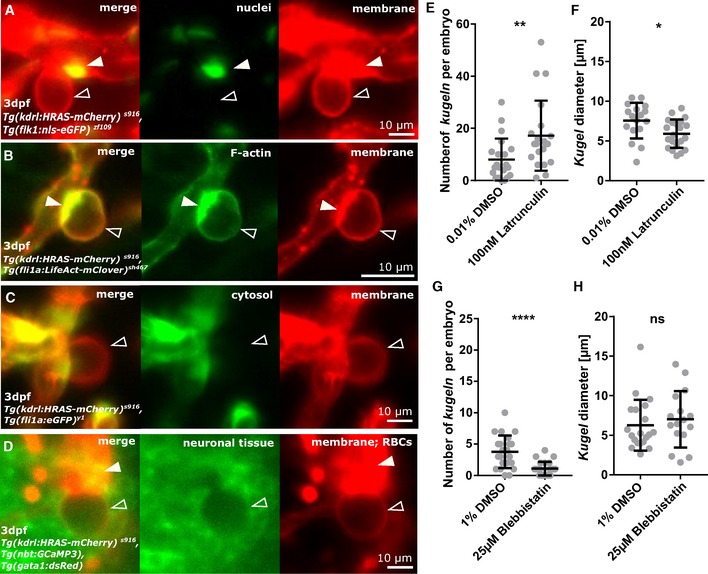
- Double transgenic visualizing endothelial membrane (red) and endothelial nuclei (green). Endothelial nuclei (arrowhead) were observed close to but never within the kugel (unfilled arrowhead).
- Double transgenic showing endothelial membrane (red) and endothelial F‐actin (green). F‐actin was found to localize at the neck of the kugeln (arrowhead; see also: Movie EV3).
- Double transgenic showing endothelial membrane (red) and endothelial cytoplasm (green). The cytoplasmic reporter was visible in the parent vessel but not in the kugel (unfilled arrowhead).
- Triple transgenic showing endothelial membrane (red), neurons (green) and erythrocytes (red). Surrounding neurons were excluded from the volume of the kugel (unfilled arrowhead), and erythrocytes were not observed inside kugeln (arrowhead).
- Treatment with the inhibitor of actin polymerization Latrunculin B statistically significantly increased number of kugeln per embryo (100 nM 1 h; **P = 0.0041; control n = 21 embryos 8.05 ± 1.76 (mean ± s.e.m.); Latrunculin n = 21 embryos 17.19 ± 2.93 (mean ± s.e.m.); 4 dpf; three experimental repeats; Mann–Whitney U‐test).
- Latrunculin B treatment statistically significantly reduced kugel diameter (*P = 0.0164; control n = 169 kugeln from 21 embryos 7.56 ± 2.25 (mean ± s.e.m.); Latrunculin n = 361 kugeln from 21 embryos 5.91 ± 1.78 (mean ± s.e.m.); 4 dpf; three experimental repeats; Student's t‐test).
- Treatment with the Myosin II inhibitor Blebbistatin statistically significantly reduced number of kugeln per embryo (25 μM 1 h; ****P < 0.0001; control n = 22 embryos 3.77 ± 0.56 (mean ± s.e.m.), Blebbistatin n = 24 embryos 1.08 ± 0.22 (mean ± s.e.m.); 3 dpf; three experimental repeats; Mann–Whitney U‐test).
- Blebbistatin treatment had no effect on kugel diameter (P = 0.3731; control n = 83 kugeln from 22 embryos 6.27 ± 0.72 (mean ± s.e.m.), Blebbistatin n = 26 kugeln from 24 embryos 7.02 ± 0.89 (mean ± s.e.m.); 3 dpf; three experimental repeats; Mann–Whitney U‐test).
Analysing the double‐transgenic line Tg(fli1a:LifeAct‐mClover) sh467, Tg(kdrl:HRAS‐mCherry) s916 labelling filamentous actin (F‐actin) and EC membrane, we observed that F‐actin co‐localized with kugeln, especially at the kugel “neck” (Fig 3B). Time‐lapse microscopy revealed this enrichment of F‐actin at the kugel neck to be highly dynamic (Movie EV3).
To further examine the contents of kugeln, we imaged the double‐transgenic Tg(fli1a:eGFP) y1 , Tg(kdrl:HRAS‐mCherry) s916 that labels the EC cytosol and membrane. We were unable to visualize cytosolic GFP within kugeln, indicating they contain little if any cytoplasm (Fig 3C).
To further characterize the content inside and tissue surrounding kugeln, we examined the triple‐transgenic Tg(gata1:dsRed), Tg(nbt:GCaMP3), Tg(kdrl:HRAS‐mCherry) s916 that labels red blood cells (RBCs), developing neurons and EC membrane. RBCs were never observed within kugeln. Examination of neurons showed an exclusion of neural tissue (Fig 3D), confirming kugeln displace rather than include neural tissue.
Kugel formation is reduced by F‐actin or Myosin II inhibition
Since F‐actin was enriched at kugel necks, we examined whether this was necessary for growth and/or maintenance of kugeln. Thus, actin polymerization was inhibited by application of Latrunculin B. This led to a significant increase of number of kugeln per embryo (Fig 3E; P = 0.0041), but a significant decrease in kugel diameter (Fig 3F; P = 0.0164).
The role of Myosin II was investigated by chemical inhibition by Blebbistatin treatment. This significantly reduced the number of kugeln per embryo (Fig 3G; P < 0.0001) but did not affect kugeln diameter (Fig 3H; P = 0.3731).
The finding that F‐actin inhibition increased, while Myosin II inhibition decreased, kugel number is consistent with the effect of these manipulations on cellular blebs 13, suggesting some shared mechanisms of kugel and bleb biogenesis.
Blood flow is not required for kugel formation, maintenance, retraction or oscillation
To study the impact of blood flow and blood pressure, we performed exsanguination by cardiac puncture to reduce blood pressure and flow to zero. Imaging the same animals pre‐ and post‐exsanguination showed no effect on kugel size or shape (Fig 4A and B), suggesting neither flow nor pressure are needed to maintain kugeln once they have formed. To confirm this, cardiac contraction was temporarily stopped by high‐dose Tricaine application and time‐lapses acquired. Despite absent blood flow, kugeln still developed, changed shape and retracted (Fig 4C and D) as seen under normal blood flow conditions.
Figure 4. The relationship between endothelial kugeln and blood flow.
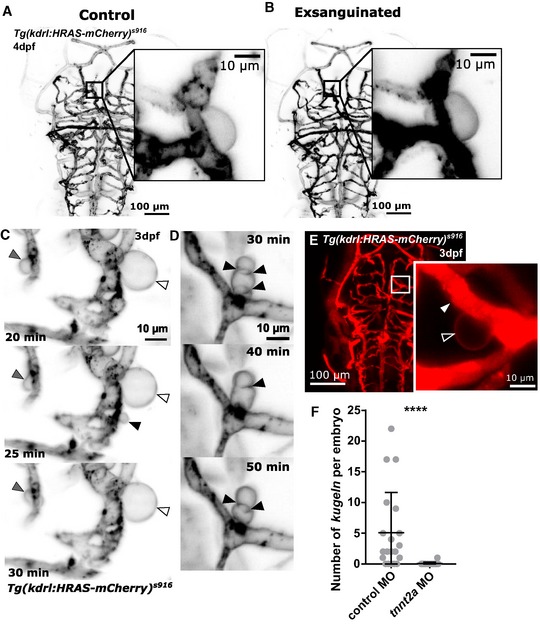
- MIP of the cerebral vessels of a 4 dpf embryo before exsanguination (grey LUT; inverted).
- MIP of the same embryo after exsanguination, which did not alter kugel size.
- Time‐lapse imaging of an embryo with transiently halted cardiac contraction (using Tricaine). Despite absent blood flow kugeln still changed shape (grey arrowhead), retained shape (white arrowhead) or protruded and retracted (black arrowhead; time post cessation of flow is indicated on micrographs; 3 dpf; grey inverted LUT).
- Time‐lapse of an embryo with transiently halted cardiac contraction as in (C) showed that kugel diameter still oscillates (time post cessation of flow).
- Dextran microangiography filled perfused vessels with dextran (arrowhead), while dextran was not observed to enter kugeln (unfilled arrowhead).
- Inhibition of cardiac contraction by tnnt2a morpholino (MO) knockdown statistically significantly reduced kugel number per embryo (****P < 0.0001; control n = 20 embryos 5.10 ± 1.47 (mean ± s.e.m.), tnnt2a MO = 18 embryos 0.06 ± 0.06 (mean ± s.e.m.); 3 dpf; three experimental repeats; Mann–Whitney U‐test).
We next performed microangiography with fluorescent dextran to investigate whether kugeln were perfused by blood or communicated with the lumen of the parent vessel. No entry of dextran into kugeln was observed (Fig 4E), suggesting no such communication existed, at least after kugeln were formed.
Lastly, we prevented the development of heart contraction by morpholino (MO) knockdown of cardiac troponin 2a (tnnt2a) 25, 26, 27. This induced a significant decrease in number of kugeln per embryo (Fig 4F; P < 0.0001).
Together, these data suggest that blood flow is not the driving mechanism of kugel formation, retraction or oscillation, but that vessels that have never experienced flow do not form kugeln.
Altered membrane permeability or osmotic pressure does not affect kugel formation or diameter
Since DMSO increases membrane permeability 28, we examined whether this had an impact on kugel formation. We incubated embryos for 24 h in 2.5% DMSO and found no significant difference in kugel number (Fig EV2A; P = 0.1596) or diameter (Fig EV2B; P = 0.3665).
Figure EV2. Kugeln number or diameter is not altered by changes of membrane permeability or osmotic pressure.
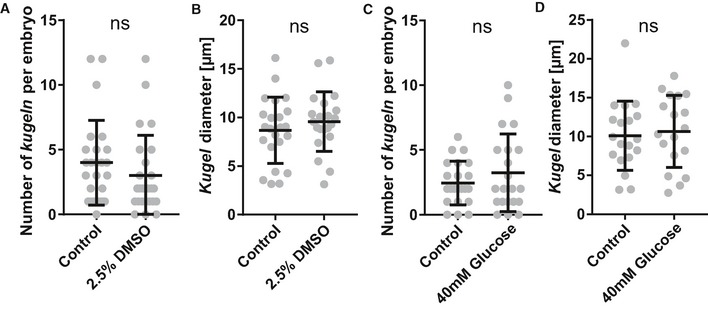
- The influence of membrane permeability increase was studied by application of DMSO; the number of kugeln was not statistically significantly changed (2.5% DMSO 24 h; P = 0.1596; control n = 25 embryos 4.00 ± 0.65 (mean ± s.e.m.), DMSO n = 25 embryos 3.00 ± 0.62 (mean ± s.e.m.); 4 dpf; three experimental repeats; Mann–Whitney U‐test).
- Diameter of kugeln was not statistically significantly different after incubation with DMSO (P = 0.3665; control n = 97 kugeln from 25 embryos 8.68 ± 0.71 (mean ± s.e.m.), DMSO n = 75 kugeln from 25 9.76 ± 0.67 (mean ± s.e.m.); 4 dpf; three experimental repeats; Student's t‐test).
- The impact of osmotic pressure on kugeln was studied by application of glucose; no statistically significant difference was observed (40 mM glucose 24 h; P = 0.7371; control n = 22 embryos 2.46 ± 0.36 (mean ± s.e.m.), glucose n = 21 embryos 3.24 ± 0.65 (mean ± s.e.m.); 4 dpf; two experimental repeats; Mann–Whitney U‐test).
- Kugel diameter was not statistically significantly different after incubation with glucose (P = 0.7060; control n = 54 kugeln from 22 embryos 10.09 ± 1.02 (mean ± s.e.m.), glucose n = 67 kugeln from 21 embryos 10.66 ± 4.65 (mean ± s.e.m.); 4 dpf; two experimental repeats; Student's t‐test).
As osmotic pressure increases cellular bleb formation and platelet ballooning 13, 16, we examined whether kugeln were altered by increased osmotic pressure. Embryos were incubated for 24 h in a 40 mM glucose solution. No significant difference was found in kugel number (Fig EV2C; P = 0.7371) or diameter (Fig EV2D; P = 0.7060).
Lymphatic cells did not interact with kugeln
Next, we investigated the relationship between kugeln and the meningeal population of lymphatic cells, brain lymphatic endothelial cells (BLECs) 14. We first investigated whether BLECs interacted physically with kugeln in the double‐transgenic Tg(kdrl:HRAS‐mCherry) s916, Tg(fli1a:Lifeact‐mClover) sh467, which allowed us to visualize BLECs as mClover‐positive but mCherry‐negative structures (Fig 5A–C). BLECs were observed to be at a different anatomical depth than kugeln (Fig 5D), and no direct interaction between BLECs and kugeln was ever observed (n = 21, 4 dpf embryos; three experimental repeats).
Figure 5. Kugeln do not interact with brain lymphatic endothelial cells (BLECs) in Tg(fli1a:LifeAct‐mClover) sh467, Tg(kdrl:HRAS‐mCherry) s916, nor do they take up injected IgG‐conjugated Alexa 674.
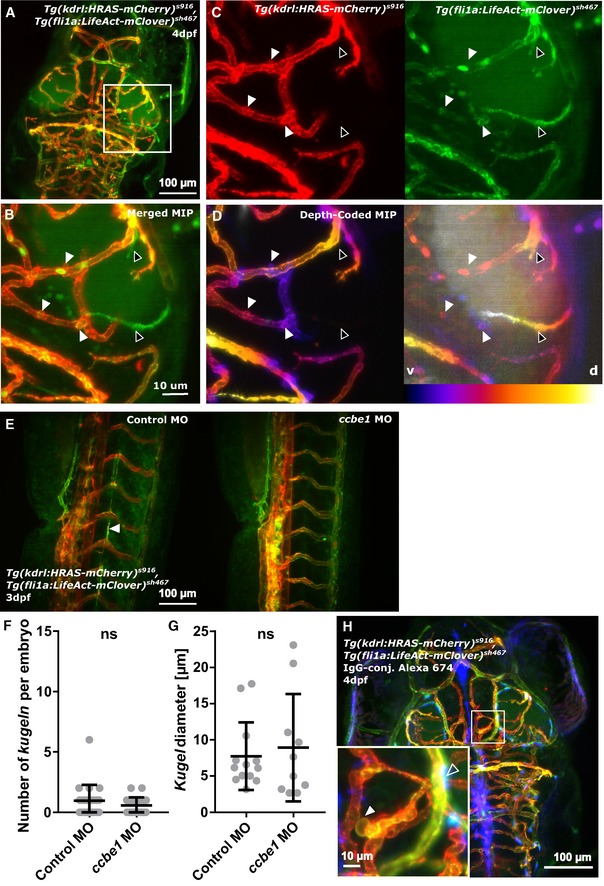
- Kugeln were studied in the double‐transgenic Tg(fli1a:LifeAct‐mClover) sh467, Tg(kdrl:HRAS‐mCherry) s916.
- BLECs were mClover‐positive and mCherry‐negative (BLECs—black arrowheads; kugeln—white arrowheads).
- No direct physical interaction between kugeln (BLECs—black arrowheads; kugeln—white arrowheads) and BLECs was observed (n = 21 4 dpf embryos; three experimental repeats).
- Depth‐coded MIPs showed that BLECs and kugeln are present on different anatomical planes (depths; purple ventral (v), white dorsal (d); BLECs—black arrowheads; kugeln—white arrowheads).
- Ccbe1 morpholino (MO) injection led to a loss of lymphatics (white arrowhead).
- Kugel number was not statistically significantly altered by ccbe1 morpholino (MO) knockdown (P = 0.3496; control MO n = 22 embryos 0.95 ± 0.28 (mean ± s.e.m.), ccbe1 MO n = 23 embryos 0.57 ± 0.14 (mean ± s.e.m.); 3 dpf; three experimental repeats; Mann–Whitney U‐test).
- Kugel diameter was not statistically significantly altered by ccbe1 MO knockdown (P = 0.8783; control MO n = 21 kugeln from 22 embryos 7.75 ± 1.29 (mean ± s.e.m.), ccbe1 MO n = 13 kugeln from 23 embryos 8.93 ± 2.35 (mean ± s.e.m.); 3 dpf; three experimental repeats; Mann–Whitney U‐test).
- Injection of IgG‐conjugated Alexa 647 into the tectum showed no uptake (blue; unfilled arrowhead) by kugeln (white arrowhead; 140 kugeln from 17 4 dpf embryos; two experimental repeats).
To examine whether lymphatics are required for kugeln formation, BLEC formation was inhibited by MO knockdown of ccbe1 (Fig 5E) 21. This had no effect on either number of kugeln per embryo (Fig 5F) or kugel diameter (Fig 5G).
These findings were confirmed by imaging the double‐transgenic Tg(kdrl:HRAS‐mCherry) s916, Tg(flt4 BAC :mCitrine) hu7135, which more specifically labels BLECs (Fig EV3A). Again, ccbe1 MO knockdown had no effect on kugel number (Fig EV3B and C; P = 0.1472) or diameter (Fig EV3D; P = 0.0962).
Figure EV3. Kugeln do not interact with BLECs in Tg(flt4 BAC :mCitrine) hu7135, Tg(kdrl:HRAS‐mCherry) s916 .
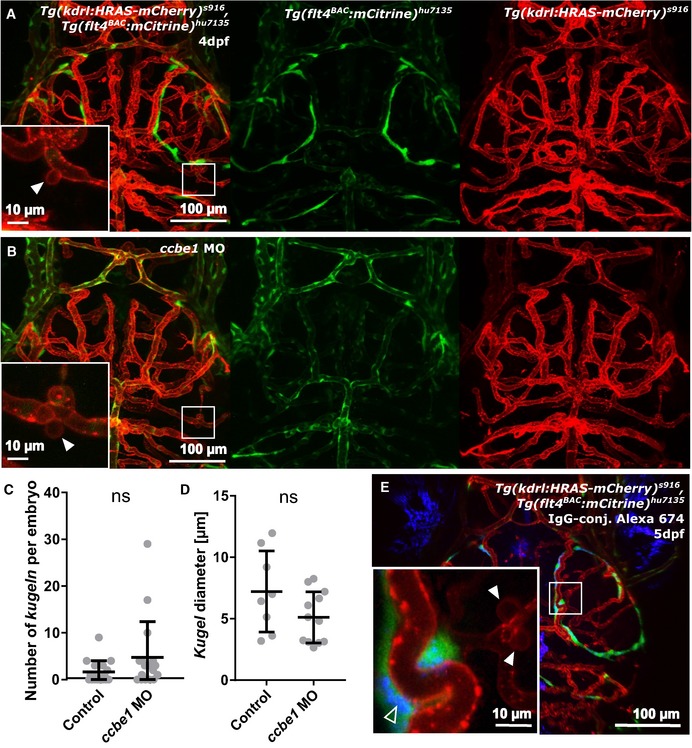
- Kugeln (white arrowhead) were additionally studied in the transgenic Tg(flt4 BAC :mCitrine) hu7135, Tg(kdrl:HRAS‐mCherry) s916, which was more specific for BLECs, using confocal microscopy to allow higher resolution imaging.
- Ccbe1 morpholino (MO) injection leads to loss of BLECs, while kugeln were still observed (white arrowhead).
- The number of kugeln was not statistically significantly altered by ccbe1 morpholino (MO) knockdown (P = 0.1472; control n = 17 embryos 1.65 ± 0.58 (mean ± s.e.m.), ccbe1 MO n = 17 embryos 4.77 ± 1.85 (mean ± s.e.m.); 4 dpf; two experimental repeats; Mann–Whitney U‐test).
- Diameter of kugeln was not statistically significantly altered upon ccbe1 MO injection (P = 0.0962; control n = 27 kugeln from 17 embryos 7.22 ± 1.17 (mean ± s.e.m.), ccbe1 MO n = 81 kugeln from 17 embryos 5.12 ± 0.60 (mean ± s.e.m.); two experimental repeats; Student's t‐test).
- Injection of IgG‐conjugated Alexa 647 into the tectum (unfilled arrowhead) showed no uptake of IgG‐conjugated Alexa 647 by kugeln (n = 52 kugeln from 5 5 dpf embryos, kugeln indicated by white arrowheads).
Next, we examined whether kugeln serve an uptake function, similar to BLECs 14. We studied whether IgG‐conjugated Alexa Fluor 674, injected into the tectum, would be taken up by kugeln. While BLECs readily took up the IgG‐conjugated Alexa, this was not observed in kugeln (Fig 5H; 140 kugeln from 17 4 dpf embryos; Fig EV3E 52 kugeln from five 5 dpf embryos).
Macrophages do not interact with kugeln
Since macrophages interact with vessels undergoing anastomosis 29, we imaged the triple‐transgenic line Tg(fms:GAL4.VP16) i186 , Tg(UAS‐E1b:nfsB.mCherry) il149 , Tg(kdrl:HRAS‐mCherry) s916, which labels both macrophages and endothelial cells with mCherry (Fig 6A). To distinguish the anatomical depth of kugeln and macrophages, depth coding was performed; also, macrophages could be identified by cell shape and movement between frames of time‐lapses (Fig 6B). We never observed direct interaction of macrophages with kugeln (Fig 6C; Movie EV4; n = 21 kugeln in eight 3 dpf embryo), suggesting that kugeln do not directly respond to local inflammation and/or recruitment of macrophages 29.
Figure 6. Kugeln do not interact with macrophages.
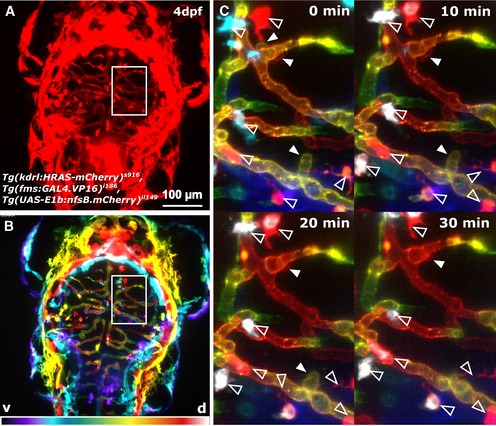
- The possible interaction of macrophages with kugeln was studied in the transgenic Tg(kdrl:HRAS‐mCherry) s916 , Tg(fms:GAL4.VP16) i186 , Tg(UAS‐E1b:nfsB.mCherry) il149, visualizing macrophages and the vasculature simultaneously.
- Coloured depth coding indicates the different anatomical depths of kugeln and macrophages (ventral purple, dorsal white).
- Maximum intensity projections of z‐stacks acquired every 10 min during a time‐lapse showed no interaction of macrophages (unfilled arrowhead) with kugeln (filled arrowhead; n = 21 kugeln in eight 3 dpf embryos). Macrophages are all white, indicating they are on a different z‐plane to the vessels seen.
Number of kugeln is increased by VEGF inhibition, and while kugeln contain NO, they do not require NO synthase for their formation
Due to the essential role of VEGF in angiogenesis, we examined the effect of VEGF signalling inhibition on kugel formation 17, 30. The VEGF inhibitor AV951 significantly increased the number of kugeln per embryo (Fig 7A; P < 0.0001), without affecting kugel diameter (Fig 7B; P = 0.7890). Since nitric oxide (NO) is induced by VEGF 31, we incubated embryos with the vital dye DAF‐FM, which fluoresces green in contact with NO 23, 33. We found 57.76% of kugeln (118 of 205 kugeln; 22 embryos) were fluorescent, indicating high levels of NO reactivity (Fig 7C). To identify at which point kugeln begin to contain NO, we performed time‐lapse microscopy of kugel formation after DAF‐FM staining and found NO reactivity was detectible at the start of kugel formation (Fig 7D). Because DAF‐FM can fluoresce in acidic environments even in the absence of NO 34, we performed LysoTracker staining, which visualizes acidic cell compartments 35, 36. Only 17.08% of kugeln were positive for LysoTracker (Fig 7E; 62 of 363 kugeln; 22 embryos), suggesting that kugeln do indeed contain NO.
Figure 7. Kugel number is increased by VEGF inhibition, and kugeln contain NO.
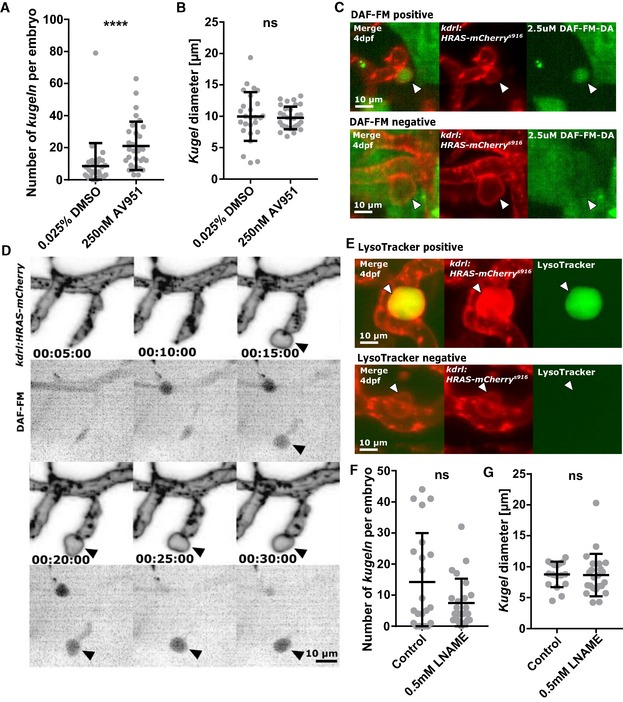
- VEGF inhibition by 2 h treatment with AV951 statistically significantly increased kugel number (****P < 0.0001; DMSO control n = 30 embryos 8.53 ± 2.62 (mean ± s.e.m.); AV951 n = 31 embryos 21.10 ± 2.71 (mean ± s.e.m.); 4 dpf; four experimental repeats; Mann–Whitney U‐test).
- Mean kugel diameter was not statistically significantly different after AV951 treatment (P = 0.7890; DMSO control n = 243 kugeln from 30 embryos 9.94 ± 0.76 (mean ± s.e.m.); AV951 n = 652 kugeln from 31 embryos 9.73 ± 0.32 (mean ± s.e.m.); 4 dpf; four experimental repeats; Student's t‐test).
- DAF‐FM staining, a vital dye for nitric oxide (NO), showed that 57.56% of kugeln were positive for nitric oxide reactivity (2.5 μM incubation from 96 to 102 hpf; n = 22 4 dpf embryos; 118 of 205 kugeln filled; three experimental repeats). Images show representative “filled” (DAF‐FM positive) and “unfilled” (DAF‐FM negative) kugeln.
- Time‐lapse acquisition with DAF‐FM revealed that kugeln contained NO early in their biogenesis (grey LUT; inverted).
- Application of LysoTracker, a vital dye that stains lysosomes or acidic compartments, showed that 17.08% of kugeln contained acidic contents (8.33 μM; 96–101 hpf; n = 22 4 dpf embryos; 62 of 363 kugeln filled; 3 experimental repeats). Images show representative “filled” (Lysotracker positive) and “unfilled” (Lysotracker negative) kugeln.
- The nitric oxide synthase (NOS) inhibitor L‐NAME had no statistically significant effect on kugel number per embryo (0.5 mM L‐NAME 18 h; P = 0.4870; control n = 22 embryos 14.27 ± 3.35 (mean ± s.e.m.), L‐NAME n = 24 embryos 7.46 ± 1.61 (mean ± s.e.m.); 4 dpf; three experimental repeats; Mann–Whitney U‐test).
- Diameter of kugeln was not affected by L‐NAME (P = 0.4161; control n = 315 kugeln from 22 embryos 8.77 ± 0.49 (mean ± s.e.m.), L‐NAME n = 179 kugeln from 24 8.66 ± 0.71 (mean ± s.e.m.); 4 dpf; three experimental repeats; Mann–Whitney U‐test).
We examined whether nitric oxide synthase (NOS) was required for kugel formation by application of the NOS inhibitor l‐NAME 31, 37. Neither kugel number (Fig 7F; P = 0.4870) nor diameter were significantly altered by L‐NAME treatment (Fig 7G; P = 0.4161).
Notch signalling is required for kugel formation
Having found that VEGF signalling negatively regulates kugel formation, we studied its counter‐part Notch 19, 38, 39. Pharmacological inhibition of Notch signalling by DAPT significantly reduced kugel number (Fig 8A; P < 0.0001), while not significantly affecting kugel diameter (Fig 8B; P = 0.0832).
Figure 8. Kugel number is decreased by Notch inhibition but increased by Wnt inhibition or activation.
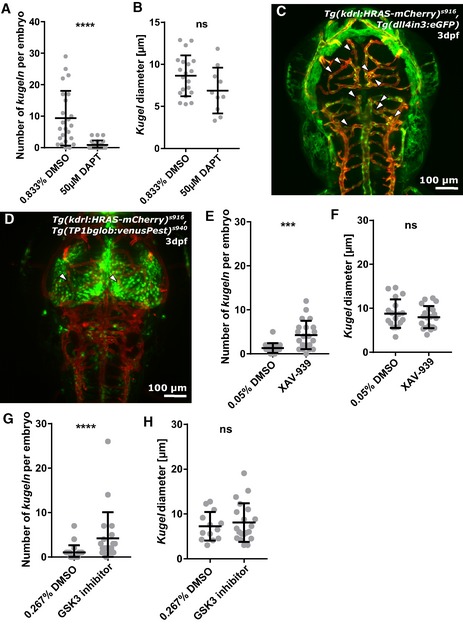
- Kugel number was significantly decreased by inhibition of Notch signalling by 12 h treatment with 50 μM DAPT (****P < 0.0001; control n = 24 embryos 9.38 ± 1.77 (mean ± s.e.m.), DAPT n = 24 embryos 0.92 ± 0.28 (mean ± s.e.m.); 4 dpf; three experimental repeats; Mann–Whitney U‐test).
- Mean kugel diameter was not statistically significantly altered by DAPT treatment (P = 0.0832; control n = 225 kugeln from 24 embryos 8.64 ± 0.54 (mean ± s.e.m.); DAPT n = 22 kugeln from 24 embryos 6.88 ± 0.86 (mean ± s.e.m.); 4 dpf; three experimental repeats; Student's t‐test).
- A transgenic dll4 reporter line showed no indication of different dll4 expression at the sites of kugeln (white arrowheads pointing to kugeln; n = 122 kugeln from 23 3 dpf embryos; two experimental repeats).
- Studying Notch signalling in the Notch reporter line Tg(TP1glob:venusPest) s940, showed high Notch levels in the midbrain, but, again, no difference in expression at the sites of kugeln (n = 45 kugeln from 19 4 dpf embryos; two experimental repeats).
- Kugel number was statistically significantly increased by inhibition of Wnt signalling by 4 h treatment with 10 μm XAV‐939 (***P = 0.0003; control n = 22 embryos 1.32 ± 0.24 (mean ± s.e.m.); XAV‐939 n = 21 embryos 4.29 ± 0.71 (mean ± s.e.m.); 3 dpf; three experimental repeats; Mann–Whitney U‐test).
- Kugel diameter was not statistically significantly altered by XAV‐939 (P = 0.4098; control n = 29 kugeln from 22 embryos 8.78 ± 0.76 (mean ± s.e.m.); XAV‐939 n = 90 kugeln from 21 embryos 7.99 ± 0.56 (mean ± s.e.m.); 3 dpf; three experimental repeats; Student's t‐test)
- Kugel number was statistically significantly increased by activation of Wnt signalling by 4 h treatment with 10 μm GSK3 inhibition XV (p 0.0359; control n = 22 embryos 1.04 ± 0.34 (mean ± s.e.m.); GSK3 inhibitor n = 21 embryos 4.24 ± 1.28 (mean ± s.e.m.); 3 dpf; three experimental repeats; Mann–Whitney U‐test).
- The diameter of kugel was not statistically significantly altered by GSK3 inhibition (p 0.5555; control n = 23 kugeln from 22 embryos 7.26 ± 0.89 (mean ± s.e.m.); GSK3 inhibitor n = 89 kugeln from 21 embryos 8.09 ± 0.97 (mean ± s.e.m.); 3 dpf; three experimental repeats; Student's t‐test).
Because these data suggested Notch signalling was required for kugel formation, we examined expression of the Notch ligand dll4 in the double‐transgenic reporter line Tg(dll4in3:eGFP), Tg(kdrl:HRAS‐mCherry) s916. Upon visual examination, no differential dll4 levels were observed in proximity to kugeln or kugel‐parent‐vessels (Fig 8C), suggesting that dll4 was not locally up‐ or down‐regulated nearby kugeln.
To further examine Notch signalling, we utilized the transgenic Tg(TP1bglob:VenusPest) s940 , Tg(kdrl:HRAS‐mCherry) s916. Expression of VenusPest is under the control of the synthetic Notch‐responsive element TP1 and was found to mainly be expressed in the midbrain and to a lesser extent in the hindbrain; but, again, no differential expression was seen adjacent to kugeln, in kugeln or in kugel‐parent‐vessels (Fig 8D).
To determine which component of the Notch pathway is required for kugeln formation, we examined the effect of morpholino knockdown of the Notch ligands dll4, notch1b, jagged‐1a and jagged‐1b. Knockdown of dll4 or jagged‐1b did not significantly affect kugel number or diameter (Fig EV4A, B, G, and H). However, morpholino knockdown of notch1b significantly reduced kugeln number (Fig EV4C; P = 0.0008), without affecting diameter (Fig EV4D). Conversely, jagged‐1a knockdown significantly increased kugel diameter (Fig EV4F; P = 0.0097) without affecting kugeln number (Fig EV4E).
Figure EV4. The effect of morpholino knockdown of dll4, notch1b, jagged‐1a and jagged‐1b on kugeln number and diameter.
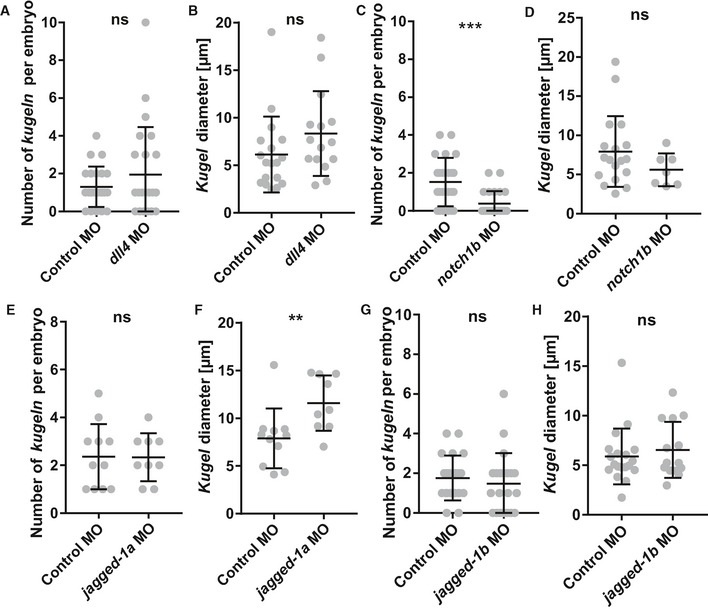
- Number of kugeln per embryo was not statistically significantly changed upon dll4 MO injection (P = 0.9639; control n = 23 embryos 1.30 ± 0.22 (mean ± s.e.m.), dll4 MO n = 23 embryos 1.96 ± 0.52 (mean ± s.e.m.); 3 dpf; three experimental repeats; Mann–Whitney U‐test).
- Kugel diameter was not statistically significantly changed upon dll4 MO injection (P = 0.0843; control n = 30 kugeln from 23 embryos 6.13 ± 0.94 (mean ± s.e.m.), dll4 MO n = 45 kugeln from 23 embryos 8.35 ± 1.15 (mean ± s.e.m.); 3 dpf; three experimental repeats; Mann–Whitney U‐test).
- Number of kugeln was statistically significantly reduced after notch1b MO injection (***P = 0.0008; control n = 23 embryos 1.52 ± 0.27 (mean ± s.e.m.), notch1b MO n = 23 embryos 0.39 ± 0.14 (mean ± s.e.m.); 3 dpf; 3 experimental repeats; Mann–Whitney U‐test).
- Kugel diameter was not statistically significantly changed upon notch1b MO injection (P = 0.3198; control n = 37 kugeln from 23 embryos 7.93 ± 1.07 (mean ± s.e.m.), notch1b MO n = 9 kugeln from 23 embryos 5.61 ± 0.79 (mean ± s.e.m.); 3 experimental repeats; Mann–Whitney U‐test).
- Number of kugeln was not statistically significantly changed after jagged‐1a MO injection (P = 0.9563; control n = 11 embryos 2.36 ± 0.41 (mean ± s.e.m.), jagged‐1a MO n = 9 embryos 2.33 ± 0.33 (mean ± s.e.m.); 3 dpf; 2 experimental repeats; Student's t‐test).
- Kugel diameter was found to be statistically significantly changed upon jagged‐1a MO injection (**P = 0.0097; control n = 23 kugeln from 11 embryos 7.89 ± 0.94 (mean ± s.e.m.), jagged‐1a MO n = 20 kugeln from 9 embryos 11.58 ± 0.97 (mean ± s.e.m.); 3 dpf; 2 experimental repeats; Mann–Whitney U‐test).
- Number of kugeln per embryo was not statistically significantly changed upon jagged‐1b MO injection (P = 0.3042; control n = 21 embryos 1.76 ± 0.25 (mean ± s.e.m.), jagged‐1b MO n = 21 embryos 1.48 ± 0.34 (mean ± s.e.m.); 3 dpf; 3 experimental repeats; Mann–Whitney U‐test).
- Kugel diameter was not statistically significantly changed upon jagged‐1b MO injection (P = 0.7060; control n = 19 kugeln from 21 embryos 5.88 ± 0.65 (mean ± s.e.m.), jagged‐1b MO n = 14 kugeln from 21 embryos 6.53 ± 0.76 (mean ± s.e.m.); 3 dpf; 3 experimental repeats; Mann–Whitney U‐test).
Both inhibition and activation of Wnt signalling increase kugel number
Since the dorsal central cranial vessels are dependent on Wnt signalling 20, 40, 41, the impact of Wnt signalling on kugeln formation was studied. Inhibition of Wnt signalling was achieved by application of XAV‐939 42. This significantly increased number of kugeln per embryo (Fig 8E; P = 0.0003), while not affecting kugel diameter (Fig 8F; P = 0.4098).
Interestingly, activation of Wnt signalling by application of the GSK3 inhibitor XV, which prevents β‐catenin phosphorylation 43, also significantly increased number of kugeln per embryo (Fig 8G; P < 0.0001), while again not affecting kugel diameter (Fig 8H).
Discussion
We here describe a previously unreported form of EC behaviour in zebrafish found only on the cerebral vessels. We believe that kugeln were not described previously due to their highly dynamic nature and low number per animal. Our discovery was greatly facilitated by state‐of‐the‐art light sheet microscopy that provided the required imaging speed and 3D imaging capability. Moreover, kugeln are only detectible in EC membrane‐tagged reporter lines, whereas many previous studies have utilized cytoplasmic reporter lines that we have shown do not label kugeln. However, retrospective analysis of multiple datasets generated in Sheffield, Münster and Dresden confirmed their existence in unrelated zebrafish colonies, and they were detected using three different approaches to label endothelial membrane. This coupled with their restriction to the cerebral vessels, and their regulation by known biological pathways such as actin polymerization as well as VEGF, Notch and Wnt signalling confirms that kugeln formation is a real phenomenon.
Kugeln are reliably observed from 3 dpf onwards but are not present at 32 hpf (2.3 dpf). The reason for this timing of onset is not presently known and may relate to a waning of VEGF signalling (which would otherwise suppress kugeln formation), increasing Notch signalling in the brain or a required state of maturity of the cerebral vessels. However, kugeln persist as late as 28 dpf, so they do not represent a purely embryonic phenomenon. Unfortunately, the increasing size and opacity of zebrafish after 28 dpf have prevented us from imaging older animals or adults.
As well as representing the first description of kugeln, our manuscript has taken important steps towards understanding their biogenesis. Although kugel diameter greatly exceeds that of previously described vesicular membrane structures, kugel biogenesis shares common features with cellular blebs such as a requirement for actin polymerization and Myosin II 13. Despite an appearance reminiscent of vascular aneurysms, unlike these kugeln are not multicellular and their formation appears not to be driven by blood flow. Because complete absence of blood flow throughout development prevents kugeln formation, we speculate that some exposure to blood flow may be needed to establish EC properties in the cerebral vessels such as polarity, shear stress responses and lumenization 4, 44, to allow kugeln to form.
Although VEGF, Notch and Wnt signalling have all been previously shown to play central roles in angiogenesis 17, 19, 20, 27, 38, 45, they have not previously been implicated in vesicle biogenesis. We find that all three of these signalling pathways influence kugel formation. VEGF signalling inhibits, while Notch signalling is required for kugel formation. Our preliminary morpholino antisense studies suggested notch1b but not dll4 is required for kugel formation, and interestingly that perhaps jagged‐1a may negatively regulate this. However, morpholinos are associated with a significant risk of off‐target effects 46 and future studies using other approaches to induce loss of function of these genes will be revealing. For example, an examination of kugel formation in stable mutants of these genes would be interesting although it may be challenging to discriminate between the effects of their loss of function on general cerebrovascular development. The recent establishment of endothelial‐specific CRISPR interference may also be informative 47.
Our finding that the majority of kugeln contained high levels of NO was unexpected as NO is generally considered to passively diffuse from NO‐producing cells and no NO‐containing organelle has previously been described 48. We speculate that accumulation of NO in kugeln may serve to establish a signalling or storage hub as described for secreted molecules such as Fgf3 49. This would provide a facility to deliver large amounts of NO rapidly. It is interesting that kugeln contain NO early in their lifetime, which, when coupled with their lack of cytoplasm, suggests that perhaps their formation might be related to “inflation” with NO, although this might be expected to be inhibited by L‐NAME which we did not observe. Furthermore, most, but not all kugeln contain NO. It is possible therefore that regression of kugeln is associated with emptying of NO from the kugel, perhaps by diffusion into the tissue or lumen.
The link between VEGF, NO and kugeln is unclear; VEGF induces NO release from ECs 31 but inhibits kugeln formation. It has been questioned whether zebrafish possess an orthologue of endothelial nitric oxide synthase (eNOS), but the balance of evidence suggests not 50. However, zebrafish do possess orthologues for inducible and neuronal NOS and it may be these isoforms that generated the NO we detected 51.
Several key questions remain unanswered by our study. Why are kugeln restricted to the cerebral vessels and what is their function? We can presently only speculate, but the dependence of kugeln on Notch signalling may provide a clue. The brain is a site of marked Notch pathway activity to which the cerebral vessels are exposed, and this may explain the localization of kugeln to these vessels. Several human genetic diseases affecting the cerebral vasculature are linked to the Notch pathway. For example, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common monogenic form of ischaemic stroke and is caused by mutations in the Notch receptor NOTCH 52, 54. Cerebral cavernous malformations (CCMs) are common large vascular cavernomas due to enlarged brain sinusoids. CCMs are caused by mutations in the CCM genes, which form a complex involved in stabilization of endothelial junctions and VEGF signalling 55, 56. Silencing CCM genes reduces Notch signalling, again implicating Notch dysregulation with human genetic cerebrovascular diseases. Since Notch inhibition impairs kugel formation, in addition to the fact that kugeln occurs in physiologically wild‐type (though transgenic) animals, it seems most likely that kugel formation plays a positive, though as yet unknown, function in maintaining cerebral vessels. To test this will require discovery of a method to specifically prevent kugel formation in order to test the effect on cerebrovascular function.
It remains to be seen whether kugeln exist in mammals. Given the conservation of endothelial behaviour between zebrafish and mammals, we consider this highly likely. Although they have not previously been described, our findings show it is clear that kugeln are easy to overlook, particularly on histological examination of mammalian brains, which would easily mistake kugeln for capillaries. However, now that their existence is known, the use of membrane‐tagged reporters or immunostaining coupled with a marker of perfusion would be expected to reveal whether kugeln exist in the mammalian brain.
We conclude that we have uncovered a striking subcellular phenomenon that appears restricted to cerebral vessels, emphasizing that our understanding of the intricate processes during vascular development is far from complete.
Material and Methods
Zebrafish strains, handling and husbandry
Experiments performed at the University of Sheffield conformed to UK Home Office regulations and were performed under Home Office Project Licence 70/8588 held by TJAC. Experiments performed at the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden and the WWU Münster Institute for Cardiovascular Organogenesis and Regeneration conformed to guidelines of the relevant German animal ethics committees.
Maintenance of adult zebrafish in all three fish facilities was conducted according to previously described husbandry standard protocols at 28°C with a 14:10 hours (h) light:dark cycle 57. Embryos, obtained from controlled pair‐ or group‐mating, were incubated in E3 buffer (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) with or without methylene blue.
The following zebrafish lines were used; Tg(kdrl:HRAS‐mCherry) s916 labels EC membrane 58 (AB background 59; Casper background 60), Tg(gata1:dsRed) labels red blood cells (RBCs) 61, Tg(fli1aep:CAAX‐eGFP) labels EC membrane 22 (kindly provided by Holger Gerhardt), Tg(fli1a:eGFP) y1 labels EC cytoplasm 8, Tg(kdr:nls‐eGFP) zf109 labels EC nuclei 62, Tg(fli1a:Lifeact‐mClover) sh467 labels endothelial filamentous actin 47 , and Tg(nbt:GCaMP3) labels neurons 63, Tg(dll4in3:GFP) is expressed at sites of expression of the Notch delta‐like ligand 4 45 (kindly provided by the De Val laboratory), Tg(TP1bglob:VenusPest) s940 labels arterial ECs 64. BLECs were studied in the double‐transgenic lines Tg(kdrl:HRAS‐mCherry) s916, Tg(flt4 BAC :mCitrine) hu7135 65 and Tg(kdrl:HRAS‐mCherry) s916, Tg(fli1a:Lifeact‐mClover) sh467. Macrophages were studied in Tg(fms:GAL4.VP16) i186 , Tg(UAS‐E1b:nfsB.mCherry) il149 and Tg(kdrl:HRAS‐mCherry) s916 66. Transient expression of Tg(fli1:myr‐mCherry), labelling EC membrane 30, 31, was achieved by injection of plasmid (50 pg) with Tol2 RNA (75 pg).
Image acquisition
Datasets in Sheffield were obtained using a Zeiss Z.1 light sheet microscope with a water‐dipping detection objective (Plan‐Apochromat 20×/1.0 Corr nd = 1.38) and a scientific complementary metal‐oxide semiconductor (sCMOS) detection unit. Data were acquired with activated pivot scan, dual‐sided illumination and online fusion; properties of acquired data are as follows: 0.7× zoom, 16bit image depth, 1,920 × 1,920px image size and minimum z‐stack interval (approx. 0.33 × 0.33× 0.5 μm). Green and red fluorophores were excited using 488 nm and 561 nm laser, respectively. Used filters in sequential tracks for multi‐colour images were LP560 for both and BP505‐545 and LP585, respectively. Samples were embedded in 1% or 2% LMP‐agarose containing 200 mg/l Tricaine (MS‐222, Sigma). The image acquisition chamber was filled with E3 plus Tricaine (200 mg/ml) and maintained at 28°C.
Light sheet datasets in Dresden were obtained with a custom‐built multidirectional SPIM (mSPIM) set‐up 67. The dorsum of the embryo head was imaged every 2.5 min over 2 days with dual illumination and 3 μm z‐spacing. The mSPIM set‐up was equipped with a Coherent Sapphire 561 nm laser, two Zeiss 10×/0.2 illumination objectives, an UMPlanFL N Olympus 20×/0.5 NA detection objective and an Andor iXon 885 EM‐CCD camera. To cover the cerebrovascular region, several regions were imaged and later stitched using custom image processing plug‐ins in Fiji 68 based on the stitching tool from Stefan Preibisch 69. Samples were mounted in fluorinated propylene ethylene (FEP) tubes according to established mounting protocols 70. The FEP tubes were coated with 3% methyl cellulose and filled with 0.1% low‐melting agarose containing 200 mg/l Tricaine to immobilize the zebrafish embryos during time‐lapse imaging.
Data acquisition for the study of BLECs was performed at the University of Münster using a Leica SP8 microscope with a 40× water immersion objective (0.75× zoom) exciting with a 514 nm argon laser, 561 nm diode‐pumped solid‐state laser, 633 nm helium‐neon and 514/561/633 beam splitters. Properties of data acquired were as follows: 16bit image depth, 1,024 × 1,024 px image size and 1 μm minimum z‐stack interval. Sample embedding was performed in 0.8% low‐melting agarose with 200 mg/l Tricaine.
Microangiography
Vascular perfusion was visualized as previously described 38, 39, using 20 μg dextran tetramethylrhodamine (2,000,000 molecular weight, Thermo Fisher) at a concentration of 10 mg/μl (2 nl injection volume).
Exsanguination and cessation of cardiac contraction
Exsanguination was achieved by mechanical opening of the heart with forceps. Temporary cessation of cardiac contraction was induced by treatment with 3.7 mM Tricaine in E3. After 20 min incubation and 30–40 min image acquisition, samples were placed in E3 without Tricaine until full recovery of cardiac contraction to confirm non‐lethality of Tricaine exposure.
Morpholino antisense oligomer‐induced gene knockdown
Inhibition of induction of cardiac contraction was achieved by injection of an ATG blocking morpholino (MO) against cardiac troponin 2 (tnnt2a) at 1.58 ng/embryo (Genetools, LLC; sequence 5′‐CATGTTTGCTCT GATCTGACACGCA‐3′) 25.
Development of BLECs was inhibited by injection of an ATG blocking MO against collagen and calcium‐binding EGF‐like domain 1 (ccbe1) at 5 ng/embryo (Genetools, LLC; sequence 5′‐CGGGTAGATCATTTCAGACACTCTG‐3′) 73.
The role of Notch signalling was investigated by injection of an ATG blocking MO against the following components: delta‐like ligand 4 (dll4; 3 ng; Genetools, LLC; sequence 5′‐GAGAAAGGTGAGCCAAGCTGCCATG‐3′) 74, jagged‐1a (jag1a; 0.1 ng; 5′‐GTCTGTCTGTGTGTCTGTCGCTGTG‐3′; Genetools, LLC) 74, jagged‐1b (jag1b; 0.8 ng; Genetools, LLC; sequence 5′‐CTGAACTCCGTCGCAGAATCATGCC‐3′) 74 and notch 1b (0.25 ng; Genetools, LLC; sequence 5′‐GTTCCTCCGGTTACCTGGCATACAG‐3′) 75.
Control MO injection was performed, according to the same protocol, with final concentrations as above (5′‐CCTCTTACCTCAGTTATTTATA‐3′; Genetools, LLC). All MO injections were conducted at one‐cell stage using phenol red as injection tracer.
Tectum injections
Possible kugel scavenging activity was examined by injection of IgG‐conjugated 150 kDa Alexa Fluor 674 (0.2 mg/ml, Thermo Fisher) into the tectum and ventricle at 3 dpf, as previously described 14.
Chemical treatments
Actin polymerization was inhibited using 100 nM Latrunculin B for 1 h between 96 and 97 hpf (Sigma‐Aldrich) 76.
Myosin II was inhibited using 25 μM Blebbistatin (with 1% DMSO in end solution for solubility) for 1 h between 75 and 76 hpf (Sigma) 77.
VEGF signalling was inhibited using VEGF receptor inhibitor AV951 78 at 250 nM for 2 h from 96 to 98 hpf (Selleckchem; S1207; Tivozanib—AVEO pharmaceuticals).
Notch signalling was inhibited using 50 μM DAPT (Sigma‐Aldrich) for 12 h from 84 to 96 hpf 79.
Wnt signalling was inhibited using 10 μM XAV‐939 for 4 h from 72 to 76 hpf (Sigma) 42. Wnt signalling activation was achieved by 10 μM GSK‐3 inhibitor XV for 4 h from 72 to 76 hpf (Merck) 43.
Control groups for the above experiments were performed using the same concentration and duration of DMSO in the active treatments.
Inhibition of nitric oxide synthase (NOS) was achieved by 0.5 mM L‐NAME (Sigma‐Aldrich) dissolved in E3 for 18 h from 3 to 4 dpf 31. Controls were incubated in E3.
The impact of osmotic pressure on kugeln was studied using 40 mM glucose treatment for 24 h from 72 to 96 hpf (Sigma). Controls were incubated in E3.
The impact of cell membrane permeability on kugeln was studied by application of 2.5% (v/v) DMSO for 24 h from 72 to 96 hpf (Sigma). Controls were incubated in E3.
Vital dye staining
In vivo visualization of nitric oxide (NO) was performed using 2.5 μM DAF‐FM‐DA (Molecular Probes; D23844) 32 for 6 h in 4 dpf embryos (96–102 hpf). DMSO control was performed at the same concentration and duration. To visualize acidic cellular compartments, LysoTracker Green (Molecular Probes; L7526 DND‐26) was applied for 5 h (96–101 hpf) at a concentration of 8.33 μM in E3 35, 36. Controls were incubated in E3.
Image analysis and representation
Images were analysed using open‐source software Fiji 68. Kymographs were produced via stack reslicing to study diameter changes of kugeln over time. To visualize data, maximum intensity projections (MIP) were generated and displayed using either grey (single channel) or red/green/blue (multi‐channel) colour representations. Z‐stack depth coding was conducted via hyper‐stack time‐coding. Intensity inversion was applied, as appropriate, to give the clearest rendering of relevant structures. Time‐lapse data were displayed using MIPs. 3D image reconstruction for 360‐degree rotation was performed using Arivis software.
Statistical analysis
All animals with sufficient image quality to be analysed were included. Animals were randomly allocated to treatment groups. Imaging and data analysis was performed unblinded to treatment allocation since the effects of treatment were usually obvious from the appearances of the micrographs.
Normality of data was tested using D'Agostino‐Pearson omnibus test. Statistical analysis of normally distributed data was performed using a one‐way ANOVA to compare multiple groups or Student's t‐test to compare two groups. Non‐normally distributed data were analysed with a Kruskal–Wallis test to compare multiple groups, or Mann–Whitney U‐test to compare two groups. Diameter of kugeln is shown as average of all kugeln per embryo, unless otherwise indicated. Analysis was performed in GraphPad Prism Version 7 (GraphPad Software, La Jolla California USA). P‐values are indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data represent mean and standard deviation (s.d.), if not otherwise stated. Image representation was performed using Inkscape (https://www.inkscape.org).
Author contribution
Funding Acquisition, RBM, JH, SS‐M, RNW, PA and TJAC; Investigation, Validation and Data Curation, ECK, ML, SD, KC, AMS, VS, KP; Formal Visualization and Analysis, ECK; Resources, RBM, JH, SS‐M, RNW, PA and TJAC; Project Administration, ECK, KP, PA, and TJAC; Writing—Original Draft, ECK and TJAC; Writing—Review and Editing, all authors contributed equally.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Review Process File
Acknowledgements
We thank the Bateson Centre aquarium facility for excellent zebrafish husbandry and guidance on experiments. We are grateful for the Tol2‐fli1a:myr‐mCherry plasmid supplied by Naoki Mochizuki. We thank Emily Noël, the Perak laboratory Sheffield and Henry Roehl for sharing chemical compounds. We thank Alex McGown for advice on tectum injections. This work was supported by a University of Sheffield, Department of Infection, Immunity and Cardiovascular Disease, Imaging and Modelling Node Studentship awarded to ECK, and by a DFG grant to SSM (SCHU 1228/2‐2). RBM is funded by a Wellcome Trust Seed award (210152/Z/18/Z). The Zeiss Z1 LSFM was funded via British Heart Foundation Infrastructure Award IG/15/1/31328 awarded to TJAC and RNW. We are grateful to Professor Holger Gerhardt and Professor Alastair Poole for discussions and advice.
EMBO Reports (2019) 20: e47047
Contributor Information
Elisabeth C Kugler, Email: ekugler1@sheffield.ac.uk.
Timothy JA Chico, Email: t.j.chico@sheffield.ac.uk.
Data availability
All data are available on request.
References
- 1. Potente M, Mäkinen T (2017) Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol 18: 477–494 [DOI] [PubMed] [Google Scholar]
- 2. Adair TH, Montani JP (2010) Angiogenesis. San Rafael, CA: Morgan & Claypool Life Sciences; [PubMed] [Google Scholar]
- 3. Davis GE, Stratman AN, Sacharidou A, Koh W (2011) Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int Rev Cell Mol Biol 288: 101–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dimmeler S, Haendeler J, Rippmann V, Nehls M, Zeiher AM (1996) Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett 399: 71–74 [DOI] [PubMed] [Google Scholar]
- 5. Gut P, Reischauer S, Stainier DYR, Arnaout R (2017) Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease. Physiol Rev 97: 889–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chico TJA, Ingham PW, Crossman DC (2008) Modeling cardiovascular disease in the zebrafish. Trends Cardiovasc Med 18: 150–155 [DOI] [PubMed] [Google Scholar]
- 7. Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EHK (2004) Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305: 1007–1009 [DOI] [PubMed] [Google Scholar]
- 8. Lawson ND, Weinstein BM (2002) In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248: 307–318 [DOI] [PubMed] [Google Scholar]
- 9. Paone S, Baxter AA, Hulett MD, Poon IKH (2018) Endothelial cell apoptosis and the role of endothelial cell‐derived extracellular vesicles in the progression of atherosclerosis. Cell Mol Life Sci 76: 1093–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289 [DOI] [PubMed] [Google Scholar]
- 11. Zamani P, Fereydouni N, Butler AE, Navashenaq JG, Sahebkar A (2018) The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc Med 29: 313–323 [DOI] [PubMed] [Google Scholar]
- 12. Bernal‐Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, de Marchena E, Ahn YS (2003) High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J 145: 962–970 [DOI] [PubMed] [Google Scholar]
- 13. Charras GT, Coughlin M, Mitchison TJ, Mahadevan L (2008) Life and times of a cellular bleb. Biophys J 94: 1836–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Lessen M, Shibata‐Germanos S, van Impel A, Hawkins TA, Rihel J, Schulte‐Merker S (2017) Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development. Elife 6: e25932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hogan BM, Schulte‐Merker S (2017) How to plumb a pisces: understanding vascular development and disease using zebrafish embryos. Dev Cell 42: 567–583 [DOI] [PubMed] [Google Scholar]
- 16. Agbani EO, van den Bosch MTJ, Brown E, Williams CM, Mattheij NJA, Cosemans JMEM, Collins PW, Heemskerk JWM, Hers I, Poole AW (2015) Coordinated membrane ballooning and procoagulant spreading in human platelets clinical perspective. Circulation 132: 1414–1424 [DOI] [PubMed] [Google Scholar]
- 17. Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D et al (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gore AV, Monzo K, Cha YR, Pan W, Weinstein BM (2012) Vascular development in the zebrafish. Cold Spring Harb Perspect Med 2: 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siekmann AF, Lawson ND (2007) Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445: 781–784 [DOI] [PubMed] [Google Scholar]
- 20. Parmalee NL, Kitajewski J (2008) Wnt signaling in angiogenesis. Curr Drug Targets 9: 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, Schulte‐Merker S (2009) Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet 41: 396–398 [DOI] [PubMed] [Google Scholar]
- 22. Gebala V, Collins R, Geudens I, Phng LK, Gerhardt H (2016) Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo. Nat Cell Biol 18: 443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kashiwada T, Fukuhara S, Terai K, Tanaka T, Wakayama Y, Ando K, Nakajima H, Fukui H, Yuge S, Saito Y et al (2015) beta‐catenin‐dependent transcription is central to Bmp‐mediated formation of venous vessels. Development 142: 497–509 [DOI] [PubMed] [Google Scholar]
- 24. Kwon H‐B, Fukuhara S, Asakawa K, Ando K, Kashiwada T, Kawakami K, Hibi M, Kwon Y‐G, Kim K‐W, Alitalo K et al (2013) The parallel growth of motoneuron axons with the dorsal aorta depends on Vegfc/Vegfr3 signaling in zebrafish. Development 140: 4081–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY (2002) Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet 31: 106–110 [DOI] [PubMed] [Google Scholar]
- 26. Packham IM, Gray C, Heath PR, Hellewell PG, Ingham PW, Crossman DC, Milo M, Chico TJ (2009) Microarray profiling reveals CXCR26a is downregulated by blood flow in vivo and mediates collateral formation in zebrafish embryos. Physiol Genomics 38: 319–327 [DOI] [PubMed] [Google Scholar]
- 27. Watson O, Novodvorsky P, Gray C, Rothman AM, Lawrie A, Crossman DC, Haase A, McMahon K, Gering M, Van Eeden FJ et al (2013) Blood flow suppresses vascular Notch signalling via dll4 and is required for angiogenesis in response to hypoxic signalling. Cardiovasc Res 100: 252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Notman R, Noro M, O'Malley B, Anwar J (2006) Molecular basis for dimethylsulfoxide (DMSO) action on lipid membranes. J Am Chem Soc 128: 13982–13983 [DOI] [PubMed] [Google Scholar]
- 29. Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C (2010) Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF‐mediated endothelial tip cell induction. Blood 116: 829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tammela T, Zarkada G, Wallgard E, Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M, Schomber T, Peltonen R et al (2008) Blocking VEGFR‐3 suppresses angiogenic sprouting and vascular network formation. Nature 454: 656–660 [DOI] [PubMed] [Google Scholar]
- 31. Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R (1997) Nitric oxide synthase lies downstream from vascular endothelial growth factor‐induced but not basic fibroblast growth factor‐induced angiogenesis. J Clin Invest 99: 2625–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T (1998) Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70: 2446–2453 [DOI] [PubMed] [Google Scholar]
- 33. Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T (1999) Fluorescent indicators for imaging nitric oxide production. Angew Chem Int Ed Engl 38: 3209–3212 [DOI] [PubMed] [Google Scholar]
- 34. Zhang X, Kim WS, Hatcher N, Potgieter K, Moroz LL, Gillette R, Sweedler JV (2002) Interfering with nitric oxide measurements 4,5‐diaminofluorescein reactd with dehydroascorbic acid and ascorbic acid. J Biol Chem 277: 48472–48478 [DOI] [PubMed] [Google Scholar]
- 35. Haller T, Dietl P, Deetjen P, Völkl H (1996) The lysosomal compartment as intracellular calcium store in MDCK cells: a possible involvement in InsP3‐mediated Ca2+ release. Cell Calcium 19: 157–165 [DOI] [PubMed] [Google Scholar]
- 36. Zucker RM, Hunter S, Rogers JM (1998) Confocal laser scanning microscopy of apoptosis in organogenesis‐stage mouse embryos. Cytometry 33: 348–354 [DOI] [PubMed] [Google Scholar]
- 37. Gray C, Packham IM, Wurmser F, Eastley NC, Hellewell PG, Ingham PW, Crossman DC, Chico TJ (2007) Ischemia is not required for arteriogenesis in zebrafish embryos. Arterioscler Thromb Vasc Biol 27: 2135–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas JL, Baker K, Han J, Calvo C, Nurmi H, Eichmann AC, Alitalo K (2013) Interactions between VEGFR and Notch signaling pathways in endothelial and neural cells. Cell Mol Life Sci 70: 1779–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bentley K, Mariggi G, Gerhardt H, Bates PA (2009) Tipping the balance: robustness of tip cell selection, migration and fusion in angiogenesis. PLoS Comput Biol 5: e1000549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N et al (2015) Tip cell‐specific requirement for an atypical Gpr124‐ and Reck‐dependent Wnt/beta‐catenin pathway during brain angiogenesis. Elife 4: 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hübner K, Cabochette P, Diéguez‐Hurtado R, Wiesner C, Wakayama Y, Grassme KS, Hubert M, Guenther S, Belting HG, Affolter M et al (2018) Wnt/beta‐catenin signaling regulates VE‐cadherin‐mediated anastomosis of brain capillaries by counteracting S1pr1 signaling. Nat Commun 9: 4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S et al (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461: 614–620 [DOI] [PubMed] [Google Scholar]
- 43. Du J, Wei Y, Liu L, Wang Y, Khairova R, Blumenthal R, Tragon T, Hunsberger JG, Machado‐Vieira R, Drevets W et al (2010) A kinesin signaling complex mediates the ability of GSK‐3beta to affect mood‐associated behaviors. Proc Natl Acad Sci USA 107: 11573–11578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ (2002) Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel‐like factor (KLF2). Blood 100: 1689–1698 [DOI] [PubMed] [Google Scholar]
- 45. Sacilotto N, Monteiro R, Fritzsche M, Becker PW, Sanchez‐Del‐Campo L, Liu K, Pinheiro P, Ratnayaka I, Davies B, Goding CR et al (2013) Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci USA 110: 11893–11898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson‐Maduro J, Kourkoulis G, Male I et al (2015) Reverse genetic screening reveals poor correlation between Morpholino‐induced and mutant phenotypes in zebrafish. Dev Cell 32: 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Savage AM, Kurusamy S, Chen Y, Jiang Z, Chhabria K, MacDonald RB, Kim HR, Wilson HL, van Eeden FJM, Armesilla AL et al (2019) tmem33 is essential for VEGF‐mediated endothelial calcium oscillations and angiogenesis. Nat Commun 10: 732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thomas DD (2015) Breathing new life into nitric oxide signaling: A brief overview of the interplay between oxygen and nitric oxide. Redox Biol 5: 225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Durdu S, Iskar M, Revenu C, Schieber N, Kunze A, Bork P, Schwab Y, Gilmour D (2014) Luminal signalling links cell communication to tissue architecture during organogenesis. Nature 515: 120–124 [DOI] [PubMed] [Google Scholar]
- 50. Syeda F, Hauton D, Young S, Egginton S (2013) How ubiquitous is endothelial NOS? Comp Biochem Physiol A Mol Integr Physiol 166: 207–214 [DOI] [PubMed] [Google Scholar]
- 51. Lepiller S, Laurens V, Bouchot A, Herbomel P, Solary E, Chluba J (2007) Imaging of nitric oxide in a living vertebrate using a diamino‐fluorescein probe. Free Radic Biol Med 43: 619–627 [DOI] [PubMed] [Google Scholar]
- 52. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cécillion M, Marechal E et al (1996) Notch3 mutations in CADASIL, a hereditary adult‐onset condition causing stroke and dementia. Nature 383: 707–710 [DOI] [PubMed] [Google Scholar]
- 53. Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier‐Lasserve E (2000) The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest 105: 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karschnia P, Nishimura S, Louvi A (2019) Cerebrovascular disorders associated with genetic lesions. Cell Mol Life Sci 76: 283–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Renz M, Otten C, Faurobert E, Rudolph F, Zhu Y, Boulday G, Duchene J, Mickoleit M, Dietrich AC, Ramspacher C et al (2015) Regulation of β1 integrin‐Klf2‐mediated angiogenesis by CCM proteins. Dev Cell 32: 181–190 [DOI] [PubMed] [Google Scholar]
- 56. You C, Sandalcioglu IE, Dammann P, Felbor U, Sure U, Zhu Y (2013) Loss of CCM3 impairs DLL4‐Notch signalling: implication in endothelial angiogenesis and in inherited cerebral cavernous malformations. J Cell Mol Med 17: 407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Westerfield M (1993) The zebrafish book: a guide for laboratory use of Zebrafish (Brachydanio rerio). Eugene, OR: University of Oregon Press; [Google Scholar]
- 58. Chi NC, Shaw RM, De Val S, Kang G, Jan LY, Black BL, Stainier DY (2008) Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev 22: 734–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Streisinger G, Walker C, Dower N, Knauber D, Singer F (1981) Production of homozygous diploid zebra fish (Brachydanio rerio). Nature 291: 293–296 [DOI] [PubMed] [Google Scholar]
- 60. White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, Bourque C, Dovey M, Goessling W, Burns CE et al (2008) Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2: 183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI (2003) Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol 4: 1238–1246 [DOI] [PubMed] [Google Scholar]
- 62. Blum Y, Belting HG, Ellertsdottir E, Herwig L, Lüders F, Affolter M (2008) Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol 316: 312–322 [DOI] [PubMed] [Google Scholar]
- 63. Bergmann K, Santoscoy PM, Lygdas K, Nikolaeva Y, MacDonald RB, Cunliffe VT, Nikolaev A (2018) Imaging neuronal activity in the optic tectum of late stage larval zebrafish. J Dev Biol 6: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ninov N, Borius M, Stainier DYR (2012) Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development 139: 1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van Impel A, Zhao Z, Hermkens DM, Roukens MG, Fischer JC, Peterson‐Maduro J, Duckers H, Ober EA, Ingham PW, Schulte‐Merker S (2014) Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development 141: 1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gray C, Loynes CA, Whyte MK, Crossman DC, Renshaw SA, Chico TJ (2011) Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb Haemost 105: 811–819 [DOI] [PubMed] [Google Scholar]
- 67. Huisken J, Stainier DYR (2007) Even fluorescence excitation by multidirectional selective plane illumination microscopy (mSPIM). Opt Lett 32: 2608–2610 [DOI] [PubMed] [Google Scholar]
- 68. Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B et al (2012) Fiji ‐ an open source platform for biological image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Preibisch S, Saalfeld S, Tomancak P (2009) Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25: 1463–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kaufmann A, Mickoleit M, Weber M, Huisken J (2012) Multilayer mounting enables long‐term imaging of zebrafish development in a light sheet microscope. Development 139: 3242–3247 [DOI] [PubMed] [Google Scholar]
- 71. Schmitt CE, Holland MB, Jin SW (2012) Visualizing vascular networks in zebrafish: an introduction to microangiography. Methods Mol Biol 843: 59–67 [DOI] [PubMed] [Google Scholar]
- 72. Weinstein BM, Stemple DL, Driever W, Fishman MC (1995) Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat Med 1: 1143–1147 [DOI] [PubMed] [Google Scholar]
- 73. Le Guen L, Karpanen T, Schulte D, Harris NC, Koltowska K, Roukens G, Bower NI, van Impel A, Stacker SA, Achen MG et al (2014) Ccbe1 regulates Vegfc‐mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 141: 1239–1249 [DOI] [PubMed] [Google Scholar]
- 74. Geudens I, Herpers R, Hermans K, Segura I, Ruiz de Almodovar C, Bussmann J, De Smet F, Vandevelde W, Hogan BM, Siekmann A et al (2010) Role of delta‐like‐4/Notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscler Thromb Vasc Biol 30: 1695–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Quillien A, Moore JC, Shin M, Siekmann AF, Smith T, Pan L, Moens CB, Parsons MJ, Lawson ND (2014) Distinct Notch signaling outputs pattern the developing arterial system. Development 141: 1544–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Morton WM, Ayscough KR, McLaughlin PJ (2000) Latrunculin alters the actin‐monomer subunit interface to prevent polymerization. Nat Cell Biol 2: 376–378 [DOI] [PubMed] [Google Scholar]
- 77. Kovács M, Tóth J, Hetényi C, Málnási‐Csizmadia A, Sellers JR (2004) Mechanism of blebbistatin inhibition of myosin II. J Biol Chem 279: 35557–35563 [DOI] [PubMed] [Google Scholar]
- 78. Nakamura K, Taguchi E, Miura T, Yamamoto A, Takahashi K, Bichat F, Guilbaud N, Hasegawa K, Kubo K, Fujiwara Y et al (2006) KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res 66: 9134–9142 [DOI] [PubMed] [Google Scholar]
- 79. Geling A, Steiner H, Willem M, Bally‐Cuif L, Haass C (2002) A gamma‐secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep 3: 688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Review Process File
Data Availability Statement
All data are available on request.


