Abstract
The competition between L (lip) and SP (sepal/petal) complexes in P‐code model determines the identity of complex perianth patterns in orchids. Orchid tetraspanin gene Auxin Activation Factor (AAF) orthologs, whose expression strongly correlated with the expansion and size of the perianth after P code established, were identified. Virus‐induced gene silencing (VIGS) of OAGL6‐2 in L complex resulted in smaller lips and the down‐regulation of Oncidium OnAAF. VIGS of PeMADS9 in L complex resulted in the enlarged lips and up‐regulation of Phalaenopsis PaAAF. Furthermore, the larger size of Phalaenopsis variety flowers was associated with higher PaAAF expression, larger and more cells in the perianth. Thus, a rule is established that whenever bigger perianth organs are made in orchids, higher OnAAF/PaAAF expression is observed after their identities are determined by P‐code complexes. Ectopic expression Arabidopsis AtAAF significantly increased the size of flower organs by promoting cell expansion in transgenic Arabidopsis due to the enhancement of the efficiency of the auxin response and the subsequent suppression of the jasmonic acid (JA) biosynthesis genes (DAD1/OPR3) and BIGPETAL gene during late flower development. In addition, auxin‐controlled phenotypes, such as indehiscent anthers, enhanced drought tolerance, and increased lateral root formation, were also observed in 35S::AtAAF plants. Furthermore, 35S::AtAAF root tips maintained gravitropism during auxin treatment. In contrast, the opposite phenotype was observed in palmitoylation‐deficient AtAAF mutants. Our data demonstrate an interaction between the tetraspanin AAF and auxin/JA that regulates the size of flower organs and impacts various developmental processes.
Keywords: Arabidopsis thaliana, auxin response, orchids, perianth, tetraspanin
1. INTRODUCTION
Orchid flowers are typically of zygomorphic symmetry and contain nearly identically shaped sepals and petals. The most marvelous feature of orchid perianths is the conversion of the upper medial petal into a well‐differentiated labellum (lip), the evolution of which is thought to be to provide a platform for potential pollinators (Cozzolino & Widmer, 2005; Kocyan, Conti, Qiu, & Endress, 2004; Rudall & Bateman, 2002). We have found a conserved principle known as the perianth (P) code, which states that the determination of lips and sepals/petals in orchids is controlled by L (lip) and SP (sepal/petal) complexes, respectively (Hsu et al., 2015). The higher‐order heterotetrameric L (lip) complex is exclusively required for lip determination, while the SP (sepal/petal) complex specifies sepal/petal formation (Hsu et al., 2015). How exactly orchid perianth formation and characteristics, such as morphological features and size, are regulated after P‐code complexes are established remains obscure. For example, lips are much larger than sepals/petals in Oncidium orchids. By contrast, lips are much smaller than sepals/petals in Phalaenopsis orchids. It is therefore a reasonable assumption that these two orchid genera have opposing mechanisms for regulating perianth size in the lips and sepals/petals.
TETRASPANIN genes have been identified in multicellular eukaryotes (Huang et al., 2005; Lambou et al., 2008; Wang et al., 2015) but were absent in yeast (Garcia‐España et al., 2008). The structures of tetraspanin family proteins were evolutionary conserved (Zuidscherwoude et al., 2015) and contained five distinct regions, including a large extracellular domain, a small extracellular domain, transmembrane domains, palmitoylation sites, and cytoplasmic domains (Stipp, Kolesnikova, & Hemler, 2003). In mammalian cells, tetraspanins form a tetraspanin‐enriched microdomain (TEM), known as a tetraspanin web, by interacting with one another, specific lipids and other transmembrane proteins, including integrins and other adhesion receptors (Charrin, Jouannet, Boucheix, & Rubinstein, 2014; Hemler, 2005; Reimann, Kost, & Dettmer, 2017; Zuidscherwoude et al., 2015). The organization of the integrin–tetraspanin microdomain and modulation of adhesion‐dependent signaling were mediated by a posttranslation modification of tetraspanins via palmitoylation (Berditchevski, Odintsova, Sawada, & Gilbert, 2002). The mutation of juxtamembrane cysteines, the palmitoylation site of a human tetraspanin CD81, reduced the ability of CD81 to interact with other proteins (Delandre, Penabaz, Passarelli, Chapes, & Clem, 2009). Additionally, the palmitoylation‐deficient human tetraspanin CD82 lost the function of inhibition of cancer cell migration and invasion (Zhou, Liu, Reddivari, & Zhang, 2004).
In plants, tetraspanins have been reported to have diverse functions during growth and development (Reimann et al., 2017). In rice and Arabidopsis, 15 and 17 TETRASPANIN genes were identified, respectively (Boavida, Qin, Broz, Becker, & McCormick, 2013; Cnops et al., 2006). Arabidopsis TETRASPANIN1(TET1)/TORNADO2/EKEKO has a function in leaf morphogenesis and root patterning (Cnops et al., 2006; Lieber, Lora, Schrempp, Lenhard, & Laux, 2011; Olmos, Reiss, & Dekker, 2003). TET1 also involved in modulating auxin homeostasis and the transition from floral meristem termination to gynoecium development (Yamaguchi, Huang, Xu, Tanoi, & Ito, 2017). TET5 and TET6 have redundant functions in restricting cell proliferation during root and leaf growth (Wang et al., 2015). TET13 has a function in the primary root, affecting apical meristem size and root length, and in lateral root initiation (Wang et al., 2015). However, the functions of most plant TETRASPANIN genes still remain to be investigated (Reimann et al., 2017).
It is well known that phytohormone auxin plays a critical role in plant growth, including photo‐ and gravitropism, root formation, embryo development, petal development, and anther dehiscence (Cecchetti et al., 2013; Lampugnani, Kilinc, & Smyth, 2012; Lavenus et al., 2013; Mironova, Teale, Shahriari, Dawson, & Palme, 2017; Moller et al., 2017; Overvoorde, Fukaki, & Beeckman, 2010; Péret et al., 2013; Sauret‐Gueto, Schiessl, Bangham, Sablowski, & Coen, 2013; Teale, Paponov, & Palme, 2006). The gradients and distributions of auxin determine organ initiation, tissue patterning, and differential growth during tropic responses (Armengot, Marques‐Bueno, & Jaillais, 2016; Finet & Jaillais, 2012; Zhao, 2010). During root formation, auxin is synthesized in the root apex (Petersson et al., 2009), then effluxed by PIN‐FORMED proteins (PIN), and influxed by AUX1/LIKE AUX1 proteins (AUX1/LAX) that generate a longitudinal gradient of auxin in roots (Armengot et al., 2016). When roots are under 90‐degree gravitropic stimulation, auxin redistributes to the lower side of the roots and inhibits the elongation of epidermal cells, causing root bending (Sato, Hijazi, Bennett, Vissenberg, & Swarup, 2015). In addition, lateral root primordia are triggered by gravitropic stimulus and develop in eight stages (Péret et al., 2012).
Auxin has been thought to regulate petal development since the disrupted expression of an auxin‐inducible indicator DR5 were observed in petal loss (PTL) mutant plants (Lampugnani, Kilinc, & Smyth, 2013). The sizes of petals and the lengths of stamens were significantly reduced in Arabidopsis auxin response factor 6 (arf6) and arf8 double‐mutant plants (Nagpal et al., 2005). Auxin may regulate petal development and anther dehiscence through the regulation of jasmonate (JA) activity, since it has been shown that exogenous auxin inhibits the expressions of the JA biosynthesis gene DEFECTIVE IN ANTHER DEHISCENCE1 (DAD1) and 12‐oxophytodienoate reductase (OPR3) and results in anther indehiscence (Cecchetti et al., 2013). Interestingly, petal size is increased in opr3 mutants due to larger cell sizes at developmental stage 14 instead of stage 9, indicating that JA controls petal size by suppressing cell expansion at late stages (Brioudes et al., 2009). This assumption was supported by the fact that JA regulates an alternative splicing event in the ubiquitously expressed gene BIGPETAL (BPE) to translate a basic helix‐loop‐helix (bHLH) transcription factor BPEp that inhibits cell expansion in late development stages of petal growth (Brioudes et al., 2009; Varaud et al., 2011).
Auxin is also involved in response to abiotic stresses such as drought, salt, and cold. Exogenous auxin enhanced tolerance to drought (Shi et al., 2014) and rescued the salt hypersensitivity phenotype of plants overexpressing TTG2/WRKY44 (Li et al., 2015). Exogenous or enhanced production of endogenous auxin improved salt tolerance in sorghum and mung bean (Azooz, Shaddad, & Abdel‐Latef, 2004; Zahir, Shah, Naveed, & Akhter, 2010). Higher drought tolerance was observed in auxin‐overexpressing mutants yuc6‐1D and yuc7‐1D. Furthermore, yuc6‐1D mutant plants also showed delayed senescence (Kim et al., 2011; Lee et al., 2012).
In this study, we identified the tetraspanin gene Auxin Activation Factor (AAF) from Oncidium and Phalaenopsis orchids and demonstrated that the expression of orchid AAF orthologs is associated with the regulation of the perianth size after its identity is determined by the P‐code complexes. Further analysis of an Arabidopsis AAF gene reveals that AAF controls not only flower organ size but also various developmental processes such as anther dehiscence, drought tolerance, and lateral root formation by enhancing the efficiency of the auxin response in plants. Our results suggest a novel interaction between the tetraspanin gene AAF and auxin in regulating plant growth and development.
2. MATERIALS AND METHODS
2.1. Plant materials and growth conditions
Seeds for Arabidopsis were sterilized and placed on agar plates containing Murashige and Skoog medium (Murashige & Skoog, 1962) at 4°C for 2 days. The seedlings were then grown in growth chambers under long‐day conditions (16‐hr light/8‐hr dark) at 22°C for 10 days before being transplanted to soil. The light intensity of the growth chambers was 150 μE m−2 s−1. Species, cultivars, and peloric mutants of orchids used in this study, including the Oncidium (O. Lemon Heart and the associated peloric mutants O. Lemon Heart Trilips) and moth orchids (Phalaenopsis Sogo Yukidian “V3,” P. Red Bell, P. Gold Diamond, and the associated peloric mutants P. Big‐Lip), were maintained in the greenhouse of National Chung‐Hsing University, Taichung, Taiwan.
2.2. Cloning of orchid AAF cDNAs
The transcriptomic RNA‐Seq for Oncidium (O. Lemon Heart) and Phalaenopsis (P. Sogo Yukidian “V3”) floral organs (lip and sepal/petal) at floral bud stage (6 mm for Oncidium and 15 mm for Phalaenopsis) was performed and data analyzed. An Oncidium Auxin Activation Factor (OnAAF) gene which expressed specifically higher in lip than in sepal/petal of Oncidium was identified. PaAAF which showed highest sequence identity/similarity to OnAAF and expressed specifically higher in petal than in lip of Phalaenopsis was also identified. The cDNA contained the 3′‐end of OnAAF was obtained by 3′‐RACE using the BD SMART RACE cDNA Amplification Kit (Clontech Laboratories) following the 5′ gene‐specific primer OnAAF‐1. The full‐length cDNA of OnAAF was amplified by PCR using 5′ primer, OnAAF‐1, and the 3′ primer, OnAAF‐2. The full‐length cDNA of Phalaenopsis PaAAF was amplified by PCR using 5′ primer, PaAAF‐1, and the 3′ primer, PaAAF‐2. Sequences for the primers are listed in Table S1.
2.3. Cloning of Arabidopsis AtAAF cDNA
Arabidopsis Auxin Activation Factor (AtAAF) (At4g30430), contains two exons separated by an intron, was identified on chromosome 4. cDNA containing an open reading frame of AtAAF was amplified by PCR using the 5' primer, MSIF‐3, and the 3' primer, MSIF‐4. The amplified fragment containing the cDNA of AtAAF gene was cloned into the linker region in binary vector pEpyon‐12K (CHY Lab) under the control of cauliflower mosaic virus (CaMV) 35S promoter (35S::AtAAF) and used for further plant transformation. Sequences for the primers are listed in Table S1.
2.4. Construction of 35S::AtAAFpalm
To generate the AtAAFpalm fragment, two fragments were amplified for mega PCR using cDNA of AtAAF as the template with primers, 5′MSIF‐3‐XbaI, 3′MSIF‐18‐C65S/C66S, 5′MSIF‐17‐C65S/C66S, and 3′MSIF‐20. The 3′MSIF‐18‐C65S/C66S primer (5′‐GAAGCCACGTCACTCTGGAAGAAGATC‐3′) contained two 1 bp substitution from GCAACA to GGAAGA and 5′MSIF‐17‐C65S/C66S primer (5′‐GATCTTCTTCCAGAGTGACGTGGCTTC‐3′) contained two 1 bp substitution from TGTTGC to TCTTCC that convert Cys65/Cys66 of the AtAAF protein to Ser65/Ser66. Another two fragments were amplified with primers 5′‐MSIF‐19, 3′‐MSIF‐21‐C252S/C253S, 5′‐MSIF‐22‐C252S/C253S, and 3′‐MSIF‐4‐KpnI. The 3′‐MSIF‐21‐C252S/C253S primer (5′‐GAAAGCGGAAGATCCCATAGCGTAGAC‐3′) contained two 1 bp substitution from GCAACA to GGAAGA and 5′‐MSIF‐22‐ C252S/C253S primer (5′‐GTCTACGCTATGGGATCTTCCGCTTTC‐3′) contained two 1 bp substitution from TGTTGC to TCTTCC that convert Cys252/Cys253 of the AtAAF protein to Ser252/Ser253. There is an endogenous XhoI recognition site in the first fragment. The XbaI‐XhoI and XhoI‐KpnI PCR fragments were inserted in the binary vector pEpyon‐12K (CHY Lab), by endogenetic XhoI (5′‐CTCGAG‐3′) digestion site ligation. A multiple point mutation fragment was generated that contained two bp substitution from TGTTGC to TCTTCC that converts Cys65, Cys66, Cys252, and Cys253 of the AtAAF protein to Ser65, Ser66, Ser252, and Ser253. The product contained the generated XbaI recognition site (5′‐TCTAGA‐3′) and KpnI recognition site (5′‐GGTACC‐3′) to facilitate the cloning of AtAAF cDNA. Sequences for the primers are listed in Table S1.
2.5. AtAAF::GUS fusion construct
For the AtAAF::GUS construct, the AtAAF promoter (2.0 kb) was obtained by PCR amplification from the genomic DNA using the pMSIF‐1 and pMSIF‐2 primers and then cloned into pGEM‐T easy vector (Promega). The full‐length promoter for AtAAF (2.0 kb) was then subcloned into the linker region before the β‐Glucuronidase (GUS) coding region in binary vector pEpyon‐01K (CHY Lab). The primers contained the generated PstI (5′‐CTGCAG‐3′) recognition site and SalI (5′‐GTCGAC‐3′) recognition site to facilitate the cloning of the promoter. Sequences for the primers are listed in Table S1.
2.6. Construction of AtAAF+GFP construct
AtAAF cDNAs were subcloned into the multiple cloning site of binary vector pEpyon‐12K (CHY Lab) upstream of the mGFP5 sequence and under the control of the CaMV 35S promoter. The fragments contained the generated XbaI and KpnI recognition site to facilitate the cloning of the AtAAF. This construct was used for plant transformation. The sequences for the primers are listed in Table S1.
2.7. Real‐time PCR analysis
For real‐time quantitative RT‐PCR, the reaction was performed on an MJ Opticon system (MJ Research) using SYBR Green Real‐Time PCR Master Mix (TOYOBO Co., LTD.). The amplification conditions were 95°C for 10 min, followed by 40 cycles of amplification (95°C for 15 s, 58°C for 15 s, and 72°C for 30 s, followed by plate reading) and melting (50–95°C with plate readings every 1°C). The sequences for the primers that were used for the real‐time quantitative RT‐PCR for OnAAF, OAGL6‐2, PaAAF, PeMADS9, AtAAF, EDF1, EDF2, ERF1, SAG12, GFP, DAD1, OPR3, MYB26, NST1, NST2, and BPEp are listed in Table S1. The Arabidopsis housekeeping gene UBQ10 was used as a normalization control with the following primers: RT‐UBQ10‐1 and RT‐UBQ10‐2. The transcript levels for orchid genes were determined using three replicates and were normalized using reference genes ACTIN (primers: RT‐PACT4‐1 and RT‐PACT4‐F) for Phalaenopsis (Hsu et al., 2015) and α‐tubulin (primers: RT‐OTUB‐1 and RT‐OTUB‐2) for Oncidium (Chang et al., 2010) as described previously (Hsu et al., 2015). The data were analyzed using CFX Manager™ software (version 3.1; Bio‐Rad) according to the manufacturer's instructions. The “delta–delta method” formula 2−[△CP sample‐△CP control], where represents perfect PCR efficiency, was used to calculate the relative expression of the genes. To calculate the statistical significance, unpaired t tests were used.
2.8. Plant transformation and transgenic plant analysis
Constructs made in this study were introduced into Agrobacterium tumefaciens strain GV3101 and transformed into Arabidopsis plants using the floral dip method as described elsewhere (Clough & Bent, 1998). Transformants that survived in the medium containing kanamycin (50 μg/ml) were further verified by RT‐PCR analysis.
2.9. Stress treatments
For drought treatment, 1‐week‐old Arabidopsis seedling was removed from the MS medium and exposed to a stream of air for various times. Subsequently, seedlings were weighted, the pictures of seedlings were taken and the RNA of seedlings was extracted for gene expression analysis. For salt stress treatment, 1‐week‐old seedlings were removed from the MS medium and NaCl were added to the liquid MS medium to a final concentration of 150 mM. Seedlings were dipped for various times, and the RNA of seedlings was extracted for gene expression analysis. Plants were grown in MS medium plate with 150 mM NaCl for survival rate calculation.
2.10. Alexander's staining
For pollen analysis, the pollen grains were mounted with Alexander's stain as previously described (Alexander, 1969).
2.11. Cryo‐scanning electron microscopy
Cryo‐scanning electron microscopy (SEM) was performed according to the method as previously described (Hsu et al., 2015).
2.12. Confocal laser scanning microscopy
The flower tissues were imaged using an Olympus FV1000 confocal microscope as described previously (Chang et al., 2010; Peng et al., 2013). The plant cell walls were stained with 40 μg/ml propidium iodide (PI; Molecular Probes). PI was excited by a 543‐nm He/Ne laser line, and the emission was collected at 555–655 nm.
2.13. Lignin staining
For lignin analysis, fresh anthers were stained with 0.01% auramine O (Pesquet et al., 2005) and observed with a confocal microscope (Olympus FV1000). The lignified cells were observed under 488 nm excitation/510–560 nm emission. Cellulose of cell walls was stained with 0.07% calcofluor white and observed under 370 nm excitation/420 nm emission.
2.14. Application of jasmonate
All opened flowers (after stage 14) were removed from the inflorescence, and the remaining flower bud clusters were dipped into 50 µM (±) Jasmonate (Sigma) dissolved in 0.05% aqueous Tween 20.
2.15. Histochemical GUS assay
Histochemical staining was performed under the standard method described previously (Jefferson, Kavanagh, & Bevan, 1987).
2.16. Measurement of the concentration of the IAA
Flower buds of Arabidopsis were ground under liquid nitrate then resuspended in 90 μl phosphate‐buffered saline (PBS) buffer for total IAA extraction. The IAA quantification was performed by enzyme‐linked immunosorbent assay (ELISA) kit (LYBDBio).
2.17. Biotin switch assay of S‐acylation
The biotin switch assay was performed as previously described (Hemsley, Taylor, & Grierson, 2008) with minor modification. Briefly, about 0.8 mg total proteins were solubilized and incubated with 25 mM N‐ethylmaleimide (Thermo Fisher Scientific) to block free sulfhydryls. Free N‐ethylmaleimide was removed by concentration tube (Millipore) then divide into two equal aliquots. One aliquot was incubated with 1 M hydroxylamine (NH2OH) (Thermo Fisher Scientific) to cleave thioester bonds and with 1 mM EZ‐link™ biotin‐HPDP (Thermo Fisher Scientific) to label liberated sulfhydryls. Hydroxylamine was replaced by Tris‐HCl buffer in the remaining aliquot as a control. Free reagent was removed as mentioned above, and 12 μl of the solution was removed as a loading control. Biotinylated proteins were then purified with 15 μl NeutrAvidin‐agarose (Thermo Fisher Scientific) and analyzed by Western blotting using GFP‐specific antibodies.
2.18. Virus‐induced gene silencing experiment
The virus‐induced gene silencing (VIGS) experiments in orchids were performed as described previously (Hsu et al., 2015). DNA fragments for OAGL6‐2 and PeMADS9 (OAGL6‐2 homolog) for insertion into the VIGS vector pCymMV‐Gateway (Lu et al., 2012) were obtained by PCR amplification using the following primers: OAGL6‐2 (in the C domain, 160 bp), O1‐gateway‐F, 5′‐GGGGACAAGTTTGTACAAAAAAGCAGGCTAGCAAATGGTGGGTCATC‐3′; O1‐gateway‐R, 5′‐GGGGACCACTTTGTACAAGAAAGCTGGGTAAATGGTTGCTTCAGAAG‐3′. PeMADS9 (in the C domain, 150 bp), PeM9‐VIGS‐F, 5′‐GGGGACAAGTTTGTACAAAAAAGCAGGCTCAGGTGATATTAACAAGCAGCTTAAACA‐3′; PeM9‐VIGS‐R, 5′‐GGGGACCACTTTGTACAAGAAAGCTGGGTCTTTGAAGAGTGGGTTCTGTATCCATG‐3′. The underlined sequences are attB sites for in vitro recombination with attP sites in the VIGS vector pCymMV‐Gateway to generate recombinant clones using Gateway® BP Clonase II Enzyme Mix (Invitrogen™, Life Technologies). pCymMV‐Gateway‐OAGL6‐2, pCymMV‐Gateway‐PeMADS9, and the empty pCymMV‐Gateway as a control were transformed into Agrobacterium tumefaciens EHA105 for further inoculation. For Oncidium Lemon Heart leaf infiltration, suspensions were injected into the third leaf (L3) just below the site where the inflorescence emerged. For Phalaenopsis Sogo Yukidian “V3” leaf infiltration, suspensions were injected into the leaf just above the site where the inflorescence emerged. For every infiltration, at least three plants were inoculated with each pCymMV‐Gateway construct. Flower samples were collected and analyzed at 45 DPI (days postinoculation), when the buds at the end of the Oncidium inflorescences or the last bud of the Phalaenopsis inflorescences bloomed.
3. RESULTS
3.1. The expression of Oncidium OnAAF is positively correlated with the growth of lip and is down‐regulated in OAGL6‐2 VIGS small lips
To explore how orchid perianth formation and characteristics, such as morphological features and size, are regulated after P‐code complexes are established, NGS analysis was used to identify differentially expressed genes in the lips or sepals/petals of Oncidium orchids. An Oncidium TET protein (Figures S1 and S2) was selected for further analysis because it was (a) predominantly expressed in the lips rather than in the sepals/petals (Figure 1a) and (b) was positively correlated with the expression of the L complex gene OAGL6‐2 during different stages of lip development (Figure 1b). We renamed the protein "Auxin Activation Factor" (AAF) for data presented in this study.
Figure 1.

Functional analysis of OnAAF and PaAAF in Oncidium and Phalaenopsis orchids. (a) Total RNA samples isolated from the lips (L), petals (P), dorsal sepals (DS), and lateral sepals (LS) of mature Oncidium flowers were used as templates to detect the expression of OnAAF by quantitative real‐time PCR. (b) Detection of OAGL6‐2 and OnAAF expression in the lips of Oncidium floral bud (FB), open flower (OF), and mature flower (MF). (c) The flowers of Oncidium Lemon Heart peloric mutant (Trilips) with two petals transformed into lips (left), the OAGL6‐2 VIGS flower (right) containing green sepal/petal‐like sectors in the lips (arrow), which are smaller than those in the wild‐type control (Mock, middle). Bar = 10 mm. Top and bottom rows indicate the adaxial and abaxial side of the flower, respectively. (d) Detection of OnAAF expression in L, P, DS, and LS of mature flowers of Oncidium Lemon Heart peloric mutant (Trilips). (e) OnAAF expression was higher and promoted more cell division/expansion in lips than sepals/petals, resulting in the production of larger lips than sepals/petals in Oncidium orchids. (f) Detection of OAGL6‐2 and OnAAF expression in the lips of OAGL6‐2 VIGS (O6‐2‐V‐1 and 2) and wild‐type control (Mock) flowers of Oncidium Lemon Heart. (g) The flower of PeMADS9 (OAGL6‐2 ortholog) VIGS Phalaenopsis Sogo Yukidian “V3” (Pe9‐VIGS, right) contains lips, which are larger and more spread out than in the wild‐type control flower (Mock, left) Bar = 20 mm. (h) Detection of PaAAF expression in lips (Lip) and P of Phalaenopsis Sogo Yukidian “V3” FB and OF. (i) The front lobe (L–f) and side lobe (L‐s) of the lips from (g). Bar = 10 mm. (j) Detection of PeMADS9 (PeM9) and PaAAF expression in lips of PeMADS9 VIGS (PM9‐V) and wild‐type control (Mock) flowers of Phalaenopsis Sogo Yukidian “V3.” (k) The flowers of a Phalaenopsis peloric mutant (Big‐Lip) have much larger sepal/petal‐like L. (l) Detection of PaAAF expression in L, P, DS, and LS from the Big‐Lip mutant flower in (k). (m) Higher PaAAF expression promoted greater cell division/expansion in sepals/petals than in lips, resulting in the production of larger petals than lips in Phalaenopsis orchids. (n) Two varieties of Phalaenopsis orchids with relatively large (P. Red Bell) and small (P. Gold Diamond) mature flower sizes. Bar = 20 mm. (o) The size comparison of the P of P. Red Bell (RB) and P. Gold Diamond (GD) from (n). (p) The comparison of the epidermal cell size in the petals of P. Red Bell (RB) and P. Gold Diamond (GD) from (n). (q, r) SEM of the epidermal cells in the petals of P. Red Bell (q) and P. Gold Diamond (r) from (n). Bar = 100 μm. (s) The comparison of the total cell number in the petals of P. Red Bell (RB) and P. Gold Diamond (GD) from (n). (t) Detection of PaAAF expression in P. Red Bell (RB) and P. Gold Diamond (GD) FB, OF, and MF
The Oncidium Auxin Activation Factor (OnAAF) is a member of the tetraspanin family in the TET7/8/9 group (Figures S1 and S2) encoding a protein of 270 amino acids (Figure S1) which showed the 57% identity and 75% similarity to its Arabidopsis TETRASPANIN orthologue AtAAF (At4g30430) (originally known as TET9) (Figures S1 and S2). The OnAAF protein contained four transmembrane domains, two extracellular loops, and cytoplasmic N and C terminals (Figure S1). When the sequence of the OnAAF protein was further analyzed, four palmitoylation sites were predicted by CSS‐Palm (Zhou, Xue, Yao, & Xu, 2006) (Figure S1).
In the Oncidium Trilips mutant (Figure 1c), OnAAF was clearly up‐regulated in the enlarged lip‐like petals (Figure 1d), indicating that OnAAF likely promoted lip expansion after the P code was established (Figure 1e). To further test this hypothesis, we attempted to suppress the activity of the L complex by VIGS of OAGL6‐2 in the lips of Oncidium Lemon Heart. VIGS of OAGL6‐2 reduced the size of the lip in flowers (Figure 1c), resulting in a smaller sepal/petal‐like lip structure (Figure 1c) that was correlated with the down‐regulation of OAGL6‐2 expression (Figure 1f). Interestingly, expression of OnAAF decreased significantly in the sepal/petal‐like lips of flowers with OAGL6‐2 VIGS (Figure 1f).
Furthermore, the expression of OnAAF in lips was found to be expressed higher during early developmental stage (10 mm bud), gradually decreased in open flower (15 mm) and decreased significantly in mature flower (Figure 1b). The size of the lips was much smaller in 10 mm bud than in mature flower (Figure S3A,B,E) (Chang et al., 2010). The cell size in lips was also smaller in 10 mm bud than in mature flower (Figure S3C,D,F) (Chang et al., 2010). However, the total cell number was similar in these two stages of lips (Figure S3G). This result indicated that cell expansion rather than cell division was occurred in lip from early stage of flower buds to later stage of mature flower. Thus, the high level of the OnAAF expression in early lip development revealed that OnAAF expression is associated with the regulation of the lip size primarily due to the control of cell division rather than cell expansion during lip development.
3.2. The expression of Phalaenopsis PaAAF is positively correlated to the growth of perianth and is up‐regulated in PeMADS9 (OAGL6‐2 ortholog) VIGS‐enlarged lips
To investigate the function of orchid AAF, an OnAAF ortholog PaAAF which showed highest sequence identity/similarity to OnAAF, was identified through the analysis of NGS data and characterized in Phalaenopsis Sogo Yukidian “V3” (Figure 1g). The PaAAF protein encodes 271 amino acids and shows 86% identity and 95% similarity to OnAAF (Figures S1 and S2).
Compared with Oncidium orchids, Phalaenopsis orchids have significantly smaller lips relative to their sepals/petals (Figure 1g). Not surprisingly, the expression of PaAAF was lower in the lips than in the petals during the same developmental stages (Figure 1h). These results suggest that PaAAF expression is also associated with the promotion of perianth organ growth in Phalaenopsis orchids. In petals, the expression of PaAAF was found to be expressed higher during early developmental stage (15 mm bud) and decreased in open flower (Figure 1h). The size of the petals was much smaller in 15 mm bud than in open flower (Figure S4A,B,E). The cell size in petals was much smaller in 15 mm bud than in open flower (Figure S4C,D,F). The total cell number was however similar in these two stages of petals (Figure S4G). This result indicated that cell expansion rather than cell division was correlated with the increasing size of petal from early stage to later stage of flower development. Thus, the high level of the PaAAF expression in early petal development revealed that PaAAF expression is associated with the regulation of the petal size primarily due to the control of cell division rather than cell expansion during lip development.
A VIGS strategy was further used to suppress PeMADS9 (OAGL6‐2 ortholog) expression in Phalaenopsis Sogo Yukidian “V3.” The PeMADS9‐silenced lips were much more spread out and expanded to a larger size compared with the sepals/petals (Figure 1g,i). The enlarged sepal/petal‐like lip structure that resulted from PeMADS9 VIGS was associated with significant down‐regulation of PeMADS9 and up‐regulation of PaAAF (Figure 1j). Analysis of the Phalaenopsis big‐lip mutants in which the lip was converted into a petal‐like structure (Figure 1k) revealed that PaAAF was clearly up‐regulated in these petal‐like lips to a level similar to that in the petals/sepals (Figure 1l). This result indicates that the expression level of PaAAF is correlated with the expansion and size of the perianth (Figure 1m). Greater PaAAF expression is associated with the production of larger perianth organs. This observation was further supported by analyzing two varieties of Phalaenopsis orchids that significantly differed in the size of their mature flowers but were otherwise morphologically similar (Figure 1n). This analysis indicated that the larger size of Phalaenopsis Red Bell flowers (Figure 1o) was associated with larger cells (Figure 1p,q,r), more cells (Figure 1s) and higher PaAAF expression (Figure 1t) in the perianth as compared to Phalaenopsis Gold Diamond flowers. These results suggest that AAF orthologs are regulators of perianth size in orchids.
3.3. AtAAF expression is high in flower buds and AtAAF protein is localized at the plasma membrane and can be palmitoylated
To further validate the function of the AAF ortholog, Arabidopsis AtAAF/TET9 (At4g30430) was characterized extensively in this study. Based on Arabidopsis eFP browser data (Schmid et al., 2005; Winter et al., 2007) (http://www.bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi), AtAAF (will be referred throughout this work) showed the most similar expression pattern in flower (higher in early than in late flower development) to OnAAF/PaAAF than other Arabidopsis TETRASPANIN genes. AtAAF contains two exons separated by a 425 base‐pair intron and encodes a protein of 272 amino acids which showed 62%/67% identity and 80%/83% similarity to the two most closely related TETRASPANIN proteins, At2g23810 (TET8) and At4g28050 (TET7), respectively (Figures S1 and S2).
AtAAF transcript was high in flower buds (Figure 2a) and was higher in sepals/petals/stamens during early flower development (stage 11) and in carpels during late flower development (stage 14) (Figure 2b). In AtAAF::GUS Arabidopsis flowers, GUS activity was detected in the developing sepals, petals and anthers before stage 10 (Figure 2c,d). GUS activity decreased in the sepals/petals and was absent in the anthers after stage 12 (Figure 2c,e,f). GUS activity was detected in developing ovules of the carpel from stage 12 until the maturation of the siliques (Figure 2e, f).
Figure 2.
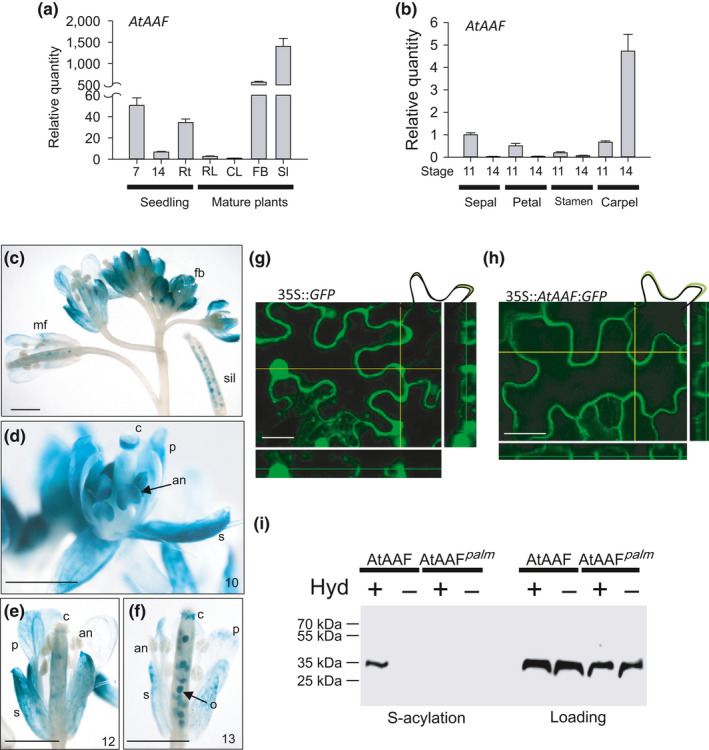
Analysis of the gene expression, protein localization, and palmitoylation for AtAAF in Arabidopsis. (a) The detection of AtAAF expression at 7 days after germination (DAG), 14 DAG, roots (Rt), rosette leaves (RL), cauline leaves (CL), floral buds (FB), and siliques (Sl). (b) The detection of AtAAF expression in sepal, petal, stamen, and carpel of stages 11 and 14 Arabidopsis flowers. (c) GUS staining pattern in floral buds (fb), mature flower (mf), and silique (sil) from an AtAAF::GUS transgenic plant. Bar = 1 mm. (d–f) GUS was detected in sepals (s), petals (p), and anther (an) of stage 10 (d) AtAAF::GUS flowers. GUS activity decreased in s and p and was absent in the anther after stage 12 (e, f). GUS was detected in ovules (o) after pollination (f). c, carpel. Bar = 1 mm. (g, h) Transient expression of 35S::GFP and 35S::AtAAF+GFP in tobacco cells. AtAAF+GFP fusion protein accumulated at the plasma membrane (h) whereas GFP protein accumulated in the cytosol (g). Bar = 30 μm. (i) Assaying palmitoylation of AtAAF and dominant negative mutant AtAAFpalm. Proteins were prepared from the 35S::AtAAF+GFP and 35S::AtAAFpalm +GFP plants by the biotin switch palmitoylation assay. Western blot analysis of proteins with the presence (+) and absence (−) of hydroxylamine treatment using a GFP antibody
An Agrobacterium infiltration‐mediated transient expression assay was applied in identification of the localization of AtAAF protein. The cDNA of AtAAF fused with GFP driven by the cauliflower mosaic virus (CaMV) 35S promoter (35S::AtAAF+GFP) was transformed into Agrobacterium and infiltrated into the leaf epidermis of Nicotiana benthamiana. The result showed that AtAAF+GFP fusion proteins accumulated at the plasma membrane (Figure 2h). In contrast, GFP proteins accumulated mainly in the cytosol (Figure 2g).
Palmitoylation of tetraspanins in mammalian cells has been reported previously (Charrin et al., 2002; Hua, Green, Wong, Warsh, & Li, 2001; Israels & McMillan‐Ward, 2010; Yang et al., 2002). Similar to OnAAF and PaAAF in orchids, AtAAF is predicted to be a member of the tetraspanin family and contains four palmitoylation sites, C65, C66, C252 and C253 (Figure S1), predicted by CSS‐alm (Ren et al., 2008). It is possible that AtAAF may also require palmitoylation to perform its function and that a mutation in the palmitoylation sites may generate a palmitoylation‐deficient mutant for AtAAF. Therefore, we generated transgenic plants that ectopically expressed AtAAF (35S::AtAAF) or a palmitoylation‐deficient mutant form of AtAAF (AtAAFpalm ) with multiple cysteine point mutations at C65S, C66S, C252S, and C253S (35S::AtAAFpalm ) fused with GFP. To determine whether the AtAAF protein was palmitoylated, AtAAF+GFP or AtAAFpalm+GFP was isolated from 35S::AtAAF+GFP or 35S::AtAAFpalm+GFP transgenic plants and a biotin switch assay (Hemsley et al., 2008) was performed using anti‐GFP antibody (Figure 2i). The results indicated that the AtAAF protein was indeed palmitoylated, whereas the AtAAFpalm protein was not (Figure 2i).
3.4. Ectopic expression of AtAAF increases the size of flower organs and seeds
To investigate function of the AtAAF gene, 35S::AtAAF transgenic Arabidopsis were generated and analyzed. 35S::AtAAF transgenic plants showed abnormal phenotypes including sterility (see below) and produced larger flowers than did the wild‐type plants (Figure 3a), increasing petal size by approximately 42% (Figure 3a–c) in transgenic Arabidopsis. 35S::AtAAF petals contained similar numbers of cells (Figure 3d), but they were 40% larger (Figure 3e–g) than cells in wild‐type petals (Figure 3h,i). These results suggest that AtAAF promoted petal cell expansion. The length of mature siliques on 35S::AtAAF plants after manual self‐pollination was also increased compared with that on wild‐type siliques (Figure 3l). 35S::AtAAF seeds (Figure 3m) were clearly larger and heavier than wild‐type seeds (Figure 3n), increasing by approximately 74% (Figure 3p). Furthermore, the altered phenotype for the 35S::AtAAF plants was correlated with high level of AtAAF expression (Figure 3q).
Figure 3.
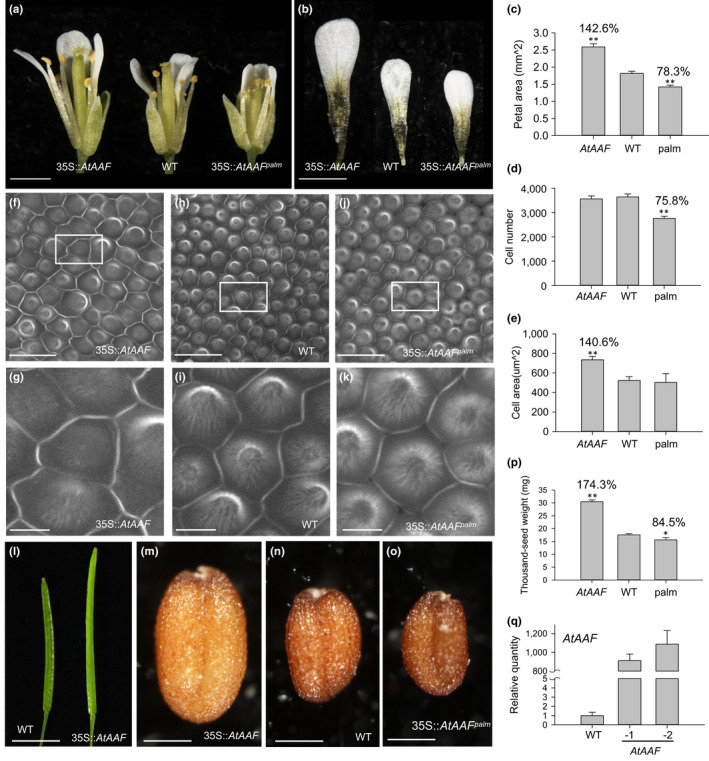
Functional analysis of flower and seed size for 35S::AtAAF and 35S::AtAAFpalm Arabidopsis. (a, b) The flower (a) and petals (b) of 35S::AtAAF (left), wild‐type (middle), and 35S::AtAAFpalm (right) Arabidopsis. Bar = 1 mm. (c) Size comparison of the petals from 35S::AtAAF (AtAAF), WT, and 35S::AtAAFpalm (palm) Arabidopsis. Petals from three flowers for each plant (AtAAF, WT, and palm) were used to measure the average size. The size of the wild‐type petals is set at 100%. (d, e) Comparison of total cell number (d) and the epidermal cell size (e) in petals from 35S::AtAAF (AtAAF), wild‐type (WT), and 35S::AtAAFpalm (palm) Arabidopsis. (f–k) Confocal laser scanning microscopy of the epidermal cells in the petals from 35S::AtAAF (f, g), WT (h, i), and 35S::AtAAFpalm (j, k) flowers. (g), (i), and (k) are close‐ups of (f), (h), and (j), respectively. Bar = 20 μm in (f, h, j) and 5 μm in (g, i, k). (l) The mature siliques of 35S::AtAAF (right) and wild‐type (WT, left) Arabidopsis. Bar = 5 mm. (m–o) The seeds of 35S::AtAAF (m), WT (n), and 35S::AtAAFpalm (o) Arabidopsis. Bar = 0.2 mm. (p) Comparison of the weight for 1,000 seeds from 35S::AtAAF (AtAAF), WT, and 35S::AtAAFpalm (palm) Arabidopsis. (q) Detection of AtAAF expression for one WT and two 35S::AtAAF (−1, −2) plant. The asterisks in (c, d, e, p) indicate a significant difference from the WT value (*p ≤ .05, **p ≤ .01). Statistic analysis was measured by Student's t test
3.5. Ectopic expression of palmitoylation‐deficient C65S, C66S, C252S, and C253S multiple point mutations in AtAAF causes early senescence and reduced size of flower organs and seeds
To further determine the role of AtAAF, AtAAF loss‐of‐function T‐DNA insertion lines, SALK_018161 and SALK_115646 (both containing an insertion in the 5′ promoter region) (Figure S5A), were analyzed. The result indicated that these AAF mutants were phenotypically indistinguishable from wild‐type plants in both vegetative and reproductive development. Further analysis indicated that the expression of AAF in these T‐DNA insertion mutants was completely abolished (Figure S5B). This finding indicates a possible functional redundancy between AAF and other unknown genes in Arabidopsis.
Based on the data from mammalian cells, tetraspanins form a tetraspanin‐enriched microdomain (TEM), a tetraspanin web, by interacting with one another and other transmembrane proteins such as integrins and other adhesion receptors (Charrin et al., 2014; Hemler, 2005; Reimann et al., 2017; Zuidscherwoude et al., 2015). The organization of the integrin–tetraspanin microdomain and modulation of adhesion‐dependent signaling were mediated by palmitoylation of tetraspanins (Berditchevski et al., 2002). It has been shown that ectopic expression of the human palmitoylation‐deficient tetraspanin CD151 in Rat‐1 cells impaired the interactions of the endogenous tetraspanins CD63 and CD81 and weakened the association of integrin with the tetraspanin‐enriched microdomains and affected integrin‐dependent signaling (Berditchevski et al., 2002). It also showed that the mutation in palmitoylation site reduced the ability of human tetraspanin CD81 to interact with other proteins (Delandre et al., 2009) and tetraspanin CD82 to inhibit cancer cell migration and invasion (Zhou et al., 2004). These results indicated that overexpression of a palmitoylation‐deficient tetraspanin could occupy the positions in the tetraspanin‐enriched microdomain and impaired the interactions for all other tetraspanins with the redundant function. This resulted in the production of a palmitoylation‐deficient mutant in which the function of all the redundant tetraspanins can be altered in the same time. Thus, a palmitoylation‐deficient AtAAF (AtAAFpalm ) with multiple cysteine point mutations at C65S, C66S, C252S, and C253S was ectopically expressed in Arabidopsis to generate palmitoylation‐deficient mutant of AtAAF.
Pronounced small‐leaf and early‐senescence phenotypes were observed in the severe 35S::AtAAFpalm transgenic plants (Figure 4a). Early senescence was observed in the rosette leaves (Figure 4a,b), developing cauline leaves (Figure 4c), and young flower buds (Figure 4d,e) of the severe 35S::AtAAFpalm plants. The senescence‐marker gene SAG12 (Swartzberg, Dai, Gan, Amasino, & Granot, 2006) and ethylene response genes EDF1, EDF2, and ERF1 (Alonso et al., 2003; Stepanova & Alonso, 2005) were clearly up‐regulated in these severe 35S::AtAAFpalm plants (Figure 4f). In the medium–severe 35S::AtAAFpalm transgenic plants, flower senescence was slightly promoted (at number 2–3) (Figure 4g) compared with wild‐type plants (at number 3–4) (Figure 4h). The severity of the phenotype for the 35S::AtAAFpalm plants was correlated with AtAAFpalm expression level (Figure 4i). The early senescence of flowers and the up‐regulation of SAG12 were opposite to those observed in 35S::AtAAF plants (Figure 4h,j).
Figure 4.
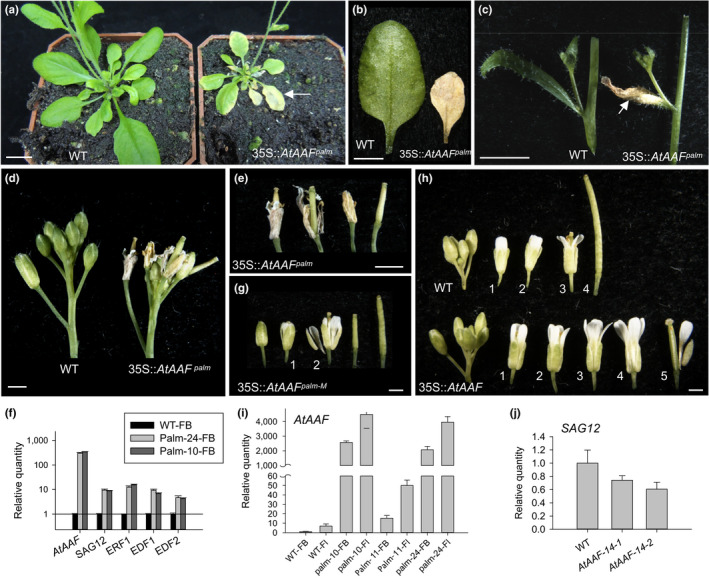
Phenotypic analysis of 35S::AtAAFpalm dominant negative mutant Arabidopsis. (a) Five‐week‐old 35S::AtAAFpalm Arabidopsis (right) produced smaller rosette leaves which showed earlier senescence (arrowed) than the wild‐type plants (left). Bar = 10 mm. (b, c) 35S::AtAAFpalm transgenic plants showed earlier senescence of rosette (b, right) and cauline leaves (c, right, arrow) than the wild‐type (WT, left). Bar = 5 mm. (d) Flowers from a severe 35S::AtAAFpalm inflorescence which showed earlier senescence than WT flowers. Bar = 1 mm. (e) Close‐up of the flowers from a severe 35S::AtAAFpalm inflorescence shown in (d). Bar = 1 mm. (f) Detection of expression for AtAAF, senescence‐related gene (SAG12), and the ethylene response genes (ERF1, EDF1, and EDF2) in floral buds (FB) before stage 12 from one WT plant and two severe 35S::AtAAFpalm plants (palm‐24 and palm‐10). Transcript levels in 35S::AtAAFpalm plants are presented relative to those in the wild‐type plant, which were set to 1. (g) Close‐up of the flowers from a medium–severe 35S::AtAAFpalm‐M inflorescence. The numbers indicate the positions of the open flowers. Bar = 1 mm. (h) Close‐up of the flowers from wild‐type (top row) and 35S::AtAAF (bottom row) inflorescences. The 35S::AtAAF flowers are clearly larger and abscised later than wild‐type flowers. The numbers indicate the positions of the open flowers. Bar = 1 mm. (i) Detection of AtAAF expression in FB and mature flowers (Fl) of one WT, two severe 35S::AtAAFpalm (palm‐10, ‐24), and one medium–severe 35S::AtAAFpalm (palm‐11) plants. (j) Detection of SAG12 expression in WT and two 35S::AtAAF (AtAAF‐14‐1 and AtAAF‐14‐2) plants
Interestingly, both flowers (Figure 3a) and petals (Figure 3b) of the medium–severe 35S::AtAAFpalm plants were ~22% smaller than those of wild‐type plants (Figure 3c). The number of epidermal cells in 35S::AtAAFpalm petals was ~24% less than in the wild type (Figure 3d), but the average cell size (Figure 3e) and morphology (Figure 3j,k) were comparable to that in the wild type (Figure 3e,h,i). In addition to petals, seeds produced by medium–severe 35S::AtAAFpalm plants (Figure 3o) were slightly smaller and approximately 15.5% lighter than wild‐type seeds (Figure 3n,p).
3.6. Ectopic expression of AtAAF shows male sterility and indehiscence of the anther which can be rescued by exogenic jasmonic acid
35S::AtAAF Arabidopsis plants also produced sterile flowers in which the siliques failed to elongate (Figure 5a,b). 35S::AtAAF anthers were indehiscent at all stages of flower development (Figure 5e,f). In contrast, wild‐type anthers were completely dehiscent, and pollen was released after stage 14 of flowering (Figure 5c,d). When further examined by SEM, the wild‐type anther dehisced at stage 14 (Figure 5g,h), and the pollen grains that were released from wild‐type anthers exhibited an egg shape, 30 × 5 μm in size (Figure 5k,l). When the indehiscent anthers from the stage 12 (Figure 5i) and 14 (Figure 5j) flowers of the 35S::AtAAF plants were opened manually and compared with wild‐type pollen, normal pollen grains were observed (Figure 5m,n).
Figure 5.
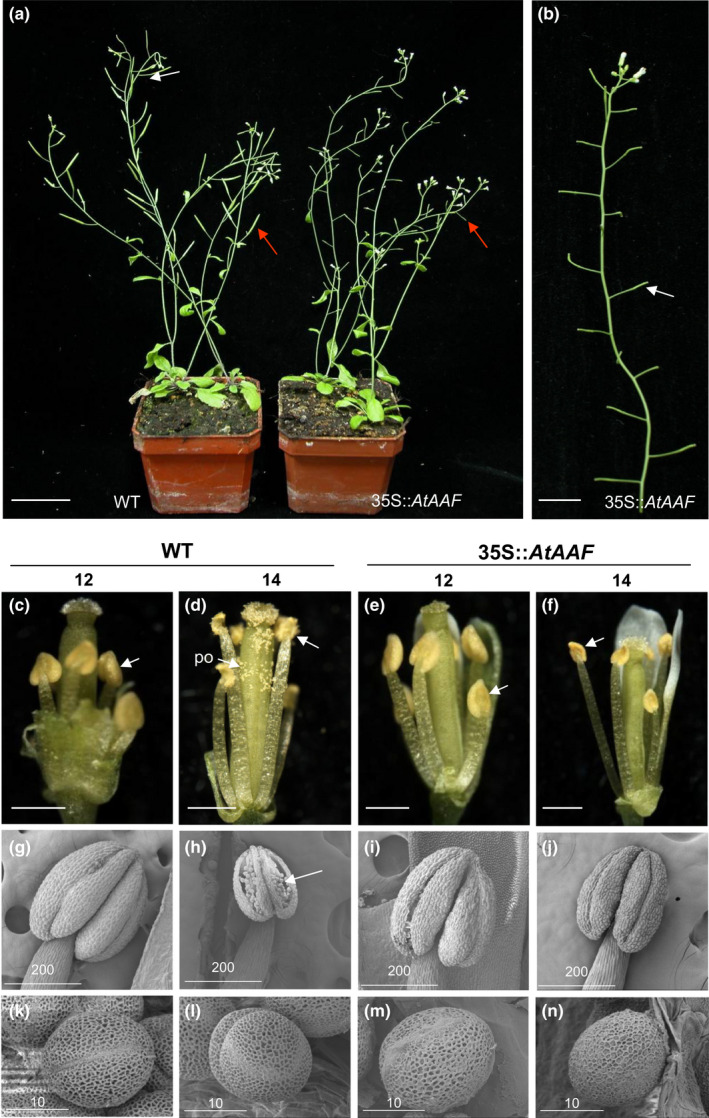
Phenotypic analysis of Arabidopsis plants ectopically expressing AtAAF. (a) A 35S::AtAAF plant (right) was sterile and produced short siliques (arrow), whereas wild‐type plants (WT, left) produced long, well‐developed siliques (arrow). Bar = 30 mm. (b) Inflorescences from a 35S::AtAAF plant that showed sterility with short siliques (arrow). Bar = 10 mm. (c–f) Indehiscent anthers (arrow) were observed at stage 12 in WT plants (c), stages 12 (e), and 14 (f) 35S::AtAAF flowers compared with stage 14 wild‐type flower (d), which showed normal anther dehiscence (arrow) and pollen (po) release. Bar = 0.5 mm. (g–j) Close‐up of stage 12 (g) wild‐type, stages 12 (i), and 14 (j) 35S::AtAAF anther compared with stage 14 wild‐type anther (h) by SEM, which showed normal anther dehiscence and pollen (arrow) release. Bar = 200 μm. (k–n) Close‐up of the stage 12 (k), 14 (l) wild‐type and stage 12 (m), 14 (n) 35S::AtAAF pollen grains by SEM. Bar = 10 µm
Further examination of the pollen by Alexander's stain, which can distinguish viable pollen from nonviable pollen (Alexander, 1969), normal viability (dark red staining) similar to that of wild‐type pollen (Figure S6A) was observed in the 35S::AtAAF pollen (Figure S6B). The pollen from 35S::AtAAF flowers was viable, as silique elongation and seed maturation were observed (Figure S6C,D) after the 35S::AtAAF flowers were manually self‐pollinated. The 35S::AtAAF pistil was normal, as fully developed siliques with normal seed development (Figure S6E,F) were observed after manual pollination with wild‐type pollen.
One of the reasons for defects in anther dehiscence is the mutation of JA biosynthesis genes such as DAD1 and OPR3 (Ishiguro, Kawai‐Oda, Ueda, Nishida, & Okada, 2001; Sanders et al., 2000). We thus investigated whether an external application of JA rescued the sterility of the 35S::AtAAF plants in a similar manner to that in JA‐treated dad1 flowers (Ishiguro et al., 2001). Dehiscence of anthers (Figure S6G) and elongation of siliques (Figure S6I) were observed in JA‐treated 35S::AtAAF flowers. These phenotypes were clearly distinguished from those observed in the JA‐untreated 35S::AtAAF flowers, which did not show anther dehiscence (Figure S6H) or silique elongation (Figure S6I). Further analysis indicated that the expressions of genes involved in JA biosynthesis, such as DAD1 and OPR3, were all significantly down‐regulated in the flowers of 35S::AtAAF plants (Figure S6J).
3.7. The secondary wall thickness is deficient and the expression of NST1/2 that participate in lignin accumulation of anther secondary wall is down‐regulated in 35S::AtAAF transgenic Arabidopsis
In wild‐type plants, secondary thickening occurs in the endothecium before anther dehiscence, and the surrounding cell layers of the anther do not undergo secondary thickening (Cecchetti et al., 2013; Yang et al., 2007). To examine the formation of secondary wall thickness, cellulose staining with calcofluor white and lignin staining with auramine O were performed in the endothecium of the developing anther in 35S::AtAAF and wild‐type plants. Secondary thickening in the endothecium was observed in wild‐type anthers at stage 11 (Figure S7A–C), just prior to anther dehiscence, as well as at stage 13 (Figure S7D–F), while the anthers dehisced completely. In contrast, no secondary thickening or lignification was observed in the anther endothecium of 35S::AtAAF plants at either stage 11 (Figure S7G–I) or stage 13 (Figure S7J–L). When the expression of MYB26, NST1, and NST2, which are necessary for secondary wall thickening in the anther wall (Mitsuda, Seki, Shinozaki, & Ohme‐Takagi, 2005; Steiner‐Lange et al., 2003), was examined, a clear down‐regulation for these genes was observed in 35S::AtAAF transgenic plants (Figure S7M). This result clearly indicates that altered anther dehiscence in 35S::AtAAF plants is correlated with altered expression of genes participating in the regulation of secondary wall thickening in anthers.
3.8. Ectopic expression of AtAAF enhances drought/salt tolerance and auxin response
35S::AtAAF expression also enhanced the drought and salt tolerance of transgenic Arabidopsis. One‐week‐old wild‐type seedlings clearly withered 15–30 min after they were removed from the MS medium and exposed to a stream of air (Figure 6a) and subsequently lost approximately 20%–40% of their weight (Figure 6c). By contrast, the 35S::AtAAF seedlings did not show signs of wilt 30 min after treatment, and their weight was similar to that of untreated seedlings (Figure 6b,c). The 35S::AtAAF seedlings started to show signs of wilt 60 min after treatment, and they lost approximately 30% of their weight (Figure 6b,c). During this same time, wild‐type seedlings severely withered and lost approximately 70% of their weight (Figure 6a,c). Unlike the 35S::AtAAF plants, the 35S::AtAAFpalm palmitoylation‐deficient mutant seedlings clearly showed lower drought tolerance, withered earlier and lost more weight than wild‐type seedlings from 15 to 60 min following drought treatment (Figure 6c). The wild‐type seeds barely germinated into seedlings (Figure 6d,f) and had a survival rate as low as 10% (Figure 6g) in MS medium containing 150 mM NaCl. By contrast, more than 60%–80% of the 35S::AtAAF seeds germinated and produced leaves (Figure 6d,e,g). Unsurprisingly, a clear up‐regulation of AtAAF expression was observed 15 min after drought or salt treatments (Figure 6h,i).
Figure 6.
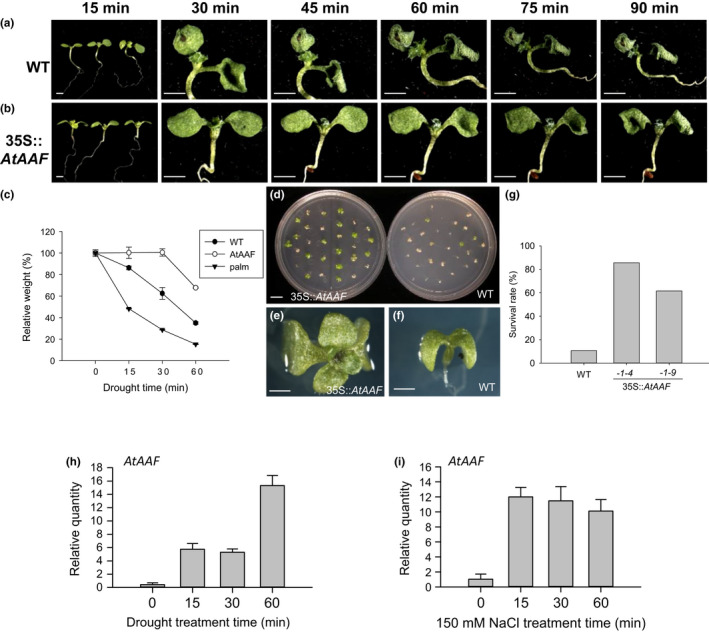
The analysis of drought and salt tolerance for 35S::AtAAF plants. (a, b) One‐week‐old wild‐type (a) and 35S::AtAAF (b) seedlings were tested for drought tolerance by removing them from MS medium and exposing them to a stream of air for 15, 30, 45, 60, 75, or 90 min. Bar = 5 mm. (c) The 35S::AtAAF (AtAAF) seedlings clearly withered later and lost less weight than wild‐type (WT) seedlings at 15, 30, and 60 min after drought treatment. In contrast, the 35S::AtAAFpalm (palm) seedlings clearly withered earlier and lost more weight than WT seedlings at 15, 30, and 60 min after drought treatment. (d) Comparison of germination for the 35S::AtAAF and WT seedlings grown on MS medium containing 150 mM NaCl. Bar = 10 mm. (e, f) Close‐up of the 35S::AtAAF (e) and WT (f) seedlings grown on MS medium containing 150 mM NaCl. Bar = 5 mm. (g) The survival rate for WT and two lines of 35S::AtAAF (‐1‐4, ‐1‐9) seedlings grown on MS medium containing 150 mM NaCl. (h, i) Detection of AtAAF expression after 15, 30, or 60 min of drought (h) or salt (i) treatment in wild‐type plants
It has been reported that auxin regulates flower organ development, since the elongation of petals and stamens is defective in auxin response factor 6 (arf6) and arf8 double‐mutant plants (Tabata et al., 2010). Auxin also regulates anther dehiscence by controlling the timing of endothecium secondary cell wall lignification and JA biosynthesis (Cecchetti et al., 2013), and auxin is involved in the response to abiotic stresses such as drought and salt (Li et al., 2015; Shi et al., 2014). Since 35S::AtAAF altered flower organ size, anther dehiscence, and the response to drought and salt stresses, AtAAF may function in auxin regulation. To investigate this assumption, 35S::AtAAF was introduced into DR5::GFP Arabidopsis, which contains a highly active synthetic auxin response element DR5 with a GFP reporter. The level of GFP transcript was clearly up‐regulated after 1–4 hr of external IAA treatment in both DR5::GFP and DR5::GFP/35S::AtAAF plants (Figure 7a). Interestingly, the increased amount of GFP transcript was much higher in DR5::GFP/35S::AtAAF than in DR5::GFP plants after IAA treatment (Figure 7a). A similar amount of IAA was detected in wild‐type, 35S::AtAAF, and 35S::AtAAFpalm plants (Figure 7b), suggesting that 35S::AtAAF enhanced efficiency of the auxin response rather than increasing auxin production.
Figure 7.

Investigation of the relationship between AtAAF and Auxin response in Arabidopsis. (a) Detection of GFP expression in DR5::GFP and DR5::GFP/35S::AtAAF transgenic Arabidopsis after 1, 2, and 4 hr of external IAA treatment. (b) Detection of the amount of IAA in wild‐type (WT), 35S::AtAAF (AAF), and 35S::AtAAFpalm (palm) flower buds (FB). (c) Number of lateral roots produced in WT and 35S::AtAAF (AtAAF) Arabidopsis seedlings grown on MS medium with or without IAA/2‐NOA (auxin influx inhibitor) treatments. (d) The development of the primordia (arrowhead) for lateral roots of the WT and 35S::AtAAF roots of 14 DAG seedlings 18 hr post‐gravitropic induction with (d‐3 and d‐4) or without (d‐1 and d‐2) IAA treatment. Stage I (d‐1), VII (d‐2), VI (d‐3), and beyond stage VIII (d‐4) primordia of lateral roots were formed. Bar = 50 μm. (e) Root gravitropic assays for DR5::GFP roots with 5 days of a 90‐degree gravitropic stimulus without (mock, e‐1) or with IAA (e‐3) or 2‐NOA (e‐5) treatment. Bar = 0.5 mm. The detection of GFP in the lower (L) and upper (U) parts of the DR5::GFP root tips 5 hr after 90‐degree gravitropic stimulus without (mock, e‐2) or with IAA (e‐4) or 2‐NOA (e‐6) treatment. Bar = 50 μm. (f) The detection of GFP integrated intensity in lower and upper parts of the DR5::GFP root tips from (e‐2), (e‐4) and (e‐6). (g) Root gravitropic assays for 35S::AtAAF/DR5::GFP roots after 5 days of a 90‐degree gravitropic stimulus without mock (g‐1) or with IAA (g‐3) or 2‐NOA (g‐5) treatment. Bar = 0.5 mm. The detection of GFP in lower (L) and upper (U) parts of the 35S::AtAAF/DR5::GFP root tips 5 hr after 90‐degree gravitropic stimulus without (mock, g‐2) or with IAA (g‐4) or 2‐NOA (g‐6) treatment. Bar = 50 μm. (h) The detection of GFP integrated intensity in lower and upper parts of the 35S::AtAAF/DR5::GFP root tips from (g‐2), (g‐4), and (g‐6)
Since auxin is a key signal that coordinates lateral root primordia outgrowth (Benková et al., 2003; Péret et al., 2012; Swarup & Bennett, 2009), lateral root production was measured in 2‐week‐old wild‐type and 35S::AtAAF seedlings. The results indicated that 35S::AtAAF plants produced significantly more lateral roots than did wild‐type plants (Figure 7c). Furthermore, IAA‐treated 35S::AtAAF plants produced three times more lateral roots than did IAA‐treated wild‐type plants (Figure 7c). This result supports an enhanced auxin response in 35S::AtAAF that could stimulate the formation of lateral roots. Formation of lateral roots was significantly prohibited in 35S::AtAAF and wild‐type seedlings grown at medium containing 2‐NOA (auxin influx inhibitor) as controls (Figure 7c).
3.9. Lateral root primordia of 35S::AtAAF develops faster than wild type
It has been reported that a 90‐degree gravitropic stimulus can induce lateral root primordia (LRP) to form on the outer side of bending roots (Lucas, Godin, Jay‐Allemand, & Laplaze, 2008; Péret et al., 2009). In addition, auxin plays a key role as a signal that coordinates lateral root primordia outgrowth, outer tissue deformation, and cell separation (Benková et al., 2003; Lucas et al., 2008; Swarup & Bennett, 2009). There are eight LRP stages previously described (Péret et al., 2012). When grown on a 90‐degree gravitropic stimulus, wild‐type seedlings produced stage I (Figure 7d‐1) lateral root primordia 18 hr post‐gravitropic induction (pgi), while 35S::AtAAF seedlings produced stage VII LRP (Figure 7d‐2) in the same time frame. Following auxin treatment, the wild‐type seedlings developed stage VI LRP (Figure 7d‐3), while the LRP produced by 35S::AtAAF seedlings were beyond stage VIII (Figure 7d‐4) at 18 hr pgi. These results again suggest that 35S::AtAAF could enhance the auxin response and hasten the formation of LRP on the roots.
3.10. 35S::AtAAF root tip retains gravitropism under auxin treatment
The movement of auxin in gravitropic response in roots is well understood (Chen, Rosen, & Masson, 1999). Exogenous auxin treatment on roots disrupts the endogenous auxin gradient and prevents gravitropism (Chen et al., 1999; Ishikawa & Evans, 1995). Auxin‐induced asymmetric gravitropism was observed in DR5::GFP transgenic roots after 5 days of a 90‐degree gravitropic stimulus (Figure 7e‐1). GFP intensity was greater in the lower parts than in the upper parts of DR5::GFP root tips 5 hr after 90‐degree gravitropic stimulus (Figure 7e‐2,f). The reaction to this asymmetric gravitropism was impaired in DR5::GFP transgenic roots 5 days after auxin treatment (Figure 7e‐3) due to the equal distribution of GFP in the lower and upper parts of the auxin‐treated DR5::GFP root tips 5 hr after 90‐degree gravitropic stimulus (Figure 7e‐4,f). As expected, the GFP signal was clearly higher in auxin‐treated than in untreated DR5::GFP root tips (Figure 7f).
Similar to DR5::GFP transgenic roots, the auxin‐induced asymmetric gravitropism was also retained in DR5::GFP/35S::AtAAF double transgenic roots under the same treatment (Figure 7g‐1). GFP fluorescence was also brighter in the lower parts of the DR5::GFP/35S::AtAAF root tips than in the upper parts (Figure 7g‐2,h). Unlike DR5::GFP transgenic roots, asymmetric gravitropism was still retained in DR5::GFP/35S::AtAAF double transgenic roots 5 days after auxin treatment (Figure 7g‐3). GFP intensity was higher in the lower parts than in the upper parts of the auxin‐treated DR5::GFP/35S::AtAAF root tips 5 hr after 90‐degree gravitropic stimulus (Figure 7g‐4,h) and was also higher in auxin‐treated than in untreated DR5::GFP/35S::AtAAF root tips (Figure 7h). Furthermore, the GFP signal was higher in DR5::GFP/35S::AtAAF than in DR5::GFP either with or without IAA treatment (Figure 7f,h). In controls, asymmetric gravitropism was absent in both DR5::GFP and DR5::GFP/35S::AtAAF transgenic roots 5 days after 2‐NOA (auxin influx inhibitor) treatment (Figure 7e‐5,g‐5). GFP was undetectable in both the lower and upper parts of the 2‐NOA‐treated DR5::GFP (Figure 7e‐6) and DR5::GFP/35S::AtAAF (Figure 7g‐6) root tips 5 hr after 90‐degree gravitropic stimulus.
4. DISCUSSION
In this study, orthologs of the tetraspanin gene AAF, which are associated with the regulation of perianth size, were identified and characterized in Oncidium and Phalaenopsis orchids. The results obtained indicate that the higher the expression level of orchid AAF orthologs, the larger size of the perianth organ produced. In Oncidium orchids, after perianth identity was determined by P‐code complexes, OnAAF was expressed at a higher level in lips than in sepals/petals and was associated with the production of larger lips. By contrast, PaAAF was more highly expressed in sepals/petals than in lips and was associated with the production of relatively small lips in Phalaenopsis orchids. This assumption was further supported by functional analysis of orchid OAGL6‐2 orthologs, a key component in L complex of P code (Hsu et al., 2015), through VIGS experiments. VIGS of OAGL6‐2 resulted in the production of smaller lips and down‐regulation of Oncidium OnAAF. By contrast, VIGS of PeMADS9 (an OAGL6‐2 ortholog) resulted in enlarged lips and up‐regulation of Phalaenopsis PaAAF. Our findings clearly reveal that although Oncidium and Phalaenopsis orchids do take different approaches to determining lip and sepal/petal size after P‐code complexes are established, the mechanism and the genes involved are the same. The rule is whenever bigger perianth organs are made in orchids, higher tetraspanin OnAAF/PaAAF expression is associated. Thus, high OnAAF expression in lips is associated with the production of large size of lips in Oncidium orchid whereas high PaAAF expression in petals is associated with the production of large size of petals in Phalaenopsis orchid after their organ identity was determined by P‐code complexes.
One interesting and critical issue that must be explored is the exact role of AAF orthologs in positively regulating perianth size and other plant developmental processes. The answer is largely revealed by the analysis of Arabidopsis AAF ortholog AtAAF. It is interestingly to note that AtAAF has been reported to be possibly regulated by MADS box gene AGL15 in Arabidopsis (Wang et al., 2015). Two putative MADS protein binding site of CArG boxes consensus sequence (CC(A/T)6GG) were identified in the promoter region of AtAAF (Figure S8). These results indicated that AAF orthologues could be regulated by MADS box genes in plants. Thus, it supported the assumption that MADS protein OAGL6‐2 orthologues may be able to regulate AAF orthologues in orchids after P code established.
Not surprisingly, ectopic expression of AtAAF increased the size of the flower organs in transgenic Arabidopsis. This result revealed a conserved role of the tetraspanin gene AAF in the regulation of flower perianth size in monocot orchids and dicot. 35S::AtAAF petals contained similar numbers of cells but were 40% larger than cells in wild‐type petals, suggesting that AtAAF enlarged petal size primarily by promoting petal cell expansion. In addition, 35S::AtAAF also showed a phenotype of male sterility and indehiscence of the anther resembling that found in plants with mutations in genes that participate in JA biosynthesis. The possible involvement of AtAAF in regulating JA activity was further evidenced by exogenic JA rescue of the anther indehiscence phenotype and the down‐regulation of genes (DAD1 and OPR3) that participate in JA biosynthesis in 35S::AtAAF transgenic Arabidopsis. It is interesting to explore the linkage between the regulation of petal size and JA activity controlled by AtAAF. Mutation in OPR3 produced large petals by increasing cell size at developmental stage 14, indicating that JA regulates petal size by suppressing cell expansion at late stages (Brioudes et al., 2009). Thus, AtAAF likely controls petal size and anther dehiscence simultaneously by negatively regulating JA activity. This assumption can be supported by the expression pattern of AtAAF that is predominantly expressed in sepals/petals/stamens during early flower development. AtAAF might suppress JA activity and prohibit early anther dehiscence and cell expansion at these early stages of flower development. When AtAAF expression significantly decreased after stage 12, JA activity was promoted, resulting in anther dehiscence and the suppression of petal growth by activating genes, such as BPEp (Figure S9), that are involved in limiting petal size by suppressing postmitotic cell expansion (Varaud et al., 2011) (Figure 8). In 35S::AtAAF Arabidopsis, the activity of JA was suppressed due to the high level of AtAAF expression during all stages of flower development, subsequently causing anther indehiscence throughout flower development and an increase in petal size by promotion of cell expansion due to the suppression of BPEp gene (Figure S9) during late flower development. Since AtAAF is highly expressed in early developmental stage and may be involved in controlling cell division, ectopic expression of AtAAF by 35S::AtAAF during early stage should not affect the function of the AtAAF in promoting cell division during early developmental stage. Thus, the cell number will not be affected in 35S::AtAAF flowers as seen in our result.
Figure 8.
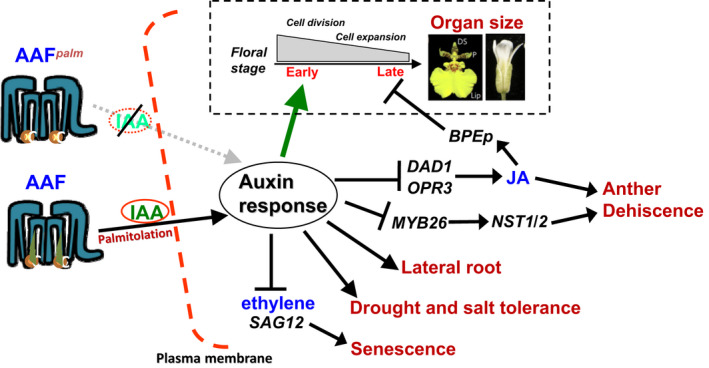
Model for the function of AAF orthologues in regulating auxin response and development in plants. In plants, the targeting of palmitoylated AAF proteins to the plasma membrane promotes auxin uptake and enhances (→) the auxin response. In flowers, AAF is more highly expressed in early than in late developmental stages (gray bar). Thus, it mainly enhances the auxin response and promotes (green arrow) cell division/expansion in the flower organs at an early stage. Ectopic expression of AAF extends its affect to the whole flower and results in an increase in the size of the flower organs. By contrast, a palmitoylation‐deficient mutant of AAF reduces ([ ]) the auxin response and alters its ability to promote cell division/expansion in early flowering stages resulting in a decrease in flower organ size. In addition, the enhancement of the auxin response by ectopic expression of AAF could suppress anther dehiscence during whole flower development by suppressing the expression of MYB26/NST1/2, DAD1/OPR3, and JA activity (which also causes the suppression of the BPEp and resulted in the expansion of the flower organs). The enhancement of the auxin response by AAF also suppresses ethylene signaling and organ senescence, promotes drought/salt tolerance and lateral root formation, and retains root tip gravitropism in plants. Dominant negative mutation of AAF suppresses auxin response and causes the opposite effect on the processes described above
]) the auxin response and alters its ability to promote cell division/expansion in early flowering stages resulting in a decrease in flower organ size. In addition, the enhancement of the auxin response by ectopic expression of AAF could suppress anther dehiscence during whole flower development by suppressing the expression of MYB26/NST1/2, DAD1/OPR3, and JA activity (which also causes the suppression of the BPEp and resulted in the expansion of the flower organs). The enhancement of the auxin response by AAF also suppresses ethylene signaling and organ senescence, promotes drought/salt tolerance and lateral root formation, and retains root tip gravitropism in plants. Dominant negative mutation of AAF suppresses auxin response and causes the opposite effect on the processes described above
In contrast, in the AtAAFpalm palmitoylation‐deficient mutant, AtAAF was suppressed throughout flower development, resulting in the suppression of the cell division during early flower development. No or low effect on cell expansion was observed during late flower development since AtAAF is normally expressed low during late developmental stage (Figure 8). This caused the production of small petal with fewer cells of normal size in AtAAFpalm palmitoylation‐deficient mutant when compared to those in wild‐type petals.
The next question we propose is in what mechanisms AtAAF participates in regulating JA activity. It is worth noting that auxin could inhibit the expression of JA biosynthesis genes DAD1 and OPR3 to result in anther indehiscence (Cecchetti et al., 2013). In addition, petal size was increased in opr3 mutants (Brioudes et al., 2009) and was significantly reduced in arf6/arf8 double‐mutant plants (Tabata et al., 2010). These results suggest a possibly functional correlation between AtAAF and auxin in regulating JA activity. Interestingly, auxin‐controlled phenotypes, such as the enhancement of drought and salt tolerance, the development of fast growing lateral root primordia, and the production of significantly more lateral roots, were also observed in 35S::AtAAF plants. Thus, we propose that AtAAF is likely participating in auxin regulation by either increasing auxin production or enhancing the efficiency of the auxin response. It is clear that a similar amount of IAA was detected in wild‐type, 35S::AtAAF, and 35S::AtAAFpalm plants, indicating that AtAAF is likely functioning to enhance the efficiency of the auxin response rather than to increase auxin production. This conclusion is supported by three lines of evidence. First, exogenous auxin treatment increased the amount of GFP transcript much higher in DR5::GFP/35S::AtAAF than in DR5::GFP plants. Second, 35S::AtAAF plants produced three times more lateral roots than did wild‐type plants, and the formation of LRP on the roots was much faster in 35S::AtAAF than in wild‐type seedlings after auxin treatment. Third, after auxin treatment, unlike the asymmetric gravitropism impairment in DR5::GFP roots, asymmetric gravitropism was still retained in DR5::GFP/35S::AtAAF roots. All these results indicate that AtAAF could function to increase efficiency of the auxin response, directly or indirectly, in controlling JA activity and plant developmental processes.
As illustrated in Figure 8, our results reveal a possible model for the interaction of the tetraspanin AAF orthologues and auxin in regulating plant growth and development. In Arabidopsis, the targeting of palmitoylated AtAAF proteins to the plasma membrane promotes auxin uptake and enhances the auxin response, which may affect signaling by other plant hormones (JA, ethylene) and control several developmental processes, such as size of flower organs and seeds, anther dehiscence, lateral root formation, and root tip gravitropism, drought and salt tolerance, and organ senescence. Ectopic expression of AtAAF enhanced the auxin response, whereas a palmitoylation‐deficient mutation of AtAAF suppressed the auxin response and the processes described above. In Oncidium orchids, after perianth identity was determined by P‐code complexes, OnAAF was expressed at a higher level in lips than in sepals/petals, enhancing auxin response and subsequently promoting cell division/expansion in lips more than in sepals/petals. This was associated with the production of larger lips. By contrast, PaAAF was more highly expressed in sepals/petals than in lips, which was associated with the production of relatively small lips in Phalaenopsis orchids. To further unravel the molecular role for AAF orthologues in auxin response in the future, the analysis of protein–protein interaction between AAF and the transporters of auxin such as PIN proteins or AUX proteins is necessary. The analysis of signal transduction of cells via AAF from the plasma membrane to nucleus is also needed.
CONFLICT OF INTEREST
The authors declare no conflict of interest associated with the work described in this manuscript.
AUTHOR CONTRIBUTIONS
C‐H. Y. developed the overall strategy, designed experiments, and coordinated the project. W‐H. C. performed orchid genes cloning, expression analyses, VIGS experiments, and all the Arabidopsis AtAAF experiments. H‐F. H. performed the cryo‐scanning electron microscopy and orchid gene expression analyses. C‐H. Y. collected the orchid samples. W‐H. H. performed the confocal analysis. C‐H. Y. prepared and revised the manuscript.
Supporting information
ACKNOWLEDGMENTS
This work was supported by grants to C‐H Y from the Ministry of Science and Technology, Taiwan, ROC, grant numbers: MOST 103‐2313‐B‐005‐001‐MY3 and MOST 106‐2321‐B‐005‐010. This work was also financially supported (in part) by the Advanced Plant Biotechnology Center from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Chen W‐H, Hsu W‐H, Hsu H‐F, Yang C‐H. A tetraspanin gene regulating auxin response and affecting orchid perianth size and various plant developmental processes. Plant Direct. 2019;3:1–20. 10.1002/pld3.157
REFERENCES
- Alexander, M. P. (1969). Differential staining of aborted and nonaborted pollen. Stain Technology, 44, 117–122. 10.3109/10520296909063335 [DOI] [PubMed] [Google Scholar]
- Alonso, J. M. , Stepanova, A. N. , Leisse, T. J. , Kim, C. J. , Chen, H. , Shinn, P. , … Ecker, J. R. (2003). Genome‐wide insertional mutagenesis of Arabidopsis thaliana . Science, 301, 653–657. 10.1126/science.1086391 [DOI] [PubMed] [Google Scholar]
- Armengot, L. , Marques‐Bueno, M. M. , & Jaillais, Y. (2016). Regulation of polar auxin transport by protein and lipid kinases. Journal of Experimental Botany, 67, 4015–4037. 10.1093/jxb/erw216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azooz, M. M. , Shaddad, M. A. , & Abdel‐Latef, A. A. (2004). Leaf growth and K+/Na+ ratio as an indication of the salt tolerance of three sorghum cultivars grown under salinity stress and IAA treatment. Acta Agronomica Hungarica, 52, 287–296. [Google Scholar]
- Benková, E. , Michniewicz, M. , Sauer, M. , Teichmann, T. , Seifertová, D. , Jürgens, G. , & Friml, J. (2003). Local, efflux‐dependent auxin gradients as a common module for plant organ formation. Cell, 115, 591–602. 10.1016/S0092-8674(03)00924-3 [DOI] [PubMed] [Google Scholar]
- Berditchevski, F. , Odintsova, E. , Sawada, S. , & Gilbert, E. (2002). Expression of the palmitoylation‐deficient CD151 weakens the association of alpha 3 beta 1 integrin with the tetraspanin‐enriched microdomains and affects integrin‐dependent signaling. Journal of Biological Chemistry, 277, 36991–37000. [DOI] [PubMed] [Google Scholar]
- Boavida, L. C. , Qin, P. , Broz, M. , Becker, J. D. , & McCormick, S. (2013). Arabidopsis tetraspanins are confined to discrete expression domains and cell types in reproductive tissues and form homo‐ and heterodimers when expressed in yeast. Plant Physiology, 163, 696–712. 10.1104/pp.113.216598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioudes, F. , Joly, C. , Szecsi, J. , Varaud, E. , Leroux, J. , Bellvert, F. , … Bendahmane, M. (2009). Jasmonate controls late development stages of petal growth in Arabidopsis thaliana . Plant Journal, 60, 1070–1080. 10.1111/j.1365-313X.2009.04023.x [DOI] [PubMed] [Google Scholar]
- Cecchetti, V. , Altamura, M. M. , Brunetti, P. , Petrocelli, V. , Falasca, G. , Ljung, K. , … Cardarelli, M. (2013). Auxin controls Arabidopsis anther dehiscence by regulating endothecium lignification and jasmonic acid biosynthesis. Plant Journal, 74, 411–422. 10.1111/tpj.12130 [DOI] [PubMed] [Google Scholar]
- Chang, Y. Y. , Kao, N. H. , Li, J. Y. , Hsu, W. H. , Liang, Y. L. , Wu, J. W. , & Yang, C. H. (2010). Characterization of the possible roles for B class MADS box genes in regulation of perianth formation in orchid. Plant Physiology, 152, 837–853. 10.1104/pp.109.147116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrin, S. , Jouannet, S. , Boucheix, C. , & Rubinstein, E. (2014). Tetraspanins at a glance. Journal of Cell Science, 127, 3641–3648. 10.1242/jcs.154906 [DOI] [PubMed] [Google Scholar]
- Charrin, S. , Manie, S. , Oualid, M. , Billard, M. , Boucheix, C. , & Rubinstein, E. (2002). Differential stability of tetraspanin/tetraspanin interactions: Role of palmitoylation. FEBS Letters, 516, 139–144. 10.1016/S0014-5793(02)02522-X [DOI] [PubMed] [Google Scholar]
- Chen, R. , Rosen, E. , & Masson, P. H. (1999). Gravitropism in higher plants. Plant Physiology, 120, 343–350. 10.1104/pp.120.2.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S. J. , & Bent, A. F. (1998). Floral dip: A simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . Plant Journal, 16, 735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Cnops, G. , Neyt, P. , Raes, J. , Petrarulo, M. , Nelissen, H. , Malenica, N. , … Van Lijsebettens, M. (2006). The TORNADO1 and TORNADO2 genes function in several patterning processes during early leaf development in Arabidopsis thaliana . The Plant Cell, 18, 852–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino, S. , & Widmer, A. (2005). Orchid diversity: An evolutionary consequence of deception? Trends in Ecology & Evolution, 20, 487–494. 10.1016/j.tree.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Delandre, C. , Penabaz, T. R. , Passarelli, A. L. , Chapes, S. K. , & Clem, R. J. (2009). Mutation of juxtamembrane cysteines in the tetraspanin CD81 affects palmitoylation and alters interaction with other proteins at the cell surface. Experimental Cell Research, 315, 1953–1963. 10.1016/j.yexcr.2009.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finet, C. , & Jaillais, Y. (2012). Auxology: When auxin meets plant evo‐devo. Developmental Biology, 369, 19–31. 10.1016/j.ydbio.2012.05.039 [DOI] [PubMed] [Google Scholar]
- Garcia‐España, A. , Chung, P. J. , Sarkar, I. N. , Stiner, E. , Sun, T. T. , & Desalle, R. (2008). Appearance of new tetraspanin genes during vertebrate evolution. Genomics, 91, 326–334. 10.1016/j.ygeno.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Hemler, M. E. (2005). Tetraspanin functions and associated microdomains. Nature Reviews Molecular Cell Biology, 6, 801–811. 10.1038/nrm1736 [DOI] [PubMed] [Google Scholar]
- Hemsley, P. A. , Taylor, L. , & Grierson, C. S. (2008). Assaying protein palmitoylation in plants. Plant Methods, 4, 2. 10.1186/1746-4811-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, H. F. , Hsu, W. H. , Lee, Y. I. , Mao, W. T. , Yang, J. Y. , Li, J. Y. , & Yang, C. H. (2015). Model for perianth formation in orchids. Nature Plants, 1, 15046. 10.1038/nplants.2015.46 [DOI] [Google Scholar]
- Hua, L. V. , Green, M. , Wong, A. , Warsh, J. J. , & Li, P. P. (2001). Tetraspan protein CD151: A common target of mood stabilizing drugs? Neuropsychopharmacol, 25, 729–736. 10.1016/S0893-133X(01)00269-X [DOI] [PubMed] [Google Scholar]
- Huang, S. , Yuan, S. , Dong, M. , Su, J. , Yu, C. , Shen, Y. , … Xu, A. (2005). The phylogenetic analysis of tetraspanins projects the evolution of cell‐cell interactions from unicellular to multicellular organisms. Genomics, 86, 674–684. 10.1016/j.ygeno.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Ishiguro, S. , Kawai‐Oda, A. , Ueda, J. , Nishida, I. , & Okada, K. (2001). The defective in anther dehiscience gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. The Plant Cell, 13, 2191–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, H. , & Evans, M. L. (1995). Specialized zones of development in roots. Plant Physiology, 109, 725–727. 10.1104/pp.109.3.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israels, S. J. , & McMillan‐Ward, E. M. (2010). Palmitoylation supports the association of tetraspanin CD63 with CD9 and integrin alphaIIbbeta3 in activated platelets. Thrombosis Research, 125, 152–158. [DOI] [PubMed] [Google Scholar]
- Jefferson, R. A. , Kavanagh, T. A. , & Bevan, M. W. (1987). GUS fusions: Beta‐glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal, 6, 3901–3907. 10.1002/j.1460-2075.1987.tb02730.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. I. , Murphy, A. S. , Baek, D. , Lee, S. W. , Yun, D. J. , Bressan, R. A. , & Narasimhan, M. L. (2011). YUCCA6 over‐expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana . Journal of Experimental Botany, 62, 3981–3992. 10.1093/jxb/err094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocyan, A. , Conti, E. , Qiu, Y. L. , & Endress, P. K. (2004). A phylogenetic analysis of Apostasioideae (Orchidaceae) based on ITS, trn L‐F and mat K sequences. Plant Systematics and Evolution, 247, 203–213. 10.1007/s00606-004-0133-3 [DOI] [Google Scholar]
- Lambou, K. , Tharreau, D. , Kohler, A. , Sirven, C. , Marguerettaz, M. , Barbisan, C. , … Lebrun, M.‐H. (2008). Fungi have three tetraspanin families with distinct functions. BMC Genomics, 9, 63. 10.1186/1471-2164-9-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani, E. R. , Kilinc, A. , & Smyth, D. R. (2012). Petal loss is a boundary gene that inhibits growth between developing sepals in Arabidopsis thaliana . Plant Journal, 71, 724–735. 10.1111/j.1365-313X.2012.05023.x [DOI] [PubMed] [Google Scholar]
- Lampugnani, E. R. , Kilinc, A. , & Smyth, D. R. (2013). Auxin controls petal initiation in Arabidopsis. Development, 140, 185–194. 10.1242/dev.084582 [DOI] [PubMed] [Google Scholar]
- Lavenus, J. , Goh, T. , Roberts, I. , Guyomarc'h, S. , Lucas, M. , De Smet, I. , … Laplaze, L. (2013). Lateral root development in Arabidopsis: Fifty shades of auxin. Trends in Plant Science, 18, 450–458. 10.1016/j.tplants.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Lee, M. , Jung, J. H. , Han, D. Y. , Seo, P. J. , Park, W. J. , & Park, C. M. (2012). Activation of a flavin monooxygenase gene YUCCA7 enhances drought resistance in Arabidopsis. Planta, 235, 923–938. 10.1007/s00425-011-1552-3 [DOI] [PubMed] [Google Scholar]
- Li, Q. , Yin, M. , Li, Y. , Fan, C. , Yang, Q. , Wu, J. , … Zhou, Y. (2015). Expression of Brassica napus TTG2, a regulator of trichome development, increases plant sensitivity to salt stress by suppressing the expression of auxin biosynthesis genes. Journal of Experimental Botany, 66, 5821–5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber, D. , Lora, J. , Schrempp, S. , Lenhard, M. , & Laux, T. (2011). Arabidopsis WIH1 and WIH2 genes act in the transition from somatic to reproductive cell fate. Current Biology, 21, 1009–1017. 10.1016/j.cub.2011.05.015 [DOI] [PubMed] [Google Scholar]
- Lu, H. C. , Hsieh, M. H. , Chen, C. E. , Chen, H. H. , Wang, H. I. , & Yeh, H. H. (2012). A high‐throughput virus‐induced gene‐silencing vector for screening transcription factors in virus‐induced plant defense response in orchid. Molecular Plant‐Microbe Interactions, 25, 738–746. 10.1094/MPMI-10-11-0266 [DOI] [PubMed] [Google Scholar]
- Lucas, M. , Godin, C. , Jay‐Allemand, C. , & Laplaze, L. (2008). Auxin fluxes in the root apex co‐regulate gravitropism and lateral root initiation. Journal of Experimental Botany, 59, 55–66. 10.1093/jxb/erm171 [DOI] [PubMed] [Google Scholar]
- Mironova, V. , Teale, W. , Shahriari, M. , Dawson, J. , & Palme, K. (2017). The systems biology of auxin in developing embryos. Trends in Plant Science, 22, 225–235. 10.1016/j.tplants.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Mitsuda, N. , Seki, M. , Shinozaki, K. , & Ohme‐Takagi, M. (2005). The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. The Plant Cell, 17, 2993–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller, B. K. , Ten Hove, C. A. , Xiang, D. , Williams, N. , Lopez, L. G. , Yoshida, S. , … Weijers, D. (2017). Auxin response cell‐autonomously controls ground tissue initiation in the early Arabidopsis embryo. Proceedings of the National Academy of Sciences USA, 114, E2533–E2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T. , & Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- Nagpal, P. , Ellis, C. M. , Weber, H. , Ploense, S. E. , Barkawi, L. S. , Guilfoyle, T. J. , … Reed, J. W. (2005). Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development, 132, 4107–4118. 10.1242/dev.01955 [DOI] [PubMed] [Google Scholar]
- Olmos, E. , Reiss, B. , & Dekker, K. (2003). The ekeko mutant demonstrates a role for tetraspanin‐like protein in plant development. Biochemical and Biophysical Research Communications, 310, 1054–1061. 10.1016/j.bbrc.2003.09.122 [DOI] [PubMed] [Google Scholar]
- Overvoorde, P. , Fukaki, H. , & Beeckman, T. (2010). Auxin control of root development. Cold Spring Harbor Perspectives in Biology, 2, a001537. 10.1101/cshperspect.a001537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y. J. , Shih, C. F. , Yang, J. Y. , Tan, C. M. , Hsu, W. H. , Huang, Y. P. , … Yang, C. H. (2013). A RING‐type E3 ligase controls anther dehiscence by activating the jasmonate biosynthetic pathway gene defective in anther dehiscence1 in Arabidopsis. Plant Journal, 74, 310–327. [DOI] [PubMed] [Google Scholar]
- Péret, B. , De Rybel, B. , Casimiro, I. , Benkova, E. , Swarup, R. , Laplaze, L. , … Bennett, M. J. (2009). Arabidopsis lateral root development: An emerging story. Trends in Plant Science, 14, 399–408. 10.1016/j.tplants.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Péret, B. , Li, G. , Zhao, J. , Band, L. R. , Voß, U. , Postaire, O. , … Bennett, M. J. (2012). Auxin regulates aquaporin function to facilitate lateral root emergence. Nature Cell Biology, 14, 991–998. 10.1038/ncb2573 [DOI] [PubMed] [Google Scholar]
- Peret, B. , Middleton, A. M. , French, A. P. , Larrieu, A. , Bishopp, A. , Njo, M. , … Bennett, M. J. (2013). Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Molecular Systems Biology, 9, 699–713. 10.1038/msb.2013.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesquet, E. , Ranocha, P. , Legay, S. , Digonnet, C. , Barbier, O. , Pichon, M. , & Goffner, D. (2005). Novel markers of xylogenesis in zinnia are differentially regulated by auxin and cytokinin. Plant Physiology, 139, 1821–1839. 10.1104/pp.105.064337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson, S. V. , Johansson, A. I. , Kowalczyk, M. , Makoveychuk, A. , Wang, J. Y. , Moritz, T. , … Ljung, K. (2009). An auxin gradient and maximum in the Arabidopsis root apex shown by high‐resolution cell‐specific analysis of IAA distribution and synthesis. The Plant Cell, 21, 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann, R. , Kost, B. , & Dettmer, J. (2017). TETRASPANINs in plants. Frontiers in Plant Science, 8, 545. 10.3389/fpls.2017.00545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, J. , Wen, L. , Gao, X. , Jin, C. , Xue, Y. , & Yao, X. (2008). CSS‐Palm 2.0: An updated software for palmitoylation sites prediction. Protein Engineering, Design & Selection, 21, 639–644. 10.1093/protein/gzn039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudall, P. J. , & Bateman, R. M. (2002). Roles of synorganisation, zygomorphy and heterotopy in floral evolution: The gynostemium and labellum of orchids and other lilioid monocots. Biological Reviews of the Cambridge Philosophical Society, 77, 403–441. 10.1017/S1464793102005936 [DOI] [PubMed] [Google Scholar]
- Sanders, P. M. , Lee, P. Y. , Biesgen, C. , Boone, J. D. , Beals, T. P. , Weiler, E. W. , & Goldberg, R. B. (2000). The Arabidopsis delayed dehiscence1 gene encodes an enzyme in the jasmonic acid synthesis pathway. The Plant Cell, 12, 1041–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, E. M. , Hijazi, H. , Bennett, M. J. , Vissenberg, K. , & Swarup, R. (2015). New insights into root gravitropic signalling. Journal of Experimental Botany, 66, 2155–2165. 10.1093/jxb/eru515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauret‐Gueto, S. , Schiessl, K. , Bangham, A. , Sablowski, R. , & Coen, E. (2013). JAGGED controls Arabidopsis petal growth and shape by interacting with a divergent polarity field. PLoS Biology, 11, e1001550. 10.1371/journal.pbio.1001550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, M. , Davison, T. S. , Henz, S. R. , Pape, U. J. , Demar, M. , Vingron, M. , … Lohmann, J. U. (2005). A gene expression map of Arabidopsis thaliana development. Nature Genetics, 37, 501–506. 10.1038/ng1543 [DOI] [PubMed] [Google Scholar]
- Shi, H. , Chen, L. , Ye, T. , Liu, X. , Ding, K. , & Chan, Z. (2014). Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiology and Biochemistry, 82, 209–217. 10.1016/j.plaphy.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Steiner‐Lange, S. , Unte, U. S. , Eckstein, L. , Yang, C. , Wilson, Z. A. , Schmelzer, E. , … Saedler, H. (2003). Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non‐dehiscent anthers. Plant Journal, 34, 519–528. 10.1046/j.1365-313X.2003.01745.x [DOI] [PubMed] [Google Scholar]
- Stepanova, A. N. , & Alonso, J. M. (2005). Arabidopsis ethylene signaling pathway. Science’s STKE, 2005, cm4. [DOI] [PubMed] [Google Scholar]
- Stipp, C. S. , Kolesnikova, T. V. , & Hemler, M. E. (2003). Functional domains in tetraspanin proteins. Trends in Biochemical Sciences, 28, 106–112. 10.1016/S0968-0004(02)00014-2 [DOI] [PubMed] [Google Scholar]
- Swartzberg, D. , Dai, N. , Gan, S. , Amasino, R. , & Granot, D. (2006). Effects of cytokinin production under two SAG promoters on senescence and development of tomato plants. Plant Biol, 8, 579–586. 10.1055/s-2006-924240 [DOI] [PubMed] [Google Scholar]
- Swarup, R. , & Bennett, M. J. (2009). Root gravitropism. Annual Palnt Reviews, 37, 157–174. [Google Scholar]
- Tabata, R. , Ikezaki, M. , Fujibe, T. , Aida, M. , Tian, C. E. , Ueno, Y. , … Ishiguro, S. (2010). Arabidopsis auxin response factor6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX genes. Plant and Cell Physiology, 51, 164–175. 10.1093/pcp/pcp176 [DOI] [PubMed] [Google Scholar]
- Teale, W. D. , Paponov, I. A. , & Palme, K. (2006). Auxin in action: Signalling, transport and the control of plant growth and development. Nature Reviews Molecular Cell Biology, 7, 847–859. 10.1038/nrm2020 [DOI] [PubMed] [Google Scholar]
- Varaud, E. , Brioudes, F. , Szecsi, J. , Leroux, J. , Brown, S. , Perrot‐Rechenmann, C. , & Bendahmane, M. (2011). Auxin response factor8 regulates Arabidopsis petal growth by interacting with the bHLH transcription factor BIGPETALp. The Plant Cell, 23, 973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. , Muto, A. , Van de Velde, J. , Neyt, P. , Himanen, K. , Vandepoele, K. , & Van Lijsebettens, M. (2015). Functional analysis of the Arabidopsis TETRASPANIN gene family in plant growth and development. Plant Physiology, 169, 2200–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, D. , Vinegar, B. , Nahal, H. , Ammar, R. , Wilson, G. V. , & Provart, N. J. (2007). An "Electronic Fluorescent Pictograph" browser for exploring and analyzing large‐scale biological data sets. PLoS ONE, 2, e718. 10.1371/journal.pone.0000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, N. , Huang, J. , Xu, Y. , Tanoi, K. , & Ito, T. (2017). Fine‐tuning of auxin homeostasis governs the transition from floral stem cell maintenance to gynoecium formation. Nature Communications, 8, 1125. 10.1038/s41467-017-01252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C. , Xu, Z. , Song, J. , Conner, K. , Vizcay Barrena, G. , & Wilson, Z. A. (2007). Arabidopsis MYB26/MALE STERILE35 regulates secondary thickening in the endothecium and is essential for anther dehiscence. The Plant Cell, 19, 534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Claas, C. , Kraeft, S. K. , Chen, L. B. , Wang, Z. , Kreidberg, J. A. , & Hemler, M. E. (2002). Palmitoylation of tetraspanin proteins: Modulation of CD151 lateral interactions, subcellular distribution, and integrin‐dependent cell morphology. Molecular Biology of the Cell, 13, 767–781. 10.1091/mbc.01-05-0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahir, Z. A. , Shah, M. K. , Naveed, M. , & Akhter, M. J. (2010). Substrate‐dependent auxin production by Rhizobium phaseoli improves the growth and yield of Vigna radiata L. under salt stress conditions. Journal of Microbiology and Biotechnology, 20, 1288–1294. 10.4014/jmb.1002.02010 [DOI] [PubMed] [Google Scholar]
- Zhao, Y. (2010). Auxin biosynthesis and its role in plant development. Annual Review of Plant Biology, 61, 49–64. 10.1146/annurev-arplant-042809-112308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B. , Liu, L. , Reddivari, M. , & Zhang, X. A. (2004). The palmitoylation of metastasis suppressor KAI1/CD82 is important for its motility‐ and invasiveness‐inhibitory activity. Cancer Research, 64, 7455–7463. 10.1158/0008-5472.CAN-04-1574 [DOI] [PubMed] [Google Scholar]
- Zhou, F. , Xue, Y. , Yao, X. , & Xu, Y. (2006). CSS‐Palm: Palmitoylation site prediction with a clustering and scoring strategy (CSS). Bioinformatics, 22, 894–896. 10.1093/bioinformatics/btl013 [DOI] [PubMed] [Google Scholar]
- Zuidscherwoude, M. , Gottfert, F. , Dunlock, V. M. , Figdor, C. G. , van den Bogaart, G. , & van Spriel, A. B. (2015). The tetraspanin web revisited by super‐resolution microscopy. Scientific Reports, 5, 12201. 10.1038/srep12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


