Abstract
Endophthalmitis is one of the most feared complications after cataract surgery. The aim of this systematic review was to evaluate the effect of intracameral and topical antibiotics on the prevention of endophthalmitis after cataract surgery. A systematic literature review in the MEDLINE, CINAHL, Cochrane Library and EMBASE databases revealed one randomized trial and 17 observational studies concerning the prophylactic effect of intracameral antibiotic administration on the rate of endophthalmitis after cataract surgery. The effect of topical antibiotics on endophthalmitis rate was reported by one randomized trial and one observational study. The quality and design of the included studies were analysed using the Cochrane risk of bias tool. The quality of the evidence was evaluated using the GRADE approach. We found high‐to‐moderate quality evidence for a marked reduction in the risk of endophthalmitis with the use of intracameral antibiotic administration of cefazolin, cefuroxime and moxifloxacin, whereas no effect was found with the use of topical antibiotics or intracameral vancomycin. Endophthalmitis occurred on average in one of 2855 surgeries when intracameral antibiotics were used compared to one of 485 surgeries when intracameral antibiotics were not used. The relative risk (95% CI) of endophthalmitis was reduced to 0.12 (0.08; 0.18) when intracameral antibiotics were used. The difference was highly significant (p < 0.00001). Intracameral antibiotic therapy is the best choice for preventing endophthalmitis after cataract surgery. We did not find evidence to conclude that topical antibiotic therapy prevents endophthalmitis.
Keywords: antibiotic therapy, cataract surgery, cefuroxime, endophthalmitis, prevention
Introduction
Cataract surgery is the most frequently performed elective surgical procedure in many Westernized countries. Although cataract surgery is generally considered a safe procedure resulting in a favourable visual outcome, surgical complications do occur. The most feared complication is postoperative endophthalmitis which is an infectious condition caused by micro‐organisms introduced to the interior of the eye during or after the surgical procedure. The visual outcome after endophthalmitis is often very poor. Seventeen per cent of patients in the European Society of Cataract and Refractive Surgeons (ESCRS) study had a final visual acuity ≤ 20/200 and 48.3% had a final visual acuity ≤ 20/40 (Barry et al. 2009). Treatment of endophthalmitis often requires further surgery and hospitalization; thus, every case of endophthalmitis puts a heavy burden on the healthcare system (Fongsre et al. 2004; Schmier et al. 2007), not to mention the distress and loss of quality of life experienced by the patient (Clark et al. 2008).
During cataract surgery, an incision is made in the anterior segment of the eye to remove the cataractous lens. Corneal incisions may allow inflow of ocular surface fluid even after hydrosealing (Herretes et al. 2005). The use of clear corneal incisions has been found to be a risk factor for endophthalmitis (Cao et al. 2013), but the evidence is not conclusive (Lundstrom 2006). Microbiological examinations have shown that the rate of contamination of the surgical fluids is high (up to 50%) in spite of preoperative cleaning of the conjunctiva with povidone‐iodine and preoperative use of topical antibiotic (Balestrazzi et al. 2012). The rate of positive samples from the anterior chamber is usually <5% (Parmar et al. 2006; Cornut et al. 2010; Baillif et al. 2012; Kumar et al. 2012) but has been reported to be as high as 14% (Das et al. 2009). The rate of anterior chamber contamination is similar after manual small‐incision cataract surgery and phacoemulsification (Kumar et al. 2012). Contaminated surgical equipment (Malathi et al. 2006), IOLs (Ramappa et al. 2012) and viscoelastic material (Voss et al. 2012) may also cause outbreaks of endophthalmitis.
The risk of endophthalmitis is higher in older patients (West et al. 2005; Wejde et al. 2005b; Kamalarajah et al. 2007; Freeman et al. 2010; Cao et al. 2013), in patients with wound dehiscence (Wejde et al. 2005b), after posterior capsule rupture (Wong & Chee 2004a; Wejde et al. 2005b; Kamalarajah et al. 2007), when face masks are not worn in theatre (Kamalarajah et al. 2007) and in patients using immunosuppressants (Kamalarajah et al. 2007). Surgeons and clinics that perform a large number of cataract surgeries annually have a lower rate of endophthalmitis than those who perform fewer surgeries (Fang et al. 2006). The incidence of endophthalmitis has by some been reported to drop with technical advancement from intracapsular cataract extraction (ICCE) over extracapsular cataract extraction (ECCE) to the phacoemulsification technique and the use of small incisions that is possible due to foldable IOLs (Mayer et al. 2003; Wejde et al. 2005b; Freeman et al. 2010), whereas others have found a steady rate of endophthalmitis cases in spite of changing surgical procedures (Semmens et al. 2003).
As endophthalmitis is an infection, it should be preventable by antibiotic treatment. The question is which type of antibiotic treatment provides the best prevention against postcataract endophthalmitis? Prophylactic antibiotic treatment can be given as topical treatment preoperatively to reduce the bacterial load on the conjunctiva before surgery. It can be given during surgery directly into the anterior chamber or as a subconjunctival injection. Finally, it can be given as topical treatment postoperatively. Globally, we face increasing problems concerning resistance of micro‐organisms to antibiotic treatment probably because of a too liberal use. Thus, prophylactic antibiotic treatment should be given wisely to offer the best possible protection against endophthalmitis whilst protecting the patient and the society against selection of multiresistant bacterial strains. The aim of this study was to evaluate the available scientific data on the efficacy of the prophylactic effect of intracameral, peri‐operative antibiotic delivery and topical antibiotic treatment as these two regimes are the most widely used. The present work was undertaken after an initiative by the Danish National Health and Medicines Authorities to formulate evidence‐based guidelines on surgery for age‐related cataract. A 2013 Cochrane review analysed the use of preoperative, intra‐operative or postoperative antibiotics of any delivery route, but only randomized trials were included resulting in a conclusion based upon the recent ESCRS study and three older randomized trials using various routes of antibiotic administration (Gower et al. 2013). A great number of non‐randomized studies have been published as the ESCRS study reporting endophthalmitis prevalence with and without the use of intracameral antibiotics and we decided to include this information in our analysis.
Material and Methods
This systematic review and resulting meta‐analyses were performed based on the principles described in the Grades of Recommendation, Assessment, Develop‐ment and Evaluation (GRADE) approach (Guyatt et al. 2011f). First, we defined the topic of the systematic review using the Patient, Intervention, Comparison and Outcome (PICO) approach (Guyatt et al. 2011a).
We formulated two PICO questions to examine both the prophylactic efficacy of topical antibiotic treatment and intracameral antibiotic therapy on postoperative endophthalmitis in patients with age‐related cataract undergoing phacoemulsification:
Is the risk of endophthalmitis (O) lower in patients with age‐related cataract undergoing phacoemulsification (P) with the use of intracameral antibiotic administration (I) or in patients not receiving intracameral antibiotics (C)?
Does topical antibiotic treatment (I) or no topical antibiotic treatment (C) result in the lowest number of endophthalmitis cases (O) in patients with age‐related cataract undergoing phacoemulsification (P)?
Randomized clinical trials and non‐randomized trials were considered for inclusion if they reported on the incidence of postoperative endophthalmitis in patients undergoing surgery for age‐related cataract. Furthermore, the study should compare endophthalmitis rates in two comparable populations receiving/not receiving antibiotic therapy, either intracamerally or topically. For a non‐randomized trial to be included, the study had to compare endophthalmitis rates in the same institution(s) in two different time periods: one time period using antibiotic prevention of endophthalmitis and one time period not using antibiotic prevention. Studies that only reported cases and not the prevalence of endophthalmitis were excluded.
The outcome measure was endophthalmitis. Endophthalmitis was defined as clinical cases of postoperative endophthalmitis, that is both culture‐positive and culture‐negative cases.
A systematic literature search was conducted in July 2014 in the EMBASE, MEDLINE, Cochrane Library and CINAHL databases. A schematic presentation of the literature search is provided in Fig. 1A for intracameral antibiotic prophylaxis and in Fig. 1B for topical antibiotic prophylaxis. The search was limited to references published within the last 10 years in the English or Scandinavian languages. The time limit was chosen to ensure that the studies used surgical methods comparable to modern surgical methods, that is phacoemulsification. The search strategy is shown in Fig. 1.
Figure 1.
Schematic presentation of the literature search (A) literature search profile for intracameral antibiotic prophylaxis of postoperative endophthalmitis (B) literature search profile for topical antibiotic prophylaxis of postoperative endophthalmitis Both search profiles were limited to the publications published in English or the Scandinavian languages published within the last 10 years.
According to Danish law, no institutional review board approval was needed for the study.
All included studies were reviewed by two reviewers independently (LK and PF), and the quality of the studies was evaluated using the Cochrane risk of bias tool (Higgins & Green 2011). In short, the Cochrane risk of bias tool assesses the risk of bias associated with the selection of patients (randomization or patient allocation and concealment of allocation), study performance (blinding of patients and personnel), detection of outcomes (blinding of outcome assessment), attrition of data (such as missing patients or drop‐outs), reporting of study findings (selective outcome reporting) or other types of bias. Data from each included study were extracted independently by two reviewers (LK and PF). Data were entered into a meta‐analysis providing relative risk ratios based on the available scientific data. Disagreement was resolved by discussion and consensus. This part of the systematic review was carried out using the Review Manager Software [Review Manager (RevMan) 2012].
The quality of the evidence for each prespecified outcome was evaluated across the included studies using the GRADE system by two reviewers independently (LK and PF). Each outcome was analysed for study limitations (risk of bias, e.g. lack of allocation concealment or lack of blinding of patients or outcome assessors, incomplete accounting of patients and outcome, selective outcome reporting or other limitations) (Guyatt et al. 2011g), inconsistency (different results between studies) (Guyatt et al. 2011d), indirectness (was the study population and intervention comparable to the patient population and intervention that is relevant to the readers of meta‐analysis, use of surrogate measures) (Guyatt et al. 2011c), imprecision (large confidence intervals or the lack of statistical strength by included studies to answer the posed question) (Guyatt et al. 2011b) and risk of publication bias (e.g. lack of reporting of negative findings) (Guyatt et al. 2011e). The quality of the evidence for the prespecified outcome (endophthalmitis rate) could be up or downgraded based on the assessment of each of the limitations mentioned above. Finally, tables summarizing the findings and the quality of the evidence were prepared using the Grade Profiler software (GRADE profiler 2011).
Dichotomous outcome data were analysed by calculating risk ratios. The Review Manager 5 Software [Review Manager (RevMan) 2012] was used for the estimation of overall treatment effects. Random‐effect models were used to calculate pooled estimates of effects.
Results
Endophthalmitis epidemiology
The systematic literature search revealed several studies that reported the rate of endophthalmitis after cataract surgery (Table 1). Reported endophthalmitis rates after cataract surgery vary greatly between different continents, between neighbouring countries and even within the same country. The average rate of endophthalmitis ranges from a high of one case per 315–368 surgeries in Africa and South America, respectively, over one case per ~700 surgeries in Asia, Australia and North America to a low of one case per 1418 surgeries in Europe. Even in Europe, the rate varies greatly from 0.3 per 1000 surgeries in Sweden (Friling et al. 2013) to seven per 1000 surgeries in a French report (Barreau et al. 2012).
Table 1.
Overview on the global prevalence of endophthalmitis
| Study | Country | Incidence of endophthalmitis (%) |
|---|---|---|
| Africa | 26/8190 (0.32%) | |
| van der Merwe et al. (2012) | South Africa | 26/8190 (0.32%) |
| Asia | 1108/763 690 (0.15%) | |
| Lin et al. (2011) | China | 9/94 650 (0.01%) |
| Yao et al. (2013) | China | 66/201 757 (0.03%) |
| Lalitha et al. (2005) | India | 19/22 294 (0.09%) |
| Ravindran et al. (2009) | India | 38/42 426 (0.09%) |
| Haripriya et al. (2012) | India | 21/79 777 (0.03%) |
| Matsuura et al. (2013) | Japan | 11/34 762 (0.03%) |
| Nagaki et al. (2003) | Japan | 15/11 595 (0.13%) |
| Al‐Mezaine et al. (2009) | Saudi Arabia | 20/29 509 (0.07%) |
| Wong & Chee (2004b) | Singapore | 34/44 803 (0.08%) |
| Tan et al. (2012) | Singapore | 21/50 177 (0.04%) |
| Wu et al. (2006a) | Taiwan | 46/21 562 (0.21%) |
| Wu et al. (2006b) | Taiwan | 12/10 614 (0.11%) |
| Fang et al. (2006) | Taiwan | 772/108 705 (0.71%) |
| Trinavarat et al. (2006) | Thailand | 24/11 059 (0.22%) |
| Australia | 723/504 471 (0.14%) | |
| Ellis (2003) | Australia | 5/633 (0.79%) |
| Semmens et al. (2003) | Australia | 188/94 653 (0.20%) |
| Li et al. (2004) | Australia | 210/117 083 (0.18%) |
| Rosha et al. (2006) | Australia | 92/162 120 (0.06%) |
| Clark et al. (2011) | Australia | 228/129 982 (0.18%) |
| Europe | 1253/1 777 045 (0.07%) | |
| ESCRS 2007 (ESCRS Endophthalmitis Study Group 2007) | Europe | 29/16 603 (0.17%) |
| Eurequo 2012 (Lundstrom et al. 2012) | Europe | 148/406 703 (0.04%) |
| Haapala et al. (2005) | Finland | 47/29 350 (0.16%) |
| Barreau et al. (2012) | France | 36/5115 (0.70%) |
| Ness et al. (2011) | Germany | 16/26 566 (0.06%) |
| Krikonis et al. (2009) | Greece | 7/8393 (0.08%) |
| Khan et al. (2005) | Ireland | 43/8763 (0.49%) |
| Rahman & Murphy (2014) | Ireland | 5/8239 (0.06%) |
| Kessner et al. (2014) | Israel | 40/13 284 (0.30%) |
| Råen et al. (2013) | Norway | 9/15 954 (0.06%) |
| Beselga et al. (2014) | Portugal | 16/15 689 (0.10%) |
| Garat et al. (2005) | Spain | 31/18 579 (0.17%) |
| Garcia‐Saenz et al. (2010) | Spain | 42/13 652 (0.31%) |
| Romero‐Aroca et al. (2012) | Spain | 83/25 001 (0.33%) |
| Rodriguez‐Caravaca et al. (2013) | Spain | 44/19 463 (0.23%) |
| Montan et al. (2002a) | Sweden | 20/32 180 (0.06%) |
| Wejde et al. (2005a) | Sweden | 112/188 151 (0.06%) |
| Lundstrom et al. (2007) | Sweden | 109/225 471 (0.05%) |
| Friling et al. (2013) | Sweden | 135/464 996 (0.03%) |
| Mayer et al. (2003) | UK | 30/18 191 (0.16%) |
| Patwardhan et al. (2006) | UK | 44/12 362 (0.36%) |
| Kelly et al. (2007) | UK | 7/12 831 (0.05%) |
| Mollan et al. (2007) | UK | 101/101 920 (0.10%) |
| Yu‐Wai‐Man et al. (2008) | UK | 46/38 819 (0.12%) |
| Carrim et al. (2009) | UK | 25/12 500 (0.20%) |
| Anijeet et al. (2010) | UK | 14/16 606 (0.08%) |
| Myneni et al. (2013) | UK | 14/21 664 (0.06%) |
| North America | 6935/5 122 623 (0.14%) | |
| Shorstein et al. (2013) | California | 19/16 264 (0.12%) |
| Lloyd & Braga‐Mele (2009) | Canada | 6/13 931 (0.04%) |
| Hatch et al. (2009) | Canada | 617/422 177 (0.15%) |
| Freeman et al. (2010) | Canada | 754/490 690 (0.15%) |
| Rudnisky et al. (2014) | Canada | 23/75 318 (0.03%) |
| Miller et al. (2005) | Florida | 7/15 920 (0.04%) |
| Wykoff et al. (2010) | Florida | 8/28 568 (0.03%) |
| Thoms et al. (2007) | Michigan | 5/815 (0.61%) |
| Buzard & Liapis (2004) | Nevada | 0/5131 (0%) |
| Wallin et al. (2005) | Utah | 27/15 254 (0.18%) |
| Jensen et al. (2005) | Utah | 26/9079 (0.29%) |
| Moshirfar et al. (2007) | Utah | 14/20 013 (0.07%) |
| Jensen et al. (2008) | Utah | 40/29 276 (0.14%) |
| West et al. (2005) | USA | 1026/477 627 (0.21%) |
| Stein et al. (2011) | USA | 357/221 594 (0.16%) |
| Keay et al. (2012) | USA | 4006/3 280 966 (0.12%) |
| South America | 74/27 264 (0.27%) | |
| Melo et al. (2010) | Brazil | 73/24 590 (0.30%) |
| Galvis et al. (2014) | Colombia | 1/2674 (0.04%) |
We also identified 21 studies reporting the microbiological findings in endophthalmitis cases (Table 2). The reader should note that only studies reporting more than 50 cases of endophthalmitis were included in Table 2 as the finding of uncommon bacteria in small studies could skew the prevalence of causative micro‐organisms. In up to half of the clinically diagnosed endophthalmitis cases, no causative micro‐organism was found. The predominant causative micro‐organism was coagulase‐negative staphylococci with Staphylococcus aureus ranking second. In Sweden, where the national rate of endophthalmitis is the lowest reported globally possibly due to a nearly universal use of intracameral cefuroxime, the rate of infection caused by enterococci is high (Wejde et al. 2005a; Lundstrom et al. 2007; Friling et al. 2013). Fungi are a rare cause of endophthalmitis after cataract surgery and were only reported in large numbers from India (Gupta et al. 2003).
Table 2.
Causative micro‐organisms in endophthalmitis after cataract surgery
| Study id | Country | Years | No. of cases | Culture negative | Gram + | Coagulase‐negative staphylococci | Staphylococcus aureus | Enterococcus | Gram − | Fungi |
|---|---|---|---|---|---|---|---|---|---|---|
| Asia | 311 | 54% | 48% | 23% | 7% | 1% | 39% | 13% | ||
| Yao et al. (2013) | China | 2006–2011 | 64 | 39 | 14 | 8 | 3 | 1 | 11 | 0 |
| Gupta et al. (2003) | India | 1996–2001 | 124 | 77 | 5 | 0 | 2 | 0 | 7 | 27 |
| Joseph et al. (2012) | India | 2008–2010 | 64 | 27 | 20 | 14 | 4 | 0 | 17 | – |
| Jindal et al. (2014) | India | 2006–2013 | 248 | – | 124 | 60 | 9 | 0 | 89 | 20 |
| Cheng et al. (2010) | Taiwan | 2002–2008 | 59 | 25 | 15 | 1 | 8 | 4 | 19 | 0 |
| Australia | 213 | 46% | 86% | 47% | 18% | 0% | 12% | 2% | ||
| Ng et al. (2005) | Australia | 1980–2000 | 213 | 99 | 113 | 61 | 24 | – | 16 | 2 |
| Europe | 1282 | 30% | 88% | 45% | 10% | 13% | 11% | 0% | ||
| Kodjikian et al. (2009) | France | 2003–2004 | 95 | 50 | 39 | 26 | 6 | 0 | 3 | 0 |
| Cornut et al. (2012) | France | 2004–2005 | 100 | 30 | 66 | 33 | 14 | 0 | 4 | 0 |
| Sandvig & Dannevig (2003) | Norway | 1996–1998 | 111 | 23 | 75 | 32 | 7 | 11 | 4 | 1 |
| Romero‐Aroca et al. (2012) | Spain | 1996–2002 | 83 | 28 | 44 | 37 | 5 | 0 | 6 | 0 |
| Wejde et al. (2005a) | Sweden | 1999–2001 | 112 | 14 | 77 | 30 | 6 | 23 | 14 | 0 |
| Lundstrom et al. (2007) | Sweden | 2002–2004 | 109 | 20 | 79 | 34 | 9 | 25 | 9 | 0 |
| Friling et al. (2013) | Sweden | 2005–2010 | 135 | 20 | 94 | 35 | – | 42 | 19 | 0 |
| Kamalarajah et al. (2004) | UK | 1999–2000 | 199 | 88 | 103 | 54 | 10 | 3 | 8 | 0 |
| Pijl et al. (2010) | The Netherlands | 1996–2006 | 250 | 84 | 152 | 89 | 20 | 3 | 10 | 0 |
| Altan et al. (2009) | Turkey | 2000–2007 | 88 | 31 | 35 | 18 | 8 | 1 | 22 | 0 |
| North America | 911 | 25% | 92% | 64% | 12% | 3% | 6% | 1% | ||
| Recchia et al. (2005) | USA | 1989–2000 | 497 | 175 | 304 | 180 | 43 | 13 | 17 | 7 |
| Mollan et al. (2007) | USA | 1996–2004 | 103 | 44 | 98 | 38 | 3 | 2 | 2 | 0 |
| Lalwani et al. (2008) | USA | 1996–2005 | 73 | 7 | 66 | 50 | 5 | 0 | 7 | 0 |
| Shirodkar et al. (2012); Shirodkar et al. (2012)a | USA | 2000–2009 | 92 | – | – | 57 | 11 | – | – | – |
| South America | 73 | 37% | 83% | 57% | 4% | 2% | 17% | 0% | ||
| Melo et al. (2010) | Brazil | 2002–2008 | 73 | 27 | 38 | 26 | 2 | 1 | 8 | 0 |
Only studies reporting more than 50 cases of endophthalmitis after cataract surgery are included in the table Bacterial species are expressed as number of a given species demonstrated after culture For each continent, the total number of endophthalmitis cases and the percentage of culture‐negative cases and the percentage of Gram‐positive and Gram‐negative species, fungi, coagulase‐negative staphylococci, staphylococcus aureus and enterococci are reported – not reported.
Publication excluded from calculation of percentages of culture‐positive species and culture‐negative samples because of too few data.
Intracameral antibiotic and endophthalmitis risk
A systematic literature search identified one randomized controlled trial evaluating the effect of intracameral cefuroxime on the prevention of post‐phacoemulsification endophthalmitis (Seal et al. 2006; ESCRS Endophthalmitis Study Group 2007). Furthermore, we found 17 observational studies describing the prevalence of endophthalmitis in the same institution(s) before and after introducing intracameral delivery of an antibiotic agent at the conclusion of surgery (Wejde et al. 2005a; ESCRS Endophthalmitis Study Group 2007; Lundstrom et al. 2007; Yu‐Wai‐Man et al. 2008; Garat et al. 2009; Anijeet et al. 2010; Barreau et al. 2012; van der Merwe et al. 2012; Romero‐Aroca et al. 2012; Tan et al. 2012; Beselga et al. 2014; Friling et al. 2013; Matsuura et al. 2013; Myneni et al. 2013; Rodriguez‐Caravaca et al. 2013; Shorstein et al. 2013; Galvis et al. 2014; Rudnisky et al. 2014). Of those 17 studies, 10 studies reported the rates of endophthalmitis with and without intracameral cefuroxime (Wejde et al. 2005a; Lundstrom et al. 2007; Yu‐Wai‐Man et al. 2008; Barreau et al. 2012; van der Merwe et al. 2012; Beselga et al. 2014; Friling et al. 2013; Myneni et al. 2013; Rodriguez‐Caravaca et al. 2013; Shorstein et al. 2013). Three studies looked at cefazolin (Garat et al. 2009; Romero‐Aroca et al. 2012; Tan et al. 2012). Five studies looked at moxifloxacin (Friling et al. 2013; Matsuura et al. 2013; Shorstein et al. 2013; Galvis et al. 2014; Rudnisky et al. 2014; ). Finally, three studies reported the rates of endophthalmitis with and without intracameral vancomycin (Anijeet et al. 2010; Rudnisky et al. 2014; Shorstein et al. 2013).
The characteristics of included studies are provided in Appendix S1. Characteristics of studies excluded from the analysis as well as reason for exclusion are presented in Appendix S2.
The randomized controlled trial was conducted as a European multicenter trial after an initiative by the European Society of Cataract and Refractive surgeons (Seal et al. 2006; ESCRS Endophthalmitis Study Group 2007). It was planned to include 35 000 participants, but the study was stopped after recruitment of 16 603 patients as the treatment effect was so marked that it was deemed unethical to continue the study. A total of 29 cases of clinically suspected endophthalmitis were detected. The rate of endophthalmitis was 0.6 per 1000 surgeries when intracameral cefuroxime was used at the conclusion of surgery versus 3.0 per 1000 surgeries when intracameral cefuroxime was not used. The difference was highly statistically significant, RR 0.21 (95% CI: 0.08; 0.55) (Fig. 2).
Figure 2.
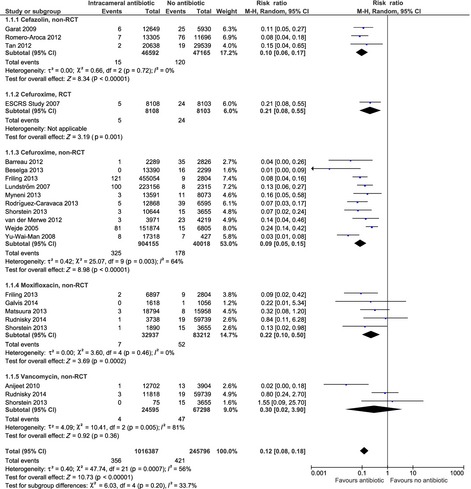
Forest plot showing the effect of peri‐operative, prophylactic intracameral antibiotic treatment as reported in the randomized trial and the 17 observational studies reporting endophthalmitis rate in patients receiving intracameral antibiotic prophylaxis (cefazolin, cefuroxime, moxifloxacin or vancomycin) versus no intracameral antibiotic prophylaxis.
Since the publication of the ESCRS trial, several institutions have adopted the prophylactic intracameral administration of antibiotic. In total, 17 publications describing the rate of endophthalmitis reported by single institutions or countries before and after changing prophylactic regimes were identified. The majority of these studies are from Europe (Wejde et al. 2005a; Lundstrom et al. 2007; Garat et al. 2009; Anijeet et al. 2010; Barreau et al. 2012; van der Merwe et al. 2012; Romero‐Aroca et al. 2012; Beselga et al. 2014; Friling et al. 2013; Myneni et al. 2013; Rodriguez‐Caravaca et al. 2013), but findings from Asia (Tan et al. 2012; Matsuura et al. 2013), Africa (van der Merwe et al. 2012), North America (Shorstein et al. 2013; Rudnisky et al. 2014) and South America (Galvis et al. 2014) are also included in the analysis.
Based on the non‐randomized studies, the risk of endophthalmitis was significantly lower in patients treated with intracameral cefazolin, cefuroxime and moxifloxacin, whereas no significant effect was found for intracameral vancomycin (see Fig. 2). The relative risk [RR (95% confidence interval)] of endophthalmitis was reduced to 0.10 (0.06; 0.17) in patients receiving cefazolin, 0.09 (0.05; 0.15) in patients receiving cefuroxime, 0.22 (0.10; 0.50) in patients receiving moxifloxacin and 0.30 (0.02; 3.90) in patients receiving vancomycin.
In total, 1 192 330 cataract surgeries and 719 cases of endophthalmitis were included in the analysis. There were 356 cases of endophthalmitis in the 1 016 387 surgeries where intracameral antibiotics were used compared to 363 cases of endophthalmitis in the 175 943 surgeries where intracameral antibiotics were not used. Thus, endophthalmitis occurred in one of 2855 surgeries when intracameral antibiotics was used compared to one of 485 surgeries when no intracameral antibiotic was used. None of the studies included in the meta‐analyses above reported adverse events associated with the use of intracameral antibiotic treatment.
The quality of evidence was high for the randomized trial and moderate for the observational studies concerning cefuroxime and cefazolin and low to very low for the observational studies concerning moxifloxacin and vancomycin, respectively (Table 3). The quality of the evidence for the observational studies concerning cefazolin and cefuroxime was upgraded because of the very large effect of intracameral antibiotic treatment.
Table 3.
Summary of findings and quality of evidence concerning the prophylactic role of intracameral antibiotic administration
| Outcomes: post‐phacoemulsification endophthalmitis rates | No of Participants (studies) | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk without intracameral antibiotic | Risk difference with intracameral antibiotic (95% CI) | ||||
| Cefazolin, non‐RCT | 93 757 (3 studies) |
⊕⊕⊕⊝ moderatea
Due to large effect |
RR 01 (006–017) | 3 per 1000 | 2 fewer per 1000 (from 2 fewer to 2 fewer) |
| Cefuroxime, RCT | 16 211 (1 study) |
⊕⊕⊕⊕ high |
RR 021 (008–055) | 3 per 1000 | 2 fewer per 1000 (from 1 fewer to 3 fewer) |
| Cefuroxime, non‐RCT | 944 173 (10 studies) |
⊕⊕⊕⊝ moderatea Due to large effect |
RR 009 (005–015) | 4 per 1000 | 4 fewer per 1000 (from 4 fewer to 4 fewer) |
| Moxifloxacin, non‐RCT | 116 149 (5 studies) |
⊕⊕⊝⊝ low |
RR 022 (01–05) | 1 per 1000 | 0 fewer per 1000 (from 0 fewer to 1 fewer) |
| Vancomycin, non‐RCT | 91 893 (3 studies) |
⊕⊝⊝⊝ very lowb , c Due to inconsistency, imprecision |
RR 03 (002–39) | 1 per 1000 | 0 fewer per 1000 (from 1 fewer to 2 more) |
CI = confidence interval; RR = risk ratio.
GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect; Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality: We are very uncertain about the estimate.
Randomized trials begin as high‐quality evidence and can be upgraded or downgraded Observational studies begin as low quality of evidence and can be upgraded or downgraded.
The basis for the assumed risk (e.g the median control group risk across studies) is provided in footnotes The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Upgraded because of marked effect of intracameral antibiotic.
Large differences in estimates and confidence intervals between studies.
Too few events (endophthalmitis cases) and included patients for a definite conclusion to be drawn plus confidence interval cross RR 1.0.
Topical antibiotics and risk of endophthalmitis
After a systematic review of the literature, we found one randomized trial (Seal et al. 2006; ESCRS Endophthalmitis Study Group 2007) and one retrospective study (Råen et al. 2013) evaluating the effect of topical antibiotic treatment on the rate of endophthalmitis. Characteristics of the included studies are presented in Appendix S3, and characteristics of excluded studies are presented in Appendix S4.
The ESCRS study was designed in a 2 × 2 factorial design, and besides examining the prophylactic effect of intracameral cefuroxime, the study also evaluated the prophylactic effect of 1 hr preoperative topical 0.5% levofloxacin treatment versus placebo (Seal et al. 2006; ESCRS Endophthalmitis Study Group 2007). In addition to the preoperative randomized treatment, all patients received topical 0.5% levofloxacin for 6 days after cataract surgery. The rate of endophthalmitis was 1.5 per 1000 patients in those treated preoperatively with levofloxacin versus 2.1 per 1000 patients in those not treated with topical levofloxacin preoperatively. The difference between the two groups was not statistically significant, RR 0.71 (95% CI: 0.34; 1.48) (Fig. 3).
Figure 3.
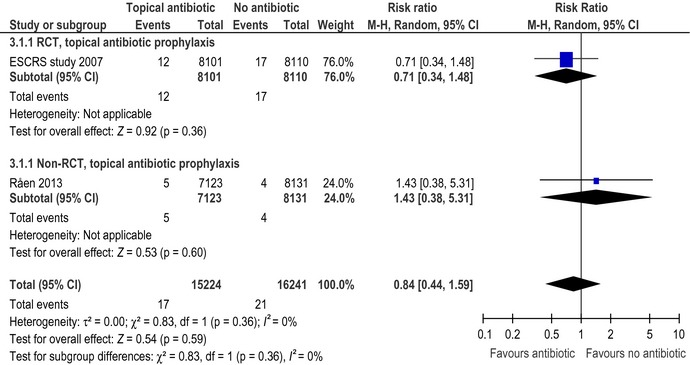
Forest plot showing the effect of prophylactic topical antibiotic therapy as reported in the randomized trial and in the observational study.
The effect of postoperative topical chloramphenicol treatment was reported in a retrospective Norwegian study (Råen et al. 2013). In Sweden, topical antibiotic treatment is not used routinely and yet the reported rates of endophthalmitis are among the lowest reported worldwide (see Table 1). This prompted the Department of Ophthalmology at the University Hospital of Oslo in Norway to stop using postoperative antibiotic topical treatment, and they evaluated the rate of endophthalmitis in the years preceding and following the change in postoperative topical antibiotic treatment. All patients received intracameral cefuroxime unless they had a history of penicillin allergy. The rate of endophthalmitis was 0.7 per 1000 patients in the time period where topical chloramphenicol was used versus 0.5 per 1000 patients in the time period where no topical antibiotic treatment was used postoperatively. The difference between time periods was not significant, RR 1.43 (95% CI: 0.38; 5.31) (Fig. 3).
The summary of findings and the quality of evidence concerning the prophylactic use of topical antibiotic treatment is presented in Table 4. The quality of evidence was high for the randomized trial evaluating the rate of endophthalmitis in patients randomized to preoperative levofloxacin or placebo. The quality of evidence was graded as low according to the GRADE guidelines for the retrospective study (Guyatt et al. 2011g).
Table 4.
Summary of findings and quality of evidence for the prophylactic use of topical antibiotic treatment
| Outcomes: endophthalmitis rates after phacoemulsification using topical antibiotics | No of Participants (studies) | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk without topical antibiotic | Risk difference with topical antibiotic (95% CI) | ||||
| Endophthalmitis rate, RCT | 16 211 (1 study) |
⊕⊕⊕⊕ HIGH |
RR 071 (034–148) | 2 per 1000 | 1 fewer per 1000 (from 1 fewer to 1 more) |
| Endophthalmitis rate, observational study | 15 254 (1 study) |
⊕⊕⊝⊝ LOW |
RR 143 (038–531) | 0 per 1000 | 0 more per 1000 (from 0 fewer to 2 more) |
CI = confidence interval; RR = risk ratio.
GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect; Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality: We are very uncertain about the estimate.
The basis for the assumed risk (e.g the median control group risk across studies) is provided in footnotes The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Discussion
Endophthalmitis is the most feared complication after cataract surgery. There are striking global differences in the prevalence of endophthalmitis. The risk of endophthalmitis is more than doubled in the USA compared to Europe even when comparing nationwide data covering the period of time, 0.05% in the years 2002–2004 in Sweden (Lundstrom et al. 2007) versus 0.12% in the years 2003–2004 in the USA (Keay et al. 2012). Although the populations covered by the reports may not be directly comparable, both aforementioned reports are based on a very large number of patients, 225 000 in the Swedish report and 3 280 000 in the US report, and both report from publically funded healthcare systems (the Medicare in the US). A direct comparison between the different reports of endophthalmitis rates is not always possible as important information on major risk factors, for example age, gender, capsule rupture or the use of intracameral antibiotics, is not always available. Nevertheless, the data bring us one important message: the rate of endophthalmitis can be reduced if prophylactic actions are taken.
This raises the important question: What is the most effective prophylactic regime? The aim of the present systematic review was to evaluate the effect of antibiotic treatment alone, but the role of cleaning the conjunctiva by povidone‐iodine, keeping the eye lashes out of the surgical field and treating blepharitis prior to surgery, is also of importance. It is, however, beyond the scope of the present study to provide evidence‐based recommendations for non‐antibiotic prophylactic regimes.
We examined the evidence for a prophylactic role of intracameral cefuroxime and found high‐quality evidence that it significantly reduces the rate of endophthalmitis (ESCRS Endophthalmitis Study Group 2007; Seal et al. 2006). Two to four cases of endophthalmitis can be avoided per 1000 cataract surgeries performed when intracameral cefuroxime is used. The finding of the randomized trial was confirmed by several retrospective, observational studies (Wejde et al. 2005a; ESCRS Endophthalmitis Study Group 2007; Lundstrom et al. 2007; Yu‐Wai‐Man et al. 2008; Garat et al. 2009; Anijeet et al. 2010; Barreau et al. 2012; van der Merwe et al. 2012; Romero‐Aroca et al. 2012; Tan et al. 2012; Beselga et al. 2014; Friling et al. 2013; Galvis et al. 2014; Matsuura et al. 2013; Myneni et al. 2013; Rodriguez‐Caravaca et al. 2013; Shorstein et al. 2013; Rudnisky et al. 2014). The ESCRS study has been criticized for a high rate of endophthalmitis in the non‐cefuroxime group, but as the present meta‐analysis demonstrates, comparable rates of endophthalmitis in the non‐cefuroxime group was found in the ESCRS study and in the observational studies. Furthermore, the rate in the non‐cefuroxime group is comparable to that reported in many of the studies summarized in Table 1. Thus, the authors of the present systematic review have found that the ESCRS reports high‐quality and reliable data that undisputedly demonstrate a significant prophylactic effect of intracameral cefuroxime. Several studies have shown that intracameral cefuroxime at a dose of 1 mg in 0.1 ml is safe for the human eye (Montan et al. 2002b; Gupta et al. 2005; Lam et al. 2010).
Surveys on the use of prophylactic antibiotic regimes published after the publication of the ESCRS trial show that there are large global differences in the use of intracameral antibiotic therapy. In Sweden, nearly all patients receive intracameral antibiotic (99%) (Friling et al. 2013). The ESCRS 2012 survey found that 74% always used intracameral antibiotics (Barry 2014). A Greek study found that 50% of surgeons use intracameral cefuroxime (Mataftsi et al. 2011). In the UK, 40% (Murjaneh et al. 2010) to 54% (Nanavaty & Wearne 2010) of ophthalmology units and 63% of the United Kingdom and Ireland Society of Cataract and Refractive surgeons (Gore et al. 2009) use intracameral antibiotic as standard. The ASCRS survey showed that 30% used intracameral antibiotic; of those who did, half used it as injection and the other half in the irrigation fluid, whereas nearly all surgeons (98%) used topical antibiotic postoperatively (Chang et al. 2007). In Singapore, 30% of surgeons use intracameral antibiotic (Han & Chee 2012). In New Zealand, 24% use intracameral antibiotic and 92% use postoperative topical antibiotic (Pick et al. 2008). The main reason for not using intracameral antibiotic reported in the above‐mentioned studies has been a fear of risks associated with the use and the lack of a commercially available preparation.
Around 5% of patients who are allergic to penicillin may respond with cross‐reactivity to cephalosporins. Serious systemic anaphylactic reactions have been reported after the use of intracameral cefuroxime (Villada et al. 2005). However, a study based on 36 patients with penicillin allergy (ranging from rash to loss of consciousness) did not find any adverse effects after subconjunctival cefuroxime injection (Mitra & McElvanney 2006). Each surgeon must make his or her own choice when it comes to the use of intracameral cefuroxime in patients with a history of allergic reaction to penicillin or cephalosporin.
One of the practical problems associated with intracameral cefuroxime has been the lack of a commercially available ready‐to‐use drug. This has caused fear of dilution errors. Erroneous injection of 3 mg in 0.1 ml in six patients did not result in adverse effects (Sakarya & Sakarya 2010), whereas 62.5 mg resulted in macular infarction (Qureshi & Clark 2011). A larger case series from Finland showed that erroneously high amounts of cefuroxime (between 10 and 100 mg intracamerally) resulted in severe ocular toxicity with corneal oedema and lowering of visual field sensitivity but that half of the patients ended with a reasonable (>0.5 Snellen) visual acuity (Olavi 2012). In Europe, a ready‐to‐mix solution of cefuroxime has been approved, thus minimizing the risk of dilution errors. It is hoped that a ready‐to‐mix cefuroxime formulation will also be available in the rest of the world in the future. Using intracameral cefuroxime is not cost‐free, but studies have shown that intracameral cefuroxime is cost‐effective, whereas the topical use of ciprofloxacin, ofloxacin, moxifloxacin and gatifloxacin is not (Sharifi et al. 2009).
So far, no international ophthalmological society has advocated strongly for the use of intracameral cefuroxime. A joint European initiative aimed at improving the quality of cataract surgery, the EUREQUO, reports lower incidence of postoperative endophthalmitis after intracameral cefuroxime but does not recommend the use/no use of intracameral antibiotic (Lundstrom et al. 2012). The Canadian Ophthalmological Society has a consensus statement saying that if the surgeon has a higher endophthalmitis rate than published norms, consideration should be given to change to intracameral or subconjunctival antibiotic supplementation (Canadian Ophthalmological Society Cataract Surgery Clinical Practise Guideline Expert Committee 2008). The recommendations from the British Royal College of Ophthalmologists are similar to the Canadian (The Royal College of Ophthalmologists 2010). The American Association of Ophthalmologists recommends ‘It would appear that antibiotic use on the day of surgery is important rather than waiting until the next day. Any additional prophylactic antibiotic strategy in the perioperative period is up to the ophthalmologist to determine’ (American Academy of Ophthalmology 2011). The findings reported in the present study should, however, lead all ophthalmological societies to make a strong recommendation to use intracameral cefuroxime.
The second part of the present systematic review deals with the use of topical antibiotics in the prevention of endophthalmitis. Surveys have shown that nearly all surgeons prescribe topical antibiotics to be administered after cataract surgery (Rosha et al. 2006; Chang et al. 2007; Pick et al. 2008). In theory, topical antibiotics may work by reducing the number of bacteria on the conjunctiva, thus lowering the risk of intraocular contamination either during surgery or through a leaking wound postoperatively. Three days of topical antibiotic treatment reduces the number of positive conjunctival samples by approximately 50% (Inoue et al. 2008; He et al. 2009). In other words, even after several days of antibiotic treatment, a high number of bacteria remain on the conjunctiva lowering the theoretical rationale for topical antibiotic prophylaxis. Topical antibiotic therapy was not found to lower the rate of endophthalmitis in the ESCRS study (ESCRS Endophthalmitis Study Group 2007) nor in a retrospective Norwegian study (Råen et al. 2013). Unnecessary antibiotic therapy carries a risk of selecting drug‐resistant bacterial strains. An American study found that five of 31 endophthalmitis cases treated with perioperative gatifloxacin or moxifloxacin were resistant to gatifloxacin and moxifloxacin (Deramo et al. 2006). We did not find a protective effect of topical antibiotics. In addition, we did not find evidence that postoperative use of topical antibiotics increases the risk of endophthalmitis as has been described after anti‐VEGF injections (Cheung et al. 2012; Storey et al. 2014).
Conclusions and recommendations
In conclusion, we found strong and consistent evidence that intracameral cefuroxime administered at the conclusion of cataract surgery significantly lowers the risk of endophthalmitis. Two to four cases of endophthalmitis per 1000 surgeries can be avoided if surgeons adopt the use of intracameral cefuroxime and the authors of the review strongly recommend its use. We could not find any evidence that topical antibiotic treatment after cataract surgery lowers the risk of endophthalmitis. As there is no documented effect of topical antibiotic treatment and its use may be associated with concern for selection of resistant bacterial strains, we cannot recommend using it.
Supporting information
Appendix S1. Characteristics of included studies, intracameral antibiotic prophylaxis.
Appendix S2. Characteristics of excluded studies, intracameral antibiotic prophylaxis.
Appendix S3. Characteristics of included studies.
Appendix S4. Characteristics of excluded studies.
The authors would like to thank information specialist Birgitte Holm Petersen at the Danish Health and Medicines Authority for assistance in the literature search. The study was initiated and financed by the National Danish Health and Medicines Authority.
References
- Al‐Mezaine HS, Al‐Assiri A & Al‐Rajhi AA (2009): Incidence, clinical features, causative organisms, and visual outcomes of delayed‐onset pseudophakic endophthalmitis. Eur J Ophthalmol 19: 804–811. [DOI] [PubMed] [Google Scholar]
- Altan T, Acar N, Kapran Z, Unver YB, Yurttaser S, Kucuksumer Y & Eser I (2009): Acute‐onset endophthalmitis after cataract surgery: success of initial therapy, visual outcomes, and related factors. Retina 29: 606–612. [DOI] [PubMed] [Google Scholar]
- American Academy of Ophthalmology (2011): Cataract in the adult eye. Preferred Practice Pattern 1‐89. [DOI] [PubMed]
- Anijeet DR, Palimar P & Peckar CO (2010): Intracameral vancomycin following cataract surgery: an eleven‐year study. Clin Ophthalmol 4: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asencio MA, Huertas M, Carranza R, Tenias JM, Celis J & Gonzalez‐Del VF (2014): Impact of changes in antibiotic prophylaxis on postoperative endophthalmitis in a Spanish hospital. Ophthalmic Epidemiol 21: 45–50. [DOI] [PubMed] [Google Scholar]
- Aslan O, Teberik K, Yucel M, Gur N & Karakoc AE (2008): Effect of topical netilmicin on the reduction of bacterial flora on the human conjunctiva. Eur J Ophthalmol 18: 512–516. [DOI] [PubMed] [Google Scholar]
- Baillif S, Roure‐Sobas C, Le‐Duff F & Kodjikian L (2012): Aqueous humor contamination during phacoemulsification in a university teaching hospital. J Fr Ophtalmol 35: 153–156. [DOI] [PubMed] [Google Scholar]
- Balestrazzi A, Malandrini A, Montagnani F et al. (2012): Phacoemulsificator and sterile drapes contamination during cataract surgery: a microbiological study. Eur J Ophthalmol 22: 188–194. [DOI] [PubMed] [Google Scholar]
- Barreau G, Mounier M, Marin B, Adenis JP & Robert PY (2012): Intracameral cefuroxime injection at the end of cataract surgery to reduce the incidence of endophthalmitis: French study. J Cataract Refract Surg 38: 1370–1375. [DOI] [PubMed] [Google Scholar]
- Barry P (2014): Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg 40: 138–142. [DOI] [PubMed] [Google Scholar]
- Barry P, Seal DV, Gettinby G, Lees F, Peterson M & Revie CW (2006): ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: preliminary report of principal results from a European multicenter study. J Cataract Refract Surg 32: 407–410. [DOI] [PubMed] [Google Scholar]
- Barry P, Gardner S, Seal D, Gettinby G, Lees F, Peterson M & Revie C (2009): Clinical observations associated with proven and unproven cases in the ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery. J Cataract Refract Surg, 35: 1523–1531, 1531. [DOI] [PubMed] [Google Scholar]
- Behndig A, Cochener B, Guell JL et al. (2013): Endophthalmitis prophylaxis in cataract surgery: overview of current practice patterns in 9 European countries. J Cataract Refract Surg 39: 1421–1431. [DOI] [PubMed] [Google Scholar]
- Beselga D, Campos A, Castro M et al. (2014): Postcataract surgery endophthalmitis after introduction of the ESCRS protocol: a 5‐year study. Eur J Ophthalmol 24: 516–519. [DOI] [PubMed] [Google Scholar]
- Bhoomibunchoo C, Ratanapakorn T, Sinawat S, Sanguansak T, Moontawee K & Yospaiboon Y (2013): Infectious endophthalmitis: review of 420 cases. Clin Ophthalmol 7: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci FA Jr (2004): An in vivo study comparing the ocular absorption of levofloxacin and ciprofloxacin prior to phacoemulsification. Am J Ophthalmol 137: 308–312. [DOI] [PubMed] [Google Scholar]
- Buzard K & Liapis S (2004): Prevention of endophthalmitis. J Cataract Refract Surg 30: 1953–1959. [DOI] [PubMed] [Google Scholar]
- Cagini C, Piccinelli F, Lupidi M, Messina M, Cerqualglia A, Manes S, Fiore T & Pellegrino RM (2013): Ocular penetration of topical antibiotics: study on the penetration of chloramphenicol, tobramycin and netilmicin into the anterior chamber after topical administration. Clin Experiment Ophthalmol 41: 644–647. [DOI] [PubMed] [Google Scholar]
- Camesasca FI, Bianchi C, Beltrame G, Caporossi A, Piovella M, Rapisarda A, Tassinari G & Zeppa L (2007): Control of inflammation and prophylaxis of endophthalmitis after cataract surgery: a multicenter study. Eur J Ophthalmol 17: 733–742. [DOI] [PubMed] [Google Scholar]
- Canadian Ophthalmological Society Cataract Surgery Clinical Practise Guideline Expert Committee (2008): Canadian Ophthalmological Society evidence‐based clinical practise guidelines for cataract surgery in the adult eye. Can J Ophthalmol 43: S1–S57. [DOI] [PubMed] [Google Scholar]
- Cao H, Zhang L, Li L & Lo S (2013): Risk factors for acute endophthalmitis following cataract surgery: a systematic review and meta‐analysis. PLoS ONE 8: e71731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrim ZI, Richardson J & Wykes WN (2009): Incidence and visual outcome of acute endophthalmitis after cataract surgery–the experience of an eye department in Scotland. Br J Ophthalmol 93: 721–725. [DOI] [PubMed] [Google Scholar]
- Chang DF, Braga‐Mele R, Mamalis N, Masket S, Miller KM, Nichamin LD, Packard RB & Packer M (2007): Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member survey. J Cataract Refract Surg 33: 1801–1805. [DOI] [PubMed] [Google Scholar]
- Cheng JH, Chang YH, Chen CL, Chen YH, Lu DW & Chen JT (2010): Acute endophthalmitis after cataract surgery at a referral centre in Northern Taiwan: review of the causative organisms, antibiotic susceptibility, and clinical features. Eye (Lond) 24: 1359–1365. [DOI] [PubMed] [Google Scholar]
- Cheung CS, Wong AW, Lui A, Kertes PJ, Devenyi RG & Lam WC (2012): Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology 119: 1609–1614. [DOI] [PubMed] [Google Scholar]
- Clark A, Ng JQ, Morlet N, Tropiano E, Mahendran P, Spilsbury K, Preen D & Semmens JB (2008): Quality of life after postoperative endophthalmitis. Clin Experiment Ophthalmol 36: 526–531. [DOI] [PubMed] [Google Scholar]
- Clark A, Morlet N, Ng JQ, Preen DB & Semmens JB (2011): Whole population trends in complications of cataract surgery over 22 years in Western Australia. Ophthalmology 118: 1055–1061. [DOI] [PubMed] [Google Scholar]
- Colin J, Simonpoli S, Geldsetzer K & Ropo A (2003): Corneal penetration of levofloxacin into the human aqueous humour: a comparison with ciprofloxacin. Acta Ophthalmol Scand 81: 611–613. [DOI] [PubMed] [Google Scholar]
- Cornut PL, Vandenesch F, Lina G et al. (2010): Bacterial contamination rate of the anterior chamber during cataract surgery using conventional culture and eubacterial PCR. Eur J Ophthalmol 20: 365–369. [DOI] [PubMed] [Google Scholar]
- Cornut PL, Thuret G, Creuzot‐Garcher C et al. (2012): Relationship between baseline clinical data and microbiologic spectrum in 100 patients with acute postcataract endophthalmitis. Retina 32: 549–557. [DOI] [PubMed] [Google Scholar]
- Coskun M, Altintas AG, Anayol MA, Raza S, Celikbilek N & Simsek S (2011): Evaluation of efficacy of topical povidone‐iodine and different types of fluoroquinolones in the sterilization of bacterial flora on the conjunctiva. J Ocul Pharmacol Ther 27: 589–592. [DOI] [PubMed] [Google Scholar]
- Das D, Das S, Bandyopadhyay S, Mondal KK, Ray B, Das A, Chakrabarti A & Dey AK (2009): A prospective evaluation of anterior chamber contamination following cataract surgery. J Indian Med Assoc, 107: 30, 32–33. [PubMed] [Google Scholar]
- De Kaspar HM, Chang RT, Shriver EM, Singh K, Egbert PR, Blumenkranz MS & Ta CN (2004): Three‐day application of topical ofloxacin reduces the contamination rate of microsurgical knives in cataract surgery: a prospective randomized study. Ophthalmology 111: 1352–1355. [DOI] [PubMed] [Google Scholar]
- Deramo VA, Lai JC, Fastenberg DM & Udell IJ (2006): Acute endophthalmitis in eyes treated prophylactically with gatifloxacin and moxifloxacin. Am J Ophthalmol 142: 721–725. [DOI] [PubMed] [Google Scholar]
- Diez MR, De la Rosa G, Pascual R, Giron C & Arteta M (2009): [Prophylaxis of postoperative endophthalmitis with intracameral cefuroxime: a five years’ experience]. Arch Soc Esp Oftalmol 84: 85–89. [DOI] [PubMed] [Google Scholar]
- Donnenfeld ED, Nichamin LD, Hardten DR et al. (2011): Twice‐daily, preservative‐free ketorolac 045% for treatment of inflammation and pain after cataract surgery. Am J Ophthalmol 151: 420–426. [DOI] [PubMed] [Google Scholar]
- Ellis MF (2003): Topical anaesthesia: a risk factor for post‐cataract‐extraction endophthalmitis?. Clin Experiment Ophthalmol 31: 125–128. [DOI] [PubMed] [Google Scholar]
- ESCRS Endophthalmitis Study Group (2007): Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg 33: 978–988. [DOI] [PubMed] [Google Scholar]
- Fang YT, Chien LN, Ng YY, Chu HF, Chen WM, Cheng CY & Wu SC (2006): Association of hospital and surgeon operation volume with the incidence of postoperative endophthalmitis: Taiwan experience. Eye (Lond) 20: 900–907. [DOI] [PubMed] [Google Scholar]
- Fongsre T, Aiempanakit K, Ratanalerdwee T, Leelaprasasne S, Kanok‐Kantapong S & Jamulitrat S (2004): Extra charge and extra length of hospital stay attributable to postcataract surgery endophthalmitis. Am J Infect Control 32: 374–375. [DOI] [PubMed] [Google Scholar]
- Freeman EE, Roy‐Gagnon MH, Fortin E, Gauthier D, Popescu M & Boisjoly H (2010): Rate of endophthalmitis after cataract surgery in quebec, Canada, 1996–2005. Arch Ophthalmol 128: 230–234. [DOI] [PubMed] [Google Scholar]
- Friling E, Lundstrom M, Stenevi U & Montan P (2013): Six‐year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg 39: 15–21. [DOI] [PubMed] [Google Scholar]
- Galvis V, Tello A, Sanchez MA & Camacho PA (2014): Cohort study of intracameral moxifloxacin in postoperative endophthalmitis prophylaxis. Ophthalmol Eye Dis 6: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garat M, Moser CL, Alonso‐Tarres C, Martin‐Baranera M & Alberdi A (2005): Intracameral cefazolin to prevent endophthalmitis in cataract surgery: 3‐year retrospective study. J Cataract Refract Surg 31: 2230–2234. [DOI] [PubMed] [Google Scholar]
- Garat M, Moser CL, Martin‐Baranera M, Alonso‐Tarres C & Alvarez‐Rubio L (2009): Prophylactic intracameral cefazolin after cataract surgery: endophthalmitis risk reduction and safety results in a 6‐year study. J Cataract Refract Surg 35: 637–642. [DOI] [PubMed] [Google Scholar]
- Garcia‐Saenz MC, Arias‐Puente A, Rodriguez‐Caravaca G & Banuelos JB (2010): Effectiveness of intracameral cefuroxime in preventing endophthalmitis after cataract surgery Ten‐year comparative study. J Cataract Refract Surg 36: 203–207. [DOI] [PubMed] [Google Scholar]
- Ghazi‐Nouri SM, Lochhead J, Mearza AA et al. (2003): Penetration of oral and topical ciprofloxacin into the aqueous humour. Clin Experiment Ophthalmol 31: 40–43. [DOI] [PubMed] [Google Scholar]
- Gore DM, Angunawela RI & Little BC (2009): United Kingdom survey of antibiotic prophylaxis practice after publication of the ESCRS Endophthalmitis Study. J Cataract Refract Surg 35: 770–773. [DOI] [PubMed] [Google Scholar]
- Gower EW, Lindsley K, Nanji AA, Leyngold I & Mcdonnell PJ (2013): Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database Syst Rev 7: CD006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADE profiler (2011) Grade working group 2004‐2007, www.gradeworkinggroup.org.
- Gungor SG, Akova YA, Bozkurt A, Yasar U, Babaoglu MO, Cetinkaya A & Colak M (2011): Aqueous humour penetration of moxifloxocin and gatifloxacin eye drops in different dosing regimens before phacoemulsification surgery. Br J Ophthalmol 95: 1272–1275. [DOI] [PubMed] [Google Scholar]
- Gupta A, Gupta V, Gupta A, Dogra MR, Pandav SS, Ray P & Chakraborty A (2003): Spectrum and clinical profile of post cataract surgery endophthalmitis in north India. Indian J Ophthalmol 51: 139–145. [PubMed] [Google Scholar]
- Gupta MS, McKee HD, Saldana M & Stewart OG (2005): Macular thickness after cataract surgery with intracameral cefuroxime. J Cataract Refract Surg 31: 1163–1166. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist G et al. (2011a): GRADE guidelines: 4 Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 64: 407–415. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R et al. (2011b): GRADE guidelines: 2 Framing the question and deciding on important outcomes. J Clin Epidemiol 64: 395–400. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R et al. (2011c): GRADE guidelines 6 Rating the quality of evidence–imprecision. J Clin Epidemiol 64: 1283–1293. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R et al. (2011d): GRADE guidelines: 8 Rating the quality of evidence–indirectness. J Clin Epidemiol 64: 1303–1310. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R et al. (2011e): GRADE guidelines: 7 Rating the quality of evidence–inconsistency. J Clin Epidemiol 64: 1294–1302. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Montori V et al. (2011f): GRADE guidelines: 5 Rating the quality of evidence–publication bias. J Clin Epidemiol 64: 1277–1282. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P & Knottnerus A (2011g): GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 64: 380–382. [DOI] [PubMed] [Google Scholar]
- Haapala TT, Nelimarkka L, Saari JM, Ahola V & Saari KM (2005): Endophthalmitis following cataract surgery in southwest Finland from 1987 to 2000. Graefes Arch Clin Exp Ophthalmol 243: 1010–1017. [DOI] [PubMed] [Google Scholar]
- Halachmi‐Eyal O, Lang Y, Keness Y & Miron D (2009): Preoperative topical moxifloxacin 05% and povidone‐iodine 50% versus povidone‐iodine 50% alone to reduce bacterial colonization in the conjunctival sac. J Cataract Refract Surg 35: 2109–2114. [DOI] [PubMed] [Google Scholar]
- Han DC & Chee SP (2012): Survey of practice preference pattern in antibiotic prophylaxis against endophthalmitis after cataract surgery in Singapore. Int Ophthalmol 32: 127–134. [DOI] [PubMed] [Google Scholar]
- Haripriya A, Chang DF, Reena M & Shekhar M (2012): Complication rates of phacoemulsification and manual small‐incision cataract surgery at Aravind Eye Hospital. J Cataract Refract Surg 38: 1360–1369. [DOI] [PubMed] [Google Scholar]
- Hatch WV, Cernat G, Wong D, Devenyi R & Bell CM (2009): Risk factors for acute endophthalmitis after cataract surgery: a population‐based study. Ophthalmology 116: 425–430. [DOI] [PubMed] [Google Scholar]
- He L, Ta CN, Hu N, Sinnar S & Mino de Kaspar H (2009): Prospective randomized comparison of 1‐day and 3‐day application of topical 05% moxifloxacin in eliminating preoperative conjunctival bacteria. J Ocul Pharmacol Ther 25: 373–378. [DOI] [PubMed] [Google Scholar]
- Herretes S, Stark WJ, Pirouzmanesh A, Reyes JM, Mcdonnell PJ & Behrens A (2005): Inflow of ocular surface fluid into the anterior chamber after phacoemulsification through sutureless corneal cataract wounds. Am J Ophthalmol 140: 737–740. [DOI] [PubMed] [Google Scholar]
- Higgins JPT & Green S (2011): Cochrane Handbook for Systematic Reviews of Interventions The Cochrane Collaboration, www.cochrane-handbook.org.
- Inoue Y, Usui M, Ohashi Y, Shiota H & Yamazaki T (2008): Preoperative disinfection of the conjunctival sac with antibiotics and iodine compounds: a prospective randomized multicenter study. Jpn J Ophthalmol 52: 151–161. [DOI] [PubMed] [Google Scholar]
- Ishida M, Kataoka T, Niwa K, Iwaki M & Zako M (2011): Efficient penetration into aqueous humor by administration of oral and topical levofloxacin. J Ocul Pharmacol Ther 27: 247–250. [DOI] [PubMed] [Google Scholar]
- Jensen MK, Fiscella RG, Crandall AS, Moshirfar M, Mooney B, Wallin T & Olson RJ (2005): A retrospective study of endophtalmitis rates comparing quinolone antibiotics. Am J Ophthalmol 139: 141–148. [DOI] [PubMed] [Google Scholar]
- Jensen MK, Fiscella RG, Moshirfar M & Mooney B (2008): Third‐ and fourth‐generation fluoroquinolones: retrospective comparison of endophthalmitis after cataract surgery performed over 10 years. J Cataract Refract Surg 34: 1460–1467. [DOI] [PubMed] [Google Scholar]
- Jindal A, Pathengay A, Mithal K et al. (2014): Microbiologic spectrum and susceptibility of isolates in acute postcataract surgery endophthalmitis: are they same as they were more than a decade ago?. Br J Ophthalmol 98: 414–416. [DOI] [PubMed] [Google Scholar]
- Joseph CR, Lalitha P, Sivaraman KR, Ramasamy K & Behera UC (2012): Real‐time polymerase chain reaction in the diagnosis of acute postoperative endophthalmitis. Am J Ophthalmol 153: 1031–1037. [DOI] [PubMed] [Google Scholar]
- Kamalarajah S, Silvestri G, Sharma N, Khan A, Foot B, Ling R, Cran G & Best R (2004): Surveillance of endophthalmitis following cataract surgery in the UK. Eye (Lond) 18: 580–587. [DOI] [PubMed] [Google Scholar]
- Kamalarajah S, Ling R, Silvestri G, Sharma NK, Cole MD, Cran G & Best RM (2007): Presumed infectious endophthalmitis following cataract surgery in the UK: a case‐control study of risk factors. Eye (Lond) 21: 580–586. [DOI] [PubMed] [Google Scholar]
- Katz HR, Masket S, Lane SS, Sall K, Orr SC, Faulkner RD, McCue BA & Dahlin DC (2005): Absorption of topical moxifloxacin ophthalmic solution into human aqueous humor. Cornea 24: 955–958. [DOI] [PubMed] [Google Scholar]
- Keay L, Gower EW, Cassard SD, Tielsch JM & Schein OD (2012): Postcataract surgery endophthalmitis in the United States: analysis of the complete 2003 to 2004 Medicare database of cataract surgeries. Ophthalmology 119: 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Mathews D, Mathews J & Vail A (2007): Reflective consideration of postoperative endophthalmitis as a quality marker. Eye (Lond) 21: 1419–1426. [DOI] [PubMed] [Google Scholar]
- Kessner R, Golan S & Barak A (2014): Changes in the etiology of endophthalmitis from 2003 to 2010 in a large tertiary medical center. Eur J Ophthalmol 24: 918–924. [DOI] [PubMed] [Google Scholar]
- Khan RI, Kennedy S & Barry P (2005): Incidence of presumed postoperative endophthalmitis in Dublin for a 5‐year period (1997–2001). J Cataract Refract Surg 31: 1575–1581. [DOI] [PubMed] [Google Scholar]
- Khan S, Khan KR & Aasi NA (2011): Efficacy of prophylactic intracameral moxifloxacin in cataract surgery. Pak J Med Health Sci 5: 553–557. [Google Scholar]
- Kobayakawa S, Tochikubo T & Tsuji A (2003): Penetration of levofloxacin into human aqueous humor. Ophthalmic Res 35: 97–101. [DOI] [PubMed] [Google Scholar]
- Koch HR, Kulus SC, Roessler M, Ropo A & Geldsetzer K (2005): Corneal penetration of fluoroquinolones: aqueous humor concentrations after topical application of levofloxacin 05% and ofloxacin 03% eyedrops. J Cataract Refract Surg 31: 1377–1385. [DOI] [PubMed] [Google Scholar]
- Kodjikian L, Salvanet‐Bouccara A, Grillon S, Forestier F, Seegmuller JL & Berdeaux G (2009): Postcataract acute endophthalmitis in France: national prospective survey. J Cataract Refract Surg 35: 89–97. [DOI] [PubMed] [Google Scholar]
- Krikonis TS, Panagiotoglou TD, Tsika C, Alegakis A, Pallikaris IG & Tsilimbaris MK (2009): Endophthalmitis after cataract extraction: incidence, treatment, and outcome in Crete, Greece, during period 2000‐2008. Semin Ophthalmol 24: 234–238. [DOI] [PubMed] [Google Scholar]
- Kumar MA, Kurien SS, Selvaraj S, Devi U & Selvasundari S (2012): Comparison of different techniques of cataract surgery in bacterial contamination of the anterior chamber in diabetic and non‐diabetic population. Indian J Ophthalmol 60: 41–44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalitha P, Rajagopalan J, Prakash K, Ramasamy K, Prajna NV & Srinivasan M (2005): Postcataract endophthalmitis in South India incidence and outcome. Ophthalmology 112: 1884–1889. [DOI] [PubMed] [Google Scholar]
- Lalwani GA, Flynn HW Jr, Scott IU et al. (2008): Acute‐onset endophthalmitis after clear corneal cataract surgery (1996‐2005) Clinical features, causative organisms, and visual acuity outcomes. Ophthalmology 115: 473–476. [DOI] [PubMed] [Google Scholar]
- Lam PT, Young AL, Cheng LL, Tam PM & Lee VY (2010): Randomized controlled trial on the safety of intracameral cephalosporins in cataract surgery. Clin Ophthalmol 4: 1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Morlet N, Ng JQ, Semmens JB & Knuiman MW (2004): Significant nonsurgical risk factors for endophthalmitis after cataract surgery: EPSWA fourth report. Invest Ophthalmol Vis Sci 45: 1321–1328. [DOI] [PubMed] [Google Scholar]
- Lin M, Zhang W, Liu Y et al. (2011): Nosocomial acute‐onset postoperative endophthalmitis at a university teaching hospital in China. J Hosp Infect 79: 323–327. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang NL, Wang YL et al. (2010): Determination of drug concentration in aqueous humor of cataract patients administered gatifloxacin ophthalmic gel. Chin Med J (Engl) 123: 2105–2110. [PubMed] [Google Scholar]
- Lloyd JC & Braga‐Mele R (2009): Incidence of postoperative endophthalmitis in a high‐volume cataract surgicentre in Canada. Can J Ophthalmol 44: 288–292. [DOI] [PubMed] [Google Scholar]
- Lundstrom M (2006): Endophthalmitis and incision construction. Curr Opin Ophthalmol 17: 68–71. [DOI] [PubMed] [Google Scholar]
- Lundstrom M, Wejde G, Stenevi U, Thorburn W & Montan P (2007): Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology 114: 866–870. [DOI] [PubMed] [Google Scholar]
- Lundstrom M, Barry P, Henry Y, Rosen P & Stenevi U (2012): Evidence‐based guidelines for cataract surgery: guidelines based on data in the European Registry of Quality Outcomes for Cataract and Refractive Surgery database. J Cataract Refract Surg 38: 1086–1093. [DOI] [PubMed] [Google Scholar]
- Malathi J, Madhavan HN, Therese KL & Margarita S (2006): Phacoemulsification probe as a source of postoperative endophthalmitis following phacoemulsification cataract extraction (PKE) surgery‐DNA sequencing‐based study. J Hosp Infect 62: 117–119. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Gira J, Berdy GJ & Brusatti R (2012): Safety of besifloxacin ophthalmic suspension 06% as a prophylactic antibiotic following routine cataract surgery: results of a prospective, parallel‐group, investigator‐masked study. Clin Ophthalmol 6: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataftsi A, Tsinopoulos IT, Tsaousis KT & Dimitrakos SA (2011): Perioperative antibiotic prophylaxis during cataract surgery in Greece. J Cataract Refract Surg 37: 1732–1733. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Miyoshi T, Suto C, Akura J & Inoue Y (2013): Efficacy and safety of prophylactic intracameral moxifloxacin injection in Japan. J Cataract Refract Surg 39: 1702–1706. [DOI] [PubMed] [Google Scholar]
- Mayer E, Cadman D, Ewings P, Twomey JM, Gray RH, Claridge KG, Hakin KN & Bates AK (2003): A 10 year retrospective survey of cataract surgery and endophthalmitis in a single eye unit: injectable lenses lower the incidence of endophthalmitis. Br J Ophthalmol 87: 867–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo GB, Bispo PJ, Regatieri CV, Yu MC, Pignatari AC & Hofling‐Lima AL (2010): Incidence of endophthalmitis after cataract surgery (2002‐2008) at a Brazilian university‐hospital. Arq Bras Oftalmol 73: 505–507. [DOI] [PubMed] [Google Scholar]
- van der Merwe J, Mustak H & Cook C (2012): Endophthalmitis prophylaxis with intracameral cefuroxime in South Africa. J Cataract Refract Surg 38: 2054. [DOI] [PubMed] [Google Scholar]
- Miller JJ, Scott IU, Flynn HW Jr, Smiddy WE, Newton J & Miller D (2005): Acute‐onset endophthalmitis after cataract surgery (2000‐2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol 139: 983–987. [DOI] [PubMed] [Google Scholar]
- Mino de Kaspar H, Kreutzer TC, Aguirre‐Romo I, Ta CN, Dudichum J, Bayrhof M, Klauss V & Kampik A (2008): A prospective randomized study to determine the efficacy of preoperative topical levofloxacin in reducing conjunctival bacterial flora. Am J Ophthalmol 145: 136–142. [DOI] [PubMed] [Google Scholar]
- Mitra A & McElvanney A (2006): Prophylactic subconjunctival cefuroxime during cataract surgery in patients with a penicillin allergy. Ann Ophthalmol (Skokie) 38: 293–295. [DOI] [PubMed] [Google Scholar]
- Mizuki N, Watanabe Y, Miyamoto M et al. (2005): Flomoxef sodium and levofloxacin concentrations in aqueous humor. Ocul Immunol Inflamm 13: 229–234. [DOI] [PubMed] [Google Scholar]
- Mollan SP, Gao A, Lockwood A, Durrani OM & Butler L (2007): Postcataract endophthalmitis: incidence and microbial isolates in a United Kingdom region from 1996 through 2004. J Cataract Refract Surg 33: 265–268. [DOI] [PubMed] [Google Scholar]
- Montan PG, Wejde G, Koranyi G & Rylander M (2002a): Prophylactic intracameral cefuroxime Efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg 28: 977–981. [DOI] [PubMed] [Google Scholar]
- Montan PG, Wejde G, Setterquist H, Rylander M & Zetterstrom C (2002b): Prophylactic intracameral cefuroxime Evaluation of safety and kinetics in cataract surgery. J Cataract Refract Surg 28: 982–987. [DOI] [PubMed] [Google Scholar]
- Moshirfar M, Feiz V, Vitale AT, Wegelin JA, Basavanthappa S & Wolsey DH (2007): Endophthalmitis after uncomplicated cataract surgery with the use of fourth‐generation fluoroquinolones: a retrospective observational case series. Ophthalmology 114: 686–691. [DOI] [PubMed] [Google Scholar]
- Moss JM, Nguyen D, Liu YI, Singh K, Montague A, Egbert PR, Kaspar HM & Ta CN (2008): Comparison of one‐day versus one‐hour application of topical gatifloxacin in eliminating conjunctival bacterial flora. Ophthalmology 115: 2013–2016. [DOI] [PubMed] [Google Scholar]
- Moss JM, Sanislo SR & Ta CN (2009): A prospective randomized evaluation of topical gatifloxacin on conjunctival flora in patients undergoing intravitreal injections. Ophthalmology 116: 1498–1501. [DOI] [PubMed] [Google Scholar]
- Murjaneh S, Waqar S, Hale JE, Kasmiya M, Jacob J & Quinn AG (2010): National survey of the use of intraoperative antibiotics for prophylaxis against postoperative endophthalmitis following cataract surgery in the UK. Br J Ophthalmol 94: 1410–1411. [DOI] [PubMed] [Google Scholar]
- Murphy CC, Nicholson S, Quah SA, Batterbury M, Neal T & Kaye SB (2007): Pharmacokinetics of vancomycin following intracameral bolus injection in patients undergoing phacoemulsification cataract surgery. Br J Ophthalmol 91: 1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myneni J, Desai SP & Jayamanne DG (2013): Reduction in postoperative endophthalmitis with intracameral cefuroxime. J Hosp Infect 84: 326–328. [DOI] [PubMed] [Google Scholar]
- Nagaki Y, Hayasaka S, Kadoi C et al. (2003): Bacterial endophthalmitis after small‐incision cataract surgery effect of incision placement and intraocular lens type. J Cataract Refract Surg 29: 20–26. [DOI] [PubMed] [Google Scholar]
- Nanavaty MA & Wearne MJ (2010): Perioperative antibiotic prophylaxis during phaco‐emulsification and intraocular lens implantation: national survey of smaller eye units in England. Clin Experiment Ophthalmol 38: 462–466. [DOI] [PubMed] [Google Scholar]
- Ness T, Kern WV, Frank U & Reinhard T (2011): Postoperative nosocomial endophthalmitis: is perioperative antibiotic prophylaxis advisable? A single centre's experience. J Hosp Infect 78: 138–142. [DOI] [PubMed] [Google Scholar]
- Ng JQ, Morlet N, Pearman JW, Constable IJ, McAllister IL, Kennedy CJ, Isaacs T & Semmens JB (2005): Management and outcomes of postoperative endophthalmitis since the endophthalmitis vitrectomy study: the Endophthalmitis Population Study of Western Australia (EPSWA)'s fifth report. Ophthalmology 112: 1199–1206. [DOI] [PubMed] [Google Scholar]
- Ng JQ, Morlet N, Bulsara MK & Semmens JB (2007): Reducing the risk for endophthalmitis after cataract surgery: population‐based nested case‐control study: endophthalmitis population study of Western Australia sixth report. J Cataract Refract Surg 33: 269–280. [DOI] [PubMed] [Google Scholar]
- Norcross EW, Sanders ME, Moore Q III, Sanfilippo CM, Hesje CK, Shafiee A & Marquart ME (2010): Comparative efficacy of besifloxacin and other fluoroquinolones in a prophylaxis model of penicillin‐resistant Streptococcus pneumoniae rabbit endophthalmitis. J Ocul Pharmacol Ther 26: 237–243. [DOI] [PubMed] [Google Scholar]
- Olavi P (2012): Ocular toxicity in cataract surgery because of inaccurate preparation and erroneous use of 50 mg/ml intracameral cefuroxime. Acta Ophthalmol 90: e153–e154. [DOI] [PubMed] [Google Scholar]
- Ong‐Tone L (2008): Aqueous humor penetration of gatifloxacin and moxifloxacin eyedrops given in different concentrations in a wick before cataract surgery. J Cataract Refract Surg 34: 819–822. [DOI] [PubMed] [Google Scholar]
- Parmar P, Salman A, Kaliamurthy J, Prasanth DA, Thomas PA & Jesudasan CA (2006): Anterior chamber contamination during phacoemulsification and manual small‐incision cataract surgery. Am J Ophthalmol 141: 1160–1161. [DOI] [PubMed] [Google Scholar]
- Patwardhan A, Rao GP, Saha K & Craig EA (2006): Incidence and outcomes evaluation of endophthalmitis management after phacoemulsification and 3‐piece silicone intraocular lens implantation over 6 years in a single eye unit. J Cataract Refract Surg 32: 1018–1021. [DOI] [PubMed] [Google Scholar]
- Pea F, Ferrari E, Pavan F, Roman‐Pognuz D, Bandello F & Furlanut M (2005): Levofloxacin disposition over time in aqueous humor of patients undergoing cataract surgery. Antimicrob Agents Chemother 49: 2554–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick ZS, Leaming DV & Elder MJ (2008): The fourth New Zealand cataract and refractive surgery survey: 2007. Clin Experiment Ophthalmol 36: 604–619. [DOI] [PubMed] [Google Scholar]
- Pijl BJ, Theelen T, Tilanus MA, Rentenaar R & Crama N (2010): Acute endophthalmitis after cataract surgery: 250 consecutive cases treated at a tertiary referral center in the Netherlands. Am J Ophthalmol 149: 482–487. [DOI] [PubMed] [Google Scholar]
- Qureshi F & Clark D (2011): Macular infarction after inadvertent intracameral cefuroxime. J Cataract Refract Surg 37: 1168–1169. [DOI] [PubMed] [Google Scholar]
- Råen M, Sandvik GF & Drolsum L (2013): Endophthalmitis following cataract surgery: the role of prophylactic postoperative chloramphenicol eye drops. Acta Ophthalmol 91: 118–122. [DOI] [PubMed] [Google Scholar]
- Rahman N & Murphy CC (2014): Impact of intracameral cefuroxime on the incidence of postoperative endophthalmitis following cataract surgery in Ireland. Ir J Med Sci [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Ramappa M, Majji AB, Murthy SI et al. (2012): An outbreak of acute post‐cataract surgery Pseudomonas sp endophthalmitis caused by contaminated hydrophilic intraocular lens solution. Ophthalmology 119: 564–570. [DOI] [PubMed] [Google Scholar]
- Ravindran RD, Venkatesh R, Chang DF, Sengupta S, Gyatsho J & Talwar B (2009): Incidence of post‐cataract endophthalmitis at Aravind Eye Hospital: outcomes of more than 42,000 consecutive cases using standardized sterilization and prophylaxis protocols. J Cataract Refract Surg 35: 629–636. [DOI] [PubMed] [Google Scholar]
- Recchia FM, Busbee BG, Pearlman RB, Carvalho‐Recchia CA & Ho AC (2005): Changing trends in the microbiologic aspects of postcataract endophthalmitis. Arch Ophthalmol 123: 341–346. [DOI] [PubMed] [Google Scholar]
- Review Manager (RevMan) (2012): The Nordic Cochrane Centre, The Cochrane Collaboration.
- Rodriguez‐Caravaca G, Garcia‐Saenz MC, Villar‐Del‐Campo MC, Andres‐Alba Y & Arias‐Puente A (2013): Incidence of endophthalmitis and impact of prophylaxis with cefuroxime on cataract surgery. J Cataract Refract Surg 39: 1399–1403. [DOI] [PubMed] [Google Scholar]
- Romero P, Mendez I, Salvat M, Fernandez J & Almena M (2006): Intracameral cefazolin as prophylaxis against endophthalmitis in cataract surgery. J Cataract Refract Surg 32: 438–441. [DOI] [PubMed] [Google Scholar]
- Romero‐Aroca P, Mendez‐Marin I, Salvat‐Serra M, Fernandez‐Ballart J, Almena‐Garcia M & Reyes‐Torres J (2012): Results at seven years after the use of intracamerular cefazolin as an endophthalmitis prophylaxis in cataract surgery. BMC Ophthalmol 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosha DS, Ng JQ, Morlet N, Boekelaar M, Wilson S, Hendrie D & Semmens JB (2006): Cataract surgery practice and endophthalmitis prevention by Australian and New Zealand ophthalmologists. Clin Experiment Ophthalmol 34: 535–544. [DOI] [PubMed] [Google Scholar]
- Rudnisky CJ, Wan D & Weis E (2014): Antibiotic choice for the prophylaxis of post‐cataract extraction endophthalmitis. Ophthalmology 121: 835–841. [DOI] [PubMed] [Google Scholar]
- Sakarya Y & Sakarya R (2010): Cefuroxime dilution error. Eur J Ophthalmol 20: 460–461. [DOI] [PubMed] [Google Scholar]
- Sandvig KU & Dannevig L (2003): Postoperative endophthalmitis: establishment and results of a national registry. J Cataract Refract Surg 29: 1273–1280. [DOI] [PubMed] [Google Scholar]
- Schimel AM, Miller D & Flynn HW Jr (2013): Endophthalmitis isolates and antibiotic susceptibilities: a 10‐year review of culture‐proven cases. Am J Ophthalmol 156: 50–52. [DOI] [PubMed] [Google Scholar]
- Schmier JK, Halpern MT, Covert DW, Lau EC & Robin AL (2007): Evaluation of Medicare costs of endophthalmitis among patients after cataract surgery. Ophthalmology 114: 1094–1099. [DOI] [PubMed] [Google Scholar]
- Seal DV, Barry P, Gettinby G, Lees F, Peterson M, Revie CW & Wilhelmus KR (2006): ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: Case for a European multicenter study. J Cataract Refract Surg 32: 396–406. [DOI] [PubMed] [Google Scholar]
- Semmens JB, Li J, Morlet N & Ng J (2003): Trends in cataract surgery and postoperative endophthalmitis in Western Australia (1980‐1998): the Endophthalmitis Population Study of Western Australia. Clin Experiment Ophthalmol 31: 213–219. [DOI] [PubMed] [Google Scholar]
- Sharifi E, Porco TC & Naseri A (2009): Cost‐effectiveness analysis of intracameral cefuroxime use for prophylaxis of endophthalmitis after cataract surgery. Ophthalmology 116: 1887–1896. [DOI] [PubMed] [Google Scholar]
- Sheng Y, Sun W, Gu Y, Lou J & Liu W (2011): Endophthalmitis after cataract surgery in China, 1995‐2009. J Cataract Refract Surg 37: 1715–1722. [DOI] [PubMed] [Google Scholar]
- Shirodkar AR, Pathengay A, Flynn HW Jr et al. (2012): Delayed‐ versus acute‐onset endophthalmitis after cataract surgery. Am J Ophthalmol 153: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorstein NH, Winthrop KL & Herrinton LJ (2013): Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg 39: 8–14. [DOI] [PubMed] [Google Scholar]
- Solomon R, Donnenfeld ED, Perry HD, Snyder RW, Nedrud C, Stein J & Bloom A (2005): Penetration of topically applied gatifloxacin 03%, moxifloxacin 05%, and ciprofloxacin 03% into the aqueous humor. Ophthalmology 112: 466–469. [DOI] [PubMed] [Google Scholar]
- Stein JD, Grossman DS, Mundy KM, Sugar A & Sloan FA (2011): Severe adverse events after cataract surgery among medicare beneficiaries. Ophthalmology 118: 1716–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey P, Dollin M, Pitcher J, Reddy S, Vojtko J, Vander J, Hsu J & Garg SJ (2014): The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmology 121: 283–289. [DOI] [PubMed] [Google Scholar]
- Sundelin K, Seal D, Gardner S, Ropo A, Geldsetzer K, Lokkila J & Stenevi U (2009): Increased anterior chamber penetration of topical levofloxacin 05% after pulsed dosing in cataract patients. Acta Ophthalmol 87: 160–165. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Tanaka H, Toriyama K, Okamoto S, Urabe K, Hashida M, Shinkai Y & Ohashi Y (2013): Prospective clinical evaluation of 15% levofloxacin ophthalmic solution in ophthalmic perioperative disinfection. J Ocul Pharmacol Ther 29: 887–892. [DOI] [PubMed] [Google Scholar]
- Ta CN, Egbert PR, Singh K, Shriver EM, Blumenkranz MS & Mino de Kaspar H (2002): Prospective randomized comparison of 3‐day versus 1‐hour preoperative ofloxacin prophylaxis for cataract surgery. Ophthalmology 109: 2036–2040. [DOI] [PubMed] [Google Scholar]
- Ta CN, Chan I, Dhatt HS et al. (2008): Prospective comparison of topical moxifloxacin in eliminating conjunctival bacterial flora following a one‐day or one‐hour application. J Ocul Pharmacol Ther 24: 427–431. [DOI] [PubMed] [Google Scholar]
- Tan CS, Wong HK & Yang FP (2012): Epidemiology of postoperative endophthalmitis in an Asian population: 11‐year incidence and effect of intracameral antibiotic agents. J Cataract Refract Surg 38: 425–430. [DOI] [PubMed] [Google Scholar]
- Teshigawara T, Hata S, Hayashi T, Watanabe Y, Itoh Y, Hitoi K & Mizuki N (2007): Penetration of gatifloxacin eye drops into the aqueous humor in humans. Ocul Immunol Inflamm 15: 309–313. [DOI] [PubMed] [Google Scholar]
- The Royal College of Ophthalmologists (2010): Cataract surgery guidelines, September 2010 Scientific Department The Royal College of Ophthalmologists: 1–104.
- Thoms SS, Musch DC & Soong HK (2007): Postoperative endophthalmitis associated with sutured versus unsutured clear corneal cataract incisions. Br J Ophthalmol 91: 728–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinavarat A, Atchaneeyasakul LO, Nopmaneejumruslers C & Inson K (2006): Reduction of endophthalmitis rate after cataract surgery with preoperative 5% povidone‐iodine. Dermatology 212(Suppl 1): 35–40. [DOI] [PubMed] [Google Scholar]
- Vasavada AR, Gajjar D, Raj SM, Vasavada V & Vasavada V (2008): Comparison of 2 moxifloxacin regimens for preoperative prophylaxis: prospective randomized triple‐masked trial Part 2: residual conjunctival flora. J Cataract Refract Surg 34: 1383–1388. [DOI] [PubMed] [Google Scholar]
- Villada JR, Vicente U, Javaloy J & Alio JL (2005): Severe anaphylactic reaction after intracameral antibiotic administration during cataract surgery. J Cataract Refract Surg 31: 620–621. [DOI] [PubMed] [Google Scholar]
- Voss M, la CM, Friis M & Tr H (2012): Epidemic bacillus endophthalmitis after uncomplicated cataract surgery due to contaminated viscoelastic Milan: ESCRS congress. [Google Scholar]
- Wallin T, Parker J, Jin Y, Kefalopoulos G & Olson RJ (2005): Cohort study of 27 cases of endophthalmitis at a single institution. J Cataract Refract Surg 31: 735–741. [DOI] [PubMed] [Google Scholar]
- Wejde G, Montan P, Lundstrom M, Stenevi U & Thorburn W (2005a): Endophthalmitis following cataract surgery in Sweden: national prospective survey 1999‐2001. Acta Ophthalmol Scand 83: 7–10. [DOI] [PubMed] [Google Scholar]
- Wejde G, Samolov B, Seregard S, Koranyi G & Montan PG (2005b): Risk factors for endophthalmitis following cataract surgery: a retrospective case‐control study. J Hosp Infect 61: 251–256. [DOI] [PubMed] [Google Scholar]
- West ES, Behrens A, Mcdonnell PJ, Tielsch JM & Schein OD (2005): The incidence of endophthalmitis after cataract surgery among the US Medicare population increased between 1994 and 2001. Ophthalmology 112: 1388–1394. [DOI] [PubMed] [Google Scholar]
- Wong TY & Chee SP (2004a): Risk factors of acute endophthalmitis after cataract extraction: a case‐control study in Asian eyes. Br J Ophthalmol 88: 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY & Chee SP (2004b): The epidemiology of acute endophthalmitis after cataract surgery in an Asian population. Ophthalmology 111: 699–705. [DOI] [PubMed] [Google Scholar]
- Wu PC, Kuo HK, Li M et al. (2006a): Nosocomial postoperative endophthalmitis: a 14‐year review. Graefes Arch Clin Exp Ophthalmol 244: 920–929. [DOI] [PubMed] [Google Scholar]
- Wu PC, Li M, Chang SJ, Teng MC, Yow SG, Shin SJ & Kuo HK (2006b): Risk of endophthalmitis after cataract surgery using different protocols for povidone‐ iodine preoperative disinfection. J Ocul Pharmacol Ther 22: 54–61. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Parrott MB, Flynn HW Jr, Shi W, Miller D & Alfonso EC (2010): Nosocomial acute‐onset postoperative endophthalmitis at a university teaching hospital (2002‐2009). Am J Ophthalmol 150: 392–398. [DOI] [PubMed] [Google Scholar]
- Yao K, Zhu Y, Zhu Z et al. (2013): The incidence of postoperative endophthalmitis after cataract surgery in China: a multicenter investigation of 2006‐2011. Br J Ophthalmol 97: 1312–1317. [DOI] [PubMed] [Google Scholar]
- Yu‐Wai‐Man P, Morgan SJ, Hildreth AJ, Steel DH & Allen D (2008): Efficacy of intracameral and subconjunctival cefuroxime in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg 34: 447–451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Characteristics of included studies, intracameral antibiotic prophylaxis.
Appendix S2. Characteristics of excluded studies, intracameral antibiotic prophylaxis.
Appendix S3. Characteristics of included studies.
Appendix S4. Characteristics of excluded studies.


