Abstract
Objectives
To evaluate the feasibility, safety, and efficacy of the Occlutech® PDA occluder for closure of patent ductus arteriosus (PDA).
Background
The Occlutech® PDA occluder is novel, self‐shaping Nitinol wire device with PET (polyethylene terephthalate) patches integrated into the shank of the device to assure a better obturation of the ductus. The Occlutech® PDA occluder has undergone two design modifications.
Methods
A prospective, non‐randomized pilot study was started in November 2011. Thirty‐three patients were included until April 2013. Patients weighing <6 kg or those with associated cardiac anomalies that required surgery were excluded. All patients were followed up by transthoracic echocardiography at 24 hr, 30 days, 90 days, 180 days, and 360 days after implantation. Residual shunt, left pulmonary artery (LPA) and descending aortic velocities were among the parameters assessed. All occluders were delivered via 6–8 F long sheaths and PDA closures were performed following standard techniques.
Results
Thirty three patients (20 female/13 male), with a median age of 2 years (6 month to 38 years), and median weight of 9.3 kg (6–69.2 kg) were included. The narrowest median PDA diameter was 3mm (1.8–5.8 mm). All the 33 patients were closed successfully using Occlutech ductal occluder, 16 patients (48.4%) had immediate and complete closure on angiography. Within 24 hr, color Doppler revealed complete closure in 27patients (81.8%), 32patients (97%) at 30 days, and in 100% of patients at 90 days. All patients with a large PDA had immediate residual shunt which was closed at the 90‐day follow‐up. There was no device embolization, hemolysis, or obstruction to the LPA or descending aorta.
Conclusion
The new Occlutech® PDA is safe and effective. In patients with a large PDA complete closure tended to take longer time. © 2015 Wiley Periodicals, Inc.
Keywords: patent ductus arteriosus, Occlutech® PDA occluder
INTRODUCTION
Patent ductus arteriosus (PDA) is one of the most common congenital heart defects and it is usually identified in childhood. Premature babies are more likely to have a PDA, and the condition occurs twice as often in girls as in boys. Since the first successful attempt of transcatheter closure by Porstmann in 1967 1 a number of different devices have been developed and evaluated. Today, transcatheter closure of PDAs using nitinol‐based, self‐expanding occluder devices or coils has become the gold standard and the Amplatzer Duct Occluder (ADO) is currently among the most widely used devices 2, 3. While device closure of PDAs in the current era has become a simple procedure with low risks, possible complications such as device embolization or protrusion of device into the left pulmonary artery and descending aorta, particularly in the younger age group 4, 5 can occur. Although PDA anatomies vary in shape, currently available devices are mainly suited for conically shaped PDAs (i.e., Krichenko type A morphology) 6. The Occlutech® PDA occluder has been developed to better address anatomic heterogeneities encountered among PDA patients and to further improve shunt closure. The objective of this study was to evaluate the feasibility, safety, and efficacy of the new Occlutech® PDA occluder for PDA closure.
Occlutech® PDA Occluder
The Occlutech® PDA occluder is made of a flexible and self‐shaping nitinol wire mesh. The main body of the device consists of a tapered “shank” and a retention disk on the distal aortic side of the device. The shank is meant to be positioned across the narrowest part of the PDA. The proximal end of the shank (pulmonic end) has a diameter that is 1.5–2.0 mm larger than that of its aortic end and the retention disc has a diameter which exceeds the size of aortic end of the shank by 5.5–8.0 mm (Fig. 1). Finally, PET patches are integrated into the shank to facilitate initial sealing and closure of the shunt (Fig. 1).
Figure 1.
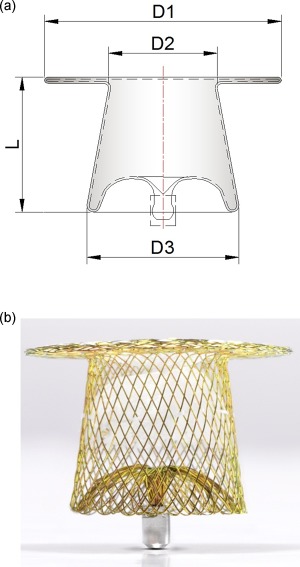
(a and b) Schematic representation and actual photograph of the Occlutech® PDA occluder design. (D1) The aortic disc diameter. (D2) The distal (aortic) end diameter. (D3) The proximal (pulmonary) end diameter. (L) The shank length, note the long tether between the aortic disc and the shank.
The Occlutech® PDA occluder has underwent two designs modifications. It is available in two different shank lengths, each coming in various sizes (Table 1).
Table 1.
Occlutech Ductal Occluder Sizes and Lengths
| Ductal occluder short shank | Size | 3.5/5 | 4/6 | 5/7 | 6/8 | 8/10 | 10/12 | 12/15 | 14/18 |
| Length | 4.25 | 5 | 6.05 | 6.3 | 7 | 12 | 14 | 16 | |
| Ductal occluder longer shank | Size | 3.5/5 | 4/6L | 5/7L | 6/8L | 8/10L | |||
| Length | 7 | 7.5 | 8.5 | 9 | 10.5 |
METHODS
A prospective, non‐randomized, 33 patients pilot study was started in November 2011. While recruiting of patients into the study has ended, patient follow up continues and this interim report includes patient data collected from study start until the end of April 2013. Approval of the Institutional Review Board (IRB) and informed consent were obtained prior to patient enrollment. All patients enrolled had clinical and echocardiographic evidence of PDA and each study participant underwent attempted transcatheter PDA closure with the Occlutech® PDA occluder. Patients weighing <6 kg, those with associated cardiac anomalies that required cardiac surgery, and subjects with pulmonary vascular resistance (PVR) >8 Woods U m−2 were excluded from the study.
Closure Protocol
All PDAs were closed from the venous side according to the Instruction for Use (IFU) provided with the Occlutech® PDA occluder 7 and as described elsewhere 8; fluoroscopy‐and procedure‐times were recorded. Hemodynamic data collected included pulmonary artery (PA) pressure, Q P:Q S ratio, PVR and left ventricular end diastolic pressure (LVEDP). Aortography was performed in straight lateral and right anterior oblique (RAO) 30° views to evaluate the position, shape, size, and length of the PDA and the aortic ampulla. Each PDA was classified according to Krichenko 6. In addition, PDAs were defined as “large” if the narrowest PDA diameter was ≥3.5 mm (in patients with symptomatic heart failure), or ≥4 mm (in patients without symptoms). Small PDA was defined if measuring ≤2 mm. PDAs measuring between 2 and 3.5 mm were defined as “moderate.”
Size selection of the Occlutech® PDA occluder was based on the narrowest diameter of the PDA. The aortic end of the occluder shank (diameter D2, Fig. 1a) was sized at least 2.0 mm larger than the narrowest diameter of the ductus. For small to medium size PDAs device diameter A of 1.5–2.0 mm lager than the narrowest ductus diameter was selected.
Patient Evaluation
Post implantation angiograms were performed at 0 and 10 min after release to confirm device position and to evaluate residual shunts (Fig. 2b,d, and e). If a device was malpositioned (prior to release), it was recaptured and redeployed.
Figure 2.
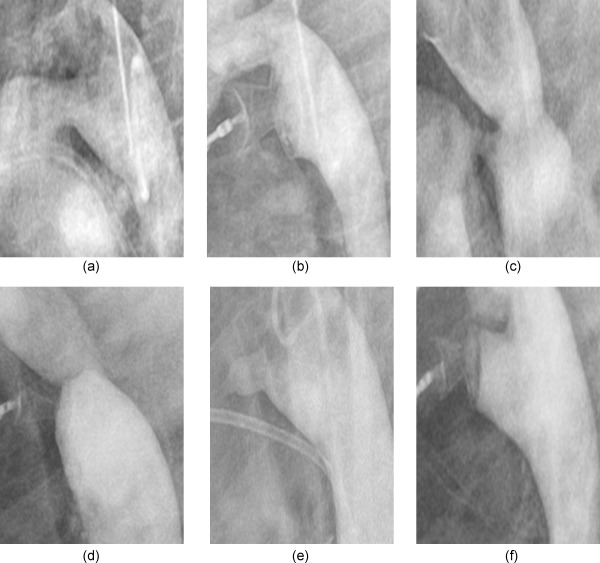
(a) Descending aortic angiography in lateral projection. Large type A PDA measuring 4.8 mm at its narrowest diameter in an asymptomatic 20‐month old, 7.8 kg Down syndrome female. (b) The 8/10 short shank version 3 Occlutech® PDA Occluder was implanted. Small residual shunt shown on angiography, and significant residual shunt 24‐hr post implant was shown by echocardiography together with audible murmur but achieved complete closure in 30 days. (c) Descending aortic angiography in lateral projection. Small type C PDA measuring 2.1 mm in a 4‐year‐old male. Note mild coarctation. (d) The 4/6 short shank Occlutech® PDA Occluder was implanted, no residual shunt with no increase in descending aorta velocity pre and post‐procedure. (e) Type E PDA measured 2.75 mm at its narrowest diameter in a 3‐years old, 11.2 kg, female. (f) 4/6 short shank Occlutech® PDA occluder was implanted with no residual shunt.
Residual shunts shown by angiography were graded as follows: Grade 1—none; Grade 2—small (dye filling only proximal pulmonary artery branches); Grade 3—moderate (dye filling the main pulmonary artery and extending to the distal branches); and Grade 4—significant (dye filling the main pulmonary artery and branches extending to the peripheral vessels with contrast return to the left atrium).
Patients were followed up at 24 hr, 30 days (or earlier for those with a significant residual shunt), 90 days, 180 days, and at 360 days by clinical examination and echocardiography. All patients were evaluated by clinical examination for the presence of murmur at 24 hr and in accordance with standard clinical care. Chest radiographs in the posteroanterior and lateral projections, detailed two‐dimensional echocardiography examination to assess device position and residual shunts, and Doppler flow velocities of the left pulmonary artery and the descending aorta were performed at 24 hr, 30 days, 90 days, 180 days, and at 360 days. Residual shunts detected by echocardiography were graded as follows: Grade 1—none; Grade 2—trivial (a small color Doppler jet limited to the device); Grade 3—moderate (Doppler jet beyond the device but no audible murmur); and Grade 4—significant shunt (large Doppler jet with an audible murmur and continuous Doppler flow).
Statistical Analysis
Results are expressed as mean value ± SD or median and range, using Statistical Package for the Social Sciences (SPSS version 20).
RESULTS
The median age of the 33 patients was 2 years (range, 0.5–38 years) and median weight was 9.3 kg (range, 6.0–69.2 kg). Thirty‐one patients (93.9%) had isolated PDAs. One patient (3%) had a mild coarctation of the aorta; and one had a hypoplastic left pulmonary artery with left lung hypoplasia that was confirmed by thoracic multi‐slice computed tomography. All patients had continuous cardiac murmur on examination. PDAs were type A in 30 patients (90.9%), type E in 2 patients (6.1%) (Fig. 2b) and type C in 1 patient (3%) (Fig. 2e). Additional patient demographics, clinical parameters, and procedure related data for the 33 patients are summarized in (Table 2).
Table 2.
Demographic and Hemodynamic Data of All Patients Underwent Attempted PDA Closure
| No. of patients | 33 patients |
| Female 20(60.6%)/male 13 (39.4%) | |
| Age (years) | Median = 2 (0.5–38) years |
| Weight (kg) | Median = 9.3 (6–69.2) kg |
| PDA size (narrowest diameter) (mm) | Mean = 2.97 ± 1.03 |
| Q P:Q S | Mean = 2±1.1 |
| PVR | Mean = 2.06 ± 0.89 |
| MEAN PAP (mm Hg) | Median = 21 (12–52) |
| Mean PA//AO ratio | Median = 0.33 (0.17–0.81) |
| Large | 9 (27.3%) |
| Moderate | 18 (54.5%) |
| Small | 6 (18.1%) |
| PDA morphology | |
| Type A | 30 (90.9%) |
| Type E | 2 (6.1%) |
| Type C | 1 (3%) |
| Fluoroscopy time (min) | 8.76 ± 3.81 |
| Procedure time (min) | Median = 60 (20–165) |
All 33 patients underwent successful PDA closure with the Occlutech® PDA Occluder, and have completed the 360‐days follow‐up. Of the 33 patients who received the Occlutech® PDA occluder, final angiography immediately following the procedure showed complete PDA closure in 16 patients (48.5%). A small residual shunt was seen in 10 patients (30.3%), a moderate residual shunt in 4 patients (12.1%), and 3 patients (9.1%) had a significant residual shunt with a soft systolic murmur. All 9 patients with large PDAs had residual shunts as assessed by angiography immediately after implantation.
Color Doppler data at 24‐hr post‐implantation showed that complete closure (no, or trivial residual shunt, Grades 1 and 2) was achieved in 24 patients (81.8%). Moderate residual shunt (Grade 3) was seen in 4 patients (12.1%) and 2 patients (6%) had significant residual shunt (Grade 4) with a soft systolic murmur. Thirty‐two patients (97%) achieved complete closure at 30‐day follow‐up. One patient who had residual shunt at 30‐day follow‐up, showed complete closure at day 90 (Table III).
The patient with a mild coarctation of aorta continued to have the same peak systolic Doppler velocity in the descending aorta of 2.6 m sec−1 (Fig. 2c and d). The patient with a hypoplastic left pulmonary artery and with a hypoplastic left lung underwent successful immediate closure without the device affecting the right pulmonary artery.
Complications
There was no device embolization or hemolysis. None of the patients treated required blood transfusion. There were no femoral arterial or venous complications except for the patient with a pre‐existing, mild coarctation, none of the patients showed LPA or descending aorta velocities >1.5 m sec−1 during follow up.
DISCUSSION
Patent ductus arteriosus (PDA) is among the most common congenital heart defects. Because virtually all PDAs, regardless of size and clinical presentation require closure, transcatheter closure has gained wide acceptance with excellent long term results and the procedure has become one of the most common procedures in the treatment of congenital heart disease 3, 9, 10, 11. Despite its remarkable safety, complications such as device embolization to the pulmonary artery and aorta have been reported, as well as mild obstruction to the LPA and descending aorta, especially in small infants with large PDAs who require a relatively large device 4, 5.
In this article, we report prospective, non‐randomized interim data on 33 patients, who underwent an attempt of transcatheter closure of PDA to assess the feasibly, safety and efficacy of the new Occlutech® PDA occluder. As shown by fluoroscopy and procedure times, PDA closure with this novel PDA occluder was generally straight forward and simple procedure. The occluder exhibited a good safety profile without incidence of device embolization, hemolysis, or trauma to adjacent structures. Short‐and medium‐term follow up showed no significant increased Doppler velocities in the LPA and descending aorta. When compared to the data collected from much larger series of Amplatzer Ductal Occluder studies 3, 9, 10, 11, the immediate closure rates obtained with the Occlutech® PDA occluder was comparable. While the incidence of significant residual shunts in patients with a large PDA was somewhat higher with the Occlutech® PDA occluder, over the course of weeks the novel Occlutech device demonstrated excellent efficacy in achieving complete closure.
Comparable to other PDA occluders, the Occlutech® PDA occluder tested here is constructed from an expandable nitinol wire mesh with PET fabric patches incorporated to seal and to effect closure of the PDA. In addition, the Occlutech® PDA occluder is fitted with a retention disc on its distal, aortic end to prevent device embolization to the pulmonary artery. However a number of features, distinguish the Occlutech® PDA occluder from the others. First, and fundamentally different to the Amplatzer Duct Occluder, the shank of the Occlutech® PDA occluder is 2 mm larger at the pulmonic end than the aortic end. Our experience suggest that this feature provides additional stability of the device position and that it may reduce the risk of device embolization, particularly to the aorta in instances where a device needs to be retrieved post‐release. The hub housing pointing out of the shank of the Occlutech® PDA occluder is another positive feature which makes retrieval of the Occlutech® PDA occluder by snaring the hub housing to the screw attachment and to the delivery cable easier than by having the hub housing placed in a recess (Fig. 1). Finally, the Occlutech® PDA occluder has a lower profile such that even the largest device (8/10) could be implanted using a 6F long sheath.
Complete occlusion at 10‐min post implantation was seen in 16 patients (48.5%). Patients categorized as having a large PDA tended to have a residual shunt at 10 minutes post implant and on echocardiography at 24 hr. Of the nine patients with large PDA, three patients had a significant residual shunt, four patients had a moderate shunt, and two patients had a small residual shunt at 10 min. While shunt closure rates in these patients was an initial concern, all subjects with a significant residual shunt achieved complete closure at 90 days.
Study Limitations
This is a non‐randomized prospective study that recruited a relatively small number of patients. The great majority of PDAs (90.9%) were of type A morphology (conical). A study enrolling a higher number of patients with less frequent PDA morphologies/shapes would serve well to demonstrate the device's efficacy for the closure of PDA morphologies especially the large, long tubular PDA without constriction (type C).
CONCLUSIONS
The new Occlutech® PDA Occluder is safe and effective for the closure of type A PDAs. In large PDAs there is a tendency for residual shunts and delayed complete closure. Larger multicenter studies are required to further establish the safety and efficacy profile of Occlutech® PDA Occluder and to more comprehensively assess its suitability for closure of less frequently seen non‐type A PDAs.
Conflict of interest: Nothing to report.
REFERENCES
- 1. Porstmann W, Wierny L, Warnke H. Catheter closure of patent ductus arteriosus: 62 cases treated without thoracotomy. Radiol Clin North Am 1971;9:203–218. [PubMed] [Google Scholar]
- 2. Cambier PA, Kirby WC, Moore JW. Percutaneous closure of small (less than 2.5 mm) patent ductus arteriosus using coil embolization. Am J Cardiol 1992;69:815–816. [DOI] [PubMed] [Google Scholar]
- 3. Sheridan BJ, Ward CJ, Anderson BW, Justo RN. Transcatheter closure of the patent ductus arteriosus: An intention to treat analysis. Heart Lung Circ 2013;22:428–432. [DOI] [PubMed] [Google Scholar]
- 4. Vijayalakshmi IB, Chitra N, Praveen J, Prasanna SR. Challenges in device closure of a large patent ductus arteriosus in infants weighing less than 6 kg. J Interven Cardiol 2013;26:69–76. [DOI] [PubMed] [Google Scholar]
- 5. Liddy S, Oslizlok P, Walsh KP. Comparison of the results of transcatheter closure of patent ductus arteriosus with newer amplatzer devices. Catheter Cardiovasc Interv 2013;82:253–259. [DOI] [PubMed] [Google Scholar]
- 6. Krichenko A, Benson LN, Burrows P, Möes CA, McLaughlin P, Freedom RM. Angiographic classification of isolated persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol 1989;63:877–880. Apr1; [DOI] [PubMed] [Google Scholar]
- 7. Masura J, Walsh KP, Thanopoulous B, Chen Chan Bas J, Goussous Y, Gavora P, Hijazi ZM. Catheter closure of moderate to large patent ductus arteriosus using the new amplatz duct occluder: Immediate and short term results. J Am Coll Cardiol 1998;31:878–882. [DOI] [PubMed] [Google Scholar]
- 8. Alwi M. Patent ductus arteriosus occlusion with the Amplatzer devices In: Sievert H, Qureshi SA, Wilson N, Hijazi ZM, editors. Percutaneous Interventions for Congenital Heart Disease. Informa UK Ltd; 2007. pp 377–384. [Google Scholar]
- 9. Bentham JR, Thomson JDR, Gibbs JL. Transcatheter closure of persistent patent ductus arteriosus in adults. J Interven Cardiol 2012;25:501–504. [DOI] [PubMed] [Google Scholar]
- 10. Pass RH, Hijazi ZM, Hsu T, Lewis D, Hellenbr VWE. Multicenter USA amplatzer patent ductus arteriosus occlusion device trial initial and one‐year results. J Am Coll Cardiol 2004;44:513–519. [DOI] [PubMed] [Google Scholar]
- 11. Thanopoulos B, Eleftherakis N, Tzannos K, Stefanadis C, Giannopoulos A. Further experience with transcatheter closure of the patent ductus arteriosus using the new amplatz duct occluder. Am J Cardiol 2010;105:1005–1009. [DOI] [PubMed] [Google Scholar]
- 12. Tometzki AJ, Arnold R, Peart I, Sreeram N, Abdul Hamed J, Goodman M, Patel R, Kitchiner D, Bu'Lock F, Walsh K. Transcatheter occlusion of the patent ductus arteriosus with cook detachable coils. Heart 1996;76:531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ata JA, Arfi AM, Hussain A, et al. The efficacy and safety of the amplatzer ductal occluder in young children and infants. Cardiol Young 2005;15:279–285. [DOI] [PubMed] [Google Scholar]
- 14. Baspinar O, Irdem A, Sivasli E, Sahin DA, Kilinc M. Comparison of the efficacy of different‐sized amplatzer duct occluders (I, II and II AS) in children weighing less than 10 kg. Pediatr Cardiol 2013;34:88–94. [DOI] [PubMed] [Google Scholar]
- 15. Bilkis AA, Alwi M, Hasri S, Haifa AL, Geetha K, Rehman MA, Hasanah I. The amplatzer duct occluder experience. J Am Coll Cardiol 2001;37:258–261. [DOI] [PubMed] [Google Scholar]
- 16. Faella HJ, Hijazi ZM. Closure of the patent ductus arteriosus with the amplatzer PDA device: Immediate results of the international clinical trial. Cathet Cardiovasc Interv 2000;51:50–54. [DOI] [PubMed] [Google Scholar]


