Abstract
Objective
This study aimed to evaluate the effectiveness of expressive writing intervention (EWI) for improving psychological and physical health in cancer patients and survivors.
Methods
We searched databases and existing reviews for randomized controlled studies published between 1986 and 2014 that evaluated the effects of EWI on psychological and physical health outcomes. We computed and combined effect sizes and examined the role of methodological characteristics.
Results
From 223 unique citations, we identified 16 independent randomized controlled trials published from 1999 to 2014, examining the effect of EWI on a range of psychological and physical health outcomes. No statistically significant effects were found for any of the individual or combined psychological (Hedges's g: 0.04; 95% CI, −0.06 to 0.14; p = 0.42), physical (0.08; 95% CI, −0.05 to 0.20; p = 0.22), or quality‐of‐life outcomes (0.09; 95% CI, −0.05 to 0.24; p = 0.22). The results were unaffected by differences in study characteristics, for example, type of control condition, study setting, cancer type, and overall study quality ratings. Results from a subset of studies indicated a possible moderating effect of social constraints, suggesting that participants experiencing low levels of emotional support may be more likely to benefit from EWI.
Conclusions
Our results do not support the general effectiveness of EWI in cancer patients and survivors. However, given the practical and inexpensive intervention, it is possible that even small effects in subgroups of patients could be clinically relevant, and future studies are recommended to test the effects of potential moderators, including pre‐intervention distress levels and context‐dependent factors such as emotional support. © 2015 The Authors. Psycho‐Oncology Published by John Wiley & Sons Ltd.
Introduction
A cancer diagnosis is a stressful and potentially traumatic event 1, and even after successful treatment, the cancer diagnosis and treatment may continue to be a source of considerable distress [2, 3, 4, 5]. Research suggests that the willingness, ability, and opportunity to express cancer‐related concerns and emotions—or lack thereof—may influence cancer patients' adjustment to the stressors associated with cancer and cancer treatment [6, 7, 8, 9]. This may have consequences not only for their psychological health but also perhaps even for physical health outcomes, including prognosis [10, 11, 12]. Exploring and expressing thoughts and feelings are considered core aspects of psychotherapy 13, 14, and there is evidence to suggest that supportive‐expressive interventions, helping cancer patients express their cancer‐related thoughts and emotions, may improve both psychological and physical health outcomes 15, 16. One mode of emotional expression linked with beneficial health outcomes is writing 17, and the early research by Pennebaker and colleagues 18, 19 demonstrated that writing as little as 15–20 min for 3 days about emotions associated with a traumatic event could lead to improvements in both psychological 20, 21 and biological health 22, 23.
A growing number of controlled trials of expressive writing intervention (EWI) with both healthy and clinical populations have found a wide range of benefits. The first meta‐analysis of 13 studies of EWI 24 reported a medium overall effect size (ES) for healthy participants (Cohen's d = 0.47). Two later meta‐analyses have revealed more modest effects of EWI in clinical samples (d = 0.19) 25 and across samples of healthy and clinical participants (d = 0.15) 26. Although the existing meta‐analyses have included studies with cancer patients, they have not reported separate results for EWI with cancer patients. A recently published systematic review 27 identified 13 controlled studies of the effects of EWI in cancer patients, reviewed the results, and evaluated study quality. While the majority of results were null findings, there were some positive results for effects on pain, sleep, and general physical and psychological symptoms. Furthermore, a number of identified moderator effects suggested that the efficacy of EWI may depend on contextual factors such as levels of social support 6, *28. The authors conclude that although the available studies are limited in their methodological quality and heterogeneous with respect to various aspects of their design, EWI appears to be generally feasible and could represent a safe, simple, accessible, and inexpensive intervention that may offer some relief 27. However, as the authors did not subject the results to quantitative analysis, their conclusion must be considered preliminary.
To evaluate the possible efficacy of EWI in cancer patients, we therefore conducted a systematic review and meta‐analysis of randomized controlled trials of EWI with cancer patients and survivors. Our primary aim was to evaluate the overall effects of EWI on psychological and physical health outcomes. Secondary aims were to quantitatively evaluate possible associations between the effects and variations in methodological quality and study design and to review possible moderating effects, for example, of social constraints.
Methods
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) recommendations 29.
Search strategy
A keyword‐based search in the electronic databases of PubMed and PsychINFO was conducted. Keywords related to the population (cancer OR neoplasm) were combined with keywords related to the intervention ((expressive OR emotion* OR disclosure) AND (writing OR written)) AND (intervention OR therapy OR treatment). The search was conducted independently by the two authors for the period from 1986 (the year of the first article on the expressive writing paradigm 18) to December 2014. In addition, a backward search (snowballing) was conducted using reference lists of identified articles and earlier systematic reviews together with a forward search (citation tracking) until no additional relevant articles were found.
Selection procedure and data extraction
Only English language reports published in peer‐reviewed journals were considered eligible for the present study. Studies were selected using the PICO (Patient, Intervention, Comparison, Outcomes) approach 30. Eligible studies had to (a) use a study population of adult cancer patients or survivors, (b) use an EWI following the original Pennebaker paradigm 18, instructing the participants in three or four home‐based or lab‐based writing sessions to disclose their emotions about their cancer or another traumatic event, (c) randomize participants to EWI or one or more control conditions, consisting of either non‐writing or an active neutral, non‐emotional writing control condition, (d) present data for both the EWI and control group(s) for either psychological health (e.g., distress, depression, anxiety, and perceived stress), physical health (e.g., physical symptoms and health care utilization), or combined mental and physical health outcomes (e.g., health‐related quality of life (QoL)), and (e) report results as pre–post means and standard deviation (SD)/standard error (SE) in all groups, change scores in all groups, ESs (e.g., Cohen's d and η 2), or other relevant statistics (e.g., p‐values, F‐values, and N).
First, the authors independently removed duplicates and screened the titles and abstracts of the identified references with the purpose of excluding irrelevant studies. Then, full texts of the remaining references were evaluated and ineligible reports excluded on the basis of the criteria described earlier and reasons for exclusion registered. Disagreements were discussed until a negotiated conclusion was reached.
Quality assessment
Methodological quality was assessed using a modified version of the original 11 Jadad criteria 31 together with an item from the Cochrane assessment of bias tool 32 and additional EWI‐relevant criteria yielding a total quality score (range: 0–15). The 15 criteria were as follows: (a) study designed as randomized, (b) randomization procedure clearly described, (c) attempts to mask the condition to the participants, (d) allocation concealed for the researchers during the intervention, (e) clear description of withdrawals and dropouts, (f) study objectives clearly defined, (g) outcome measures clearly defined, (h) inclusion and exclusion criteria clearly described, (i) sample size justified (e.g., power calculation), (j) clear description of intervention(s), (k) at least one control group, (l) statistical methods clearly described, (m) study report free of suggestion of selective outcome reporting (e.g., results for all included outcomes are described), (n) manipulation check included (e.g., measuring responses immediately before and after intervention and interviewing participants about their writing and assessing emotional content), and (o) an active control condition included (neutral writing controls). To obtain valid quality scores, the quality ratings were first conducted independently by the two authors. Disagreements and uncertainties were then discussed until a negotiated final score was reached for each study. Quality ratings were not used as weights when calculating aggregated ESs, as this is discouraged because of the risk of inducing bias 33. Instead, possible associations between ESs and specific design characteristics and study quality scores were explored with meta‐analysis of variance (ANOVA) and meta‐regression 34.
Heterogeneity
Heterogeneity was explored using Q and I 2 statistics. Heterogeneity tests are aimed at determining whether results reflect systematic between‐study differences (heterogeneity) or whether the variation is due to random error (homogeneity) 35. Because of the generally low statistical power of heterogeneity tests, a more liberal p‐value of ≤0.10 was used to determine significant heterogeneity 36. The I 2 statistic is an estimate of the amount of variance in a pooled ES accounted for by heterogeneity in the sample of studies and is unaffected by the number of studies (K) 37. An I 2 value of 0% indicates no observed heterogeneity. Values of 25%, 50%, and 75% are considered low, moderate, and high, respectively.
Computing effect sizes
Hedges's g, a variation of Cohen's d 38, correcting for possible bias due to small sample size 39 was used as the standardized ES. Whenever possible, ESs were computed using pre‐intervention and post‐intervention means and their standard deviations. If these data were unavailable, ESs were based on those reported by the authors or estimated on the basis of N and other reported statistics, for example, p‐value, F‐value, or b‐value. Pooled ESs were weighted by the inverse standard error, taking into account the precision of each study. When available and relevant, the N used in the calculation was the N in the final analysis for each outcome. As statistical power to detect heterogeneity may always be optimal, a random effects model was chosen for all analyses. A positive value was chosen to indicate an ES in the hypothesized direction. If studies reported results for more than one measure per outcome, independence of results was ensured by averaging ESs across all outcomes, so that only one result per study was used for each quantitative data synthesis.
Analytical strategy
First, pooled ESs for the effect of EWI on all individual psychological and physical health outcomes reported in a sufficient number of studies were calculated separately. Then, the pooled overall ESs for the combined psychological health, physical health, and QoL outcomes were calculated together with the overall combined ES for all outcomes. If studies had allocated participants to more than one control group, for example, both non‐writing and neutral writing *40, EWI was compared with each group separately. If a study had included more than one EWI group, for example, standard emotional writing and writing about helping others *41, only the data for the group allocated to a writing intervention similar to the standard Pennebaker EWI approach—the focus of the present meta‐analysis—were used. If studies had included more than one assessment time point, the time points chosen for the analysis were those closest in time to post‐treatment. The possible influence of time to post‐treatment assessment was subsequently analyzed with meta‐regression using the time (in weeks) as a continuous variable. Additional between‐study differences in ESs were explored by comparing the ESs of studies according to the following study characteristics: (a) active (neutral writing) versus passive control (non‐writing), (b) studies of breast cancer patients versus studies of patients with other cancers, (c) lab‐based versus home‐based intervention, (d) daily versus weekly writing sessions, (e) three versus four writing sessions, and (f) quality rating. This was carried out with either meta‐ANOVA or meta‐regression. The calculations were conducted with Comprehensive Meta‐Analysis version 2 and IBM spss version 21. A statistical power analysis 42 indicated that to detect a statistically significant small ES (0.20) similar to that previously found for clinical samples 25, with an alpha of 5%, a statistical power of 80% and an average sample size of 100 would require 8 and 13 studies using fixed and random effects models, respectively.
Publication bias
Publication bias, a widespread problem when conducting meta‐analyses 43, was visually inspected with funnel plots and statistically tested with Egger's test 44. If the results were suggestive of publication bias, we planned to calculate an adjusted ES using Duval and Tweedie's trim and fill method 45, which imputes ESs of missing studies and recalculates the ES accordingly. In case of statistically significant results, we planned to calculate the fail‐safe number 46, 47, that is, the number of unpublished studies with null findings that would reduce the result to statistical non‐significance (p > 0.05).
Results
The study selection process with reasons for exclusion is described in Figure 1. The initial search yielded 223 papers, out of which 39 were read in full during the second round of assessment. After excluding further 23 papers, 16 individual research papers describing results of 16 independent randomized controlled trials published in the years from 1999 to 2014 were included and subjected to meta‐analytic evaluation.
Figure 1.
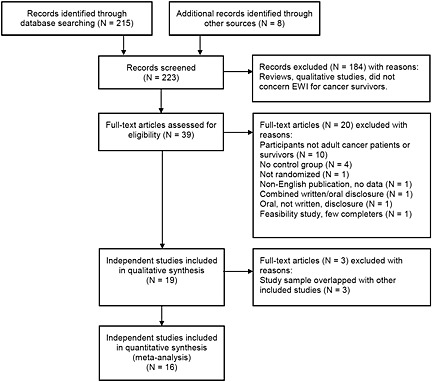
Flow diagram of reviewed studies from identification to inclusion
Study characteristics
The characteristics of the included studies are summarized in Table 1. The 16 studies had recruited a total of 2392 cancer patients or survivors, and we analyzed the final data for 1797 participants with a mean study sample size of 112. Eight studies investigated breast cancer patients or survivors, with the remaining eight studies investigating participants with renal, prostate, colorectal, ovarian, and mixed cancers. Most studies (N = 14) instructed EWI participants to disclose their emotions about their cancer in three (N = 5) or four (N = 11) daily (N = 7), weekly (N = 8), or biweekly (N = 1) sessions. Three studies had participants write in a lab‐based setting, 12 had used a home‐based design, and one study had used a mixed lab‐based and home‐based design. Four studies used non‐writing controls, and 12 studies used neutral non‐emotional writing, for example, about their daily activities or facts about their cancer. Post‐treatment assessment time points also varied, with eight studies collecting outcome data at two or more time points and eight presenting data for one time point only. Post‐treatment assessments varied from 2 to 24 weeks after the intervention. As seen in Tables 1 and 2, the included studies assessed a broad range of psychological and physical health outcomes. Fourteen studies included one or more psychological health outcome measures; 11 studies included one or more measures of physical symptoms, physical function, or health care utilization; and six studies included a combined generic or cancer‐related QoL measure.
Table 1.
Characteristics of the included studies
| Author | Year | N a (N)b | Cancer | EWI writing topicc | Controlc (N)b | Number of writing sessions | Schedule (days between sessions) | Study design, settingd | Post‐intervention assessment time points (weeks) | Quality rating e | Psychological outcomesf and moderators g | Physical health outcomesf | Combined or global QoL outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Walker et al.*48 | 1999 | 44 (28) | Breast, stages I and II | 1. Cancer (1 session) (N = 11) 2. Cancer (3 sessions) (N = 14) | 3. Non‐writing (N = 14) | 1 and 3 | Consecutive days | RCT, lab based and home based | 4–6, 16, and 28 | 9 | Intrusive thoughts, avoidance (IES), and mood (POMS) | — | |
| de Moor et al.*49 | 2002 | 42 (35) | Renal | 1. Cancer (N = 18) | 2. Neutral writing (N = 17) | 4 | Over 4 weeks | RCT, lab based | 4, 6, 8, and 10 | 9 | Stress (PSS), intrusive thoughts, avoidance (IES), and mood (POMS) | Sleep (PSQI) | |
| Rosenberg et al.*50 | 2002 | 30 (30) | Prostate | 1. Cancer (N = 15) | 2. Non‐writing (N = 15) | 4 | Consecutive days | RCT, home based (phone, once) | 12, 24 | 9 | General distress (SCL‐90), mood (POMS) Rumination, Ways of Coping | QoL (SF‐36; FACT), physical symptoms, health care utilization, health behaviors | |
| Stanton et al.*51 | 2002 | 63 (60) | Breast, stages I and II | 1. Cancer (N = 21) 2. Positive thoughts about cancer (N = 21) | 3. Facts about cancer (N = 18) | 4 | Over 3 weeks | RCT, lab based | 4 and 12 | 15 | Avoidance (COPE and IES), mood (POMS), and avoidance | QoL (FACT), physical symptoms, and health care utilization | |
| Zakowski et al.*28 | 2004 | 127 (104) | Gynecological and prostate | 1. Cancer (N = 62) | 2. Neutral writing (N = 42) | 3 | Consecutive days | RCT, home based (phone) | 24 | 11 | General distress (BSI) intrusive thoughts, avoidance (IES), and SCS | — | |
| Cepeda et al.*52 | 2008 | 234 (178) | Mixed, with pain | 1. Cancer (N = 42) | 2. Questionnaire, non‐writing (N = 66) 3. Non‐writing (N = 70) | 3 | Over 3 weeks | RCT, home based (phone once) | 4 and 8 | 11 | Pain (VAS) | Total well‐being (Likert scale) | |
| de Moor et al.*53 | 2008 | 64 (38) |
Breast, stages II and III in neo‐adjuvant chemotherapy |
1. Cancer (before surgery) (N = 16) | 2. Neutral writing (N = 22) | 4 | Over 1 week | RCT, home based (mail) | 2 and 4 | 8 | General distress (BSI), stress (PSS), intrusion, avoidance (IES), and SCS | Pain (BPI) and sleep (PSQI) | |
| Gellaitry et al.*54 | 2010 | 93 (80) | Breast, stages I and II | 1. Cancer (N = 38) | 2. Non‐writing (N = 42) | 4 | Consecutive days | RCT, home based (mail) | 4, 12 and, 24 | 8 | Mood (POMS) and social support (SOS) | Health care utilization | QoL (FACT‐B) |
| Low et al.*55 | 2010 | 76 (62) | Breast, stage IV | 1.Cancer (N = 31) | 2. Facts about cancer (N = 31) | 4 | Over 3 weeks | RCT, home based (phone) | 12 | 13 | Depressive symptoms (CES‐D), intrusive thoughts (IES), and social support | Physical symptoms and sleep (PSQI) | |
| Mosher et al.*56 | 2012 | 87 (86) | Breast, stage IV | 1. Cancer (N = 44) | 2. Neutral writing (N = 42) | 4 | Over 4–7 weeks | RCT, home based (phone) | 8 | 14 | Depression (CES‐D), anxiety (HADS‐A), and existential well‐being (FACIT‐sp) | Sleep (PSQI), fatigue (FACIT‐F), and use of mental health services | |
| Craft et al.*40 | 2013 | 120 (97) | Breast | 1. Cancer (N = 21) 2. Self‐selected (N = 19) | 3. Facts about cancer (N = 16) 4. No writing (N = 26) | 4 | Consecutive days | RCT, lab based | 4 and 24 | 11 | — | QoL (FACT‐B) | |
| Arden‐Close et al.57 | 2013 | 120 (80) | Ovarian (and partners) | 1. Cancer (N = 41) | 2. Neutral writing (N = 39) | 3 | Consecutive days | RCT, home based (phone) | 12 | 12 | Perceived stress (PSS) and intrusive thoughts (IES) | QoL (FACT‐G) | |
| Jensen‐Johansen et al.*58 | 2013 | 507 (417) | Breast, stages I and II | 1. Free choice (cancer or other) (N = 198) | 2. Neutral writing (N = 219) | 3 | Over 3 weeks | RCT, home based (phone) | 12, 36 | 15 | Depressive symptoms (BDI‐SF), intrusive thoughts, avoidance (IES), mood (POMS, PPMS), SCS, and Alexithymia (TAS‐20) | ||
| Rini et al.h *41 | 2013 | 315 (136) | Various cancer survivors treated with hemato‐poietic stem cell transplants | 1. Cancer (N = 67) 3) Cancer + peer helping (N = 69) (not included) | 2. Neutral writing (N = 69) 4) Peer helping writing (N = 59) (not included) | 4 | Over 4 weeks | RCT, home based (phone) | 12 | 13 | General distress (BSI‐GSI) | Inventory of physical symptoms | QoL (FACT‐BMT) |
| Milbury et al.*59 | 2014 | 277 (173) | Renal cancer | 1. Cancer (N = 87) | 2. Neutral writing (N = 86) | 4 | 1 and 5 days | RCT, home based (phone) | 4 | 12 | Depression (CES‐D), cancer‐related distress (IES), and QoL (SF‐36 mental component) | Cancer symptoms (MDASI), fatigue (BFI), sleep (PSQI), and physical symptoms (SF‐36 physical component) | |
| Lepore et al.*60 | 2014 | 193 (193) | Colorectal cancer, stages I–III | 1. cancer (N = 101) | 2. Neutral writing (N = 92) | 4 | Biweekly | RCT, home based (phone) | 4 | 15 | Depression (CES‐D), emotional function (QLQ‐C30), and SCS | Sleep (PSQI) and physical function (QLQ‐C30) | QoL (QLQ‐C30) |
BDI, Beck's Depression Inventory; BDI‐SF, Beck's Depression Inventory‐Short Form; BFI, Brief Fatigue Inventory; BPI, Brief Pain Inventory; BSI, Brief Symptom Inventory; BSI‐GSI, Brief Symptom Inventory—Global Severity Index; CES‐D, Center for Epidemiological Studies—Depression Scale; COPE, COPE Inventory; EWI, expressive writing intervention; FACT, Functional Assessment of Canter Therapy; FACT‐B, Functional Assessment of Canter Therapy—Breast Cancer; FACT‐BMT; Functional Assessment of Canter Therapy—Bone Marrow Transplantation; FACT‐G, Functional Assessment of Canter Therapy—General; FACIT‐F, Functional Assessment of Chronic Illness—Fatigue; FACIT‐sp, Functional Assessment of Chronic Illness—spiritual well‐being; HADS, Hospital Anxiety and Depression Scale; IES, Impact of Event Scale; MDASI, M.D. Anderson Symptom Inventory; PHQ, Patient Health Questionnaire; POMS, Profile of Mood State; PPMS, Passive Positive Mood Scale; PSQI, Pittsburgh Sleep Quality Index; PSS, Perceived Stress Scale; QLQ‐C30, EORTC Quality of Life Questionnaire‐Core 36; QoL, quality of life; RCT, randomized controlled trial; SCL‐90, Symptom Checklist; SCS, Social Constraints Scale; SF‐36, Short Form (36) Health Survey; SOS, Significant Others Scale; TAS‐20, Toronto Alexithymia Scale‐20 item version; VAS, Visual Analog Scale.
Initial N allocated to intervention.
Final N included in analyses, including intention‐to‐treat (ITT).
Number refers to group number.
Home‐based phone, home‐based mail, or lab based.
Modified Jadad rating scale (score range: 0–15).
Only outcomes relevant for the analyses are described, and studies may have reported on other outcomes not listed.
Potential moderators in italics.
Not reported (n.r.).
Only emotional writing (group 3) and neutral writing (group 4) included in the present analysis.
Table 2.
Pooled effects of EWI for psychological health, physical health, and QoL outcomes in cancer patients
| Outcome | Sample size | Heterogeneitya | Pooled effect sizesc, d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K | N | Q | df | p | I2 | Hedges's g b | 95% CI | P (two‐tailed) | |
| Overall effect | 16 | 1749 e | 4.47 | 15 | 0.996 | 0.00 | 0.04 | −0.05 to 0.14 | 0.377 |
| Neutral writing control | 12 | 1429 | 6.97 | 11 | 0.801 | 0.00 | 0.03 | −0.08 to 0.13 | 0.616 |
| Non‐writing controlf | 5 | 371 | 3.82 | 4 | 0.307 | 16.91 | 0.12 | −0.11 to 0.36 | 0.304 |
| Between groups g | 15 | 1704 | 0.02 | 1 | 0.921 | ||||
| Breast cancer | 8 | 822 | 1.41 | 7 | 0.985 | 0.00 | 0.001 | −0.14 to 0.14 | 0.992 |
| Other cancers | 8 | 929 | 2.39 | 7 | 0.935 | 0.00 | 0.08 | −0.05 to 0.21 | 0.229 |
| Between groups | 16 | 1749 | 0.67 | 1 | 0.414 | ||||
| Home based | 12 | 1577 | 3.41 | 11 | 0.984 | 0.00 | 0.04 | −0.06 to 0.14 | 0.459 |
| Lab based h | 3 | 144 | 0.20 | 2 | 0.905 | 0.00 | 0.12 | −0.22 to 0.45 | 0.495 |
| Between groups | 14 | 1721 | 0.65 | 1 | 0.421 | ||||
| Daily sessions | 8 | 606 | 1.88 | 7 | 0.966 | 0.00 | 0.05 | −0.11 to 0.22 | 0.531 |
| Weekly sessions | 7 | 974 | 2.56 | 6 | 0.862 | 0.00 | 0.04 | −0.09 to 0.17 | 0.531 |
| Between groups | 15 | 1560 | 0.01 | 1 | 0.913 | ||||
| Three sessions | 5 | 809 | 0.46 | 4 | 0.977 | 0.00 | −0.02 | −0.16 to 0.12 | 0.782 |
| Four sessions | 11 | 942 | 2.56 | 10 | 0.990 | 0.00 | 0.09 | −0.03 to 0.23 | 0.143 |
| Between groups | 16 | 1749 | 1.45 | 1 | 0.229 | ||||
| Psychological health combined | 14 | 1522 | 4.68 | 13 | 0.982 | 0.00 | 0.04 | −0.06 to 0.14 | 0.419 |
| Physical health combined | 11 | 1071 | 7.96 | 10 | 0.632 | 0.00 | 0.08 | −0.05 to 0.20 | 0.221 |
| QoL | 6 | 716 | 4.75 | 5 | 0.447 | 0.00 | 0.09 | −0.05 to 0.24 | 0.215 |
| Intrusive thoughts | 6 | 732 | 2.03 | 5 | 0.845 | 0.00 | −0.05 | −0.19 to 0.10 | 0.517 |
| Avoidance | 4 | 590 | 1.89 | 3 | 0.595 | 0.00 | −0.01 | −0.17 to 0.15 | 0.900 |
| Depression | 5 | 932 | 0.09 | 4 | 0.999 | 0.00 | 0.02 | −0.11 to 0.15 | 0.722 |
| Perceived stress | 3 | 153 | 2.72 | 2 | 0.256 | 26.59 | 0.23 | −0.16 to 0.61 | 0.246 |
| Other distress measuresi | 8 | 705 | 2.11 | 7 | 0.954 | 0.00 | 0.11 | −0.04 to 0.26 | 0.158 |
| Positive mood | 4 | 709 | 0.13 | 3 | 0.998 | 0.00 | −0.02 | −0.17 to 0.13 | 0.770 |
| Fatigue | 2 | 259 | 0.48 | 1 | 0.490 | 0.00 | 0.16 | −0.08 to 0.41 | 0.191 |
| Pain | 3 | 246 | 3.46 | 2 | 0.177 | 42.18 | 0.03 | −0.36 to 0.43 | 0.875 |
| Sleep disturbance | 5 | 587 | 4.66 | 5 | 0.458 | 0.00 | 0.00 | −0.16– 0.16 | 0.990 |
| Other physical symptoms | 5 | 624 | 2.56 | 4 | 0.635 | 0.00 | 0.11 | −0.05 to 0.27 | 0.160 |
| Healthcare utilization | 3 | 170 | 1.88 | 2 | 0.391 | 0.00 | 0.21 | −0.10 to 0.52 | 0.177 |
EWI, expressive writing intervention; QoL, quality of life; df, degrees of freedom; 95% CI, confidence interval; ES, effect size.
Q statistic: p‐values < 0.1 taken to suggest heterogeneity. I 2 statistic: 0% (no heterogeneity), 25% (low heterogeneity), 50% (moderate heterogeneity), and 75% (high heterogeneity).
Random effects model.
ES, Hedges's g. Standardized mean difference, adjusting for small sample bias. A positive value indicates an ES in the hypothesized direction, that is, reduced pain or relatively small increase in pain in the intervention group. All ESs were combined using a random effects model. To ensure independency, if a study reported results for more than one measure, the ESs were combined (mean), ensuring that only one ES per study was used in the calculation. Conventions: small (<0.3); medium (0.5); and large (0.8>).
In case of statistically significant ESs, it was planned to examine the robustness of findings by calculating the fail‐safe N (number of non‐significant studies that would bring the p‐value to non‐significant (p > 0.05) 46. No ESs reached statistical significance (p > 0.05), and fail‐safe N was not calculated.
Numbers do not necessarily add up to the total N analyzed (1797), as some studies that have included more than two groups are excluded from certain analyses to ensure independency.
The number for neutral writing and non‐writing exceeds the total, as one study *40 had included both neutral and no writing control groups.
Meta‐ANOVA.
One study used mixed lab‐based and home‐based settings.
For example, Brief Symptom Inventory (BSI) and Symptom Checklist (SCL‐90).
Possible publication bias was examined with funnel plots and Egger's test. If statistically significant (p < 0.05), this was to be followed by imputation of missing studies 45. However, no analyses suggested publication bias.
The initial study quality ratings of the two raters showed good inter‐rater agreement with the raters agreeing on 90.4% of the 240 individual ratings and a high correlation between the total quality ratings of the two raters (r = 0.87, p < 0.001). Each of the ratings on which the raters had initially disagreed was discussed in depth and a final rating negotiated. The mean final total quality rating was 11.6 (SD = 2.5; range: 8–15). The primary methodological limitations were that researchers had not attempted to blind or mask the experimental conditions and related hypotheses to participants (N = 12), allocation was not concealed to researchers during intervention (N = 8), sample size had not been based on statistical power calculations (N = 7), a clear description of the randomization procedure was not provided (N = 7), and a clearly described manipulation check of writing instruction adherence had not been included (N = 6).
Pooled effect sizes
As seen in Table 2 and Figure 2, no statistically significant effects were found for any of the individual or combinations of outcomes. All pooled ESs (Hedges's g) were small, ranging from −0.05 (intrusive thoughts) to 0.23 (perceived stress). The largest, albeit non‐significant, ESs were found for the individual outcomes of perceived stress (0.23; N = 3), healthcare utilization (0.21; N = 3), fatigue (0.16; N = 2), other general distress measures (0.11; N = 8), other physical symptoms (0.11; N = 5), and the combined or global QoL outcomes (0.09; N = 6). To explore the issue of statistical power, a series of post hoc statistical power analyses 42 were conducted. The number of independent studies with similar sample sizes (N = 112) needed to detect statistically significant ESs corresponding to those found in the present analysis with a random effects model, a p‐value of 0.05, and a statistical power of 0.80 were 58, 73, and 292 for the combined QoL, physical, and psychological outcomes, respectively.
Figure 2.
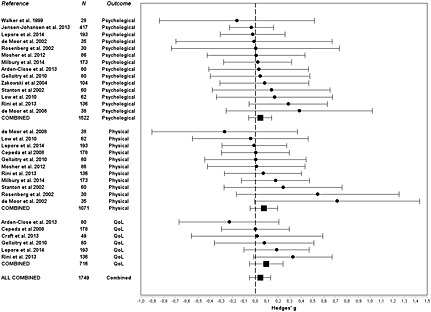
Forest plot of effects (Hedges's g) of randomized controlled studies of expressive writing intervention (EWI) on psychological, physical, and quality‐of‐life (QoL) outcomes in cancer patients and survivors
Exploring between‐study differences
As seen in Table 2, the results of the heterogeneity analyses indicated that between‐study differences in ESs were highly likely to be due to random, rather than systematic error. No Q statistics reached statistical significance (range of p: 0.177–0.999), and I 2 values indicated no (22 ESs) or low heterogeneity (three ESs) 37. Although all planned comparisons with meta‐ANOVA's failed to reach statistical significance, they suggested that studies using non‐writing controls had larger effects (g = 0.12) than studies with active (neutral) writing control (0.03), studies of patients with other cancers had larger effects (0.08) than studies with breast cancer patients (0.001), studies using a lab‐based setting had larger effects (0.12) than home‐based studies (0.04), and studies with four writing sessions (0.10) showed larger effects than studies with three sessions (−0.02). Using daily (0.05) versus weekly sessions (0.04) did not suggest a differential effect. When analyzing the possible influence of post‐treatment assessment time on ESs with meta‐regression, no statistically significant effects were found for either the overall combined ES (coefficient = −0.002; p = 0.768), combined psychological outcomes (0.01; p = 0.549), combined physical outcomes (−0.001; p = 0.852), or combined QoL outcomes (−0.01; p = 0.134). Likewise, when exploring the association between study quality ratings and ESs, the associations were small and did not reach statistical significance (coefficient = −0.008 to 0.03; range of p: 0.350–0.775).
Moderators
Five studies had explored the possible moderating role of emotional support or perceived social constraints 61 on the effect of EWI. Three studies found evidence suggesting that participants with high levels of social constraints or low levels of emotional support experienced greater reductions in general distress and avoidance *28, lower average daily pain *53, and fewer intrusive thoughts *55 than participants high in emotional support or low in social constraints. In contrast, two studies *58, *60 found no moderating effect of social constraints. Additional moderating effects were reported for avoidance *51, with EWI being relatively effective for psychological outcomes for participants low in avoidance, while more positive writing, focused on benefit finding, was more effective for women high in avoidance. Moderation effects were also found for aspects of Alexithymia *58, with lower scores on externally oriented thinking in the EWI group, but not controls, being associated with greater reductions in cancer‐related distress, and higher scores on difficulties describing feelings being associated with greater increases in controls, but not the EWI group. In one study, participants in the EWI group were free to write about their cancer or another traumatic experience *58, with results indicating fewer depressive symptoms and more positive mood when participants wrote about their cancer than when writing about other traumatic experiences. Finally, one study *60 explored but failed to find a moderating effect of gender.
Discussion
Our meta‐analysis of the 16 randomized controlled studies of EWI with data for 1797 cancer patients and survivors revealed no statistically significant main effects for any individual or combined psychological or physical health outcomes. Taken together, the results suggest that instructing cancer patients and survivors to write emotionally about their cancer and cancer treatment has no beneficial effects on their psychological or physical health. This finding is in contrast to results of earlier meta‐analyses of EWI studies with healthy and clinical samples [24, 25, 26].
Null findings raise the possibility of type 2 errors, and one reason for our failure to find any effects could be insufficient statistical power of the meta‐analysis. However, with 16 studies with an average sample size of 112, our meta‐analysis was sufficiently powered to detect a small ES (0.2) 38 similar to that found in a previously published meta‐analysis of EWI studies with clinical samples (0.19) 25 and to the average effects found in the lower quartile of 302 (0.3) 62 and 64 (0.2) 63 meta‐analyses of behavioral interventions.
Another reason could be that the available studies were limited in their methodological quality and heterogeneous in their design 27. However, when examining the role of study quality, we found no associations between ESs and study quality scores. Quality scores are often used to contrast, model, or modify meta‐analysis results, but generally appear to be poor predictors of study results 33, 64, the reason being that while some indicators of less‐than‐optimal methodological quality, for example, insufficient blinding or masking of study conditions, could theoretically lead to larger effects, other indicators, for example, an active neutral writing control condition, could be associated with smaller effects. It could thus be more informative to explore variation in individual methodological characteristics, which could have influenced the results, for example, intervention setting, number of sessions, adherence to writing instructions with manipulation checks, and control condition.
A third reason could thus be that the majority of studies used a home‐based writing setting, which could be less effective than the lab‐based setting used in many of the early studies of expressive writing, and several studies had only used three writing sessions, while others had used four, which could be more effective. Likewise, studies which had, in accordance with the original EWI paradigm 18, used neutral, non‐emotional writing as active control condition could be expected to yield smaller effects than a non‐writing control condition. Although the effects found for lab‐based studies, four‐session studies, and studies with non‐writing controls were larger than for home‐based studies, three‐session studies, and studies using neutral writing as control, no differences reached statistical significance (p‐values: 0.23–0.92). Even more importantly, when assessing study heterogeneity, our results generally indicated that any between‐study variation in ESs was much more likely due to random error than systematic between‐study differences. Although statistical power for testing study heterogeneity is often limited 42, not only the Q statistic but also the I 2 statistic, which is unaffected by the number of studies, indicated the ESs to be highly homogeneous.
Finally, a limitation could be that ESs for a subset of studies were estimated on the basis of sample size and secondary statistics, for example, p‐values. However, the number was limited, ESs based on reported ESs or other statistics were comparable with ESs based on means and SDs (p = 0.66), and both types of results were highly homogenous (I 2 = 0.00).
A more likely reason for our null findings could therefore be that EWI, at least when conducted as in the available studies, is not particularly effective as a psychotherapeutic intervention for cancer patients and survivors. There could be several possible explanations for the lacking efficacy in this group.
First, several studies may not have adhered completely with the original Pennebaker paradigm. Although there were exceptions *40, *58, participants in the experimental groups were generally instructed to write about their cancer. In the original study by Pennebaker and colleagues 18 and several subsequently conducted studies, participants were asked to write about the most stressful or traumatic experience in their lives, and it is possible that for some participants, especially in later stages of recovery, their cancer was insufficiently traumatic to elicit a beneficial effect of EWI. Other deviations from the original EWI paradigm include a study in which participants were not explicitly instructed to write about their emotions but about ‘how cancer affected their lives’, which may explain the finding that 20 out of 79 patients had no emotional disclosure *52. In a second study, the participants were not instructed to write about their emotions on the first of the three writing days 57. However, excluding these studies from the analyses did not alter the result (overall effect: g = 0.05; p = 0.31).
Second, although the present results could seem paradoxical given the earlier positive findings, cancer patients, who agree to participate in psychological intervention studies, may be relatively well‐adjusted 65, and it is theoretically possible that the participants have not been sufficiently distressed for the intervention to be effective (‘floor effect’). Although the many different psychological outcome measures and patient groups investigated restricted us in establishing whether this was the case, in one study *58, participants were compared with a comparable national reference group and were in fact found to report significantly lower levels of distress. Furthermore, a large proportion of studies investigated breast cancer patients and survivors, and as the pooled ES approached zero for participants with breast cancer (g = 0.001) and was larger for other cancers (g = 0.08), we cannot completely exclude the possibility that EWI may be more effective for patients with other cancers.
Third, the degree of psychological adjustment to cancer may depend on the severity of disease as well as time since diagnosis and treatment. Unfortunately, the studied samples were mixed with respect to disease severity and time since diagnosis, not only between studies but also within studies, thus limiting our ability to conduct moderation analyses.
Fourth, as time increases from intervention to post‐treatment assessment, one would generally expect the effect of the intervention to decrease. Again, the included studies showed considerable variation in the time points assessed, and some studies had included several time points. To reduce the likelihood of type 2 error, we therefore chose the time closest to post‐treatment, and when exploring the associations between ESs and post‐treatment assessment time with meta‐regression, the coefficients approached zero and did not reach statistical significance. It should, however, be noted that two studies found indications of long‐term improvements over time in EWI participants. Rosenberg *50 thus found increased pain report in controls from 3 to 6 months with no change in the EWI group. Likewise, in the study by Stanton *51, EWI participants reported fewer somatic symptoms from 1 to 3 months after intervention compared with controls. It could be hypothesized that EWI might catalyze other positive changes, which could in turn strengthen effects over time.
Fifth, a specific challenge in EWI, especially for home‐based interventions, could be whether participants adhere to the writing instructions. However, several studies had included manipulation checks, which indicated that the participants had followed the writing instructions, and that EWI, compared with neutral writing, was successful in inducing the brief increases in negative mood generally associated with emotional disclosure 66. Still, most studies failed to find any main effects post‐treatment, and the pooled ESs were generally homogenous, small, and statistically non‐significant, regardless of the outcomes investigated.
Finally, it is possible that the extent to which expression of emotion is helpful or adaptive depends on the context. It has thus been demonstrated that expressive flexibility, that is, the ability to both upregulate and downregulate emotional expression, was associated with long‐term psychological adjustment 67. Together with other findings showing that healthy individuals manage their emotions in different ways depending on the intensity level 68, this suggests that it can be adaptive to both engage and disengage from emotions depending on the context. This finds some support in studies showing that patients with low levels of emotional support or high levels of experienced social constraints were more likely to benefit from EWI than patients with high levels of emotional support *28, *53, *55. Another study showed EWI to be relatively effective for participants low in avoidance, while more positive writing focused on benefit finding was more effective for women high in avoidance *51.
Conclusion
Despite the modest effects previously found in healthy students and other clinical samples [24, 25, 26], our results do not support the general effectiveness of EWI in cancer patients and survivors for any of the psychological or physical health outcomes studied. This finding is unlikely to be due to insufficient statistical power, and the ESs were furthermore quite homogenous and unassociated with any of the key methodological aspects explored. A reason for the null finding could be that effects of emotional expression are context dependent, as supported by a small number of studies showing differential effects depending on the perceived availability of emotional support. Although EWI does not appear to work well for all cancer patients, given the very practical and inexpensive intervention, even small effects in subgroups of patients could be clinically relevant, and future studies are recommended to test the effects of potential moderators, including pre‐intervention distress levels and context‐dependent factors such as emotional support. Further studies of other approaches, for example, by instructing participants to focus on benefit finding *51, multimodal interventions combining verbal and written ‘healthy expressions’ 69, or helping others *41 are also needed.
Conflict of interest
The authors have no conflicts of interest to disclose.
Supporting information
Supporting Info Item
Acknowledgements
The authors thank Mikael Jensen‐Johansen, PhD, for the assistance in identifying reviews and eligible studies.
Zachariae, R. , and O'Toole, M. S. (2015) The effect of expressive writing intervention on psychological and physical health outcomes in cancer patients—a systematic review and meta‐analysis. Psycho‐Oncology, 24: 1349–1359. doi: 10.1002/pon.3802.
The copyright line for this article was changed on 9 February 2016 after original online publication.
References
* References included in the meta‐analysis.
- 1. Lepore SJ. A social‐cognitive processing model of emotional adjustment to cancer In Psychosocial interventions for cancer, Baum A, Andersen BL. (eds.), American Psychological Association: Washington D.C., 2001;99–116. [Google Scholar]
- 2. Koopman C, Butler LD, Classen C, et al Traumatic stress symptoms among women with recently diagnosed primary breast cancer. J Trauma Stress 2002;15(4):277–287. [DOI] [PubMed] [Google Scholar]
- 3. Gallagher J, Parle M, Cairns D. Appraisal and psychological distress six months after diagnosis of breast cancer. Br J Health Psychol 2002;7(Part 3):365–376. [DOI] [PubMed] [Google Scholar]
- 4. Christensen S, Zachariae R, Jensen AB, et al Prevalence and risk of depressive symptoms 3–4 months post‐surgery in a nationwide cohort study of Danish women treated for early stage breast‐cancer. Breast Cancer Res Treat 2009;113(2):339–355. [DOI] [PubMed] [Google Scholar]
- 5. O'Connor M, Christensen S, Jensen AB, et al How traumatic is breast cancer? Post‐traumatic stress symptoms (PTSS) and risk factors for severe PTSS at 3 and 15 months after surgery in a nationwide cohort of Danish women treated for primary breast cancer. Br J Cancer 2011;104(3):419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lepore SJ, Helgeson VS. Social constraints, intrusive thoughts, and mental health after prostate cancer. Journal of Social & Clinical Psychology 1998;17(1):89–106. [Google Scholar]
- 7. Stanton AL, Danoff‐Burg S, Cameron CL, et al Emotionally expressive coping predicts psychological and physical adjustment to breast cancer. J Consult Clin Psychol 2000;68(5):875–882. [PubMed] [Google Scholar]
- 8. Hack TF, Degner LF. Coping responses following breast cancer diagnosis predict psychological adjustment three years later. Psycho‐Oncology 2004;13(4):235–247. [DOI] [PubMed] [Google Scholar]
- 9. Manne SL, Rubin S, Edelson M, et al Coping and communication‐enhancing intervention versus supportive counseling for women diagnosed with gynecological cancers. J Consult Clin Psychol 2007;75(4):615–628. [DOI] [PubMed] [Google Scholar]
- 10. Epping‐Jordan JE, Compas BE, Howell DC. Predictors of cancer progression in young adult men and women: avoidance, intrusive thoughts, and psychological symptoms. Health Psychol 1994;13:539–547. [DOI] [PubMed] [Google Scholar]
- 11. Butow PN, Brown JE, Coates AS, et al Psychosocial predictors of outcome IV: patients with early‐stage breast cancer. Breast 2001;10:182–189. [Google Scholar]
- 12. Giese‐Davis J, DiMiceli S, Sephton S, et al Emotional expression and diurnal cortisol slope in women with metastatic breast cancer in supportive‐expressive group therapy: a preliminary study. Biol Psychol 2006;73(2):190–198. [DOI] [PubMed] [Google Scholar]
- 13. Stiles WB. Disclosure as a speech act: is it psychotherapeutic to disclose? In Emotion, Disclosure, and Health, Pennebaker JW. (ed.), American Psychological Association:: Washington D.C., 1995;71–91. [Google Scholar]
- 14. Smyth J, Helm R. Focused expressive writing as self‐help for stress and trauma. J Clin Psychol 2003;59(2):227–235. [DOI] [PubMed] [Google Scholar]
- 15. Kissane DW, Grabsch B, Clarke DM, et al Supportive‐expressive group therapy for women with metastatic breast cancer: survival and psychosocial outcome from a randomized controlled trial. Psycho‐Oncology 2007;16(4):277–286. [DOI] [PubMed] [Google Scholar]
- 16. Butler LD, Koopman C, Neri E, et al Effects of supportive‐expressive group therapy on pain in women with metastatic breast cancer. Health Psychol 2009;28(5):579–587. [DOI] [PubMed] [Google Scholar]
- 17. Zachariae R, Jensen‐Johansen MB. Written emotional disclosure In Handbook of Psychotherapy in Cancer Care,Watson M, Kissane D (eds), Wiley‐Blackwell: New York, 2011; pp 89–103 [Google Scholar]
- 18. Pennebaker JW, Beall SK. Confronting a traumatic event: toward an understanding of inhibition and disease. J Abnorm Psychol 1986;95(3):274–281. [DOI] [PubMed] [Google Scholar]
- 19. Francis ME, Pennebaker JW. Putting stress into words: the impact of writing on physiological, absentee, and self‐reported emotional well‐being measures. Am J Health Promot 1992;6(4):280–287. [DOI] [PubMed] [Google Scholar]
- 20. Pennebaker JW, Francis ME. Cognitive, emotional, and language processes in disclosure. Cognition & Emotion 1996;10:601–626. [Google Scholar]
- 21. Richards JM, Beal WE, Seagal JD, et al Effects of disclosure of traumatic events on illness behavior among psychiatric prison inmates. J Abnorm Psychol 2000;109(1):156–160. [DOI] [PubMed] [Google Scholar]
- 22. Petrie KJ, Booth RJ, Pennebaker JW, et al Disclosure of trauma and immune response to a hepatitis B vaccination program. J Consult Clin Psychol 1995;63:787–792. [DOI] [PubMed] [Google Scholar]
- 23. Esterling BA, Antoni MH, Fletcher MA, et al Emotional disclosure through writing or speaking modulates latent Epstein‐Barr virus antibody titers. J Consult Clin Psychol 1994;62(1):130–140. [DOI] [PubMed] [Google Scholar]
- 24. Smyth JM. Written emotional expression: effect sizes, outcome types, and moderating variables. Journal of Consulting & Clinical Psychology 1998;66(1):174–184. [DOI] [PubMed] [Google Scholar]
- 25. Frisina PG, Borod JC, Lepore SJ. A meta‐analysis of the effects of written emotional disclosure on the health outcomes of clinical populations. J Nerv Ment Dis 2004;192(9):629–634. [DOI] [PubMed] [Google Scholar]
- 26. Frattaroli J. Experimental disclosure and its moderators: a meta‐analysis. Psychol Bull 2006;132(6):823–865. [DOI] [PubMed] [Google Scholar]
- 27. Merz EL, Fox RS, Malcarne VL. Expressive writing interventions in cancer patients: a systematic review. Health Psychology Review 2014;8(3):339–361. [DOI] [PubMed] [Google Scholar]
- *28. Zakowski SG, Ramati A, Morton C, et al Written emotional disclosure buffers the effects of social constraints on distress among cancer patients. Health Psychol 2004;23(6):555–563. [DOI] [PubMed] [Google Scholar]
- 29. Liberati A, Altman DG, Tetzlaff J, et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sackett DL, Richardson WS, Rosenberg W, et al Evidence‐based Medicine: How to Practice and Teach EBM, Churchill Livingstone: New York, 1997. [Google Scholar]
- 31. Jadad AR, Moore RA, Carroll D, et al Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 32. Higgins JP, Altman DG, Gotzsche PC, et al The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greenland S, O'Rourke K. On the bias produced by quality scores in meta‐analysis, and a hierarchical view of proposed solutions. Biostatistics 2001;2(4):463–471. [DOI] [PubMed] [Google Scholar]
- 34. Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sterne JAC, Egger M, Moher D. Addressing reporting biases In Cochrane Handbook for Systematic Reviews of Intervention, Higgins JPT , Green S. (eds.), Wiley‐Blackwell: Chichester, 2008;297–333. [Google Scholar]
- 36. Poole C, Greenland S. Random‐effects meta‐analyses are not always conservative. Am J Epidemiol 1999;150(5):469–475. [DOI] [PubMed] [Google Scholar]
- 37. Higgins JP, Thompson SG, Deeks JJ, et al Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cohen J. Statistical Power Analysis for the Behavioral Sciences, Lawrence Erlbaum Associates: Hillsdale, N.J., 1988. [Google Scholar]
- 39. Hedges L, Olkin I. Statistical Methods for Meta‐analysis, Academic Press: New York, 1985. [Google Scholar]
- *40. Craft MA, Davis GC, Paulson RM . Expressive writing in early breast cancer survivors. J Adv Nurs 2013;69(2):305–315. [DOI] [PubMed] [Google Scholar]
- *41. Rini C, Austin J, Wu LM, et al Harnessing benefits of helping others: a randomized controlled trial testing expressive helping to address survivorship problems after hematopoietic stem cell transplant. Health Psychol 2014;33(12):1541–1551. [DOI] [PubMed] [Google Scholar]
- 42. Hedges LV, Pigott TD. The power of statistical tests in meta‐analysis. Psychol Methods 2001;6(3):203–217. [PubMed] [Google Scholar]
- 43. Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. CMAJ 2007;176(8):1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Egger M, Davey SG, Schneider M, et al Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 46. Rosenthal R. The “file‐drawer problem” and tolerance for null results. Psychol Bull 1979;86:638–641. [Google Scholar]
- 47. Copas J, Shi JQ. Meta‐analysis, funnel plots and sensitivity analysis. Biostatistics 2000;1(3):247–262. [DOI] [PubMed] [Google Scholar]
- *48. Walker BL, Nail LM, Croyle RT. Does emotional expression make a difference in reactions to breast cancer? Oncol Nurs Forum 1999;26(6):1025–1032. [PubMed] [Google Scholar]
- *49. de Moor C, Sterner J, Hall M, et al A pilot study of the effects of expressive writing on psychological and behavioral adjustment in patients enrolled in a Phase II trial of vaccine therapy for metastatic renal cell carcinoma. Health Psychol 2002;21(6):615–619. [DOI] [PubMed] [Google Scholar]
- *50. Rosenberg HJ, Rosenberg SD, Ernstoff MS, et al Expressive disclosure and health outcomes in a prostate cancer population. Int J Psychiatry Med 2002;32(1):37–53. [DOI] [PubMed] [Google Scholar]
- *51. Stanton AL, Danoff‐Burg S, Sworowski LA, et al Randomized, controlled trial of written emotional expression and benefit finding in breast cancer patients. J Clin Oncol 2002;20(20):4160–4168. [DOI] [PubMed] [Google Scholar]
- *52. Cepeda MS, Chapman CR, Miranda N, et al Emotional disclosure through patient narrative may improve pain and well‐being: results of a randomized controlled trial in patients with cancer pain. J Pain Symptom Manage 2008;35(6):623–631. [DOI] [PubMed] [Google Scholar]
- *53. de Moor JS, Moye L, Low MD, et al Expressive writing as a presurgical stress management intervention for breast cancer patients. J Soc Integr Oncol 2008;6(2):59–66. [PubMed] [Google Scholar]
- *54. Gellaitry G, Peters K, Bloomfield D, et al Narrowing the gap: the effects of an expressive writing intervention on perceptions of actual and ideal emotional support in women who have completed treatment for early stage breast cancer. Psycho‐Oncology 2010;19(1):77–84. [DOI] [PubMed] [Google Scholar]
- *55. Low CA, Stanton AL, Bower JE, et al A randomized controlled trial of emotionally expressive writing for women with metastatic breast cancer. Health Psychol 2010;29(4):460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *56. Mosher CE, DuHamel KN, Lam J, et al Randomised trial of expressive writing for distressed metastatic breast cancer patients. Psychol Health 2012;27(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arden‐Close E, Gidron Y, Bayne L, et al Written emotional disclosure for women with ovarian cancer and their partners: randomised controlled trial. Psycho‐Oncology 2013;22:2262–2269. [DOI] [PubMed] [Google Scholar]
- *58. Jensen‐Johansen MB, Christensen S, Valdimarsdottir H, et al Effects of an expressive writing intervention on cancer‐related distress in Danish breast cancer survivors ‐ results from a nationwide randomized clinical trial. Psycho‐Oncology 2013;22(7):1492–1500. [DOI] [PubMed] [Google Scholar]
- *59. Milbury K, Spelman A, Wood C, et al Randomized controlled trial of expressive writing for patients with renal cell carcinoma. J Clin Oncol 2014;32(7):663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *60. Lepore SJ, Revenson TA, Roberts KJ, et al Randomised controlled trial of expressive writing and quality of life in men and women treated for colon or rectal cancer. Psychol Health 2014;1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lepore SJ, Silver RC, Wortman CB, et al Social constraints, intrusive thoughts, and depressive symptoms among bereaved mothers. J Pers Soc Psychol 1996;70(2):271–282. [DOI] [PubMed] [Google Scholar]
- 62. Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioral treatment confirmation from meta‐analysis. Am Psychol 1993;48(12):1181–1209. [DOI] [PubMed] [Google Scholar]
- 63. Butler AC, Chapman JE, Forman EM, et al The empirical status of cognitive‐behavioral therapy: a review of meta‐analyses. Clin Psychol Rev 2006;26(1):17–31. [DOI] [PubMed] [Google Scholar]
- 64. Juni P, Witschi A, Bloch R, et al The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282(11):1054–1060. [DOI] [PubMed] [Google Scholar]
- 65. Stanton AL, Danoff‐Burg S. Emotional expression, expressive writing, and cancer In The Writing Cure: How Expressive Writing Promotes Health and Emotional Well‐being, Lepore SJ , Smyth JM. (eds.), American Psychological Association: Washington, DC., 2002;31–52. [Google Scholar]
- 66. Smyth JM. Written disclosure: evidence, potential mechanism, and potential treatment. Adv Mind Body Med 1999;15(3):179–184. [DOI] [PubMed] [Google Scholar]
- 67. Bonanno GA, Papa A, Lalande K, et al The importance of being flexible: the ability to both enhance and suppress emotional expression predicts long‐term adjustment. Psychol Sci 2004;15(7):482–487. [DOI] [PubMed] [Google Scholar]
- 68. Sheppes G, Scheibe S, Suri G, et al Emotion‐regulation choice. Psychol Sci 2011;22(11):1391–1396. [DOI] [PubMed] [Google Scholar]
- 69. Carmack CL, Basen‐Engquist K, Yuan Y, et al Feasibility of an expressive‐disclosure group intervention for post‐treatment colorectal cancer patients: results of the healthy expressions study. Cancer 2011;117(21):4993–5002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Info Item


