Summary
Bovine tuberculosis (BTB), caused by Mycobacterium bovis, has an annual incidence in cattle of 0.5% in the Republic of Ireland and 4.7% in the UK, despite long‐standing eradication programmes being in place. Failure to achieve complete eradication is multifactorial, but the limitations of diagnostic tests are significant complicating factors. Previously, we have demonstrated that Fasciola hepatica infection, highly prevalent in these areas, induced reduced sensitivity of the standard diagnostic tests for BTB in animals co‐infected with F. hepatica and M. bovis. This was accompanied by a reduced M. bovis‐specific Th1 immune response. We hypothesized that these changes in co‐infected animals would be accompanied by enhanced growth of M. bovis. However, we show here that mycobacterial burden in cattle is reduced in animals co‐infected with F. hepatica. Furthermore, we demonstrate a lower mycobacterial recovery and uptake in blood monocyte‐derived macrophages (MDM) from F. hepatica‐infected cattle which is associated with suppression of pro‐inflammatory cytokines and a switch to alternative activation of macrophages. However, the cell surface expression of TLR2 and CD14 in MDM from F. hepatica‐infected cattle is increased. These findings reflecting the bystander effect of helminth‐induced downregulation of pro‐inflammatory responses provide insights to understand host‐pathogen interactions in co‐infection.
Keywords: co‐infection, Fasciola hepatica, monocyte‐derived macrophages, Mycobacterium bovis, uptake
Introduction
The control of infectious diseases within populations, whether through vaccination programmes and/or identification and removal of infected individuals, is dependent on immune responsiveness to the pathogens concerned. Immune responsiveness to a particular pathogen within a population is subject to modulation by a multitude of factors including host genetics 1, 2, 3, reproductive cycles and the presence of co‐infections 4. Such factors are easily controlled in experimental studies, but they may have a significant impact in the real world of naturally occurring infection 5, 6, 7, 8. Parasitic helminths, ubiquitous in grazing livestock and common in humans in many parts of the world, typically use immunoregulatory strategies to achieve persistent infections, while reducing inflammatory pathology in the host 9, 10. They also have the potential, through bystander immunoregulation, to alter the pathology and course of other infections in the same host 11, 12, 13, 14, 15, 16. Here, we model some aspects of the interaction between a helminth and a bacterial pathogen, both of which cause economically important diseases of livestock.
The liver fluke, Fasciola hepatica, is a highly prevalent helminth parasite of farmed ruminants 17, estimated to cause annual losses of about €2.5 billion annually to the livestock industry worldwide. F. hepatica also affects a wide range of other mammals, including humans, with approximately 170 million people at risk of infection 18. Mycobacterium bovis, the causative agent of bovine tuberculosis (BTB), also has zoonotic implications; as an estimate, 3.1% of human tuberculosis cases globally are caused by M. bovis 19. In some developed countries, eradication programmes have reduced or eliminated BTB. However, reservoirs of M. bovis in wildlife 20, 21, 22, together with the limitations of diagnostic tests 23, have made complete eradication difficult in others 24.
In previous studies, we have shown that two diagnostic tests used in BTB eradication schemes, the single intradermal comparative cervical tuberculin test (SICCT) and the in vitro interferon (IFN)‐γ assay, were between 50% and 80% less effective in detecting M. bovis‐infected cattle when the animals were co‐infected with F. hepatica 25, 26, leading to a lower positive predictive value of the tests. This was also shown in a large‐scale epidemiological study in England and Wales, where dairy herds with a high prevalence of F. hepatica infection had a lower number of BTB reactors 6. This reduced sensitivity of the diagnostic tests was hypothesized to be due to an immunosuppression induced by F. hepatica infection. A downregulation of the Th1 immune response in other co‐infection models has been associated with an increase in bacterial loads 14, 15, 16, 27. Thus, we proposed that these changes in F. hepatica‐infected animals might also result in an increase in the M. bovis numbers in co‐infected animals.
Previously, it has been shown that CD14 played a major role in phagocytosis of M. bovis BCG by macrophages, and optimal uptake required the presence of both CD14 and complement receptor 3 (CR3). In addition, co‐expression of CD14 and TLR2 markedly enhanced uptake of BCG 28. Other studies have shown the importance of these receptors in mycobacterial phagocytosis 29, 30 and immune responsiveness 31, 32. Thus, given that expression of these receptors might vary after infection with helminths 33, we proposed that TLR2 and CD14 could be modified in macrophages from F. hepatica‐infected cattle, leading to alterations in mycobacterial uptake and recovery from such macrophages. Next, we investigated the cytokine profile and the activation of macrophages in vitro after F. hepatica infection of cattle. Alternatively activated macrophages, known to develop in F. hepatica infections 34, 35, are not efficient at bacterial phagocytosis 36. In addition, these alternatively activated macrophages are characterized by the production of Th2 and Treg cytokines and a reduced production of Th1 cytokines. Some of these pro‐inflammatory cytokines such as IL‐12, IL‐1β and IL‐6 directly affect IFN‐γ production 37, 38, 39 and the immune response in the SICCT 40, the two standard tests for the diagnosis of BTB in Europe. Thus, we hypothesized that an alteration in the pro‐inflammatory cytokine profile in MDM from F. hepatica‐infected cattle could relate to a modified bacterial uptake as well as explaining the lower response to the diagnostic tests for BTB in animals co‐infected with M. bovis and F. hepatica.
Materials and Methods
Experimental design
For the co‐infection experiments (experiments 1 and 2), male castrated Holstein–Friesian cattle aged between 3 and 7 weeks of age (12 animals for each experiment) were purchased from farms known to be TB‐free, as determined by the official tuberculosis eradication programme in the UK, and where fasciolosis had not been reported. Animals were housed in Containment Level 3 (CL3) facilities at AFBI (Stormont, Belfast, UK) as described by Flynn et al. 26. Animals were then randomly divided into two groups and assigned to M. bovis‐only‐infected group (n = 6) or the F. hepatica–M. bovis‐infected group (n = 6), in order to compare mycobacterial burden. Blood was collected weekly to measure IFN‐γ production until week 14 (Experiment 1) and week 22 (Experiment 2).
For the in vitro experiment (Experiment 3), six male castrated Holstein–Friesian calves aged between 6 and 8 months of age were purchased from herds free of BTB and where F. hepatica infection was not reported. Animals were maintained under uniform housing conditions at University College Dublin (UCD) Lyons Research Farm (Newcastle, County Kildare, Ireland). All six animals were infected with F. hepatica, and mycobacterial recovery and uptake, TLR2 and CD14 receptor expression and cytokine production from MDM, compared prior to and after F. hepatica infection.
Experiments 1 and 2 were approved/licensed by the Animal Scientific Procedures Act 1986 (DHSSPS) under the scrutiny of the Animal Welfare and Ethical Review Body (AWERB), PPL2728, Agri‐Food and Biosciences Institute, Belfast, UK. Experiment 3 was approved/licensed by the UCD Animal Research Ethics Committee/Health Products Regulatory Agency (AE18982/P023), University College Dublin, Ireland.
Experimental F. hepatica infection of cattle
In all experiments, to ensure that animals were free from F. hepatica infection before starting the study, animals were serologically screened by ELISA using recombinant mutant F. hepatica cathepsin L1 (rmFhCL1), as previously described 35, and by faecal egg examination. For F. hepatica infection, 150 F. hepatica metacercariae (Baldwin Aquatics, (Oregon)), dispersed in 10 mL of dH2O, were administered orally via a syringe onto the back of the tongue. For confirmation of infection, numbers of parasites in the liver of each animal in Experiment 1 26 and Experiment 2 (Table S1) were recorded. In addition, specific antibody responses to F. hepatica infection were measured and no effect of M. bovis infection on such antibody levels to F. hepatica was observed in Experiment 1 26 or Experiment 2 (data not shown). For Experiment 3, post‐mortem examinations were not carried out, but seroconversion for F. hepatica infection using recombinant cathepsin‐like 1 (rmFhCL1) by ELISA (Figure S1) was monitored as previously described 35.
Bacterial strains and bacterial stocks
In all three experiments, Mycobacterium bovis AF2122/97, a UK isolate, was used. For Experiment 3, in addition, the nonvirulent M. bovis BCG‐GFP Pasteur was used. All the work involving virulent M. bovis was performed in a Biosafety Containment Level 3 (CL3) laboratory and conformed to guidelines on the use of Hazard Group 3 infectious organisms.
M. bovis was cultured in Middlebrook 7H9 medium (DifcoTM, Becton Dickinson) containing 10% Middlebrook albumin–dextrose–catalase (ADC) enrichment 5% BSA (Fisher Chemical), 111 mm glucose (GPR), 145.4 mm NaCl (Fluka BioChemika)) and 10 mm sodium pyruvate (Sigma–Aldrich) at 37°C. Bacterial cultures were grown to logarithmic phase (O.D = 1). To achieve this, 1 mL of bacterial frozen aliquot was diluted in 4 mL of Middlebrook 7H9 medium in a 50‐mL Falcon tube and incubated at 37°C in static conditions for 5 days. Then, cultures were scaled up by adding the 5 mL of bacterial culture to 45 mL of Middlebrook 7H9 medium into an Erlenmeyer flask (Corning) and incubated at 37°C for further 2 weeks.
Experimental M. bovis infection of cattle
For co‐infection experiments (experiments 1 and 2), all animals were prescreened for immunological responses to the M. bovis antigens PPDA, PPDB, ESAT‐6 and CFP‐10 to ensure that there was no pre‐existing exposure. All animals were housed in Containment Level 3 facilities under negative air pressure at AFBI Veterinary Sciences Division, Stormont. At 4 weeks post‐F. hepatica infection, animals were exposed to low‐dose aerosol challenge with M. bovis using a Madison chamber modified for use with cattle as previously described 41. The intended target infection dose was 5 × 102 colony‐forming units (CFU) of M. bovis strain AF2122/99, which has been demonstrated to be infective in animals while mimicking the natural infection 42. Actual doses delivered were calculated by side‐flow sampling from the Madison chambers, and there were no statistically significant differences between delivered doses between groups (See Table S2 of Supporting Information).
IFN‐γ test
For the co‐infection experiments (experiments 1 and 2), IFN‐γ production was measured in whole blood cultures after stimulation with PPDB‐PPDA and ESAT‐6 antigens, using the Bovigam Kit as per manufacturer's instructions.
Post‐mortem examination
Fourteen (Experiment 1) or 22 (Experiment 2) weeks after F. hepatica infection, the animals were euthanized and samples from each lung lobe, right and left bronchial lymph nodes, along with hepatic and cervical lymph nodes, were collected. Lungs were palpated by hand and then sliced at 1‐cm intervals to reveal any tuberculous tissue. Each tissue sample was dissected carefully to reveal lesions indicative of tuberculosis, which were counted and measured. Lesions or suspect lesions, together with the other tissues, were sliced into cubes measuring approximately 5 mm in diameter and sent for bacteriology for preparation of cultures (see details below) and for histology processing as previously described 43, 44.
For confirmation of F. hepatica infection, each liver was carefully sliced at 1‐cm intervals. The bile ducts were squeezed to extrude any contained fluke. After dissection, the cubes of liver tissue were placed inside two layers of muslin cloth, soaked in water and squeezed several times. The inside of the cloth was then rinsed in water to remove flukes. All flukes were fixed in formaldehyde for microscopic verification of fluke morphology and counting.
Bacterial recovery from tissues
For experiments 1 and 2, the cubed tissue samples were placed into double‐thickness stomacher bags with between 4 and 15 mL PBS and homogenized in a stomacher at maximum speed for 2 min. Tissue homogenates were decanted into sterile universal bottles for inoculation into media for qualitative and quantitative cultures.
Qualitative culture
For qualitative culture, tissue homogenates were inoculated into the BACTEC MGIT 960 culture system and incubated for up to 56 days. Samples identified as positive by the BACTEC MGIT 960 system were stained using the Ziehl–Neelsen method for confirmation purposes. Samples containing typical acid‐fast rods were presumed to be positive for M. bovis and analysed further by VNTR to identify the infection strain (AF2122/97).
Quantitative culture
For quantitative culture, tissue homogenates were decontaminated in 0.075% hexadecylpyridinium chloride (HPC). Hundred microlitres of this was serially diluted and inoculated in quadruplicate onto 7H11‐OADC agar plates and incubated at 37°C for up to 6 weeks. M. bovis colonies were counted weekly from 3 weeks onwards and the average colony counts at 6 weeks post‐inoculation used to calculate the total number of CFU per gram of sample. M. bovis colonies were initially identified on the basis of colony morphology and a selection of colonies was prepared for VNTR typing to confirm isolation of AF2122/97.
Isolation of monocyte‐derived macrophages (MDM)
For Experiment 3, 300 mL of blood was collected per each of the 6 animals, into lithium heparin vacutainers (Cruinn Diagnostics), pre‐F. hepatica infection and at 4, 8 and 10 weeks post‐F. hepatica infection. Then, peripheral blood mononuclear cells (PBMC) were isolated by the density gradient centrifugation method using Histopaque (Sigma–Aldrich). Next, anti‐CD14+ microbeads (Miltenyi Biotec) were used to isolate monocytes as per manufacturer's instructions (detailed protocol in Supporting Information Page 17). Cell viability was checked with trypan blue (Sigma–Aldrich), and selected CD14+ cells were adjusted to 1 × 106 cells/mL in complete medium (RPMI‐1640 [Bio‐Sciences], 1% glutamine [Sigma–Aldrich], 1% nonessential amino acids [Sigma–Aldrich], 15% heat‐inactivated foetal calf serum [Bio‐Sciences], 1% penicillin–streptomycin [Sigma–Aldrich]) and plated in 12‐, 24‐ or 96‐well plates (Greiner), depending on the experiment.
MDM culture, stimulation and infection
For Experiment 3, plates were incubated for 7 days at 37°C, 5% CO2 to allow the maturation of monocytes into macrophages as previously described 45. The culture medium was changed every 2–3 days. From Day 3 onwards, antibiotic‐free medium was used. At Day 6, selected wells containing MDM were stimulated with F. hepatica antigen by adding 20 μg/mL of FhES. For FhES preparation, see Method S2 in Supporting Information. At Day 7, M. bovis or M. bovis BCG was added at a ratio of 1 : 1 45 to selected wells containing either FhES or PBS and then incubated for a further 24 h. PBS was used as a negative control.
For flow cytometry experiments, at Day 7 PPDB (1 μg/mL) (kindly provided by Prof. Eamon Gormley, UCD) was added to selected wells, TLR2 as a positive control 46. In all the cases, at least 3 wells were used for each in vitro condition, with 3 unstimulated controls (PBS), 3 mycobacteria, 3 mycobacteria plus FhES and 3 FhES wells, per animal.
Confirmation of bacterial infection of MDM
Ziehl–Neelsen staining was used to confirm intracellular macrophage infection with M. bovis or M. bovis BCG (see Method S2 and Figure S2 in Supporting Information).
Bacterial recovery from MDM (CFU)
In Experiment 3, for bacterial recovery from MDM, mycobacterial colony‐forming units (CFU) were calculated. When culturing M. bovis, agar plates were made using Middlebrook 7H11 medium (DifcoTM, Becton Dickinson) containing 10 mm of sodium pyruvate (Sigma–Aldrich) and 10% ADC. For BCG‐GFP culture in agar, glycerol 1% (Sigma) was added instead of sodium pyruvate.
After removal of medium and extracellular bacteria from the 96‐well plates, cells were lysed by the addition of 65 μL of 0.1% Triton X‐100 (Sigma–Aldrich) for 5 min. Next, the lysates were serially diluted in Middlebrook 7H9 medium at concentrations from 10−2 to 10−4 before plating out in triplicate on agar plates. In parallel, 3 wells for each condition and animal were used to count the number of total macrophages in each well. After incubation of agar plates for 4 weeks, colony counts were performed and colony‐forming units (CFU)/macrophage were calculated using the formula:
CFU per macrophage = average of CFU in 3 agar plates × 200 μL (total volume of the dilution)/50 μL (volume used to plate out bacteria) × 103 (dilution factor) × 65 μL (volume of lysis buffer added to the well)/20 μL (volume used to do the dilution)/number of macrophages in a well.
Bacterial uptake and receptor expression in MDM by flow cytometry
Quality control measures
In Experiment 3, titration of antibodies for flow cytometry was carried out by performing serial dilutions (1/20 to 1/10 000) and selecting the optimal concentration in pilot experiments. Instrument quality control was carried out using Cytometer Setup and Tracking Beads (BD CS&T beads), which were also used to standardize fluorescence across samples acquired on different days. Final voltage settings were set using single‐stained cell controls. For the gating strategy and confirmation of consistency of the results, fluorescence minus one controls (FMO) (cells containing all fluorochromes except one) were used. Unstained samples, as well as stained controls noninfected and nonstimulated for each animal and each time point, were included.
Harvesting of macrophages and viability staining
At Day 8 post‐MDM culture (24 h post‐BCG‐GFP stimulation), medium and extracellular bacteria were washed off and macrophages were harvested by incubating cells for 10 min at 37°C with Accutase® (Sigma). For the single positive control for viability dye, a mixture of dead/alive cells was prepared, as per manufacturer's instructions. Next, 50 μL of previously titrated viability dye (Fixable Viability Dye eFluor® 780, eBiosciences) (1/1000 in D‐PBS) was added to 50 μL of D‐PBS containing the cells. Then, tubes were incubated for 30 min at 2–8°C in the dark. After this, the excess dye was washed off (D‐PBS [cell culture grade, Gibco], 3% heat‐inactivated foetal calf serum [Gibco] and 0.09% sodium azide [Sigma–Aldrich], pH 7.5) and cell pellets were incubated in 50 μL of blocking buffer (0.05 m Tris‐buffered saline, pH 7.4, containing 1% normal bovine serum, 0.1% azide [Sigma–Aldrich] and 2% heat‐inactivated foetal calf serum [Gibco]) for 20 min at 4°C.
Antibody staining and cell fixation
TLR2 antibody CD282, a bivalent human recombinant Fab antibovine antibody (AF 647 CD282 (AbD Serotec)), was prepared at 1/10 dilution in staining buffer. CD14 antibody, a mouse anti‐human antibody cross‐reactive with bovine (PerCP‐Cy5.5 CD14 (BioLegend)), was diluted 1/25 in staining buffer. Twenty‐five microlitres from each diluted antibody was added to 50 μL of blocking buffer containing cells, to have a final concentration of 1/40 for TLR2 and 1/100 for CD14 antibody. For detailed antibody description, see Table S5 included in Document S1 of Supporting Information. Then, tubes were incubated at 4°C for 1 h. After washing cell pellets, those were fixed in 100 μL of 1% paraformaldehyde in D‐PBS for 1 h. Then, 550 μL of staining buffer was added and tubes were left overnight at 4°C until flow cytometry processing of samples.
Instrument and data analysis details
A Beckman Coulter model Cyan ADP Lx flow cytometry machine was used. The 488‐nm laser was selected for FL1 (530/40‐GFP) and FL4 (680/30 – PerCP‐Cy5.5). The 635‐nm laser was used for FL8 (665/20 – Alexa Fluor 647) and FL9 (750LP – eFluor 780).
Data were analysed with Beckman Coulter Summit V4.03.02 Build 2451 software, and re‐analysis was carried out with Summit and De Novo Software FCS Express 4 RUO. Data files were kept as FCS data files, compensation was not required, and the gating applied was carried out by including FMO controls which were the same cells as used in the experiment. Gating was applied by selecting target cells based on FSC vs. SSC plot, pulse width vs. FSC area, SSC line vs. SSC area and LD (live death cells) vs. FSC (Figure S3). The purity of monocyte‐derived macrophages was 99% as indicated by CD14 labelling. The median of fluorescence intensities of each sample obtained was normalized to its own unstimulated control, resulting in a fold change value, so that BCG‐stimulated samples were comparable with Pre‐F.hep and Post‐F.hep. For further information, see MIFlowCyt Standard in Document S1.
mRNA analysis of MDM
mRNA extraction and cDNA transcription
In Experiment 3, for RNA extraction, the EZNA Total RNA Kit 1 (OMEGA Bio‐Tek) was used as per manufacturer's instructions. For reverse transcription into cDNA, 500 ng of RNA was added to 12 μL of RNase‐free water (Sigma–Aldrich), containing 1 μL of oligo DT (0.5 μg/μL) and 1 μL of 10 mm dNTP mix (Promega). A master mix containing 1 μL of Moloney murine leukaemia virus reverse transcriptase (MMLV‐RT) (Biosciences), 4 μL of first‐strand buffer (5×), 2 μL of 0.1 m DTT and 1 μL of RNase OUT (40 U/μL) (Biosciences), per sample, was prepared. Nonenzyme controls (no MMLV) and a nontemplate control (RNase‐free water) were also prepared. Once samples were incubated at 65°C for 5 min and at 4°C for 2 min, 8 μL of the master mix per vial was added. Samples were then incubated at 37°C for 50 min followed by 15 min at 70°C, and from then until the cDNA was fully transcribed, kept at 4°C. cDNA samples were stored at −20°C until further processing.
qPCR
For real‐time qPCR, all samples and controls were included in duplicate. Nontemplate control and nonenzyme control cDNA template were included for each set of primers. Five nanograms of cDNA from each sample was added to the qPCR plate containing 10 μL of SYBR Green PCR (2×) (Applied Biosystems), 0.3 μL of the forward primer and 0.3 μL of the reverse primer, and these were adjusted to a final volume of 20 μL with RNase‐free water. A standard qPCR program was used to run the reactions: 95°C for 1 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. A dissociation step was included for all reactions to confirm the presence of a single PCR product of the expected size. Reference genes were established by carrying out a GeneNorm analysis of 10 potential candidates for internal controls. Reference target stability was assessed across a set of 12 samples selected at random. Results were analysed using the GeNorm qbasePLUS software package (Biogazelle NV, Belgium) as described by Hellemans et al. 47. Analysis determined peptidylprolyl isomerase A (PPIA) to be the most stable target gene for our samples with an M (gene expression stability value) <0.02, and PPIA was included as internal control on all plates analysed. Intron‐spanning primer was designed for arginase I, iNOS, IL‐6 and TGF‐β using the Primer3Plus package and synthesized commercially (Eurofins MWG Operon, Ebersberg, Germany) 48:
(Arg1_fwd_180:5′‐CCAAGGTGTGTGGGAAAAGC‐3′;
Arg1_rev_180: 5′‐GATGTCCGTGTGAGCATCCA‐3′),
(iNOS_fwd: 5′‐AGAGACGGGGAGATCGGAAA‐3′;
iNOS_rev: 5′‐CATGCAGAGAACCTTGGGGT‐3′),
(IL‐6_fwd: 5′‐ACGAAAGAGAGCTCCATCTGC‐3′;
IL‐6_rev: 5′‐AATGGAGTGAAGGCGCTTGT‐3′),
(TGF‐β_fwd: 5′‐ACAATTCCTGGCGCTACCTC‐3′;
TGF‐β_rev: 5′‐ATTTCCTCTCTGCGGGTCAG‐3′)
Nitric oxide and cytokine production
In Experiment 3, nitric oxide (NO) production in MDM supernatants was measured using the Griess reagent system (Promega), according to the manufacturer's instructions. IL‐6, IL‐1β and TGF‐β were measured using the ELISA kits for Bovine IL‐6 (Cat: ESS0029, Thermo Scientific), IL‐1β (Cat: ESS0027, Thermo Scientific) and TGF‐β (Cat: G7590, Promega Corporation), according to the manufacturer's instructions. IL‐12 was measured using paired antibodies. A standard curve (Cat: RP0077B, Kingfisher Biotech) was included starting from 5000 ng/mL. The working volumes were 100 μL/well, washing steps were carried out with PBS‐Tween three washes, and incubation times were 1 h at room temperature unless otherwise indicated. The capture antibody for IL‐12 (MCA1782EL, AbD Serotec) was coated onto a 96‐well plate (8 μg/mL) and incubated at RT overnight. After this, plates were washed and blocked using 1% BSA (Sigma–Aldrich) in PBS‐T. After the addition of the samples and incubation, the detection antibody (MCA2173B, AbD Serotec) was added (5 μg/mL). For the next step, streptavidin‐HRP (1 : 5000 dilution) (Invitrogen) was added and incubated for 45 min and subsequently washed 5 times. TMB was used to develop the colour under dark conditions for 15 min. Finally, the reaction was stopped by the addition of 1 N H2SO4 (Fisher Chemical), and the absorbance was read at 450 nm.
Statistical analysis
For co‐infection studies (experiments 1 and 2), the response variable Y for each animal (CFU) was defined as Y = log (1 + total CFU) where total CFU denotes the sum of bacterial CFU recovered from the left bronchial lymph node, the right bronchial lymph node, the caudal mediastinal lymph node, the cranial mediastinal lymph node and the cervical lymph node. The treatment effect is the difference between the mean of Y in animals co‐infected vs. infected with M. bovis only. Data from experiments 1 and 2 were combined, and analysis was carried out by randomized block ANOVA, with ‘experiment’ as the blocking factor on two levels. Firstly, the hypothesis that fluke infection reduces mycobacterial load was tested. Secondly, we tested the hypothesis that in co‐infected animals, fluke count was negatively associated with intensity of M. bovis infection. A simple linear regression analysis adjusted for ‘experiment’ as a blocking factor on two levels was used to determine the association between fluke count and M. bovis load and the number of M. bovis‐positive tissues.
For Experiment 3, data were analysed using one‐way anova for comparison of more than 2 groups and t‐test for comparison of 2 groups, using the software GraphPad Prism 5. For qPCR data, relative gene expression fold changes for each sample were calculated according to calibrated normalized relative quantities (CNRQ) utilizing the qbasePLUS package (Eurofins MWG Operon).
Results
Effect of F. hepatica infection on bacterial load in cattle following aerosol challenge with M. bovis
For experiments 1 and 2, statistical analysis of bacterial burden revealed that although the numbers of bacteria recovered differed between the two experiments, there was no interaction between treatment (co‐infected or M. bovis only infected) and experiment (P = 0.657). Combining the results of both experiments, the total M. bovis bacterial load was significantly lower in co‐infected animals (P = 0.0031), allowing for a significant difference (P = 0.017) between experiments (Figure 1a). In Experiment 1, all animals were confirmed positive for M. bovis infection with either the qualitative or quantitative technique, with the exception of 1 animal in the co‐infected group 26. In Experiment 2, all animals were confirmed positive in at least one tissue. The number of culture‐positive tissues was lower in the co‐infected group; however, this was not statistically significant (Figure 1b). We also compared the number of liver flukes and bacterial load between animals; however, there was no correlation (data not shown).
Figure 1.
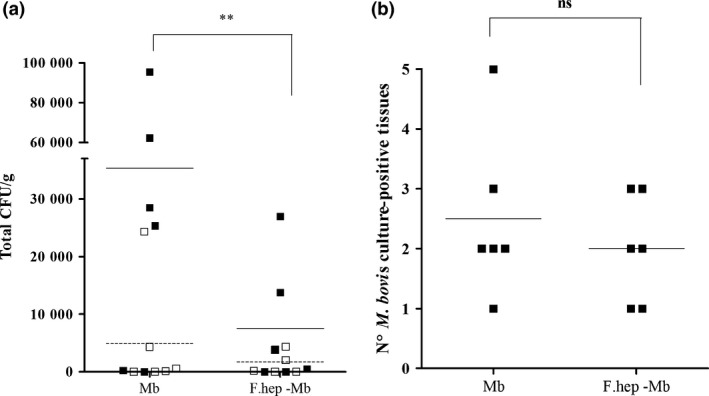
Effect of F. hepatica Infection on Bacterial Recovery in M. bovis‐infected Cattle. For experiments 1 and 2, one group of 6 animals in each was infected with 5 × 102 CFU M. bovis and the other 6 animals with 5 × 102 CFU M. bovis and 150 metacercariae from F. hepatica. Tissues were recovered, homogenates were cultured onto 7H11‐OADC agar plates, and CFU were counted per gram of tissue, to obtain the quantitative value. CFU were transformed to Y = (1 + total CFU). Data from experiments 1 and 2 were combined, and analysis was carried out by randomized block anova, with ‘experiment’ as the blocking factor on two levels. Co‐infected animals (F. hep‐Mb) showed a reduced total bacterial recovery in comparison with those infected with M. bovis only (Mb). ** means P = 0.0031 with a 95% confidence interval −9.083 ± 5.446 (a). For qualitative culture, tissue homogenates were cultured into the BACTEC MGIT 960 system, which gave a positive or negative result. In Experiment 1, all animals were culture positive with the exception of 1 in the M. bovis–F. hepatica group (Flynn et al. (2009)). In Experiment 2, all animals were confirmed infected with M. bovis with either the qualitative or quantitative technique (b). Co‐infected animals showed a reduction in the number of M. bovis culture‐positive tissues; however, the differences were not statistically significant (ns) (b). White or black squares correspond to each animal of Experiment 1 or Experiment 2, respectively. Dashed lines (Experiment 1) or black lines (Experiment 2) indicate the mean CFU per gram of tissue (a) or mean number of M. bovis culture‐positive tissues (b). See Table S3 and Table S4 for detailed information in the bacterial counts.
The distribution of infection identified through bacterial culture was similar in both the M. bovis and the co‐infected groups with the majority of bacteria recovered from the bronchial, mediastinal and cervical lymph nodes (Tables S3 and S4). A small number of additional isolates were recovered from lung tissues (Experiment 2) in which small (<1 cm in diameter) early‐stage lesions were visible projecting from pleural surfaces with central cores of caseous necrosis. Overall, no qualitative or quantitative differences were observed in tuberculous lesions between the M. bovis‐infected and F. hepatica–M. bovis‐infected animals. In both treatment groups, after microscopical examination, lesions exhibited varying degrees of central caseation necrosis with contained foci of dystrophic mineralization. Necrotic cores were bounded by layers of closely apposed macrophages, macrophage giant cells and interspersed lymphocytes. This layer in turn was surrounded by bands of fibrosis. While there was some minor variation in the extent of these lesion components, there was no major difference in these features between the two groups (data not shown).
Effect of F. hepatica infection on IFN‐γ production in animals co‐infected with M. bovis
In co‐infection experiments (experiments 1 and 2), IFN‐γ production was measured in whole blood cultures after stimulation in vitro with PPDB‐PPDA and ESAT‐6. In Experiment 2, both groups responded with distinct increases in IFN‐γ release after week 6 (2 weeks post‐M. bovis infection) in response to PPDB‐PPDA stimulation and these increases were sustained throughout the experimental period. In the group co‐infected with F. hepatica and M. bovis, there was a noticeably reduced IFN‐γ response being statistically significant at 7 and 20 weeks post‐infection (P < 0.05) (Figure 2a). Similarly, co‐infected animals showed a reduced IFN‐γ production in response to ESAT‐6 which was statistically significant at 7, 17 and 22 weeks post‐infection (P < 0.05) (Figure 2b). These results are similar to those of Flynn et al. 26 (Experiment 1).
Figure 2.
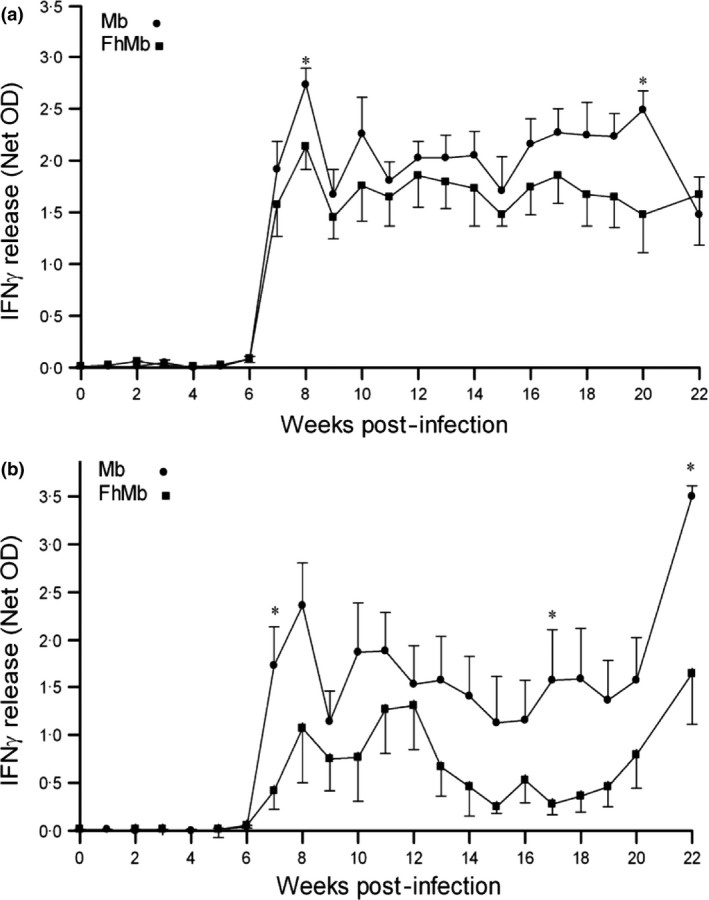
Effect of F. hepatica Infection on IFN‐γ production in M. bovis‐infected Cattle. For co‐infection experiments IFN‐γ release was measured from whole blood cultures following stimulation with PPDB‐PPDA or ESAT‐6. Results (Net OD) were calculated from PPDB minus PPBA, with positives indicated by the value for PPDB greater than 0.1 OD and PPDB‐PPDA greater or equal to 0.05 OD (a). In (b), Net OD was calculated from ESAT‐6 minus PBS, with positives indicated by a value >0.05 OD. Co‐infected animals with F. hepatica and M. bovis (squares) showed a reduced response to the mycobacterial antigens in comparison with only M. bovis‐infected animals (circles). * = P < 0.05.
The effect of F. hepatica infection on mycobacterial recovery in monocyte‐derived macrophages infected with M. bovis and M. bovis BCG in vitro
In parallel, we used an in vitro study (Experiment 3) to examine the effect of F. hepatica infection on bovine monocyte‐derived macrophages (MDM) in response to M. bovis, M. bovis BCG‐GFP and F. hepatica excretory/secretory antigen (FhES). First, acid‐fast (ZN) staining was used to confirm mycobacterial infection of MDM (Figure S2). Next, cultures of mycobacteria in agar plates, obtained from lysed stimulated MDM were analysed. The mean number of M. bovis recovered from cultures prior to F. hepatica infection was 6 colony‐forming units (CFU) per macrophage, which decreased to 4 CFU per macrophage 10 week post‐F. hepatica infection. At this time point, the numbers of M. bovis recovered from MDM co‐stimulated with FhES decreased further to 2 CFU per macrophage, which was significantly different in comparison with before F. hepatica infection (P < 0.05) (Figure 3a). Four weeks post‐F. hepatica infection, bacterial recovery tended to be lower than prior to F. hepatica infection also; however, differences were not statistically significant. In the same way as described for M. bovis, the nonvirulent BCG‐GFP was cultured in agar plates. A reduction in the numbers of BCG‐GFP recovered (CFU) was seen 10 weeks post‐F. hepatica infection (P < 0.05); in this case, the addition of FhES did not alter these numbers (Figure 3b).
Figure 3.
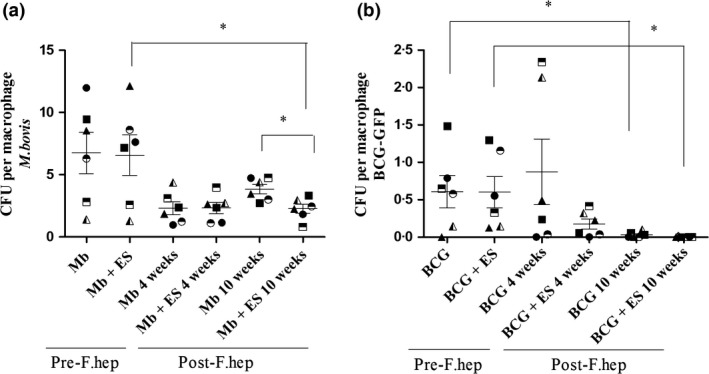
Effect of F. hepatica Infection on Recovery of M. bovis and M. bovis BCG‐GFP from MDM. For Experiment 3, MDM were isolated from cattle before, and 4 week and 10 week after F. hepatica infection and then incubated with M. bovis (Mb) (a) or BCG‐GFP (b) (MOI 1 : 1) in vitro for 24 h or added to MDM previously stimulated with FhES (ES) (20 μg/mL) for 24 h. Cultures in 7H11‐OADC agar plates of mycobacteria show that 10 weeks post‐F. hepatica infection, M. bovis recovery per macrophage was lower than before F. hepatica infection, although this was not statistically significant until after co‐stimulation with FhES (*=P < 0.05) (a). Similarly, lower numbers of BCG‐GFP were recovered 10 weeks post‐F. hepatica infection either with or without FhES restimulation (*=P < 0.05) (b). The CFU per macrophage were calculated, with three replicates per animal (represented as different symbols). Horizontal lines represent the mean CFU per macrophage ± SEM, n = 6.
The Effect of F. hepatica Infection on Bacterial Uptake and TLR2–CD14 Expression in Macrophages Infected with BCG‐GFP in vitro
Next, we used flow cytometry to investigate whether bacterial uptake and surface receptor expression in MDM would be modified by F. hepatica infection. At 8 weeks post‐F. hepatica infection, the fluorescence intensity of BCG‐GFP‐infected MDM was shifted towards the left, indicating a reduction in fluorescence intensity (Figure 4a). However, the fluorescence intensity of TLR2 and CD14 receptors from BCG‐GFP‐infected MDM moved to the right after F. hepatica infection, indicating an increase in fluorescence intensity (Figure 4b, c).
Figure 4.
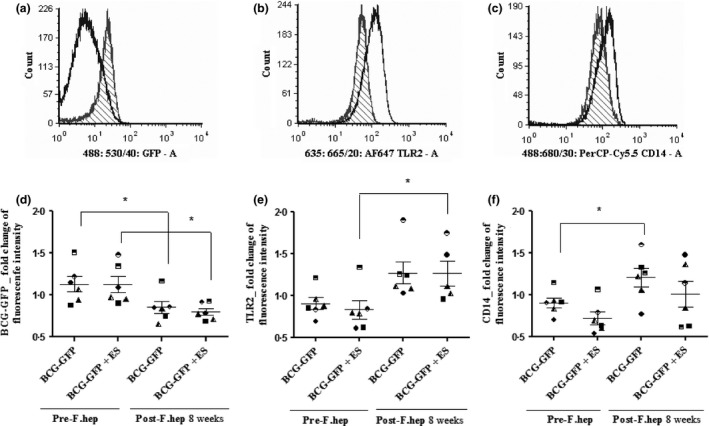
Effect of F. hepatica Infection on Bacterial Uptake, TLR2 and CD14 Receptor Expression by MDM. In Experiment 3, MDM isolated from cattle prior to and 8 weeks post‐F. hepatica infection were stimulated in vitro with BCG‐GFP (MOI 1 : 1) for 24 h or FhES (20 μg/mL) (ES) for 48 h. Then, MDM were collected and stained with the antibodies Alexa Fluor 643 TLR2 and with PerCP‐Cy5.5 CD14 and analysed with flow cytometry. In the overlaid univariate histograms, a representative sample stimulated with BCG‐GFP shows a shift towards the left in fluorescence intensity after F. hepatica infection of cattle (a). However, there is a shift towards the right when the CD14 and TLR2 receptors were measured after F. hepatica infection (b, c). Y‐axis indicates cell count and the x‐axis indicates median fluorescence intensity. Nonfilled histogram = Post‐F. hepatica infection; filled line histogram = Pre‐F. hepatica infection. A minimum of 6 × 104 single cells have been recorded and analysed per sample (sample = biological replicate) (a–c). Statistical analysis of all samples from the 6 animals shows that at 8 week post‐F. hepatica infection, bacterial uptake is reduced in MDM (d); however, TLR2 (e) and CD14 (f) receptor expression was maintained or increased. Each symbol with the same shape represents the average of 3 biological replicates of each animal. The median of fluorescence intensity obtained from the histograms was normalized to the unstimulated controls, and results were expressed as the mean ± S.E.M (d–f). *P < 0.05, n = 6. For gating strategy, see Figure S3.
Statistical analysis of the data from all animals showed that after F. hepatica infection, bacterial uptake was lower than before F. hepatica infection (P < 0.05) (Figure 4d). On the contrary, TLR2 expression following F. hepatica infection was increased and statistically significant when MDM were restimulated with FhES in vitro (Figure 4e). In the case of CD14, its increased expression after F. hepatica infection was statistically significant (P < 0.05) regardless of the addition of FhES (Figure 4f). Nevertheless, these surface receptors and bacterial uptake did not show a statistically significant inverse correlation (data not shown).
The effect of F. hepatica infection on macrophage activation and cytokine profile
We hypothesized that reduced mycobacterial recovery after F. hepatica infection of cattle could be associated with an alteration in the activation of macrophages and their cytokine profile. To study such modification in macrophage phenotype, we measured arginase and nitric oxide production, markers of alternative and classical activation, respectively. MDM showed upregulated arginase I mRNA expression at 10 week after F. hepatica infection (P < 0.05) (Figure 5a). However, as expected, there was a downregulation in iNOS mRNA expression (fold change of 5500) (P < 0.05) and nitric oxide production (P < 0.01) in M. bovis‐stimulated MDM after F. hepatica infection (Figure 5b, c). Next, we investigated the levels of a panel of cytokines. The levels of pro‐inflammatory cytokines IL‐1b, IL‐12 and IL‐6 were reduced after F. hepatica infection (P < 0.001, P < 0.01 and P < 0.05, respectively), whereas TGF‐β was increased after F. hepatica infection (P < 0.05) (Figure 6).
Figure 5.
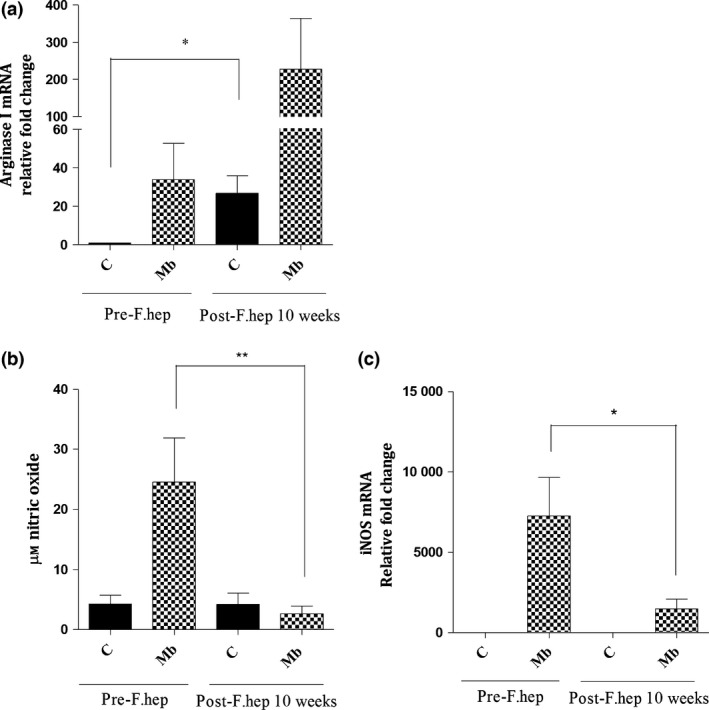
Effect of F. hepatica Infection on Macrophage Activation. For Experiment 3, MDM were isolated from cattle before and 10 week after F. hepatica infection and then incubated with M. bovis (Mb) (MOI 1 : 1) in vitro for 24 h or left unstimulated. qPCR results show that arginase I mRNA in MDM was upregulated after F. hepatica infection of cattle, in both unstimulated MDM and after Mb stimulation. However, there was a downregulation in iNOS mRNA expression in Mb‐MDM after F. hepatica infection (c) which coincided with reduction in nitric oxide production (b). * = P < 0.05, ** = P < 0.01. Results express mean ± SEM of relative fold change of mRNA (a,c) or μm nitric oxide (b), n = 6.
Figure 6.
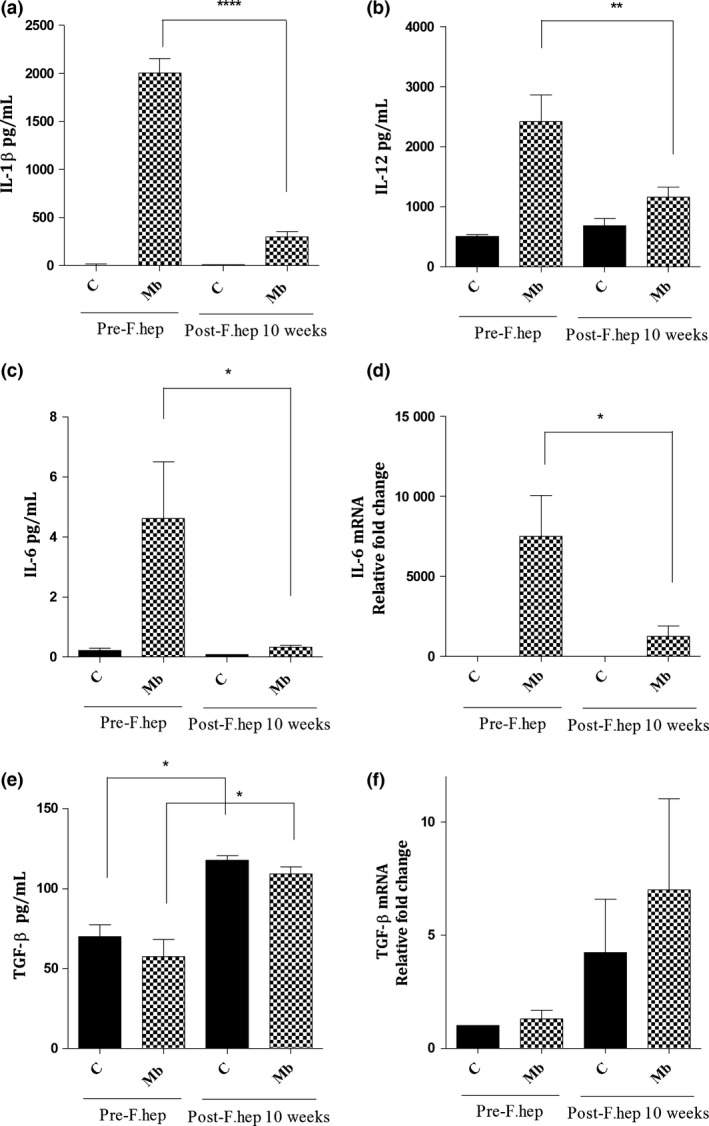
Effect of F. hepatica Infection on Macrophage Cytokine Profile. For Experiment 3, MDM were isolated from cattle before and 10 week after F. hepatica infection of cattle and then incubated with M. bovis (Mb) (MOI 1 : 1) in vitro for 24 h or left unstimulated. The levels of cytokines IL‐1β, IL‐12, IL‐6 and TGF‐β in MDM cultures were measured by ELISA (a–c, e) or qPCR (d, f). There was a decrease in the production of IL‐1β (a), IL‐12 (b) and IL‐6 in Mb‐MDM after F. hepatica infection (c). Similarly, IL‐6 mRNA expression in Mb‐MDM was downregulated (d). However, TGF‐β production (e) and TGF‐β mRNA expression (f) in MDM were increased after F. hepatica infection. * = P < 0.05, ** = P < 0.01, **** = P < 0.001. Results express mean ± SEM of pg/mL (a–c, e) or relative fold change of mRNA (d, f). n = 6.
Discussion
We have previously described reduced responsiveness to M. bovis diagnostic tests (SICCT test and the IFN‐γ test) in cattle co‐infected with F. hepatica 26. However, to date, there has been no evidence to link this effect to bacterial load or disease dynamics. Given the substantial resources expended in national control programmes for bovine tuberculosis, which rely on those immunodiagnostic tests, any such interactions warrant further study.
Thus, one of our objectives was to study the effect of F. hepatica on bacterial load from M. bovis‐infected animals. Helminth‐mediated immunoregulation is a multifactorial phenomenon, with macrophage‐mediated effects one of the principal facets involved. As macrophages are also key elements in the progress of and response to mycobacterial infections, we chose also to study changes in macrophages. Previous co‐infection studies have shown that Schistosoma mansoni 27 and Nippostrongylus brasiliensis 14 co‐infection in mice increased the bacterial burden and growth of M. tuberculosis, respectively. F. hepatica infection in cattle induced higher excretion rates in concurrent infection with Salmonella Dublin and more disseminated bacterial infection in the organs 15. Similarly, another study in mice showed that co‐infection with F. hepatica and Bordetella pertussis delayed bacterial clearance in the co‐infected groups 16. Thus, based on our previous 26 results demonstrating reduced Th1 responses, with reduction in M. bovis‐induced IFN‐γ production in animals co‐infected with F. hepatica (Figure 2), we hypothesized that mycobacterial recovery and uptake would be enhanced in the presence of the helminth infection. In contrast, however, the opposite was observed, with reduced mycobacterial burden directly recorded from the tissues of co‐infected animals (Figure 1). Our data also show that bacterial recovery from MDM infected with M. bovis in vitro was reduced after helminth infection (Figure 3). Additionally, given that F. hepatica ES antigen (FhES) is able to induce the production of Th2 and Treg cytokines as well as arginase 25, 49, we suggested that FhES could further influence such mycobacterial uptake and survival. In this case, the addition of FhES to macrophages further decreased M. bovis survival (Figure 3a). However, when the nonvirulent M. bovis BCG was used for the experiments (Figure 3b and Figure 4d), the addition of FhES did not result in any further differences in bacterial uptake or recovery in comparison with the BCG‐only‐stimulated cells. This could be explained because M. bovis BCG uptake and growth is much lower than when M. bovis is used 45, so a subtle further difference added with FhES may not have been detected.
Nevertheless, the finding of lower mycobacterial uptake and recovery after F. hepatica infection of cattle is in line with some previous studies. It has been shown that rats co‐infected with the nematode Litomosoides sigmodontis and M. tuberculosis had lower bacterial loads than rats with M. tuberculosis infection only 50. Likewise, another study demonstrated that acute Nippostrongylus brasiliensis (Nb) infection in mice reduced early pulmonary mycobacterial colonization 11. Additionally, other studies have shown decreased M. tuberculosis–GFP uptake in human DC when they were pre‐exposed to microfilaria 51. Next, we investigated a potential cause of this lowered bacterial uptake, the receptors TLR2 and CD14. TLR2 is one of the pathogen‐recognition receptors (PRRs) on the surface of macrophages which recognize pathogen‐associated molecular patterns (PAMPs) from mycobacteria 52, 53. TLR2 together with its co‐receptor CD14 has been associated with recognizing and triggering mycobacterial immune responses 31, 32. Sendide et al. (2005) showed that in THP‐1 and CHO cell lines, optimal M. bovis BCG uptake required the presence of both CD14 and complement receptor 3 (CR3) and co‐expression of CD14 and TLR2 markedly enhanced such uptake 28. In bovine macrophages, Souza et al. (2007) demonstrated that mannose receptors and CD14 contributed to Mycobacterium avium phagocytosis 29. Blocking TLR2 receptors in bovine macrophages enhanced killing of Mycobacterium avium paratuberculosis by enabling phagosome maturation within the cell 30. Hence, we might have expected that lower bacterial uptake would be associated with reduced expression of TLR2 and CD14 after F. hepatica infection of animals. However, expression of TLR2 and CD14 was conserved or enhanced in F. hepatica‐infected animals (Figure 4b, c, e, f). In agreement with those findings, however, other studies have also shown enhancement of TLR2 expression following helminth infection 33, 54. For example, schistosome‐specific phosphatidylserine (PS) activated TLR2 in dendritic cells 55. In another study, dendritic cells and B cells from Schistosoma mansoni infected patients with multiple sclerosis, stimulated with soluble egg Ag (SEA), resulted in significant TLR2 upregulation 33. Given this inverse correlation between TLR2–CD14 expression and bacterial uptake, we examined other mechanisms involved in lower mycobacterial uptake by macrophages in the context of F. hepatica infection. For this, we studied the activation phenotype of macrophages after F. hepatica infection. Our results show that M. bovis‐stimulated MDM had upregulated mRNA expression of arginase I but downregulated mRNA expression of iNOS, which was associated with lowered production of nitric oxide (Figure 5). Previously, it has been demonstrated that F. hepatica infection and F. hepatica products elicit alternative activation of peritoneal macrophages 56 and of a ruminant macrophage cell line 34. These alternatively activated macrophages, known to develop in F. hepatica infections 34, 35, are not as efficient in phagocytosing bacteria as classically activated macrophages. For example, Varin et al. (2010) showed low uptake of Neisseira meningitidis after IL‐4 and IL‐13 stimulation of murine macrophages which was related to alternative activation of macrophages 36. As our study shows such phenotype switching after F. hepatica infection (Figure 5), alternative activation of macrophages could be the reason for a lower bacterial uptake. Additionally, another group found that F. hepatica fatty acid binding protein (FABP) induced alternative activation of human macrophages and reduced production of the pro‐inflammatory cytokine IL‐1β by MDM after LPS stimulation 57. Likewise, we have demonstrated a diminution in IL‐1β in MDM stimulated with M. bovis after F. hepatica infection (Figure 6a). In mycobacterial infections, IL‐1β has been described as essential for recruiting immune cells to the site of infection 58. In addition, IL‐1β is involved in direct activation of humoral immune responses 59, antigen presentation, and expansion and recruitment of T cells to the skin, which in consequence promotes local inflammation in delayed‐type hypersensitivity responses 40. As our data show that IL‐1β production by M. bovis‐infected MDM is reduced when animals are infected with F. hepatica, this could indicate that in co‐infected animals, the responsiveness to the SICCT, which is based on a delayed‐type hypersensitivity reaction, would be reduced. Moreover, IL‐12 together with IL‐1β is able to induce IFN‐γ production in Th1 lymphocytes 37, 38. As we have found that M. bovis‐stimulated MDM produced less IL‐12 after F. hepatica infection (Figure 6b), this could explain why blood cultures from co‐infected animals with F. hepatica had lower IFN‐γ production than those infected with M. bovis only (Figure 2) 25, 26. Our results also show a reduction in IL‐6 production by MDM after F. hepatica infection of cattle. Among the multiple functions of IL‐6, it has been described to be a marker for M. tuberculosis infection in mouse peritoneal macrophages 60. In addition, IL‐6 has been associated with inhibition of type I interferon, which is involved in disease progression 61 and with differentiation of T cells to the Th17 phenotype 62, 63, which contributes to Th1 response expansion 64, 65, 66. A reduction in IL‐6 production after F. hepatica infection, as shown in our study (Figure 6c, d), would potentially affect bacterial burden, and granuloma formation in M. bovis and BCG infections 64, 65, 66. On the other hand, our results show an increase of TGF‐β in MDM after F. hepatica infection. This regulatory cytokine has been found to be produced in F. hepatica‐infected cattle, in PBMC stimulated with F. hepatica LFH (liver fluke homogenate) 67. In another study, peritoneal macrophages from naïve mice produced TGF‐β, IL‐10 and arginase I after in vitro stimulation with F. hepatica ES as well as after F. hepatica infection of mice 68. In the context of F. hepatica and mycobacterial co‐infection, TGF‐β would downregulate the type 1 responses elicited against the bacterial infection. Indeed, a study with humans immunized with M. bovis BCG showed that concurrent helminth infection produced greater levels of TGF‐β, which was associated with reduced response to the immunization, affecting vaccine efficacy 69.
In our studies, in summary, bovine MDM infected ex vivo with M. bovis showed strikingly reduced production of IL‐6, IL‐1β, IL‐12 and NO after donor animals were infected with F. hepatica infection. As it has been shown that INF‐γ production in response to mycobacterial infection is dependent on IL‐6, IL‐1β and IL‐12 37, 38, 70, 71, this could explain the reduced IFN‐γ production observed when animals were infected with F. hepatica in the co‐infection experiments (Figure 2) 25, 26. Additionally, the fact that IL‐1β promotes local inflammation in delayed‐type hypersensitivity responses 40 indicates that a reduced production of IL‐1β in F. hepatica‐infected animals could cause a lowered SICCT test reaction in those animals 25, 26. Based on these results, we propose that early in the course of M. bovis infection, alternatively activated macrophages limit the uptake of mycobacteria, setting the stage for a slower rate of bacterial proliferation and lesion development, leading to a reduced pro‐inflammatory response. In fact, Atkinson et al. (2000) showed that production of TNF‐α, IL‐12, IL‐6 and IL‐10 by human MDM was dependent on the M. bovis BCG dose used, so that lowered bacterial dose reduced cytokine production 72. As macrophages produce cytokines upon stimulation 73, a reduced stimulus elicited by an inefficient phagocytosis (in our case lowered M. bovis uptake) could decrease a pro‐inflammatory response 74. Therefore, we hypothesize that this immune downregulation may induce a latency‐like state in co‐infected animals, which would explain why cattle herds with high prevalence of fasciolosis would have lower reactivity in tests for BTB. This hypothesis is consistent with the data on an epidemiological scale shown by Claridge et al. (2012) who showed the under‐ascertainment rate of BTB was about one‐third in herds infected with F. hepatica 6.
Latent infections with M. tuberculosis in humans are extremely common, with some estimates indicating that up to one‐third of the world's population are latently infected 19, representing a reservoir from which clinically apparent infections can emerge, given appropriate environmental circumstances. Latent infection is characterized by asymptomatic, chronic infection, with lowered responsiveness to skin testing 75. Latent M. bovis infections in cattle have not been described. However, the presence of such infections in cattle is suggested by findings such as IFN‐γ test 23, 76 and skin test positivity 77, 78 in animals that lack lesions at post‐mortem; the positive culture of bacteria in animals without lesions 44, 79; and IFN‐γ positive and skin test negative animals, which later convert to positive, that have lesions at post‐mortem examination 78. Studies on the influence of concurrent helminth infections on latent/active tuberculosis in humans have given contradictory results. Babu et al. (2009) observed a reduction in mycobacterial‐specific TLR2 and TLR4 expression as well as a decrease in pro‐inflammatory production in individuals with filariasis/TB in comparison with patients with TB only 8. However, subsequent studies by this group observed no alteration in the incidence of progression from latent to active tuberculosis in populations co‐infected with gastrointestinal and/or filarial nematodes 80.
Such apparent paradoxes can be resolved by considering that helminth infection limits the pro‐inflammatory environment, favouring a slower development of mycobacteria, with reduced bacterial loads, but a higher diagnostic barrier for immune‐based detection assays. This may ensure a strategy to maintain M. bovis in the population by increasing the reservoir of undetected infection. Therefore, these findings have significant implications for the control of bovine TB and especially for eradication programmes based on detection and slaughter of infected animals.
Author Contributions
LG carried out the design of the experiments and the experimental work and wrote the manuscript. JOS contributed to the design of experimental methodology and carried out the experimental work. AB designed and supervised the flow cytometry experiments. JMN and MW designed and carried out the co‐infection studies. RF contributed to interpretation of the data and revision of the manuscript. DW and PD suggested and carried out the statistical analysis of bacterial recovery following in vivo experiments. JC analysed the pathology of the lesions. GM conceived the study, edited the manuscript and managed the collaborations. All authors contributed to the intellectual discussion and structure of the manuscript.
Supporting information
Table S1. Fasciola hepatica numbers. At post‐mortem examination of cattle (Experiment 2), livers were processed and liver flukes counted, confirming F. hepatica infection in all 6 animals.
Table S2. Mycobacterial dose obtained by side‐flow sampling in Madison Chambers. During infection with M. bovis, side‐stream samples of air were taken from the Madison Chamber, and then M. bovis was cultured to determine the actual dose (CFU) delivered to each individual animal. There were no statistically significant differences between the bacterial doses delivered to the calves between groups. In brackets is represented the standard deviation.
Table S3. Experiment 1_Isolation of M. bovis from tissues recovered from calves infected with M. bovis only or M. bovis and F. hepatica. There was a decrease in the total bacterial load and total number of positive tissues recovered in the M. bovis–F. hepatica co‐infected group in comparison with the M. bovis infected group only. Results are expressed as 101 × CFU/g (quantitative counting).
Table S4: Experiment 2_Isolation of M. bovis from tissues recovered from calves infected with M. bovis and F. hepatica (a) or M. bovis only (b). There was a reduced bacterial load recovery and number of positive tissues (by quantitative or qualitative bacteriology) in the M. bovis–F. hepatica co‐infected group vs. the only infected with M. bovis. When the same tissue was positive in both techniques, it was counted as positive only once. aQL – Qualitative bacteriology (BACTEC MGIT system). bQN – Quantitative bacteriology (7H11‐OADC agar plates). cResults expressed as × 101 CFU/g
Figure S1. Seroconversion of F. hepatica Infected Cattle.
Figure S2. Confirmation of MDM infected with mycobacteria in vitro
Figure S3. Gating strategy.
Method S1. F. hepatica Excretory/Secretory Products (FhES)
Method S2. Acid Fast Staining
Document S1. Experiment Annotation Using MIFlowCyt Standard
Table S5. Description of antibodies and lasers used.
Acknowledgements
This work was supported by Science Foundation Ireland grant 09/IN.1/B2625 The authors are very grateful to Eddie Jordan and Frank Griffith for providing valuable assistance in sourcing, housing and handling cattle in UCD Lyons Farm, to Dr Lyanne McCallan and David Corbett for their expertise with bacteriology and experimental infections in the co‐infection experiments in AFBI and to Dr Dave Magee and Dr Claire Healy for their advice in the in vitro bacterial work in UCD. Alfonso Blanco acknowledges the assistance of the ISAC Scholars Program.
Disclosures and competing interests: None.
References
- 1. Thompson‐Crispi KA, Sargolzaei M, Ventura R, et al A genome‐wide association study of immune response traits in Canadian Holstein cattle. BMC Genom 2014; 15: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deshpande P, Lucas M & Gaudieri S. Analysis of Genetic Variation in Animals. Caliskan M, editor. InTech; 2012. [cited 2015 Mar 20]. Available from: http://www.intechopen.com/books/analysis-of-genetic-variation-in-animals/genetic-variation-of-host-immune-response-genes-and-their-effect-on-hepatitis-c-infection-and-treatm [Google Scholar]
- 3. Allen AR, Minozzi G, Glass EJ, et al Bovine tuberculosis: the genetic basis of host susceptibility. Proc Biol Sci 2010; 277: 2737–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garza‐Cuartero L, Garcia‐Campos A, Zintl A, et al The worm turns: trematodes steering the course of co‐infections. Vet Pathol 2014; 51: 385–392. [DOI] [PubMed] [Google Scholar]
- 5. Li X‐X & Zhou X‐N. Co‐infection of tuberculosis and parasitic diseases in humans: a systematic review. Parasit Vectors 2013; 6: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Claridge J, Diggle P, McCann CM, et al Fasciola hepatica is associated with the failure to detect bovine tuberculosis in dairy cattle. Nat Commun Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. 2012; 3: 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thigpen MC, Filler SJ, Kazembe PN, et al Associations between peripheral Plasmodium falciparum malaria parasitemia, human immunodeficiency virus, and concurrent helminthic infection among pregnant women in Malawi. Am J Trop Med Hyg 2011; 84: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Babu S, Bhat SQ, Kumar NP, et al Attenuation of toll‐like receptor expression and function in latent tuberculosis by coexistent filarial infection with restoration following antifilarial chemotherapy. PLoS Negl Trop Dis 2009; 3: 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McSorley HJ & Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev 2012; 25: 585–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Girgis NM, Gundra UM & Loke P. Immune regulation during helminth infections. Knoll LJ, editor. PLoS Pathog Public Library of Science; 2013;9:e1003250 Available from: http://dx.plos.org/10.1371/journal.ppat.1003250 Jan [cited 2014 May 8]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du Plessis N, Kleynhans L, Thiart L, et al Acute helminth infection enhances early macrophage mediated control of mycobacterial infection. Mucosal Immunol 2013; 6: 931–941. [DOI] [PubMed] [Google Scholar]
- 12. Abruzzi A & Fried B. Coinfection of Schistosoma (Trematoda) with bacteria, protozoa and helminths. Adv Parasitol 2011; 77: 1–85. [DOI] [PubMed] [Google Scholar]
- 13. Bucher K, Dietz K, Lackner P, et al Schistosoma co‐infection protects against brain pathology but does not prevent severe disease and death in a murine model of cerebral malaria. Int J Parasitol 2011; 41: 21–31. [DOI] [PubMed] [Google Scholar]
- 14. Potian JA, Rafi W, Bhatt K, McBride A, Gause WC & Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti‐tuberculosis defense by engaging the IL‐4 receptor pathway. J Exp Med 2011/08/10 ed 2011; 208: 1863–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aitken MM, Jones PW, Hall GA, Hughes DL & Collis KA. Effects of experimental Salmonella dublin infection in cattle given Fasciola hepatica thirteen weeks previously. J Comp Pathol 1978/01/01 ed. 1978; 88: 75–84. [DOI] [PubMed] [Google Scholar]
- 16. Brady MT, O'Neill SM, Dalton JP & Mills KH. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect Immun 1999; 67: 5372–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Selemetas N, Phelan P, O'Kiely P & Waal T de. Weather and soil type affect incidence of fasciolosis in dairy cow herds. Vet Rec BMJ Publishing Group Limited; 2014; 175: 371. [DOI] [PubMed] [Google Scholar]
- 18. European Commission . Research – International Co‐operation in Food, Agriculture and Biotechnology Research. 2012. Available from: http://ec.europa.eu/research/biosociety/inco/projects/0047_en.html. [cited 2012 Dec 19]
- 19. WHO . WHO | Tuberculosis. World Health Organization; 2014. Available from: http://www.who.int/mediacentre/factsheets/fs104/en/ [cited 2014 Oct 29] [Google Scholar]
- 20. Gilbert M, Mitchell A, Bourn D, Mawdsley J, Clifton‐Hadley R & Wint W. Cattle movements and bovine tuberculosis in Great Britain. Nature 2005; 435: 491–496. [DOI] [PubMed] [Google Scholar]
- 21. De la Rua‐Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH & Clifton‐Hadley RS. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, gamma‐interferon assay and other ancillary diagnostic techniques. Res Vet Sci 2006; 81: 190–210. [DOI] [PubMed] [Google Scholar]
- 22. Palmer MV, Thacker TC, Waters WR, Gortázar C & Corner LAL. Mycobacterium bovis: A Model Pathogen at the Interface of Livestock, Wildlife, and Humans. Vet Med Int 2012; 2012: 236–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alvarez J, de Juan L, Bezos J, et al Effect of paratuberculosis on the diagnosis of bovine tuberculosis in a cattle herd with a mixed infection using interferon‐gamma detection assay. Vet Microbiol 2009; 135: 389–393. [DOI] [PubMed] [Google Scholar]
- 24. Corner LAL. The role of wild animal populations in the epidemiology of tuberculosis in domestic animals: How to assess the risk. Vet Microbiol 2006; 112: 303–312. [DOI] [PubMed] [Google Scholar]
- 25. Flynn RJ, Mannion C, Golden O, Hacariz O & Mulcahy G. Experimental Fasciola hepatica infection alters responses to tests used for diagnosis of bovine tuberculosis. Infect Immun 2007; 75: 1373–1381 2006/12/30 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flynn RJ, Mulcahy G, Welsh M, et al Co‐Infection of cattle with Fasciola hepatica and Mycobacterium bovis‐ immunological consequences. Transbound Emerg Dis 2009; 56: 269–274. 2009/07/07 ed. [DOI] [PubMed] [Google Scholar]
- 27. Frantz FG, Rosada RS, Peres‐Buzalaf C, et al Helminth coinfection does not affect therapeutic effect of a DNA vaccine in mice harboring tuberculosis. PLoS Negl Trop Dis 2010; 4: e700 2010/06/15 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sendide K, Reiner NE, Lee JSI, Bourgoin S, Talal A & Hmama Z. Cross‐talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria: regulation by phosphatidylinositol 3‐kinase and cytohesin‐1. J Immunol. American Association of Immunologists; 2005; 174: 4210–4219. [DOI] [PubMed] [Google Scholar]
- 29. Souza CD, Evanson OA, Sreevatsan S & Weiss DJ. Cell membrane receptors on bovine mononuclear phagocytes involved in phagocytosis of Mycobacterium avium subsp paratuberculosis. Am J Vet Res 2007; 68: 975–980. [DOI] [PubMed] [Google Scholar]
- 30. Weiss DJ, Souza CD, Evanson OA, Sanders M & Rutherford M. Bovine monocyte TLR2 receptors differentially regulate the intracellular fate of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. J Leukoc Biol 2008; 83: 48–55. [DOI] [PubMed] [Google Scholar]
- 31. Janot L, Secher T, Torres D, et al CD14 works with toll‐like receptor 2 to contribute to recognition and control of Listeria monocytogenes infection. J Infect Dis 2008; 198: 115–124. [DOI] [PubMed] [Google Scholar]
- 32. Raby A‐C, Holst B, Le Bouder E, et al Targeting the TLR co‐receptor CD14 with TLR2‐derived peptides modulates immune responses to pathogens. Sci Transl Med 2013; 5: 185ra64. [DOI] [PubMed] [Google Scholar]
- 33. Correale J & Farez M. Helminth antigens modulate immune responses in cells from multiple sclerosis patients through TLR2‐dependent mechanisms. J Immunol 2009; 183: 5999–6012. [DOI] [PubMed] [Google Scholar]
- 34. Flynn RJ, Irwin JA, Olivier M, Sekiya M, Dalton JP & Mulcahy G. Alternative activation of ruminant macrophages by Fasciola hepatica. Vet Immunol Immunopathol 2007; 120: 31–40. [DOI] [PubMed] [Google Scholar]
- 35. Golden O, Flynn RJ, Read C, et al Protection of cattle against a natural infection of Fasciola hepatica by vaccination with recombinant cathepsin L1 (rFhCL1). Vaccine 2010; 28: 5551–5557 2010/07/06 ed. [DOI] [PubMed] [Google Scholar]
- 36. Varin A, Mukhopadhyay S, Herbein G & Gordon S. Alternative activation of macrophages by IL‐4 impairs phagocytosis of pathogens but potentiates microbial‐induced signalling and cytokine secretion. Blood American Society of Hematology 2010; 115: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tominaga K, Yoshimoto T, Torigoe K, et al IL‐12 synergizes with IL‐18 or IL‐1beta for IFN‐gamma production from human T cells. Int Immunol 2000; 12: 151–160. [DOI] [PubMed] [Google Scholar]
- 38. Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L & O'Shea JJ. Signaling by IL‐12 and IL‐23 and the immunoregulatory roles of STAT4. Immunol Rev 2004; 202: 139–156. [DOI] [PubMed] [Google Scholar]
- 39. McLoughlin RM, Witowski J, Robson RL, et al Interplay between IFN‐gamma and IL‐6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest 2003; 112: 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nambu A, Nakae S & Iwakura Y. IL‐1beta, but not IL‐1alpha, is required for antigen‐specific T cell activation and the induction of local inflammation in the delayed‐type hypersensitivity responses. Int Immunol 2006; 18: 701–712. [DOI] [PubMed] [Google Scholar]
- 41. Rodgers JD, Connery NL, McNair J, et al Experimental exposure of cattle to a precise aerosolised challenge of Mycobacterium bovis: a novel model to study bovine tuberculosis. Tuberculosis 2007; 87: 405–414. [DOI] [PubMed] [Google Scholar]
- 42. Buddle BM, Aldwell FE, Pfeffer A, de Lisle GW & Corner LA. Experimental Mycobacterium bovis infection of cattle: Effect of dose of M. bovis and pregnancy on immune responses and distribution of lesions. N Z Vet J 1994; 45: 2. [DOI] [PubMed] [Google Scholar]
- 43. Cassidy JP, Bryson DG, Pollock JM, Evans RT, Forster F & Neill SD. Early lesion formation in cattle experimentally infected with Mycobacterium bovis. J Comp Pathol 1998; 119: 27–44. [DOI] [PubMed] [Google Scholar]
- 44. Cassidy JP, Bryson DG, Pollock JM, Evans RT, Forster F & Neill SD. Lesions in cattle exposed to Mycobacterium bovis‐inoculated calves. J Comp Pathol 1999; 121: 321–337. [DOI] [PubMed] [Google Scholar]
- 45. Denis M, Wedlock DN & Buddle BM. IFN‐gamma enhances bovine macrophage responsiveness to Mycobacterium bovis: Impact on bacterial replication, cytokine release and macrophage apoptosis. Immunol Cell Biol 2005; 83: 643–650 2005/11/04 ed. [DOI] [PubMed] [Google Scholar]
- 46. Werling D, Piercy J & Coffey TJ. Expression of TOLL‐like receptors (TLR) by bovine antigen‐presenting cells‐potential role in pathogen discrimination? Vet Immunol Immunopathol 2006; 112: 2–11. [DOI] [PubMed] [Google Scholar]
- 47. Hellemans J, Mortier G, De Paepe A, Speleman F & Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real‐time quantitative PCR data. Genome Biol 2007; 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R & Leunissen JAM. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 2007;35:W71–W74 2007/05/09 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Flynn RJ & Mulcahy G. Possible role for Toll‐like receptors in interaction of Fasciola hepatica excretory/secretory products with bovine macrophages. Infect Immun 2008; 76: 678–684 2007/12/12 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hübner MP, Killoran KE, Rajnik M, et al Chronic helminth infection does not exacerbate Mycobacterium tuberculosis infection. PLoS Negl Trop Dis 2012; 6: e1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Talaat KR, Bonawitz RE, Domenech P & Nutman TB. Preexposure to Live Brugia malayi Microfilariae Alters the Innate Response of Human Dendritic Cells to Mycobacterium tuberculosis. J Infect Dis 2006; 193: 196–204. [DOI] [PubMed] [Google Scholar]
- 52. Miyauchi M, Murata M, Shibuya K, et al Phagocytosis plays a dual role in activating dendritic cells; digestive production of active Toll‐like receptor ligands and cooperation with Toll‐like receptor signaling. Drug Discov Ther 2010; 4: 135–143. [PubMed] [Google Scholar]
- 53. Almeida PE, Roque NR, Magalhães KG, et al Differential TLR2 downstream signaling regulates lipid metabolism and cytokine production triggered by Mycobacterium bovis BCG infection. Biochim Biophys Acta 2014; 1841: 97–107. [DOI] [PubMed] [Google Scholar]
- 54. Correale J & Farez MF. Does helminth activation of toll‐like receptors modulate immune response in multiple sclerosis patients? Front Cell Infect Microbiol 2012; 2: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Van Riet E, Everts B, Retra K, et al Combined TLR2 and TLR4 ligation in the context of bacterial or helminth extracts in human monocyte derived dendritic cells: molecular correlates for Th1/Th2 polarization. BMC Immunol 2009; 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Donnelly S, O'Neill SM, Sekiya M, Mulcahy G & Dalton JP. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect Immun 2005; 73: 166–173 2004/12/25 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Figueroa‐Santiago O & Espino AM. Fasciola hepatica fatty acid binding protein induces the alternative activation of human macrophages. Infect Immun 2014; 82: 5005–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Juffermans NP, Florquin S, Camoglio L, et al Interleukin‐1 signaling is essential for host defense during murine pulmonary tuberculosis. J Infect Dis 2000; 182: 902–908. [DOI] [PubMed] [Google Scholar]
- 59. Martinon F, Mayor A & Tschopp J. The Inflammasomes: Guardians of the Body. Annu Rev Immunol Annual Reviews; 2009; 27: 229–265. [DOI] [PubMed] [Google Scholar]
- 60. Singh PP & Goyal A. Interleukin‐6: a potent biomarker of mycobacterial infection. Springerplus Springer; 2013; 2: 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martinez AN, Mehra S & Kaushal D. Role of interleukin 6 in innate immunity to Mycobacterium tuberculosis infection. J Infect Dis 2013; 207: 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghoreschi K, Laurence A, Yang X‐P, et al Generation of pathogenic T(H)17 cells in the absence of TGF‐β signalling. Nature. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. 2010; 467: 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bettelli E, Carrier Y, Gao W, et al Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441: 235–238. [DOI] [PubMed] [Google Scholar]
- 64. Khader SA, Bell GK, Pearl JE, et al IL‐23 and IL‐17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007; 8: 369–377. [DOI] [PubMed] [Google Scholar]
- 65. Wozniak TM, Ryan AA & Britton WJ. Interleukin‐23 restores immunity to Mycobacterium tuberculosis infection in IL‐12p40‐deficient mice and is not required for the development of IL‐17‐secreting T cell responses. J Immunol 2006; 177: 8684–8692. [DOI] [PubMed] [Google Scholar]
- 66. Happel KI, Lockhart EA, Mason CM, et al Pulmonary interleukin‐23 gene delivery increases local T‐cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect Immun 2005; 73: 5782–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Flynn RJ & Mulcahy G. The roles of IL‐10 and TGF‐beta in controlling IL‐4 and IFN‐gamma production during experimental Fasciola hepatica infection. Int J Parasitol 2008; 38: 1673–1680 2008/07/04 ed. [DOI] [PubMed] [Google Scholar]
- 68. Guasconi L, Serradell MC, Garro AP, Iacobelli L & Masih DT. C‐type lectins on macrophages participate in the immunomodulatory response to Fasciola hepatica products. Immunology 2011; 133: 386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Elias D, Britton S, Aseffa A, Engers H & Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF‐beta production. Vaccine 2008; 26: 3897–3902. [DOI] [PubMed] [Google Scholar]
- 70. Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M & Kaufmann SH. Lethal tuberculosis in interleukin‐6‐deficient mutant mice. Infect Immun 1997; 65: 4843–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Saunders BM, Frank AA, Orme IM & Cooper AM. Interleukin‐6 Induces Early Gamma Interferon Production in the Infected Lung but Is Not Required for Generation of Specific Immunity to Mycobacterium tuberculosis Infection. Infect Immun 2000; 68: 3322–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Atkinson S, Valadas E, Smith SM, Lukey PT & Dockrell HM. Monocyte‐derived macrophage cytokine responses induced by M. bovis BCG. Tuber Lung Dis 2000; 80: 197–207. [DOI] [PubMed] [Google Scholar]
- 73. Murray RZ & Stow JL. Cytokine Secretion in Macrophages: SNAREs, Rabs, and Membrane Trafficking. Front Immunol 2014; 5: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Leelahavanichkul A, Bocharov AV, Kurlander R, et al Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J Immunol 2012; 188: 2749–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pollock JM & Neill SD. Mycobacterium bovis infection and tuberculosis in cattle. Vet J 2002; 163: 115–127. [DOI] [PubMed] [Google Scholar]
- 76. Jones GJ, Pirson C, Gideon HP, et al Immune responses to the enduring hypoxic response antigen Rv0188 are preferentially detected in Mycobacterium bovis infected cattle with low pathology. PLoS One 2011; 6: e21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Neill SD, Hanna J, Pollock JM, et al The diagnosis of bovine tuberculosis by blood testing. Proc Soc Vet Epidemiol Prev Med 1994: 1–8. [Google Scholar]
- 78. Monaghan M, Quinn PJ, Kelly AP, et al A pilot trial to evaluate the γ‐interferon assay for the detection of Mycobacterium bovis infected cattle under Irish conditions. Ir Vet J 1997; 50: 229–232. [Google Scholar]
- 79. Whipple DL, Bolin CA & Miller JM. Distribution of lesions in cattle infected with Mycobacterium bovis. J Vet Diagn Invest 1996; 8: 351–354. [DOI] [PubMed] [Google Scholar]
- 80. Chatterjee S, Kolappan C, Subramani R, et al Incidence of active pulmonary tuberculosis in patients with coincident filarial and/or intestinal helminth infections followed longitudinally in South India. PLoS One Public Library of Science 2014; 9: e94603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Fasciola hepatica numbers. At post‐mortem examination of cattle (Experiment 2), livers were processed and liver flukes counted, confirming F. hepatica infection in all 6 animals.
Table S2. Mycobacterial dose obtained by side‐flow sampling in Madison Chambers. During infection with M. bovis, side‐stream samples of air were taken from the Madison Chamber, and then M. bovis was cultured to determine the actual dose (CFU) delivered to each individual animal. There were no statistically significant differences between the bacterial doses delivered to the calves between groups. In brackets is represented the standard deviation.
Table S3. Experiment 1_Isolation of M. bovis from tissues recovered from calves infected with M. bovis only or M. bovis and F. hepatica. There was a decrease in the total bacterial load and total number of positive tissues recovered in the M. bovis–F. hepatica co‐infected group in comparison with the M. bovis infected group only. Results are expressed as 101 × CFU/g (quantitative counting).
Table S4: Experiment 2_Isolation of M. bovis from tissues recovered from calves infected with M. bovis and F. hepatica (a) or M. bovis only (b). There was a reduced bacterial load recovery and number of positive tissues (by quantitative or qualitative bacteriology) in the M. bovis–F. hepatica co‐infected group vs. the only infected with M. bovis. When the same tissue was positive in both techniques, it was counted as positive only once. aQL – Qualitative bacteriology (BACTEC MGIT system). bQN – Quantitative bacteriology (7H11‐OADC agar plates). cResults expressed as × 101 CFU/g
Figure S1. Seroconversion of F. hepatica Infected Cattle.
Figure S2. Confirmation of MDM infected with mycobacteria in vitro
Figure S3. Gating strategy.
Method S1. F. hepatica Excretory/Secretory Products (FhES)
Method S2. Acid Fast Staining
Document S1. Experiment Annotation Using MIFlowCyt Standard
Table S5. Description of antibodies and lasers used.


