Abstract
Poorly controlled pain is a global public health issue. The personal, familial and societal costs are immeasurable. Only a minority of European patients have access to a comprehensive specialist pain clinic. More commonly the responsibility for chronic pain management and initiating opioid therapy rests with the primary care physician and other non‐specialist opioid prescribers. There is much confusing and conflicting information available to non‐specialist prescribers regarding opioid therapy and a great deal of unjustified fear is generated. Opioid therapy should only be initiated by competent clinicians as part of a multi‐faceted treatment programme in circumstances where more simple measures have failed. Throughout, all patients must be kept under close clinical surveillance. As with any other medical therapy, if the treatment fails to yield the desired results and/or the patient is additionally burdened by an unacceptable level of adverse effects, the overall management strategy must be reviewed and revised. No responsible clinician will wish to pursue a failed treatment strategy or persist with an ineffective and burdensome treatment. In a considered attempt to empower and inform non‐specialist opioid prescribers, EFIC convened a European group of experts, drawn from a diverse range of basic science and relevant clinical disciplines, to prepare a position paper on appropriate opioid use in chronic pain. The expert panel reviewed the available literature and harnessed the experience of many years of clinical practice to produce these series of recommendations. Its success will be judged on the extent to which it contributes to an improved pain management experience for chronic pain patients across Europe.
Significance
This position paper provides expert recommendations for primary care physicians and other non‐ specialist healthcare professionals in Europe, particularly those who do not have ready access to specialists in pain medicine, on the safe and appropriate use of opioid medications as part of a multi‐faceted approach to pain management, in properly selected and supervised patients.
1. Introduction
Uncontrolled pain is a major public health concern. Despite multiple pain management guideline documents and initiatives by the WHO and others, there is still a reluctance to apply evidence‐based and validated strategies in pain management. A major element contributing to this unacceptable situation is the pervasive negative bias that exists in respect of opioid use. In this regard, it is important to state that we are solely concerned with the legitimate use of opioid medication by competent and responsible clinicians, in carefully selected and supervised patients. Opioids are only considered in circumstances where non‐opioid and adjuvant therapies have failed. The correct dose of an opioid is the lowest possible dose that achieves the desired outcome. The purpose of opioid use, as part of a multi‐faceted approach to pain management, is to achieve and maintain an optimal level of pain and symptom relief with the minimal level of side effects. Ultimately, this is intended to rehabilitate the pain patient so that he/she may again engage more fully with life across a range of domains – family, work, social etc.
This guideline document is produced by the European Pain Federation (EFIC) in order to provide a fair, balanced and evidence‐based summary regarding the role of opioid use in pain management. The guideline is intended primarily for non‐specialist prescribers and summarises all of the relevant data where such exists. Where data are lacking, the views and expert recommendations presented reflect the extensive clinical experience of a diverse range of European clinicians and scientists who have collaborated on this project. In so doing, we hope to better inform clinicians, regulators, legislators, administrators and the general public. We all have a shared responsibility to improve the care experience for patients experiencing pain across Europe.
2. Background
Pain is an unpleasant sensory and emotional experience. (IASP Taxonomy 2012) The individual experience and manifestation of pain is influenced by a complex series of interactions involving sensory, pathophysiological, affective, socio‐cultural, behavioural and cognitive elements (Fig. 1; Dalal and Bruera 2012).
Figure 1.
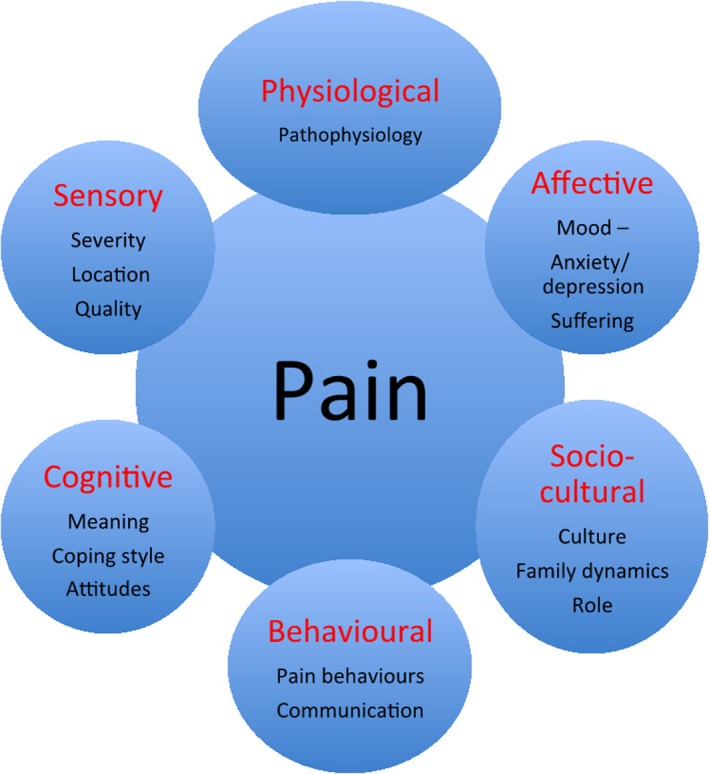
The multi‐dimensional concept of ‘total pain’.
Both chronic non‐cancer pain and cancer pain remain significant public health concerns (Breivik et al., 2006; Ripamomti et al., 2012). The personal and socio‐economic impact of chronic pain is considered to be at least as great as that of other established healthcare priorities, including cardiovascular disease and cancer (Breivik et al., 2013). Pain reduces patient quality of life, preventing many from leading an independent lifestyle. This can also negatively affect the lives of their family, friends and co‐workers (West et al., 2012). The Declaration of Montreal states that pain management is inadequate across most of the world (IASP, 2012).
Opioid analgesics are indispensable for the management of pain (Gilson et al., 2011). Opioids are highly effective and safe analgesics and their appropriate use by competent clinicians is a crucial element in modern pain management. Opioids are not a panacea for all painful conditions and are only introduced when strictly necessary and with due regard to an ongoing risk benefit analysis. Opioids are not used in isolation, but form part of a multi‐faceted strategy that includes all necessary adjuvant analgesics, non‐drug interventions, psychological support and rehabilitation. All healthcare professionals must be adequately trained in basic pain assessment and management, and must take steps to maintain the essential competencies and skills required. When deemed medically necessary by a senior responsible clinician, opioids must be readily accessible under supervision for those who legitimately require such therapy (Box 1).
Box 1. Chronic pain in Europe – the facts.
Chronic pain
Affects 20% of European citizens
Disrupts the lives of millions of European citizens and their families
More common in women
More common with increasing age
Negative impact on quality of life, physical and psychological well‐being
-
Major economic cost:
-
○
Indirect (inability to work)
-
○
Direct (treatment‐related costs)
-
○
Grossly under‐recognised and under‐treated
Major public health concern
Access to comprehensive pain assessment and management is a basic human right
Coordinated and collaborative approach is urgently required, particularly in patients whose pain does not respond to standard therapeutic interventions
3. Pain assessment and screening
In many instances, the absence of systematic screening of patients for pain results in the under‐recognition and under‐treatment of pain. It is over twenty years since the American Pain Society recommended routine assessment and recording of patients’ pain as a first step in improving pain management (APSQoCC, 1995). Whilst identifying pain is no guarantee of improved pain management, it is a vital first step (Mularski et al., 2006), and failure to do so means that pain will not be addressed at all.
All patients undergoing medical assessment for whatever purpose should be screened for pain. However, clinicians do not routinely seek a pain history and patients do not always volunteer one, even when asked. The reasons for this are summarised in Boxes 2 and 3 below (Dar et al., 1992; Anderson et al., 2000, 2002; Cleeland et al., 2000; Anderson, 2010).
Box 2. Why do clinicians not routinely seek a pain history?
1.
Lack of training and expertise
Time constraints
Excessive focus on disease indices
Not part of standard clinical assessment
Gender and ethnic differences
Box 3. Why do patients not always report pain, even if asked?
1.
May believe that pain is inevitable
Pain may not be the primary symptom
Cognitive impairment
Regard pain as an unavoidable consequence of their disease and/or treatment
Wish to be seen in a positive way
Do not wish to challenge the position of the clinician
-
Fearful of the anticipated consequences:
-
○
Hospitalisation/investigations
-
○
Anticipated therapies, including opioid medications
-
○
4. Barriers to appropriate opioid use
Worldwide, one of the most significant barriers to optimal pain management is lack of access to vital opioid medications, due to inappropriate restrictions on their availability and accessibility (INCB, 2016). Opioids are clearly not a panacea for all pains and this position paper is solely concerned with the scientific use of opioid medications in selected and supervised chronic pain patients as part of a comprehensive, multi‐modality, multi‐disciplinary approach to treatment. In this context alone, opioid therapy is an essential and indispensable tool in achieving and maintaining an optimal level of pain control in selected patients. Due regard must be paid to the patients psychological and emotional status – there is always more to achieving and maintaining analgesia than simply prescribing analgesics. The overall objective is to enable rehabilitation of the pain patient in order for them to resume their usual work and leisure activities.
There are enormous variations in opioid use across the globe, and even within regions, similar variations are observed. Key barriers to responsible and appropriate opioid use identified by the International Narcotics Control Board are shown in Box 4 (INCB, 2016).
Box 4. Barriers to responsible and appropriate opioid use.
1.
Unnecessarily strict rules and regulations have created an impediment to providing adequate access of populations to certain controlled medications
The negative perception about controlled drugs among medical professionals and patients has limited their rational use
Lack of economic means and insufficient resources have resulted in inadequate medical treatment, including the use of narcotic drugs
Major differences in opioid use (class, type, administration and dose) between countries and clinicians, as well as lack of uniform guidelines in Europe, have restricted the use in some countries to selected drugs. This neglects the individual differences in response to different opioids
According to the World Health Organization (WHO): ‘the central principle of balance represents a dual obligation of governments to establish a system of control that ensures adequate availability of controlled substances for medical and scientific purposes, while simultaneously preventing abuse, diversion and trafficking. While misuse of controlled substances poses a risk to society, the system of control is not intended to be a barrier to their availability for medical and scientific purposes, nor interfere in their legitimate medical use for patient care’ (WHO, 2011).
A measure of the average per capita consumption of opioids in a given country is not in itself a reliable quality indicator of pain management in that country, yet it does provide some indirect evidence of the awareness among healthcare professionals of the role of opioids in pain management. Although a significant cumulative increase in opioid use has been reported in recent years, this has mainly occurred in a selected limited number of developed countries. Access to opioids varies enormously with the greatest use noted in North America, Western Europe and Oceania. Ninety‐two per cent of the world's morphine is consumed by 17% of the world's population, leaving a mere 8% for distribution amongst 83% of the world's population. Consequently, millions of people are allowed to suffer pain unnecessarily. (INCB, 2016). Cherny et al. note that throughout Europe there are ‘excessively zealous or poorly considered laws and regulations to restrict the diversion of medicinal opioids into illicit markets profoundly interfere with the medical availability of opioids for the relief of pain. Often, the logistics of the treatment of pain with opioids is so burdensome or complex for physicians, nurses or pharmacists as to be a major disincentive to involvement’ (Cherny et al., 2010).
The solution to this major public health issue does not rest solely with any one country, professional group or other constituency. All concerned must work together to implement in a meaningful way the recommendations of the WHO (WHO, 2011) and the Council of Europe (CoE, 2003) in respect of opioid availability and accessibility for legitimate medical and scientific purposes. The European Pain Federation (EFIC) believes that a clearly focused, balanced and coordinated approach at local, regional, national and international level is required if we are to address the gross inequities that currently exist. There is an urgent need to shift the focus from a predominantly opioid‐centric approach to a more patient‐centric approach.
5. Opioids: separating fact from fallacy
Unfortunately, despite all of the available research, extensive clinical experience and published evidence, unjustified and exaggerated concerns regarding the safety of opioids continue to serve as a significant impediment to their appropriate availability, accessibility and rational use (INCB, 2016).
For absolute clarity, the observations made in this article concerning the use of opioids relate exclusively to the medical use of opioid medications in adequately assessed and supervised patients for the sole purpose of achieving and maintaining a satisfactory level of pain and symptom control and are based on the strict understanding and implementation of the core principles of opioid prescribing that are summarised in Box 5 below.
Box 5. Clarification of medical use of opioid medications in adequately assessed and supervised patients.
1.
All patients presenting with pain are adequately assessed by competent clinicians and a management strategy is devised and implemented with due regard to best international practice
All prescribing clinicians are familiar with pain assessment techniques and management guidelines, including the safe and effective use of opioid medications
Non‐specialist prescribers must be able to refer patients for specialist opinion, that will be undertaken within a reasonable time‐frame by a specialist multi‐disciplinary pain team
Opioids are prescribed by competent and responsible clinicians acting solely in the best interests of patient care
The correct dose of any opioid is the lowest possible dose that achieves the desired clinical effect with the minimal side‐effect profile
If a satisfactory outcome is achieved, the patient will remain under close medical surveillance for the duration of opioid therapy
Opioids, as in the case of all other medications, are initiated on a trial basis. If a satisfactory response in not achieved because of inadequate pain control and/or unacceptable burden of side effects, the specific opioid will be safely withdrawn and alternative options actively explored
Patients and families are fully informed regarding the use and storage of opioids and are fully supported throughout the duration of therapy
Opioids are dispensed by competent and responsible pharmacists with due regard to local and national regulations and in accordance with best international practice.
Patients/family members and health care professionals are expected to engage with each other in a truthful and mutually respectful manner
Table 1 summarises some of the most commonly encountered misconceptions concerning opioid use and highlights some of the key facts necessary to understanding the unique role of opioid medications in clinical practice.
Table 1.
Common misconceptions and hard facts about opioids
| Opioid Misconceptions ✗ |
|
| Opioid facts ✓ |
|
There is marked inter‐individual variability in responsiveness to different opioids, both in terms of analgesic benefit and toxicity (de Stoutz et al., 1995; Drewes et al., 2013). Aside from molecular, pharmacological, genetic and phenotypic factors there are various μ‐opioid receptors sub‐types, which may explain, at least in part, the variation in observed clinical responses (Gretton and Droney, 2014).
6. Clinical pharmacology of opioids
There are three classical types of G‐protein coupled opioid receptors: δ‐, κ‐ and μ‐receptors. Opioids act at these receptors as agonists, antagonists or partial agonists. Opioid agonists bind to the receptors to induce cellular hyperpolarisation. The majority of clinically relevant opioid analgesics are agonists of μ‐receptors in the central nervous system (Pathan and Williams, 2012).
Opioids mainly elicit their analgesic action through binding to central μ‐opioid receptors. Opioid‐related side effects are mediated via both central and peripheral μ‐opioid receptors. Genetic variability in μ‐opioid receptors, complex regulation of receptor expression, different binding affinities of the opioids, and additional κ‐ and δ‐opioid receptors all contribute to the need for individualisation of pain treatment (Brunton et al., 2011).
The majority of patients respond to low or moderate doses of opioids. The non‐specialist prescriber should seek expert opinion if a patient needs rapidly escalating doses and/or if the effective dose is in excess of oral morphine equivalent of 120 mg/24 h. Table 2 presents pharmacological data on common opioids, including the dose equivalent to 30 mg morphine (Brunton et al., 2011; DrugBank 2015). Please note that tables of equivalence are intended as a guideline only and do not represent clinically precise equivalence. Further dosing guidance can be obtained using one of a number of opioid dosage conversion apps that are available for smartphones and tablets (Haffey et al., 2013).
Table 2.
Clinical pharmacology of common opioids and approximate dose equivalent to oral morphine 30 mg
| Drug | Absorption fraction (F*) (%) | Protein Binding (%) | Clearance (mL/min/kg) | Half‐life (h) | Volume of distribution (L/kg) | Equivalent dose to 30 mg oral morphine Oral dose unless stated otherwise |
|---|---|---|---|---|---|---|
| Morphine | 24 | 35 | 24.0 | 1.9 | 3.3 | 30 mg |
| Codeine | 50 | 7 | 11.0 | 2.9 | 2.6 | 300 mg |
| Tramadol | 70–75 | 20 | 8.0 | 5.5 | 2.7 | 300 mg |
| Fentanyl | 50 | 84 | 13.0 | 3.7 | 4.0 | 12.5 μg/h (transdermal) |
| Hydromorphone | 42 | 7 | 14.6 | 2.4 | 2.9 | 4 mg |
| Buprenorphine | 28–90 | 96 | 13.3 | 2.3 | 1.4 | 12.5 μg/h (transdermal) |
| Oxycodone | 60–87 | 45 | 12.4 | 2.6 | 2.0 | 15 mg |
| Methadone | 92 | 89 | 1.7 | 27.0 | 3.6 | Variable |
| Tapentadol | 32 | 20 | 20.4 | 5.0 | 7.2 | 100 mg |
7. Guidelines for initiating opioid analgesia
The decision to initiate opioid therapy is made by a senior responsible clinician following a comprehensive assessment of the individual patient and a detailed analysis of the nature of the pain and its impact. For chronic non‐cancer pain, opioid therapy is only initiated when more simple strategies have failed following a reasonable trial. All patients must be fully informed on the proposed therapeutic strategy, including all potential risks and benefits and must be educated on the appropriate use and storage of the opioid medication. It is good practice at the outset to identify and document the expected therapeutic outcomes, both favourable in terms of pain relief and restoration of function, and potential adverse effects. This will be important when determining if the opioid therapy is successful or not.
The recommended practice involves use of a μ‐receptor agonist administered in a slow‐release formulation from the WHO analgesic ladder, based on a single‐prescriber policy. Patients are kept under close medical surveillance and may be encouraged to keep a pain and activity diary. The aim of this section is to outline a practical, step‐by‐step guide to the clinical processes and considerations involved in initiating analgesic therapy with opioids. A flowchart summarizing this algorithm is presented in Fig. 2.
Figure 2.
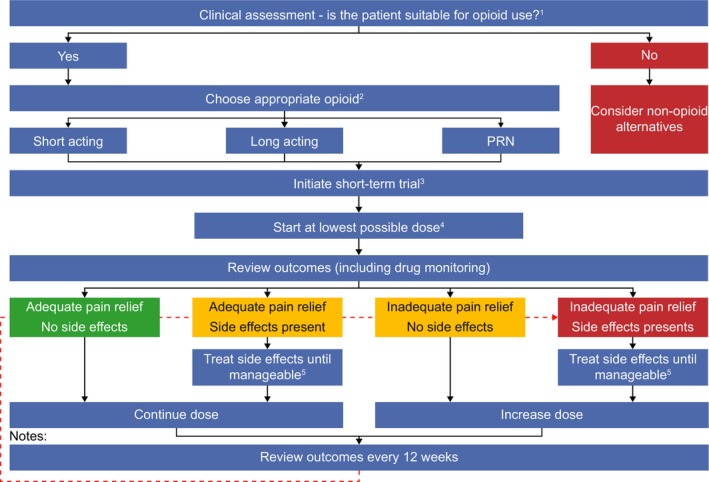
A step‐by‐step guide to the initiation of opioid analgesia.
7.1. Clinical assessment
-
Patient suitability for opioid treatment should be assessed prior to initiation of therapy and consider:
Nature of the pain(s) and documented response to previous treatments
Pain intensity and its likely sensitivity to opioids
Impact of the pain(s) on the patient's life – occupational, social, recreational
Psychosocial assessment including mood, family/social supports, psychiatric morbidities and addiction risk including previous opioid use, if any
Significant co‐morbidities such as gastro‐intestinal, hepatic, renal, respiratory disease etc.
7.2. Definition of therapeutic goals
Agreement on expected therapeutic goals – pain intensity scores and restoration of function/activities
Planned management of anticipated opioid related side effects, particularly in respect of bowel dysfunction
Likelihood of achieving therapeutic goals such as the maximum pain score that is acceptable to an individual patient and functional goals such as resuming hobbies and/or returning to work etc.
7.3. Choose an appropriate opioid
-
Opioid selection should be individualized to the patient. There is no single opioid that is superior to all other opioids on the individual level and therefore opioid therapy is initiated on a trial‐and‐error basis. As response cannot be predicted in advance, physician preference in terms of familiarity and availability is important and also patient preference (where identified) should be respected where possible. Transdermal opioid delivery systems are not suitable for patients with acute, uncontrolled pain. The following shall be taken into consideration when selecting an opioid:
Physician/patient preference in terms of familiarity, availability
Drug–drug interactions and comorbidities identified e.g. avoid methadone and buprenorphine if history of prolonged QT interval/structural heart disease/arrhythmia etc.
No direct clinical trial evidence to suggest any one opioid is superior to any other in initial therapy on individual basis. However, some patients may experience severe side effects to one opioid and not to another and this cannot be predicted (opioid trial phase).
Initiate therapy at the outset to minimize opioid induced bowel dysfunction – laxatives and/or peripherally acting opioid receptor antagonists
7.4. Initiate a short‐term trial
Single‐prescriber, single‐dispenser policy if possible.
Close medical surveillance particularly during the initial titration phase
Monitor with pain, activity and side‐effect diary
Initial course of opioid treatment should be considered as a short‐term therapeutic trial of between several weeks and a few months.
-
Outcomes to consider include:
Progress towards pre‐defined therapeutic goals.
Presence, absence and tolerability of opioid‐related side effects.
Changes in daily physical and social activity.
Changes in underlying pain condition.
Changes in comorbidities or psychiatric health status.
Identification of misuse, abuse or addictive behaviours (e.g. loss of control and/or preoccupation with obtaining opioids despite adequate analgesia and presence of side effects).
7.5. Start at lowest possible dose and up‐titrate stepwise
Initially all patients who are prescribed opioids for the first time should start on the lowest available convenient dose schedule using a long‐acting oral formulation (for good compliance and ease of administration)
Particular caution is required in frail patients in the setting of significant co‐morbidities, e.g. renal dysfunction
Caution is required in patients using other centrally acting drugs such as benzodiazepines – ideally, the co‐prescription of opioids and benzodiazepines should be avoided if possible
-
Long‐acting opioids offer a more convenient option for the patient resulting in:
Enhanced compliance.
Reduction in breakthrough pain episodes.
Reduced likelihood of addiction/abuse or misuse.
7.6. Treatment of side effects
From the beginning, treatment of predictable opioid‐induced side effects is an integral part of effective opioid administration
This is particularly relevant in terms of reducing the burden of opioid induced bowel dysfunction
All patients should receive laxative medications and/or peripherally acting opioid antagonists when initiating opioid therapy
A smaller proportion of patients will experience short‐term nausea at the initiation of opioid therapy. A dopamine receptor antagonist is useful in such circumstances.
In summary, the main objective of the short‐term opioid therapy is to find the best balance between analgesic efficacy and tolerability. That is, to provide the patient with pain relief, while at the same time ensuring that they are comfortable in terms of side effects, with minimal impairment of physical and psychosocial functional status and no aberrant drug‐related behaviour. Patients need to be reviewed regularly for signs of inefficacy, continuous dose escalation, noncompliance, unapproved co‐medications, deterioration in functional status or addiction/abuse or misuse. If any such signs are seen, or there is an unfavourable balance between side effects and analgesia, a full re‐evaluation must be undertaken and an alternative therapeutic strategy pursued.
Key points.
There are six main steps for initiating opioid therapy:
Step 1: Determine whether opioid therapy is suitable for the patient.
Step 2: Define the desired goals of opioid treatment.
Step 3: Choose an appropriate opioid therapy.
Step 4: Initiate a short‐term trial with the chosen opioid and concurrently introduce bowel protection
Step 5: Titrate the opioid dose up from the lowest possible starting dose.
Step 6: Aim to achieve a favourable balance between analgesia and side effects.
8. Opioid switching
In circumstances where an individual patient fails to achieve satisfactory pain control and/or they are troubled by unacceptable side effects, a trial of an alternative opioid is indicated. In clinical practice, one of two strategies to affect the switch may be employed as follows:
The equi‐analgesic dose of the current opioid and the proposed new opioid is established by reference to published equivalence tables. Once the equivalent dose is established, it is further reduced by a factor of 25–50% to establish the new baseline starting dose (Drewes et al., 2013). This reduction is necessary to allow for incomplete cross‐tolerance and inter‐individual variation in response. Equivalence tables are for guidance only as they may underestimate the potency of the new opioid (Fine and Portenoy, 2009). Fatal outcomes can occur during opioid rotation, even when prescribers have not deviated from published guidelines (Webster and Fine, 2012). Once the previous opioid is withdrawn and the new opioid introduced, the clinician will continue to monitor the patient and will titrate the dose as required, based on observed clinical response.
Alternatively, the dose of the established opioid may be reduced sequentially over a number of days and stopped. Simultaneously, the new opioid is introduced at the lowest possible dose and gradually titrated over a number of days to the optimal dose that achieves a satisfactory outcome with an acceptable side‐effect burden.
Key points.
When switching opioids, safe use of an opioid equi‐analgesic dose table involves two steps:
Step 1: Establish the equi‐analgesic dose of the two opioids by reference to published guidelines
Step 2: Reduce the starting dose of the new opioid by a further factor of 25–50% to allow for inter‐individual variation in response and the phenomenon of incomplete cross‐tolerance. An additional dose adjustment may be necessary based on the individual patient characteristics.
9. Assessment and management of short‐term opioid side effects
9.1. Opioid‐induced constipation or opioid‐induced bowel dysfunction
In humans, μ‐, δ‐ and κ‐opioid receptors are present in the gastrointestinal tract, and enteric neurones synthesize endogenous opioid peptides (Holzer, 2009). Activation of enteric opioid receptors by exogenous opioid analgesics results in increased intestinal tone together with decreased and uncoordinated motility, decreased secretion from the intestine and associated organs, and increased tone of the sphincters (Holzer, 2004). This is known as opioid‐induced constipation (OIC), and defined as a change when initiating opioid therapy from baseline bowel habits (over 7 days) characterized by any of the following (Camilleri et al., 2014):
Reduced bowel movement frequency
Development or worsening of straining to pass bowel movements
Sense of incomplete rectal evacuation
Harder stool consistency
However, because opioid receptors are present throughout the gastrointestinal tract, these symptoms are not restricted to the colon. Hence, opioid‐induced bowel dysfunction (OIBD) is a more accurate description. OIBD includes symptoms such as dry mouth, gastro‐oesophageal reflux, vomiting, bloating, abdominal pain, anorexia, hard stool, delayed digestion (constipation) and incomplete evacuation (Pappagallo, 2001; Brock et al., 2012). In placebo trials, OIC occurs in 11% of patients, whereas in non‐cancer and cancer patients treated with opioids prevalence ranges from 33–94% (Kalso et al., 2004; Bell et al., 2009; Tuteja et al., 2010; Dorn et al., 2014), and unlike other side effects of opioids, OIBD does not resolve over time (Akbarali et al., 2014).
Prophylaxis against OIBD is the recommended treatment because there is little evidence that lifestyle changes and fibres improve symptoms (Dorn et al., 2014). Laxatives are recommended as first‐line treatment although their efficacy is generally minimal (Brenner and Chey, 2014) and may explain the high opioid discontinuation rates in patients with GI effects (Bell et al., 2009). Opioid rotation may be helpful in reducing OIBD (Webster and Fine, 2012; Drewes et al., 2013), and some opioids with effects on the noradrenergic system (e.g. tapentadol) may preserve analgesic effects with fewer side effects (Xu et al., 2012).
Another approach is to use opioid antagonists whose effects are strictly limited or ‘compartmentalised’ to the gut. Because there are no significant levels of the anatagonist in the systemic circulation, central analgesia is maintained (Diego et al., 2011; Brenner and Chey, 2014; Leppert, 2014). One such example is an oral, prolonged‐release formulation that combines the agonist oxycodone in a fixed 2:1 ratio with naloxone. Negligible amounts of naloxone reach the systemic circulation because of extensive first‐pass metabolism in the liver. Hence, this formulation is not suitable for patients with significant hepatic impairment. (Leppert, 2014). The effect has been shown to be superior to placebo and laxatives in four RCTs totalling 974 patients, and in which the primary outcome was the Bowel Function Index (Simpson et al., 2008; Lowenstein et al., 2009; Meissner et al., 2009; Ahmedzai et al., 2012).
Another approach is the use of peripherally acting μ‐opioid receptor antagonists such as the subcutaneously administered methylnaltrexone and the oral tablet naloxegol. Naloxegol was investigated in two RCTs where bowel function improved, whereas pain intensity and opioid requirements were unchanged, and no withdrawal symptoms or serious cardiovascular events were observed. Moreover, upper gastrointestinal dysfunctions improved (Webster et al., 2013; Chey et al., 2014; Poulsen et al., 2015).
A Cochrane review on the use of laxatives and peripherally acting mu opioid receptor antagonists for the management of constipation in palliative care patients found insufficient evidence to support laxative use because of a paucity of randomized controlled trials. However, there is evidence demonstrating the efficacy of peripherally acting mu opioid receptor antagonists in inducing laxation in palliative care patients with opioid induced constipation where laxatives have failed. As this is a relatively new therapeutic strategy, longer term safety data are required (Candy et al., 2011). A recent consensus review from Scandinavia has outlined the pitfalls in OIBD (Drewes et al., 2016) and an adopted treatment algorithm is shown in Fig. 3.
Figure 3.
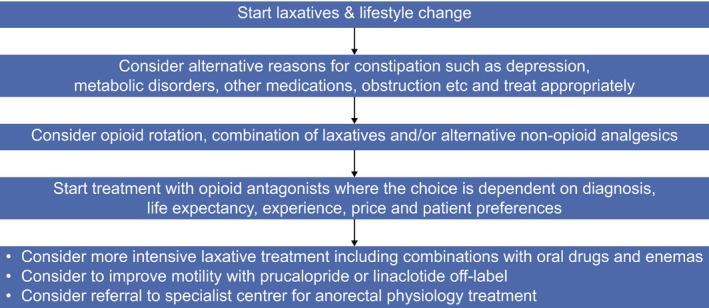
Algorithm to treat opioid induced constipation and bowel dysfunction. The arrows indicate failure of the first recommendation and thus continuation to next step. Treatment goals are to establish regular bowel function and eliminate upper gastrointestinal symptoms, improve QoL and avoid complications, such as haemorrhoids, rectal prolapse and faecal impaction. As support for clinical evaluation questionnaires such as the Bowel Function Index may be used, where a score >30 should lead to more intensive treatment.
9.2. Opioid‐induced emesis
Opioid‐induced nausea and vomiting are experienced by up to 40% of pain patients with no history of emesis. However, because this is an inconsistent consequence of opioid therapy, prophylactic antiemetics are not generally prescribed. A systematic review (Laugsand et al., 2011) found nine studies with relief of opioid‐induced nausea and vomiting as the primary outcome, only two of which reported efficacy following high‐dose metoclopramide treatment. Fifty studies of low quality included nausea and/or vomiting as secondary outcomes; these indicated that emesis could be alleviated by opioid dose reduction, switching opioid therapy, or changing route of administration (e.g. oral to transdermal or parenteral). It is recommended that some antidopaminergic drugs (e.g. haloperidol, domperidone) and other agents with antidopaminergic and additional modes of action (e.g. metoclopramide) are used to treat opioid‐induced emesis. However, it should be borne in mind that domperidone can cause cardiotoxic effects that prolong the QT interval (GOV.UK, 2014). Moreover, high‐dose and/or long‐term use of metoclopramide may cause short‐term extrapyramidal disorders and tardive dyskinesia (GOV.UK, 2013). It is therefore recommended that domperidone and metoclopramide are administered at the lowest effective dose for the shortest possible time.
9.3. Opioid‐related central nervous system side effects
Opioid‐related CNS side effects can be separated into symptoms and signs associated with a lowering level of consciousness (e.g. sedation and drowsiness), cognitive and psychomotor impairment, and hyperexcitability reactions (e.g. hallucinations, myoclonus and hyperalgesia) (Vella‐Brincat and Macleod, 2007). In all cases, it is recommended that the opioid dose is lowered, and also to check for precipitating causes such as renal dysfunction. If a dose reduction is ineffective or poorly tolerated, then a careful re‐evaluation of opioid choice and other analgesic options is required. In patients with opioid‐related neurotoxicity (delirium, hallucination, myoclonus and hyperalgesia), dose reduction or opioid switching should be considered. Recent case reports suggest a possible neurotoxic effect of opioids that manifests as a subjective disturbance in auditory function. This is typically described as a subjective sensation of ‘blocked ears’ or an experience reported as similar to what sometimes occurs when descending in an aircraft (Cran et al., 2014).
Key points.
OIBD is caused by blockade of different enteric opioid receptors, resulting in decreased gut motility and secretion and reduced sphincter tone.
OIBD is a whole gut syndrome. Symptoms include constipation, as well as dry mouth, gastro‐oesophageal reflux, vomiting, bloating, abdominal pain, anorexia, hard stool, delayed digestion and incomplete evacuation.
OIBD can be managed prophylactically with laxatives, by opioid rotation, or by opioid antagonists with effects restricted to the gut (e.g. a combination of prolonged‐release oxycodone plus naloxone).
Up to 40% of pain patients treated with opioids experience emesis.
Opioid‐induced emesis is alleviated by opioid dose reduction, opioid rotation, changing route of administration or treatment with an antidopaminergic drug (e.g. haloperidol) or an antidopaminergic drug with additional modes of action (e.g. metoclopramide).
Opioid‐related CNS side effects include sedation, drowsiness, cognitive and psychomotor impairment, hallucinations, myoclonus and hyperalgesia.
Opioid‐related CNS side effects should be managed by dose reduction and/or opioid switching, and patients checked for underlying causes (e.g. renal dysfunction).
10. Managing long‐term use of opioids
Patients on long‐term opioid therapy must be kept under close clinical surveillance. At all times, the opioid dose is kept to the minimal level that achieves the desired outcome. Equally, side effects must be kept under review, especially in terms of bowel dysfunction (which is not dose‐dependent and is the only opioid side effect with a mechanism‐based therapy). As with any other medication, opioid therapy should only continue if it is clinically beneficial, with an acceptable side‐effect profile that does not further compromise patient quality of life.
Long‐term opioid treatment of up to 6 months will benefit approximately 25% of patients with painful conditions such as osteoarthritis, diabetic polyneuropathy, postherpetic neuralgia and chronic low back pain (Häuser et al., 2014). In such patients, regular clinical reviews are required to assess pain control, impact on lifestyle (daily activities, sleep disturbance and participation), physical and psychological well‐being, side effects and continued need for treatment (Häuser et al., 2014).
After 6 months of opioid therapy, a dose reduction (or ‘drug holiday’) should be considered and discussed with the patient, to determine whether continued opioid treatment is appropriate (Häuser et al.,2015). The response to non‐pharmacological treatment, such as pain‐related physiotherapy, psychotherapy and systematic lifestyle modification, should also be assessed (Häuser et al.,2015). Discontinuation of opioid therapy should be considered if the individual goals of treatment are not met; if side effects are intolerable and/or untreatable; if the individual goals are reached by additional treatment (e.g. surgery or physiotherapy); or if the patient shows signs of opioid misuse, abuse or addiction (Häuser et al.,2014). In relation to the latter point, using opioids for pain control in drug‐dependent patients is complex and should always prompt referral to a specialist service.
A situation may arise where the clinician decides that discontinuing opioids is required, but the patient disagrees and seeks to continue the therapy. It may be difficult for the clinician to determine if the patient's insistence represents a genuine desire for pain relief, inappropriate drug seeking behaviour or a combination of both (Alford, 2016). In such circumstances, the clinician is the responsible prescriber and must be fully satisfied that continued opioid prescription is the correct management strategy. If not, the decision must be respectfully explained to the patient that opioid prescriptions are to be withdrawn. Other therapeutic options and more specialist multi‐disciplinary assessment should be undertaken in such circumstances.
Key points.
Regular clinical reviews are required for long‐term (≥26 weeks) opioid therapy, to assess pain control, impact on lifestyle, physical and psychological well‐being, side effects and continued need for treatment.
A ‘drug holiday’ should be considered after 6 months of opioid therapy, and additional non‐pharmacological treatments should be explored.
Discontinuation of opioid therapy should be considered if treatment goals are not met, side effects are intolerable/untreatable, if additional non‐pharmacological treatments are effective alone, or there are signs of opioid misuse, abuse or addiction.
Using opioids for pain control in drug‐dependent patients is complex and should always prompt referral to a specialist service.
11. The use of opioids in special populations
Opioids should always be considered in selected chronic pain patients, irrespective of their underlying co‐morbidities. However, additional care must be taken when choosing the appropriate type and dose of opioid, particularly in patients with renal or hepatic insufficiency.
11.1. Renal insufficiency
In patients with renal insufficiency, changes in response to opioids can result from impaired excretion (and thus accumulation of the parent opioid and/or its metabolites), changes in acid base balance, protein levels, volume of distribution and absorption. The impairment of excretion increases in line with renal dysfunction and might be predicted by estimates of the glomerular filtration rate.
Opioids that are transformed to analgesic active or toxic metabolites and are dependent on the kidneys for excretion should be avoided. Based on available pharmacokinetic data, the opioids that are least likely to cause harm to patients with real insufficiency are: fentanyl, buprenorphine and oxymorphone. Opioids to be used with caution in this special population are hydromorphone and oxycodone. Patients with renal insufficiency should generally not be prescribed codeine, morphine, pethidine, dextropropoxyphene and tramadol (Coller et al., 2009; King et al., 2015; Tawfic and Bellingham, 2015).
11.2. Hepatic insufficiency
In patients with hepatic insufficiency, changes in response to opioids can result from altered pharmacokinetics. Portosystemic shunting may decrease first‐pass metabolism and increase systemic bioavailabilty, and distribution may alter due to decreased production of drug‐binding enzymes, or changes in body composition. Furthermore, rate of metabolism may decrease due to altered activity and capacity of the metabolizing enzymes, cytochrome P450 (CYP) and uridine 5′‐diphospho‐glucuronosyltransferase (UGT); CYP activity is affected more than UGT activity.
The impairment of opioid metabolism increases in line with increased liver dysfunction. Changes are most pronounced in cirrhotic patients. Little if any changes are seen in patients with chronic liver diseases without a significant fibrosis component. Dose adjustment and other precautions are therefore only relevant in severe liver diseases. Of note, however, is the fact routine liver function tests are not necessarily a reliable index of the severity of underlying liver fibrosis and portosystemic shunting. Opioids that require a prolonged dose interval or a dose reduction are tramadol, tapentadol, morphine, hydromorphone and oxycodone. Codeine and pethidine should be avoided in patients with hepatic impairment (Tegeder et al., 1999; Bosilkovska et al., 2012).
Key points.
-
1
In patients with renal insufficiency:
Avoid: Codeine, morphine, pethidine, dextropropoxyphene and tramadol.
Use with caution: Hydromorphone and oxycodone.
Least likely to cause harm: Fentanyl, buprenorphine and oxymorphone.
-
2
In patients with hepatic insufficiency, dose adjustment and other precautions are normally only relevant in severe liver disease, as follows:
Avoid: Codeine and pethidine.
Prolong dose interval or reduce dose: Tramadol, tapentadol, morphine and oxycodone.
Reduce dose: Hydromorphone.
11.3. Opioid use in patients at risk of drug abuse
Numerous assessment tools are available to assess drug‐seeking behaviour (Chou et al., 2009; Atluri et al., 2012), although the majority have methodological flaws making them unsuitable for screening patients before initiating chronic opioid therapy. However, the Screener and Opioid Assessment for Patients with Pain‐Revised (SOAPP‐R) tool is reasonably effective in conjunction with clinical assessment (Chou et al., 2009).
Three other useful tools to assess risk of inappropriate prescription opioid use are the Addiction Behaviours Checklist (ABC) (Wu et al., 2006), the Diagnosis, Intractability, Risk and Efficacy (DIRE) score (Belgrade et al., 2006), and the six‐criteria screening tool created by Atluri and Sudarshan (Atluri and Sudarshan, 2004). All three are valid tests based on highly objective questions (Atluri et al., 2012). For patients suspected of being at high risk of inappropriate opioid use, urine drug screens, prescription monitoring programs and dose limitations are options to prevent abuse/misuse (Atluri et al., 2012). The most important aspect of this discussion is the fact that doctors are not treating pain per se, but are treating patients. Hence, it is vital that doctors take time to get to know and understand their patient, so that their therapeutic relationship is built on mutual respect, honesty and trust. Legitimate opioids treatment in patients at risk of abuse should generally be considered a specialist task.
11.4. Opioid use while driving or working
There is a clear relationship between the illicit use or abuse of opioids and fatal car crashes (Dubois et al., 2010; Corsenac et al., 2012; Reguly et al., 2014; Wilson et al., 2014), which has led to laws across Europe limiting opioid use while driving or working. However, research conducted over the last 20 years indicates that patients on stable doses of prescribed opioids for legitimate clinical purposes show little, if any, impairment to their driving skills (Vainio et al., 1995; Sabatowski et al., 2003, 2014; Dagtekin et al., 2007; Amato et al., 2013). The key is for patients to be on stable opioid therapy of at least 5–7 days’ duration, and ensuring that no other psychoactive drugs are being taken concomitantly (Kress and Kraft, 2005). The International Association for the Study of Pain (IASP) advises that opioid analgesics should be used with caution when combined with CNS depressant drugs such as benzodiazepines. (IASP, 2015).
Physicians prescribing opioids should ensure that their patients are well‐informed of the risks and benefits. Despite the scientific data suggesting minimal impact on driving ability, a patient's decision to drive or use machinery remains theirs alone, and caution is advised. With long‐term opioid use, prescription changes should be handled by a single physician or team who should reiterate the risks and benefits and explain the need for additional caution in the days and weeks following the change. Use of a well‐informed, written consent form is recommended for these purposes.
Key points.
PCPs must clearly explain the potential risks of driving or working while taking opioids.
Avoid co‐prescription of other psychoactive drugs, or a change in current prescribed dosage, without a further explanation of the potential risks to the patients
Regularly monitor therapy and ensure any changes in the prescription is made by the same physician, or the same team.
11.5. Educational requirements to prescribe opioids
Education on pain management and opioid use must begin at undergraduate level. In Europe there appears to be a lack of education about pain, both at undergraduate level in medical schools and during residency training. The APPEAL study, which involved 242 undergraduate medical schools in 15 EU countries, found that 82% of schools did not have dedicated pain management courses that were compulsory for all students (EFIC, 2013a). To begin to address this, in 2013 the EFIC published the Pain Management Core Curriculum for European Medical Schools, which encourages both students and medical schools to be gain in‐depth knowledge and training about pain management (EFIC, 2013b). In conjunction with this curriculum, the EFIC offers a Diploma of Pain Medicine in order to better provide up‐to‐date knowledge, thinking and management, as well as to allow sharing of best practice amongst European clinicians involved in pain treatment. The Diploma is multidisciplinary, recognising that no single healthcare professional will have all the necessary training and skills to treat all patients across the pain spectrum. Knowledge of the curriculum, pain assessment and treatment skills are first evaluated by a multiple choice examination questionnaire. This is followed by a series of objective structured clinical examinations, which test clinical skill performance and competence in modalities such as communication, clinical examination and diagnosis and treatment planning, including referral to colleagues for appropriate management. The present review will be included in the recommended reading list for the EFIC's curriculum.
However, more needs to be done in this direction. A positive and comprehensive educational programme is needed to change attitudes on the medical use of opioids, extending from the core curriculum of medical students to patients taking opioids, as well as their families and employers. This education should cover the rules governing opioid use, the management of their side‐effects and, most importantly, an unequivocal, evidence‐based message from governments highlighting issues relating to abuse and misuse, but distinguishing them from appropriate medical use. The need for training in the correct use of opioids is multifaceted. Education would include drug–drug interactions, safe dosing, how to transition from one medication to another, how to monitor and look for signs of abuse, pharmacokinetics, pharmacodynamics, and how to use one's state prescription monitoring program. The EFIC organises Pain Schools, which are continuing medical education (CME) courses aimed at young European medical doctors. These would be the ideal setting to provide specialist training to enable PCPs to confidently and safely prescribe opioid analgesics to patients with chronic pain conditions.
Alford (Alford, 2016) highlights the need for prescriber education which he believes will ‘empower clinicians to make appropriate, well‐informed decisions about whether to initiate, continue, modify or discontinue opioid treatment for individual patients at each clinical encounter. Education has the power to both reduce overprescribing and ensure that patients in need retain access to opioids’.
Key points.
In Europe there is currently a lack of education about pain in medical schools or during residency training.
Training should include drug–drug interactions, safe dosing, how to transition from one medication to another, how to monitor and look for signs of abuse, pharmacokinetics, pharmacodynamics, and how to use one's state prescription monitoring program.
Policies need to be developed to encourage education of practitioners to ensure they take the necessary precautions to prescribe opioid analgesics responsibly.
The EFIC is committed to providing such education through its Pain Schools CME courses.
12. Conclusions
Pain is a global public health problem. In Europe, the reasons are many and varied but ultimately relate to a lack of understanding of the nature and impact of chronic pain and a failure to apply evidence based strategies to pain management. The situation is further compounded by an inappropriate and exaggerated fear concerning the legitimate scientific use of opioid medications as part of a comprehensive pain management strategy in carefully selected and supervised patients. In many countries, overly stringent and ill‐considered restrictions and regulations intended to prevent illicit, non‐medical use of opioids, results in patients suffering unnecessarily. Opioids should only be introduced when less potent analgesics and adjuvant therapies have failed to achieve and maintain adequate pain relief and rehabilitation. A positive educational programme is required to change attitudes towards the proper medical use of opioids, both in the medical community and for patients, their families and wider society. The European Pain Federation (EFIC) is committed to improving the understanding of opioids and their role in pain management, and believes that appropriate education and training will enable PCPs to prescribe opioids responsibly.
The medical profession is compassionate enough and bright enough to learn how to prescribe opioids when they are indicated in ways that maximise benefit and minimise harm. Though managing chronic pain is complicated and time‐consuming, we owe it to our patients to ensure access to comprehensive pain management, including the medically appropriate use of opioids (Alford, 2016).
Author contributions
Each of the authors made a substantial contribution to the development of the paper by informing its content, collaborating in the drafting of the various sections and reviewing the final draft. TO'B served as chairperson of the group and played a major role in the coordination and editing of the various contributions and in liaising with the publisher.
Acknowledgements
We gratefully acknowledge the editorial support received from Mr Neil Morrison and Mr Kevin West of GKPharmacomm in the preparation of this position paper.
Funding sources
The project was funded in full by EFIC – European Federation of IASP Chapters.
Conflicts of interest
TOB has received fees and grant support from Archimedes, Astra Zeneca, Cephalon, Grunenthal, Janssen‐Cilag, Mundipharma, Roche and Teva; LLC has received an unrestricted grant from Norpharma and has participated in advisory boards for Grünenthal; AMD has received unrestricted grants from Mundipharma, AstraZeneca and Grünenthal; MTF has received research grants from Mundipharma, Pfizer and GW Pharmaceuticals; has participated in advisory boards for GW Pharmaceuticals, Grunenthal, Pfizer and Mundipharma and has received speaker fees from Mundipharma, Pfizer and Astellas; HGK has received honoraria as a speaker or consultant from Angelini/CSC Pharma, Astellas, Bionorica SE, Boehringer Ingelheim, Teva ratiopharm, Grunenthal, IBSA, Linde Group, Mundipharma Int., Nevro, Philips. St Jude; HJM has received consultancy fees from Menarini; GM has participated in advisory boards for AstraZeneca, Mundipharma GmbH and has received speaker fees from Archimedes, Astra Zeneca, Indivior, MSD, Mundipharma GmbH and ProStrakan; BJM has provided consultancy services to Astellas, Boehringer Ingelheim, Grunenthal, Mundipharma, Teva and served on Mundipharma Speakers’ bureau; JPC; EPZ has received financial support from Mundipharma GmbH, lectured on behalf of MSD Sharp & Dohme GmbH, Merck, Mundipharma GmbH, Mundipharma International, Pfizer Deutschland GmbH, Janssen‐Cilag GmbH, Fresenius Kabi, Grünenthal and The Medicines Company, and acted as advisor for MSD Sharp & Dohme GmbH; Merck; Mundipharma GmbH; Janssen‐Cilag GmbH, Grünenthal and The Medicines Company; GV has received unrestricted funds for research by Dompé and has participated in advisory boards of Abbott, Dompé and Menarini International. JCDW has received speaker and consultancy fees from Grünenthal and Indivior.
References
Web References
- Ahmedzai, S.H. , Nauck, F. , Bar‐Sela, G. , Bosse, B. , Leyendecker, P. , Hopp, M. (2012). A randomized, double‐blind, active‐controlled, double‐dummy, parallel‐group study to determine the safety and efficacy of oxycodone/naloxone prolonged‐release tablets in patients with moderate/severe, chronic cancer pain. Palliat Med 26, 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarali, H.I. , Inkisar, A. , Dewey, W.L. (2014). Site and mechanism of morphine tolerance in the gastrointestinal tract. Neurogastroenterol Motil 26, 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford, D.P. (2016). Opioid prescribing for chronic pain – achieving a right balance through education. N Engl J Med 374, 301–303. [DOI] [PubMed] [Google Scholar]
- Amato, J.N. , Marie, S. , Lelong‐Boulouard, V. , Paillet‐Loilier, M. , Berthelon, C. , Coquerel, A. , Denise, P. , Bocca, M.L. (2013). Effects of three therapeutic doses of codeine/paracetamol on driving performance, a psychomotor vigilance test, and subjective feelings. Psychopharmacology 228, 309–320. [DOI] [PubMed] [Google Scholar]
- American Pain Society Quality of Care Committee (1995). Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA 274, 1874–1880. [DOI] [PubMed] [Google Scholar]
- Anderson, K.O. (2010). The assessment of cancer pain: Measurement strategy In Cancer Pain: Assessment and Management, Bruera E.D., Protenoy R.K., eds. (Cambridge: Cambridge University Press; ) pp. 89–104. [Google Scholar]
- Anderson, K.O. , Mendoza, T.R. , Valero, V. , Richman, S.P. , Russell, C. , Hurley, J. , DeLeon, C. , Washington, P. , Palos, G. , Payne, R. , Cleeland, C.S. (2000). Minority cancer patients and their providers: Pain management attitudes and practice. Cancer 88, 1929–1938. [PubMed] [Google Scholar]
- Anderson, K.O. , Richman, S.P. , Hurley, J. , Palos, G. , Valero, V. , Mendoza, T.R. , Gning, I. , Cleeland, C.S. (2002). Cancer 94, 2295–2304. [DOI] [PubMed] [Google Scholar]
- Atluri, S.L. , Sudarshan, G. (2004). Development of a screening tool to detect the risk of inappropriate prescription opioid use in patients with chronic pain. Pain Physician 7, 333–338. [PubMed] [Google Scholar]
- Atluri, S. , Akbik, H. , Sudarshan, G. (2012). Pain prevention of opioid abuse in chronic non‐cancer pain: An algorithmic, evidence based approach. Pain Physician 15, ES177–ES189. [PubMed] [Google Scholar]
- Belgrade, M.J. , Schamber, C.D. , Lindgren, B.R. (2006). The DIRE score: Predicting outcomes of opioid prescribing for chronic pain. J Pain 7, 671–681. [DOI] [PubMed] [Google Scholar]
- Bell, T.J. , Panchal, S.J. , Miaskowski, C. , Bolge, S.C. , Milanova, T. , Williamson, R. (2009). The prevalence, severity, and impact of opioid‐induced bowel dysfunction: Results of a US and European Patient Survey (PROBE 1). Pain Med 10, 35–42. [DOI] [PubMed] [Google Scholar]
- Bosilkovska, M. , Walder, B. , Besson, M. , Daali, Y. , Desmeules, J. (2012). Analgesics in patients with hepatic impairment. Drugs 72, 1645–1669. [DOI] [PubMed] [Google Scholar]
- Breivik, H. , Collett, B. , Ventafridda, V. , Cohen, R. , Gallacher, D. (2006). Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 10, 287–333. [DOI] [PubMed] [Google Scholar]
- Breivik, H. , Eisenberg, E. and O'Brien, T. , OPENMinds . (2013). The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 13, 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, D.M. , Chey, W.D. (2014). An evidence‐based review of novel and emerging therapies for constipation in patients taking opioid analgesics. Am J Gastroenterol Suppl 2, 38–46. [Google Scholar]
- Brock, C. , Olesen, S.S. , Olesen, A.E. , Frøkjær, J.F. , Andresen, T. , Drewes, A.M. (2012). Opioid‐induced bowel dysfunction: Pathophysiology and management. Drugs 72, 1847–1865. [DOI] [PubMed] [Google Scholar]
- Brunton L., Chabner B., Knollmann B., eds. (2011). Goodman & Gilman's The Pharmacological Basis of Therapeutics 12th Edition. (New York, NY: McGraw‐Hill; ). Chapter 119. Opiates and Opioids; Chapter 207. Morphine. [Google Scholar]
- Camilleri, M. , Drossman, D.A. , Becker, G. , Webster, L.R. , Davies, N. , Mawe, G.M. (2014). Emerging treatments in neurogastroenterology: A multidisciplinary working group consensus statement on opioid‐induced constipation. Neurogastroenterol Motil 26, 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candy, B. , Jones, L. , Goodman, M.L. , Drake, R. , Tookman, A. (2011). Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev 1, CD003448. [DOI] [PubMed] [Google Scholar]
- Cherny, N.I. , Baselga, J. , de Conno, F. , Radbruch, L. (2010). Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Europe – a report from ESMO and EAPC opioid policy initiative. Ann Oncol 21, 615–626. [DOI] [PubMed] [Google Scholar]
- Chey, W.D. , Webster, L. , Sostek, M. , Lappalainen, J. , Barker, P.N. , Tack, J. (2014). Naloxegol for opioid‐induced constipation in patients with noncancer pain. N Engl J Med 370, 2387–2396. [DOI] [PubMed] [Google Scholar]
- Chou, R. , Fanciullo, G.J. , Fine, P.G. , Adler, J.A. , Ballantyne, J.C. , Davies, P. , Donovan, M.I. , Fishbain, D.A. , Foley, K.M. , Fudin, J. , Gilson, A.M. , Kelter, A. , Mauskop, A. , O'Connor, P.G. , Passik, S.D. , Pasternak, G.W. , Portenoy, R.K. , Rich, B.A. , Roberts, R.G. , Todd, K.H. , Miaskowski, C. ; American Pain Society‐American Academy of Pain Medicine Opioids Guidelines Panel (2009). Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 10, 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland, C.S. , Janjan, N.A. , Scott, C.B. , Seiferheld, W.F. , Curran, W.J. (2000). Cancer pain management by radiotherapists: A survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys 47, 203–208. [DOI] [PubMed] [Google Scholar]
- Coller, J.K. , Christrup, L.L. , Somogyi, A. (2009). Role of active metabolites in the use of opioids. Eur J Clin Pharmacol 65, 121–139. [DOI] [PubMed] [Google Scholar]
- Corsenac, P. , Lagarde, E. , Gadegbeku, B. , Delorme, B. , Tricotel, A. , Castot, A. , Moore, N. , Philip, P. , Laumon, B. , Orriols, L. (2012). Road traffic crashes and prescribed methadone and buprenorphine: A French registry‐based case‐control study. Drug Alcohol Depend 123, 91–97. [DOI] [PubMed] [Google Scholar]
- Cran, A. , Kiely, F. , O'Brien, T. (2014). Auditory symptoms as an unrecognised manifestation of opioid toxicity: Two case reports. J Pain Palliat Care Pharmacother 28, 378–381. [DOI] [PubMed] [Google Scholar]
- Dagtekin, O. , Gerbershagen, H.J. , Wagner, W. , Petzke, F. , Radbruch, L. , Sabatowski, R. (2007). Assessing cognitive and psychomotor performance under long‐term treatment with transdermal buprenorphine in chronic non‐cancer pain patients. Anesth Analg 105, 1442–1448. [DOI] [PubMed] [Google Scholar]
- Dalal, S. , Bruera, E. (2012). Assessing cancer pain. Curr Pain Headache Rep 16, 314–324. [DOI] [PubMed] [Google Scholar]
- Dar, R. , Beach, C.M. , Barden, P.L. , Cleeland, C.S. (1992). Cancer pain in the marital system: A study of patients and their spouses. J Pain Symptom Manage 7, 87–93. [DOI] [PubMed] [Google Scholar]
- Diego, L. , Atayee, R. , Helmons, P. , Hsiao, G. , von Gunten, C.F. (2011). Novel opioid antagonists for opioid‐induced bowel dysfunction. Expert Opin Investig Drugs 20, 1047–1056. [DOI] [PubMed] [Google Scholar]
- Dorn, S. , Lembo, A. , Cremonini, F. (2014). Opioid‐Induced bowel dysfunction: Epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol Suppl 2, 31–37. [DOI] [PubMed] [Google Scholar]
- Drewes, A.M. , Jensen, R.D. , Nielsen, L.M. , Droney, J. , Christrup, L.L. , Arendt‐Nielsen, L. , Riley, J. , Dahan, A. (2013). Differences between opioids: Pharmacological, experimental, clinical and economical perspectives. Br J Clin Pharmacol 75, 60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes, A.M. , Munkholm, P. , Simrén, M. , Breivik, H. , Kongsgaard, U.E. , Hatlebakk, J.G. , Agreus, L. , Christrup, L.L. (2016). Definition, diagnosis and treatment strategies for opioid‐induced bowel dysfunction – recommendations of the Nordic Working Group. Scand J Pain 16, 11–22. [DOI] [PubMed] [Google Scholar]
- Dubois, S. , Bédard, M. , Weaver, B. (2010). The association between opioid analgesics and unsafe driving actions preceding fatal crashes. Accid Anal Prev 42, 30–37. [DOI] [PubMed] [Google Scholar]
- Fine, P.G. , Portenoy, R.K. ; Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation (2009). Establishing “best practices” for opioid rotation: Conclusions of an expert panel. J Pain Symptom Manage 38, 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson, A.M. , Maurer, M.A. , Ryan, K.M. , Skemp‐Brown, M. , Husain, A. , Cleary, J. (2011). Ensuring patient access to essential medicines while minimising harmful use: A revised World Health Organization tool to improve national drug control policy. J Pain Palliat Care Pharmacother 25, 246–251. [DOI] [PubMed] [Google Scholar]
- Gretton, S.K. , Droney, J. (2014). Splice variation of the μ receptor and its effect on the action of opioids. Br J Pain 8, 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffey, F. , Brady, R.R. , Maxwell, S. (2013). A comparison of the reliability of smartphone apps for opioid conversion. Drug Saf 36, 111–117. [DOI] [PubMed] [Google Scholar]
- Häuser, W. , Bock, F. , Engeser, P. , Tölle, T. , Willweber‐Strumpf, A. , Petzke, F. (2014). Long‐term opioid use in non‐cancer pain. Dtsch Arztebl Int 111, 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häuser, W. , Klose, P. , Welsch, P. , Petzke, F. , Nothacker, M. , Kopp, I. (2015). Methodology of the development of the updated LONTS guidelines for long‐term administration of opioids in noncancer pain]. [Article in German]. Schmerz 29, 8–34. [DOI] [PubMed] [Google Scholar]
- Holzer, P. (2004). Pharmacology of opioids and their effects on gastrointestinal function. Am J Gastroenterol Suppl 2, 9–16. [Google Scholar]
- Holzer, P. (2009). Opioid receptors in the gastrointestinal tract. Regul Pept 155, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalso, E. , Edwards, J.E. , Moore, R.A. , McQuay, H.J. (2004). Opioids in chronic non‐cancer pain: Systematic review of efficacy and safety. Pain 112, 372–380. [DOI] [PubMed] [Google Scholar]
- King, S. , Forbes, K. , Hanks, G.W. , Ferro, C.J. , Chambers, E.J. (2015). A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment. A European Palliative Care Research Collaborative opioid guidelines project. Palliat Med 25, 525–552. [DOI] [PubMed] [Google Scholar]
- Kress, H.G. , Kraft, B. (2005). Opioid medication and driving ability. Eur J Pain 9, 141–144. [DOI] [PubMed] [Google Scholar]
- Laugsand, E.A. , Kaasa, S. , Klepstad, P. (2011). Management of opioid‐induced nausea and vomiting in cancer patients: Systematic review and evidence‐based recommendations. Palliat Med 25, 442–453. [DOI] [PubMed] [Google Scholar]
- Leppert, W. (2014). Oxycodone/naloxone in the management of patients with pain and opioid–induced bowel dysfunction. Curr Drug Targets 15, 124–135. [DOI] [PubMed] [Google Scholar]
- Löwenstein, O. , Leyendecker, P. , Hopp, M. , Schutter, U. , Rogers, P.D. , Uhl, R. , Bond, S. , Kremers, W. , Nichols, T. , Krain, B. , Reimer, K. (2009). Combined prolonged‐release oxycodone and naloxone improves bowel function in patients receiving opioids for moderate‐to‐severe non‐malignant chronic pain: A randomised controlled trial. Expert Opin Pharmacoher 10, 531–543 [DOI] [PubMed] [Google Scholar]
- Meissner, W. , Leyendecker, P. , Mueller‐Lissner, S. , Nadstawek, J. , Hopp, M. , Ruckes, C. , Wirz, S. , Fleischer, W. , Reimer, K. (2009). A randomized controlled trial with prolonged‐release oral oxycodone and naloxone to prevent and reverse opioid‐induced constipation. Eur J Pain 13, 56–64. [DOI] [PubMed] [Google Scholar]
- Mularski, R.A. , White‐Chu, F. , Overbay, D. , Miller, L. , Asch, S.M. , Ganzini, L. (2006). Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med 21, 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappagallo, M. (2001). Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg 182, S11–S18. [DOI] [PubMed] [Google Scholar]
- Pathan, H. , Williams, J. (2012). Basic opioid pharmacology: An update. Br J Pain 6, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen, J.L. , Brock, C. , Olesen, A.E. , Nilsson, M. , Drewes, A.M. (2015). Evolving paradigms in the treatment of opioid‐induced bowel dysfunction. Therap Adv Gastroenterol 8, 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguly, P. , Dubois, S. , Bédard, M. (2014). Examining the impact of opioid analgesics on crash responsibility in truck drivers involved in fatal crashes. Forensic Sci Int 234, 154–161. [DOI] [PubMed] [Google Scholar]
- Ripamonti, C.I. , Santini, D. , Maranzano, E. , Berti, M. , Roila, F. (2012). Management of cancer pain: ESMO clinical guidelines. Ann Oncol 23 (Suppl 7), vii39–vii154. [DOI] [PubMed] [Google Scholar]
- Sabatowski, R. , Schwalen, S. , Rettig, K. , Herberg, K.W. , Kasper, S.M. , Radbruch, L. (2003). Driving ability under long‐term treatment with transdermal fentanyl. J Pain Symptom Manage 25, 38–47. [DOI] [PubMed] [Google Scholar]
- Sabatowski, R. , Scharnagel, R. , Gyllensvärd, A. , Steigerwald, I. (2014). Driving ability in patients with severe chronic low back or osteoarthritis knee pain on stable treatment with tapentadol prolonged release: A multicenter, open‐label, phase 3b trial. Pain Ther 3, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, K. , Leyendecker, P. , Hopp, M. , Müller‐Lissner, S. , Löwenstein, O. , De Andrés, J. , Troy Ferrarons, J. , Bosse, B. , Krain, B. , Nichols, T. , Kremers, W. , Reimer, K. (2008). Fixed‐ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid‐induced constipation in moderate‐to‐severe noncancer pain. Curr Med Res Opin 24, 3503–3512. [DOI] [PubMed] [Google Scholar]
- de Stoutz, N.D. , Bruera, E. , Suarez‐Almazor, M. (1995). Opioid rotation for toxicity reduction in terminal cancer patients. J Pain Symptom Manage 10, 378–384. [DOI] [PubMed] [Google Scholar]
- Tawfic, Q.A. , Bellingham, G. (2015). Post‐operative management in patients with chronic kidney disease. J Anaesthesiol Clin Pharmacol 31, 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder, I. , Lötsch, J. , Geisslinger, G. (1999). Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet 37, 17–40. [DOI] [PubMed] [Google Scholar]
- Tuteja, A.K. , Biskupiak, J. , Stoddard, G.J. , Lipman, A.G. (2010). Opioid‐induced bowel disorders and narcotic bowel syndrome in patients with chronic non‐cancer pain. Neurogastroenterol Motil 22, 424–430. [DOI] [PubMed] [Google Scholar]
- Vella‐Brincat, J. , Macleod, A.D. (2007). Adverse effects of opioids on the central nervous systems of palliative care patients. J Pain Palliat Care Pharmacother 21, 15–25. [PubMed] [Google Scholar]
- Webster, L.R. , Fine, P.G. (2012). Review and critique of opioid rotation practices and associated risks of toxicity. Pain Med 13, 562–570. [DOI] [PubMed] [Google Scholar]
- Webster, L. , Dhar, S. , Eldon, M. , Masuoka, L. , Lappalainen, J. , Sostek, M. (2013). A phase 2, double‐blind, randomized, placebo‐controlled, dose‐escalation study to evaluate the efficacy, safety, and tolerability of naloxegol in patients with opioid‐induced constipation. Pain 154, 1542–1550. [DOI] [PubMed] [Google Scholar]
- West, C. , Usher, K. , Foster, K. , Stewart, L. (2012). Chronic pain and the family: the experience of the partners of people living with chronic pain. J Clin Nurs 21, 3352–3360. [DOI] [PubMed] [Google Scholar]
- Wilson, F.A. , Stimpson, J.P. , Pagán, J.A. (2014). Fatal crashes from drivers testing positive from drugs in the U.S., 1993–2010. Public Health Rep 129, 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S.M. , Compton, P. , Bolus, R. , Schieffer, B. , Pham, Q. , Baria, A. , Van Vort, W. , Davis, F. , Shekelle, P. , Naliboff, B.D. (2006). The addiction behaviors checklist: Validation of a new clinician‐based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manage 32, 342–351. [DOI] [PubMed] [Google Scholar]
- Xu, S. , Etropolski, M. , Upmalis, D. , Okamoto, A. , Lin, R. , Nandy, P. (2012). Pharmacokinetic and pharmacodynamics modeling of opioid‐induced gastrointestinal side effects in patients receiving tapentadol IR and oxycodone IR. Pharm Res 29, 2555–2564. [DOI] [PubMed] [Google Scholar]
- Council of Europe (CoE) (2003). Recommendations Rec (2003) 24 of the Committee of Ministers to member states on the organisation of palliative care. Adopted by the Committee of Ministers on 12 November 2003 at the 860th meeting of the Ministers’ Deputies. Strasbourg, France. http://www.coe.int (accessed 16 March 2016).
- DrugBank . Drug & Target Database (Version 4.3) (2015). http://www.drugbank.ca (accessed 16 March 2016).
- GOV.UK . (2013). Drug Safety Update – Metoclopramide: risk of neurological adverse effects. https://www.gov.uk/drug-safety-update/metoclopramide-risk-of-neurological-adverse-effects (accessed 16 March 2016).
- GOV.UK. (2014). Drug Safety Update – Domperidone: risk of cardiac side effects. https://www.gov.uk/drug-safety-update/ domperidone‐risks‐of‐cardiac‐side‐effects (accessed 16 March 2016).
- International Association for the Study of Pain (IASP) (2012). IASP Taxonomy. http://iasp-pain.org/Education/Content.aspx?ItemNumber=1698&&navItemNumber=576 (accessed 16 March 2016).
- International Association for the Study of Pain (IASP) (2015). Pain: Clinical Updates. Volume 23, Issue 6 (November). http://www.iasp-pain.org/PublicationsNews/NewsletterIssue.aspx?ItemNumber=4962 (accessed 16 March 2016).
- The European Pain Federation (EFIC) (2013a). APPEAL (Advancing Provision of Pain Education and Learning) study. http://www.efic.org/index.asp?sub=lWaAG1sc58KESH (accessed 16 March 2016).
- The European Pain Federation (EFIC) (2013b). The Pain Management Core Curriculum for European Medical Schools – Version July 2013. http://www.efic.org/userfiles/CoreCurriculumPainManagementEFIC_Version130718.pdf (accessed 16 March 2016).
- International Narcotics Control Board (INCB) (2016). Availability of narcotic drugs for medical use/availability of opioids for pain management 2010 – 2012. https://www.incb.org/en/narcotic-drugs/Availability/availability.html (accessed 31 August 2016)
- World Health Organization (WHO) . (2011). Achieving a balance in national policies on controlled substances – guidance for availability and accessibility of controlled medicines. Geneva, Switzerland. http://www.who.int (accessed 16 March 2016).


