Abstract
Purpose
To compare the outcomes of canaloplasty and trabeculectomy in open‐angle glaucoma.
Methods
This prospective, randomized clinical trial included 62 patients who randomly received trabeculectomy (n = 32) or canaloplasty (n = 30) and were followed up prospectively for 2 years. Primary endpoint was complete (without medication) and qualified success (with or without medication) defined as an intraocular pressure (IOP) of ≤18 mmHg (definition 1) or IOP ≤21 mmHg and ≥20% IOP reduction (definition 2), IOP ≥5 mmHg, no vision loss and no further glaucoma surgery. Secondary endpoints were the absolute IOP reduction, visual acuity, medication, complications and second surgeries.
Results
Surgical treatment significantly reduced IOP in both groups (p < 0.001). Complete success was achieved in 74.2% and 39.1% (definition 1, p = 0.01), and 67.7% and 39.1% (definition 2, p = 0.04) after 2 years in the trabeculectomy and canaloplasty group, respectively. Mean absolute IOP reduction was 10.8 ± 6.9 mmHg in the trabeculectomy and 9.3 ± 5.7 mmHg in the canaloplasty group after 2 years (p = 0.47). Mean IOP was 11.5 ± 3.4 mmHg in the trabeculectomy and 14.4 ± 4.2 mmHg in the canaloplasty group after 2 years. Following trabeculectomy, complications were more frequent including hypotony (37.5%), choroidal detachment (12.5%) and elevated IOP (25.0%).
Conclusions
Trabeculectomy is associated with a stronger IOP reduction and less need for medication at the cost of a higher rate of complications. If target pressure is attainable by moderate IOP reduction, canaloplasty may be considered for its relative ease of postoperative care and lack of complications.
Keywords: canaloplasty, glaucoma surgery, open‐angle glaucoma, trabeculectomy
Introduction
The purpose of modern glaucoma surgery was to reduce intraocular pressure (IOP) to an individual target pressure without the risk of glaucoma progression while minimizing serious complications commonly associated with filtering blebs. Trabeculectomy achieves an excellent IOP reduction, remains the most frequently performed glaucoma surgery and is still considered as the gold standard in the surgical treatment of patients with glaucoma since its first introduction by Cairns (1968). Severe adverse events and the need for an intensive postoperative treatment regimen have encouraged glaucoma surgeons to develop less invasive non‐penetrating procedures avoiding filtering blebs (Godfrey et al. 2009; Francis et al. 2011; Grieshaber 2012; Jampel et al. 2012; Bettin & Di Matteo 2013; Wallin et al. 2013; Yamamoto et al. 2013). Canaloplasty is a non‐filtering, bleb‐free method combining viscocanalostomy with the placement of a 360° intracanalicular tension suture within Schlemm's canal, thus allowing aqueous humour outflow through the natural pathway (Godfrey et al. 2009; Harvey & Khaimi 2011; Grieshaber 2012). Previous studies comparing non‐penetrating with penetrating glaucoma surgeries have reported that although canaloplasty may not be as effective as filtering surgery in lowering IOP without medication, complication rate and risk of serious vision‐threatening adverse events are low (Ayyala et al. 2011; Brusini & Tosoni 2012; Bruggemann et al. 2013; Matlach et al. 2013). In addition, the combination of canaloplasty with cataract surgery provides a better IOP reduction than single canaloplasty (Lewis et al. 2007, 2011; Shingleton et al. 2008; Bull et al. 2011; Arthur et al. 2013; Tetz et al. 2013).
Although studies comparing canaloplasty with trabeculectomy have been reported, scientific evidence based on prospective, randomized trials is lacking. Therefore, our aim was to compare results of canaloplasty and trabeculectomy in patients with open‐angle glaucoma in a prospective randomized study.
Materials and Methods
Study design
The TVC study (trabeculectomy versus canaloplasty) is a prospective, randomized, interventional clinical trial of trabeculectomy versus canaloplasty in patients with open‐angle glaucoma performed at one surgical centre. Written informed consent was obtained from all participants prior to inclusion. The study protocol was approved by the ethics committee of the University of Würzburg, Germany. The described research adhered to the tenets of the Declaration of Helsinki. The trial was registered at clinicaltrials.gov before commencing recruitment (registration number: NCT01228799).
Study population and patient selection
Patients
Sixty‐two eyes of 62 Caucasian patients with uncontrolled open‐angle glaucoma were randomly assigned to undergo either trabeculectomy or canaloplasty using a permuted block randomization. The sample size determination was based on the absolute IOP reduction. The hypothesis was no difference between both surgical groups. Therefore, the null hypothesis was defined as a difference in the absolute IOP (± 3 mmHg) reduction between both groups. The power of the statistical tests is 80% (29 patients each group, total of 58 patients) and correctly rejects the null hypothesis when the null hypothesis is false which was tested using Schuirmann's two‐one‐sided tests with a p value of 0.05.
All surgeries were performed by a single experienced glaucoma surgeon (TK) at the Department of Ophthalmology, University of Würzburg, Germany. Approximately 120 canaloplasty surgeries were performed by the study surgeon in advance to the study. A total of 32 trabeculectomy and 30 canaloplasty procedures were performed between June 2010 and August 2012. Diagnosis of glaucoma was made according to typical optic nerve head appearances including glaucomatous optic disc cupping and neuroretinal rim thinning associated with nasalization and bayoneting of the blood vessels, flame‐shaped or splinter haemorrhages at the optic disc margin not related to other vascular diseases, and retinal nerve fibre layer defects. Patients with perimetric glaucoma were defined as having visual field defects corresponding to the structural change of the optic disc and were distinguished from patients with preperimetric glaucoma having characteristic glaucomatous optic nerve head changes without visual field defects. Surgery was considered in glaucoma patients with medically uncontrolled or not sufficiently lowered IOP and progression of visual field defects or structural changes to the optic disc over time.
Inclusion criteria were male or female patients aged 18 years or older with medically uncontrolled primary or secondary (pseudoexfoliative and pigmentary) open‐angle glaucoma. Patients with previous penetrating or non‐penetrating surgeries in the study eye, patients with angle closure, normal tension, congenital or other secondary types of glaucoma (uveitic, neovascular or traumatic) and more than 1 laser trabeculoplasty or more than 1 cyclodestructive procedures in the study eye were excluded. Low‐dose cyclophotocoagulation is routinely performed in patients with moderate IOP increase and failed medical or laser treatment at our department. Patients were not included if more than 1 cyclodestructive procedure was performed within at least 1 year prior inclusion.
Examinations and follow‐up
All patients underwent a baseline standard ophthalmic examination including ophthalmic history with previous ocular surgeries or laser treatments, glaucoma type and glaucoma medication use, IOP measurement using Goldmann applanation tonometry, visual acuity testing converted to the logarithm of the minimum angle of resolution (logMAR), slit‐lamp biomicroscopy for anterior segment evaluation, gonioscopy with angle grading and indirect ophthalmoscopy for fundus assessment. Relevant demographic information such as age and sex was also recorded. Follow‐up visits were scheduled at day 1 and 7, 4 weeks, 3, 6, 12 and 24 months after surgery and additional examinations whenever they seemed clinically necessary. Postoperative evaluations included all baseline examinations and additional monitoring for complications and subsequent surgical interventions. All patients were followed up prospectively and examined at the Department of Ophthalmology, University of Würzburg. Only in case of missing postoperative data, ophthalmologists in private practice or clinics were contacted for IOP, medication, visual acuity, complications and second surgeries.
Outcome measurements
Baseline IOP was calculated as the mean of several IOP measurements taken at different times of the day and on different days within 42 days before surgery or earlier if the decision for surgery was made before. This is in accordance with the principles of the World Glaucoma Association (WGA) (Heuer et al. 2008).
Primary endpoint was the success rate defined as an IOP of ≤18 mmHg (definition 1) or IOP reduction by ≥20% and to ≤21 mmHg (definition 2) without medication (complete success) and irrespective of medication (qualified success) based on the guidelines of the WGA (Heuer et al. 2008). Success was defined as eyes fulfilling the above IOP criteria after 4 weeks, IOP ≥5 mmHg after 4 weeks, no loss of light perception attributable to glaucoma, eyes not requiring subsequent glaucoma surgery (e.g. photocoagulation of ciliary body, trabeculectomy or aqueous shunt surgery) and a completed follow‐up until 2 years postoperatively. Secondary endpoints were the absolute reduction of IOP (preoperative–postoperative IOP) after 2 years, IOP and visual acuity during follow‐up, use of IOP‐lowering medication, postoperative complications, further interventions and early bleb management including laser suture lysis of scleral flap sutures and subconjunctival bleb injections of 5‐fluorouracil (5‐FU).
Surgical technique
Trabeculectomy
The 12 o′clock position was exposed using a 6 o′clock corneal traction suture technique, as previously described (Grehn & Klink 2011). A fornix‐based conjunctival flap was created, and episcleral blood vessels were cauterized. Mitomycin C (MMC) was used as an antimetabolite in all trabeculectomy patients. Four sponges soaked with MMC were placed subconjunctivally for 3 min. In all cases, MMC 0.2 mg/ml was applied. Afterwards, the site of MMC application was washed with 30 ml balanced salt solution (BSS). A rectangular scleral flap measuring 4 × 3 mm was dissected and a surgical trabeculectomy of 0.8 × 2 mm performed, followed by a peripheral iridectomy. The scleral flap was secured with 10.0 non‐absorbable nylon sutures. Four single sutures were placed at both corners of the scleral flap and to each side. Additional sutures were placed if needed based on the outflow through the scleral flap during surgery. Mean number of scleral flap sutures was 4.5 ± 1.5 (range 4–12). A 10.0 nylon running mattress suture was used to close the conjunctiva.
Canaloplasty
We favour a superonasal surgical approach to create the conjunctival flap due to a higher number of collector channels draining the superonasal part of Schlemm's canal. Additionally, superotemporal conjunctiva will be saved for further glaucoma surgeries. To unroof Schlemm's canal, a parabolic superficial flap (one‐third scleral thickness, 5 × 5 mm) was created using the Kearney parabolic marker (Duckworth & Kent, UK). A deeper scleral flap was created leaving a ledge of 1 mm to the margins of the superficial flap to achieve a watertight closure and dissected so that only some layers of the scleral tissue remained above the ciliary body. The dissection of both flaps was continued into clear cornea (1 mm) for creation of a trabeculo‐Descemet window (TDW). After Schlemm's canal was reached, a paracentesis was made and the traction released to soften the eye and reduce the risk of TDW perforation. After creation of the TDW, the endothelium of Schlemm's canal was removed using a vitreoretinal‐peeling forceps. The deeper scleral flap was removed and a flexible illuminating microcatheter (iTrack™ 250A; iScience Interventional Corporation, Menlo Park, CA, USA) inserted into Schlemm's canal. A blinking light at the tip of the microcatheter visualized the correct position of the catheter. After successful 360° catheterization, sodium hyaluronate 1.4% (Healon GV; Advanced Medical Optics, Inc., Santa Ana, CA, USA) was injected to dilate Schlemm's canal and a 10.0 non‐absorbable polypropylene suture (Prolene®; Ethicon, Johnson & Johnson Medical Corporation, New Brunswick, NJ, USA) was tied to the tip of the microcatheter while pulling the microcatheter and suture back to distend Schlemm's canal. Viscoelastics (4–6 μg) were applied every 2 o'clock hours via the catheter. The intracanalicular suture was tightened to permanently distend the trabecular meshwork. The suture was tied under tension, so that a groove appeared in the trabeculo‐Descemet window. The superficial flap was closed watertight using 10.0 non‐absorbable nylon sutures. To prevent leakage from suture penetration through the scleral flap, sutures were placed through half of the flap thickness. The superficial flap was thoroughly attached to the scleral ledge, and the sutures tied firmly using 10.0 absorbable vicryl sutures. Nine sutures were placed, 1 suture posteriorly to the flap and four sutures to each side. The closure tightness was tested during surgery by increasing the pressure in the anterior chamber to approximately 20 mmHg and applying 0.17% fluorescein (Fluoreszein SE Thilo, Alcon). Additional sutures were placed, if a leakage of fluoresceine was seen. Successful 360° catheterization with placement of a tension suture was achieved in all canaloplasty patients. Instillation of viscoelastics into Schlemm's canal to both sides to the maximum extent can be an escape strategy in case of failed 360° catheterization. Finally, the conjunctiva was fixed with two purse‐string sutures at the corners.
Postoperative treatment
Postoperatively, all trabeculectomy patients received a standard treatment regimen consisting of prednisolone acetate 1% eye drops with preservatives or dexamethasone dihydrogen phosphate 0.1% preservative‐free eye drops every 2 hr or every hour for 1 week tapering over 6–8 weeks, antibiotic eye drops (gentamicin sulphate 0.3% with preservatives or ofloxacin 0.3% preservative‐free) three times daily for 1 week or as needed and atropine 0.5% eye drops (with preservatives) or cyclopentolate hydrochloride 1% preservative‐free eye drops to prevent malignant glaucoma and posterior synechiae in case of increased anterior chamber inflammation for 1–2 weeks. If signs of scarring of the filtering bleb such as corkscrew vessels, flat bleb and increased IOP occurred, early subconjunctival injections of 5‐FU were administered or surgical bleb needling and scleral flap revision performed. In case of flat blebs which inflate after ocular massage, laser suture lysis was carried out to increase outflow. Marquardt et al. (2004) proposed an intensive postoperative bleb management protocol to control wound healing and reduce the risk of early bleb failure. If treatment failed, topical glaucoma medications were added or second glaucoma surgeries were performed.
All canaloplasty patients received a standard postoperative treatment including prednisolone acetate 1% eye drops with preservatives or dexamethasone dihydrogen phosphate 0.1% preservative‐free eye drops every 2 hr for 1 week tapering over 2 weeks, non‐steroidal anti‐inflammatory eye drops (diclofenac sodium 0.1% with or without preservatives) three times a day tapering over 4 weeks and antibiotic eye drops (gentamicin sulphate 0.3% with preservatives or ofloxacin 0.3% preservative‐free) three times daily for 1 week or as needed. Pupils were not dilated to avoid anterior synechiae of the iris to the Descemet window. A hyphaema is a common event after canaloplasty which is more likely to be seen as a positive predictor for a good IOP response to the surgery rather than a complication (Grieshaber et al. 2013). We applied topical steroids for a short period after surgery tapering over 2 weeks to achieve a faster hyphaema resolution. As canaloplasty is a bleb‐independent procedure, an intensive postoperative management was not necessary. In case of increased IOP after canaloplasty, Nd:YAG (Neodynium:yttrium aluminium garnet) laser goniopuncture of the Descemet window was performed or antiglaucomatous drugs were added if target IOP was not reached.
Statistical analysis
Statistical analyses were performed using SPSS© version 21.0 for Windows (IBM Corporation, Armonk, NY, USA). A power analysis was performed using Schuirmann's two‐one‐sided tests with a p value of 0.05 to determine the sample size. The Kolmogorov–Smirnov test was used to test for normal distribution. Preoperative demographic data were analysed with the nonparametric Mann–Whitney U‐test for continuous variables (IOP, visual acuity and number of glaucoma medications; except for age which was analysed using Student's t‐test) and the Fisher exact test or chi‐square test for categorical variables (sex, eye, glaucoma type, previous surgeries or laser treatment). To calculate a difference in absolute IOP reduction between both groups, Mann–Whitney U‐test was performed. The Fisher exact test was used to compare complications and surgical interventions between both groups. A Kaplan–Meier survival plot illustrates the cumulative probability of success. The log‐rank test was used to compare success rate between both groups. Changes of IOP, visual acuity and medications during follow‐up in each group were analysed using one‐way repeated measures analyses of variance (anova). Subgroup analyses were performed using logistic regression for success and medication (binary data, yes/no), linear regression for IOP and Cox regression for the cumulative probability of success (Kaplan–Meier). Mean, standard deviation (SD), median, interquartile range (IQR) and absolute values are displayed, as appropriate. A p ≤ 0.05 was considered statistically significant.
Results
Table 1 summarizes the preoperative data of all patients. Groups were comparable for preoperative IOP, visual acuity and number of glaucoma medications, type of glaucoma, number of previous ocular surgeries, age and sex. All patients completed the baseline visit and follow‐up visits at day 1 and 7. Sixty‐one patients (98.4%) completed the 4‐week, 58 patients (93.5%) the 3‐month, 56 patients (90.3%) the 6‐month and 54 patients (87.1%) the 12‐ and 24‐month follow‐up.
Table 1.
Preoperative patients′characteristics
| Trabeculectomy | Canaloplasty | p‐Valuea | |
|---|---|---|---|
| No. of eyes/patients | 32/32 (51.6) | 30/30 (48.4) | |
| Age, years | 67.9 ± 9.3 | 66.5 ± 11.3 | 0.58 |
| Sex, male/female | 11 (34.4)/21 (65.6) | 18 (60.0)/12 (40.0) | 0.07 |
| Eye, right/left | 19 (59.4)/13 (40.6) | 10 (33.3)/20 (66.7) | 0.05 |
| IOP, mmHg | 20.0 (19.0–25.0) | 22.0 (20.0–26.3) | 0.06 |
| BCVA, logMAR | 0.10 (0.00–0.28) | 0.22 (0.09–0.40) | 0.13 |
| Topical glaucoma medication | 3.5 (3.0–4.0) | 3.0 (1.8–4.0) | 0.09 |
| Systemic medication | 4 (12.5) | 6 (20.0) | 0.50 |
| Glaucoma type | |||
| POAG | 19 (59.4) | 13 (43.3) | 0.33 |
| PEXG | 12 (37.5) | 13 (43.3) | |
| PG | 1 (3.1) | 2 (6.7) | |
| Preperimetric POAG | 0 (0.0) | 2 (6.7) | |
| Previous ocular surgeryb | |||
| None | 19 (59.4) | 22 (73.3) | 0.45 |
| Laser trabeculoplasty | 11 (34.4) | 8 (26.7) | |
| Cyclodestructive | 2 (6.3) | 0 (0.0) | |
| Laser peripheral iridotomyc | 3 (9.4) | 2 (6.7) | |
| Phaco | 6 (18.8) | 5 (16.7) | |
n = sample size, SD = standard deviation, IOP = intraocular pressure, BCVA best‐corrected visual acuity, logMAR = log of the minimum angle of resolution, Phaco = phacoemulsification, POAG = primary open‐angle glaucoma, PEXG = pseudoexfoliative glaucoma, PG = pigmentary glaucoma.
Data are absolute values (%), median (interquartile range) or mean ± SD as appropriate.
Fisher exact test, Mann–Whitney U‐test, Student's t‐test as appropriate.
More than 1 pervious ocular surgeries or laser treatments were performed.
Laser peripheral iridotomy was performed in eyes with pigment dispersion syndrome or slightly narrow angles but no evidence of angle closure. All patients underwent gonioscopy for angle grading. Only patients with an open angle (at least scleral spur was seen), no goniosynechiae or other angle dysgenesis were included.
Results of success, IOP, visual acuity and glaucoma medication of patients receiving further glaucoma surgeries were not used for analyses from the date of the second glaucoma surgery.
Complete success (without medication)
In the trabeculectomy group, 23 patients (74.2%) were classified as successfully treated according to definition 1 (IOP ≤18 mmHg) compared to nine patients (39.1%) in the canaloplasty group after 2 years (plog‐rank = 0.01 during follow‐up). Twenty‐one (67.7%) and nine (39.1%) patients met the criteria of success without medications according to definition 2 (IOP ≤21 mmHg and ≥20% IOP reduction) in the trabeculectomy and canaloplasty group (plog‐rank = 0.04 during follow‐up) after 2 years, respectively. Complete success rate was significantly higher in the trabeculectomy group for both success criteria (p < 0.05; Table 2 and Fig. 1A).
Table 2.
Success
| Trabeculectomy | Canaloplasty | p‐Valuea | |
|---|---|---|---|
| Complete successb | |||
| IOP ≤21 mmHg and ≥20% IOP reduction | |||
| 1 month | 26/32 (81.2) | 15/29 (51.7) | 0.04 |
| 3 months | 27/32 (84.4) | 16/26 (61.5) | |
| 6 months | 25/32 (78.1) | 13/24 (54.2) | |
| 12 months | 24/31 (77.4) | 14/23 (60.9) | |
| 24 months | 21/31 (67.7) | 9/23 (39.1) | |
| IOP ≤18 mmHg | |||
| 1 month | 29/32 (90.6) | 16/29 (55.2) | 0.01 |
| 3 months | 27/32 (84.4) | 16/26 (59.3) | |
| 6 months | 25/32 (78.1) | 14/24 (58.3) | |
| 12 months | 24/31 (77.4) | 13/23 (56.5) | |
| 24 months | 23/31 (74.2) | 9/23 (39.1) | |
| Qualified successc | |||
| IOP ≤21 mmHg and ≥20% IOP reduction | |||
| 1 month | 26/32 (81.2) | 21/29 (72.4) | 0.40 |
| 3 months | 30/32 (93.8) | 25/26 (96.2) | |
| 6 months | 29/32 (90.6) | 20/24 (83.3) | |
| 12 months | 30/31 (96.8) | 23/23 (100) | |
| 24 months | 28/31 (90.3) | 19/23 (82.6) | |
| IOP ≤18 mmHg | |||
| 1 month | 29/32 (90.6) | 22/29 (75.9) | 0.01 |
| 3 months | 31/32 (96.6) | 24/26 (88.9) | |
| 6 months | 31/32 (96.9) | 23/24 (95.8) | |
| 12 months | 31/31 (100) | 22/23 (95.7) | |
| 24 months | 30/31 (96.8) | 19/23 (82.6) | |
IOP = intraocular pressure.
Data are absolute values (%).
Success was defined as an IOP of ≤18 mmHg (definition 1) or IOP‐reduction by ≥20% and to ≤21 mmHg (definition 2) without medication (complete success) and irrespective of medication (qualified success) after 4 weeks postoperatively, IOP ≥5 mmHg after 4 weeks, no loss of light perception attributable to glaucoma, eyes not requiring subsequent glaucoma surgery (e. g. photocoagulation of ciliary body) and a completed follow‐up until 2 years postoperatively.
Number of patients with success varies during follow‐up. Success rate can be higher later on as patients can fulfil success criteria again after adding topical treatment (see qualified success with or without medication).
Log‐rank test.
Without glaucoma medication.
With or without glaucoma medication.
Figure 1.
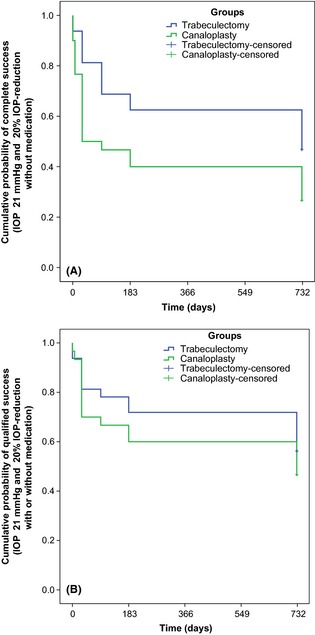
Kaplan–Meier survival plot of cumulative probability of complete success. Complete success was defined as intraocular pressure (IOP) reduction to ≤18 mmHg (A) or ≤21 mmHg and ≥20% IOP reduction (B) without glaucoma medication.
Qualified success (with or without medication)
As antiglaucomatous drugs were added in case of increased IOP after failed laser goniopuncture in canaloplasty patients and scleral flap revision or bleb needling in trabeculectomy patients, qualified success rate was higher than success rate without medications. Qualified success rate was not significantly different between both groups for definition 2 (IOP ≤21 mmHg and ≥20% IOP reduction) and was 90.3% in the trabeculectomy and 82.6% in the canaloplasty group after 2 years (plog‐rank = 0.40 during follow‐up, Table 2, Fig. 1B). For definition 1 (IOP ≤18 mmHg), qualified success rate was 96.8% and 82.6% in the trabeculectomy and canaloplasty group after 2 years, respectively (plog‐rank = 0.01 during follow‐up, Table 2).
IOP
Median baseline IOP was 20.0 mmHg (IQR 19.0–25.0) and 22.0 mmHg (20.0–26.3) in the trabeculectomy and canaloplasty groups, respectively (p = 0.06). Mean absolute IOP reduction was 10.8 ± 6.9 mmHg in the trabeculectomy and 9.3 ± 5.7 mmHg in the canaloplasty group after 2 years (p = 0.47). Only one patient in the trabeculectomy group had a higher postoperative IOP compared to the baseline value. Both procedures significantly reduced IOP during follow‐up (p < 0.001, 1–24 months compared to baseline) but was not significantly different between both groups (p = 0.56) although IOP was lower in the trabeculectomy group at all time intervals. Mean postoperative IOP was 11.5 ± 3.4 mmHg in the trabeculectomy group and 14.4 ± 4.2 mmHg in the canaloplasty group after 2 years (Table 3). Figure 2 presents the IOP results during follow‐up. A scatter plot of preoperative IOP and postoperative IOP after 2 years shows the results for each patient (Fig. 3).
Table 3.
Results of IOP and medication
| Trabeculectomy | Canaloplasty | |||||
|---|---|---|---|---|---|---|
| n | IOP in mmHg | Number of medication | n | IOP in mmHg | Number of medication | |
| Preoperative | 32 | 22.2 ± 5.3 (17–39) | 3.3 ± 1.0 (0–5) | 30 | 23.7 ± 5.1 (18–35) | 2.6 ± 1.6 (0–5) |
| 7 days | 32 | 12.9 ± 5.2 (4–28) | 0 | 30 | 15.0 ± 6.7 (5–35) | 0.3 ± 0.8 (0–3) |
| 1 month | 32 | 12.1 ± 5.1 (2–28) | 0 | 29 | 15.7 ± 5.1 (11–30) | 0.7 ± 1.2 (0–4) |
| 3 months | 32 | 11.0 ± 4.0 (5–24) | 0.2 ± 0.6 (0–3) | 26 | 13.4 ± 3.8 (10–27) | 0.8 ± 1.2 (0–4) |
| 6 months | 32 | 10.7 ± 3.7 (4–18) | 0.3 ± 0.7 (0–3) | 24 | 13.8 ± 2.6 (9–20) | 0.8 ± 1.0 (0–3) |
| 12 months | 31 | 10.8 ± 2.9 (6–17) | 0.3 ± 0.7 (0–3) | 23 | 13.8 ± 2.7 (10–20) | 0.8 ± 1.2 (0–4) |
| 24 months | 31 | 11.5 ± 3.4 (6–19) | 0.4 ± 0.8 (0–3) | 23 | 14.4 ± 4.2 (8–24) | 0.9 ± 1.1 (0–3) |
n = sample size, IOP = intraocular pressure, SD = standard deviation.
Data are means ± SD (range).
Figure 2.
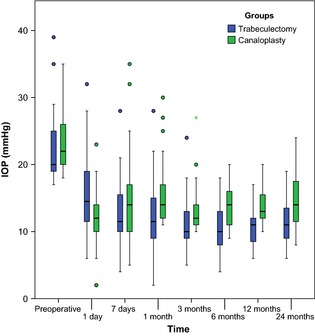
Intraocular pressure (IOP) results for canaloplasty and trabeculectomy. IOP significantly dropped to a lower level during follow‐up in both groups (p < 0.001). Overall, IOP was significantly lower in patients undergoing trabeculectomy. Box plots illustrate the median (50th percentile) as a black centre line and the 25th and 75th percentile as the lower and upper hinges of the box, 1.5 IQR as the upper and lower bards. Circles represent minor outliers.
Figure 3.
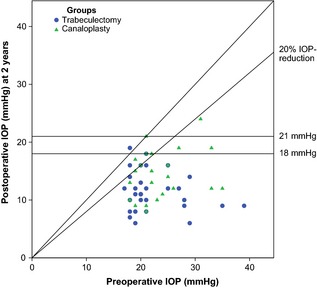
Scatter plot of preoperative and postoperative intraocular pressure (IOP) after 2 years. Each eye is illustrated as a single circle or triangle. The oblique line indicates no change of IOP. Circles or triangles above the oblique line define a higher postoperative IOP. Eyes below the line of 18 or 21 mmHg plus 20% IOP reduction fulfilled criteria of success with or without medication.
Medication
Preoperatively, median number of topical glaucoma medications was 3.5 (3.0–4.0) in the trabeculectomy group and 3.0 (1.8–4.0) in patients undergoing canaloplasty (p = 0.09). Five canaloplasty patients and 1 trabeculectomy patient were on no topical medications but most of them on systemic IOP‐lowering medication. None of the trabeculectomy patients was on topical medications up to 4 weeks after surgery, while five canaloplasty patients (16.7%) were already on antiglaucomatous drugs at day 7. After 2 years, 12 patients (52.2%) of the canaloplasty and 8 (25.8%) of the trabeculectomy group needed additional IOP‐lowering medication. The postoperative number of medications was significantly reduced during follow‐up (p < 0.001). The mean number of required medications was significantly lower in the trabeculectomy group after surgery (p = 0.01, 1–24 months compared to baseline, Table 3).
Visual acuity
Preoperatively, median visual acuity was 0.10 logMAR (0.00–0.28 logMAR) in the trabeculectomy and 0.22 logMAR (0.08–0.40 logMAR) in the canaloplasty group (p = 0.13). Six trabeculectomy patients (18.8%) and 5 canaloplasty patients (16.7%) were pseudophakic at the time of the surgery. Mean postoperative visual acuity was 0.30 ± 0.56 logMAR in trabeculectomy patients and 0.20 ± 0.26 logMAR in canaloplasty patients. Visual acuity was not significantly different between both groups during follow‐up (p = 0.08, 1–24 months compared to baseline).
Regression analysis revealed no significant difference in postoperative IOP, medication or complete and qualified success at 2 years for different glaucoma types (primary open‐angle glaucoma including preperimetric glaucoma versus secondary open‐angle glaucoma including pseudoexfoliation and pigmentary glaucoma) or previous laser trabeculoplasty.
Complications and subsequent surgeries
Intraoperative complications in the canaloplasty group included microperforation of Descemet membrane in two eyes (6.7%). One patient developed anterior synechiae which needed surgical peripheral iridotomy. Successful 360° catheterization with placement of a tension suture was achieved in all canaloplasty patients. No surgery‐specific intraoperative complications were reported in the trabeculectomy group.
Postoperative complications and further interventions are summarized in Table 4.
Table 4.
Postoperative complications and surgical interventions
| Trabeculectomy n = 32 | Canaloplasty n = 30 | p‐Valuea | |
|---|---|---|---|
| Early complications ≤90 days | |||
| Hypotony (IOP<5 mmHg) | 12 (37.5) | 6 (20.0) | 0.17 |
| Shallow anterior chamber | 2 (6.2) | 0 (0.0) | 0.49 |
| Choroidal detachment | 4 (12.5) | 1 (3.3) | 0.36 |
| Elevated IOP (IOP >25 mmHg) | 8 (25.0) | 9 (30.0) | 0.78 |
| Conjunctival leak | 3 (9.4) | 3 (10.0) | 1.00 |
| Corneal erosion | 14 (43.8) | 1 (3.3) | <0.001 |
| Hyphaema (≥ 1 mm layered blood) | 1 (3.1) | 7 (23.3) | 0.02 |
| Intracorneal hematoma after Descemet detachment | – | 1 (3.3) | |
| Secondary suture migration | – | 2 (6.7)b | |
| Iris incarceration | 1 (3.1) | – | |
| Scarring of the filtering bleb | 1 (3.1) | – | |
| Blebitis/endophthalmitis | 0 (0.0) | 0 (0.0) | |
| Early surgeries | |||
| Laser suture lysis, eyes | 24 (75.0) | – | |
| Number of sutures [median (IQR)] | 1.00 (0.25–2.00) | ||
| 5‐FU bleb injections | 29 (90.6) | – | |
| Number of injections [median (IQR)] | 7.00 (5.00–7.75) | ||
| Iris revision | 1 (3.1) | – | |
| Scleral flap revision (hypotony) | 6 (18.8) | 1 (3.3)c | 0.11 |
| Scleral flap revision (elevated IOP) | 0 (0.0) | – | |
| Bleb needling | 1 (3.1) | – | |
| Conjunctival suturing | 1 (3.1) | 2 (6.7) | 0.61 |
| Nd:YAG laser goniopuncture | – | 4 (13.3) | |
| Second surgeries | |||
| Laser cyclophotocoagulation | 0 (0.0) | 2 (6.7) | 0.19 |
| Trabeculotomy | 0 (0.0) | 1 (3.3) | |
| Late complications (>90 days) | |||
| Elevated IOP (IOP >25 mmHg) | 1 (3.1) | 1 (3.4) | 1.00 |
| Hypotony (IOP<5 mmHg) | 6 (18.8) | 0 (0.0) | 0.03 |
| Scarring of the filtering bleb | 8 (25.0) | – | |
| Blebitis/endophthalmitis | 0 (0.0) | 1 (3.4)d | 0.48 |
| Late surgeries | |||
| Scleral flap revision (hypotony) | 1 (3.1) | 0 (0.0) | 1.00 |
| Second surgeries | |||
| Laser cyclophotocoagulation | 0 (0.0) | 1 (3.4) | 0.48 |
n = sample size, IOP = intraocular pressure, 5‐FU = 5‐fluorouracil, Nd:YAG = Neodynium:yttrium aluminium garnet, IQR = interquartile range.
Data are absolute values (%) or median (interquartile range) as stated.
Fisher exact test, chi‐square test, as appropriate.
In 1 canaloplasty patient with suture displacement and penetration into the anterior chamber, suture had to be removed by paracentesis.
Scleral patch was used for external drainage in 1 patient of the canaloplasty group.
Endophthalmitis occurred after phacoemulsification and was not associated with leakage after canaloplasty.
The number of postoperative complications and second interventions was higher in the trabeculectomy group; however, complications after a filtering procedure are not truly comparable to those after non‐penetrating surgery. Following trabeculectomy, early postoperative complications were transient hypotony (37.5%), hypotony‐related choroidal detachment (12.5%) and elevated IOP (25.0%). In the early postoperative period, intensive bleb management including laser suture lysis of scleral flap sutures, subconjunctival application of 5‐FU and scleral flap revisions was performed which is essential for long‐term success. Corneal erosion (43.8%) which is usually 5‐FU‐related was the most frequent complication in trabeculectomy patients.
A common late complication was scarring of the filtering bleb in approximately 25.0% and required additional glaucoma medications.
In canaloplasty eyes, common early postoperative complications were elevated IOP in 30.0% and hyphaema in 23.3% which spontaneously resolved within the first postoperative week and is more likely to be seen as a positive predictor to reach target IOP after surgery. A displacement and penetration of the suture into the anterior chamber was seen in two patients (6.7%) necessitating suture removal and consecutive 360° trabeculotomy in one patient. Both patients with secondary suture migration into the anterior chamber had no history of laser trabeculoplasty. A strong intracanalicular suture tension or the injection of viscoelastics during catheterization causing small tears of Schlemm's canal may be possible explanations for secondary suture migration. A haemorrhagic Descemet detachment occurred in one patient (3.3%) and resolved spontaneously within 5 months. Postoperative management was limited to Nd:YAG laser goniopuncture which was performed in four patients (13.3%). Additional IOP‐lowering medications were added in case of insufficiently controlled IOP and failed laser goniopuncture.
None of the trabeculectomy patients received further glaucoma surgeries, while two patients (6.7%) of the canaloplasty group underwent laser cyclophotocoagulation within 90 days of the initial surgery.
Discussion
Non‐penetrating, bleb‐free glaucoma procedures intend to efficaciously lower IOP without the risk of severe postoperative complications and the need for an intensive postoperative care as it becomes necessary after filtration surgery (Godfrey et al. 2009; Francis et al. 2011; Grieshaber 2012; Jampel et al. 2012; Bettin & Di Matteo 2013; Wallin et al. 2013; Yamamoto et al. 2013). Canaloplasty is a relatively novel surgical method which aims at restoring the natural pathway of aqueous humour outflow via Schlemm's canal using circumferential viscodilation and a tension suture to permanently distend the trabecular meshwork without inducing a filtering bleb (Godfrey et al. 2009; Harvey & Khaimi 2011; Grieshaber 2012; Klink et al. 2012).
As prospective randomized clinical trials comparing canaloplasty to the current gold standard of trabeculectomy have been lacking and only a limited number of centres are able to offer both techniques, we were compelled to address this issue. Our data indicate that both techniques lead to significant IOP reduction, which is more pronounced and required less postoperative medications following trabeculectomy. On the other hand, complication rates and follow‐up complexity were clearly more favourable following canaloplasty.
Earlier studies addressed the outcome of canaloplasty with regard to IOP management and postoperative complications. Grieshaber et al. (2010) reported long‐term outcomes in a non‐comparative study in 60 black Africans. The authors concluded that canaloplasty provided a long‐term reduction of IOP (16.3 ± 4.2 mmHg) without glaucoma medication after 24 months. Tetz and co‐workers reported on their 3‐year results of canaloplasty in phakic or pseudophakic eyes as well as in combination with cataract surgery. In this study, phacocanaloplasty provided the best IOP reduction with the least need for medication (Tetz et al. 2013). Schoenberg et al. (2015) reported their outcomes of phacocanaloplasty and phacotrabeculectomy in a retrospective, non‐randomized longitudinal cohort study. Although both methods achieved significant reduction in IOP and visual improvement after 1 year, a greater percentage of IOP decrease was seen after phacotrabeculectomy. Recently, Barnebey (2013) studied the adjunctive use of MMC during canaloplasty to maintain the intrascleral lake and reported a good IOP reduction to 13.4 ± 4.3 mmHg without additional medication after 12 months. Complications were comparable to canaloplasty without adjunctive MMC. However, signs of subconjunctival filtration were evident in 35% of the patients, although microcysts were not observed. In our current study, we achieved comparable IOP results for canaloplasty (13.8 ± 2.7 mmHg after 12 months) without MMC treatment or concomitant cataract surgery.
With regard to complications, hyphaema is a common event after canaloplasty and is suggested as a positive predictor of surgical success as it indicates permeability of the distended trabecular meshwork (Shingleton et al. 2008; Lewis et al. 2009; Grieshaber et al. 2010, 2015; Ayyala et al. 2011; Bull et al. 2011; Arthur et al. 2013; Matlach et al. 2013; Tetz et al. 2013). An intracorneal haematoma is rare and can be removed by stab incision (Tetz et al. 2013), partial‐thickness paracentesis (Gismondi & Brusini 2011) or Nd:YAG laser Descemet membranotomy (Robert & Harasymowycz 2013). Perforation of the trabeculo‐Descemet window during dissection is an intraoperative complication during canaloplasty, which occurs in <10% of all cases (Shingleton et al. 2008; Lewis et al. 2009; Grieshaber et al. 2010; Ayyala et al. 2011; Bull et al. 2011; Arthur et al. 2013; Tetz et al. 2013). These data are in line with our observations of Descemet detachment (6.7%) and hyphaema rate (23.3%) following canaloplasty. In contrast to earlier studies, we detected a secondary suture migration into the anterior chamber in two patients (6.7%), which required suture removal in one patient.
Non‐randomized retrospective assessments comparing canaloplasty and trabeculectomy had previously suggested that both techniques yield significant IOP reductions (Ayyala et al. 2011; Matlach et al. 2013). In line with our results, Ayyala et al. (2011) observed a more pronounced IOP reduction after 12 months with fewer medications in trabeculectomy (mean 43%) than canaloplasty (mean 32%). Canaloplasty had a better IOP reduction when performed in combination with cataract surgery (Lewis et al. 2007, 2011; Shingleton et al. 2008; Bull et al. 2011; Arthur et al. 2013; Tetz et al. 2013).
In our trabeculectomy group, complications were mostly hypotony‐associated as it has been reported in retrospective studies (Gedde et al. 2012; Jampel et al. 2012). Severe bleb‐related infections, blebitis and endophthalmitis are rare but serious vision‐threatening complications after trabeculectomy (Yamamoto et al. 2013). In particular, the use of intraoperative MMC leading to thin‐walled filtering blebs is a risk factor of blebitis and associated endophthalmitis (Wallin et al. 2013). No serious bleb‐related infections were encountered in our study.
Complications and second interventions after filtering surgery are difficult to compare with those after non‐penetrating procedures. Despite an excellent IOP reduction, trabeculectomy requires an intensive postoperative care to gently adjust IOP using laser suture lysis of flap sutures, 5‐FU bleb injections or further flap revisions and to manage complications commonly associated with filtering blebs. In contrast, the aim of canaloplasty is to restore the natural outflow of aqueous humour without subconjunctival filtration reducing the risk of severe bleb‐related infections and avoiding an intensive postoperative bleb management. However, no postoperative adjustment to the scleral flap except for Nd:YAG laser goniopuncture can be performed following canaloplasty leaving IOP‐lowering medication and further glaucoma surgeries as the only options if canaloplasty failed to lower IOP sufficiently.
Potential limitations of our study deserve consideration. The limited sample size may restrict conclusions on the safety and efficacy of both methods. Trabeculectomy was routinely performed using MMC which has been shown to have a cytotoxic effect on the ciliary body and may thus also contribute to IOP reduction. However, no MMC was applied during canaloplasty as the aim of non‐penetrating canaloplasty is to restore the natural outflow pathway without creating a subconjunctival filtration. It may be inaccurate to compare trabeculectomy using MMC to canaloplasty performed without MMC. Additional limitations to the data collection include adjustment of the postoperative time‐points to the nearest possible time‐point or inclusion of postoperative data acquired by ophthalmologists in private practice or clinics if patients had failed to attend a scheduled visit at the trial centre.
In summary, our results obtained in a randomized prospective clinical trial confirm earlier case series observations. Trabeculectomy allows for a stronger IOP decrease with less need for medication than canaloplasty at the cost of a higher complication rate and far more demanding postoperative care. If a higher target IOP and supplemental topical drugs are acceptable, canaloplasty is a true alternative to filtration surgery as it is characterized by a lower complication rate and less complex postoperative follow‐up. Canaloplasty may also be reasonable for patients with high risk of bleb failure or early to moderate glaucoma with target IOP values not necessarily requiring a filtering procedure. Furthermore, canaloplasty may be favourable in healthcare settings with limited capacity for intense follow‐up. As the relative advantages of both techniques are now apparent, a meticulous definition of patient subgroups benefiting the most of canaloplasty emerges as the next challenge.
Franz Grehn receives funding for consultancy (Pharm Allergan, European Glaucoma Advisory Board). Thomas Klink received travel grants for congress fees and accommodation (Novartis). None of the remaining authors has any conflict of interests including relevant financial interests, activities, relationships and affiliations to disclose related to this work. None of the remaining authors received funding for this work. We thank Klemens Hügen for his statistical advice on the power calculation for this study.
References
- Arthur SN, Cantor LB, Wudunn D, Pattar GR, Catoira‐Boyle Y, Morgan LS & Hoop JS (2013): Efficacy, safety, and survival rates of IOP‐lowering effect of phacoemulsification alone or combined with canaloplasty in glaucoma patients. J Glaucoma 23: 316–320. [DOI] [PubMed] [Google Scholar]
- Ayyala RS, Chaudhry AL, Okogbaa CB & Zurakowski D (2011): Comparison of surgical outcomes between canaloplasty and trabeculectomy at 12 months' follow‐up. Ophthalmology 118: 2427–2433. [DOI] [PubMed] [Google Scholar]
- Barnebey HS (2013): Canaloplasty with intraoperative low dosage mitomycin C: a retrospective case series. J Glaucoma 22: 201–204. [DOI] [PubMed] [Google Scholar]
- Bettin P & Di Matteo F (2013): Glaucoma: present challenges and future trends. Ophthalmic Res 50: 197–208. [DOI] [PubMed] [Google Scholar]
- Bruggemann A, Despouy JT, Wegent A & Muller M (2013): Intraindividual comparison of canaloplasty versus trabeculectomy with mitomycin C in a single‐surgeon series. J Glaucoma 22: 577–583. [DOI] [PubMed] [Google Scholar]
- Brusini P & Tosoni C (2012): Canaloplasty after failed trabeculectomy: a possible option. J Glaucoma 23: 33–34. [DOI] [PubMed] [Google Scholar]
- Bull H, von Wolff K, Korber N & Tetz M (2011): Three‐year canaloplasty outcomes for the treatment of open‐angle glaucoma: European study results. Graefes Arch Clin Exp Ophthalmol 249: 1537–1545. [DOI] [PubMed] [Google Scholar]
- Cairns JE (1968): Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol 66: 673–679. [PubMed] [Google Scholar]
- Francis BA, Singh K, Lin SC, Hodapp E, Jampel HD, Samples JR & Smith SD (2011): Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology 118: 1466–1480. [DOI] [PubMed] [Google Scholar]
- Gedde SJ, Singh K, Schiffman JC & Feuer WJ, Tube Versus Trabeculectomy Study Group (2012): The Tube Versus Trabeculectomy Study: interpretation of results and application to clinical practice. Curr Opin Ophthalmol 23: 118–126. [DOI] [PubMed] [Google Scholar]
- Gismondi M & Brusini P (2011): Intracorneal hematoma after canaloplasty in glaucoma. Cornea 30: 718–719. [DOI] [PubMed] [Google Scholar]
- Godfrey DG, Fellman RL & Neelakantan A (2009): Canal surgery in adult glaucomas. Curr Opin Ophthalmol 20: 116–121. [DOI] [PubMed] [Google Scholar]
- Grehn F & Klink T (2011): A new 6 o'clock traction suture technique for glaucoma filtration surgery. J Glaucoma 20: 28–29. [DOI] [PubMed] [Google Scholar]
- Grieshaber MC (2012): Ab externo Schlemm's canal surgery: viscocanalostomy and canaloplasty. Dev Ophthalmol 50: 109–124. [DOI] [PubMed] [Google Scholar]
- Grieshaber MC, Pienaar A, Olivier J & Stegmann R (2010): Canaloplasty for primary open‐angle glaucoma: long‐term outcome. Br J Ophthalmol 94: 1478–1482. [DOI] [PubMed] [Google Scholar]
- Grieshaber MC, Schoetzau A, Flammer J & Orgul S (2015): Postoperative microhyphema as a positive prognostic indicator in canaloplasty. Acta Ophthalmol 91: 151–156. [DOI] [PubMed] [Google Scholar]
- Harvey BJ & Khaimi MA (2011): A review of canaloplasty. Saudi J Ophthalmol 25: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer DK, Barton K, Grehn F, Shaarawy T & Sherwood M (2008): Consensus of definitions of success In: Shaarawy T, Grehn F. & Sherwood M. (eds). Guidelines on design and reporting of glaucoma surgical trials. The Hague, Amsterdam, The Netherlands: World Glaucoma Association, Kugler Publications, pp. 15–24. [Google Scholar]
- Jampel HD, Solus JF, Tracey PA, Gilbert DL, Loyd TL, Jefferys JL & Quigley HA (2012): Outcomes and bleb‐related complications of trabeculectomy. Ophthalmology 119: 712–722. [DOI] [PubMed] [Google Scholar]
- Klink T, Panidou E, Kanzow‐Terai B, Klink J, Schlunck G & Grehn FJ (2012): Are there filtering blebs after canaloplasty? J Glaucoma 21: 89–94. [DOI] [PubMed] [Google Scholar]
- Lewis RA, von Wolff K, Tetz M, Korber N, Kearney JR, Shingleton B & Samuelson TW (2007): Canaloplasty: circumferential viscodilation and tensioning of Schlemm's canal using a flexible microcatheter for the treatment of open‐angle glaucoma in adults: interim clinical study analysis. J Cataract Refract Surg 33: 1217–1226. [DOI] [PubMed] [Google Scholar]
- Lewis RA, von Wolff K, Tetz M, Koerber N, Kearney JR, Shingleton BJ & Samuelson TW (2009): Canaloplasty: circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open‐angle glaucoma in adults: two‐year interim clinical study results. J Cataract Refract Surg 35: 814–824. [DOI] [PubMed] [Google Scholar]
- Lewis RA, von Wolff K, Tetz M, Koerber N, Kearney JR, Shingleton BJ & Samuelson TW (2011): Canaloplasty: Three‐year results of circumferential viscodilation and tensioning of Schlemm canal using a microcatheter to treat open‐angle glaucoma. J Cataract Refract Surg 37: 682–690. [DOI] [PubMed] [Google Scholar]
- Marquardt D, Lieb WE & Grehn F (2004): Intensified postoperative care versus conventional follow‐up: a retrospective long‐term analysis of 177 trabeculectomies. Graefes Arch Clin Exp Ophthalmol 242: 106–113. [DOI] [PubMed] [Google Scholar]
- Matlach J, Freiberg FJ, Leippi S, Grehn F & Klink T (2013): Comparison of phacotrabeculectomy versus phacocanaloplasty in the treatment of patients with concomitant cataract and glaucoma. BMC Ophthalmol 13: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert MC & Harasymowycz P (2013): Hemorrhagic descemet detachment after combined canaloplasty and cataract surgery. Cornea 32: 712–713. [DOI] [PubMed] [Google Scholar]
- Schoenberg ED, Chaudhry AL, Chod R, Zurakowski D & Ayyala RS (2013): Comparison of surgical outcomes between phacocanaloplasty and phacotrabeculectomy at 12 months' follow‐up: a longitudinal cohort study. J Glaucoma. 24: 543–549. [DOI] [PubMed] [Google Scholar]
- Shingleton B, Tetz M & Korber N (2008): Circumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open‐angle glaucoma and visually significant cataract: one‐year results. J Cataract Refract Surg 34: 433–440. [DOI] [PubMed] [Google Scholar]
- Tetz M, Koerber N, Shingleton BJ, von Wolff K, Bull H, Samuelson TW & Lewis RA (2013): Phacoemulsification and intraocular lens implantation before, during, or after canaloplasty in eyes with open‐angle glaucoma: 3‐year results. J Glaucoma 24: 187–194. [DOI] [PubMed] [Google Scholar]
- Wallin O, Al‐Ahramy AM, Lundstrom M & Montan P (2013): Endophthalmitis and severe blebitis following trabeculectomy. Epidemiology and risk factors; a single‐centre retrospective study. Acta Ophthalmol 92: 426–431. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kuwayama Y, Nomura E, Tanihara H & Mori K, Japan Glaucoma Society Survey of Bleb‐related Infection (2013): Changes in visual acuity and intra‐ocular pressure following bleb‐related infection: the Japan Glaucoma Society Survey of Bleb‐related Infection Report 2. Acta Ophthalmol 91: e420–e426. [DOI] [PubMed] [Google Scholar]


