ABSTRACT
The effects of the cathepsin K inhibitor odanacatib (ODN) on fracture healing were monitored for ∼6 and 15 weeks post‐fracture in two separate studies using the unilateral transverse mid‐ulnar osteotomy model in skeletally mature female rabbits. Rabbits were pre‐treated for 3–4 weeks with vehicle (Veh), ODN (2 mg/kg, po, daily), or alendronate (ALN) (0.3 mg/kg, sc, twice‐weekly) prior to osteotomy. In Study 1, the animals were maintained on the same respective treatment for ∼6 weeks. In Study 2, the animals were also continued on the same therapy or switched from Veh to ODN or ODN to Veh for 15 weeks. No treatment‐related impairment of fracture union was seen by qualitative histological assessments in the first study. Cartilage retention was detected in the calluses of ALN‐treated rabbits at week‐6, while calluses in the ODN and Veh groups contained bony tissue with significantly less residual cartilage. ODN treatment also markedly increased the number of cathepsin K‐(+) osteoclasts in the callus, indicating enhanced callus remodeling. From the second study, ex vivo DXA and pQCT confirmed that ODN treatment pre‐ and post‐osteotomy increased callus bone mineral content and bone mineral density (BMD) versus Veh (p < 0.001) and discontinuation of ODN post‐surgery returned callus BMD to Veh. Peak load of ODN‐ or ALN‐treated calluses were comparable to Veh. ODN increased callus yield load (20%, p = 0.056) and stiffness (26%, p < 0.05) versus Veh. These studies demonstrated that ODN increased mineralized callus during the early phase of fracture repair without impairing callus formation or biomechanical integrity at the fracture site. © 2015 The Authors. Journal of Orthopaedic Research published by Wiley Periodicals, Inc. on behalf of the Orthopaedic Research Society. J Orthop Res 34:72–80, 2016.
Keywords: cathepsin K, fracture healing, callus formation, osteoclast, rabbit
Cathepsin K (CatK), a lysosomal cysteine protease highly expressed by the osteoclast, is primarily responsible for the degradation of the non‐mineralized component of bone, predominantly type I collagen. Odanacatib (ODN) is an orally active, selective, and reversible CatK inhibitor being developed for the treatment of osteoporosis in postmenopausal women and men. Treatment with ODN decreases osteoclastic bone resorption efficiency without affecting other osteoclast activities, including osteoclastogenesis, migration, or survival.1 In preclinical models of estrogen deficiency and in clinical trials, ODN treatment reduces osteoclastic bone resorption while modestly or transiently reducing bone formation.2 As a novel class of therapeutics for the chronic treatment of osteoporotic patients in whom fractures may occur, it is essential to evaluate the effects of ODN on post‐fracture events.
Fracture repair is a complex sequential process of events conventionally partitioned into four overlapping stages: the initial inflammatory response, formation of fibrocartilage, mineralization of callus, and remodeling into lamellar bone.3 Inflammatory and vascular cells, osteochondral progenitors, and osteoclasts play key roles in the fracture repair process which are coordinated by pro‐inflammatory cytokines, growth factors, pro‐osteogenic, and angiogenic factors.4, 5 Following the initial inflammatory and hematoma stages associated with mesenchymal stem cell recruitment and fibrocartilaginous callus formation, the cartilaginous callus becomes mineralized through endochondral ossification to form woven bone, stabilizing the fracture site. As fracture healing progresses, the soft callus undergoes resorption and replacement with hard callus during a period of primary bone formation. This is followed by an extended period of secondary bone formation associated with remodeling of the mineralized callus back to the original structure of the injured bone.
Previous fracture studies with antiresorptive agents, such as bisphosphonates and anti‐RANK ligand antibody denosumab (D‐MAb), were reported to increase callus sizes and delay callus remodeling in animal models of fracture repair.6, 7, 8 In a canine osteotomy model, alendronate (ALN) increased callus area but did not result in significant differences in peak load or stiffness compared to non‐treated controls.7 Treatment with another bisphosphonate, zoledronic acid, increased callus area, and peak load in a rabbit tibial fracture model of distraction osteogenesis.9 Gerstenfeld et al. showed that D‐MAb delayed the removal of cartilage but enhanced bone strength in a murine fracture model.6 These antiresorptives either block osteoclastic differentiation, bone resorption, or survival, and consequently reduce bone formation. Furthermore, the long‐term skeletal retention of bisphosphonates10 and relatively extended circulating half‐life of D‐Mab have posed a theoretical concern of delaying fracture repair in patients experiencing a fracture while on therapy.11
In a murine femoral fracture model, inhibition of CatK by genetic deletion or pharmacological treatment enhanced the number of TRAP‐positive osteoclasts in the fracture calluses and bone formation, but did not fully abolish callus remodeling.12, 13 The human CatK inhibitor ODN is significantly less potent against the rodent enzyme due to the differences between human versus rodent enzymatic sites.14 Meanwhile, rabbit CatK shares high sequence identity to its human counterpart.15 Thus, the inhibitors developed against the human enzyme, including ODN, have similar efficacy in blocking the recombinant rabbit CatK and effectively preventing bone loss in the rabbit model of estrogen‐deficiency osteopenia.15, 16 Previously, the rabbit mid‐ulnar osteotomy fracture model has been used to evaluate the therapeutic agent, recombinant human bone morphogenic protein.17, 18 Using the same fracture repair model, two different studies were conducted to evaluate the effects of ODN compared to alendronate (ALN) treatment on the early phase (∼6‐weeks) of callus development (Study 1) and the late phase (15‐weeks) of callus remodeling (Study 2). Both studies were performed by pretreatment of skeletally mature female rabbits with either ODN, ALN, or vehicle for 3–4 weeks prior to osteotomy, and radiographical monitoring of bone healing in animals continued on the same respective treatment. Ex vivo assessments of the treatment‐related effects were evaluated on callus formation by histopathology (Study 1) and on callus mineralization and biomechanical properties as determined by DXA, QCT, and four‐point bending tests (Study 2). The resolution of effect on fracture healing was also evaluated in Study 2 with animals being discontinued on ODN treatment post‐surgery.
MATERIALS AND METHODS
Experimental Study Designs
The in‐life portion of these studies was conducted in accordance with recommendations of the Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Merck Research Laboratories, West Point.
Study 1
In this study, a pilot investigation was performed to assess inhibition of bone resorption by ODN and ALN on cartilage retention and osseous union by quantitative histopathological analysis in a rabbit mid‐ulnar osteotomy fracture model. Twenty‐seven female, 9‐month‐old New Zealand White rabbits were randomly divided equally (N = 9/group) into three treatment groups: vehicle (Veh, saline), ODN (0.004% in rabbit chow), or ALN (0.3 mg/kg twice‐weekly, s.c.). Rabbits were treated for 3 weeks prior to a transverse osteotomy of the left ulnar diaphysis. After an additional 41 days or ∼6‐weeks of treatment, the rabbits were euthanized and the left ulnae with attached radii were disarticulated at the humeroulnar and carporadial joints and preserved in 10% neutral buffered formalin.
Osteotomy Surgery
A complete osteotomy of the left ulna was performed with a low‐speed bone saw at ∼2.7–4.5 cm proximal to the distal end of the ulna. Ketamine/xylazine anesthesia was used to effect. A splint was applied to the fractured limb and was changed weekly for 4 weeks, after which no additional splinting or activity restriction was necessary. Lateral and antero‐posterior radiographs of left radii/ulnae were taken immediately postoperatively using a JB‐70 Dental X‐Ray System (Progeny, Lincolnshire, Illinois; 70 kVp, 7 mA) to assure proper alignment of the fracture at the start of the healing period. Additional radiographs were obtained at day 14 and 28 to monitor healing.
Histological Analysis
Histopathologic evaluation of mineralized tissue at the osteotomy bridging site was performed. The left ulnar fracture site was fast decalcified in 5% formic acid for 24 h, embedded in paraffin, sectioned, and stained with Fast Green/Safranin O for histomorphometric analysis, and hematoxylin and eosin for histological analysis. A five point grading scale (Table 1) was used for the histomorphologic evaluation of the fracture site and callus as previously described.7 For histomorphometric analysis of the paraffin‐embedded sections (N = 9 per group), cartilaginous, bony, and periosteal callus areas of the fracture site were outlined in a region 4 mm proximal and distal to the osteotomy gap and quantified using ImageScope Ver. 12.0.0.5039 (Aperio Technologies, Inc., Vista, CA). For cartilaginous (safranin red area) and bony callus (fast green) area, measurements included both the external callus and internal fracture gap regions. Periosteal callus area was defined as external callus located outside of the old cortical bone. A color deconvolution algorithm was used to define cartilage versus bone.
Table 1.
Fractured Ulnae From Study 1 at Approximately Week 6 Post‐Osteotomy: Histomorphologic Grades by Incidence
| Grade a | Control (n = 9) | ODN (n = 9) | ALN (n = 9) |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 |
| 3 | 0 | 1 | 4 |
| 4 | 9 | 8 | 5 |
| 5 | 0 | 0 | 0 |
Grade: 1: Incomplete cartilaginous union with retention of fibrous tissue in cartilaginous plate. 2: Complete cartilaginous union with a well formed plate of hyaline cartilage. 3: Osseous union with a callus containing cartilage. 4: Complete osseous union with a small callus containing no or small amount of cartilage. 5: Complete osseous union with no evidence of callus.
The fractured ulnar tissues were subjected to acid decalcification which destroyed TRAP activity, hence to detect osteoclasts in the callus, decalcified sections of the left ulna were immunostained with a CatK monoclonal antibody (Santa Cruz Biotechnologies, CA) according to a previously described method.19
Statistical Analysis
All statistical comparisons were analyzed using Statview 5.0 software (SAS Institute Inc., Cary, NC). All reported numerical data were subjected to calculation of group means and standard errors. All data in tables and figures are shown as mean plus or minus the standard error (SEM). Data were tested for normal distribution and equal variance, and the differences among three or more groups were analyzed by one way analysis of variance (ANOVA) and followed by Fisher's protected least significant difference (Fisher's PLSD), except where indicated.
Study 2
Figure 3 summarizes the design of this study including 75 skeletally mature female New Zealand White rabbits, (∼7 months old and mean body weight of 3.2 kg) received from Covance (Denver, PA). The rabbits were randomized by body weight and assigned to five groups (N = 15/group). Prior to osteotomy surgery (as described above), the rabbits were treated for 4 weeks with either Harlan Teklad 2031 (Madison, WI) vehicle diet (Veh), ODN 2 mg/kg (0.004% in the same diet, 180 g daily), or ALN at 0.3 mg/kg (s.c., 2×/week). After osteotomy, the animals were either maintained on the same respective treatment regimen (Veh→Veh, ODN→ODN, ALN→ALN), or switched from vehicle to ODN (Veh→ODN) or from ODN to Veh (ODN→Veh) for an additional 15 weeks. Radiographs were taken as described previously and at 15‐weeks post‐fracture. The selected ALN dose was previously shown to fully prevent bone loss in an ovariectomized rabbit study.16 Pharmacokinetic evaluation of the ODN 2 mg/kg dose resulted in a mean drug exposure of 9 μM·24 h in the OVX‐rabbit.16 The animals were euthanized at 15‐weeks post‐fracture. The right (intact) and left (fractured) forelimbs were removed, cleaned of soft tissue, wrapped in saline‐soaked gauze, and frozen (−20 °C). Note that in Study 2, six rabbits were excluded from the analysis. Four exclusions were due to complications associated with the osteotomy surgery. One rabbit in the Veh→Veh group was found dead 1 month post‐surgery and post‐mortem results indicated a cause of tricuspid valve endocardiosis. One rabbit in the ODN→ODN group suffered a dislocated hip 1 month post‐surgery and was euthanized.
Imaging Analyses
Dual energy X‐Ray absorptiometry (DXA) (QDR4500A; Hologic, Inc., Waltham, MA) ‐ Ex vivo DXA was used to measure bone mineral density (BMD) of the left and right radii/ulnae from Study 2. High resolution measurements of area, bone mineral content (BMC), and BMD data were captured from a region of interest approximately 1.6 (length) × 1.28 (width) cm over the region of osteotomy in the left limb as well as the osteotomy‐equivalent site in the right intact limb.
Peripheral Quantitative Computed Tomography (pQCT)
Ex vivo pQCT evaluation was done on the articulated ulna and radius. Forelimbs were thawed overnight at 4 °C prior to scanning. Peripheral QCT was used to measure BMC, BMD, and geometric parameters of the osteotomy site of each left ulna for all animals from Study 2. pQCT scans were performed using an XCT Research SA or SA+ bone scanner with software version 5.50D (Stratec Medizintechnik GmbH, Pforzheim, Germany). For the left ulna, three slices were scanned: one at the osteotomy site and the other two below and above the osteotomy site (1 mm apart from site) in order to evaluate the extent of the callus formation. The fractured left ulnae were analyzed using a threshold of 1.09 1/cm at the osteotomy site to obtain total callus area, BMC, and BMD values. Based on historical data, this threshold is arbitrarily used to detect cortical bone.
Biomechanical Testing
Four‐point bending of the fractured site of the left ulnar or fractured‐equivalent site of the right ulnae was performed using a servohydraulic test system (MTS 858 Mini Bionix, Eden Prairie, MN). The radius and ulnae were first separated, then the ulnae were tested to failure at a constant loading rate of 1 mm/sec. The upper contact points with a span length of 15 mm and the lower points with a span length of 30 mm on the fixture were centered over the osteotomy or osteotomy‐equivalent site. Load and displacement data were collected using TestWorks version 3.8A for TestStar software, version 4.0c. At the conclusion of testing, each bone was examined to determine the site at which failure occurred. Peak load was measured as the maximum height of the load–displacement curve and stiffness was measured as the slope of the linear portion of the load–displacement curve. Yield load was determined with a 0.02% offset as the height of the load–displacement curve at which permanent deformation of the specimen is achieved.
Statistical Analysis
Statistical comparisons and reporting of numerical data were performed as described above. Correlation analysis of ex vivo pQCT‐derived parameters and biomechanical strength parameters were performed using a Pearson correlation test. To account for individual animal variation with respect to bone geometry, pQCT‐derived total BMC and strength parameters were expressed as a percentage of the contralateral limb.
RESULTS
No Histopathological Abnormalities in Odanacatib‐Treated Callus
The objective of Study 1 was to assess the effects of ODN compared to that of ALN on the reparative phase and callus formation. The animals were pretreated with vehicle, ODN, or ALN for 3 weeks prior to osteotomy and continued on the same respective treatment for additional 41 days or about 6 weeks. All rabbits recovered from surgery well and tolerated the limb splints. Subsequent healing of the osteotomy site proceeded uneventfully. At ∼6‐weeks post‐fracture, all groups revealed completed osseous union as determined radiographically and showed no qualitative abnormalities by histopathological assessments of the callus (Table 1). All nine rabbits in the control group had complete osseous union with a small callus containing no or small amounts of cartilage (grade 4). There was a very slight increase in the callus cartilage content (grade 3) in one rabbit from the ODN group and four rabbits from the ALN group. Osteotomy sites in remaining treated rabbits were histomorphologically similar to rabbits in the control group (Table 1).
Histomorphologic evaluation revealed no meaningful qualitative differences in callus size and formation in rabbits from any treatment group (Fig. 1A). Mid‐sagittal sections of the callus stained with Fast Green/Safranin O revealed that at ∼6‐weeks post‐facture, the ALN group exhibited callus cartilaginous retention (Fig. 1A‐c, in red), with cartilage area significantly higher than that from Veh or ODN group (p < 0.05, Fig. 2B). Calluses from the ODN group showed mostly bony tissue, comparable to that in the Veh group (Fig. 1A‐a and b). The ODN group displayed similar amounts of cartilaginous tissue, bony callus formation, and periosteal callus formation compared to the Veh group (Fig. 1A–C).
Figure 1.
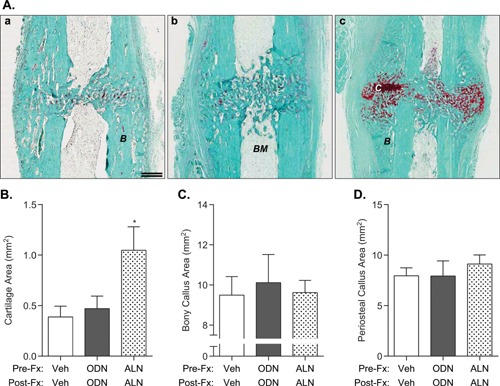
(A) Cartilage and bony callus retention in fractured calluses from Study 1 at day 41 post‐osteotomy. Representative images from paraffin‐embedded sections from the fractured ulnae of (a) vehicle‐, (b) ODN‐, and (c) ALN‐treated rabbits and stained with Safranin‐O/Fast Green to identify cartilaginous (red) and mineralized callus formation (green) at the osteotomy site. Note the presence of cartilage in the osteotomy gap in the ALN‐treated group. (B–D) Histomorphometric analysis to quantitate the area of cartilaginous region, bony callus, and periosteal callus of fractured ulnae post‐osteotomy. (B: bone, BM: bone marrow, C: cartilage). Double scale bar, 1.5 mm. N = 8–9/group; mean ± SEM.
Figure 2.
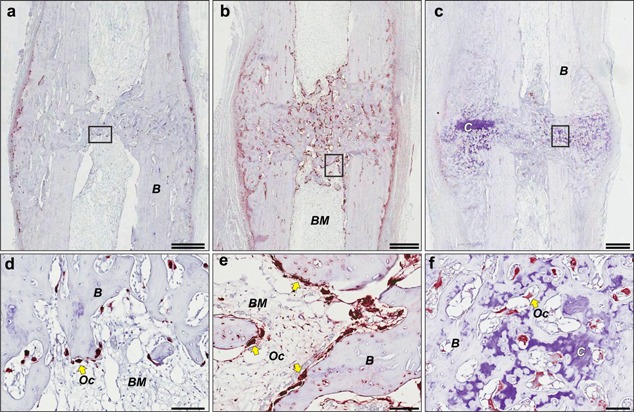
Osteoclast recruitment to fractured callus from Study 1 at day 41 post‐osteotomy. Representative images from serial paraffin‐embedded sections from the same fractured ulnae showed in Figure 1, including (a) vehicle‐, (b) ODN‐, and (c) ALN‐treated rabbits and immunostained with anti‐cathepsin K monoclonal antibody and then counterstained with toluidine‐blue. (a–c) Double scale bar, 1.5 mm. (d–f) Large multinucleated osteoclasts (Oc, yellow arrows) are shown in the respective inset from a–c, viewed at higher magnification with scale bar, 100 μm. (B: bone, BM: bone marrow, C: cartilage).
To survey the recruitment of osteoclasts to the callus during the reparative phase, representative sections from the Veh, ODN, and ALN‐treated callus at ∼6‐weeks post‐osteotomy were also subjected to immunohistological staining for CatK, a marker of osteoclasts (Fig. 2, red cells). Multinucleated CatK‐(+) osteoclasts were detected along the periosteal and trabecular‐like bony regions of the callus in the vehicle‐treated ulnae (Fig. 2a and d). Remarkedly, ODN treatment significantly increased the number of CatK‐(+) cells on the periosteal, cortical, and trabecular regions of the remodeling callus (Fig. 2b and e). While osteoclasts were almost undetectable on the periosteal surface of the ALN‐treated callus, they could be detected amongst bony as well as cartilage regions of the callus (Fig. 2c and f).
Mineralized Callus is Higher With Odanacatib Treatment
Similarly, skeletally mature female rabbits in Study 2 (Fig. 3) were pretreated with vehicle, ODN, or ALN and continued on the same respective treatment (Veh→Veh, ODN→ODN, ALN→ALN), or switched from Veh to ODN (Veh→ODN) or from ODN to Veh (ODN→Veh). Fracture healing in Study 2 was radiographically assessed at day 14 and day 28 post‐fracture. At day 28, the fracture sites were radiographically similar in bridging callus formation and size in all groups (Fig. 4, upper panels). There were no observed treatment‐related delays in osseous union. However, by study termination at week 15 post‐surgery, cortical bridging was completed in all groups (Fig. 4, lower panels). To examine the effects of CatK inhibition on mineralization during callus remodeling, we performed ex‐vivo areal densitometric analyses of the intact (right) and fractured (left) ulnae/radii (Fig. 5A and B). Note, both the fractured ulnar with the non‐fractured radius were scanned together by DXA with an assumption that there was no treatment‐related changes in BMD of the non‐fractured radius (Fig. 5A). Rabbits of the ODN→ODN group showed the greatest increases in areal BMC (20%, p < 0.01) and BMD (22%, p < 0.001) in the fractured (left) ulnae/radii (Table 2 and Fig. 5), while the ALN group also significantly increased areal BMC (11%, p < 0.05) and BMD (17%, p < 0.001) versus Veh→Veh. BMD of the ODN→Veh group was similar to Veh treatment alone (Table 2 and Fig. 5B). Additionally, starting ODN treatment after osteotomy (Veh→ODN) increased BMD by 14% versus Veh→Veh. There were no intergroup differences in BMC or BMD (Table 2 and Fig. 5A) of the osteotomy‐equivalent region in the right ulnae/radii of the ODN‐ or Veh‐treated groups; however, the area of ALN‐treated intact ulnae/radii was smaller than that in Veh controls (Table 2).
Figure 3.
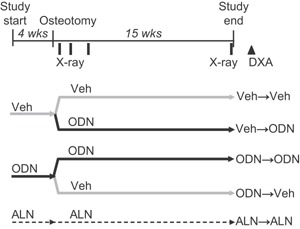
Schematic of the Study design 2. Veh: vehicle; ODN: odanacatib; ALN: alendronate. In vivo radiographic (X‐ray) scanning and ex vivo DXA are noted.
Figure 4.
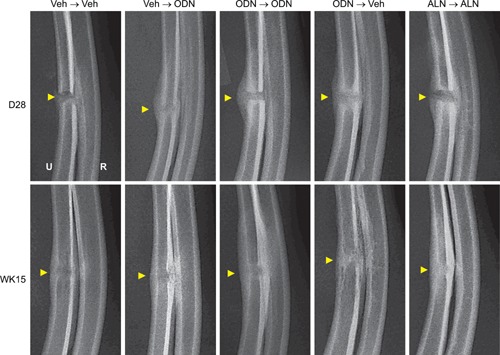
Representative radiographic images of the ulnar fracture sites from Study 2 at day 28 and week 15 post‐osteotomy. Arrows indicate the original osteotomy sites.
Figure 5.
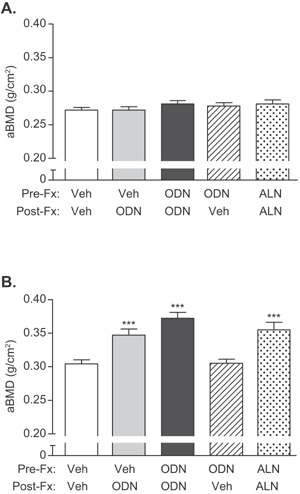
DXA‐based bone mineral density of intact (A) and fractured ulnae (B) from Study 2 at week 15 post‐osteotomy. (A) aBMD of the intact ulna did not show differences between treatment groups; (B) aBMD of the fractured ulnae showed significant increases with treatment either with ALN or with ODN administered continuously or right after fracture. N = 13–15/group. Data are expressed as mean ± SEM.
Table 2.
Ex‐vivo DXA‐Based Bone Mineral Density of Intact and Fractured Radii/Ulnae From Study 2
| Group | Veh→Veh | Veh→ODN | ODN→ODN | ODN→Veh | ALN→ALN |
|---|---|---|---|---|---|
| Intact (right) radii/ulnae | |||||
| Area (cm2) | 1.12 ± 0.02 | 1.07 ± 0.02 | 1.06 ± 0.02 | 1.070 ± 0.02 | 1.013 ± 0.02 * |
| BMC (g) | 0.30 ± 0.01 | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.28 ± 0.01 |
| BMD (g/cm2) | 0.272 ± 0.004 | 0.272 ± 0.005 | 0.281 ± 0.005 | 0.278 ± 0.005 | 0.281 ± 0.006 |
| Fractured (left) radii/ulnae | |||||
| Area (cm2) | 1.16 ± 0.02 | 1.15 ± 0.02 | 1.14 ± 0.02 | 1.10 ± 0.02 * | 1.09 ± 0.02 * |
| BMC (g) | 0.35 ± 0.01 | 0.40 ± 0.01** | 0.42 ± 0.01*** | 0.34 ± 0.01 | 0.39 ± 0.01 * |
| BMD (g/cm2) | 0.304 ± 0.006 | 0.347 ± 0.009*** | 0.372 ± 0.009*** | 0.305 ± 0.006 | 0.355 ± 0.011*** |
Mean ± SEM.
p < 0.05 versus Veh→Veh. **p < 0.01. ***p < 0.001.
After carefully separating the ulna from the radius, pQCT analysis of the healing left ulnae corroborated the areal DXA results showing increased tissue mineralization observed with ODN and ALN treatments compared to Veh controls. A significant increase in total callus area of the ODN→ODN group was noted compared to the Veh‐treated group (Table 3). The total callus BMC of the fractured ulnae in ODN‐treated rabbits was greater (40%, p < 0.001) than that in Veh controls (Table 3). Fractured ulnae from the Veh→ODN and ALN→ALN groups also showed higher total BMC compared to that in Veh→Veh (Table 3).
Table 3.
Ex vivo pQCT‐Related Density Measurements of Fractured Ulnae and Biomechanical Properties of Intact and Fractured Ulnae From Study 2
| Group | Veh→Veh | Veh→ODN | ODN→ODN | ODN→Veh | ALN→ALN |
|---|---|---|---|---|---|
| pQCT | |||||
| Fractured (left) ulnae | |||||
| Total callus area (mm2) | 18.15 ± 0.85 | 17.87 ± 0.57 | 20.83 ± 0.72 * | 17.10 ± 0.78 | 18.56 ± 1.21 |
| Total callus BMC (mg/mm) | 13.71 ± 0.56 | 19.13 ± 0.91** | 20.21 ± 0.75** | 12.91 ± 0.55 | 18.99 ± 1.13** |
| Total callus BMD (mg/cm3) | 764.96 ± 27.15 | 964.76 ± 23.02** | 971.94 ± 16.94** | 762.72 ± 23.05 | 1,029.75 ± 12.37** |
| Biomechanical properties | |||||
| Intact (right) ulnae | |||||
| Peak load (N) | 270.0 ± 12.9 | 263.0 ± 13.5 | 258.7 ± 12.8 | 278.0 ± 16.9 | 244.7 ± 11.0 |
| AUC (N‐mm) | 226.8 ± 13.5 | 230.3 ± 10.8 | 243.5 ± 14.7 | 223.4 ± 15.3 | 260.4 ± 15.8 |
| Yield load (N) | 213.8 ± 10.5 | 209.9 ± 12.7 | 202.0 ± 12.9 | 216.6 ± 13.5 | 188.5 ± 10.4 |
| Stiffness (N/mm) | 283.5 ± 16.3 | 273.6 ± 19.3 | 264.7 ± 18.4 | 291.0 ± 20.1 | 232.7 ± 15.3 |
| Ultimate stress (MPa) | 306.2 ± 10.2 | 324.6 ± 7.8 | 326.3 ± 4.8 | 315.5 ± 11.9 | 332.9 ± 6.7 |
| Modulus (MPa) | 16,907.2 ± 726 | 17,994.2 ± 589 | 18,947.2 ± 491 | 17,070.9 ± 740 | 18,218.9 ± 538 |
| Toughness (MPa) | 4.9 ± 0.3 | 5.4 ± 0.3 | 5.4 ± 0.3 | 5.1 ± 0.5 | 6.1 ± 0.3 |
| Fractured (left) ulnae | |||||
| Peak load (N) | 208.4 ± 14.1 | 242.0 ± 17.0 | 215.8 ± 13.6 | 214.8 ± 11.9 | 178.1 ± 17.3 |
| AUC (N‐mm) | 136.9 ± 21.5 | 111.5 ± 13.6 | 128.0 ± 34.5 | 109.3 ± 9.0 | 72.5 ± 13.9 |
| Yield load (N) | 173.3 ± 10.4 | 219.5 ± 15.2*** | 207.9 ± 12.8 | 181.8 ± 9.4 | 167.8 ± 13.6 |
| Stiffness (N/mm) | 267.8 ± 19.2 | 360.1 ± 35.4*** | 337.3 ± 18.9 * | 290.3 ± 19.1 | 283.7 ± 17.6 |
Mean ± SEM.
p < 0.05 versus Veh→Veh. **p < 0.001. ***p < 0.01.
Odanacatib Treatment Does Not Reduce Bone Strength at the Fracture Site 15‐Weeks Post‐Osteotomy
No treatment‐related differences were noted for bone strength results obtained by four‐point bending testing in the intact (right) ulnae (Table 3). In the fractured (left) ulnae, a trend for increased peak load and significant increases in yield load and stiffness were observed in the Veh→ODN group (27%, p < 0.01 and 37%, p < 0.05, respectively) versus Veh→Veh (Table 3). Stiffness was also significantly increased in the ODN→ODN group. After 15 weeks post‐osteotomy, the biomechanical properties of the fractured (left) ulnae of the Veh→Veh group had attained approximately 77 and 94% of the peak load and stiffness, respectively, of the intact (right) ulnae. In contrast, although the peak load of the fracture ulnae from ODN→ODN group achieved ∼83% of the intact ulna, stiffness in this group was significantly greater than that in the Veh→Veh group. Stiffness and yield load were positively and significantly correlated with total vBMC across groups (r 2 = 0.3997 and 0.2669, respectively, with p < 0.001 in both correlations). Peak load was also positively correlated to total vBMC (r 2 = 0.1222, p < 0.01). In contrast to the intact (right) ulnae, intrinsic strength parameters could not be derived for the fracture (left) ulnae due to the irregularity of callus area moment of inertia even after 15‐weeks post‐osteotomy.
DISCUSSION
Fracture repair recapitulates certain aspects of skeletal development and is conventionally partitioned into four stages of cellular and molecular events.4 At the cellular level, inflammatory cells, vascular cells, osteochondral progenitors, and osteoclasts are key players in the repair process. Events during fracture healing are also considerably influenced by numerous key factors including the injury location, biomechanical status, prevalence of infection, general skeletal health, surgical fixation, and pharmacological factors.20 Among the adjunctive therapies, several bone agents are suggested to modify the speed of callus remodeling or strength of the healing site.21, 22 Applying the concepts of anabolism and catabolism of skeletal remodeling, the balance between formation and removal of the early fibrocartilage soft callus and the hard bony callus during the late phase have been speculated to determine the speed of fracture healing.22
The standard‐of‐care antiresorptives, bisphosphonates, and denosumab (D‐MAb, an anti‐RANKL antibody), have also been studied in preclinical models of fracture repair. Both classes of agents demonstrated similar findings of increased callus retention, mineralization, and neutral to enhanced callus strength. Treatment with the bisphosphonate ALN in osteotomized radii in dogs produced larger calluses but did not inhibit bone formation or mineralization, ultimate strength or stiffness.7 Bolus zoledronic acid treatment increased callus volume, bone mineral content, and callus strength at 6 and 26 weeks post‐fracture in a rat‐closed fracture model.23 In human RANKL knock‐in mice treated with D‐MAb, bone volume and cross‐sectional area as assessed by μCT were significantly increased after 42 days of treatment, while larger callus sizes were observed but not significantly increased.6 These mice also displayed a significantly higher percentage of mineralized callus at 21 and 42 days as compared to ALN‐ or no‐treatment controls. As a result, the D‐MAb‐treated fractured mouse bones exhibited higher maximum torque and higher torsional rigidity than their contralateral limbs, while stiffness also increased over time, showing significant differences to that in the controls.6 Treatment with potent antiresorptives also delay removal of cartilage within the fracture callus. D‐MAb delayed the removal of cartilage and the remodeling of the fracture callus during fracture healing in human RANKL knock‐in mice.6 D‐MAb inhibited cartilage resorption, showing evidence of an increased number of hypertrophic chondrocytes with large areas of mesenchymal and connective tissue adjacent to areas of unresorbed cartilage matrix.6 In the same study, ALN inhibited cartilage resorption at day 21 post‐fracture compared to the control group.6
A previous study in mice investigating the effects of the CatK inhibitor L‐006235 also showed enhanced mineralization within the calluses at day 21 post‐fracture.13 The present studies demonstrated that continuous administration of ODN before and during bone fracture repair resulted in increased callus mineralization and significant increases in stiffness. The dose of ODN used in these studies has been demonstrated to fully protect against estrogen‐deficiency induced osteopenia in the ovariectomized rabbit.16 Also, note that to mimic the clinical exposure of ODN 50 mg (6–7 μM·24 h) dosed in humans as a once‐weekly tablet, the food‐formulated ODN in this rabbit study was developed to provide a mean steady state plasma exposure at 9 μM·24 h, approximately 1.2–1.5‐fold of the clinical exposure of this drug. On the other hand, ALN subcutaneously administered between 0.2 and 0.6 mg/kg/week in prevention mode for 13‐weeks was previously demonstrated to fully protect against estrogen‐deficiency‐induced bone loss in skeletal mature female rabbits.16 An ALN dose lower than 0.2 mg/kg/week showed only partial efficacy in that study. The requirement of the high doses of ALN in OVX‐rabbits may be due to differences in ALN metabolism in various species; however, based on the result of the titration study,16 we selected the ALN 0.6 mg/kg/week (s.c.) dose as the active control for subsequent studies in rabbits, particularly those associated with high callus remodeling during fracture healing.
Radiologic examination of the fractured (left) ulnae indicated that the fracture sites of the second study did not achieve full resolution of the calluses at 15 weeks post‐osteotomy across all treatment groups. The lack of post‐yield parameters derived from the four‐point bending of the fracture (left) ulnae was evident due to irregularity of the callus cross‐sectional area moment of inertia. Furthermore, the increase of mineralized bony tissue in the fracture calluses of rabbits treated with ODN was expected, given the known reduction of efficiency in osteoclastic bone resorption.19 Consistent with previous findings from pharmacological studies in OVX‐rabbits and monkeys,16, 24 ODN treatment also increased the number of osteoclasts, which may mediate subsequent bone formation through the coupling of osteoclast–osteoblast during bone remodeling.2 While significant recruitment of CatK‐positive osteoclasts were detected in the callus from ODN‐treated rabbits, a drawback in the current study design was not including sufficient number of fractured rabbits to dedicate for dynamic histomorphometry analysis. This will be a subject of future study to further our understanding on the mechanism of CatK inhibition on potentially enhancing bone formation during fracture repair.
No abnormal cartilage or bone retention was observed at osteotomy sites when rabbits were treated with ODN for 3 weeks prior to osteotomy and then continued treatment on ODN treatment for an additional 6 weeks post‐osteotomy as observed in the Study 1. These findings differ from a previous study showing that significant amounts of residual cartilaginous callus persisted in femoral fractures of CatKi treated mice compared to control mice at day 21 post‐fracture.13 The discrepancy may be due to the examination of the fracture site at different healing timepoints (day 21 in the mice and day 41 in the rabbits) or to the different remodeling rate in each model. In addition, fracture calluses in the Veh→Veh group in this study showed a modest amount of cartilage at day 41 and generally appeared to heal faster than that as previously described in a similar rabbit ulnar bilateral osteotomy model, with osteotomy‐bridged callus containing primarily cartilage even at 6 weeks after surgical fracture.18 Perhaps the additional stabilization of the limb with external splinting, the age of the rabbits, and the use of a unilateral osteotomy model may account for the different healing rate in the various studies.
The effects of discontinuation of ODN following fracture on mineralization and bone strength were also examined. Both oral and intravenous bisphosphonates are known to have long‐term skeletal retention10 and D‐MAb remains in circulation for more than 3 months after biannual subcutaneous injections.11 Even after discontinuation, these more slowly reversible therapies have been predicted to display persistent antiresorptive effects in patients fracturing during therapy. In Study 2, discontinuation of ODN following fracture did not have a detrimental effect on bone strength, and callus mineralization was generally similar to vehicle‐treated controls. We have previously examined the kinetics of reversibility of ODN in blocking osteoclast function in vitro showing that discontinuation of ODN in culture fully reverses inhibition of osteoclast activity back to control levels by 4 days.25 In an ODN phase II study in patients with postmenopausal osteoporosis, discontinuation of ODN was demonstrated to quickly increase bone biochemical markers above baseline.24 The accrued BMD gained for 2 years and the elevated bone turnover markers in the group with discontinuation of therapy largely returned toward baseline within 1 year.26 Furthermore, we previously demonstrated that ODN inhibits osteoclastic resorption without affecting osteoclast number, while maintaining bone formation rates in trabecular and endocortical bone in rabbits.1 These characteristics are different from other standard antiresorptives used to treat osteoporosis which are known to either decrease osteoclast progenitor development and recruitment or to promote osteoclast apoptosis.27
In summary, the results shown here demonstrated that ODN treatment does not impede callus formation during fracture healing. ODN treatment prior to fracture or during the reparative phase and remodeling phase of fracture repair did not delay osseous union and significantly enhanced the stiffness of the fractured sites by increasing mineralization of the callus. Furthermore, discontinuation of ODN immediately following fracture led to callus properties comparable to vehicle controls. Currently, additional long‐term studies in the rabbit osteotomy model of fracture repair are being conducted to assess the safety profile of the CatK inhibitor ODN during the later stages of hard callus remodeling when the fractured limb is restored to its original shape and function.
AUTHORS’ CONTRIBUTIONS
B.L.P. and D.B.K. designed and coordinated the in‐life study. D.G. executed osteotomy surgery. N.T.G. performed the histomorphologic bone evaluation. R.S. and S.S. contributed to QCT and bone strength testing. B.L.P. and L.T.D. performed analysis, interpretated results, and drafted the manuscript. All provided critical review and approval of the manuscript.
ACKNOWLEDGMENTS
We thank the staff from Safety Assessment and Laboratory Animal Resources for their excellent support on the surgical procedure and the in‐life study; Charles River Labs (Senneville, QU, Canada) for the bone strength testing, and Renee Gentzel for technical assistance regarding the ImageScope algorithm.
Conflicts of interest: Pennypacker BL, Gilberto D, Gatto NT, Kimmel DB, and Duong LT are current or former employees of Merck, the studies’ sponsor, and may own stock/stock options in this company. Samadfam R. and Smith S. are employees of Charles River Laboratories, which performed contract research for this study, and may own stock/stock options in this company.
REFERENCES
- 1. Gauthier JY, et al. 2008. The discovery of odanacatib (MK‐0822), a selective inhibitor of cathepsin K 1. Bioorg Med Chem Lett 18:923–928. [DOI] [PubMed] [Google Scholar]
- 2. Duong LT. 2012. Therapeutic inhibition of cathepsin K‐reducing bone resorption while maintaining bone formation. Bonekey Rep 1:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerstenfeld LC, et al. 2003. Fracture healing as a post‐natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 88:873–884. [DOI] [PubMed] [Google Scholar]
- 4. Schindeler A, et al. 2008. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol 19:459–466. [DOI] [PubMed] [Google Scholar]
- 5. Barnes GL, et al. 1999. Growth factor regulation of fracture repair. J Bone Miner Res 14:1805–1815. [DOI] [PubMed] [Google Scholar]
- 6. Gerstenfeld LC, et al. 2009. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res 24:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peter CP, et al. 1996. Effect of alendronate on fracture healing and bone remodeling in dogs. J Orthop Res 14:74–79. [DOI] [PubMed] [Google Scholar]
- 8. Bauss F, et al. 2004. New model for simulation of fracture repair in full‐grown beagle dogs: model characterization and results from a long‐term study with ibandronate. J Pharmacol Toxicol Methods 50:25–34. [DOI] [PubMed] [Google Scholar]
- 9. Little DG, Smith NC, Williams PR, et al. 2003. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J Bone Miner Res 18:1300–1307. [DOI] [PubMed] [Google Scholar]
- 10. Porras AG, Holland SD, Gertz BJ. 1999. Pharmacokinetics of alendronate. Clin Pharmacokinet 36:315–328. [DOI] [PubMed] [Google Scholar]
- 11. Kostenuik PJ. 2005. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol 5:618–625. [DOI] [PubMed] [Google Scholar]
- 12. Gentile MA, Soung do Y, Horrell C, et al. Increased fracture callus mineralization and strength in cathepsin K knockout mice. Bone 66:72–81. [DOI] [PubMed] [Google Scholar]
- 13. Soung do Y, Gentile MA, Duong LT, et al. 2013. Effects of pharmacological inhibition of cathepsin K on fracture repair in mice. Bone 55:248–255. [DOI] [PubMed] [Google Scholar]
- 14. Stroup GB, Lark MW, Veber DF, et al. 2001. Potent and selective inhibition of human cathepsin K leads to inhibition of bone resorption in vivo in a nonhuman primate 1. J Bone Miner Res 16:1739–1746. [DOI] [PubMed] [Google Scholar]
- 15. Pennypacker BL, Oballa RM, Levesque S, et al. 2013. Cathepsin K inhibitors increase distal femoral bone mineral density in rapidly growing rabbits. BMC Musculoskelet Disord 14:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pennypacker BL, et al. 2011. Cathepsin K inhibitors prevent bone loss in estrogen‐deficient rabbits. J Bone Miner Res 18:252–262. [DOI] [PubMed] [Google Scholar]
- 17. Li RH, Bouxsein M, Blake CA, et al. 2003. RhBMP‐2 injected in a calcium phosphate paste (alpha‐BSM) accelerates healing in the rabbit ulnar osteotomy model. J Orthop Res 21:997–1004. [DOI] [PubMed] [Google Scholar]
- 18. Luppen CA, Blake CA, Ammirati KM, et al. 2002. Recombinant human bone morphogenetic protein‐2 enhances osteotomy healing in glucocorticoid‐treated rabbits. J Bone Miner Res 17:301–310. [DOI] [PubMed] [Google Scholar]
- 19. Leung P, Pickarski M, Zhuo Y, et al. 2011. The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. Bone 49:623–635. [DOI] [PubMed] [Google Scholar]
- 20. Hak DJ, Fitzpatrick D, Bishop JA, et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury 45:S3–S7. [DOI] [PubMed] [Google Scholar]
- 21. Capone A, Orgiano F, Pianu F, et al. Orthopaedic surgeons’ strategies in pharmacological treatment of fragility fractures. Clin Cases Miner Bone Metab 11:105–109. [PMC free article] [PubMed] [Google Scholar]
- 22. Little DG, Ramachandran M, Schindeler A. 2007. The anabolic and catabolic responses in bone repair. J Bone Joint Surg Br 89:425–433. [DOI] [PubMed] [Google Scholar]
- 23. McDonald MM, Dulai S, Godfrey C, et al. 2008. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone 43:653–662. [DOI] [PubMed] [Google Scholar]
- 24. Masarachia PJ, Pennypacker BL, Pickarski M, et al. 2012. Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. J. Bone Miner Res 1:509–523. [DOI] [PubMed] [Google Scholar]
- 25. Zhuo Y, Gauthier JY, Black WC, et al. Inhibition of bone resorption by the cathepsin K inhibitor odanacatib is fully reversible. Bone 67:269–280. [DOI] [PubMed] [Google Scholar]
- 26. Eisman JA, Bone HG, Hosking DJ, et al. 2011. Odanacatib in the treatment of postmenopausal women with low bone mineral density: three‐year continued therapy and resolution of effect 1. J Bone Miner Res 26:251. [DOI] [PubMed] [Google Scholar]
- 27. Hughes DE, Wright KR, Uy HL, et al. 1995. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 10:1478–1487. [DOI] [PubMed] [Google Scholar]


