Abstract
Objective
To determine the effect of the introduction of combination antiretroviral treatment (cART) in the HIV-1 infected US population on the epidemiology of Kaposi’s sarcoma herpesvirus (KSHV).
Design, setting and participants
We investigated the epidemiology of KSHV in 5022 HIV-1-infected, antiretroviral naïve US persons participating in six AIDS Clinical Trials Group (ACTG) randomized clinical trials, and followed in a long-term cohort study. We tested the first and last available sera of each participant for antibodies to KSHV K8.1 and ORF73.
Main outcome measures
We studied prevalence and incidence of KSHV infection, incidence of Kaposi’s sarcoma (KS), and overall survival.
Results
KSHV prevalence was 38.1% (95% CI 36.8-39.5%). Male gender, Caucasian race, age between 30 and 49, residence in North-eastern or Western US, and enrolment after 2001 were independently associated with prevalent infection.
KSHV incidence was 4.07/100 person/years (95%CI 3.70-4.47). Male gender, Caucasian race, age below 30, and enrolment after 2001 were associated with incident infection. CD4 count increase following cART was associated with lower risk.
KS incidence was 104.05/100,000 person/years (95% CI 71.17-146.89). Higher baseline CD4 count, but not increase in CD4 count after cART, was associated with lower hazard of KS. Randomized assignment of protease inhibitors was not associated with better KSHV outcomes.
Conclusions
HIV-1 infected individuals, in particular Caucasian men, remain at significant risk for KSHV co- infection and KS. Thus, optimal management of HIV-1 infection should continue to include vigilance for manifestations of KSHV co-infection, including KS.
Video abstract at http://ncifrederick.cancer.gov/services/spgm/filedownload/pdsDownload.aspx?id=c472803f-1d66–4391-b18b-24e367b6b190
Keywords: Sarcoma, Kaposi, Herpesvirus 8, human, Antiretroviral therapy, highly active, HIV-1, epidemiology
Introduction
The prevalence of Kaposi’s sarcoma associated herpesvirus (KSHV) has marked geographical variations [1]. In general adult populations, KSHV infection is quite common in sub-Saharan Africa (seroprevalence >50%), relatively common in the Mediterranean region (20–30%), and uncommon in Western and Northern Europe, Asia and the Americas (5–10%) [2]. Prevalence is higher, however, in people of specific ethnic minorities or who have certain behavioural risk factors. Significantly, prevalence is elevated in men who have sex with men (MSM): estimates around 20–40% have been reported over the years for the US [3] and Northern Europe [4, 5].
Individuals who are coinfected with KSHV and HIV-1 are at risk of developing KSHV associated malignancies [6], i.e. Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), which is a rare, AIDS-related form of non-Hodgkin’s lymphoma, and KSHV-associated multicentric Castleman’s disease (KSHV-MCD), an infrequently diagnosed multifocal lymphoproliferative disease.
Upon introduction of combination antiretroviral therapy (cART), the incidence of KS has dramatically diminished [7-10], but not completely abated [11, 12]. In the United States, KS remains the second most common AIDS associated malignancy [11]. The most recent estimates from linked HIV and cancer registry data indicate a burden of disease of approximately 1000 cases/year, bearing almost exclusively on HIV infected men [13]. KS occurs in persons with untreated or poorly controlled HIV-1 infection; however, a third of AIDS-KS cases now arise in individuals with relatively high CD4 cell counts and low HIV-1 viral load [14, 15]. It has been speculated that such cases exemplify a clinico-pathological entity more similar to Classic KS, which occurs in elderly HIV uninfected individuals, than AIDS-associated KS. Observations such as older age of onset and evidence of associated immunosenescence [16] seem to support this hypothesis, although they are not consistently reported [17]. Regardless, KS occurring in well controlled HIV-1 infection may become an increasing clinical and public health challenge as life expectancy of HIV-1 infected individuals on cART continues to extend.
To best interpret these observations it is necessary to understand the epidemiology of KSHV infection itself in the cART era; yet, limited data are available. Studies conducted prior to the introduction of cART reported varying estimates of KSHV prevalence in HIV-1 infected US individuals, depending on the specific population[18]. Among MSM, estimates ranged most commonly from 30–40% to 60% [19], however, higher figures up to 90% [20, 21] were also reported. In contrast, estimates ranging from 10–20% to lower values, indistinguishable to those found in the HIV uninfected population, were reported in injection drug users (IDU) and women [21, 22]. This study seeks to understand if KSHV epidemiology has changed in US persons living with HIV-1 following the introduction of cART, and to further elucidate the epidemiology of KS in a context relevant to public health and clinical practice.
Specific objectives of this study were to determine the prevalence of KSHV infection in HIV-1 infected persons at the time of initiation of cART and the incidence of KSHV infection after cART initiation. Furthermore, we aimed to investigate potential associations between KSHV infection, KS, randomized cART and its outcomes. Finally, we sought to ascertain the impact of KSHV infection and KS on survival of HIV-1 infected individuals.
Methods
Study participants
The AIDS Clinical Trials Group (ACTG) longitudinal linked randomized trials (ALLRT) study is a longitudinal cohort study of HIV-infected individuals who had been enrolled into selected multicentre clinical trials conducted by the ACTG and had randomized assignment of a cART regimen. The rationale, design, and baseline characteristics of ALLRT are described elsewhere [23]. The ALLRT cohort is notable because of its diversity in gender, race, geography, and risk factors for HIV-1 acquisition, which facilitates generalizability of findings to the general population of HIV-1 infected persons in the United States (US).
We selected participants enrolled in six US ACTG studies, for a total of 5140 antiretroviral naïve HIV-1 infected individuals who initiated antiretroviral therapy as part of a randomized clinical trial during the decade 1997–2007 (Table 1S) and were co-enrolled in ALLRT. For each individual, the first and last available sera (in either the parent clinical trial or ALLRT) were tested for antibodies to KSHV ORFK8.1 and ORF73. The first (entry) serum was collected shortly prior or at the time of treatment assignment in the parent study, or if no such specimen was available, at the next available study visit. The last (exit) serum was collected at the last available study visit before June 30, 2009. On both occasions, CD4 counts were also recorded. A diagram representing the study timeline is shown in Fig.1S.
Serology testing
KSHV serodiagnosis was performed as previously described [24]. In brief, KSHV indirect ELISAs utilizing recombinant antigens (baculovirus-derived ORF73, and E.Coli derived K8.1) were used. The characteristics of the assays, assessed on validation panels composed of healthy US blood donors and KS patients, are as follows: for ORF73, 91.4% sensitivity, 98.8% specificity, area under curve (AUC) 0.99; for K8.1, 93.1% sensitivity and 98.8% specificity, AUC 0.98. Assay reproducibility is high for both assays (Cohen’s kappa, 0.89 and 0.99, respectively).
Concordance amongst the two assays is moderate: in a large international population-based study [1], Spearman’s rho was 0.57, p<0.0001. The relatively modest correlation between responses to different KSHV antigens is well known: Infected individuals can seroconvert for one antigen long before they do for others [25, 26]. For this particular assay system, utilizing an either/or algorithm maximizes sensitivity of serodiagnosis -defined as successful identification of KSHV infection in patients with detectable KSHV viral load in mononuclear cells or pathologically confirmed KSHV associated diseases, without compromising specificity. Participants were, therefore, considered KSHV seropositive if antibodies were detected to either antigen. Table 2S provides a sensitivity analysis for KSHV prevalence and incidence according to other definitions.
Covariate categorization and statistical analysis
For some analyses, continuous variables were categorised as follows: age :<30, 30–39, 40–49, ≥50; CD4 count: 0–199, 200–349, 350–499, ≥500; CD4 count change during cART: no change/decrease vs. increase. When analysed as a continuous variable, CD4 counts were scaled to 100 cells/µL.
Antiherpesvirus drugs administered by indication were pooled in two categories: anti-HSV, acyclovir-like (acyclovir, valacyclovir, famcyclovir, with no reported activity against KSHV in vitro) and anti-CMV (ganciclovir/valganciclovir, cidofovir, and foscarnet, which are reportedly active against KSHV in vitro).
To analyse the incidence of KSHV infection, study time was defined as time intervening between collection of entry and exit samples. For KS incidence and survival analyses, time at risk accrued from parent study randomization; participants were administratively censored on December 31, 2009 and June 30, 2010, respectively. Because the six parent studies were conducted over ten years, in order to account for unmeasured variability due to calendar time, two periods were defined: early (1997–2001) and late (2002–2007). Participants were assigned to either period based on entry date. Prevalence models were fitted using logistic regression; Poisson regressions were used to calculate KSHV incidence; Cox proportional hazard models were used for KS incidence and survival analysis. Because the ALLRT cohort is composed of individuals who were enrolled in separate clinical trials, sensitivity analyses with mixed effect models were performed.
This study is powered (83%−93%) to measure differences in KSHV prevalence of 3% or lower across a large range (25–75%). For each measure of association, central estimates of effect size and their precision (95% confidence interval) are reported; we consider intervals excluding the unit indicative of a statistically significant association.
All analyses were performed using STATA v11.0 (StataCorp, College Station, TX)
Ethics statement:
This work, as well as the ALLRT study and each parent randomized ACTG clinical trial were conducted according to the Declaration of Helsinki, with the understanding and the consent of each participant; all protocols were approved by the respective Institutional Review Boards.
Results
Of the 5140 eligible individuals, we were able to test 5022 individuals from 83 sites in 25 states, the District of Columbia (DC) and the Commonwealth of Puerto Rico (Table 3S). Characteristics of the study participants are shown in Table 1. At entry, study participants were predominately men in their fourth and fifth decade, and of varied racial background. Few reported current or past injection drug use (IDU). CD4 count was generally low, and HIV-1 viral load (VL) elevated. Participants in the later period were older and had higher baseline VL; the remaining baseline characteristics were not significantly different between the early and late enrolment period. Data on treatment were available for 4953 patients, randomized to receive 40 different cART regimens; nearly half of the participants received protease inhibitors (PI) as part of their initial cART regimen. Few patients were prescribed systemic antiherpesvirus drugs based on clinical indications.
Table 1.
Characteristics of the study participants. N=5022 participants
| Variable | ||
|---|---|---|
| N | % | |
| gender | ||
| Male | 4,112 | 81.9 |
| Female | 910 | 18.1 |
| age | ||
| <30 | 1015 | 20.21% |
| 30-39 | 1951 | 38.85% |
| 40-49 | 1462 | 29.11% |
| >50 | 594 | 11.83% |
| Injection drug use | ||
| Never | 4,530 | 90.2 |
| Previously | 479 | 9.5 |
| Currently | 13 | 0.3 |
| Race/ethnicity | ||
| White | 2,014 | 40.1 |
| Black | 1,793 | 35.7 |
| Hispanic | 1,069 | 21.3 |
| Asian | 88 | 1.75 |
| Native American | 29 | 0.6 |
| other/missing | 29 | 0.6 |
| calendar time | ||
| 1997-2001 | 1733 | 34.51% |
| 2002-2007 | 3289 | 65.49% |
| Randomised cART | ||
| cART regimen including PI | 2,399 | 47.77 |
| Antiherpesviral drugs | ||
| Anti HSV | 663 | 13.20 |
| Anti-CMV | 36 | 0.72 |
| Baseline CD4 count (cell/μL) | N | % |
| <200 | 2330 | 46.40% |
| 200-349 | 1477 | 29.41% |
| 350-499 | 788 | 15.69% |
| ≥500 | 423 | 8.42% |
| median | IQR | |
| Baseline Log HIV-1 VL | 4.8 | 4.4-5.3 |
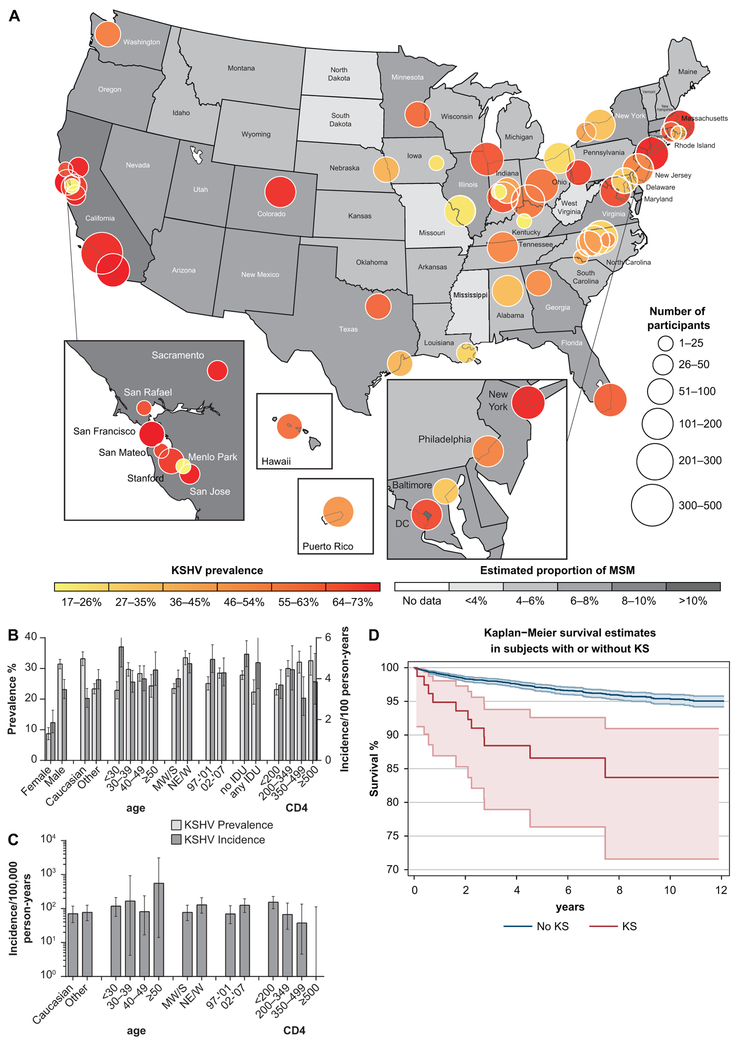
Crude KSHV prevalence is shown in Fig.1B: overall prevalence was 38.1% (95% confidence interval, CI 36.8–39.5%), and was higher in men, Caucasians, individuals in their fourth and fifth decade and recent enrolees. A significant geographical variability of KSHV prevalence was observed (Fig.1A); prevalence was high in California, DC, Massachusetts, New York, Colorado, and Washington; it was generally moderate in most of the Mid Atlantic, New England, in the Great Lakes region and in the South, and low elsewhere. In further analyses, prevalence estimates were also calculated for four US Census Bureau-designated regions (Northeast, Midwest, South, and West). Local KSHV prevalence correlated to some extent with the estimated proportion of men who have sex with men (MSM) in the source population [27]; state wise correlation: rho=0.67, p=0.0001.
Figure 1.
A) Geographical distribution of KSHV prevalence. Local KSHV prevalence is shown. Bubble position indicates the county or city location of each study site (study sites are listed in Table 3S). Bubble size indicates the number of participants; hue indicates prevalence. Estimated proportion of MSM in each state by is indicated by increasingly saturated shades of grey. B) Crude Prevalence and incidence of KSHV infection. Unadjusted prevalence and incidence of KSHV infection stratified by baseline characteristics are shown. C) Crude KS incidence. Unadjusted incidence of KS stratified by baseline characteristics is shown. MW/S indicates Midwest and South; NE/W, Northeast and West. D). Survival functions. Kaplan-Meier survival estimates are plotted for patient with (red) and without (blue) KS. Dotted lines delimit 95% confidence bands.
Multivariable models of prevalence were analysed. Estimates from a model including socio-demographic factors, baseline status (CD4 count and HIV-1 viral load) and antiherpesvirus treatment prior to study entry is shown in Table 2. Significant risk factors were male gender (odds ratio, OR 2.43, 95%CI 2.04–2.90), Caucasian race (OR 1.20, 95% CI, 1.06–1.36), age between 30 and 49 years, residence in the Northeast or West regions (OR 1.52, 95%CI 1.35–1.71), enrolment in the later period (OR 1.26, 95% CI 1.11–1.43) and higher baseline CD4 count. Lower prevalence was associated with IDU (OR 0.72, 95% CI 0.59–0.88).
Table 2.
Multivariable models of KSHV prevalence and incidence
| OR* (prevalence) |
[95% | CI#] | p value | IRR+ (incidence) | [95% | CI] | p value | |
|---|---|---|---|---|---|---|---|---|
| Socio demographic | ||||||||
| Male gender | 2.43 | 2.04 | 2.90 | <0.001 | 2.30 | 1.69 | 3.14 | <0.001 |
| Caucasian race | 1.20 | 1.06 | 1.36 | 0.004 | 1.35 | 1.11 | 1.63 | 0.003 |
| Age (years) | ||||||||
| <30 | ref. | ref. | ||||||
| 30-39 | 1.32 | 1.12 | 1.55 | 0.001 | 0.76 | 0.59 | 0.97 | 0.025 |
| 40-49 | 1.33 | 1.12 | 1.58 | 0.001 | 0.65 | 0.50 | 0.85 | 0.002 |
| ≥50 | 1.03 | 0.82 | 1.28 | 0.806 | 0.57 | 0.40 | 0.81 | 0.002 |
| IDU | 0.72 | 0.59 | 0.88 | 0.001 | 1.17 | 0.86 | 1.60 | 0.312 |
| Residence in NE or W region | 1.20 | 1.35 | 1.71 | <0.001 | 1.06 | 0.87 | 1.29 | 0.561 |
| calendar time | ||||||||
| 1997-2001 | ref. | ref. | ||||||
| 2002-2007 | 1.26 | 1.11 | 1.43 | <0.001 | 1.82 | 1.48 | 2.23 | <0.001 |
| Antiherpesvirus drugs | ||||||||
| Anti-HSV | 0.96 | 0.81 | 1.15 | 0.650 | 0.84 | 0.63 | 1.11 | 0.225 |
| Anti-CMV-like | 0.49 | 0.22 | 1.09 | 0.080 | 0.82 | 0.26 | 2.56 | 0.729 |
| Baseline CD4 count (cell/μL) | ||||||||
| <200 | ref. | ref. | ||||||
| 200-349 | 1.50 | 1.29 | 1.74 | <0.001 | 1.08 | 0.85 | 1.37 | 0.534 |
| 350-499 | 1.56 | 1.30 | 1.87 | <0.001 | 1.10 | 0.82 | 1.47 | 0.523 |
| ≥500 | 1.40 | 1.11 | 1.77 | 0.005 | 0.97 | 0.67 | 1.41 | 0.866 |
| Baseline Log HIV-1 VL | 1.09 | 0.99 | 1.19 | 0.067 | 1.07 | 1.00 | 1.15 | 0.067 |
| cART | n/a | |||||||
| assigned PI | 0.91 | 0.75 | 1.10 | 0.315 | ||||
| CD4 response | n/a | |||||||
| No CD4 increase | ref. | |||||||
| Any CD4 increase | 0.60 | 0.43 | 0.80 | 0.001 |
OR, odds ratio
CI, confidence interval
IRR, incidence rate ratio
For 4416 participants, both entry and exit samples were available for analysis. Median follow up was 3 years (IQR, 2.09–5.52). Crude KSHV incidence is shown in Fig.1B: it was 4.07/100 persons/years overall (95% CI 3.70–4.47), but higher in younger, Caucasian men enrolled in the later period. Multivariable models of KSHV incidence were analysed. Estimates from a model including socio-demographic factors, baseline CD4 count and HIV-1 VL, randomized assignment to HIV-1 protease inhibitors, antiherpesvirus treatment post-entry and cART outcome are shown in Table 3. Risk factors for KSHV acquisition were male gender (incidence rate ratio, IRR 2.30, 95% CI 1.69–3.14), Caucasian race (IRR 1.35, 95% CI 1.11–1.63), residence in the Northeast or West regions and enrolment in the later period of the study (IRR 1.82, 95% CI1.48–2.23). Lower KSHV incidence was associated with older age and increasing CD4 count upon cART initiation (IRR 0.60, 95% CI 0.43–0.80).
Table 3.
Multivariable model of incident KS
| HR | [95% | CI#] | p value | |
|---|---|---|---|---|
| Socio demographic | ||||
| Caucasian race | 2.30 | 1.05 | 5.02 | 0.036 |
| Age (years) | 0.99 | 0.95 | 1.03 | 0.684 |
| Later period | 1.12 | 0.50 | 2.56 | 0.778 |
| Residence in NE or W regions | 1.62 | 1.09 | 2.40 | 0.015 |
| Baseline status | ||||
| CD4 count (100 cell/μl) | 0.49 | 0.33 | 0.72 | <0.001 |
| Log HIV-1 VL | 0.94 | 0.51 | 1.74 | 0.851 |
| KSHV seropositivity | 9.52 | 3.58 | 25.30 | <0.001 |
| Antiherpesvirus drugs | ||||
| Anti HSV | 2.19 | 1.00 | 4.81 | 0.051 |
| Anti CMV | 3.63 | 0.46 | 29.77 | 0.221 |
| cART | ||||
| Assigned PI | 1.51 | 0.71 | 3.21 | 0.278 |
| CD4 response | ||||
| No CD4count increase | Ref. | |||
| Any CD4 count increase | 1.23 | 0.16 | 9.24 | 0.841 |
HR, hazard ratio
CI, confidence interval
Seventy-eight individuals, all men, received a diagnosis of KS. Two patients with pre-existing KS and three with incident KS remained seronegative at the end of follow up; they had a median baseline CD4 count of 27.5 (IQR 15.5–80.5) cells/µL; detailed data are provided in Table 4S. Forty-six cases were diagnosed prior to or at study entry; in a multivariate model including sociodemographic and baseline characteristics, higher CD4 counts were associated with lower risk of KS (OR:0.40, 95% CI 0.18–0.86 for CD4 counts between 200 and 349, OR:0.16, 95% CI 0.04–0.73 for CD4 counts between 350 and 499, the highest value was 445). Thirty–two cases occurred after randomization and were defined as incident: KS incidence was 104.05/100,000 person/years (95% CI 71.17–146.89), KS incidence in KSHV seropositive participants was 192.27/100,000 person/years (95% CI 128.76–276.13). Crude KS incidence is shown inFig.1C. KS onset occurred a median of 102 (IQR 25–316) days after cART randomization; 14 cases (43%) occurred after 6 months or later. At the end of follow up, participants with incident KS had a median CD4 cell count of 445 (IQR 151–578) cells/µL, not significantly lower than participants without KS (median 496, IQR 327–681 cells/µL, Wilcoxon rank sum test, p=0.054).
Multivariable models of KS incidence were analysed. Estimates from a model including socio-demographic factors, baseline status, randomised assignment to an HIV-1 protease inhibitor, post-enrolment antiherpesvirus treatment and cART outcome are shown in Table 4. Lower KS incidence was associated with higher baseline CD4 count (hazard ratio, HR 0.49, 95% CI 0.34–0.72). Neither randomized assignment to a regimen containing a HIV-1 protease inhibitor, treatment with anti-herpesvirus drugs, nor CD4 increase upon cART initiation were associated with KS risk. Calendar time was not significantly associated with KS risk in multivariable analysis.
Table 4.
Multivariate survival model
| HR | [95% | CI] | p value | |
|---|---|---|---|---|
| Socio- demographic | ||||
| Male gender | 0.98 | 0.64 | 1.50 | 0.929 |
| Caucasian race | 1.12 | 0.80 | 1.56 | 0.507 |
| IDU | 1.71 | 1.14 | 2.58 | 0.009 |
| Age | 1.05 | 1.04 | 1.07 | <0.001 |
| Later period | 1.16 | 0.82 | 1.63 | 0.383 |
| Residence in NE or W regions | 1.04 | 0.89 | 1.21 | 0.600 |
| Baseline status | ||||
| CD4 count (100 cell/μl) | 0.84 | 0.75 | 0.93 | <0.001 |
| Log HIV-1 VL | 1.47 | 1.15 | 1.88 | 0.002 |
| KSHV seropositivity | 1.17 | 0.85 | 1.63 | 0.337 |
| Clinical course | ||||
| Kaposi Sarcoma | 3.23 | 1.74 | 6.78 | <0.001 |
| No CD4count increase | Ref. | |||
| Any CD4 count increase | 0.14 | 0.10 | 0.20 | <0.001 |
One hundred and ninety deaths occurred during the study. Multivariable survival models were analysed. Estimates from a model including socio-demographic factors, baseline status, and clinical course are shown in Table 5. Besides age and IDU (HR 1.71, 95% CI 1.14–2.58), survival was negatively associated with high baseline HIV-1 viral load (HR 1.47, 95% CI1.15–1.88), and diagnosis of KS (HR 3.23, 95% CI1.74–6.78). Survival expectations of individuals with or without KS are shown in Fig.1D. Survival was positively associated with higher baseline CD4 count (HR 0.84,95% CI0.75–1.93), and increase in CD4 upon cART initiation (HR 0.13,95% CI0.10–0.20).
Discussion
This study investigates the epidemiology of KSHV, KS and associated mortality in HIV-1 infected individuals initiating cART across a ten year time span at multiple sites in the US and Puerto Rico.
In HIV-1-infected persons in the US, KSHV remains prevalent, in particular in Caucasian men who have no history of injection drug use. Presumably, this represents higher likelihood of having sex with other men as the main HIV-1 risk factor; however, such data are not collected in ALLRT and this represents a limitation of the present study. Provenance from metropolitan areas of the Northeast and the West is also a risk factor. The reasons for the geographical variability of KSHV prevalence are not completely clear; while ecological biases cannot be excluded, the pattern correlates with the estimated local proportions of MSM. The geographical variation we observed is also consistent with reports of geographical variation in AIDS-KS incidence [28, 29]. Higher baseline CD4 count appeared to be associated with a higher KSHV prevalence, perhaps reflecting the possible occurrence of false negative serology in individuals with profound immune compromise, due to loss of antibody to K8.1 and ORF73. Indeed, two patients with pre-existing KS and three with incident KS who remained seronegative had very low baseline CD4 counts. Enrolment in the later period of the study was an independent risk factor for KSHV seropositivity at entry, suggesting that KSHV prevalence is not, in fact, diminishing in the HIV infected population.
New KSHV infections were observed in our study, particularly, in younger, Caucasian men enrolled in the later period, indicating that KSHV transmission in this population is actually increasing. CD4 increase in response to cART appeared to be a protective factor for incident KSHV. While this finding may reflect an unrecognized confounder, it could denote a real biologic phenomenon, perhaps a hitherto undescribed role of the immune system in preventing establishment of infection following exposure to KSHV. CD4 count increases following cART have been shown to lower the incidence of herpesviruses reactivations and of associated diseases, but a decrease in the incidence of infection itself has not been reported so far. However, higher CD4+ T-cell counts have been found to be associated with reduced risk of infection with high-risk human papillomavirus DNA types in HIV-1 infected women [30].
As expected, the risk of KS was positively associated with pre-existing KSHV infection and lower baseline CD4 count, while initial randomly assigned protease inhibitor treatment and subsequent immunological response had no effect; individuals developing KS had CD4 count responses to cART non inferior to those of their peers without KS. Furthermore, KS incidence did not decrease with calendar time. Taken together, these data support observations indicating that KS is still occurring in HIV infected individuals, even in the context of successful cART.
Studying cART naïve persons who had been randomly assigned to their initial cART regimen permitted investigation of the potential effect of specific antiretroviral drugs on KSHV-related outcomes. Specifically, in vitro studies had reported anti- KSHV activity by protease inhibitors, most notably nelfinavir [31]. In the present study, assignment of individual PIs, including nelfinavir, and PIs overall had no significant effects on the incidence of KSHV infection or KS. Effects on other aspects of the natural history of KSHV infection such as frequency of reactivation, shedding or transmission were not evaluated. Participants in this study were enrolees of six randomized cART trials spanning over ten years and including 40 different cART regimens. Calendar time was introduced as a variable in all analyses; residual heterogeneity amongst parent studies is not likely to represent a major confounder, as sensitivity analyses conducted with hierarchical models confirmed the result obtained with single-level modelling. The reported administration of anti-herpesvirus drugs by clinical indication was not associated with decreased risk for any KSHV-related outcome, although confounding by indication cannot be excluded.
Both higher CD4 count at baseline and positive immunologic response to cART were associated with better survival, as expected. KSHV infection appeared to have no effect on survival, but KS still significantly impacted life expectancy, as did IDU and higher HIV-1 viral load prior to cART initiation.
The introduction of cART has swiftly and dramatically diminished mortality from KS [32, 33]; however, further decreases have not been recently documented. Recent literature reporting on KS associated mortality in the cART era is primarily focused on sub-Saharan Africa [34-36], although there are few reports on US persons [29, 37, 38]. Results of the present study are consistent with such findings, likely indicating the continuing contribution of KS to mortality in HIV infected individuals; however, because this study did not analyse specific causes of death, residual confounding is possible.
In conclusion, the unremittingly high prevalence and increased acquisition of KSHV in HIV-1 infected persons on cART in the US indicates the importance of KSHV co-infection in this population. This is of particular concern, given the accumulating evidence on the continuing occurrence of KS in HIV-1 infected individuals and the associated decrease in life expectancy.
Supplementary Material
Figure 1S. Study timeline diagram.
Enrolment in ACTG parent study and ALLRT are indicates by red arrows, entry and exit sample collection by green arrows and bracket, cART assignment by a black arrow. Longitudinal study time is represented by a dotted line, and follow up periods are indicated by blue brackets.
Acknowledgments.
We are especially grateful to Andrew Ellingson, MPH, Jeffrey Lavenberg, MS, and Supriya Krishna, D.Sc., for extracting and compiling ALLRT covariates; to Randall Johnson, PhD and Huilee L. Wong, PhD for discussions on modelling, and Timothy Wilkin, MD for critically reviewing the manuscript. We thank Tammy Schroyer and Joseph Meyer for help producing the figures. Finally, we particularly thank all participants in this study.
Potential conflicts of interest and sources of funding:
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E and the Intramural Research Program, as well as the AIDS Clinical Trials Group (ACTG), funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants AI68636, AI69450)
T.B.C. has served as a consultant for Gilead Sciences. C.B.is member of the Data Safety Monitoring Board for a clinical trial conducted by GlaxoSmithKline/ViiV, and member of the Scientific Advisory Board for MBio Diagnostics. None of the other authors has potential conflicts of interest to disclose.
References
- 1.de Sanjose S, Mbisa G, Perez-Alvarez S, Benavente Y, Sukvirach S, Hieu NT, et al. Geographic Variation in the Prevalence of Kaposi Sarcoma-Associated Herpesvirus and Risk Factors for Transmission. J Infect Dis 2009,199:1449–1456. [DOI] [PubMed] [Google Scholar]
- 2.Martin J The epidemiology of KSHV and its association with malignant disease In: Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. Chapter 54. Edited by Arvin A GC-F, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 3.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med 1998,338:948–954. [DOI] [PubMed] [Google Scholar]
- 4.Goudsmit J, Renwick N, Dukers NH, Coutinho RA, Heisterkamp S, Bakker M, et al. Human herpesvirus 8 infections in the Amsterdam Cohort Studies (1984–1997): analysis of seroconversions to ORF65 and ORF73. Proc Natl Acad Sci U S A 2000,97:4838–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melbye M, Cook PM, Hjalgrim H, Begtrup K, Simpson GR, Biggar RJ, et al. Risk factors for Kaposi’s-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981–1996. Int J Cancer 1998,77:543–548. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan RJ, Pantanowitz L, Casper C, Stebbing J, Dezube BJ. HIV/AIDS: epidemiology, pathophysiology, and treatment of Kaposi sarcoma-associated herpesvirus disease: Kaposi sarcoma, primary effusion lymphoma, and multicentric Castleman disease. Clin Infect Dis 2008,47:1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones JL, Hanson DL, Dworkin MS, Jaffe HW. Incidence and trends in Kaposi’s sarcoma in the era of effective antiretroviral therapy. J Acquir Immune Defic Syndr 2000,24:270–274. [DOI] [PubMed] [Google Scholar]
- 8.Schwarcz L, Chen MJ, Vittinghoff E, Hsu L, Schwarcz S. Declining incidence of AIDS-defining opportunistic illnesses: results from 16 years of population-based AIDS surveillance. AIDS 2013,27:597–605. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson LP, Yamashita TE, Detels R, Margolick JB, Chmiel JS, Kingsley LA, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 1999,21 Suppl 1:S34–41. [PubMed] [Google Scholar]
- 10.Veugelers PJ, Strathdee SA, Moss AR, Page KA, Tindall B, Schechter MT, et al. Is the human immunodeficiency virus-related Kaposi’s sarcoma epidemic coming to an end? Insights from the Tricontinental Seroconverter Study. Epidemiology 1995,6:382–386. [DOI] [PubMed] [Google Scholar]
- 11.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008,123:187–194. [DOI] [PubMed] [Google Scholar]
- 12.Hleyhel M, Belot A, Bouvier AM, Tattevin P, Pacanowski J, Genet P, et al. Risk of AIDS-Defining Cancers Among HIV-1-Infected Patients in France Between 1992 and 2009: Results From the FHDH-ANRS CO4 Cohort. Clin Infect Dis 2013. [DOI] [PubMed] [Google Scholar]
- 13.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess Cancers Among HIV-Infected People in the United States. J Natl Cancer Inst 2015,107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med 2007,357:1352–1353. [DOI] [PubMed] [Google Scholar]
- 15.Mani D, Neil N, Israel R, Aboulafia DM. A retrospective analysis of AIDS-associated Kaposi’s sarcoma in patients with undetectable HIV viral loads and CD4 counts greater than 300 cells/mm(3). J Int Assoc Physicians AIDS Care (Chic) 2009,8:279–285. [DOI] [PubMed] [Google Scholar]
- 16.Unemori P, Leslie KS, Hunt PW, Sinclair E, Epling L, Mitsuyasu R, et al. Immunosenescence is associated with presence of Kaposi’s sarcoma in antiretroviral treated HIV infection. AIDS 2013,27:1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stebbing J, Powles T, Bower M. AIDS-associated Kaposi’s sarcoma associated with a low viral load and a high CD4 cell count. AIDS 2008,22:551–552. [DOI] [PubMed] [Google Scholar]
- 18.Gnann JW Jr., Pellett PE, Jaffe HW. Human herpesvirus 8 and Kaposi’s sarcoma in persons infected with human immunodeficiency virus. Clin Infect Dis 2000,30 Suppl 1:S72–76. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Fitzpatrick L, Campbell TB, Badaro R, Schechter M, Melo M, et al. Comparison of the prevalence of antibodies to human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) in Brazil and Colorado. J Infect Dis 1998,178:1488–1491. [DOI] [PubMed] [Google Scholar]
- 20.Davis DA, Humphrey RW, Newcomb FM, O’Brien TR, Goedert JJ, Straus SE, et al. Detection of serum antibodies to a Kaposi’s sarcoma-associated herpesvirus-specific peptide. J Infect Dis 1997,175:1071–1079. [DOI] [PubMed] [Google Scholar]
- 21.Lennette ET, Blackbourn DJ, Levy JA. Antibodies to human herpesvirus type 8 in the general population and in Kaposi’s sarcoma patients. Lancet 1996,348:858–861. [DOI] [PubMed] [Google Scholar]
- 22.Kedes DH, Ganem D, Ameli N, Bacchetti P, Greenblatt R. The prevalence of serum antibody to human herpesvirus 8 (Kaposi sarcoma-associated herpesvirus) among HIV-seropositive and high-risk HIV-seronegative women. JAMA 1997,277:478–481. [PubMed] [Google Scholar]
- 23.Smurzynski M, Collier AC, Koletar SL, Bosch RJ, Wu K, Bastow B, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials 2008,9:269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mbisa GL, Miley W, Gamache CJ, Gillette WK, Esposito D, Hopkins R, et al. Detection of antibodies to Kaposi’s sarcoma-associated herpesvirus: a new approach using K8.1 ELISA and a newly developed recombinant LANA ELISA. J Immunol Methods 2010,356:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinlivan EB, Wang RX, Stewart PW, Kolmoltri C, Regamey N, Erb P, et al. Longitudinal sero-reactivity to human herpesvirus 8 (KSHV) in the Swiss HIV Cohort 4.7 years before KS. J Med Virol 2001,64:157–166. [DOI] [PubMed] [Google Scholar]
- 26.Biggar RJ, Engels EA, Whitby D, Kedes DH, Goedert JJ. Antibody reactivity to latent and lytic antigens to human herpesvirus-8 in longitudinally followed homosexual men. J Infect Dis 2003,187:12–18. [DOI] [PubMed] [Google Scholar]
- 27.Lieb S, Fallon SJ, Friedman SR, Thompson DR, Gates GJ, Liberti TM, et al. Statewide estimation of racial/ethnic populations of men who have sex with men in the U.S. Public Health Rep 2011,126:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beral V, Bull D, Jaffe H, Evans B, Gill N, Tillett H, et al. Is risk of Kaposi’s sarcoma in AIDS patients in Britain increased if sexual partners came from United States or Africa? BMJ 1991,302:624–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong AW, Lam KH, Chase EP. Epidemiology of classic and AIDS-related Kaposi’s sarcoma in the USA: incidence, survival, and geographical distribution from 1975 to 2005. Epidemiol Infect 2013,141:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konopnicki D, Manigart Y, Gilles C, Barlow P, de Marchin J, Feoli F, et al. Sustained viral suppression and higher CD4+ T-cell count reduces the risk of persistent cervical high-risk human papillomavirus infection in HIV-positive women. J Infect Dis 2013,207:1723–1729. [DOI] [PubMed] [Google Scholar]
- 31.Gantt S, Carlsson J, Ikoma M, Gachelet E, Gray M, Geballe AP, et al. The HIV protease inhibitor nelfinavir inhibits Kaposi’s sarcoma-associated herpesvirus replication in vitro. Antimicrob Agents Chemother 2011,55:2696–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer 2010,103:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pipkin S, Scheer S, Okeigwe I, Schwarcz S, Harris DH, Hessol NA. The effect of HAART and calendar period on Kaposi’s sarcoma and non-Hodgkin lymphoma: results of a match between an AIDS and cancer registry. AIDS 2011,25:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohlius J, Valeri F, Maskew M, Prozesky H, Garone D, Sengayi M, et al. Kaposi’s Sarcoma in HIV-infected patients in South Africa: Multicohort study in the antiretroviral therapy era. Int J Cancer 2014,135:2644–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson BC, Borok MZ, Mhlanga TO, Makadzange AT, Campbell TB. AIDS-associated Kaposi sarcoma: outcomes after initiation of antiretroviral therapy at a university-affiliated hospital in urban Zimbabwe. Int J Infect Dis 2013,17:e902–906. [DOI] [PubMed] [Google Scholar]
- 36.Chu KM, Mahlangeni G, Swannet S, Ford NP, Boulle A, Van Cutsem G. AIDS-associated Kaposi’s sarcoma is linked to advanced disease and high mortality in a primary care HIV programme in South Africa. J Int AIDS Soc 2010,13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maskew M, Fox MP, van Cutsem G, Chu K, Macphail P, Boulle A, et al. Treatment response and mortality among patients starting antiretroviral therapy with and without Kaposi sarcoma: a cohort study. PLoS One 2013,8:e64392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lodi S, Guiguet M, Costagliola D, Fisher M, de Luca A, Porter K, et al. Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst 2010,102:784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1S. Study timeline diagram.
Enrolment in ACTG parent study and ALLRT are indicates by red arrows, entry and exit sample collection by green arrows and bracket, cART assignment by a black arrow. Longitudinal study time is represented by a dotted line, and follow up periods are indicated by blue brackets.