Abstract
Purpose:
After failure of hypomethylating agents (HMA), patients with myelodysplastic syndromes (MDS) have dismal survival and no approved treatment options.
Patients and Methods:
We conducted a phase 1b investigator-initiated trial of ipilimumab in patients with higher risk MDS who have failed HMAs. Patients received monotherapy at two dose levels (DL; 3 and 10 mg/kg) with an induction followed by a maintenance phase. Toxicities and responses were evaluated with CTCAE.4 and IWG-2006 criteria, respectively. We also performed immunologic assays and T-cell receptor sequencing on serial samples.
Results:
Twenty-nine patients from 7 centers were enrolled. In the initial DL1 (3 mg), 3 of 6 patients experienced grade 2–4 immune-related adverse events (IRAE) that were reversible with drug discontinuation and/or systemic steroids. In DL2, 4 of 5 patients experienced grade 2 or higher IRAE; thus, DL1 (3 mg/kg) was expanded with no grade 2–4 IRAEs reported in 18 additional patients. Best responses included marrow complete response (mCR) in one patient (3.4%). Prolonged stable disease (PSD) for ≥46 weeks occurred in 7 patients (24% of entire cohort and 29% of those treated with 3 mg/kg dose), including 3 patients with more than a year of SD. Five patients underwent allografting without excessive toxicity. Median survival for the group was 294 days (95% CI, 240–671+). Patients who achieved PSD or mCR had significantly higher frequency of T cells expressing ICOS (inducible T-cell co-stimulator).
Conclusions:
Our findings suggest that ipilimumab dosed at 3 mg/kg in patients with MDS after HMA failure is safe but has limited efficacy as a monotherapy. Increased frequency of ICOS-expressing T cells might predict clinical benefit.
Introduction
In patients with higher risk (HR) myelodysplastic syndromes (MDS), hypomethylating agent (HMA) failure is associated with dismal prognosis with a median overall survival (OS) of less than 6 months (1, 2), and the development of effective therapies after HMA failure remains a significant unmet clinical need. Several innate and adaptive immune system aberrations have been described in HR-MDS, including reduced numbers of and dys-functional natural killer and cytotoxic CD8+ cells as well as increases in CD4+CD25high Foxp3+-regulatory T cells (Treg) compared with lower risk (LR) MDS patients (3–6).
In addition, immune evasion has also been proposed as an important mechanism of disease progression and/or HMA resistance with increased levels of immune checkpoint (IC) expression, including programmed death-1 (PD-1), its ligands (PD-L1 and PD-L2), and cytotoxic T lymphocyte-associated protein 4 (CTLA-4), having been observed in CD34+ cells from HR-MDS and having levels further increased in the post-HMA failure setting (7). Multiple other IC receptors have also been demonstrated to be upregulated on T cells in this patient population (7–9). The CTLA-4 and PD-1/PD-L pathways are operational at different phases of the immune response. Whereas CTLA-4 inhibits auto-reactive T cells at initial stages of naïve T-cell activation preferentially in the lymph nodes, the PD-1 pathway regulates previously activated T cells at the later phases of the immune response predominantly in peripheral tissues (9).
Augmentation of antitumor immunity by antibody-mediated CTLA-4 blockade has emerged as an effective strategy for inducing responses in preclinical models and in vitro studies of hematologic malignancies (10–12), but clinical trial evaluation has lagged behind research in solid tumors. Ipilimumab was safe and had clinical activity in small studies of lymphoid and myeloid malignancies treated after relapse following allogeneic stem cell transplantation (alloSCT; refs. 13, 14). In this investigator-initiated multicenter phase I study, we hypothesized that ipilimumab would be tolerated and lead to meaningful clinical and measurable immunologic responses in patients with MDS who experienced HMA failure.
Materials and Methods
Patients
Eligible patients included adults with pathologically confirmed MDS who experienced HMA failure. Patients were required to have high-risk features as defined by at least one of the following conditions: an intermediate-2 (INT-2) or higher International Prognostic Scoring System (IPSS; ref. 15) risk category, or intermediate-1 (INT-1) with excess blasts (≥5% bone marrow blasts), or red blood cell or platelet transfusion dependency at the time of screening for the trial. Patients must have experienced primary or secondary failure of an HMA (azacitidine or decitabine) after ≥4 cycles and were required to have adequate end organ function and an Eastern Cooperative Oncology Group performance status of ≤2. Primary HMA failure was defined as failure to achieve an objective response after at least 4 cycles of HMA, and secondary failure as loss of response/relapse after achieving an initial objective response to the HMA.
Study design
This was an investigator-initiated, NCI-Cancer Therapy Evaluation Program (NCI-CTEP)–sponsored, multi-center, dose escalation phase Ib trial. The dose escalation was conducted at Johns Hopkins University (Baltimore, MD), whereas the dose expansion included 6 additional academic centers [Yale University (New Haven, CT), Columbia University (New York, NY), Duke University (Durham, NC); University of North Carolina (Raleigh, NC); Baylor University (Waco, TX), and Washington University (St. Louis, MO)].
Single-agent ipilimumab (YERVOY, Bristol-Myers Squibb), a human IgG1 mAb specific for human CTLA-4 (CD152) was administered intravenously on an outpatient basis on day 1 of each 21-day cycle for 4 cycles of induction, followed by 4 maintenance doses at 12-week intervals for patients who did not progress for a maximum total of 8 doses (Fig. 1A). There were two dose levels [doselevel1 (DL1): 3 mg/kg andDL2: 10mg/kg]. Intrapatient dose escalation and concomitant administration of any other anticancer therapies were not allowed. The dose expansion part used the optimal dose identified during escalation (3 mg/kg) to enroll an additional 18 patients.
Figure 1.
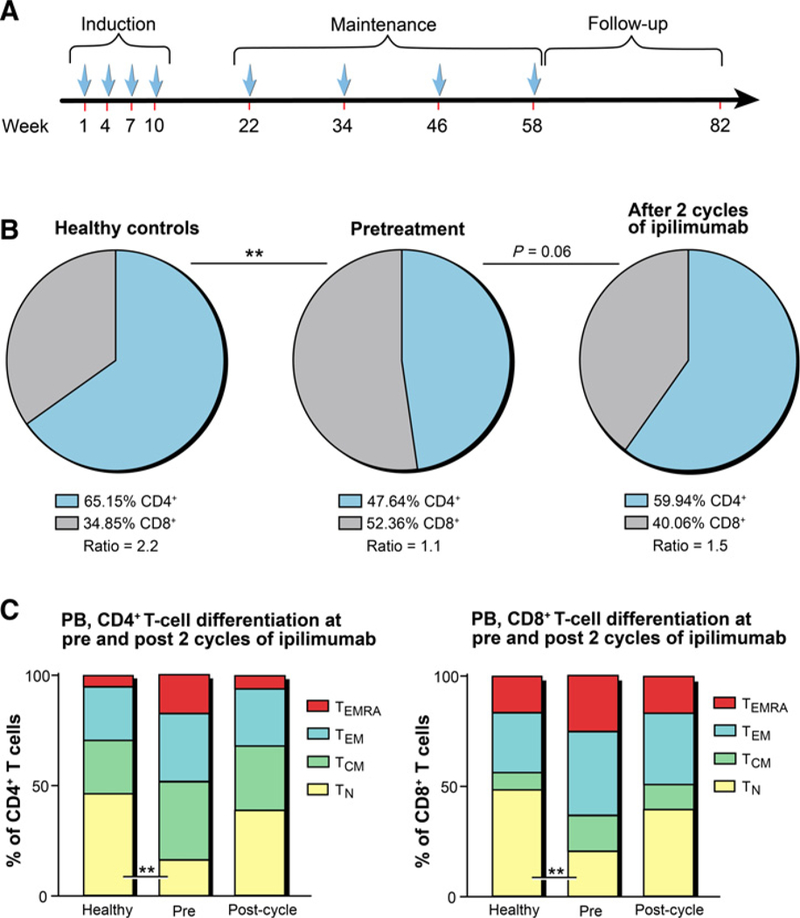
Peripheral blood T cells from patients with HR-MDS are characterized by an altered CD4:CD8 T-cell ratio and a later differentiated phenotype and can partly be restored through ipilimumab treatment. A, Ipilimumab was administered on day 1 of each 21-day cycle for 4 cycles of induction, followed by 4 maintenance doses at 3-month intervals. B and C, Freshly frozen PBMCs prospectively collected from patients with HR-MDS before and after 2 cycles of ipilimumab treatment (n = 12) and healthy donors (n = 14) were immunophenotyped. Pie charts and bar graphs represent the mean percentages of different T-cell subsets detected in peripheral blood (PB) of patients with HR-MDS and HCs. B, Pie charts showing the relative distribution of CD4+ and CD8+ T cells for healthy donors, patients before treatment, and patients after 2 cycles of ipilimumab treatment. C, Percentages of four major T-cell differentiation subsets, TN (CCR7+CD45RA+), Tcm (CCR7+CD45RA–), TEm (CCR7–CD45RA–), and TEmra (CCR7–CD45RA+). CD4+ T cells are presented on the left, and CD8+ T cells are shown on the right. Statistical comparisons were calculated in R as described in Materials and Methods with *, P ≤ 0.05 and **, P≤ 0.01.
The protocol was developed by the authors in collaboration with NCI-CTEP and was approved by the individual Institutional Review Boards. All patients provided informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Safety and efficacy assessments
Toxicities were graded and tabulated according to CTCAE 4.0 criteria (16). Immune-related adverse events (IRAE) were managed per the manufacturer’s algorithms. Bone marrow biopsies were scheduled at baseline, postinduction cycles 2 and 4, and before each maintenance dose. Continued therapy was contingent upon disease that was at least stable.
Dose-limiting toxicity (DLT) was defined as the occurrence of any of the following during the first 2 cycles of therapy: (i) any grade 3 or 4 nonhematologic toxicity with the following exceptions: (A) transient laboratory abnormalities that can be treated or resolve to grade 2 or less within 72 hours or (B) grade 3 expected and known IRAEs that resolve within 4 weeks of steroid therapy to grade 1 (17–19); and (ii) grade 4 hematologic toxicity defined as treatment-associated aplasia lasting greater than 42 days from the last dose of study drug. Given that severe cytopenias are features of advanced MDS and commonly encountered in this patient population, hematologic parameters were not used for dose interruption, discontinuation, or to define DLT, the exception being prolonged treatment–associated aplasia.
Responses were evaluated using International Working Group (IWG) 2006 criteria (20) after the second and fourth dose of induction and before each maintenance dose. Best observed responses were classified as complete response (CR), marrow CR (mCR), partial response (PR), hematologic improvement (HI), stable disease (SD), or progressive disease (PD). The overall response rate (ORR) was defined as the sum of CR, mCR, PR, and HI. We defined SD as per the IWG 2006 criteria (20) as failure to achieve at least PR, but no evidence of progression for ≥8 weeks.
Correlative studies
Correlative immunologic studies were performed on mononuclear cells from both peripheral blood (PBMC) and bone marrow (BMMC) samples that were collected at baseline and at predetermined time points after ipilimumab treatment (after induction cycle 2 and 4; after each maintenance cycle 1–4, and end of treatment or study discontinuation). Correlative assays included immunophenotyping of PBMCs and BMMCs by multicolor flow cytometry (Supplementary Table S1), as well as high-throughput sequencing of the T-cell antigen receptor (TCR) repertoire. Detailed description of the methods used in laboratory studies is provided in the Supplementary Material.
Statistical analyses
All patients with MDS who received ipilimumab were included in the safety and efficacy analyses. Clinical characteristics measured at screening and during follow-up are summarized using frequencies and percentages for discrete measures and median (range) for continuous measures. OS was calculated from first dose of ipilimumab to the date of death, last follow-up visit, or censored at alloSCT and estimated using the Kaplan–Meier method. T-cell percentages, absolute numbers, and ratios were summarized by time point (pretreatment, cycle 2, and cycle 4) for patients and overall for healthy controls (HC) using descriptive statistics (mean, SD). Differences in log-transformed T-cell values were compared between HCs and patients at pretreatment using two-sample t tests. Differences from pretreatment to cycle 2 and cycle 4 were estimated for patients and tested for positive or negative changes with paired t tests. Analyses were completed using R version 3.3.2 [R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/].
Results
Study population
A total of 29 patients with MDS from 7 centers enrolled in this trial, of whom 11 patients were treated during the dose escalation phase (6 patients at 3 mg/kg dose and 5 patients at 10 mg/kg), whereas another 18 patients were treated in the dose expansion at the 3 mg/kg dose. The mean age of enrolled patients was 67.3 years (standard deviation, 7.9 years), and 79.3% were males (Table 1). All patients received prior HMA therapy before enrolling in the study. Median number of administered HMA cycles was 6, and most patients (72%) had primary HMA failure. Most patients were in the IPSS high (17.2%) or INT-2 (37.9%) risk categories, whereas 44.8% had IPSS INT-1 with high-risk features. Median bona marrow blast count at enrollment was 8.5% (range, 0%–30%), median hemoglobin 8.9 g/dL (range, 7.2–11.3), median platelet count was 46 (range, 12–474), and median absolute neutrophil count was 0.41 (range, 0–4.72).
Table 1.
Demographics and baseline characteristics at screening
| All patients N = 29 |
|
|---|---|
| Sex - no. (%) | |
| Male | 23 (79) |
| Female | 6(21) |
| IPSS risk category | |
| INT-1 | 13 (45) |
| INT-2 | 11 (38) |
| High | 5 (17) |
| IPSS-R risk category | |
| Intermediate | 9 (31) |
| High | 8 (28) |
| Very high | 12 (41) |
| ECOG PS at screening - no. (%) | |
| 0 | 10 (36) |
| 1 | 18 (64) |
| BM blast count (%) - median (range) | 8.5 (0–30) |
| BM cellularity (%) - median (range) | 65 (9–95) |
| WBC (G/I) - median (range) | 2 (0.69–9.1) |
| Hb (g/dL) - median (range) | 8.9 (7.2–11.3) |
| PLT (G/I) - median (range) | 46 (12–474) |
| ALC (G/L) - median (range) | 1.02 (0.13–2.13) |
| ANC (G/L) - median (range) | 0.41 (0–4.72) |
| PB blast count (%) - median (range) | 0(0–23) |
Abbreviations: ANC, absolute neutrophil count; BM, bone marrow; ECOG PS, Eastern Cooperative Oncology Group performance status; PB, peripheral blood; WBC, white blood cells; PLT, platelets.
Safety evaluation
Fifteen patients (51.7%) received all 4 induction doses, of whom 7 patients proceeded to the maintenance phase and received at least one dose. All patients on the trial experienced grade 1 and 2 AEs and 82.8% experienced grade 3 or 4 AEs (Table 2). As expected in this population, the most common grade 3 and 4 AEs were hematologic and infectious in nature (Table 2). Six patients (20.7%) developed grade ≥2 IRAEs (Table 2; Supplementary Table S2) that necessitated removal from study. During the dose escalation part of the study (n = 11), 3 of 6 patients in DL1 developed grade ≥2 IRAEs, and all 3 patients received systemic steroids. At DL2 with 10 mg/kg, 4 of 5 patients developed IRAEs, of which 3 were grade ≥2 and one was grade 1. The 3 patients who developed grade ≥2 IRAEs were treated with systemic steroids. All IRAEs were reversible with stopping ipilimumab and/or initiation of systemic steroids, and all patients were successfully weaned from steroids. The spectrum of IRAEs observed was similar to that of ipilimumab use in patients with solid malignancies (rash, hepatitis, and diarrhea/colitis). On the basis of these findings, the DL1 (3 mg/kg) was chosen for dose expansion. Interestingly, no grade ≥2 IRAEs occurred during the dose expansion part of the study at the 3 mg/kg dose.
Table 2.
AEs observed during study
| AE | Number of patients affected (%) |
Grade |
|---|---|---|
| IRAE | ||
| Dermatitis | 4 (13.8) | G1–3 |
| Colitis | 2 (6.9) | G2–3 |
| Transaminitis | 2 (6.9) | G3–4 |
| Pneumonitis | 1 (3.4) | G2 |
| Myositis | 1 (3.4) | G2 |
| Bell palsy | 1 (3.4) | G2 |
| cGVHDa | 1 (3.4) | G3 |
| Nonimmune related | ||
| Hematologic | ||
| Anemia | 8 (27.6) | G3–4 |
| Thrombocytopenia | 4 (13.8) | G3–4 |
| Neutropenia | 6 20.1) | G3–4 |
| Infections | ||
| Febrile neutropenia | 7 (24.1) | G3–4 |
| Pneumonia | 5 (17.2) | G3–4 |
| Catheter-related infection | 1 (3.4) | G3–4 |
| Skin infection | 1 (3.4) | G3–4 |
| Sepsis | 1 (3.4) | G3–4 |
| Others | ||
| Fatigue | 1 (3.4) | G3–4 |
| Syncope | 1 (3.4) | G3–4 |
| Increased liver enzymes | 2 (6.9) | G3–4 |
| Generalized muscle weakness | 2 (6.9) | G3–4 |
| Upper gastrointestinal bleeding | 1 (3.4) | G3–4 |
| Arthralgia | 1 (3.4) | G3–4 |
| Intracranial bleeding | 1 (3.4) | G3–4 |
Patient with prior alloSCT.
A total of 15 of the treated patients (51.7%) died during study follow-up, all of which were related to disease progression or disease-related complications. No deaths were attributed to ipilimumab or an IRAE. Of the 15 deaths attributed to disease progression or were considered disease-related, 9 (60%) progressed during therapy and the remaining 6 patients were taken off study due to grade ≥2 IRAEs and only progressed later. In total, 3 patients (10.3%) completed all 8 doses of study therapy by last follow-up, and 4 patients (13.8%) proceeded to alloSCT without progressing on ipilimumab.
Efficacy evaluation
The clinical efficacy of single-agent ipilimumab is noted in Table 3 with an ORR of only 3.4% as one patient achieved mCR as the best response. This patient had a baseline INT-2 IPSS risk category with 12.5% blasts in the baseline marrow. There were no CR, PR, or HI observed and the duration of mCR was 3 months. We defined patients with SD for ≥46 weeks as having prolonged SD (PSD). PSD was observed in 7 patients (24% of the entire study cohort and 29% of those patients treated at the tolerated 3 mg/kg dose), including ≥54 weeks in 3 of these patients (10.3%). The three patients who had stable disease for ≥54 weeks all had INT-1 IPSS risk category. A total of 5 patients (17.2%) proceeded to alloSCT. Three of these patients underwent alloSCT after finishing the 4 doses of induction ipilimumab therapy and before receiving any maintenance therapy. A fourth patient proceeded to alloSCT after developing a grade 3 IRAE that required steroid use after 3 induction doses of ipilimumab, and the fifth patient underwent alloSCT after being removed from study after the second induction dose to rapid disease progression and after receiving salvage chemotherapy. The median OS for entire cohort (censoring at the time of alloSCT) was 294 days [95% confidence interval (CI), 240–671+]. Patients who received any maintenance therapy (n = 7) had a median OS of 400 days (95% CI, 240–671 days; Supplementary Fig. S1).
Table 3.
Treatment characteristics and responses (N = 29)
| Ipilimumab dose administered | |
| 3 mg/kg | 24 (82.8) |
| 10 mg/kg | 5 (17.2) |
| Completed 4 doses of induction therapy - no. (%) | 15 (51.7) |
| Median number of cycles of therapy (range) | 4 (1, 8) |
| Response after the induction 4 cycles - no. (%) | |
| SD | 14 (93.3) |
| PD | 1 (6.7) |
| Received at least one dose of maintenance therapy - no. (%) | 7 (24.1) |
| Best response during study - no. (%) | |
| CR | 0(0) |
| PR | 0(0) |
| mCR | 1 (3.4) |
| HI | 0 (0) |
| SD | 17 (58.6) |
| PD | 11 (37.9) |
Abbreviations: CR, complete response; HI, hematologic improvement; mCR, marrow complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Correlative studies
Studies to evaluate the dynamic changes in T-cell subsets during ipilimumab treatment were performed on 16 patients who had PBMCs and/or BMMCs available from at least two time points. We defined patients who achieved mCR or PSD (SDfor ≥46 weeks) as having achieved meaningful clinical benefit (MCB) for laboratory analyses.
Absolute lymphocyte count and T-cell subsets
We did not observe a correlation between absolute lymphocyte count (ALC) and probability of achieving MCB (Supplementary Fig. S2). Using multi-parameter flow cytometry, we performed an extensive phenotypic characterization of lymphocyte populations. Prior to treatment, our patient population showed a lower CD4:CD8 ratio of 1:1 compared with healthy, age-matched controls (HC) that were closer to 2:1. Following 2 cycles of ipilimumab, there was a trend toward normalization of this ratio, but it did not reach that of HCs (Fig. 1B). We used CD45RA and chemokine receptor 7 (CCR7) to distinguish four differentiation subsets of T cells: naïve (TN), central memory (TCM), effector memory (TEM), and terminally differentiated EM (TEMRA) T cells (21). When comparing the differentiation status of CD4+ and CD8+ T cells in peripheral blood of patients with MDS at baseline to that of HCs, we found significantly decreased median frequencies of TN (P = 0.004 and P = 0.008, respectively) and increased TEMRA cells (Fig. 1C). Interestingly, after 2 cycles of ipilimumab, frequencies of TEMRA cells decreased, whereas TN levels were partially restored toward levels of HCs. A similar but insignificant trend toward decreased TN, but increased TEMRA was observed in the bone marrow of patients with MDS versus HCs (Supplementary Fig. S3). However, there were no changes in bone marrow T-cell differentiation before and after ipilimumab treatment.
Analysis of costimulatory and coinhibitory molecules
We analyzed the expression of costimulatory molecules (ICOS; inducible T-cell co-stimulator) and two coinhibitory molecules (PD-1 and CTLA-4) in peripheral blood and determined that patients who achieved MCR following ipilimumab treatment had a higher percentage and absolute number of CD4+ and CD8+ T cells expressing ICOS (P = 0.05 and P = 0.01, respectively; Fig. 2; Supplementary Fig. S4). ICOS expression and absolute numbers peaked at cycle 2 of treatment, slowly decreased over the study period, and reached pretreatment levels during ipilimumab maintenance. In contrast, minimal increases in ICOS expression were observed for patients who did not achieve MCB. A similar pattern was observed in the bone marrow (Supplementary Fig. S5). Together, these data suggest that ICOS expression is positively correlated with achievement of MCB with single-agent ipilimumab in patients with MDS. This observation is consistent with published results with CTLA-4 blockade in patients with solid tumors, suggesting correlation between increased peripheral CD4+ and CD8+ T cells expressing ICOS and clinical benefit (22, 23). Taking data from previous studies into consideration that have shown a 2-fold higher increase in CD4+ICOShi T cells in the 10 mg/kg/dose than that seen with the 3 mg/kg/dose of antibody (22), it is plausible that increase in ICOS expression could be dose dependent. Unfortunately, we could not address this hypothesis because IRAEs precluded patients in the 10 mg/kg group from further analysis due to lack of available specimens because patients with severe IRAEs were taken off the study. Expression of the immune coinhibitory receptor CTLA-4 followed a similar pattern showing a trend of increased expression in both CD4+ and CD8+ T cells (average increase of 6% and 2.4% respectively, P = n.s.) over the first 4 cycles of ipilimumab treatment among patients who achieved MCB, followed by a gradual decrease to pretreatment levels during maintenance (Supplementary Fig. S6A), and similar but insignificant trends were observed for absolute numbers (data not shown).
Figure 2.
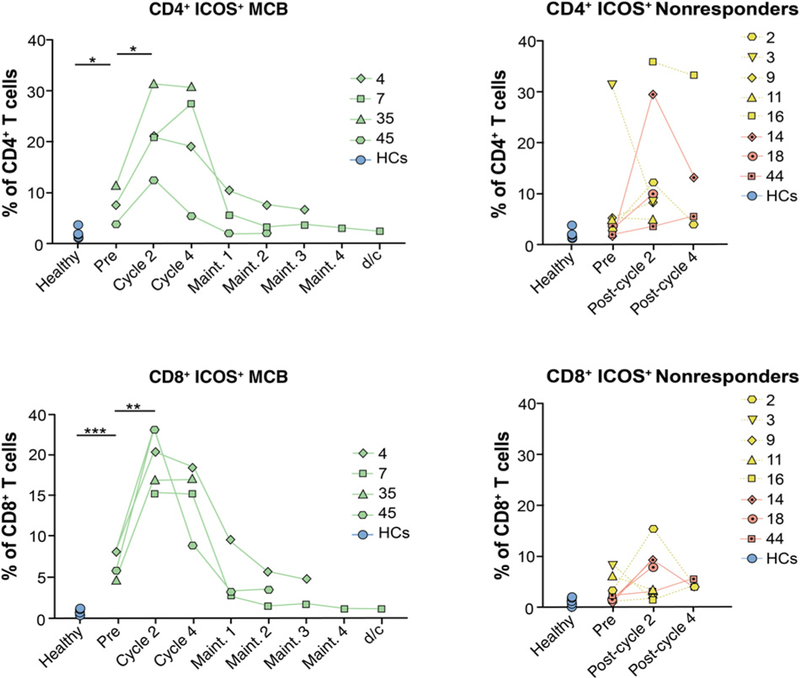
Increased frequencies of ICOS are associated with a clinical benefit to ipilimumab treatment. Immunophenotyping by flow cytometry was performed on PBMC samples collected from patients with HR-MDS (n = 12) at different time points before and after ipilimumab treatment as well as on HC PBMC samples (n = 6). CD4+ICOS+ (top) and CD8+ICOS+ (bottom) peripheral blood T cells after treatment with 3 or 10 mg/kg/dose of anti-CTLA-4. Data are presented for 4 patients who achieved MCB (left; UPIN 04/07 = prolonged SD and 35/45 = mCR) and 8 nonresponding patients (right; UPIN 14/18/44 = PD and UPIN 02/03/09/11/16 = PD with IRAE). Dotted lines indicate IRAEs/toxicity. Statistical comparisons were calculated in R as described in Materials and Methods with *, P ≤ 0.05;**, P ≤ 0.01; and ***, P ≤ 0.001.
Although it seems counterintuitive that CTLA-4 blockade would increase CTLA-4 expression, increased intracellular expression of this molecule has previously been reported after ipilimumab treatment of melanoma patients in both the helper and cytotoxic T-cell compartments (24). We hypothesize that the observed phenomenon likely reflects a physiologic counter regulation upon treatment with anti–CTLA-4 antibody. In contrast to CTLA-4, flow cytometric assessment of PD-1 demonstrated a wide range of frequencies of CD4+ and CD8+ T cells in both patients and HCs, and both frequencies and absolute numbers of PD-1+T cells remained stable throughout ipilimumab treatment (Supplementary Fig. S6B, absolute numbers not shown). This suggests that the lack of T-cell activation in nonresponders was not due to increased expression of other coinhibitory molecules.
Tregs
Tregs normally constitute 1% to 2% of circulating CD4+ T cells (25). Consistent with previous reports (6, 26, 27), flow cytometric analysis showed significantly higher frequency of CD3+CD4+Foxp3+ and activated, effector Tregs (eTreg subset; Miyara fraction II; refs. 28, 29) in both peripheral blood and bone marrow prior to treatment (Supplementary Fig. S7A). Overall enrichment of CD3+CD4+Foxp3+ and eTregs persisted throughout all of the examined time points, and neither their frequency nor the CD8 T-cell:Treg ratio were impacted by ipilimumab (Supplementary Fig. S7B). Furthermore, no correlation between Treg expression and immune toxicities was observed.
TCR sequencing
We performed high-throughput antigen receptor sequencing of the TCR Vβ CDR3 region on sorted peripheral blood CD4+ and CD8+T cells from three groups of patients: (i) those who achieved MCB; (ii) those who did not achieve MCB; and (iii) patients who were taken off study due to toxicity. TCRβ sequencing data revealed that the majority of patients had some improvement in TCR diversity as measured by clonality or Gini coefficient in either the CD4 or CD8 compartment, or for some patients in both compartments (Fig. 3A and B). Interestingly, the magnitude of improvement in diversity did not always correlate with achieving MCB (Fig. 3A and B; Supplementary Fig. S8).
Figure 3.
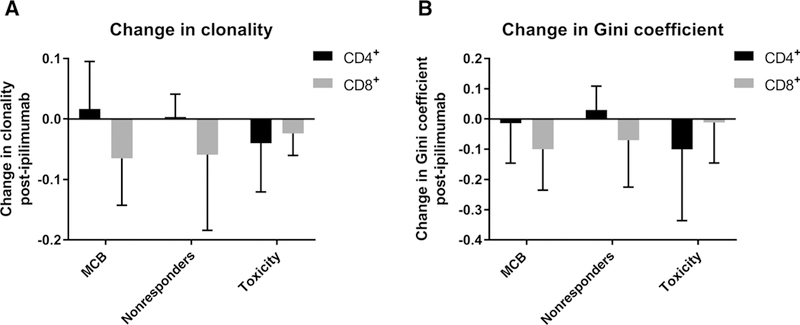
Ipilimumab treatment increases peripheral TCR diversity but does not correlate with clinical outcomes. PBMC samples from 16 patients, both at baseline and after 2 cycles of ipilimumab treatment, were sorted into CD4+ and CD8+ T cells and subjected to survey-level next-generation high-throughput sequencing of the TCR Vβ CDR3 region. Patients were chosen to represent 3 subgroups: (i) patients who achieved MCB (n = 5); (ii) patients who progressed/did not achieve MCB (nonresponders, n = 7); and (iii) patients who developed IRAEs to ipilimumab treatment and had to be taken off study (toxicity, n = 4). A-B, Clonality, Gini coefficient, and top 20 T-cell clones for CD4+ and CD8+ T cells were calculated as described in the Supplementary Appendix. Data, mean + SD. Changes in clonality (A) and Gini coefficient (B) improved after 2 cycles but did not correlate with clinical response to treatment and achieving MCB.
Discussion
The use of immune checkpoint blockade (ICB) in patients with advanced solid tumors, including melanoma, bladder, lung, and head and neck, has shown impressive clinical results leading to several approvals of these agents (30–37). Likewise, the use of mAbs directed against PD-1 has shown excellent clinical activity in relapsed/refractory Hodgkin lymphoma and has recently been approved for this indication (38). Augmentation of antitumor immunity by modulation of inhibitory signals through CTLA-4 blockade has also emerged as an effective strategy for inducing responses in preclinical models of hematologic malignancies (10–12), and blocking CTLA-4 signaling with ipilimumab demonstrated a favorable safety profile and activity in a limited number of patients with relapsed lymphomas and MDS/acute myeloid leukemia (AML) treated after alloSCT (13, 14).
This investigator-initiated multi-center phase Ib study was designed to investigate the use of anti–CTLA-4 therapy in patients with MDS after HMA failure. To our knowledge, this is the first published trial that formally tested single-agent ipilimumab in patients with MDS post-HMA failure who did not previously undergo an alloSCT. We used a similar dosing and administration schema (including induction and maintenance phases) to what has been used in the initial trials of the drug in advanced melanoma (30). In our patients, the 10 mg/kg dose had unacceptable toxicity, but we found that the 3 mg/kg dose was well tolerated and led to mCR and PSD in a subset of patients. There have been 2 studies reported in abstract format that used ipilimumab and pembrolizumab in the post-HMA failure setting that also show modest clinical activity (39, 40). The first reported 1 PR and 3 mCRs in 27 patients treated with single-agent pembrolizumab (39). The second study, which used ipilimumab at 3 mg/kg in 18 patients, reported slightly better activity with 1 CR, 2 mCRs, and 2 hematologic improvements with an ORR of 30% (40). The difference in the response rates seen by these early reports and ours that used the same agent and dose in a similar patient population remains unclear but may have to do with a difference in patient selection and small sample size of the studies. Regardless, the efficacy of ipilimumab as monotherapy appears to be limited. Unfortunately, we did not have serial molecular data available on enough patients to allow a meaningful analysis of molecular clearance in patients with SD. It thus remains unclear whether ipilimumab impacted the disease at the molecular level as most patients continued to have morphologic evidence of disease.
Ipilimumab has also been studied in the relapse post-alloSCT setting. Interestingly, a recent study in this population noted that a higher dose of 10 mg/kg was tolerated in the post-alloSCT setting with only 4 of 22 patients treated at that level developing G2–4 IRAE (including 1 death) compared with 4 of 5 patients in our study who received this dose developing significant IRAEs (13). It is not clear whether post-alloSCT changes in the immune system somehow modulate the development of IRAEs and allow the 10 mg/kg dose to be tolerated. Interestingly, lower dose ipilimumab (3 mg/kg) appeared to be well tolerated both with upfront treatment and following alloSCT; however, clinical activity appears to be limited at 3 mg/kg dose because there were no complete responses in both upfront and the post-alloSCT relapse setting. This is in comparison with 23% CR, 9% PR, and 27% reduction in tumor burden in post-alloSCT patients treated 10 mg/kg ipilimumab as the single agent. In addition, responses to single-agent Ipilimumab following transplant were common for patients whose relapse was extramedullary disease with 4 of the 5 remissions seen for AML being for granulocytic sarcoma, suggesting extramedullary disease involvement may be more susceptible to ICB than marrow blasts. This raises important questions about dose effect as well as the clinical setting.
Several pharmacodynamic markers have been reported to impact responses in patients treated with ipilimumab. Increases in ALC after two or three doses and increases in eosinophil count after the first dose have been correlated with clinical benefit and OS in metastatic melanoma (41). In our study, there was no clear correlation between serial changes in ALC and achievement of MCB from ipilimumab therapy. Earlier studies also reported on increased frequency of circulating CD4+HLA-DR+ and or CD4+CD45RO+ T cells after ipilimumab therapy (41, 42); however, the most consistent finding is an increased frequency of ICOS, a costimulatory molecule that is a member of immunoglobulin gene family and structurally related to CD28 and CTLA-4, that is expressed on CD4+ and CD8+ T cells after their activation (22,43–45). Expression of this molecule has previously been shown to be increased in CD4+ and CD8+ T cells after ipilimumab monotherapy treatment in patients with melanoma and bladder cancer (22, 23). We observed a significant increase in the number of CD4+ICOS+ and CD8+ICOS+ T cells in peripheral blood after cycles 2 and 4, compared with baseline levels. These findings indicate that the percentage of circulating ICOS+ T cells might serve as an early prognostic marker of clinical efficacy of CTLA-4 blockade treatment in MDS.
Tregs have been described to constitutively express CTLA-4, thereby making them potential targets for CTLA-4 blockade therapy. However, the effect of ipilimumab on Treg frequency across different solid tumors is inconsistent (41). Increased CD8+/Treg ratio has been proposed to correlate with ADCC of intratumoral Tregs in responding lesions of melanoma patients (46, 47). Our data demonstrate that Treg frequency or the CD8/Treg ratio did not change over the course of treatment with ipilimumab. Recent studies have also suggested that treatment with anti–CTLA-4 may lead to the compensatory upregulation of alternate checkpoint molecules and/or their ligands (48, 49); however, in our analysis, we did not find a consistent relationship between increased PD-1 expression and achievement of a clinical benefit.
One mechanism by which checkpoint inhibitors are thought to elicit their effect is by broadening the TCR repertoire (50–52). We performed high-throughput sequencing of the TCR Vβ CDR3 regions on flow cytometrically sorted T-cell subsets to assess the effect of ipilimumab on the diversity of the T-cell repertoire. Data were analyzed on the basis of achievement of MCB or those who experienced toxicity. There was heterogeneity in the clonality of the T-cell populations within each group. Overall, the clonality in the CD4 T-cell population was lower at baseline and had relatively smaller changes following ipilimumab therapy. In contrast, changes in the CD8 T-cell clonality were more dramatic but still did not correlate with achieving MCB. The explanation is not clear, but it may be that measuring high-level changes in clonality is not sensitive enough to detect what is functionally occurring with particular clones within the population or achievement of complete clinical response is required to see more significant changes with this novel technology.
In summary, we found that ipilimumab dosed at 3 mg/kg is well tolerated and led to minor clinical benefit in a subset of patients with MDS resistant to HMA therapy, whereas the 10 mg/kg dose was associated with significant immune toxicity. It is also possible that the patients who achieved prolonged periods of disease stability did so due to underlying disease biology and not due to ipilimumab therapy. Although numbers were small, there was no indication of increased toxicity in patients who subsequently underwent alloSCT. We elected to combine the IWG-2006 defined mCR and the PSD as “meaningful clinical endpoints” to help inform the interpretation of the laboratory correlative results and found that circulating ICOS+ T cells can serve as an early prognostic marker of clinical efficacy of CTLA-4 blockade treatment in MDS. It will be ofinterest to see the results of other phase I studies in MDS patients are currently underway to test checkpoint inhibitors such as anti–CTLA-4, anti–PD-1, and anti–PDL-1 antibodies in combination with HMAs as upfront therapy and in the post-HMA relapsed/refractory setting.
Supplementary Material
Translational Relevance.
Our data represent the first published clinical trial data on the use of immune checkpoint blockade therapy in patients with myelodysplastic syndromes (MDS). Our data suggest that ipilimumab as a single agent is safe but is associated with a limited clinical activity in patients with refractory and relapsed MDS after hypomethylating agent failure. Marrow complete response and prolonged stable disease were observed in 1 and 7 of 24 patients (29%) treated at the tolerated dose of 3 mg/kg, respectively. Expression of ICOS on T cells positively correlated with achievement of clinical benefit and should be further explored as a biomarker for response. Immune profiling data can contribute to the clinical development of immune checkpoint blockade–based therapies in MDS.
Acknowledgments
The authors would like to thank all the patients who enrolled in this study, and the NCI-Cancer Therapy Evaluation Program (CTEP) for supporting this trial. This work was supported by the grants UM1 CA186691, UM1 CA186689, UM1 CA186689, and LLSTRP grant # 6449–13, and by the American Society of Clinical Oncology Young Investigator Award (ASCO YIA) 2013 for A.M. Zeidan.
Footnotes
Disclosure of Potential Conflicts of Interest
J.F. Zeidner reports receiving commercial research grants from Merck, Takeda Millennium, and Tolero and is a consultant/advisory board member for Agios, Celgene, and Tolero. M.G. Frattini is a consultant/advisory board member for Lin BioScience. No potential conflicts of interest were disclosed by the other authors.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.PrebetT Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol 2011;29:3322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jabbour E, Garcia-Manero G, Batty N, Shan J, O’Brien S, Cortes J, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer 2010;116:3830–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 2007;109:4816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiladjian JJ, Bourgeois E, Lobe I, Braun T, Visentin G, Bourhis JH, et al. Cytolytic function and survival of natural killer cells are severely altered in myelodysplastic syndromes. Leukemia 2006;20:463–70. [DOI] [PubMed] [Google Scholar]
- 5.Kitagawa M, Kamiyama R, KasugaT. Increase in number of bone marrow macrophages in patients with myelodysplastic syndromes. Eur J Haematol 1993;51:56–58. [DOI] [PubMed] [Google Scholar]
- 6.Kordasti SY, Afzali B, Lim Z, Ingram W, Hayden J, Barber L, et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol 2009; 145:64–72. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014;28:1280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orskov AD, Treppendahl MB, Skovbo A, Holm MS, Friis LS, Hokland M, et al. Hypomethylation and up-regulation of PD-1 inT cells by azacytidine inMDS/AML patients: a rationale forcombined targeting ofPD-1 and DNA methylation. Oncotarget 2015;6:9612–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knaus HA, Kanakry CG, Luznik L, Gojo I. Immunomodulatory drugs II: immune checkpoint agents in acute leukemia. Curr Drug Targets 2015;18:315–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaBelle JL, Hanke CA, Blazar BR, Truitt RL. Negative effect of CTLA-4 on induction of T-cell immunity in vivo to B7–1+, but not B7–2+, murine myelogenous leukemia. Blood 2002;99:2146–53. [DOI] [PubMed] [Google Scholar]
- 11.Saudemont A, Quesnel B. In a model of tumor dormancy, long-term persistent leukemic cells have increased B7-H1 and B7.1 expression and resist CTL-mediated lysis. Blood 2004;104:2124–33. [DOI] [PubMed] [Google Scholar]
- 12.Zhong RK, Loken M, Lane TA, Ball ED. CTLA-4 blockade by a human MAb enhances the capacity of AML-derived DC to induce T-cell responses against AML cells in an autologous culture system. Cytotherapy 2006; 8:3–12. [DOI] [PubMed] [Google Scholar]
- 13.Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med 2016;375:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bashey A, Medina B, Corringham S, Pasek M, Carrier E, Vrooman L, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 2009;113:1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997;89:2079–88. [PubMed] [Google Scholar]
- 16.NCI. Common terminology criteria for adverse events v.3.0 and v.4.0 (CTCAE) [Internet]. Rockville, MD: NCI; Availablefrom:http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [Google Scholar]
- 17.Hude I, Sasse S, Engert A, Brockelmann PJ. The emerging role of immune checkpoint inhibition in malignant lymphoma. Haematologica 2017; 102:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs 2016;30:571–84. [DOI] [PubMed] [Google Scholar]
- 19.Cousin S, Italiano A. Molecular pathways: immune checkpoint antibodies and their toxicities. Clin Cancer Res 2016;22:4550–5. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006;108:419–25. [DOI] [PubMed] [Google Scholar]
- 21.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol 2012;12: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 2010;16:2861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Giacomo AM, Calabro L, Danielli R, Fonsatti E, Bertocci E, Pesce I, et al. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother 2013;62:1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjoern J, Lyngaa R, Andersen R, Rosenkrantz LH, Hadrup SR, Donia M, et al. Influence of ipilimumab on expanded tumour derived T cells from patients with metastatic melanoma. Oncotarget 2017;8:27062–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol 2001;167: 1245–53. [DOI] [PubMed] [Google Scholar]
- 26.Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood 2007;110:847–50. [DOI] [PubMed] [Google Scholar]
- 27.Zou JX, Rollison DE, Boulware D, Chen DT, Sloand EM, Pfannes LV, et al. Altered naive and memory CD4+ T-cell homeostasis and immunosenescence characterize younger patients with myelodysplastic syndrome. Leukemia 2009;23:1288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009;30:899–911. [DOI] [PubMed] [Google Scholar]
- 29.Kanakry CG, Hess AD, Gocke CD, Thoburn C, Kos F, Meyer C, et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatoryTcells. Blood 2011;117:608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109–17. [DOI] [PubMed] [Google Scholar]
- 32.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 34.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med 2016;374:2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-i blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015;372:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Manero G, Tallman MS, Martinelli G, Ribrag V, Yang H, Balakumaran A, et al. Pembrolizumab, a PD-1 inhibitor, in patients with mye-lodysplastic syndrome (MDS) after failure of hypomethylating agent treatment. Blood 2016;128:345. [Google Scholar]
- 40.Garcia-Manero G, Daver NG, Montalban-Bravo G, Jabbour EJ, DiNardo CD, Kornblau SM, et al. A phase II study evaluating the combination of nivolumab (nivo) or ipilimumab (ipi) with azacitidine in pts with previously treated or untreated myelodysplastic syndromes (MDS). Blood 2016;128:344. [Google Scholar]
- 41.Weide B, Di Giacomo AM, Fonsatti E, Zitvogel L. Immunologic correlates in the course of treatmentwith immunomodulating antibodies. Semin Oncol 2015;42:448–58. [DOI] [PubMed] [Google Scholar]
- 42.Felix J, Lambert J, Roelens M, Maubec E, Guermouche H, Pages C, et al. Ipilimumab reshapes T cell memory subsets in melanoma patients with clinical response. Oncoimmunology 2016;5:1136045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res 2010;16: 3485–94. [DOI] [PubMed] [Google Scholar]
- 44.Wang W, Yu D, Sarnaik AA, Yu B, Hall M, Morelli D, et al. Biomarkers on melanoma patient T cells associated with ipilimumab treatment. J Transl Med 2012;10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, et al. Increased frequency of ICOS+ CD4Tcells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res 2013;1:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatoryT cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci USA 2015;112:6140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santegoets SJ, Stam AG, Lougheed SM, Gall H, Scholten PE, Reijm M, et al. T cell profiling reveals high CD4+CTLA-4 + T cell frequency as dominant predictor for survival after prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother 2013;62:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016; 7:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, et al. Improved survival with T cell clonotype stability afteranti-CTLA-4 treatment in cancer patients. Sci Transl Med 2014;6:23Sra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res 2014;20:2424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh DY, Cham J, Zhang L, Fong G, Kwek SS, Klinger M, et al. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res 2017;77: 1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


