Abstract
Endothelial cell activation by proinflammatory stimuli drives leukocyte recruitment through enhanced expression of counter-receptors such as vascular cell adhesion molecule-1 (VCAM-1). We previously demonstrated that activation of the receptor tyrosine kinase EphA2 with its ligand ephrin-A1 induces VCAM-1 expression. Here, we sought to characterize the proinflammatory signaling pathways involved. Analysis of over-represented transcription factors in ephrin-A1-induced genes identified multiple potential transcriptional regulators, including the Rel family members nuclear factor-κB (NF-κB/p65) and nuclear factor of activated T-cells (NFAT). While ephrin-A1 failed to induce endothelial NF-κB activation, NF-κB inhibitors prevented ephrin-A1-induced VCAM-1 expression, suggesting basal NF-κB activity is required. In contrast, ephrin-A1 induced a robust EphA2-dependent increase in NFAT activation, and mutation of the NF-κB/NFAT-binding sites in the VCAM-1 promoter blunted ephrin-A1-induced promoter activity. NFAT activation classically occurs through calcium-dependent calcineurin activation, and inhibiting NFAT signaling with calcineurin inhibitors (cyclosporine A, FK506) or direct NFAT inhibitors (A-285222) was sufficient to block ephrin-A1-induced VCAM-1 expression. Consistent with robust NFAT activation, ephrin-A1-induced an EphA2-dependent calcium influx in endothelial cells that was required for ephrin--A1-induced NFAT activation and VCAM-1 expression. This work provides the first data showing EphA2-dependent calcium influx and NFAT activation and identifies NFAT as a novel EphA2-dependent proinflammatory pathway in endothelial activation.
Keywords: NFAT, Eph receptor, ephrin, inflammation, VCAM-1, endothelial
1. INTRODUCTION
1.1. Endothelial activation.
Endothelial activation represents a phenotypic conversion from a quiescent phenotype to a permeable, proinflammatory phenotype. Classic mediators of endothelial activation, including proinflammatory cytokines (TNFα, IL-1β) and lipopolysaccharide (LPS), promote transcription induction of leukocyte counter-receptors (endothelial-selectin (E-selectin), vascular cell adhesion molecule-1 (VCAM-1)) and chemokines [1, 2]. Several transcription factors mediate the expression of proinflammatory genes, including activated protein-1 (AP-1) and nuclear factor-κB (NF-κB) [2]. While targeting inflammatory signaling or adhesion molecules pharmacologically reduces inflammation in a variety of settings [2, 3], inhibiting common inflammatory pathways and adhesion molecules can limit general immune function. Thus, identifying molecules that promote these pathways in specific contexts may offer greater selectivity for therapeutic options.
1.2. Eph/ephrin signalling.
Eph receptors and their ephrin ligands comprise the largest family of receptor tyrosine kinases in the mammalian genome. Ephs and ephrins are denoted as either type A or B depending on sequence homology, binding affinity, and ligand anchorage. Eph receptors bind to ephrin ligands on neighboring cells upon cell-cell contact, resulting in Eph receptor tyrosine phosphorylation and kinase activation. These Eph/ephrin interactions drive signaling critical for proper regulation of tissue morphogenesis, as cell-cell adhesion and repulsion cues via control of cell anchorage and migration, and therefore Ephs and ephrins play well characterized roles in developmental tissue patterning. However, certain Eph/ephrin interactions appear to play a minimal role in development but instead regulate tissue remodeling responses in a variety of pathological conditions, including inflammation [4]. For example, Eph/ephrin interactions between leukocytes and endothelial cells regulate leukocyte homing and transmigration during inflammation [5–7], and interactions between B-cell ephrins and endotheilal EphA2 even modulates endothelial localization of ICAM-1 and VCAM-1 [8]. However, less is known about Eph/ephrin-dependent regulation of proinflammatory gene expression.
1.3. EphA2 in inflammation.
EphA2 and its major ligand ephrin-A1 regulate multiple aspects of endothelial cell function and endothelial activation. Both EphA2 and ephrin-A1 show enhanced expression in response to cytokines and LPS, concomitant with enhanced EphA2 receptor activation [9, 10]. In lung injury models, activation of EphA2 promotes dissolution of endothelial barrier function and expression of several proinflammatory chemokines [11, 12]. We previously demonstrated that ephrin-A1 stimulates expression of leukocyte adhesion receptors, such as E-selectin and VCAM-1, in human aortic endothelial cells [9], and EphA2 has been shown to regulate thrombin-induced NF-κB activation and proinflammatory gene expression in human umbilical vein endothelial cells (HUVECs). [13]. However, the mechanisms of EphA2-dependent proinflammatory signaling remain largely unknown. Herein we investigated candidate pathways to determine regulators of ephrin-A1-induced VCAM-1 expression in arterial endothelial cells.
2. MATERIALS AND METHODS
2.1. Cell culture, treatments, transfections, and transfections.
HAECs were purchased from Lonza at passage 3 and were cultured in EGM-2 growth medium supplemented with the single-quots bullet kit (Lonza), 10% fetal bovine serum, and 100 U/mL penicillin/ 100 μg/mL streptomycin or in MCDB131 medium supplemented with 24μg/mL bovine brain extract, 60 μg/mL heparin, 10% fetal bovine serum, and 2 mM Glutamax. Cells were used between passages 8–10 and were serum starved for 4–6 hours in serum free medium or overnight in 0.5% FBS medium before performing experiments. Control treatments include either vehicle, 2 μg/mL Fc (R&D) or 2 μg/mL Fc (R&D) that was pre-conjugated with anti-Fc antibody as indicated. To induce proinflammatory signaling and gene expression, cells were treated with either 2 μg/mL ephrin-A1-Fc (R&D), 2 μg/mL ephrin-A1-Fc (R&D) that was pre-conjugated with anti-Fc antibody, or with 10 ng/mL TNFα (Sigma) as indicated. To conjugate Fc or ephrin-A1-Fc with anti-Fc antibody, 2.4μg of goat-anti human antibody (Jackson ImmunoResearch) was combined with every 4μg of either Fc or ephrin-A1-Fc and incubated on ice for 30 minutes prior to treatment. The NFAT inhibitor A-285222 was provided by Abbott Laboratories (Abbott Park, IL). Other inhibitors include the calcineurin inhibitors Cyclosporin A (Calbiochem) and FK506 (Tocris Bioscience), the calcium chelator BAPTA (Tocris Bioscience), the IKK inhibitor Bay11–7821 (Tocris Bioscience), and the EphA2 inhibitor ALW-II-41–27 (gift of Dr. Nathaniel Gray, Harvard University) [14]. Transfections were facilitated with Lipofectamine2000 (Invitrogen) according to the manufacturer’s instructions with two different siRNAs directed against EphA2 or EphA4 (Dharmacon Smartpool and Sigma) with two different siRNA directed against NFAT1 (Sigma), or with luciferase assay constructs 48 hours prior to experiments. The pGL3-basic luciferase vectors driven by the VCAM-1 promoter or the mutant VCAM-1 promoter lacking NF-κB binding sites were a gift from William Aird (Harvard) and have been previously described in detail [15]. Luciferase assays were performed as per the manufacturer’s instructions (Promega). In brief, cells were lysed in reporter lysis buffer and a single freeze-thaw was performed. Luciferase activity from the lysates was quantified on a FLUOstar plate reader following incubation with luciferase assay reagent. Data were expressed as fold change normalized to control experiments. The adenovirus expression systems (Vector Biolabs), including Ad-SR-IkB (cat# 1028), Ad-CMV-GFP (cat# 1060), and Ad-DSCR1 (cat # 1539), were incubated with HAECs at an MOI of 20 for 20 hours prior to treatment.
2.2. Quantitative Real-Time PCR.
Cells were treated with Fc-ephrin-A1 (2μg/mL) for the indicated times and lysed in Trizol (Life Technologies). RNA was isolated using chloroform extraction and cDNA was synthesized using BioRad cDNA synthesis kit according to manufacturer’s instructions. qRT-PCR was performed in a Biorad CFX96 Real-Time PCR Detection System using iQSYBR Green MasterMix (Biorad). Primers were designed using online Primer3 software, and sequences are as follows: GAPDH forward primer: 5’-GAAGGTGAAGGTCGGAGTC-3’, GAPDH reverse primer: 5’-GAAGATGGTGATGGGATTTC-3′, VCAM-1 forward primer: 5’-ATGAGGGGACCACATCTACG-3’, VCAM-1 reverse primer: 5’-CACCTGGATTCCTTTTTCCA-3’. Results were normalized to the housekeeping gene GAPDH, and expressed as a fold change using the 2-[delta][delta]Ct method.
2.3. Immunocytochemistry.
Immunocytochemistry was performed as previously described [16]. Cells were seeded at confluency onto coverslips coated with 10 μg/mL human fibronectin and serum-starved prior to treatment. Cells were fixed in 2–4% formaldehyde, permeabilized in Tris-buffered saline containing 0.1% Triton-X100, and blocked in PBS containing 1% BSA and 10% goat serum for at least 1 hour. Cells were incubated with anti-p65 (Santa Cruz) or anti-NFAT1 XP (Cell Signaling Technologies) antibodies (1:200) for 2 hours, Alexa488 fluorophore-conjugated secondary antibodies at 1:2000 for 1 hour, and counterstained with DAPI to visualize the nucleus. Images were acquired on a Nikon Eclipse Ti inverted fluorescent microscope. Images were captured with a Photometrics Coolsnap 120 ES2 camera and the NIS Elements BR 3.00, SP5 imaging software. At least 100 cells were counted per experiment to determine the percent that were nuclear positive for p65 or NFAT1.
2.4. Subcellular fractionation and immunoblotting.
Following treatment with 2 μg/ml ephrin-A1-Fc and/or inhibitors, cells were rinsed with ice cold PBS and processed according to the NE-PER cytoplasmic/nuclear subcellular fractionation kit (Pierce). Nuclear pellets were rinsed with ice cold PBS after extraction of the cytoplasmic fraction and fractions were diluted with 2X Laemmli sample buffer prior to analysis by immunoblotting. Immunoblotting was performed on cell lysates following separation on 10% polyacrylamide gels by SDS-PAGE and subsequent transfer to PVDF membranes. Membranes were blocked with 5% milk in tris-buffered saline containing tween-20, and then probed with rabbit anti-p65, rabbit anti-EphA4, rabbit anti-EphA2, rabbit anti-DSCR1, mouse anti-E-selectin (Santa Cruz); rabbit anti-phospho-p65, rabbit anti-NFAT1, rabbit anti-HDAC3, rabbit anti-β-tubulin, rabbit anti-GAPDH, or rabbit IκBα (Cell Signaling Technologies).
2.5. Calcium flux measurement.
Human aortic endothelial cells were loaded with 1 μM Fluo4-AM (Invitrogen) according to the manufacturer’s instructions for 45 minutes, rinsed with 3 volumes of HBSS containing calcium and magnesium, and allowed to rest for 15 minutes prior to stimulus. At the time of stimulus with either Fc alone or Fc-Ephrin-A1, cells were maintained at 37°C and 5% CO2 on a stage-mounted environment chamber from Bioscience Tools (San Diego, CA) and monitored for changes in intracellular calcium through FITC excitation. Immediately following stimulus, images were captured in a single high-powered field with a Nikon TiE microscope controlled by NIS elements advanced research software package using a Nikon monochrome cooled digital camera (DS-Qi1) at 15-second intervals for 15 minutes. Calcium flux of each cell was considered an “event” and quantified using the ImageJ SparkMaster Plugin [17]. Event quantifications are represented as both intensity (amplitude, ΔF/F0) and duration (full duration half maximum, FDHM). Results represent data from 4 independent assays.
2.6. Statistical Analysis.
oPOSSOM 3.0 analysis of over-represented transcription factors in four EphA2-associated endothelial gene (Vcam1, F3, Sele, Ptgs2) promoters (5 kb upstream) was performed using an 85% matrix score threshold. Statistical comparisons between groups were performed with the Microsoft Excel or Graphpad Prism software using Student’s T-test, one-way ANOVA, or two-way ANOVA. Samples that required 1-way ANOVA and did not show a Gaussian distribution underwent nonparametric Kruskal Wallis 1-way ANOVA with Dunn’s Multiple Comparison Test. Samples that did show Gaussian distribution utilized 1-way ANOVA with Bonferroni posthoc analysis. All 2-way ANOVA utilized Bonferroni post-test. Error bars represent standard error of the mean.
3. RESULTS
3.1. Analysis of over-represented transcription factors in EphA2-regulated genes.
Regulation of the VCAM-1 promoter in response to inflammatory stimuli can be mediated by a variety of transcription factors, including NF-κB, NFAT, the AP-1 complex, SP-1, ETS, and GATA family members. To narrow candidate signaling pathways that might be regulated by ephrin-A1-induced EphA2 activation, we performed in silico analysis with the oPOSSUM database. The oPOSSUM database calculates the probability of transcription factor activity based on known promoters of genes included in the query. Inclusion of genes that we previously identified as ephrin-A1-induced genes including Vcam1, F3, Sele, Ptgs2 [9], implicated members of the AP1, Rel (RELA, REL, NFAT1), and basic helix-loop-helix (Myf, Arnt::Ahr, Hand1:Tcfe2a) families as ephrin-A1-activated transcription factors (Table 1).
Table 1.
| Family | Members Identified | VCAM-1 regulators | Average Taraets | Average Z-Score |
|---|---|---|---|---|
| Ets Family | SPI1, ELF5, SPIB, FEV, GABPA, ELK4 | None | 3.17 | 6.91 |
| Leucine Zipper | AP1 | AP1 | 2.57 | 2.72 |
| Rel Family | NFAT1, RELA, REL | NFAT1, RELA | 2.43 | 5.59 |
| Forkhead | FoxA2, FoxA1, FoxO3 | None | 2.69 | 4.42 |
| Helix-Loop-Helix | Myf, Arnt::Ahr, Hand1::Tcfe2a | Arnt::Ahr, | 2.45 | 2.62 |
3.2. NF-κB in EphA2-dependent VCAM-1 expression.
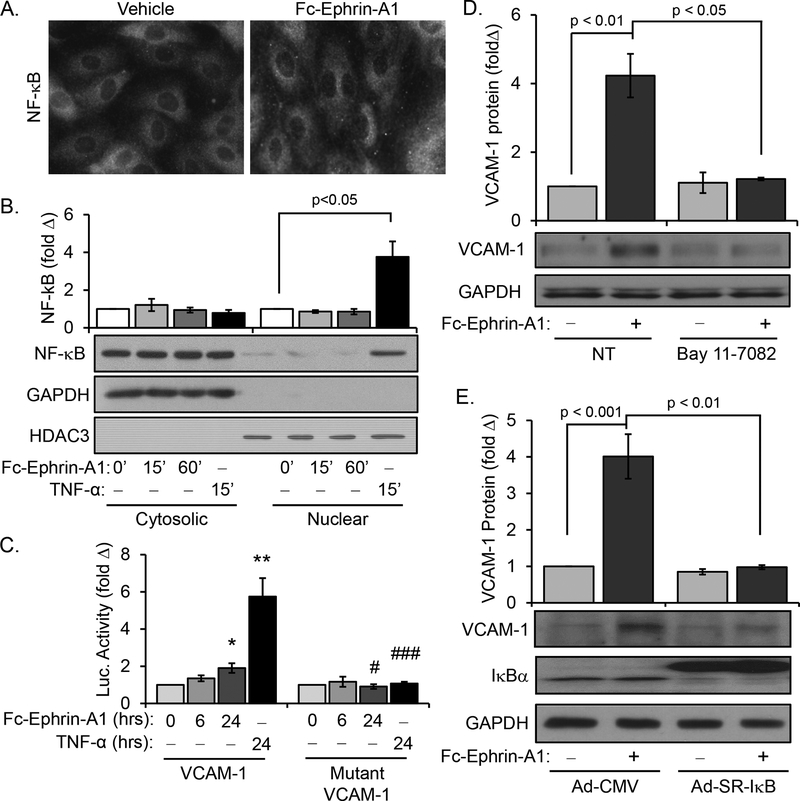
NF-κB represents the classic proinflammatory transcription factor involved in endothelial activation, and previous studies have shown EphA2 enhances NF-κB activation in HUVECs [11, 13]. However, HUVECs do not show EphA2-dependent VCAM-1 expression whereas human arterial endothelial cells do [9]. To determine if the NF-κB pathway mediates ephrin-A1-induced VCAM-1 expression in arterial endothelial cells, we treated human aortic endothelial cells (HAECs) with anti-Fc antibody-dimerized ephrin-A1-Fc and NF-κB activation was assessed by immunohistochemistry and Western blotting for the p65 subunit (hereafter NF-κB) following nuclear fractionation. Whereas TNF-α stimulus induced robust NF-κB nuclear translocation and VCAM-1 promoter activity, ephrin-A1 was unable to induce nuclear translocation of NF-κB in HAECs in the same conditions (Figure 1A–C). Consistent with this, ephrin-A1 only induced a slight but insignificant increase in p65 phosphorylation (Supplemental Figure Ia). Furthermore, cotreatment of ephrin-A1 with TNF-α did not further augment VCAM1 expression (Supplemental Figure Ib), suggesting TNF-α and ephrin-A1 do not have additive effects on VCAM-1 expression. As previously reported, Fc-ephrin-A1 treatment enhances VCAM-1 mRNA expression transiently at 3 hrs which returns to baseline levels by 6hrs treatment (Supplemental Figure 1c) [9]. To determine whether this increase is due to enhanced promoter activity, we utilized VCAM-1 luciferase promoter constructs [15]. Both TNF-α and ephrin-A1 stimulated a luciferase construct driven by the VCAM-1 promoter, but not a mutant VCAM-1 promoter lacking the Rel-binding sites and therefore incapable of interacting with NF-κB and NFAT (Figure 1C). These data suggest that ephrin-A1-induced VCAM-1 expression requires the Rel transcription factor binding sites but does not involve NF-κB activation. However, basal NF-κB activity likely contributes to this gene expression, since NF-κB inhibition with Bay 11–7082 (Figure 1D) and overexpression of a super-repressor IκB construct (Figure 1E), which is sufficient to reduce TNF-α-induced VCAM-1 expression (Supplemental Figure Id)[18], both inhibited ephrin-A1-induced VCAM-1 expression.
Figure 1.
Ephrin-A1 does not stimulate NF-κB activation in human aortic endothelial cells. A) HAECs treated with dimerized ephrin-A1-Fc (2 μg/mL, 1 hour) fail to induce NF-κB nuclear translocation as assessed by immunocytochemistry for nuclear p65 localization; n = 4. B) Endothelial cells were treated with ephrin-A1-Fc at the indicated times or with TNFα (10 ng/mL, 1 hour), and NF-κB levels in the nuclear and cytosolic fractions was assessed by Western blotting for total p65; n = 4. C) Endothelial cells expressing the luciferase reporter constructs under the control of a VCAM-1 promoter or a mutated VCAM-1 promoter were treated with ephrin-A1-Fc or TNF-α for the indicated times; n = 4. D) NF-κB inhibition with Bay 11–7082 (10 μM) prevented ephrin-A1-induced VCAM-1 expression as assessed by Western blotting. n=4. E) Adenoviral overexpression of super-repressor IκB (SR-IκB) but not a CMV control prevented VCAM-1 expression as assessed by Western blotting. n=4. * p < 0.05, ** p<0.01 compared to untreated control, # p<0.05, ### p<0.001 compared to wildtype VCAM-1 promoter construct. Statistical comparisons were made with Kruskal Wallis 1-way ANOVA with Dunn’s Multiple Comparison posttest (A) or 1-way ANOVA with Bonferroni posttest (C)
3.3. NFAT in EphA2-dependent VCAM-1 expression.
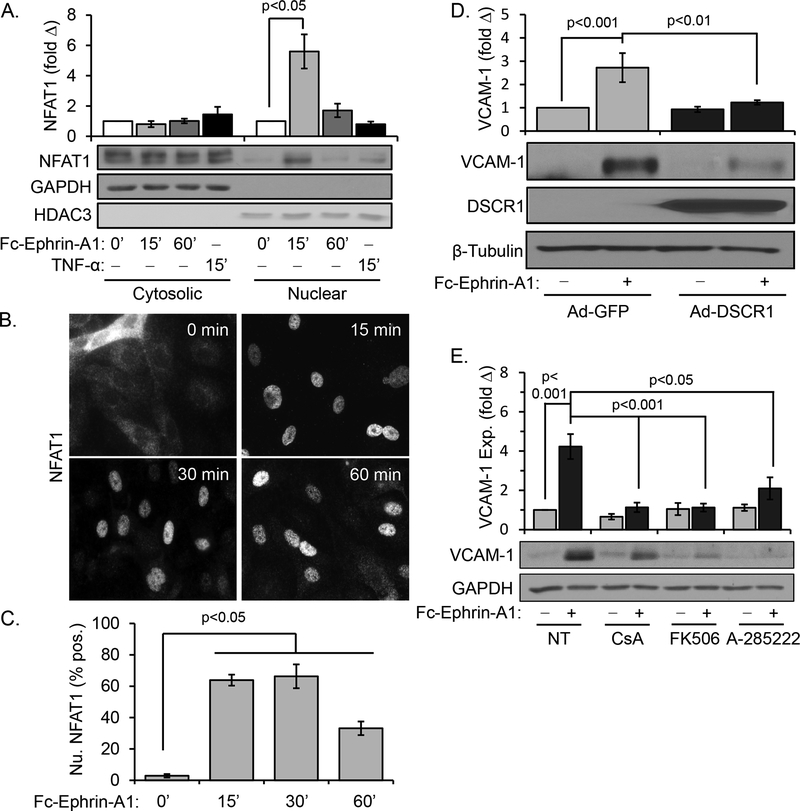
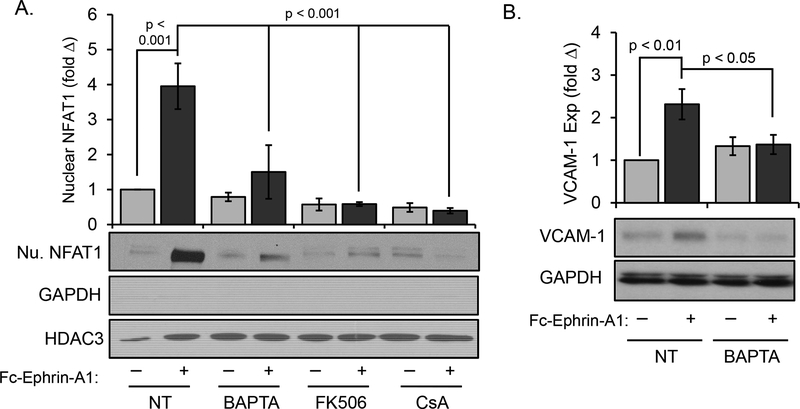
NFAT transcription factors are most well-known for regulating cytokine and chemokine expression in T-cell activation and immune function, but also mediate proinflammatory gene expression in other cell types [19, 20]. Since we did not observe changes in NF-κB activation following ephrin-A1 treatment, we next investigated a possible role for NFAT as a downstream mediator of ephrin-A1 signaling. Western blotting of subcellular fractionation preparations from ephrin-A1-treated HAECs indicated that NFAT1 nuclear translocation was robustly induced by ephrin-A1 stimulus (Figure 2A). Similarly, anti-NFAT1 immunocytochemistry revealed transient but robust NFAT1 nuclear translocation in response to ephrin-A1 (Figure 2B), peaking at 60% of nuclei between 15–30 minutes after stimulus (Figure 2C). These studies utilize a recombinant Fc-conjugated ephrin-A1 dimer, and clustering Fc-ephrin-A1 with an anti-Fc antibody did not further enhance NFAT1 activation or VCAM-1 expression (Supplemental Figure IIa/b). Additionally, neither recombinant Fc alone nor clustered Fc were sufficient to promote NFAT1 activation or VCAM expression (Supplemental Figure IIa/b) [9]. We next utilized a variety of NFAT pathway inhibitors to evaluate whether NFAT1 is required for ephrin-A1-induced VCAM-1 expression. Adenoviral overexpression of Down syndrome critical region1 (DSCR1), an endogenous NFAT inhibitor, significantly reduced ephrin-A1-induced VCAM-1 expression compared to adenoviral GFP expressing HAECs (Figure 2D). Similarly, ephrin-A1-induced VCAM-1 expression was blunted by treatment with the NFAT inhibitor A-285222 or inhibitors of the upstream NFAT activator calcineurin (cyclosporinA (CsA), tacrolimus (FK506)) (Figure 2E). Lastly, NFAT1 knockdown with two different siRNA oligonucleotides significantly blunted ephrin-A1-induced VCAM-1 expression (Supplemental Figure IIc/e). Taken together, these data demonstrate that ephrin-A1 induces VCAM-1 expression through NFAT1 activation.
Figure 2.
Ephrin-A1 promotes NFAT1 nuclear translocation in human aortic endothelial cells. A) HAECs were treated with dimerized ephrin-A1-Fc (2 μg/mL) or TNF-α (10 ng/mL) for the indicated times, and NFAT1 levels in the nuclear and cytosolic fractions was assessed by Western blotting for NFAT1. n = 4. B) Endothelial cells were treated with ephrin-A1-Fc for the indicated times, and NFAT1 nuclear translocation was assessed by immunocytochemistry for NFAT1. Representative images are shown. C) NFAT1 nuclear translocation shown in B was quantified, with at least 100 cells assessed per treatment for each experiment. n = 4. D) HAECs were transfected with a DSCR-1 expression construct 48 hours prior to treatment with dimerized ephrin-A1-Fc (2 μg/ml, 5 hours). VCAM-1 expression was assessed by Western blotting. n = 4. E) Treatment with the calcineurin inhibitors cyclosporine A (CsA, 3 μM) and FK506 (10 nM) or the NFAT nuclear translocation inhibitor A-285222 (10 μM) is sufficient to prevent ephrin-A1-induced VCAM-1 expression as assessed by Western blotting. n = 5. Statistical comparisons were made with either 2-way ANOVA with Bonferroni posttest (A,D/E) or 1-way ANOVA with Bonferroni posttest (B/C).
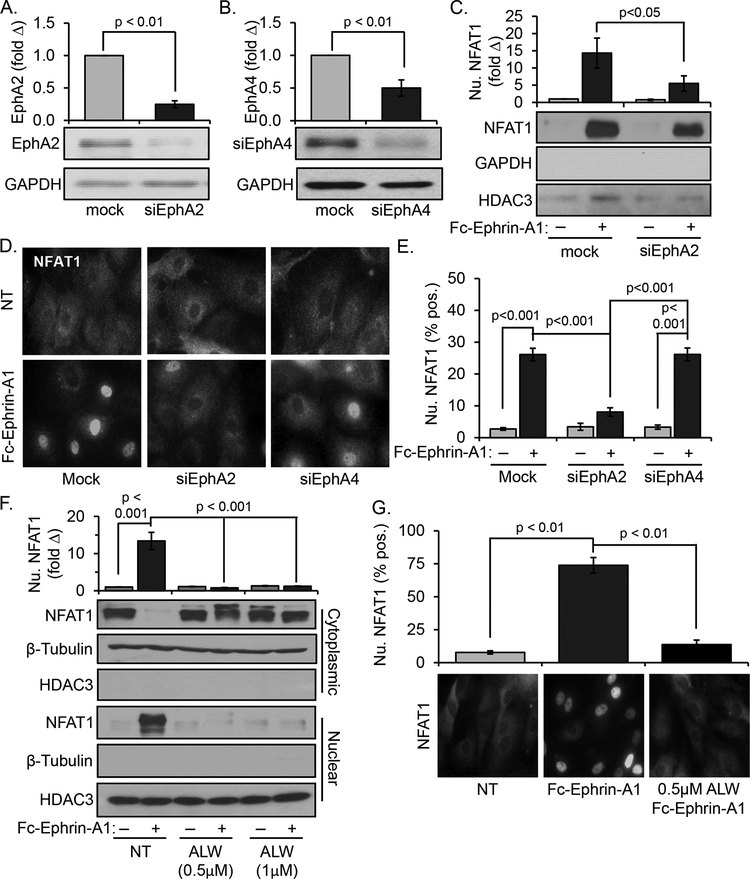
Previously we demonstrated that aortic endothelial cells express two ephrin-A1 receptors, EphA2 and EphA4, and that EphA4 was not required for ephrin-A1-induced VCAM-1 expression [9]. Consistent with our previous observations, knockdown of EphA2 prior to ephrin-A1 stimulus inhibited ephrin-A1-induced NFAT nuclear translocation, as evidenced by subcellular fractionation (Figure 3A & C). In addition, only EphA2 knockdown reduced NFAT1 nuclear accumulation as assessed by immunocytochemistry, whereas EphA4 knockdown did not (Figure 3B/D/E, Supplemental Figure III). EphA2 inhibition with the ATP-competitive EphA2 kinase inhibitor ALW-II-41–27 also completely blocked ephrin-A1-induced NFAT1 nuclear translocation (Figure 4F & G). Thus, both siRNA-mediated knockdown and kinase inhibition indicate that EphA2 mediates ephrin-A1-induced NFAT1 activation.
Figure 3.
Ephrin-A1-induced NFAT1 activation is EphA2-dependent. A) Treatment with siRNA directed against EphA2 significantly blunted EphA2 expression in HAECs after 48 hours; n = 4. B) Treatment with EphA4 siRNA significantly blunted EphA4 expression in HAECs after 48 hours; n=4. C) Ephrin-A1-induced NFAT nuclear translocation in endothelial cells transfected with siRNA directed against EphA2 was assessed by Western blotting for NFAT1 in the nuclear fraction, n = 4. D & E) Ephrin-A1-induced NFAT nuclear translocation in endothelial cells transfected with siRNA directed against EphA2 or EphA4 was assessed by immunocytochemistry for NFAT1. D) Representative images are shown. E) Nuclear translocation was quantified by scoring cells for positive nuclear NFAT1 staining. At least 100 cells were assessed for each condition per experiment. n = 4. F & G) HAECs were treated with the EphA2 kinase inhibitor ALW-II-41–27 (indicated doses) and stimulated with dimerized ephrin-A1-Fc (2 μg/mL) for 15 minutes to induce NFAT activation. NFAT nuclear translocation was measured by (F) subcellular fractionation and Western blotting and (G) by immunocytochemistry and nuclear scoring. Representative images are shown, and at least 100 cells were scored for NFAT nuclear translocation for each condition per experiment. n = 4. Statistical comparisons were made with either Student’s T-test (A/B), 2-way ANOVA with Bonferroni posttest (C,E,F) or 1-way ANOVA with Bonferroni posttest (G).
Figure 4.
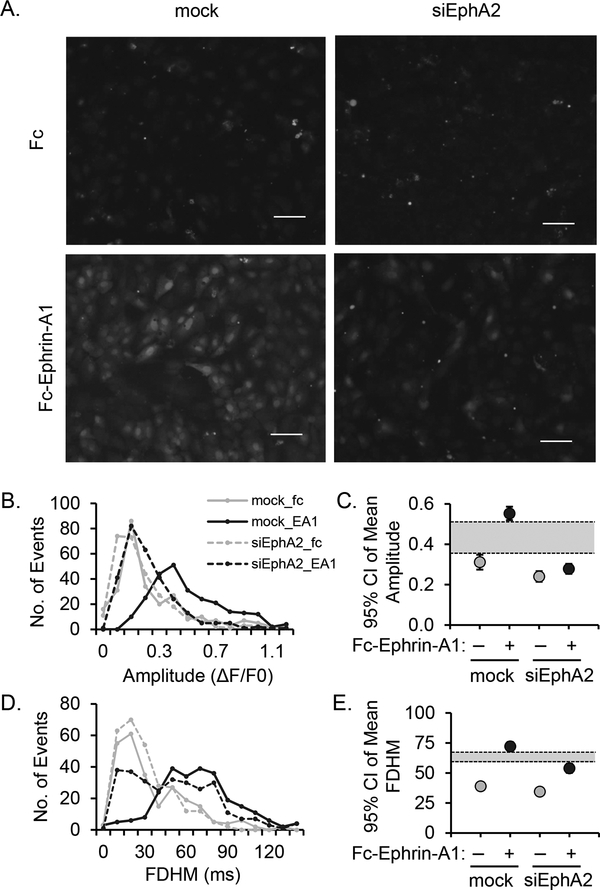
Ephrin-A1 induces EphA2-dependent calcium influx in human aortic endothelial cells. A) HAECs transfected with siRNA directed against EphA2 were labelled with 1μM Fluo4-AM (1 μM, 45 minutes) prior to treatment with dimerized ephrin-A1-Fc. Fluo4-AM fluorescence was visualized by microscopy every 15 seconds over a duration of 15 minutes. B) A histogram showing Fluo4 fluorescence intensity (amplitude) per each cell (event) by treatment. C) Mean amplitude of fluorescence intensity shown as 95% confidence interval. The shaded region indicates non-overlapping regions. D) A histogram showing duration of Fluo4 fluorescence (full duration half maximum, FDHM) for each cell (event). E) Mean duration of fluorescence intensity shown as 95% confidence interval. The shaded region indicates non-overlapping regions. n = 4.
3.4. EphA2-dependent calcium signaling in endothelial cells.
Canonical NFAT activation requires calcium-mediated activation of the serine/threonine phosphatase calcineurin [21]. To determine if EphA2 activation mediates calcium flux, HAECs were transfected with siRNA directed at EphA2 and c1alcium influx was assessed using the Fluo4 calcium indicator and visualized by live video capture epifluorescent microscopy. We measured periodic Ca2+ flux in each cell over time as shown in Figure 4A (compare mock + Fc-ephrin-A1 to mock + Fc, and to siEphA2 + Fc-ephrin-A1). Analysis of fluorescence intensity (Figure 4B and C, Supplemental Movies 1–4) and fluorescence duration (Figure 4D and E) indicate significantly lower amplitude and duration of calcium flux into ephrin-A1-treated HAEC cytosol lacking EphA2 expression. To determine if this calcium influx mediates calcineurin-dependent NFAT activation, ephrin-A1-induced NFAT activation was assessed in the presence of the calcium chelator BAPTA-AM and the calcineurin inhibitors CsA and FK506. All three inhibitors blunted ephrin-A1-induced NFAT nuclear translocation (Figure 5A), consistent with calcium/calcineurin-dependent NFAT activation. Furthermore, blunting ephrin-A1-induced calcium influx prevented VCAM-1 expression (Figure 5B), underscoring the importance of this pathway on ephrin-A1-induced proinflammatory VCAM-1 expression.
Figure 5.
Ephrin-A1-induced NFAT1 activation and VCAM-1 expression require calcium/calcineurin signaling. A) HAECs were treated with BAPTA (5 mM), cyclosporine A (CsA, 3 μM), or FK506 (10 nM) for 1 hour prior to treatment with dimerized ephrin-A1-Fc (2 μg/ml). Nuclear NFAT accumulation was assessed by subcellular fractionation and Western blotting for NFAT1; n = 4–6 B) Treatment with BAPTA (5 mM, 1 hour) blunts ephrin-A1-induced VCAM-1 expression as assessed by Western blotting; n = 7. Statistical comparisons were made with 2-way ANOVA with Bonferroni posttest.
4. DISCUSSION
4.1. Summary of results.
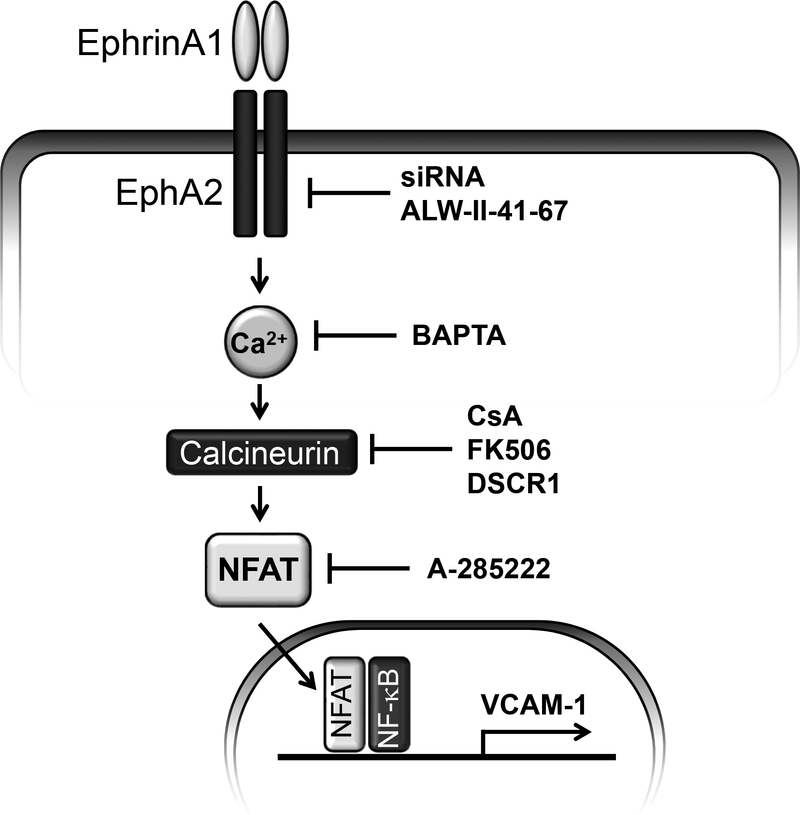
EphA2 and its ligand ephrin-A1 have been implicated in a variety of inflammatory conditions, and our previous work demonstrated an induction of pro-inflammatory genes including VCAM-1 in response to ephrin-A1-induced EphA2 signaling [4, 22]. In the current report, we demonstrate that ephrin-A1 induces VCAM-1 expression in arterial endothelial cells via calcium/calcineurin-mediated NFAT1 activity. While ephrin-A1 failed to activate the classic proinflammatory transcription factor NF-κB, ephrin-A1 induced a strong induction of NFAT1 activation that was blunted by EphA2 knockdown or inhibition. Inhibitors of NFAT or its upstream activator calcineurin were sufficient to prevent ephrin-A1-induced VCAM-1 expression. Surprisingly, preventing NF-κB signaling also blunted ephrin-A1-induced VCAM-1 expression despite no observed changes in NF-κB nuclear translocation or NF-κB-driven luciferase activity, suggesting basal NF-κB activity may cooperate with NFAT to induce VCAM-1 expression. Consistent with this, mutation of the known NF-κB/NFAT-binding sites in the VCAM-1 promoter [19] abolished ephrin-A1-induced VCAM-1 promoter activity. In addition, we demonstrate a novel role for EphA2 in the induction of endothelial calcium signaling, and show that preventing this increase in cytosolic calcium blunts both ephrin-A1-induced NFAT1 activation and VCAM-1 expression. Collectively these data describe a novel pathway of endothelial cell activation through EphA2-dependent calcium flux and NFAT activation (Figure 6).
Figure 6.
Model for EphA2 proinflammatory signaling through NFAT.
4.2. Multifaceted role of EphA2 in inflammation.
Multiple reports suggest a role for ephrin-A/EphA2 signaling in endothelial activation and inflammation. Ephrin-A1 was first identified as a TNF-α-inducible gene [23], and both ephrin-A1 and EphA2 show enhanced expression in the liver, lungs, and brain in an LPS-injection model of sepsis [10]. EphA2 deletion decreases leukocyte accumulation following ischemic stroke [24], bleomycin-induced lung injury [11], and in the ApoE model of atherosclerosis [22]. The reduction in atherosclerosis was associated with reduced ICAM-1 and VCAM-1 expression [22], and ephrin-A1 treatment promotes EphA2-dependent mRNA expression of VCAM-1, E-selectin, and tissue factor in human arterial endothelial cells [9]. However, monocyte rolling was unaffected by endothelial EphA2 knockdown [22], and E-selectin protein was unaltered by ephrin-A1 treatment or NFAT-1 depletion (Supplemental Figure IIe). Therefore, EphA2 expression primarily regulates leukocyte homing by modulating the interaction between leukocyte integrins and ICAM-1/VCAM-1. EphA2 activation stimulates its co-localization with ICAM-1 and VCAM-1 at the endothelial cell surface [8], whereas lack of EphA2 limits leukocyte adhesion to activated endothelial cells and recombinant ICAM-1/VCAM-1 [22, 25]. These data suggest that EphA2 may activate ephrin-A ligands on leukocytes to induce integrin activation and integrin-dependent adhesion to ICAM-1 and VCAM-1. In addition to EphA2, activation of EphA4 has also been shown to affect leukocyte adhesion through RhoA-dependent cytoskeletal stiffening and enhanced ICAM-1/VCAM-1 surface expression [6, 26]. Taken together, current data suggest that ephrin-A/EphA signaling regulates endothelial activation at multiple steps, including ICAM-1/VCAM-1 expression, ICAM-1/VCAM-1 surface targeting, and interaction of leukocyte counter-receptors with ICAM-1/VCAM-1.
4.3. Cross-talk between NF-κB and NFAT in proinflammatory gene expression.
While best characterized in leukocyte function and cancer [27], NFAT drives the expression of multiple genes involved in endothelial activation, including VCAM-1, E-selectin, tissue factor, and COX-2 [19, 28–30]. Regulation of the VCAM-1, tissue factor, and E-Selectin gene promoters can be independently regulated by NF-κB and NFAT depending on the stimulus [19, 28, 29]. While previous reports suggested that EphA2 stimulates NF-κB activation following treatment with thrombin and ephrin-A1 [11, 13], we did not observe any effect of ephrin-A1 on NF-κB nuclear translocation or luciferase activity. Rather, our work identifies a major role for NFAT1 activation in the induction of VCAM-1 following ephrin-A1 treatment. Previous studies of VCAM-1 expression in response to thrombin, VEGF, and TNF-α revealed that the VCAM-1 promoter requires basal activity of NF-κB, but can be augmented by enhanced NF-κB activation or by induction of NF-κB-independent NFAT binding sites thereby inducing transcriptional synergism [15, 19]. The tissue factor promoter contains NF-κB and NFAT binding sites that overlap, and can thus respond to pathway-specific NF-κB or NFAT activity, but not synergistically. Similarly, E-selectin and ICAM-1 expression appear to be more potently inhibited by NF-κB inhibition than NFAT inhibition in response to thrombin [19]. Thus, our failure to find an induction of ICAM-1, a gene potently induced by NF-κB but modestly by NFATs, in aortic endothelial cells by ephrin-A1 is also in line an NFAT-dependent/NF-κB independent paradigm. While ephrin-B2 signaling has been shown to inhibit NFAT expression [31], this represents the first data linking Eph receptor signaling to activation of the NFAT pathway.
4.4. Eph receptors and calcium signaling.
Calcium signaling contributes to multiple aspects of endothelial activation, including proinflammatory gene expression, release of Weibel-Palade bodies, cytoskeletal stiffening, and induction of endothelial permeability [2]. Our data represent the first to show enhanced cytosolic calcium following EphA2 receptor stimulation, identifying an important role for this calcium influx in NFAT activation and VCAM-1 expression. While the mechanisms mediating this calcium influx remain unknown, there are precedents in other systems for Eph/ephrin signaling regulating cytosolic calcium. Both ephrin-A3 treatment and EphB2 activation have been shown to potentiate neuronal calcium influx [32]. In addition, Eph receptor signaling in D. melanogaster and C. elegans regulates calcium influx through both cell surface receptors (Cav2.1, NMDA receptor) and endoplasmic reticulum release (IP3 receptor) [33, 34]. Since EphA2 activation promotes endothelial cytoskeletal stiffening and enhanced endothelial permeability [35, 36], the role of calcium signaling in this response and the mechanisms of EphA2-medicated calcium influx will provide interesting directions for future research.
Supplementary Material
5. Acknowledgements.
We would like to thank Patrick Albert and Sean Mathews (LSU Health – Shreveport) for assistance with endothelial cell culture studies.
6. Funding Sources.
This work was supported by the National Institute of Health R01 grants HL098435 and HL133497 to AWO and the P20 GM121307 COBRE Center for Redox Biology and Cardiovascular Disease to AWO and CBP, by the American Heart Association Grant-In-Aid 13GRNT17050093 to AWO, Scientist Development Grant 15SDG25710038 to CBP, Predoctoral Fellowship 14PRE18660003 to AYJ and 17PRE33440111 to ACF, and by a Malcolm Feist Predoctoral Fellowship to ACF and SDF.
Abbreviations:
- AP1
activated protein 1
- CsA
cyclosporine A
- ephrin
Eph receptor interacting protein
- HAEC
human aortic endothelial cells
- HUVEC
human umbilical vein endothelial cells
- NF-κB
nuclear factor κB
- LPS
lipopolysaccharide
- NFAT
nuclear factor of activated T cells
- VCAM-1
vascular cell adhesion molecule-1
7. References
- 1.Ley K, et al. , Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol, 2007. 7(9): p. 678–89. [DOI] [PubMed] [Google Scholar]
- 2.Pober JS and Sessa WC, Evolving functions of endothelial cells in inflammation. Nat Rev Immunol, 2007. 7(10): p. 803–15. [DOI] [PubMed] [Google Scholar]
- 3.Collins T and Cybulsky MI, NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest, 2001. 107(3): p. 255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funk SD and Orr AW, Ephs and ephrins resurface in inflammation, immunity, and atherosclerosis. Pharmacol Res, 2013. 67(1): p. 42–52. [DOI] [PubMed] [Google Scholar]
- 5.Braun J, et al. , Endothelial cell ephrinB2-dependent activation of monocytes in arteriosclerosis. Arterioscler Thromb Vasc Biol, 2011. 31(2): p. 297–305. [DOI] [PubMed] [Google Scholar]
- 6.Jellinghaus S, et al. , Ephrin-A1/EphA4-mediated adhesion of monocytes to endothelial cells. Biochim Biophys Acta, 2013. 1833(10): p. 2201–11. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, et al. , EphrinB-mediated reverse signalling controls junctional integrity and pro-inflammatory differentiation of endothelial cells. Thromb Haemost, 2014. 112(1): p. 151–63. [DOI] [PubMed] [Google Scholar]
- 8.Trinidad EM, et al. , An impaired transendothelial migration potential of chronic lymphocytic leukemia (CLL) cells can be linked to ephrin-A4 expression. Blood, 2009. 114(24): p. 5081–90. [DOI] [PubMed] [Google Scholar]
- 9.Funk SD, et al. , EphA2 activation promotes the endothelial cell inflammatory response: a potential role in atherosclerosis. Arterioscler Thromb Vasc Biol, 2012. 32(3): p. 686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanov AI, et al. , Expression of Eph receptors and their ligands, ephrins, during lipopolysaccharide fever in rats. Physiol Genomics, 2005. 21(2): p. 152–60. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter TC, et al. , Eph-A2 promotes permeability and inflammatory responses to bleomycin-induced lung injury. Am J Respir Cell Mol Biol, 2012. 46(1): p. 40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cercone MA, et al. , EphA2 receptor mediates increased vascular permeability in lung injury due to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol, 2009. 297(5): p. L856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan B and Sukhatme VP, Receptor tyrosine kinase EphA2 mediates thrombin-induced upregulation of ICAM-1 in endothelial cells in vitro. Thromb Res, 2009. 123(5): p. 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amato KR, et al. , Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC. J Clin Invest, 2014. 124(5): p. 2037–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minami T and Aird WC, Thrombin stimulation of the vascular cell adhesion molecule-1 promoter in endothelial cells is mediated by tandem nuclear factor-kappa B and GATA motifs. J Biol Chem, 2001. 276(50): p. 47632–41. [DOI] [PubMed] [Google Scholar]
- 16.Funk SD, et al. , Matrix-specific protein kinase A signaling regulates p21-activated kinase activation by flow in endothelial cells. Circ Res, 2010. 106(8): p. 1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picht E, et al. , SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol, 2007. 293(3): p. C1073–81. [DOI] [PubMed] [Google Scholar]
- 18.Kao DD, et al. , Tumor necrosis factor-alpha-mediated suppression of dual-specificity phosphatase 4: crosstalk between NFkappaB and MAPK regulates endothelial cell survival. Mol Cell Biochem, 2013. 382(1–2): p. 153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minami T, et al. , Thrombin-induced autoinhibitory factor, Down syndrome critical region-1, attenuates NFAT-dependent vascular cell adhesion molecule-1 expression and inflammation in the endothelium. J Biol Chem, 2006. 281(29): p. 20503–20. [DOI] [PubMed] [Google Scholar]
- 20.Hesser BA, et al. , Down syndrome critical region protein 1 (DSCR1), a novel VEGF target gene that regulates expression of inflammatory markers on activated endothelial cells. Blood, 2004. 104(1): p. 149–58. [DOI] [PubMed] [Google Scholar]
- 21.Oh-hora M and Rao A, The calcium/NFAT pathway: role in development and function of regulatory T cells. Microbes Infect, 2009. 11(5): p. 612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finney AC, et al. , EphA2 Expression Regulates Inflammation and Fibroproliferative Remodeling in Atherosclerosis. Circulation, 2017. 136(6): p. 566–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey A, et al. , Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science, 1995. 268(5210): p. 567–9. [DOI] [PubMed] [Google Scholar]
- 24.Thundyil J, et al. , Evidence that the EphA2 receptor exacerbates ischemic brain injury. PLoS One, 2013. 8(1): p. e53528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharfe N, et al. , EphA and ephrin-A proteins regulate integrin-mediated T lymphocyte interactions. Mol Immunol, 2008. 45(5): p. 1208–20. [DOI] [PubMed] [Google Scholar]
- 26.Ende G, et al. , TNF-alpha-mediated adhesion of monocytes to endothelial cells-The role of ephrinA1. J Mol Cell Cardiol, 2014. 77: p. 125–35. [DOI] [PubMed] [Google Scholar]
- 27.Muller MR and Rao A, NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol, 2010. 10(9): p. 645–56. [DOI] [PubMed] [Google Scholar]
- 28.Cockerill GW, et al. , Regulation of granulocyte-macrophage colony-stimulating factor and E-selectin expression in endothelial cells by cyclosporin A and the T-cell transcription factor NFAT. Blood, 1995. 86(7): p. 2689–98. [PubMed] [Google Scholar]
- 29.Armesilla AL, et al. , Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression. Mol Cell Biol, 1999. 19(3): p. 2032–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez GL, et al. , Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med, 2001. 193(5): p. 607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao C, et al. , Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab, 2006. 4(2): p. 111–21. [DOI] [PubMed] [Google Scholar]
- 32.Pasquale EB, Eph-ephrin bidirectional signaling in physiology and disease. Cell, 2008. 133(1): p. 38–52. [DOI] [PubMed] [Google Scholar]
- 33.Frank CA, Pielage J, and Davis GW, A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron, 2009. 61(4): p. 556–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corrigan C, Subramanian R, and Miller MA, Eph and NMDA receptors control Ca2+/calmodulin-dependent protein kinase II activation during C. elegans oocyte meiotic maturation. Development, 2005. 132(23): p. 5225–37. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M, Kamata R, and Sakai R, EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J Biol Chem, 2005. 280(51): p. 42375–82. [DOI] [PubMed] [Google Scholar]
- 36.Larson J, et al. , Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol, 2008. 295(3): p. L431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.