Abstract
By investigating the neurochemical mechanisms through which alcohol activates the brain reward systems, novel treatment strategies for alcohol use disorder (AUD), a chronic relapsing disease, can be developed. In contrast to the common view of the function of gut–brain peptides, such as neuromedin U (NMU), to regulate food intake and appetite, a novel role in reinforcement mediation has been implied. The anorexigenic effects of NMU are mediated via NMU2 receptors, preferably in the arcuate nucleus and paraventricular nucleus. The expression of NMU2 receptors is also expressed in several reward‐related areas in the brain, suggesting a role in reward regulation. The present experiments were therefore set up to investigate the effect of intracerebroventricular administration of NMU on alcohol‐mediated behaviors in rodents. We found that central administration of NMU attenuated alcohol‐induced locomotor stimulation, accumbal dopamine release and the expression of conditioned place preference in mice. In addition, NMU dose dependently decreased alcohol intake in high, but not in low, alcohol‐consuming rats. Central NMU administration did not alter the blood alcohol concentrations nor change the corticosterone levels in rodents. Given that AUD is a major health‐care challenge causing an enormous cost to society and novel treatment strategies are warranted, our data suggest that NMU analogues deserve to be evaluated as novel treatment of AUD in humans.
Keywords: Addiction, appetite regulation, dopamine, food intake, gut–brain axis, obesity, reward
Introduction
Alcohol use disorder (AUD), a heterogeneous, chronic and relapsing brain disorder, affects 5 percent of the population. Given that AUD is one of our societies major public health problems causing mortality and morbidity (Koob & Le Moal 2001) and that the clinical efficacy of the available pharmaceutical agents is limited (Anton et al. 2006), the need for novel treatment strategies is substantial. By investigating the indirect neurochemical mechanisms through which alcohol activates the brain reward systems, specifically the mesolimbic dopamine system, potential therapeutics for AUD can be developed (for a review, see Engel & Jerlhag 2014; Soderpalm, Lof & Ericson 2009). In contrast to the common view of the function of gut–brain peptides to regulate food intake and appetite, a novel role in reinforcement mediation has been suggested (Thiele et al. 2004). Indeed, the endocrine signals ghrelin and glucagon‐like peptide 1 (GLP‐1) have in recent studies been pinpointed as reward regulators (for a review, Engel & Jerlhag 2014). The present series of experiments was designed to evaluate the possibility that neuromedin U (NMU), an anorexigenic peptide produced both in the gastrointestinal tract and in the brain, could modulate alcohol‐mediated behaviors in rodents.
Neuromedin U is the endogenous ligand for two NMU receptors (NMUR1 and NMUR2, respectively) (Mitchell, Maguire & Davenport 2009). In peripheral tissues, NMU predominantly acts via NMUR1 to regulate smooth muscle contraction, increase stress responses, control body temperature and modulate nociceptive reflexes (for a review, Martinez & O'Driscoll 2015). Albeit NMU has been attributed countless of functions, most reports reflect its role in feeding and energy balance. Indeed, central administration of NMU reduces food intake as well as feeding‐associated behaviors in rodents (Egecioglu et al. 2009; Howard et al. 2000; Ida et al. 2005; Kim & Mizuno 2010; Nakahara et al. 2004). In support for a role of NMU in food intake regulation are the data showing that mice overexpressing NMU are hypophagic and lean (Kowalski et al. 2005), NMU knockout mice display elevated food intake a severe obese phenotype (Hanada et al. 2004) and NMU antiserum increases food intake in rats (Kojima et al. 2000). The anorexigenic effects of NMU involve NMUR2 (Peier et al. 2009; Zeng et al. 2006), preferentially those expressed in the arcuate nucleus and paraventricular nucleus (Howard et al. 2000; Ida et al. 2005; Nakahara et al. 2010). The findings that the NMUR2 is expressed in reward areas such as nucleus accumbens (NAc) (Gartlon et al. 2004) and that NMU‐like immunoreactivity within the brain is detected in the NAc (Domin et al. 1987) as well as ventral tegmental area (VTA) (Maderdrut et al. 1996) provide a possibility that NMU may affect reward related behaviors. We therefore initially investigated the effects of central NMU treatment on the rewarding properties of alcohol, as measured by locomotor stimulation, accumbal dopamine release and the expression of conditioned place preference in mice. Thereafter, the ability of central NMU administration, at two different doses, to influence alcohol intake in high as well as low alcohol‐consuming rats was explored. Finally, to exclude the possibility that NMU alters the metabolism of alcohol or stress responses, the effect of central NMU administration on blood alcohol concentrations and serum corticosterone levels was investigated.
Material and Methods
Animals
Adult post‐pubertal age‐matched male NMRI mice (8–12 weeks old and 25–35 g body weight; Charles River, Susfeldt, Germany) were used for the locomotor activity, the in vivo microdialysis, the conditioned place preference and the blood alcohol concentration studies. Mice were used for the present experiments because we have extensive experience with mice and that we previously have obtained a robust locomotor stimulation, conditioned place preference and accumbal dopamine releases in response to alcohol and other addictive drugs in mice (Jerlhag et al. 2009). The mice were group housed and maintained on a 12/12‐hour light/dark cycle. They were kept in rooms at 20°C with 50 percent humidity. Tap water and food (normal chow; Harlan Teklad, Norfolk, England) were supplied ad libitum. In addition, adult post‐pubertal age‐matched male outbred Rcc Han Wistar rats (Harlan, Horst, Netherlands) were used for the intermittent access 20 percent alcohol two‐bottle‐choice drinking paradigm, blood alcohol concentration and corticosterone studies. These rats were selected because they display a voluntary high and stable alcohol intake causing pharmacologically relevant blood alcohol concentrations in this drinking model (Simms et al. 2008). The rats in the intermittent access paradigm were during the entire protocol maintained on a 12‐hour reversed light/dark cycle (lights off at 8 am), whereas the other rats were kept on a 12/12‐hour light/dark cycle. Food and water were available ad libitum. The rats were housed individually in high Macrolon III cages covered with filter tops (Tecniplast, Italy) in rooms at 20°C and 50 percent humidity. All experiments were approved by the Swedish Ethical Committee on Animal Research in Gothenburg. All efforts were made to minimize animal suffering and to reduce the number of animals used. Each experiment used an independent set of animals. All animals were allowed to acclimatize at least 1 week before the start of the experiments.
Drugs
For studies investigating alcohol‐induced activation of the mesolimbic dopamine system in mice, 96 percent alcohol (VWR International AB, Stockholm, Sweden) was diluted in saline (0.9 percent NaCl) to 15 percent vol/vol for intraperitoneal (IP) injections and was administered at a dose of 1.75 g/kg 5 minutes prior to initiation of the experiments. For the intermittent access alcohol two‐bottle‐choice drinking paradigm, alcohol was diluted to a 20 percent vol/vol solution using tap water. NMU (Bionuclear, Bromma, Sweden) was diluted in Ringer solution (NaCl 140 mM, CaCl2 1.2 mM, KCl 3.0 mM and MgCl2 1.0 mM; Merck KGaA, Darmstadt, Germany), and a dose of 1 µg in 1 µl was administrated intracerebroventricularly (ICV). ICV administration of the selected dose of NMU has previously been shown to reduce food intake in rats (Nakahara et al. 2010) and was a dose without any effect per se on locomotor activity in a dose–response study in mice (data not shown). The effect of a lower NMU dose, 0.3 µg in 1 µl, on alcohol intake in rats was also studied. NMU was always administered 20 minutes prior to behavioral test or alcohol injection.
Guide cannula and probe implantation
The rodent was anesthetized with isoflurane (Isofluran Baxter; Univentor 400 Anaesthesia Unit, Univentor Ldt., Zejtun, Malta), placed in a stereotaxic frame (David Kopf Instruments; Tujunga, CA, USA) and kept on a heating pad to prevent hypothermia. Xylocain adrenalin (5 µg/ml; Pfizer Inc; New York, NY, USA) was used as local anesthetics, and carprofen (Rimadyl®, 5 mg/kg IP, Astra Zeneca; Gothenburg, Sweden) was used to relieve pain. The skull bone was exposed, and holes for the guide cannula, probe and anchoring screw were drilled. In order to administer NMU or vehicle solution, guide cannulas (stainless steel, length 10 mm, with an o.d./i.d. of 0.6/0.45 mm) were implanted. The coordinates for the third ventricle relative to bregma in mice were posterior −0.9 mm and lateral ±0.0 mm (Franklin & Paxinos 1997). For rats, the coordinates for third ventricle were posterior −1.3 mm and lateral ±0.0 mm (Paxinos & Watson 1998). The guide cannula was placed 1 mm below the surface of the brain and anchored to the screw and the skull bone with dental cement (DENTALON® plus; Agntho's AB, Lidingö, Sweden). At the time of the experiment, the cannula was extended another 1.1 mm or 3.2 mm ventrally beyond the tip of the guide cannula aiming for drug administration in the third ventricle for mice and rats, respectively. For measurements of extracellular dopamine levels, mice were implanted with a microdialysis probe positioned in NAc. The probe was randomly alternated to either the left or right side of the brain. The coordinates of 1.4 mm anterior to the bregma, ±0.6 mm lateral to the midline and 4.7 mm below the surface of the brain surface were used (Franklin & Paxinos 1997). The mice or rats were kept in individual cages for 4 days until the experiment.
Locomotor activity experiments
Locomotor activity was performed as previously described (Jerlhag et al. 2009). In brief, locomotor activity was registered in eight sound attenuated, ventilated and dim lit locomotor boxes (420 × 420 × 200 mm, Kungsbacka mät‐ och reglerteknik AB, Fjärås, Sweden). Five‐by‐five rows of photocell beams, at the floor level of the box, allowed a computer‐based system to register the activity of the mice. Locomotor activity was defined as the accumulated number of new photocell beams interrupted during a 60‐minute period. In the experiments, the mice were allowed to habituate to the locomotor activity box 1 hour prior to drug challenge.
The first experiment was designed to select a dose of NMU without any effect per se. Following habituation, the mice were challenged with either vehicle or NMU (1, 2 or 8 µg, ICV), and the cumulative activity was recorded.
In the second experiment in separate mice, the effects of NMU (1 µg, ICV) on alcohol‐induced (1.75 g/mg, IP) locomotor stimulation were investigated. NMU was administrated 20 minutes prior to alcohol, and the activity registration started 5 minutes following the alcohol injection. Each mouse received one treatment combination (Veh‐Veh, Veh‐Alc, NMU‐Veh or NMU‐Alc; n = 16 per treatment combination).
In vivo microdialysis and dopamine release measurements
The present microdialysis experiment, in freely moving mice, was design to establish an initial response to alcohol as well as to explore the effect of NMU on alcohol‐induced dopamine release.
On the day of the experiment, the probe was connected to a microperfusion pump (U‐864 Syringe Pump; AgnThós AB) and perfused with Ringer solution at a rate of 1.5 µl/minute. After 1 hour of habituation to the microdialysis setup, perfusion samples were collected every 20 minutes during the entire experimental protocol (from −40 to 240 minutes). The dopamine release was determined as the percent increase from baseline. The baseline dopamine level was defined as the average of three consecutive samples before the first alcohol (1.75 g/kg, IP) or vehicle (saline, IP) challenge (time 0). This initial alcohol challenge was given to establish that the mice respond with an accumbal dopamine release to alcohol compared with vehicle treatment. Seven consecutive 20‐minute samples were collected after this initial challenge. At 140 minutes, NMU (1 µg, ICV) or an equal volume of vehicle (Ringer solution, ICV) was administered. Twenty minutes later, vehicle (saline, IP) or alcohol (1.75 g/kg, IP) was administered (160 minutes). Thereafter, four additional samples were collected (experiment terminated at 240 minutes). Collectively, the following treatment groups (n = 12 in each group) were created: alcohol–vehicle–alcohol (Alc‐Veh‐Alc), alcohol–NMU–alcohol (Alc‐NMU‐Alc), vehicle–NMU–vehicle (Veh‐NMU‐Veh) and alcohol–vehicle–vehicle (Alc‐Veh‐Veh). This design is identical to previous studies (e.g. Egecioglu et al. 2013c; Jerlhag et al. 2009; Vallof et al. 2015).
Dopamine was separated and quantified using two different high‐performance liquid chromatography columns followed by electrochemical detection as described previously (Clarke et al. 2014). In brief, a pump (UltiMate 3000 Pump; Thermo Scientific, Darmstadt, Germany), an ion exchange column (Nucleosil SA, 2.0 × 150 mm, 5 µm diameter, pore size 100 Å; Phenomenex Scandinavia, Västra Frölunda, Sweden) and a detector (Decade, Kovalent AB, Sweden) operated at 400 mV versus the cell were used. The mobile phase was delivered at 0.3 ml/minute and consists of 58 mM citric acid, 135 mM NaOH, 0.107 mM Na2–EDTA and 20 percent methanol. The second system consists of a pump (UltiMate 3000 Pump; Thermo Scientific), a reversed phase column (2.0 × 50 mm, 3 µm diameter; pore size 100 Å; Phenomenex Scandinavia) and a detector (Dionex, Västra Frölunda, Sweden) operated at 220 mV versus the cell. The mobile phase was delivered at 0.3 ml/minute and consists of 150 mM NaH2PO4, 4.76 mM citric acid, 3 mM sodium dodecyl sulfate, 50 μM EDTA, and 10 percent MeOH and 15 percent acetonitrile.
Conditioned place preference
To evaluate the effects of NMU on the rewarding effects of alcohol, conditioned place preference tests were performed in mice as previously described (Jerlhag et al. 2009). In brief, a two‐chambered conditioned place preference apparatus (45 lux) and distinct visual and tactile cues were used. In this apparatus, the mice have no specific side preference for any of the two chambers. In addition, mice with a tendency for an unbalance initial preference were excluded (more than 60 percent of the time spend in one of the compartments). Although this exclusion criterion is strict, no mice were excluded because of this reason. The procedure consisted of preconditioning (day 1), conditioning (days 2–5) and postconditioning (day 6). At preconditioning, mice were placed in the chamber with free access to both compartments during 20 minutes to determine the initial place preference. Conditioning (20 minutes per session) was carried out using a biased procedure in which alcohol (1.75 g/kg, IP) was paired with the least preferred compartment and vehicle with the preferred compartment. The rational for selecting a biased protocol are the findings that nicotine causes a conditioned place preference when a biased, but not unbiased, model is used (Calcagnetti & Schechter 1994). All mice received one alcohol and one vehicle injection every day, and the injections were altered between morning and afternoon in a balanced design. At postconditioning, mice were injected with NMU (1 µg, ICV, n = 13) or an equal volume of vehicle solution (Ringer, n = 13) and, 20 minutes later, placed on the midline between the two compartments with free access to both compartments for 20 minutes (creating the following treatment groups; Alc‐Veh and Alc‐NMU). This design investigates the expression of conditioned place preference in mice, which may reflect human drug craving (Sanchis‐Segura & Spanagel 2006). In addition, in a control experiment for NMU, separate mice were subjected to the same procedure but received vehicle injections instead of alcohol throughout the conditioning (non‐alcohol conditioned control group; creating the following treatment groups; Veh‐Veh and NMU‐Veh, n = 8 per treatment group). Conditioned place preference was calculated as the difference in percent of total time spent in the drug‐paired (i.e. less preferred) compartment during the postconditioning and the preconditioning sessions.
Intermittent access 20 percent alcohol two‐bottle‐choice drinking paradigm
In brief, the rats were given free access to one bottle of 20 percent alcohol and one bottle of water during three 24‐hour sessions per week (Mondays, Wednesdays and Fridays), approximately 10 minutes after the lights went out in a reversed light/dark cycle room (Simms et al. 2008). The rats had unlimited access to two bottles of water between the alcohol access periods. Bottles were weighed at 24 hours after the fluids were presented, and measurements were taken to the nearest 0.1 g. The body weight of each rat was measured daily prior to bottle presentation, to allow for calculating the grams of alcohol intake per kilogram of body weight (g/kg). The preference for alcohol over water (the ratio of alcohol to total fluid intake) was calculated at all time points. In addition, water and food intake was measured.
Effects of central NMU administration on alcohol intake in outbred rats
The effects of central treatment of NMU at a dose of either 0.3 µg or 1 µg on alcohol intake were investigated in outbred rats. The rats (n = 15) voluntarily consumed alcohol for 11 weeks (Simms et al. 2008) and were based on their baseline alcohol intake divided into high and low alcohol‐consuming rats (cutoff was 3.5 g/kg per 24 hours). Thereafter, all rats were subjected to central administration of 0.3 µg NMU, 1 µg NMU or an equal volume of vehicle on an alcohol‐drinking day (Monday, Wednesday or Friday) in a balance design. There was 1 day between each injection (water drinking days, Tuesday and Thursday), and each animal served as its own control. The effect of central NMU administration on alcohol, water and food intake was registered all three treatment days 1, 4 and 24 hours after bottle presentation.
Blood alcohol concentration
Rats and mice were injected with NMU (1 µg, ICV) or an equal volume of vehicle solution (Ringer) (n = 8 per treatment group). Twenty minutes later, all animals were injected with alcohol (1.75 g/kg for mice and 2.5 g/kg for rats, IP). The animals were decapitated 20 minutes later, and trunk blood was collected in microtubes (Vacuette; Greiner Bio‐one, Florence, Italy). The analysis of the blood alcohol concentration from experiment in mice and rats was outsourced to Sahlghrenska University Hospital (Gothenburg, Sweden; study agreement BML‐NEURO) as described previously (Jerlhag et al. 2013).
Serum levels of corticosterone
Rats were injected with NMU (1 µg, ICV) or an equal volume of vehicle solution (Ringer) (n = 8 per treatment group). Twenty minutes later, capillary blood from the tail was collected in microvettes (Sarstedt, Helsingborg, Sweden). The blood was centrifuged (5 minutes, 10 000 g), and corticosterone was thereafter measured in serum with an Enzo Corticosterone Eliza kit (AH Diagnostic, Stockholm, Sweden).
Verification of probe and guide cannulas placement
Following each experiment, the location of the probe and/or guide cannulas were verified. The rodents were decapitated, and the brains were mounted on a vibroslice device (752 M Vibroslice; Campden Instruments Ltd., Loughborough, UK). The brains were cut in 50 µm sections, and the location was determined (Franklin & Paxinos 1997; Paxinos & Watson 1998) by observation using light microscopy. Only rodents with correct placement of the probe and/or guide cannula were used in the statistical analysis.
Statistical analysis
The locomotor activity and conditioned place preference experiments were evaluated by a one‐way ANOVA followed by Bonferroni post hoc test for comparisons between different treatments. The microdialysis experiments were evaluated by a two‐way ANOVA followed by Bonferroni post hoc test for comparisons between different treatments and specifically at given time points. The blood alcohol concentration and corticosterone data were evaluated by an unpaired t‐test. The effects of NMU treatment on alcohol intake in the intermittent access 20 percent alcohol two‐bottle‐choice drinking paradigm were evaluated by a two‐way ANOVA followed by Newman–Keuls multiple comparison test. Data are presented as mean ± standard error of the mean. A probability value of P < 0.05 was considered as statistically significant.
Results
Effects of NMU on alcohol‐induced locomotor stimulation, accumbal dopamine release and the expression of conditioned place preference in mice
An overall main effect of treatment was found on locomotor activity in mice following systemic administration of alcohol (1.75 g/kg) and local injection of NMU (1 µg) (F(3, 55) = 4.52, P = 0.0067; n = 12 for Veh‐Veh, n = 15 for Veh‐Alc and n = 16 for NMU‐Veh as well as NMU‐Alc). As shown in Fig. 1a, post hoc analysis revealed that alcohol‐induced locomotor stimulation (P < 0.05, Veh‐Alc versus Veh‐Veh) was significantly reduced by pre‐treatment with a single injection of NMU (P < 0.05, Veh‐Alc versus NMU‐Alc). NMU had no effect per se on locomotor activity (P > 0.05, Veh‐Veh versus NMU‐Veh). There was no difference in locomotor activity response in vehicle‐treated mice and NMU–alcohol‐treated mice (P > 0.05).
Figure 1.
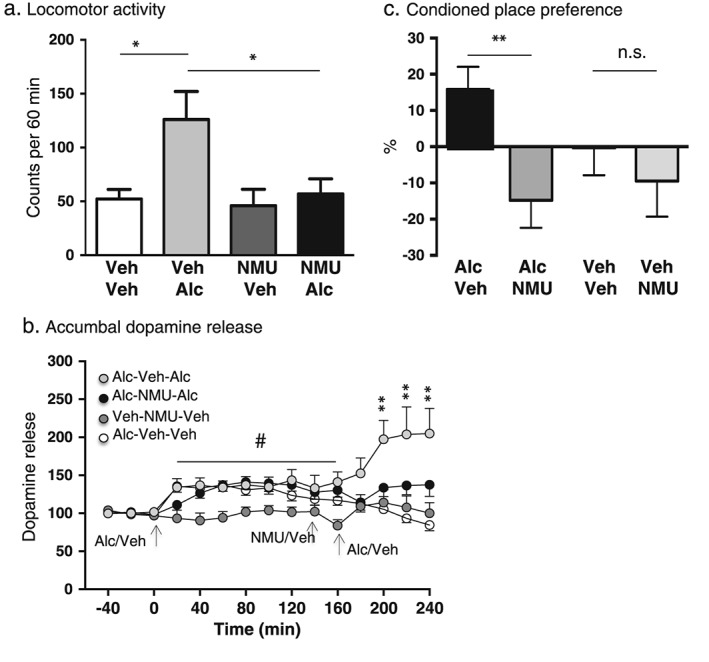
Central administration of NMU attenuates alcohol‐induced locomotor stimulation, accumbal dopamine release and expression of conditioned place preference in mice. (a) Alcohol‐induced (1.75 g/kg, IP) locomotor stimulation was attenuated by a single injection of NMU (1 µg ICV), at a dose with no effect per se (*P < 0.05, one‐way ANOVA followed by a Bonferroni post hoc test). (b) Initial injections of alcohol (1.75 g/kg, IP) caused a significant increase in accumbal dopamine release compared with vehicle treatment in all three alcohol‐treated groups (indicated by # in Fig. 1b). The subsequent part of the experiment showed that NMU (1 µg ICV at 160 minutes, a dose with no effect per se) 20 minutes prior to the second alcohol injection (at 180 minutes) significantly attenuated the alcohol‐induced accumbal dopamine release (Alc‐NMU‐Alc, black circle) compared with vehicle pre‐treatment (Alc‐Veh‐Alc, light gray circle) (P < 0.01). There was no effect per se of NMU treatment (Veh‐NMU‐Veh, dark gray circle) compared with vehicle treatment (Alc‐Veh‐Veh, white circle). (c) Central administration of NMU (1 µg ICV) (Alc‐NMU) attenuated the alcohol‐induced (1.75 g/kg) (Alc‐Veh) expression of conditioned place preference. NMU (Veh‐NMU) had no effect per se compared with vehicle treatment (Veh‐Veh). Data are presented as mean ± standard error of the mean (*P < 0.05, **P < 0.01)
Accumbal microdialysis measurements of dopamine in mice revealed an overall main effect of treatment (F(3, 405) = 38.25, P < 0.0001), time (F(14, 135) = 4.097, P < 0.0001) and a significant interaction of treatment × time (F(42, 405) = 2.985, P < 0.0001). In the first part of the experiment, the responsiveness to alcohol (1.75 g/kg) per se was investigated (alcohol injection at time point 0 minutes). This initial injection of alcohol caused a significant increase in accumbal dopamine release compared with vehicle treatment (Veh‐NMU‐Veh) in all three groups that received alcohol (Alc‐Veh‐Alc, Alc‐NMU‐Alc and Alc‐Veh‐Veh). Specifically, in the Alc‐Veh‐Alc group, alcohol significantly increased accumbal dopamine at time point 20–60 (P < 0.05), 120 (P < 0.05) and 160 minutes (P < 0.01). Moreover, alcohol increased dopamine in NAc at time point 60 (P < 0.05) and 160 minutes (P < 0.01) in the Alc‐NMU‐Alc group. In addition, alcohol increased accumbal dopamine at time point 20–60 (P < 0.05) in the Alc‐Veh‐Veh group (Fig. 1b). The subsequent part of the experiment aimed at investigating the ability of NMU to affect alcohol‐induced dopamine release as well as to study the effect of NMU per se on accumbal dopamine release. Administration of NMU (1 µg ICV at 160 minutes) 20 minutes prior to the second alcohol injection (1.75 g/kg, at 180 minutes) significantly attenuated the alcohol‐induced accumbal dopamine release (Alc‐NMU‐Alc) compared with vehicle pre‐treatment (Alc‐Veh‐Alc) at time point 220–260 (P < 0.01). The analysis also showed that the second alcohol injection significantly increased accumbal dopamine release (Alc‐Veh‐Alc) compared with vehicle treatment (Alc‐Veh‐Veh) at time point 220–260 (P < 0.001). There was no difference in dopamine response in mice treated with NMU and a second alcohol injection (Alc‐NMU‐Alc) compared with vehicle treatment (Alc‐Veh‐Veh) 200–260 (P > 0.05). There was no effect per se of NMU administration (Alc‐Veh‐Veh compared with Alc‐NMU‐Veh) 200–260 (P > 0.05) (Fig. 1b) (n = 10 in each group).
An overall main effect of treatment was found on conditioned place preference in mice following systemic administration of alcohol (1.75 g/kg) and local injection of NMU (1 µg) (F(3, 34) = 3.76, P = 0.0197; n = 13 for Alc‐Veh, n = 11 for Alc‐NMU and n = 7 for Veh‐Veh as well as Veh‐NMU). As shown in Fig. 1c, post hoc analysis revealed that NMU attenuates the alcohol‐induced conditioned place preference (P < 0.01, Alc‐Veh versus Alc‐NMU). In addition, NMU had no effect per se (P > 0.05, Veh‐Veh versus Veh‐NMU).
Effects of central NMU administration on alcohol intake in high alcohol‐consuming rats
The effect of NMU (0.3 µg, 1 µg, ICV) or an equal volume of vehicle (Ringer, ICV) on voluntary alcohol intake was evaluated in high alcohol‐consuming rats (cutoff was >3.5 g/kg per 24 hours, n = 8). There was a significant overall effect of treatment on alcohol intake at 24 hours (F(2, 14) = 4.684, P = 0.0277) (Fig. 2a). Post hoc test revealed that NMU treatment, at a dose of 1 µg (P < 0.05) as well as of 0.3 µg (P < 0.05), significantly decreased alcohol intake at 24‐hour time point compared with vehicle treatment. There was a significant overall effect of treatment on alcohol intake at 4‐hour time points (F(2, 14) = 4.893, P = 0.0245) (Fig. 2b). Post hoc analysis showed that NMU (1 µg) significantly decreased alcohol intake compared with vehicle treatment (P < 0.05). There was a tendency of an overall effect of treatment on alcohol intake at 1‐hour time point (F(2, 14) = 2.988, P = 0.0830) (Fig. 2c). No overall effect of treatment was observed on water intake at 24‐hour (F(2, 14) = 0.2831, P = 0.7576) (Fig. 2d), 4‐hour (F(2, 14) = 0.5244, P = 0.6031) (Fig. 2e) nor at 1‐hour (F(2, 14) = 0.1680, P = 0.8470) (Fig. 2f) time points. There was no overall effect of treatment on total fluid intake following NMU treatment at 24‐hour (F(2, 14) = 2.249, P = 0.1422) (Fig. 2g), 4‐hour (F(2, 14) = 0.7155, P = 0.5060) (Fig. 2h) nor at 1‐hour (F(2, 14) = 0.9071, P = 0.4261) (Fig. 2i) time points. There was a significant overall effect of treatment on food intake at 24‐hour time point (F(2, 14) = 5.543, P = 0.0169) (Fig. 2j). Post hoc test showed that NMU (0.3 µg) treatment significantly decreased food intake at 24‐hour time point compared with vehicle treatment (P < 0.05) as well as compared with NMU (1 µg) treatment (P < 0.05). There was a significant overall effect of treatment on food intake at 4‐hour time point (F(2, 14) = 6.777, P = 0.0087) (Fig. 2k). Post hoc test revealed that the food intake was lower in mice treated with NMU (0.3 µg) compared with that in mice treated with NMU (1 µg) (P < 0.05). There was no overall effect of treatment on food intake at 1‐hour time point (F(2, 14) = 2.188, P = 0.1490) (Fig. 2l). No overall main effect on body weight of the rats was found following NMU treatment (F(2, 14) = 2.867, P = 0.0904) (vehicle: 440 ± 13 g, NMU (0.3 µg): 448 ± 12 g, NMU (1 µg): 446 ± 12 g).
Figure 2.
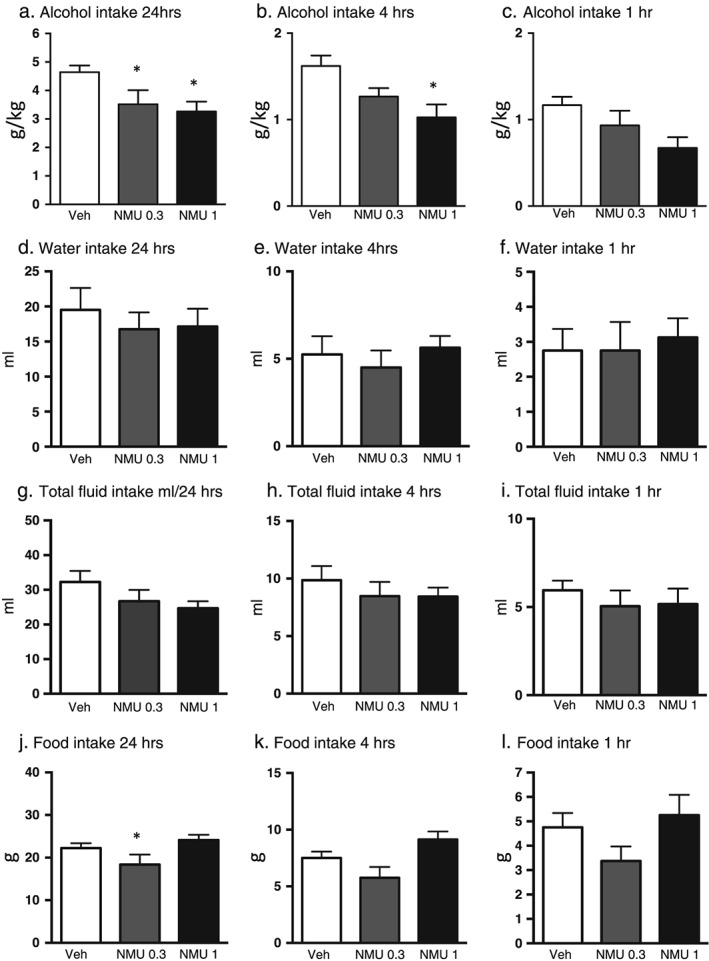
Central administration of NMU decreases alcohol intake in high alcohol‐consuming outbred rats. (a) Central administration of NMU (1 µg ICV) reduced alcohol intake (g/kg) in outbred rats at time points (a) 24 hours and (b) 4 hours. (c) There was a tendency at 1‐hour time points. NMU had no effect on water intake (ml) at 24‐hour (d), 4‐hour (e) or 1‐hour (f) time point. NMU did not affect total fluid (ml) intake at any time point (g, h and i). Central infusion of NMU reduced food intake (g) at 24‐hour time point (j) but not at 4‐hour (k) or 1‐hour (l) time point. All values represent mean ± standard error of the mean (*P < 0.05)
There was no overall effect on water consumption following termination of treatment (F(2, 14) = 0.2114, P = 0.8120), (vehicle: 34 ± 6 ml, NMU (0.3 µg): 34 ± 5 ml, NMU (1 µg): 31 ± 3 ml).
Effects of central NMU administration on alcohol intake in low alcohol‐consuming rats
The effect of NMU (0.3 µg, 1 µg, ICV) or an equal volume of vehicle (Ringer, ICV) on voluntary alcohol intake was evaluated in the low alcohol‐consuming rats (cutoff was <3.5 g/kg per 24 hours, n = 6). No overall effect of treatment was observed on alcohol intake (gram per kilogram) at 24‐hour (F(2, 10) = 2.637, P = 0.1203, Fig. 3a), 4‐hour (F(2, 10) = 0.6568, P = 0.5395, Fig. 3b) nor at 1‐hour (F(2, 10) = 0.0708, P = 0.9321, Fig. 3c) time points. There was no overall effect of treatment on water intake at 24‐hour (F(2, 10) = 1.646, P = 0.2410, Fig. 3d), 4‐hour (F(2, 10) = 0.3858, P = 0.6896, Fig. 3e) nor at 1‐hour (F(2, 10) = 0.1765, P = 0.8408, Fig. 3f) time points. No overall effect of treatment was observed on total fluid intake at 24‐hour (F(2, 10) = 1.698, P = 0.2319, Fig. 3g), 4‐hour (F(2, 10) = 0.5662, P = 0.5849, Fig. 3h) nor at 1‐hour (F(2, 10) = 0.4417, P = 0.6549, Fig. 3i) time points. There was no overall effect of treatment on the food intake at 24‐hour (F(2, 10) = 0.7421, P = 0.5006, Fig. 3j), 4‐hour (F(2, 10) = 2.713, P = 0.1145, Fig. 3k) nor at 1‐hour (F(2, 10) = 0.3670, P = 0.7018, Fig. 3l) time points. There was no overall main effect of treatment on body weight (F(2, 10) = 0.4146, P = 0.6715; vehicle: 433 ± 17 g, NMU (0.3 µg): 431 ± 18 g, NMU (1 µg):433 ± 19 g).
Figure 3.
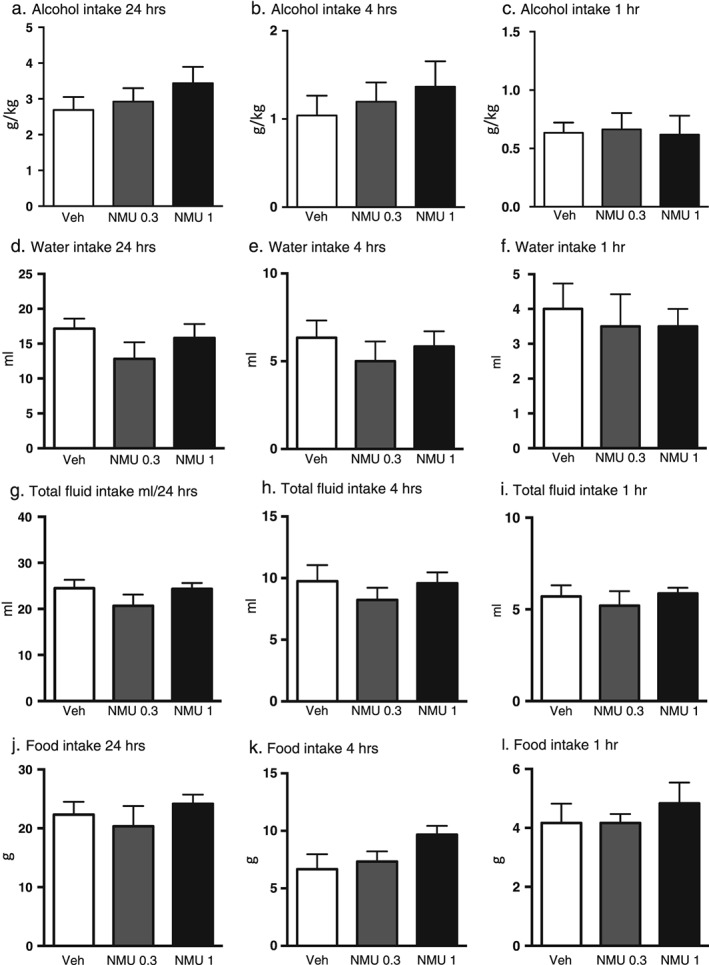
Central administration of NMU does not affect alcohol intake in low alcohol‐consuming outbred rats. (a) Central administration of NMU (1 µg ICV) does not affect alcohol intake (g/kg) in outbred rats at any time points (a) 24 hours, (b) 4 hours or 1 hour (c). NMU had no effect on water intake (ml) at 24‐hour (d), 4‐hour (e) or 1‐hour (f) time point. NMU did not affect total fluid (ml) intake at any time point (g, h and i). Central infusion of NMU did not alter food intake (g) at 24‐hour (j), 4‐hour (k) or 1‐hour (l) time point. All values represent mean ± standard error of the mean (*P < 0.05)
There was no overall effect on water consumption following termination of treatment (F(2, 10) = 0.3259, P = 0.7293), (vehicle: 28 ± 4 ml, NMU (0.3 µg): 27 ± 4 ml, NMU (1 µg): 31 ± 3 ml).
Effects of central NMU administration on blood alcohol concentration in mice and rats
Central administration of NMU (1 µg, ICV, n = 7) did not alter the blood alcohol concentration induced by an injection of alcohol (1.75 g/kg, IP) in mice compared with vehicle (n = 8) treatment (P = 0.3899) (Fig. 4a). Central administration of NMU (1 µg, ICV, n = 7) did not alter the blood alcohol concentration induced by an injection of alcohol (2.5 g/kg, IP) in rats compared with vehicle (n = 7) treatment (P = 0.3043) (Fig. 4b).
Figure 4.
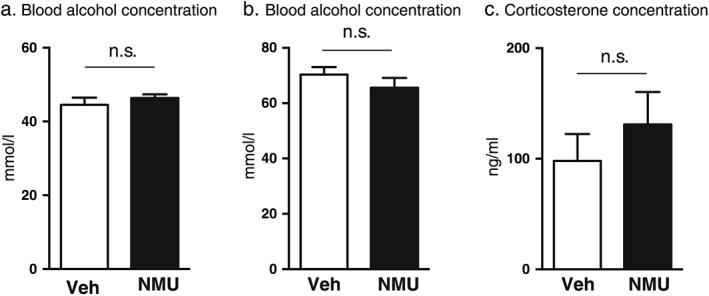
Central administration of NMU does not affect the blood alcohol concentration or the corticosterone levels in rodents. Central administration of NMU (1 µg ICV) did not affect the blood alcohol concentrations compared with vehicle treatment in mice (a) or in rats (b). Central administration of NMU (1 µg ICV) did not affect the corticosterone levels in rats compared with vehicle treatment (c)
Effects of central NMU administration on serum levels of corticosterone in rats
Central administration of NMU (1 µg, ICV, n = 7) did not alter the serum levels of corticosterone in rats compared with vehicle (n = 8) treatment (P = 0.3990) (Fig. 4c).
Discussion
The present study provides the first evidence that the anorexigenic peptide NMU, via central mechanisms, regulates alcohol‐mediated behaviors in rodents. Firstly, we showed that ICV administration of NMU, at doses with no effect per se on reward‐related parameters or gross behavior, blocked the well‐documented effects of alcohol on the mesolimbic dopamine system (Sanchis‐Segura & Spanagel 2006), namely, locomotor stimulation, accumbal dopamine release and expression of conditioned place preference in mice. Secondly, we showed that central NMU infusion dose dependently reduces alcohol intake in high alcohol‐consuming rats at both 24‐ and 4‐hour time points in the intermittent access model. Given that this alcohol two‐bottle‐choice drinking paradigm induces voluntary intake of high amounts of alcohol as well as pharmacologically relevant blood alcohol concentrations (Simms et al. 2008), the present data may suggest that NMU could be used as a pharmacological agent to treat AUD in humans. Thirdly, we showed that central NMU administration did not alter the blood alcohol concentrations in mice or in rats, indicating that NMU alters reward induced by alcohol rather than metabolism. In support for a modulatory role of NMU in alcohol reinforcement are the findings from a genome‐wide allelic association study showing that polymorphisms in the NMUR2 gene are associated with AUD in humans (Lydall et al. 2011). The findings that mice with a conditional knockdown of paraventricular NMUR2 display a hyperphagic phenotype, increased preference for high fat foods and binge eating feeding behavior when fed a high fat diet (Benzon et al. 2014) further support a role for NMU in reward processes. In contrast to the common view of endocrine signals as regulators of food intake, the present findings contribute to the contention that gut–brain peptides signals constitute additional mechanisms for reward regulation (for a review, see Engel & Jerlhag 2014). Indeed, the hunger hormones ghrelin, orexin and galanin regulate various alcohol‐mediated behaviors as well as drug reinforcement in rodents (Borgland et al. 2006; Engel & Jerlhag 2014; Lewis et al. 2004). In addition, animal studies show that the anorexic peptides cholecystokinin and leptin reduce alcohol consumption (Blednov, Walker & Harris 2004; Kulkosky 1984). Furthermore, it was recently shown that peripheral administration of analogues of the anorexic peptide GLP‐1 attenuates reward‐related behaviors (Egecioglu, Engel & Jerlhag 2013a,2013b; Egecioglu et al. 2013c; Erreger et al. 2012; Graham et al. 2013; Suchankova et al. 2015).
In the present study, we showed that central NMU administration dose dependently reduces alcohol intake in high, but not low, alcohol‐consuming rats. Similar findings have been shown for other pharmacological agents of interest for treatment of AUD, where a ghrelin receptor (GHS‐R1A) antagonist, a partial nicotinic acetylcholine agonist and a glycine transporter 1 inhibitor have been found to reduce alcohol intake in high, but not low, alcohol‐consuming rats (Molander et al. 2007; Steensland et al. 2007; Suchankova et al. 2013). Collectively, this suggests that there is a difference in sensitivity to alcohol between high and low alcohol‐consuming rats. In addition, different neurobiological mechanisms in reward‐related areas might underlie high and low alcohol intake in rats. This is further substantiated by the findings showing that the expression of ventral tegmental GHS‐R1A is downregulated in high compared with low alcohol‐comparing rats (Suchankova et al. 2013). The possibility that the expression of NMUR2 in reward‐related areas is different between low and high alcohol‐consuming rats should be explored in upcoming studies.
Even though the present study provides compelling evidence for a role of NMU in regulating alcohol reward, the areas involved in NMU‐mediated attenuation of alcohol‐induced locomotor stimulation, accumbal dopamine release, conditioned place preference and alcohol intake in rodents need to be further elucidated. Given that the expression of NMUR2 has been identified in the NAc (Gartlon et al. 2004) and that NMU‐like fibers are detected in the NAc as well as the VTA (Domin et al. 1987; Maderdrut et al. 1996), we suggest that NMU may regulate alcohol‐mediated behaviors via NMUR2 in reward‐related areas such as the VTA and/or NAc. Although this needs to be explored in detail in upcoming experiments, previous studies have reported that other gut–brain peptides regulate reinforcement directly via the mesolimbic dopamine system (for a review, Engel & Jerlhag 2014). Indeed, local administration of the GHS‐R1A antagonists into the VTA attenuates ghrelin‐induced reward as well as ghrelin‐mediated sucrose intake in rodents (for a review, Engel & Jerlhag 2014). This is further substantiated by the findings demonstrating that local VTA infusion of a GLP‐1 analogue decreases alcohol intake in rats (Shirazi, Dickson & Skibicka 2013). The possibility that NMU modulates alcohol reinforcement via NMUR2 in other areas, including hypothalamus, should also be considered because the anorexigenic properties of NMU involve arcuate nucleus and paraventricular nucleus (Egecioglu et al. 2009; Hanada et al. 2004; Howard et al. 2000; Ida et al. 2005; Kowalski et al. 2005; Nakahara et al. 2004).
A tentative explanation for the obtained results might be that NMU induces aversion rather than attenuates reward. The selected doses of NMU had no effect on water or total fluid intake or on conditioned place preference per se, suggesting that the reduced alcohol intake is not driven by aversion to drug treatment. Supportively, central NMU administration did not reduce water intake following discontinuation of drug treatment or alcohol intake in low alcohol‐consuming rats. In the present study, we showed that central NMU administration did not alter the blood alcohol concentrations in mice and rats, excluding the possibility that differences in alcohol metabolism influence the obtained results. On the other hand, the findings that intermittent access model induces a post‐dependent stressful state, which is known to increase corticosterone levels in rodents, raise the possibility that NMU attenuates alcohol reinforcement via reduction of corticosterone. The findings that corticosterone increases anxiety‐like behavior whereas low doses of NMU reduce anxiety‐like behavior in rodents (Mitra & Sapolsky 2008; Telegdy & Adamik 2013) indicate that anxiolytic effects may influence the obtained data. In addition, a biased model of conditioned place preference may capture the anxiolytic effects of alcohol. However, the ability of NMU to reduce alcohol reinforcement does not appear to involve stress responses or anxiolytic‐like behavior, because we here show that central NMU administration does not reduce the corticosterone levels in rats. Furthermore, others have reported that the selected dose of NMU does not alter anxiolytic‐like behavior in mice (Telegdy & Adamik 2013). In support for this contention are the data showing that GHS‐R1A antagonist consistently reduces drug reinforcement in rodents but depending on the experimental setup could either increase or decrease stress and anxiety‐like behavior (Skibicka & Dickson 2013).
Pharmacological and genetic studies collectively show that NMU reduces food intake and that this involves NMUR2 in the arcuate nucleus and paraventricular nucleus (Egecioglu et al. 2009; Hanada et al. 2004; Howard et al. 2000; Ida et al. 2005; Kowalski et al. 2005; Nakahara et al. 2004). Supportively, we herein report that central administration of a low‐dose NMU reduces food intake in high, but not low, alcohol‐consuming rats. A recent study showed that rats with a conditional knockdown of paraventricular NMUR2 display increased preference for high fat foods when fed a high fat diet, in contrast to standard chow (Benzon et al. 2014). Collectively, these data may suggest that the anorexigenic effects of NMU are more pronounced in rodents that have been exposed to a diet that can be considered reinforcing. Given that alcohol contains calories, the possibility should be considered that the ability of NMU to attenuate alcohol‐mediated behaviors is due to reduced intake of calories rather than attenuated reward. Therefore, the effect of NMU on drug‐induced reward, intake of sucrose and saccharine should be investigated in upcoming studies.
Collectively, the present study reports that NMU attenuates several alcohol‐related behaviors including locomotor stimulation, accumbal dopamine release, expression of conditioned place preference and alcohol intake in rodents. AUD is a major health‐care challenge, an enormous cost to society, and novel treatment strategies are warranted. Given that models reflect different aspects of AUD in humans, our data suggest that centrally acting NMU analogues deserve to be evaluated as novel treatment of AUD in humans.
Acknowledgements
Britt‐Mari Larsson and Kenn Johannessen are gratefully acknowledged for expert and valuable technical assistance. The study is supported by grants from the Swedish Research Council (2009‐2782 and 2011‐4646), Swedish Society for Medical Research, The Swedish Brain Foundation, LUA/ALF (grant no. 148251) from the Sahlgrenska University Hospital, Torsten Söderberg, Alcohol Research Council of the Swedish Alcohol Retailing Monopoly and the Foundations of Adlerbertska, Fredrik and Ingrid Thuring, Tore Nilsson, Längmanska, Wilhelm and Martina Lundgren, Knut and Alice Wallenberg, Magnus Bergvall, Anérs, Jeansons, Åke Wiberg, NovoNordisk, Gothenburg Psychiatry Research Foundation and the Swedish Society of Medicine. The funding sources had no role in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the article for publication.
Conflict of Interest
EJ has received financial support from the NovoNordisk Foundation. This does not alter the authors' adherence to any of the journals policies on sharing data and materials. The remaining authors declare no conflict of interest.
Authors Contribution
JAE contributed to the conception and interpretation and wrote the manuscript; DV designed and performed the hands‐on work, analyzed data and wrote the manuscript; LU performed hands‐on work; EE revised the content and contributed to the conception; and EJ designed the study, contributed to the conception and interpretation, managed literature search, analyzed and undertook statistical analysis and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Vallöf, D. , Ulenius, L. , Egecioglu, E. , Engel, J. A. , and Jerlhag, E. (2017) Central administration of the anorexigenic peptide neuromedin U decreases alcohol intake and attenuates alcohol‐induced reward in rodents. Addiction Biology, 22: 640–651. doi: 10.1111/adb.12355.
References
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–2017. [DOI] [PubMed] [Google Scholar]
- Benzon CR, Johnson SB, McCue DL, Li D, Green TA, Hommel JD (2014) Neuromedin U receptor 2 knockdown in the paraventricular nucleus modifies behavioral responses to obesogenic high‐fat food and leads to increased body weight. Neuroscience 258:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Harris RA (2004) Blockade of the leptin‐sensitive pathway markedly reduces alcohol consumption in mice. Alcohol Clin Exp Res 28:1683–1692. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A (2006) Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49:589–601. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD (1994) Nicotine place preference using the biased method of conditioning. Prog Neuropsychopharmacol Biol Psychiatry 18:925–933. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Adermark L, Chau P, Soderpalm B, Ericson M (2014) Increase in nucleus accumbens dopamine levels following local ethanol administration is not mediated by acetaldehyde. Alcohol and Alcoholism 49:498–504. [DOI] [PubMed] [Google Scholar]
- Domin J, Ghatei MA, Chohan P, Bloom SR (1987) Neuromedin U—a study of its distribution in the rat. Peptides 8:779–784. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E (2013a) The glucagon‐like peptide 1 analogue Exendin‐4 attenuates the nicotine‐induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One 8, e77284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E (2013b) The glucagon‐like peptide 1 analogue, Exendin‐4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One 8, e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Ploj K, Xu X, Bjursell M, Salome N, Andersson N, Ohlsson C, Taube M, Hansson C, Bohlooly YM, Morgan DG, Dickson SL (2009) Central NMU signaling in body weight and energy balance regulation: evidence from NMUR2 deletion and chronic central NMU treatment in mice. Am J Physiol Endocrinol Metab 297:E708–716. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E (2013c) The glucagon‐like peptide 1 analogue Exendin‐4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 38:1259–1270. [DOI] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E (2014) Role of Gut–Brain Hormones in the Pathophysiology of Alcoholism: Implications for Pharmacotherapy. CNS Drugs: Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A (2012) Exendin‐4 decreases amphetamine‐induced locomotor activity. Physiol Behav 106:574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (1997) The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press. [Google Scholar]
- Gartlon J, Szekeres P, Pullen M, Sarau HM, Aiyar N, Shabon U, Michalovich D, Steplewski K, Ellis C, Elshourbagy N, Duxon M, Ashmeade TE, Harrison DC, Murdock P, Wilson S, Ennaceur A, Atkins A, Heidbreder C, Hagan JJ, Hunter AJ, Jones DN (2004) Localisation of NMU1R and NMU2R in human and rat central nervous system and effects of neuromedin‐U following central administration in rats. Psychopharmacology (Berl) 177:1–14. [DOI] [PubMed] [Google Scholar]
- Graham DL, Erreger K, Galli A, Stanwood GD (2013) GLP‐1 analog attenuates cocaine reward. Mol Psychiatry 18:961–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada R, Teranishi H, Pearson JT, Kurokawa M, Hosoda H, Fukushima N, Fukue Y, Serino R, Fujihara H, Ueta Y, Ikawa M, Okabe M, Murakami N, Shirai M, Yoshimatsu H, Kangawa K, Kojima M (2004) Neuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nat Med 10:1067–1073. [DOI] [PubMed] [Google Scholar]
- Howard AD, Wang R, Pong SS, Mellin TN, Strack A, Guan XM, Zeng Z, Williams DL Jr, Feighner SD, Nunes CN, Murphy B, Stair JN, Yu H, Jiang Q, Clements MK, Tan CP, McKee KK, Hreniuk DL, McDonald TP, Lynch KR, Evans JF, Austin CP, Caskey CT, Van der Ploeg LH, Liu Q (2000) Identification of receptors for neuromedin U and its role in feeding. Nature 406:70–74. [DOI] [PubMed] [Google Scholar]
- Ida T, Mori K, Miyazato M, Egi Y, Abe S, Nakahara K, Nishihara M, Kangawa K, Murakami N (2005) Neuromedin S is a novel anorexigenic hormone. Endocrinology 146:4217–4223. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA (2009) Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A 106:11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag, E , Ivanoff, L , Vater, A , Engel, JA , 2013. Peripherally circulating ghrelin does not mediate alcohol‐induced reward and alcohol intake in rodents. ACER, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ER, Mizuno TM (2010) Role of neurotensin receptor 1 in the regulation of food intake by neuromedins and neuromedin‐related peptides. Neurosci Lett 468:64–67. [DOI] [PubMed] [Google Scholar]
- Kojima M, Haruno R, Nakazato M, Date Y, Murakami N, Hanada R, Matsuo H, Kangawa K (2000) Purification and identification of neuromedin U as an endogenous ligand for an orphan receptor GPR66 (FM3). Biochem Biophys Res Commun 276:435–438. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 24:97–129. [DOI] [PubMed] [Google Scholar]
- Kowalski TJ, Spar BD, Markowitz L, Maguire M, Golovko A, Yang S, Farley C, Cook JA, Tetzloff G, Hoos L, Del Vecchio RA, Kazdoba TM, McCool MF, Hwa JJ, Hyde LA, Davis H, Vassileva G, Hedrick JA, Gustafson EL (2005) Transgenic overexpression of neuromedin U promotes leanness and hypophagia in mice. J Endocrinol 185:151–164. [DOI] [PubMed] [Google Scholar]
- Kulkosky PJ (1984) Effect of cholecystokinin octapeptide on ethanol intake in the rat. Alcohol 1:125–128. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Johnson DF, Waldman D, Leibowitz SF, Hoebel BG (2004) Galanin microinjection in the third ventricle increases voluntary ethanol intake. Alcohol Clin Exp Res 28:1822–1828. [DOI] [PubMed] [Google Scholar]
- Lydall GJ, Bass NJ, McQuillin A, Lawrence J, Anjorin A, Kandaswamy R, Pereira A, Guerrini I, Curtis D, Vine AE, Sklar P, Purcell SM, Gurling HM (2011) Confirmation of prior evidence of genetic susceptibility to alcoholism in a genome‐wide association study of comorbid alcoholism and bipolar disorder. Psychiatr Genet 21:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderdrut JL, Lazar G, Kozicz T, Merchenthaler I (1996) Distribution of neuromedin U‐like immunoreactivity in the central nervous system of Rana esculenta. J Comp Neurol 369:438–450. [DOI] [PubMed] [Google Scholar]
- Martinez VG, O'Driscoll L (2015) Neuromedin U: a multifunctional neuropeptide with pleiotropic roles. Clin Chem 61:471–482. [DOI] [PubMed] [Google Scholar]
- Mitchell JD, Maguire JJ, Davenport AP (2009) Emerging pharmacology and physiology of neuromedin U and the structurally related peptide neuromedin S. Br J Pharmacol 158:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM (2008) Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A 105:5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander A, Lido HH, Lof E, Ericson M, Soderpalm B (2007) The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male Wistar rats. Alcohol and Alcoholism 42:11–18. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hanada R, Murakami N, Teranishi H, Ohgusu H, Fukushima N, Moriyama M, Ida T, Kangawa K, Kojima M (2004) The gut–brain peptide neuromedin U is involved in the mammalian circadian oscillator system. Biochem Biophys Res Commun 318:156–161. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Katayama T, Maruyama K, Ida T, Mori K, Miyazato M, Kangawa K, Murakami N (2010) Comparison of feeding suppression by the anorexigenic hormones neuromedin U and neuromedin S in rats. J Endocrinol 207:185–193. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The Brain Stereotaxic Coordinates. New York: Academic Press. [Google Scholar]
- Peier A, Kosinski J, Cox‐York K, Qian Y, Desai K, Feng Y, Trivedi P, Hastings N, Marsh DJ (2009) The antiobesity effects of centrally administered neuromedin U and neuromedin S are mediated predominantly by the neuromedin U receptor 2 (NMUR2). Endocrinology 150:3101–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis‐Segura C, Spanagel R (2006) Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol 11:2–38. [DOI] [PubMed] [Google Scholar]
- Shirazi RH, Dickson SL, Skibicka KP (2013) Gut peptide GLP‐1 and its analogue, Exendin‐4, decrease alcohol intake and reward. PLoS One 8, e61965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long‐Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Dickson SL (2013) Enteroendocrine hormones—central effects on behavior. Curr Opin Pharmacol 13:977–982. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Lof E, Ericson M (2009) Mechanistic studies of ethanol's interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry 42:S87–94. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE (2007) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A 104:12518–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Steensland P, Fredriksson I, Engel JA, Jerlhag E (2013) Ghrelin receptor (GHS‐R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long‐term voluntary alcohol consumption. PLoS One 8, e71284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R, Jerlhag E, Engel JA, Hodgkinson CA, Egli M, Lopez MF, Becker HC, Goldman D, Heilig M, Ramchandani VA, Leggio L (2015) The glucagon‐like peptide‐1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Translational Psychiatry 5:e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telegdy G, Adamik A (2013) Anxiolytic action of neuromedin‐U and neurotransmitters involved in mice. Regul Pept 186:137–140. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Stewart RB, Badia‐Elder NE, Geary N, Massi M, Leibowitz SF, Hoebel BG, Egli M (2004) Overlapping peptide control of alcohol self‐administration and feeding. Alcohol Clin Exp Res 28:288–294. [DOI] [PubMed] [Google Scholar]
- Vallof D, Maccioni P, Colombo G, Mandrapa M, Jornulf JW, Egecioglu E, Engel JA, Jerlhag E (2015) The glucagon‐like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addiction Biology. doi: 10.1111/adb.12295. [e‐pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Gragerov A, Hohmann JG, Pavlova MN, Schimpf BA, Xu H, Wu LJ, Toyoda H, Zhao MG, Rohde AD, Gragerova G, Onrust R, Bergmann JE, Zhuo M, Gaitanaris GA (2006) Neuromedin U receptor 2‐deficient mice display differential responses in sensory perception, stress, and feeding. Mol Cell Biol 26:9352–9363. [DOI] [PMC free article] [PubMed] [Google Scholar]


