Abstract
Objective
Rheumatoid arthritis (RA) is characterized by the presence of autoantibodies, including seropositivity for rheumatoid factor (RF) and anti–citrullinated protein antibodies (ACPAs). In addition, antibodies to carbamylated proteins (anti‐CarP) are present in patients with RA and are associated with joint damage. This study was undertaken to assess the presence of anti‐CarP antibodies in indigenous North Americans (First Nations [FN] populations) with RA compared to their at‐risk first‐degree relatives (FDRs) and healthy controls.
Methods
Anti‐CarP IgG and ACPAs (specifically, anti–cyclic citrullinated peptide [anti‐CCP] antibodies) were measured by enzyme‐linked immunosorbent assay in the sera of FN patients with RA (n = 95), their unaffected FDRs (n = 109), and healthy FN controls (n = 85). Antibodies to additional citrullinated peptides were measured using a multiplex ACPA array, and the number of peptides recognized was reported as an ACPA score. Groups were compared using the chi‐square test and Mann‐Whitney U test. Associations between RA and seropositivity for RF, ACPAs, and anti‐CarP antibodies were determined by logistic regression.
Results
Anti‐CarP antibodies were more frequent in FN patients with RA (44.3%) compared to FDRs (18.3%) and FN controls (4.7%) (both P < 0.0001 versus RA). Moreover, anti‐CarP antibodies were more frequent in FDRs than in FN controls (P = 0.008). The ACPA score was higher in anti‐CCP–positive FN patients with RA than in anti‐CCP–positive FN FDRs (median score 7 [interquartile range (IQR) 7] versus median score 1 [IQR 4]; P = 0.04). The association with RA was strongest when all 3 autoantibodies (RF, anti‐CCP, and anti‐CarP) were present in the patients’ serum (odds ratio 194, 95% confidence interval 23–1,609, P < 0.0001).
Conclusion
Anti‐CarP antibodies are prevalent in FN patients with RA and also more common in their at‐risk FDRs compared to healthy controls. The results indicate an association of RF, ACPAs, and anti‐CarP with RA that is strongest when all 3 autoantibodies are present. These findings may provide new insights into the evolution of autoimmunity in preclinical RA.
Indigenous North Americans have some of the highest prevalence rates of rheumatoid arthritis (RA) worldwide 1, 2. These indigenous peoples, particularly the Cree, Ojibway/Ojibwe, and Oji‐Cree populations, hereafter referred to as First Nations (FN) populations 3, develop RA at an earlier age, have more large joint involvement, and are more likely to be seropositive with high titers of both rheumatoid factor (RF) and anti–citrullinated protein antibodies (ACPAs), as compared to Caucasian populations 4. The high frequency of HLA shared epitope (SE) alleles in the general FN population, combined with high rates of smoking and other environmental exposures, may contribute to the increased risk of developing RA 4, 5, 6, 7. Thus, this is an ideal population to study the earliest stages of RA and to assess factors that might play a role in precipitating autoimmunity.
ACPAs and RF are present in the majority of patients with RA, and both are associated with more aggressive disease and extraarticular features 8, 9. ACPAs and RF can be detected in the serum several years prior to the development of clinical disease, and the magnitude of the antibody effect often increases with imminent arthritis 10, 11, 12, 13, 14, 15. These observations and other evidence suggest that ACPAs and/or ACPA‐producing B cells likely play a role in disease pathogenesis. Clinically, ACPAs are usually detected with commercially available anti–cyclic citrullinated peptide (anti‐CCP) assays; anti‐CCP seropositivity is included in the updated American College of Rheumatology/European League Against Rheumatism classification criteria for RA 16. However, up to one‐quarter of RA patients remain seronegative for both anti‐CCP and RF 17, 18, 19. Moreover, although it has been reported that sensitive multiplex arrays can detect additional antibodies to multiple citrullinated residues in up to 10% of anti‐CCP–negative cases 20, some RA patients remain seronegative.
Antibodies to carbamylated proteins (anti‐CarP), also known as antihomocitrulline antibodies, have recently have been described in RA 11, 21, 22, 23. Anti‐CarP antibodies recognize homocitrulline, which is structurally similar to citrulline. Homocitrulline is generated through carbamylation, a posttranslational modification of lysine by cyanate, which occurs during the urea cycle, and by myeloperoxidase, which acts as a key enzyme in inflammatory cells 24. Carbamylated antigens have been reported to enable activation of T cells, and thereby could generate T cell help for antibody production 25. Studies have shown that antibody responses to citrulline and homocitrulline do not completely overlap in patients with RA 21, 23, which suggests that anti‐CarP antibodies could play a role in RA as a separate antibody system.
Data with regard to the role of anti‐CarP antibodies in RA are limited. Anti‐CarP antibodies are relatively specific for RA and are seen in ACPA‐negative patients with polyarticular juvenile idiopathic arthritis 23, 26. These autoantibodies are associated with more severe disease 21 and, thus, may be used to identify a subset of ACPA‐negative arthritis patients whose prognosis is poor 27. Anti‐CarP antibodies can be detected in the serum of patients with RA prior to disease onset 11, 28, 29. In patients with arthralgia, these antibodies may be used to predict future RA development, independent of the presence of anti‐CCP 30. The objectives of the present study were to determine the presence of anti‐CarP antibodies and their association with other RA autoantibodies in a predisease population of subjects who are known to have a substantially increased risk of developing RA.
PATIENTS AND METHODS
Subject recruitment and serum samples
Participants from the Cree, Ojibway/Ojibwe, and Oji‐Cree FN populations were recruited from urban and rural rheumatology clinics in Manitoba, Canada, as previously described 5, 10, 31. The subjects recruited from these FN populations were required to have at least 3 grandparents of FN ethnicity. Patients with RA met the American College of Rheumatology 1987 classification criteria for RA 32 and were asked to approach their first‐degree relatives (FDRs) about participation in the study. Consenting FDRs were examined by a rheumatologist (DBR or HEG) to confirm that they did not have RA and were followed up annually. Control subjects from these same FN populations and Caucasian North American controls were recruited from the same geographic areas as the relatives and did not have a personal or family history of autoimmune disease 31.
All subjects completed questionnaires assessing demographic characteristics, health habits, and musculoskeletal symptoms. Serum was collected, spun, aliquoted, and stored at − 20°C. For this analysis, participants (95 FN patients with RA, 109 FN FDRs, 85 FN controls, and 38 Caucasian North American controls) were selected, and the groups were matched by age and sex. Serum samples were tested for anti‐CarP antibodies. An overlapping group of serum samples from 52 FN controls, 99 FN patients with RA, and 96 FN FDRs were tested for ACPA reactivity using a multiplex bead‐based assay 15, 20.
Rheumatoid factor (RF) and anti‐CCP assays
RF was measured by nephelometry in our hospital clinical laboratory. For samples in which nephelometry testing was not available, RF IgM and IgA isotypes were tested by enzyme‐linked immunosorbent assay (ELISA). Using larger healthy control populations of FN subjects (n = 200) and Caucasian North American subjects (n = 150), which included the FN and Caucasian North American controls in this analysis, and using an RF ELISA calibrated to standards of known IU (measured by nephelometry), we previously identified the cutoff value for RF positivity in this FN population to be 50 IU. At this level, 95% of Caucasian North American subjects are considered to be seronegative for RF 33.
Anti‐CCP was measured by CCP‐2 and/or CCP‐3 ELISAs (Inova), and positive test results were assigned according to the manufacturer's recommendations. Samples testing positive for either anti–CCP‐2 or anti–CCP‐3 were considered anti‐CCP positive.
Anti‐CarP antibodies were measured by ELISA using a modification of the methods developed by Shi et al 21. Briefly, Nunc Maxisorb plates were coated with fetal calf serum (FCS) or carbamylated FCS (manufactured at Leiden University Medical Center using potassium cyanate). Serum samples were added, and bound human IgG recognizing carbamylated protein was captured using a rabbit anti‐human IgG antibody containing a horseradish peroxidase (HRP) label. Rather than converting the HRP using ABTS, a method that has been described previously, we used tetramethylbenzidine (TMB) and added 50 μl of H2SO4 to stop the reaction. The absorbance was read at 450 nm (suitable for both TMB and ABTS). Standard dilutions were included on each plate to generate a standard curve. The sample concentrations based on absorbance values were calculated via nonlinear fit and adjusted for the dilution factor. The cutoff level for defining anti‐CarP–positive serum samples in the FN groups was established based on the mean (plus 2 SD) anti‐CarP level in the 85 FN control samples. The absorbance values of anti‐CarP varied in independent experiments, although a sample found to be positive for anti‐CarP in one measurement remained positive in all other experiments, and a sample found to be negative remained negative.
ACPA multiplex array
Sera were tested for antibody reactivity to multiple citrullinated peptides and proteins using a bead‐based multiplex array, as previously described 15, 20. Briefly, the serum was incubated with beads coated with 16 different citrullinated proteins, some with multiple citrullinated peptides, or with beads coated with 3 native proteins not targeted in RA, which served as controls (see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39664/abstract). Bound human IgG autoantibodies were conjugated to phycoerythrin (PE) and passed through a Luminex 200 detection laser to determine the extent of PE fluorescence. Similar to the findings in previous studies 15, 20, seropositivity against each component autoantigen in the array was calculated based on a preestablished cutoff level, defined as the mean plus 3 SD value exhibited by an FN control population, which consisted of 52 of the FN control serum samples used for the anti‐CarP ELISA. Seropositivity for each component autoantigen contributed to an aggregate ACPA score, which was based on the total number (sum) of ACPA peptides recognized in each serum sample. These scores were used as a proxy for the breadth of ACPA epitope spreading.
HLA typing
HLA–DRB1 typing was performed by polymerase chain reaction using sequence‐specific oligonucleotide primers, as previously described 5. The following DRB1 alleles were included as SE‐bearing alleles: DRB1*0101, 0102, 0401, 0404, 0405, 0408, 0410, 1001, and 1402.
Statistical analysis
Results are reported as the number (percentage) for categorical variables, median (interquartile range [IQR]) for nonparametric continuous variables, and mean ± SD for normally distributed continuous variables. Statistical comparisons of continuous anti‐CarP levels between groups were tested by Kruskal‐Wallis test (for 3‐group comparisons) and Mann‐Whitney U test (for 2‐group comparisons). Categorical variables were tested using chi‐square tests, and results were reported as the Mantel‐Haenszel common odds ratio (OR) (with 95% confidence interval [95% CI]) and P values. Correlations between the ACPA score and anti‐CarP level were tested using Spearman's correlation analyses. The contribution of antibodies to the risk of RA was determined with logistic regression models, which were controlled for age, sex, smoking, and SE status. P values less than 0.05 were considered statistically significant. All statistical comparisons were performed using IBM SPSS software, version 22.
Ethics considerations
All study participants provided informed consent in accordance with the Declaration of Helsinki. The research ethics board of the University of Manitoba approved the study. All samples and data were anonymized. Consistent with the guidelines of the Canadian Institutes of Health Research for conducting research involving indigenous populations in Canada, we established an advisory committee to provide oversight, and we entered into research agreements with the FN communities participating in the study.
RESULTS
Study subjects
The clinical characteristics of the study subjects were similar to those in previous studies 5, 34 (Table 1). Age varied between the groups (P < 0.0001, by Kruskal‐Wallis test). FN patients with RA were older than their FDRs and FN controls (both P < 0.05 versus RA). Smoking was more common in the FN patients with RA and their relatives compared to FN controls (P = 0.02 among all 3 groups, by chi‐square test). As previously reported, the prevalence of SE positivity in the FN control population was high, and an even higher rate of SE positivity was found in FN patients with RA and their relatives (P < 0.0001 among all 3 groups, by chi‐square test; P = 0.001 for FN patients with RA and FN FDRs versus FN controls).
Table 1.
Clinical characteristics of the First Nations (FN) subjects with rheumatoid arthritis (RA) compared to FN controls and FN first‐degree relatives (FDRs) at the time of enrollment
| FN controls (n = 85) | FN FDRs (n = 109) | FN patients with RA (n = 95) | |
|---|---|---|---|
| Age at enrollment, mean ± SD years | 36 ± 12 | 38 ± 14 | 45 ± 14a |
| Sex, % female | 79 | 73 | 82 |
| Current smoker, % | 69 | 85b | 79 |
| RFc | |||
| Positive, no./total (%)d | 5/77 (6) | 11/105 (10) | 69/94 (73) |
| Titer, median (IQR) | 70 (216) | 103 (133) | 275 (581) |
| Anti‐CCPc | |||
| Positive, no./total (%)d | 4/85 (5) | 14/108 (13) | 67/93 (72) |
| Titer, median (IQR) | 82 (344) | 77 (40) | 200 (86) |
| RF or anti‐CCP positive, no./total (%)d | 14/78 (18) | 25/106 (24) | 76/93 (82) |
| RF and anti‐CCP positive, no./total (%)d | 2/77 (3) | 3/105 (3) | 61/93 (66) |
| SE positive, no./total (%)c | 41/69 (59) | 71/86 (83)e | 57/65(88)e |
P < 0.05 versus FN controls and versus FN FDRs; P < 0.0001 among all 3 groups, by Kruskal‐Wallis independent‐samples test.
P = 0.01 versus FN controls; P = 0.02 among all 3 groups, by chi‐square test.
Data on seropositivity for rheumatoid factor (RF), anti–cyclic citrullinated peptide (anti‐CCP) antibodies, and shared epitope (SE) were available from a subset of subjects. An RF titer of >50 AU was defined as positive. An anti‐CCP titer of >20 AU was defined as positive. Titers (expressed as median with interquartile range [IQR]) are reported only for antibody‐positive subjects.
P < 0.0001 among all 3 groups, by chi‐square test.
P = 0.001 versus FN controls.
Determination of cutoff levels for anti‐CarP antibody positivity
The cutoff level for anti‐CarP antibody seropositivity in FN subjects was established to be 419 arbitrary units (AU)/ml, a value that was 2 SD above the mean value in the FN controls. Two serum samples from the FN control group exhibited very high anti‐CarP antibody levels (Figure 1). These 2 samples were also strongly positive for anti‐CCP, and 1 was positive for RF, suggesting that the condition in these individuals likely represented preclinical RA. These 2 subjects were not included in the cutoff calculation but were included as FN controls in the other analyses.
Figure 1.
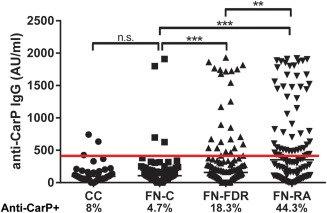
Absolute levels and distribution of anti–carbamylated protein (anti‐CarP) IgG antibodies among First Nations (FN) patients with rheumatoid arthritis (RA) and their first‐degree relatives (FN‐FDRs) compared to Caucasian North American controls (CC) and FN controls (FN‐C). Symbols represent individual subjects; bars indicate the median. Horizontal line indicates the cutoff level for anti‐CarP positivity. ∗∗ = P = 0.02; ∗∗∗ = P < 0.0001, by Mann‐Whitney U test. NS = not significant. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/doi/10.1002/art.39664/abstract.
Since RF titers are affected by ethnicity 33, we compared anti‐CarP antibody levels in FN controls to those in Caucasian North American controls. The RF titers were found to be similar between the 2 groups (median 108.3 AU [IQR 137] in FN controls versus median 95.6 AU [IQR 118] in Caucasian North American controls; P = 0.98, by Mann‐Whitney test). Furthermore, the percentage of subjects positive for anti‐CarP was similar between the 2 control groups (4.7% of FN controls versus 8% of Caucasian North American controls; P = 0.5, by Fisher's exact test). Thus, all subsequent comparisons used only FN subjects as controls.
Levels and distribution of anti‐CarP antibodies in FN subjects
The levels and distribution of anti‐CarP antibodies in FN patients with RA, FN FDRs, and FN controls are shown in Figure 1. In analyses in which the predetermined cutoff level for anti‐CarP antibody positivity was used, the prevalence of anti‐CarP antibodies differed across the 3 FN groups (χ2 = 41; P < 0.0001, by Pearson's chi‐square test). The prevalence of anti‐CarP antibodies in FN patients with RA was 44.3%, which was significantly higher than that in the FN control subjects, in whom the prevalence of anti‐CarP was 4.7% (OR 16.05, 95% CI 5.47–47.37, P < 0.0001), and also significantly higher than that in the FDRs, in whom the prevalence of anti‐CarP was 18.3% (OR 3.53, 95% CI 1.88–6.63, P < 0.0001). Furthermore, FN FDRs had a higher prevalence of anti‐CarP antibodies than did FN controls (OR 4.55, 95% CI 1.49–13.88, P = 0.008). Thus, in these FN populations, the sensitivity of antiCarP antibody positivity for predicting the presence of RA was 44% and the specificity was 88%. Among subjects positive for anti‐CarP antibodies, titers of anti‐CarP antibodies were similar across the 3 groups (median 1,249 AU [IQR 1,239] in FN controls, median 1,034 AU [IQR 1,100] in FN FDRs, and median 809 AU [IQR 1,062] in FN patients with RA; P = 0.6, by Kruskal‐Wallis test).
We further analyzed the distribution of anti‐CarP seropositivity according to anti‐CCP and RF status (Figures 2A and B). Anti‐CarP antibodies were detected in 17 (9.8%) of 172 subjects who were seronegative for both RF and anti‐CCP (among all 3 FN groups) and in 21 (10.4%) of 201 subjects who were seronegative for anti‐CCP and either positive or negative for RF (among all FN groups). In FN patients with RA, the association between the presence of anti‐CarP autoantibodies and seropositivity for both anti‐CCP and RF was significant.
Figure 2.
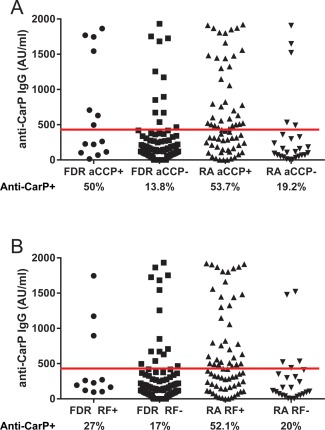
Association of anti‐CarP antibodies with seropositivity for anti–cyclic citrullinated peptide (anti‐CCP) antibodies (A) and rheumatoid factor (RF) (B) in First Nations patients with RA and their FDRs. Symbols represent individual subjects. Horizontal line indicates the cutoff level for anti‐CarP positivity. P = 0.003 between groups, by Mann‐Whitney U test. See Figure 1 for other definitions. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/doi/10.1002/art.39664/abstract.
In analyses stratified according to anti‐CCP status, 53.7% of anti‐CCP–positive RA patients were also positive for anti‐CarP, compared to 19.2% of anti‐CCP–negative RA patients (OR 4.88, 95% CI 1.65–14.47, P = 0.006) (Figure 2A). In analyses stratified according to RF status, 52.1% of RF‐positive RA patients were positive for anti‐CarP, compared to 20% of RF‐negative RA patients (OR 4.4, 95% CI 1.5–13, P = 0.01) (Figure 2B). The association between the presence of anti‐CarP and presence of anti‐CCP was also seen in FN FDRs (50% of anti‐CCP–positive FDRs were also positive for anti‐CarP versus only 13.8% of anti‐CCP–negative FDRs) (OR 6.23, 95% CI 1.88–20.69, P = 0.004). In contrast, there was no association between the presence of anti‐CarP antibodies and positivity for RF in the FDRs (27% of RF‐positive FDRs were also anti‐CarP positive compared to 17% of RF‐negative FDRs; OR 1.8, 95% CI − 0.83, − 2.03, P = 0.7). In general, the at‐risk FDRs were positive for either 1 autoantibody (22 [21%] of 105 subjects) or 2 autoantibodies (9 [9%] of 105 subjects), if found to be positive at all (73 [70%] of 105 FDRs being seronegative for all 3 autoantibodies and 1 FDR being seropositive for all 3 autoantibodies). In contrast, among the FN patients with RA, 33 (36%) of 92 patients were positive for all 3 autoantibodies (Figure 3).
Figure 3.
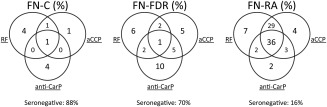
Distribution of seropositivity for rheumatoid factor (RF), anti–cyclic citrullinated peptide (anti‐CCP) antibodies, and anti‐CarP antibodies in FN controls (n = 77), FN FDRs (n = 105), and FN patients with RA (n = 92). Each circle represents a different autoantibody, and the numbers represent the percentage of subjects positive for the autoantibody or autoantibody combination. Only samples with data on all 3 autoantibodies were used to calculate the percentages (3 FN patients with RA, 4 FDRs, and 8 FN controls did not have all 3 antibodies measured). The percentage of subjects who were seronegative for all 3 antibodies is also shown. See Figure 1 for other definitions.
In models analyzing FN patients with RA compared to FN FDRs and FN controls, which were controlled for age, sex, SE status, and smoking, the odds of being in the RA patient group increased when multiple autoantibodies (RF, anti‐CCP, and anti‐CarP) were present: with 1 autoantibody, OR 2.7, 95% CI 0.99–7.6, P = 0.05; with 2 autoantibodies, OR 23.6, 95% CI 7.6–73.3, P < 0.0001; with all 3 autoantibodies, OR 193.8, 95% CI 23.4–1,609.3, P < 0.0001. In the group of FN patients with RA compared to FN FDRs and FN controls, seropositivity for RF (OR 8, 95% CI 2.8–23.2, P < 0.0001) and seropositivity for ACPAs (OR 12.5, 95% CI 3.9–39.7, P < 0.0001) were the two most important predictors of RA, and there was no multiplicative interaction between RF, ACPAs, and anti‐CarP antibodies in logistic regression models that included sex, age, SE status, and smoking as confounders (see Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39664/abstract). These results suggest that the association between autoantibodies and RA is stronger when more antibodies are present.
Associations of anti‐CarP antibodies with presence of the SE and smoking
We next evaluated the association of anti‐CarP antibodies with the presence of the SE and smoking. Anti‐CarP antibodies were more frequent in SE‐positive subjects (positivity for anti‐CarP, 43 [25.4%] of 169 SE‐positive subjects versus 5 [9.8%] of 51 SE‐negative subjects; OR 3.1, 95% CI 1.2–8.4, P = 0.03). However, the association between SE positivity and anti‐CarP positivity was no longer significant when the presence of ACPAs (as determined by anti‐CCP ELISAs) and RF status in each subject group (FN controls versus FN FDRs versus FN patients with RA) were included in multivariable models controlled for smoking status (see Supplementary Table 3, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39664/abstract). These results suggest that the association between seropositivity for anti‐CarP and presence of the SE was likely attributable to overlap with seropositivity for anti‐CCP or RF. We were unable to find an independent association with smoking, possibly because the rates of smoking in these FN populations are high. Consistent with the findings from previous studies, anti‐CCP positivity was significantly associated with the presence of the SE in these subjects (data not shown).
Associations of anti‐CarP antibodies with ACPA reactivity
Since we demonstrated an association between the presence of anti‐CarP antibodies and the presence of anti‐CCP antibodies in RA patients and their FDRs, and since previous studies have demonstrated a broader ACPA response in patients with RA than in ACPA‐positive individuals without the disease 12, we assessed the breadth of the ACPA response using a multiplex assay and examined its relationship to the anti‐CarP antibody response. The total number of citrullinated epitopes recognized in the multiplex assay was converted to an ACPA score. The ACPA score differed across the groups (P < 0.0001, by Kruskal‐Wallis test) and was significantly higher in FN patients with RA (n = 92; median score 5 [IQR 8]) compared to FN FDRs (n = 97; median score 0 [IQR 0]; P < 0.001) and FN controls (n = 52; median score 0 [IQR 0]; P < 0.0001) (Figure 4A). The ACPA score was also higher in anti‐CCP–positive FN patients with RA than in anti‐CCP–positive FN FDRs (median score 7 [IQR 7] versus median score 1 [IQR 4]; P = 0.04). Furthermore, the ACPA score was higher with increasing titers of anti‐CarP antibodies in FN patients with RA (Figure 4B).
Figure 4.
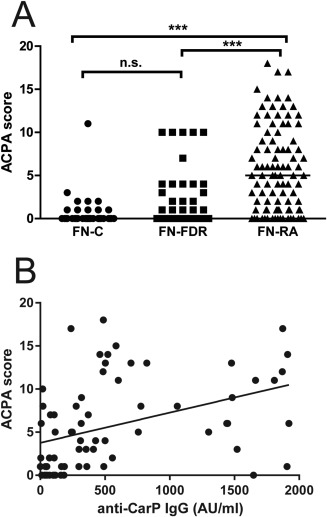
Relationship between anti–citrullinated protein antibody (ACPA) score and anti‐CarP seropositivity. A, ACPA scores at baseline in FN controls, FN FDRs, and FN patients with RA. Symbols represent individual subjects; bars indicate the median. ∗∗∗ = P < 0.0001. B, Correlation between median anti‐CarP level and median ACPA score in FN patients with RA (n = 72) (Spearman's rho = 0.53, P < 0.0001). See Figure 1 for other definitions.
Results of the multiplex array demonstrated that at least 1 citrullinated epitope was recognized in 34 (21.6%) of the 157 anti‐CCP–negative serum samples (8 [16%] of 50 FN controls, 13 [15.8%] of 82 FDRs, and 13 [52%] of 25 FN patients with RA). Of the FN patients with RA, only 13% were negative for ACPAs (being both anti‐CCP negative and having an ACPA score of 0). In 9 of the serum samples from anti‐CCP–positive subjects (5 FDRs and 4 FN patients with RA) with low to moderately positive anti‐CCP titers, ranging from 38 units to 109 units, no citrullinated epitopes were recognized in the ACPA array. ACPA scores were similar between the FN FDRs and FN controls, suggesting that the ACPA response in the FDRs was limited.
Finally, we assessed the risk of being in the RA group, as compared to the FDR or FN control group, in relation to increasing numbers of autoantibodies (RF, anti‐CCP or ACPA score >0, and anti‐CarP); in these analyses, ACPA positivity was defined using data from both the multiplex ACPA array and the anti‐CCP assays. In subjects who were positive for RF, anti‐CarP, anti‐CCP, and ACPAs (in analyses including the ACPA multiplex array data and controlling for age, sex, SE status, and smoking), the odds of having RA (versus being in the FDR or FN control group) were further increased with increasing numbers of autoantibodies (with 1 autoantibody, OR 4.3, 95% CI 1.6–12, P = 0.005; with 2 autoantibodies, OR 17.4, 95% CI 6.1–49.8, P < 0.0001; with all 3 autoantibodies, OR 287.7, 95% CI 33.4–2,478.1, P < 0.0001) (see Supplementary Table 4, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39664/abstract).
DISCUSSION
In this study, we determined the presence and distribution of anti‐CarP antibodies among FN patients with RA, their FDRs, and FN healthy control subjects without evidence of autoimmune disease. We also examined the association of anti‐CarP antibodies with anti‐CCP, RF, and ACPA epitope reactivity. We found a higher prevalence of anti‐CarP antibodies in FN patients with RA and their at‐risk relatives than in FN subjects without autoimmune disease. In RA patients, the presence of anti‐CarP antibodies was associated with a greater degree of ACPA reactivity. The association between autoantibodies and RA was strongest when all 3 antibody serotypes were present.
Anti‐CarP antibodies were most common in the setting of RA but were also present in individuals at risk for developing the disease (the FDRs) and, to a lesser extent, in FN controls. We previously reported a similar pattern with regard to associations with RF and ACPA seropositivity in this population 5. In FN patients with RA, anti‐CarP antibodies were associated with the presence of both anti‐CCP and RF, and yet in the FDRs, anti‐CarP antibodies were associated with the presence of anti‐CCP only. Although all 3 autoantibodies increased in titer in the presence of disease, we are unable to confirm the sequence of autoantibody development in this study. However, these findings are consistent with other reports describing increasing autoantibody positivity preceding the onset of RA, and suggest that the pre‐RA autoantibody responses may evolve, with ACPAs or ACPA subtypes and RF being particularly prevalent with disease onset 10, 11, 13, 14, 15, 18, 21, 28, 29. We were unable to detect significant multiplicative interactions between the 3 autoantibody serotypes, although trends toward a significant interaction were suggested.
Although the findings were not significant, our multivariable model suggested that there was a potential inverse association between anti‐CarP antibodies and RA (OR 0.3, P not significant). This unexpected finding may be related to the small numbers of seropositive subjects in the FDR and control groups, the greater contribution of ACPAs and RF to the risk of being in the RA group, the small sample size of the subset of anti‐CarP–positive RA subjects, or, alternatively, the fact that anti‐CarP antibody production may be associated with events prior to clinical disease. We can also speculate that the effect of RF on RA risk occurs mainly in the presence of ACPAs and anti‐CarP antibodies, thereby supporting recent data indicating that RF can exacerbate ACPA immune complex–mediated macrophage activation 35. Although positivity for ACPAs and positivity for anti‐CarP antibodies were closely associated in the same serum, anti‐CarP antibodies were present in some ACPA‐negative subjects, and not all ACPA‐positive subjects had anti‐CarP antibodies. This finding suggests that while there is overlap between the pathways generating these 2 antibodies in some individuals, 1 of the pathways may be more relevant to the development of autoimmunity and subsequent RA.
We further investigated the association of anti‐CarP antibodies and the extent of ACPA reactivity using a multiplex ACPA array, and calculated an ACPA score for each sample as a proxy for the degree of ACPA epitope spreading. Similar to the findings in previous studies in which this ACPA array was used, serum samples from 21% of the anti‐CCP–negative subjects had reactivity to at least 1 of the citrullinated epitopes in the multiplex array 20. Compared to controls and FDRs, RA patients had an increased ACPA score, which we attributed to an increase in ACPA epitope spreading. FN subjects without autoimmune disease can be positive for anti‐CCP antibodies; however, the increased ACPA score was seen primarily in FN patients with RA. This supports the hypothesis that ACPAs and/or ACPA‐producing B cells undergo quantitative and/or qualitative changes, which thereby provide them with pathogenic properties that can lead to clinical disease (i.e., onset of RA). The origin or cause of the epitope spreading was not the focus of this study; however, it is of interest to note that epitope spreading goes hand in hand with changes in glycosylation patterns of ACPA Fc regions prior to disease onset, suggesting that the underlying ACPA‐producing B cell population undergoes intrinsic changes 36.
Environmental factors, including infection and smoking, have been implicated in the production of ACPAs, particularly in a setting in which the HLA–DRB1 SE is present, and may also influence anti‐CarP antibody production. Smokers have elevated serum levels of thiocyanate, a byproduct of tobacco use, and smoking can theoretically enhance myeloperoxidase activity, which promotes carbamylation of thiocyanate in animal models. However, similar to the findings in previous reports 22, we were unable to demonstrate an association between smoking and anti‐CarP autoantibodies that was independent of the presence of ACPAs. Cross‐reactivity between ACPAs and anti‐CarP may occur if, for example, peptides can be both citrullinated and carbamylated 23. In silico modeling studies have shown that the SE can accommodate carbamylated peptides without steric interference 23. However, it has been shown that while anti‐CarP antibodies may be weakly associated with HLA–DR3, they are not found to be associated with the SE independent of ACPAs 22.
Furthermore, in this study, the association between anti‐CarP reactivity and the presence of the SE appeared to be dependent on the associations between the SE and ACPA production. Alternatively, our inability to find robust associations of anti‐CarP with smoking or the presence of the SE independent of ACPAs may be related to the small sample size or the high prevalence of SE and smoking in this population.
Our study has some limitations. We did not have complete data on disease activity, erosion status, or current treatment of our FN patients with RA, all of which are factors that may influence the production of anti‐CarP or ACPAs, given that inflammation potentially enhances carbamylation and, thus, may affect the propensity to develop autoimmune responses. However, results of previous studies have indicated that anti‐CCP levels do not change considerably after diagnosis 27. We cannot determine whether the different autoantibody systems have any phenotypic relevance to clinical disease from this study, and to date, the number of study subjects who have developed RA has not been sufficient to allow a robust evaluation of the role of anti‐CarP prior to disease onset. We carefully defined seropositivity for anti‐CarP antibodies in this population using only unaffected control subjects from the FN population, and did not find a significant difference in anti‐CarP titers between the FN controls and Caucasian North American controls in this study. Our calculated cutoff value (419 AU/ml) is comparable to that found in another study of Caucasian populations by Gan et al 28 but does differ from that in a study by Brink et al 29, thus highlighting the importance of potential ethnic variation in autoantibody production. This FN population and the increased risk of RA in these subjects, due to a high prevalence of genetic risk alleles and high exposure rates to potential environmental triggers, is unique, and therefore the results may not be applicable to other populations.
In summary, anti‐CarP, anti‐CCP, and RF were all prevalent in FN patients with RA, although overlap in seropositivity for these 3 autoantibodies was incomplete. The incremental clustering of RF, ACPAs, and anti‐CarP in the setting of RA as compared to at‐risk relatives and healthy controls provides new insights into the evolution of autoimmunity in the preclinical period of RA, and suggests that citrullination and carbamylation are separate pathways for the formation of autoantibodies in patients with RA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Hitchon had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Koppejan, Trouw, Smolik, Robinson, El‐Gabalawy, Toes, Hitchon.
Acquisition of data
Koppejan, Sokolove, Lahey, Huizinga, Smolik, Robinson, El‐Gabalawy, Hitchon.
Analysis and interpretation of data
Koppejan, Trouw, Sokolove, Lahey, Huizinga, El‐Gabalawy, Toes, Hitchon.
Supporting information
Supplementary Table 1 Peptides and proteins used as part of the ACPA multiplex array
Supplementary Table 2 RF and ACPA (anti‐CCP) are more important than anti‐CarP antibodies in predicting RA group versus First Degree Relatives (FDR) or FN controls in logistic regression models.
Supplementary Table 3 Anti‐CarP antibodies are associated with ACPA and RF but not smoking or shared epitope in First Nations.
Supplementary Table 4 Distribution of autoantibodies in First Nations with RA their unaffected first degree relatives and controls without autoimmune disease with ACPA defined as positive anti‐CCP or positive ACPA score.
ACKNOWLEDGMENT
We thank the Chief and Band councils of Norway House and St Theresa Point Manitoba.
Supported by the Canadian Institutes of Health Research (Institute of Musculoskeletal Health and Arthritis operating grant MOP‐77700), the European Union Seventh Framework Programme (project Euro‐TEAM; HEALTH‐2012‐INNOVATION‐1‐305549), and the Innovative Medicine Initiative Joint Undertaking–funded project BeTheCure (contract 115142‐2). Dr. Trouw's work was supported by a ZonMW Vidi grant from The Netherlands Organization for Scientific Research and by a fellowship from Janssen Biologics.
Drs. Trouw, Huizinga, and Toes have submitted a patent application for the use of anti‐CarP antibodies as a diagnostic test.
REFERENCES
- 1. Ferucci ED, Schumacher MC, Lanier AP, Murtaugh MA, Edwards S, Helzer LJ, et al. Arthritis prevalence and associations in American Indian and Alaska Native people. Arthritis Rheum 2008;59:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peschken CA, Esdaile JM. Rheumatic diseases in North America's indigenous peoples. Semin Arthritis Rheum 1999;28:368–91. [DOI] [PubMed] [Google Scholar]
- 3.Words first: an evolving terminology relating to Aboriginal peoples in Canada. Government of Canada, Communications Branch, Indian and Northern Affairs; 2002. URL: http://publications.gc.ca/collections/Collection/R2-236-2002E.pdf.
- 4. Peschken CA, Hitchon CA, Robinson DB, Smolik I, Barnabe CR, Prematilake S, et al. Rheumatoid arthritis in a North American native population: longitudinal followup and comparison with a white population. J Rheumatol 2010;37:1589–95. [DOI] [PubMed] [Google Scholar]
- 5. El‐Gabalawy HS, Robinson DB, Hart D, Elias B, Markland J, Peschken CA, et al. Immunogenetic risks of anti‐cyclical citrullinated peptide antibodies in a North American Native population with rheumatoid arthritis and their first‐degree relatives. J Rheumatol 2009;36:1130–5. [DOI] [PubMed] [Google Scholar]
- 6. Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan‐Facsinay A, van der Woude D, et al. Antibodies to porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol 2010;37:1105–12. [DOI] [PubMed] [Google Scholar]
- 7. Peschken CA, Robinson DB, Hitchon CA, Smolik I, Hart D, Bernstein CN, et al. Pregnancy and the risk of rheumatoid arthritis in a highly predisposed North American Native population. J Rheumatol 2012;39:2253–60. [DOI] [PubMed] [Google Scholar]
- 8. Krol A, Garred P, Heegaard NH, Christensen AF, Hetland ML, Stengaard‐Pedersen K, et al. Interactions between smoking, increased serum levels of anti‐CCP antibodies, rheumatoid factors, and erosive joint disease in patients with early, untreated rheumatoid arthritis. Scand J Rheumatol 2015;44:8–12. [DOI] [PubMed] [Google Scholar]
- 9. Vencovsky J, Machacek S, Sedova L, Kafkova J, Gatterova J, Pesakova V, et al. Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis 2003;62:427–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ioan‐Facsinay A, Willemze A, Robinson DB, Peschken CA, Markland J, van der Woude D, et al. Marked differences in fine specificity and isotype usage of the anti–citrullinated protein antibody in health and disease. Arthritis Rheum 2008;58:3000–8. [DOI] [PubMed] [Google Scholar]
- 11. Shi J, van de Stadt LA, Levarht EW, Huizinga TW, Hamann D, van Schaardenburg D, et al. Anti‐carbamylated protein (anti‐CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis 2014;73:780–3. [DOI] [PubMed] [Google Scholar]
- 12. Suwannalai P, van de Stadt LA, Radner H, Steiner G, El‐Gabalawy HS, Jol‐van der Zijde CM, et al. Avidity maturation of anti–citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum 2012;64:1323–8. [DOI] [PubMed] [Google Scholar]
- 13. Rantapaa‐Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. [DOI] [PubMed] [Google Scholar]
- 14. Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst‐Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380–6. [DOI] [PubMed] [Google Scholar]
- 15. Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre‐clinical phase predicts progression to rheumatoid arthritis. PloS One 2012;7:e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 17. Barra L, Pope JE, Orav JE, Boire G, Haraoui B, Hitchon C, et al, and the CATCH Investigators . Prognosis of seronegative patients in a large prospective cohort of patients with early inflammatory arthritis. J Rheumatol 2014;41:2361–9. [DOI] [PubMed] [Google Scholar]
- 18. Innala L, Kokkonen H, Eriksson C, Jidell E, Berglin E, Dahlqvst SR. Antibodies against mutated citrullinated vimentin are a better predictor of disease activity at 24 months in early rheumatoid arthritis than antibodies against cyclic citrullinated peptides. J Rheumatol 2008;35:1002–8. [PubMed] [Google Scholar]
- 19. Kastbom A, Strandberg G, Lindroos A, Skogh T. Anti‐CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 2004;63:1085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner CA, Sokolove J, Lahey LJ, Bengtsson C, Saevarsdottir S, Alfredsson L, et al. Identification of anticitrullinated protein antibody reactivities in a subset of anti‐CCP‐negative rheumatoid arthritis: association with cigarette smoking and HLA‐DRB1 ‘shared epitope’ alleles. Ann Rheum Dis 2015;74:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GM, van Veelen PA, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A 2011;108:17372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang X, Trouw LA, van Wesemael TJ, Shi J, Bengtsson C, Källberg H, et al. Anti‐CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies. Ann Rheum Dis 2014;73:1761–8. [DOI] [PubMed] [Google Scholar]
- 23. Scinocca M, Bell DA, Racape M, Joseph R, Shaw G, McCormick JK, et al. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J Rheumatol 2014;41:270–9. [DOI] [PubMed] [Google Scholar]
- 24. Odobasic D, Yang Y, Muljadi RC, O'Sullivan KM, Kao W, Smith M, et al. Endogenous myeloperoxidase is a mediator of joint inflammation and damage in experimental arthritis. Arthritis Rheumatol 2014;66:907–17. [DOI] [PubMed] [Google Scholar]
- 25. Mydel P, Wang Z, Brisslert M, Hellvard A, Dahlberg LE, Hazen SL, et al. Carbamylation‐dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol 2010;184:6882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muller PC, Anink J, Shi J, Levarht EW, Reinards TH, Otten MH, et al. Anticarbamylated protein (anti‐CarP) antibodies are present in sera of juvenile idiopathic arthritis (JIA) patients. Ann Rheum Dis 2013;72:2053–5. [DOI] [PubMed] [Google Scholar]
- 27. Barra L, Bykerk V, Pope JE, Haraoui BP, Hitchon CA, Thorne JC, et al, the CATCH Investigators . Anticitrullinated protein antibodies and rheumatoid factor fluctuate in early inflammatory arthritis and do not predict clinical outcomes. J Rheumatol 2013;40:1259–67. [DOI] [PubMed] [Google Scholar]
- 28. Gan RW, Trouw LA, Shi J, Toes RE, Huizinga TW, Demoruelle MK, et al. Anti‐carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. J Rheumatol 2015;42:572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brink M, Verheul MK, Ronnelid J, Berglin E, Holmdahl R, Toes RE, et al. Anti‐carbamylated protein antibodies in the pre‐symptomatic phase of rheumatoid arthritis, their relationship with multiple anti‐citrulline peptide antibodies and association with radiological damage. Arthritis Res Ther 2015;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi J, van de Stadt LA, Levarht EW, Huizinga TW, Toes RE, Trouw LA, et al. Anti–carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum 2013;65:911–5. [DOI] [PubMed] [Google Scholar]
- 31. Bernstein CN, El‐Gabalawy H, Sargent M, Landers C, Rawsthorne P, Elias B, et al. Assessing inflammatory bowel disease‐associated antibodies in Caucasian and First Nations cohorts. Can J Gastroenterol 2011;25:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 33. El‐Gabalawy HS, Robinson DB, Smolik I, Hart D, Elias B, Wong K, et al. Familial clustering of the serum cytokine profile in the relatives of rheumatoid arthritis patients. Arthritis Rheum 2012;64:1720–9. [DOI] [PubMed] [Google Scholar]
- 34. Newkirk MM. Rheumatoid factors: what do they tell us? J Rheumatol 2002;29:2034–40. [PubMed] [Google Scholar]
- 35. Sokolove J, Johnson DS, Lahey LJ, Wagner CA, Cheng D, Thiele GM, et al. Rheumatoid factor as a potentiator of anti–citrullinated protein antibody–mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol 2014;66:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rombouts Y, Ewing E, van de Stadt LA, Selman MH, Trouw LA, Deelder AM, et al. Anti‐citrullinated protein antibodies acquire a pro‐inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis 2015;74:234–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Peptides and proteins used as part of the ACPA multiplex array
Supplementary Table 2 RF and ACPA (anti‐CCP) are more important than anti‐CarP antibodies in predicting RA group versus First Degree Relatives (FDR) or FN controls in logistic regression models.
Supplementary Table 3 Anti‐CarP antibodies are associated with ACPA and RF but not smoking or shared epitope in First Nations.
Supplementary Table 4 Distribution of autoantibodies in First Nations with RA their unaffected first degree relatives and controls without autoimmune disease with ACPA defined as positive anti‐CCP or positive ACPA score.


