Abstract
Aim
The aim of this study was to determine whether the concentration of cytokines in the gastric fluid at birth was associated with chorioamnionitis or funisitis and with the white blood cell counts of very premature newborns.
Methods
We retrieved gastric fluid from 27 preterm infants with a gestational age of <29 weeks within 1 h of birth and used enzyme‐linked immunosorbent assay to measure the concentrations of interleukin (IL)‐1beta, epithelial cell‐derived neutrophil‐activating peptide (ENA)‐78, IL‐8 and growth‐related oncogene (Gro)‐alpha. The presence of histologic chorioamnionitis or funisitis in the placentas and the highest white blood cell count of the infants during the first week of life were compared to the cytokine concentrations.
Results
Gastric fluid concentrations of IL‐1beta, ENA‐78, IL‐8 and Gro‐alpha were strongly associated with chorioamnionitis and funisitis. In addition, chorioamnionitis and funisitis and gastric aspirate cytokine levels were associated with the highest white blood cell counts of the infants during the first week of life.
Conclusion
This study suggests that levels of inflammatory cytokines in the gastric fluid of premature infants at birth can be used to assess the exposure of the infants to antenatal inflammation.
Keywords: Chorioamnionitis, Cytokines, Inflammation, Preterm birth, White blood cell count
Abbreviations
- BPD
Bronchopulmonary dysplasia
- CRP
C‐reactive protein
- ENA‐78
Epithelial cell‐derived neutrophil‐activating peptide
- GRO
α growth‐regulated oncogene alpha
- IL
Interleukin
- IUGR
Intrauterine growth restriction
- MCP
Monocyte chemotactic protein
- TNF
α tumour necrosis factor alpha
- WBC
White blood cell
Key notes.
This study showed that concentrations of inflammatory cytokines in the gastric fluid of premature infants at birth were increased in preterm infants exposed to chorioamnionitis and funisitis.
Chorioamnionitis and funisitis were also associated with high white blood cell counts during the first week of life, indicating a systemic inflammatory response.
Evaluation of gastric aspirate fluid may be valuable for screening for the exposure of premature infants for antenatal inflammation.
Introduction
Inflammation of the chorioamniotic membrane is strongly associated with preterm birth, the main cause of neonatal mortality and morbidity 1. Although chorioamnionitis may cause symptoms in the pregnant mother, such as fever and uterine tenderness, it is usually subclinical. In histologic chorioamnionitis, the presence of bacteria may be identified by culture of the placenta, foetal membranes or of the amniotic fluid, depending on the sensitivity of the methods used 1.
At birth, the gastric fluid of the newborn infant is likely to contain amniotic fluid and lung secretions swallowed by the foetus, as well as potentially secretions from its upper gastrointestinal tract and stomach 2. Gastric fluid at birth could therefore reflect inflammation both in the amniotic cavity and in the foetal lung or upper gastrointestinal tract.
Amniotic fluid levels of the inflammatory cytokines interleukins (IL) 6, 8 and 18 and of the monocyte‐attractant chemokines monocyte chemotactic proteins (MCP) 1 and 3 correlate with histologic chorioamnionitis 3. IL‐1β is produced mainly in monocytes and macrophages, but also in neutrophils, epithelial cells, endothelial cells and fibroblasts in response to microbial products 4. The CXC chemokines ENA‐78, GRO‐α and IL‐8 (also called CXCL5, CXCL1 and CXCL8, respectively) are potent attractants and activators of neutrophils. Production of CXC chemokines is stimulated by IL‐1β 5, 6. Previously, cytokine levels have been found to be elevated at birth in the gastric fluid of infants who develop moderate or severe bronchopulmonary dysplasia (BPD) compared with those with no or mild BPD 7.
We hypothesised that the levels of inflammatory cytokines in the gastric fluid of preterm infants at birth are associated with the presence and severity of chorioamnionitis. As inflammatory cytokines attract and activate leucocytes, we also studied whether histologic chorioamnionitis or the levels of inflammatory cytokines in gastric aspirate were associated with the white blood cell counts of newborn infants.
Patients and Methods
Twenty‐four infants born at the Sahlgrenska University Hospital in Gothenburg at a gestational age of <29 weeks between the years 2005 and 2008 and their mothers participated in the study. Twenty‐one infants were singletons, whereas three mothers had twins. Of the twins, only the firstborn infants were included in the study. Maternal and neonatal medical records were reviewed and outcome data collected. This study was approved by the Institutional Review Board for Human Studies of the University of Gothenburg.
Maternal clinical data included age, previous obstetric history, parity, complications of pregnancy, time of beginning of labour or rupture of membranes, use of antibiotics before delivery, glucocorticoid and tocolytic therapy and delivery type. Clinical chorioamnionitis was diagnosed on the basis of maternal fever with supporting clinical evidence, which included foetal tachycardia, uterine tenderness, and/or malodorous vaginal discharge, and the absence of another source of infection 8.
The newborns' clinical data included birthweight, gestational age, sex, requirement of supplemental oxygen, continuous positive airway pressure (CPAP) or respirator therapy, and the presence of sepsis, patent ductus arteriosus, retinopathy of prematurity, intraventricular haemorrhage, periventricular leukomalacia and other complications of prematurity. The highest white blood cell counts of the infants during the first week of postnatal life were retrieved from the infants' charts.
BPD was diagnosed according to the National Institute of Child Health and Human Development definition of BPD. Thus, mild BPD was defined as a need for supplemental oxygen for at least 28 days but not at 36 weeks of gestational age. Moderate BPD was defined as an oxygen requirement for at least 28 days but at <30% at 36 weeks of postmenstrual age. Severe BPD was defined as the need for supplemental oxygen for at least 28 days and at more than 30% and/or the need for positive pressure at 36 weeks of postmenstrual age 9.
Histopathologic examination of all the placentas was performed by a single pathologist who was not aware of the clinical information. The placentas were categorised on the basis of the pathologist's reports into three categories: no chorioamnionitis, chorioamnionitis without funisitis or chorioamnionitis with funisitis. Chorioamnionitis was defined as inflammatory changes and infiltration by neutrophils in the chorioamniotic membranes or the chorionic plate 10. Funisitis was defined as the presence of neutrophils around the blood vessels of the umbilical cord or in the Wharton's jelly 11. The characteristics of the placentas, including weight, diameter, thrombosis, number of blood vessels in the cord, and presence of haemorrhage were examined.
Gastric fluid was collected from the newborn infants within 1 h after birth, when a nasogastric tube was inserted, and frozen immediately. None of the samples were contaminated with blood. Concentrations of IL‐8, GRO‐α, ENA‐78, IL‐1β were measured using enzyme‐linked immunosorbent assays (R&D Systems, Minneapolis, MN, USA). These assays are specific for the human proteins. Assay sensitivities were 3.9 pg/mL (IL‐1β), 31.3 pg/mL (IL‐8 and GRO‐α) and 15.6 pg/mL (ENA‐78). Total protein was determined using the bicinchoninic acid method according to the manufacturer's instructions (Sigma, St. Louis, Missouri, USA).
Statistical analysis
Statistical analysis was carried out with Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and Prism (GraphPad Software Inc., San Diego, CA, USA). Student's t‐test was used to compare two groups of observations of a continuous variable. The relation between categorical variables was analysed using Fischer's exact test. Spearman's rank correlation coefficient was used to analyse the relationship between two continuous variables. A p‐value of <0.05 was regarded as significant.
Results
The clinical characteristics of the mothers and the deliveries are shown in Table 1. The age and average parity of the mothers in the two groups were not significantly different.
Table 1.
Clinical characteristics of mothers and deliveries
| Total n = 24 n (%) | Histologic chorioamnionitis n = 16 n (%) | No chorioamnionitis n = 8 n (%) | p‐value | |
|---|---|---|---|---|
| Age at delivery (years) | 30.1 ± 6.5 | 30.1 ± 7.4 | 30.0 ± 4.7 | 0.97a |
| Mean parity | 0.4 ± 0.8 | 0.55 ± 0.9 | 0.11 ± 0.3 | 0.18a |
| Antenatal steroids | 24 (100%) | 16 (100%) | 8 (100%) | 1b |
| Clinical chorioamnionitis | 9 (37.5%) | 8 (50%) | 1 (12.5%) | 0.18b |
| CRP elevation before delivery | 11 (45.8%) | 10 (62.5%) | 1 (12.5%) | 0.033b |
| Preterm premature rupture of membranes | 7 (29%) | 6 (37.5%) | 1 (12.5%) | 0.35b |
| Preterm labour | 13 (54.2%) | 10 (62.5%) | 3 (37.5%) | 0.39b |
| Contractions before delivery | 16 (66.7%) | 14 (87.5%) | 2 (25%) | 0.0047b |
| Received tocolysis | 16 (66.7%) | 13 (81.3%) | 3 (37.5%) | 0.065b |
| Pre‐eclampsia | 3 (12.5%) | 0 (0%) | 3 (37.5%) | 0.028b |
| Caesarean delivery | 11 (46%) | 5 (31%) | 6 (75%) | 0.082b |
| Received antibiotics before delivery | 12 (50%) | 10 (62.5%) | 2 (25%) | 0.19b |
Student's t‐test, two‐tailed.
Fischer's exact test.
All mothers were given antenatal steroids to promote surfactant production and stability of the foetal lung. Nine mothers (37.5%) were diagnosed with clinical chorioamnionitis. Of these, one did not have histologic chorioamnionitis. CRP elevation was more common in the group with histologic chorioamnionitis. Contractions before delivery occurred in 87% of the patients in the chorioamnionitis group, compared to only 25% of those in the other group. This difference was highly significant (p‐value 0.005) and was likely due to the fact that intrauterine inflammation induces labour, whereas other complications of pregnancy such as pre‐eclampsia do not. Pre‐eclampsia was only present in the group with no chorioamnionitis. A disproportionate number of the Caesarian sections were in the nonchorioamnionitis group.
Characteristics of the placentas are shown in Table 2. Of the 24 placentas, 16 (66.7%) showed features of chorioamnionitis. Funisitis was present in 10 umbilical cords (40.7%). Other pathological findings were infarctions (n = 3, 12.5%) and haemorrhages (n = 8, 33%) in the placental parenchyma. All of the umbilical cords had the normal three blood vessels, and none had thrombosis of the cord vessels.
Table 2.
Histologic characteristics of placentas, n = 24
| Mean weight (g) (±SD) | 268.8 ± 89 |
| Mean diameter (cm) (±SD) | 13.5 ± 2.0 |
| Inflammation of the membranes | 16 (66.7%) |
| Inflammation of the chorionic plate | 13 (54.2%) |
| Funisitis | 10 (41.7%) |
| Umbilical cord insertion | |
| Central | 5 (20.8%) |
| Marginal | 15 (62.5%) |
| Velamentous | 4 (16.7%) |
| Three vessels in umbilical cord | 24 (100%) |
| Thrombosis of cord vessels | 0 (0%) |
| Haemorrhage in placenta | 8 (33.3%) |
| Infarction | 3 (12.5%) |
The characteristics of the infants are shown in Table 3. Gestational age was similar in the groups with or without chorioamnionitis. Although the no chorioamnionitis group contained three cases (37.5%) of intrauterine growth retardation (IUGR) whereas no infant in the group with histologic chorioamnionitis had IUGR (p = 0.027), the mean birthweights were not significantly different between the two groups. All three (11%) of the newborns who died before 28 days of age had histologic chorioamnionitis (Table 3): one died of severe intraventricular haemorrhage, one of severe respiratory distress syndrome, and one of asphyxia‐related causes. The incidence of sepsis was similar in the two groups (Table 3). However, most of the mothers (62.5%) in the group with histologic chorioamnionitis group and 25% of those in the no chorioamnionitis group had received antenatal antibiotics (Table 1). The differences between the groups were not significant for any of the common complications of prematurity, including BPD, intraventricular haemorrhage, periventricular leukomalacia or patent ductus arteriosus, possibly because of the small number of patients (Table 3).
Table 3.
Clinical characteristics of infants
| All n (%) | Histologic chorioamnionitis n (%) | No chorioamnionitis n (%) | p‐value | |
|---|---|---|---|---|
| Mean gestational age (days) (±SD) | 181 ± 9.7 | 179 ± 8.4 | 186 ± 11 | 0.09a |
| Mean birthweight (g) (±SD) | 778 ± 154 | 811 ± 133 | 708 ± 181 | 0.13a |
| IUGR | 3/24 (12.5%) | 0/16 (0%) | 3/8 (37.5%) | 0.027b |
| Female sex | 12/24 (50%) | 7/16 (43.75%) | 5/8 (62.5%) | 0.67b |
| Death | 3/24 (12.5%) | 3/16 (18.75%) | 0/8 (0%) | 0.53b |
| Sepsis | 13/24 (54.2%) | 8/16 (50%) | 5/8 (62.5%) | 0.68b |
| BPD | 19/21 (90.5%) | 12/13 (92.3%) | 7/8 (87.5%) | 1b |
| Moderate or severe BPD | 11/21 (52.4%) | 8/13 (61.5%) | 3/8 (37.5%) | 0.39b |
| Moderate or severe BPD or death | 14/24 (58.3%) | 11/16 (68.8%) | 3/8 (37.5%) | 0.20b |
| Intraventricular haemorrhage | 4/24 (16.7%) | 4/16 (25%) | 0/8 (0%) | 0.26b |
| Periventricular leukomalacia | 3/24 (12.5) | 3/16 (18.8%) | 0/8 (0%) | 0.53b |
| Patent ductus arteriosus | 13/24 (54.2%) | 9/16 (56.3%) | 4/8 (50.0%) | 1b |
| Necrotising enterocolitis | 3/24 (12.5%) | 2/16 (12.5%) | 1/8 (12.5%) | 1b |
Student's t‐test, two‐tailed.
Fischer's exact test.
The concentrations of the different cytokines in the different groups are shown in Figure 1. The correlations between concentrations of the cytokines and the grade of chorioamnionitis were found to be statistically significant (p < 0.0001 for IL‐1β; p < 0.0001 for Gro‐α; p < 0.0001 for IL‐8; p = 0.0010 for ENA‐78). Infants whose placentas had no signs of chorioamnionitis had low cytokines levels. The cytokine levels were elevated mostly in the patients with funisitis (Fig. 1).
Figure 1.
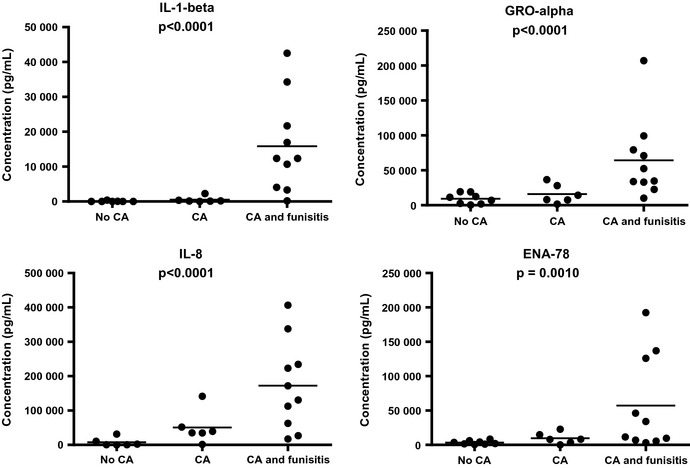
Concentrations of IL‐1β, Gro‐α, IL‐8 and ENA‐78 in the gastric aspirate of newborn infants in the groups with no chorioamnionitis (no CA), chorioamnionitis without funisitis (CA) or chorioamnionitis with funisitis (CA with funisitis). Each dot represents one patient. The straight lines indicate the mean concentration in each group. IL‐1 β, p < 0.0001; Gro‐α, p < 0.0001; IL‐8, p < 0.0001; ENA‐78 p = 0.0010 (Spearman's test).
The newborn infants' highest white blood cell (WBC) count during the first week of postnatal life was associated with chorioamnionitis and funisitis (p = 0.0067) (Fig. 2). High WBC counts indicated a systemic inflammatory response in the infants. Some of the infants had very high WBC counts (Fig. 2): two (8.3%) had a WBC count >50*109/L and seven (29%) had a WBC count ≥35*109/L. All of these infants had funisitis.
Figure 2.

The highest WBC counts during the first week of postnatal life by severity of chorioamnionitis. p = 0.0067, analysed with the Spearman's test.
Maternal pre‐eclampsia and IUGR in very preterm infants are associated with low WBC counts in preterm infants 12, 13. There were three infants in our study who had maternal pre‐eclampsia and three infants had IUGR. One of these infants had both conditions. None of these infants were exposed to antenatal chorioamnionitis. Thus, most (62.5%) of the infants in the no chorioamnionitis group had either IUGR or maternal pre‐eclampsia. Not surprisingly, when these infants were removed from the analysis, the association of high WBC with chorioamnionitis or funisitis was no longer significant, due to the small number of remaining infants.
The infants' highest WBC during the first week of life correlated significantly with the gastric fluid levels of IL‐1β (p = 0.0017), GRO‐α (p = 0.02) and ENA‐78 (p = 0.041), but barely with the levels of IL‐8 (p = 0.051) when analysed with Spearman's rank correlation coefficient (data not shown).
Discussion
Our results demonstrate that the levels of inflammatory cytokine IL‐1β and of the neutrophil chemokines ENA‐78, IL‐8 and GRO‐α in the gastric aspirate at birth were associated with the presence of histologic chorioamnionitis and of funisitis. Measuring cytokines in the gastric aspirate could therefore be a way of assessing whether a newborn has been exposed to inflammation in utero. Although not all the patients with chorioamnionitis had elevated cytokine levels, none of the patients without chorioamnionitis had elevated cytokine levels, suggesting that the test is fairly specific for chorioamnionitis. The cytokine that best differentiated between the presence and absence of funisitis was IL‐1β.
The cytokine levels correlated especially well with funisitis, which suggests that they reflected an inflammatory response in the foetus. The foetal inflammatory response indicated that inflammatory activity had spread to the foetus and may have continued after delivery and affected the newborn's outcome.
Gastric aspirate is a convenient sample to take, as nasogastric tubes are routinely inserted in infants in intensive care and gastric fluid is aspirated to check the position of the tube. Not all infants are intubated, so it is not possible to take tracheal aspirate samples from all of them. In addition, the presence of inflammatory cytokines in the gastric fluid is likely to represent exposure of the infant to inflammation at the time of delivery, whereas intubation and ventilation per se may cause inflammation in the airway after birth. Gastric fluid has been studied less often than other fluids such as amniotic fluid and tracheal aspirate to detect exposure of the foetus to inflammation. A previous study showed that levels of IL‐8 in tracheal aspirate taken soon after birth are associated with histologic chorioamnionitis 14. Levels of IL‐6 and IL‐8 in tracheal aspirate correlated with the development of BPD and greater need for mechanical ventilation 14.
The cytokines in the gastric fluid may originate in the amniotic fluid or in the foetal lungs, as the foetus swallows both amniotic fluid and its own lung secretions. Production of cytokines may also occur in the oesophagus or locally in the stomach. Miralles et al. found that the presence of bacterial rRNA genes in gastric fluid in premature infants correlated strongly with the presence of chorioamnionitis and funisitis 15. Neutrophils in the stomach and lungs of newborns have been found to be associated with chorioamnionitis 16. Likewise, the presence of bacteria in the gastric fluid at birth has been associated with chorioamnionitis 17.
In our study, the highest WBC counts of the newborns during the first week of life after birth were associated with chorioamnionitis and funisitis and with the gastric fluid concentrations of IL‐1β and of the neutrophil‐attractant chemokines IL‐8 and GRO‐α.
Our observation that some of the infants had very high WBC counts is consistent with a previous study that demonstrated very high WBC counts after birth in extremely low birthweight infants, with about 15% having WBC counts >50*109/L 18. Our results further showed that all the infants with very high WBC counts (≥35*109/L) had funisitis. High WBC counts are seen in the amniotic fluid during chorioamnionitis 19. Earlier studies have shown that the neutrophils in amniotic fluid in chorioamnionitis are mainly of foetal origin 20. De Dooy et al. found significantly higher WBC counts in the blood during the first few days after delivery in infants exposed to chorioamnionitis than in infants not exposed, but did not look at funisitis separately 21. High WBC counts in the blood indicate a systemic inflammatory response in the infant. Chorioamnionitis also leads to elevated levels of IL‐6, IL‐8, IL‐10 and G‐CSF in the cord blood of infants exposed to severe chorioamnionitis, and these have predicted the risk of BPD 22. The elevated cytokine levels decreased shortly after birth 22.
Conclusion
Histologic chorioamnionitis and particularly funisitis are associated with elevated levels of inflammatory cytokines in the gastric fluid of preterm infants at birth and with high WBC counts in the blood of newborn infants, indicating a systemic inflammatory response. This study suggests that the levels of inflammatory cytokines in the gastric fluid of premature infants at birth can be used to assess antenatal exposure of the infant to inflammation.
Funding
This study was supported by grants from the Swedish Heart and Lung Foundation, the Frimurare Barnhus Foundation and the Swedish Governments Grants for Medical Research.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000; 342: 1500–7. [DOI] [PubMed] [Google Scholar]
- 2. Brace RA. Physiology of amniotic fluid volume regulation. Clin Obstet Gynecol 1997; 40: 280–9. [DOI] [PubMed] [Google Scholar]
- 3. Holst RM, Laurini R, Jacobsson B, Samuelsson E, Sävman K, Doverhag C, et al. Expression of cytokines and chemokines in cervical and amniotic fluid: relationship to histological chorioamnionitis. J Matern Fetal Neonatal Med 2007; 20: 885–93. [DOI] [PubMed] [Google Scholar]
- 4. Dinarello CA. Biologic basis for interleukin‐1 in disease. Blood 1996; 87: 2095–147. [PubMed] [Google Scholar]
- 5. Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today 1999; 20: 254–7. [DOI] [PubMed] [Google Scholar]
- 6. Walz A, Schmutz P, Mueller C, Schnyder‐Candrian S. Regulation and function of the CXC chemokine ENA‐78 in monocytes and its role in disease. J Leukoc Biol 1997; 62: 604–11. [DOI] [PubMed] [Google Scholar]
- 7. Stichel H, Bäckman E, Hafström O, Nilsson S, Lappalainen U, Bry K. Inflammatory cytokines in gastric fluid at birth and the development of bronchopulmonary dysplasia. Acta Paediatr 2011; 100: 1206–12. [DOI] [PubMed] [Google Scholar]
- 8. Alexander JM, Gilstrap LC, Cox SM, McIntire DM, Leveno KJ. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol 1998; 91: 725–9. [DOI] [PubMed] [Google Scholar]
- 9. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005; 116: 1353–60. [DOI] [PubMed] [Google Scholar]
- 10. Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin‐6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995; 172: 960–70. [DOI] [PubMed] [Google Scholar]
- 11. Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002; 11: 18–25. [DOI] [PubMed] [Google Scholar]
- 12. Wirbelauer J, Thomas W, Rieger L, Speer CP. Intrauterine growth retardation in preterm infants ≤32 weeks of gestation is associated with low white blood cell counts. Am J Perinatol 2010; 27: 819–24. [DOI] [PubMed] [Google Scholar]
- 13. Koenig JM, Christensen RD. Incidence, neutrophil kinetics, and natural history of neonatal neutropenia associated with maternal hypertension. N Engl J Med 1989; 321: 557–62. [DOI] [PubMed] [Google Scholar]
- 14. De Dooy J, Ieven M, Stevens W, De Clerck L, Mahieu L. High levels of CXCL8 in tracheal aspirate samples taken at birth are associated with adverse respiratory outcome only in preterm infants younger than 28 weeks gestation. Pediatr Pulmonol 2007; 42: 193–203. [DOI] [PubMed] [Google Scholar]
- 15. Miralles R, Hodge R, McParland PC, Field DJ, Bell SC, Taylor DJ, et al. Relationship between antenatal inflammation and antenatal infection identified by detection of microbial genes by polymerase chain reaction. Pediatr Res 2005; 57: 570–7. [DOI] [PubMed] [Google Scholar]
- 16. Heller DS. Evaluation of fetal lungs and stomach in the determination of amniotic sac infection. Fetal Pediatr Pathol 2011; 30: 394–6. [DOI] [PubMed] [Google Scholar]
- 17. Oue S, Hiroi M, Ogawa S, Hira S, Hasegawa M, Yamaoke S, et al. Association of gastric fluid microbes at birth with severe bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed 2009; 94: F17–22. [DOI] [PubMed] [Google Scholar]
- 18. Rastogi S, Rastogi D, Sundaram R, Kulpa J, Parekh AJ. Leukemoid reaction in extremely low‐birth weight infants. Am J Perinatol 1999; 16: 93–7. [DOI] [PubMed] [Google Scholar]
- 19. Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra‐amniotic inflammation. J Matern Fetal Neonatal Med 2006; 19: 693–7. [DOI] [PubMed] [Google Scholar]
- 20. Sampson JE, Theve RP, Blatman RN, Shipp TD, Bianchi DW, Ward BE, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol 1997; 176: 77–81. [DOI] [PubMed] [Google Scholar]
- 21. De Dooy J, Colpaert C, Schuerwegh A, Bridts C, Van Der Planken M, Ieven M, et al. Relationship between histologic chorioamnionitis and early inflammatory variables in blood, tracheal aspirates, and endotracheal colonization in preterm infants. Pediatr Res 2003; 54: 113–9. [DOI] [PubMed] [Google Scholar]
- 22. Paananen R, Husa AK, Vuolteenaho R, Herva R, Kaukola T, Hallman M. Blood cytokines during the perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J Pediatr 2009; 154: 39–43. [DOI] [PubMed] [Google Scholar]


