Abstract
The aim of the present study was to assess the efficacy and safety of teneligliptin in combination with metformin in Korean patients with type 2 diabetes mellitus who were inadequately controlled with metformin monotherapy. Patients [glycated haemoglobin (HbA1c) 7.0–10.0%, on stable metformin ≥1000 mg/day] were randomized 2 : 1 to receive 20 mg teneligliptin plus metformin (n = 136) or placebo plus metformin (n = 68). The primary endpoint was the change in HbA1c levels from baseline to week 16. The mean baseline HbA1c was 7.9% in the teneligliptin group and 7.8% in the placebo group. The differences between the teneligliptin and placebo groups regarding changes in HbA1c and fasting plasma glucose levels were −0.78 % and −1.24 mmol/l (22.42 mg/dl), respectively, at week 16. The incidence of adverse events was similar between the groups. The addition of teneligliptin once daily to metformin was effective and generally well tolerated in Korean patients with type 2 diabetes.
Keywords: DPP‐4 inhibitors, Korean, metformin, teneligliptin
Introduction
Teneligliptin, characterized by a considerably rigid structure formed by five consecutive rings, is a novel dipeptidyl peptidase‐4 (DPP‐4) inhibitor for the treatment of type 2 diabetes 1. Introduction of the 1‐(1‐phenylpyrazol‐5‐yl) piperazine moiety (anchor lock domain), which binds to the S2 extensive subsite, increased the activity by 1500‐fold over the corresponding fragment that binds to S1 and S2 only 2. As the metabolites of this drug are excreted through the hepatic (∼35%) and renal (∼65%) routes, no dose adjustment is necessary in patients with renal impairment 1. Particularly because of its long half‐life, this drug has been shown to stabilize glucose fluctuations throughout the day 3. We conducted the present study to confirm the efficacy and safety of teneligliptin combined with metformin in Korean patients with type 2 diabetes inadequately controlled with metformin monotherapy.
Methods
The present study was a randomized, double‐blind, placebo‐controlled, parallel‐group, phase III study, designed to confirm the efficacy and safety of teneligliptin combined with metformin. The protocol was approved by the institutional review boards at each participating site. This trial was registered with ClinicalTrials.gov (no. NCT01805830).
Patients with type 2 diabetes were eligible to participate if they had inadequate glycaemic control [glycated haemoglobin (HbA1c) levels 7.0–10.0%] on stable‐dose metformin monotherapy (≥1000 mg/day) for at least 8 weeks. Patients who had type 1 diabetes, current or a history of significant comorbidities, such as cardiovascular, hepatic and renal conditions, were excluded from the study. After the 2‐week run‐in period, eligible patients were assigned 2 : 1 to a 20 mg teneligliptin once daily or a placebo once daily group, respectively. The metformin dose was kept constant throughout the study period. Rescue therapy was not permitted during the study period. Patients were withdrawn from the study if they met the predefined fasting plasma glucose thresholds during any subsequent visit.
A change from baseline in patients' HbA1c levels after 16 weeks of treatment was used as the primary efficacy endpoint. Safety and tolerability were assessed throughout the study.
Efficacy analyses were performed using the full population set (≥1 dose of study medication and baseline and post‐baseline efficacy data) with last observation carried forward methodology. The changes from baseline to week 16 were compared between the two groups using analysis of covariance, with site as a fixed effect and baseline HbA1c level as a covariate. The point estimate and the 95% confidence interval for the difference between the two groups were calculated based on the least squares mean ± standard error.
Results
Of the 317 subjects screened, 204 eligible subjects were randomized to treatment: 136 received teneligliptin plus metformin and 68 received placebo plus metformin. A total of 177 (86%) patients completed 16 weeks of treatment (Figure S1). Treatment groups were balanced with respect to demographics and disease characteristics (Table S1). The mean baseline HbA1c was 7.9% in the teneligliptin plus metformin group and 7.8% in the placebo plus metformin group. The mean metformin dose over the study period was ∼1400 mg for both treatment groups.
The adjusted mean changes from baseline values were −0.90% for the teneligliptin plus metformin group compared with −0.12% for the placebo plus metformin group (p < 0.0001; Table 1). A greater decrease in HbA1c was observed in the teneligliptin plus metformin group compared with the placebo plus metformin group at week 4 and throughout the randomized treatment period (Figure 1). The adjusted mean change in fasting plasma glucose from baseline to week 16 was −0.93 mmol/l (16.79 mg/dl) for the teneligliptin plus metformin group versus +0.32 mmol/l (5.69 mg/dl) for the placebo plus metformin group (p < 0.0001). A significantly greater proportion of patients achieved a therapeutic glycaemic response (HbA1c < 7%) with teneligliptin plus metformin than with placebo plus metformin (64.71 vs. 13.24%, respectively; p < 0.001).
Table 1.
Effects of teneligliptin and placebo on glucose metabolism
| Teneligliptin | Placebo | |
|---|---|---|
| n = 136 | n = 68 | |
| HbA1c, % | ||
| Baseline | ||
| Mean (s.d.) | 7.79 (0.80) | 7.72 (0.65) |
| At week 16 | ||
| Mean (s.d.) | 6.93 (0.84) | 7.65 (0.80) |
| Change from baseline to week 16 | ||
| Mean (s.d.) | −0.87 (0.65) | −0.06 (0.55) |
| p value for within treatment group | <0.0001* | 0.3384† |
| Difference vs. placebo | ||
| Adjusted mean (s.e.) | −0.90 (0.07) | −0.12 (0.09) |
| Adjusted mean for difference (s.e.) | −0.78 (0.09) | — |
| 95% CI of adjusted mean | −0.95, −0.61 | — |
| p value‡ | <0.0001 | — |
| FPG (mmol/l) | ||
| Baseline | ||
| Mean (s.d.) | 8.39 (1.97) | 8.39 (1.43) |
| At week 16 | ||
| Mean(s.d.) | 7.47 (1.78) | 8.71 (1.78) |
| Change from baseline to week 16 | ||
| Mean (s.d.) | −0.93 (1.37) | 0.32 (1.44) |
| p‐value for within treatment group | <0.0001† | 0.0749† |
| Difference vs. placebo | ||
| Adjusted mean (s.e.) | −1.10 (0.15) | 0.15 (0.19) |
| Adjusted mean for difference (s.e.) | −1.24 (0.18) | — |
| 95% CI of adjusted mean | (−1.61, −0.88) | — |
| p‐value‡ | <0.0001 | — |
| HOMA‐β | ||
| Baseline | ||
| Mean (s.d.) | 35.68 (26.15) | 33.39 (22.47) |
| At week 16 | ||
| Mean (s.d.) | 46.89 (38.86) | 33.57 (24.75) |
| Change from baseline to week 16 | ||
| Mean (s.d.) | 11.22 (24.29) | 0.19 (13.19) |
| p value for within treatment group | <0.0001* | 0.8385* |
| Difference vs. placebo | ||
| Adjusted mean (s.e.) | 12.76 (2.59) | 2.17 (3.19) |
| Adjusted mean for difference (s.e.) | 10.59 (3.11) | — |
| 95% CI of adjusted mean | 4.46, 16.72 | — |
| p value‡ | 0.0008 | — |
| HOMA‐IR | ||
| Baseline | ||
| Mean (s.d.) | 3.10 (2.52) | 2.87 (1.94) |
| At week 16 | ||
| Mean (s.d.) | 2.81 (2.27) | 3.01 (1.93) |
| Change from baseline to week 16 | ||
| Mean (s.d.) | −0.29 (1.81) | 0.13 (1.43) |
| p value for within treatment group | 0.0533* | 0.4430† |
| Difference vs. placebo | ||
| Adjusted mean (s.e.) | −0.29 (0.18) | 0.02 (0.23) |
| Adjusted mean for difference (s.e.) | −0.30 (0.22) | — |
| 95% CI of adjusted mean | −0.74, 0.14 | — |
| p value‡ | 0.1754 | — |
HbA1c, glycated haemoglobin; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; s.d., standard deviation; s.e., standard error; CI, confidence interval.
Change from baseline (Wilcoxon signed‐rank test).
Change from baseline (paired t‐test).
Difference in change from baseline between treatment groups (analysis of covariance model included site as a fixed effect and baseline as a covariate).
Figure 1.
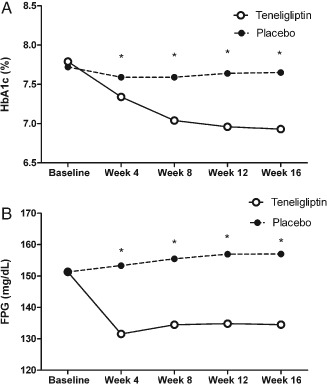
Glycaemic control in patients treated with teneligliptin plus metformin or placebo plus metformin during the randomized treatment period: (A) Mean glycated haemoglobin (HbA1c) and (B) mean fasting plasma glucose (FPG) values during the randomized treatment period. *p value < 0.001. p values are for comparisons between the teneligliptin and placebo groups.
Greater increases in β‐cell function based on homeostasis model assessment of β‐cell function (HOMA‐β) were observed in patients treated with teneligliptin plus metformin compared with those treated with placebo plus metformin at week 16 (p = 0.0008). Homeostasis model assessment of insulin resistance (HOMA‐IR) showed an improving trend in patients treated with teneligliptin plus metformin compared with placebo plus metformin (p = 0.1754). No differences were observed between treatment groups in the exploratory efficacy endpoints of body weight, fasting insulin, fasting C‐peptide, high‐sensitivity C‐reactive protein or lipid variables.
Teneligliptin combined with metformin was well tolerated compared with placebo added to metformin (Table S2). All of the events were classified as mild and did not result in study discontinuation.
Discussion
The mean reduction in HbA1c level after 16 weeks of treatment with teneligliptin combined with ongoing metformin therapy was ∼0.9%, a result similar to or slightly higher than the results of previous studies performed with other DPP‐4 inhibitors 4, 5, 6, 7. A meta‐analysis by Kim et al. 7 showed a greater blood glucose‐lowering efficacy of DPP‐4 inhibitors in Asian than in non‐Asian people. In trials evaluating oral combination therapy, the overall difference in HbA1c was −0.85% in Asian‐dominant studies (≥50% Asian participants), whereas it changed by −0.66% in non‐Asian‐dominant studies. The changes in HbA1c observed in the present study for the teneligliptin plus metformin group were similar to those achieved with the addition of teneligliptin to pioglitazone 8 or glimepiride 9.
The HOMA‐β value was improved significantly by the addition of teneligliptin in the present study. HOMA‐IR values showed an improving trend after the addition of teneligliptin; however, the possibility cannot be excluded that this may have been an indirect action through the improvement in glycaemia. Pancreatic α‐cell glucagon secretion, which accelerates hepatic glucose delivery, is suppressed by DPP‐4 inhibitors 10. Vildagliptin significantly lowered the plasma levels of postprandial glucagon and endogenous glucose 10. Teneligliptin also suppressed fasting and postprandial glucagon 3. Teneligliptin‐induced suppression of glucagon could also be responsible for improved insulin sensitivity. No safety or tolerability concerns were observed with teneligliptin as an add‐on to metformin treatment in this study.
Although the maximum recommended daily dose for metformin is 2500 mg, the percentage of patients receiving metformin >1500 mg/day in Korea was relatively small 11. We chose a dose of metformin ≥1000 mg/day. No clinically relevant interaction was observed with the co‐administration of teneligliptin with metformin because of the concurrent involvement of renal excretion and multiple metabolic pathways in its elimination 12.
In conclusion, the addition of teneligliptin to metformin treatment was effective and well tolerated in Korean patients with type 2 diabetes.
Conflict of Interest
The authors have no conflicts of interest relevant to this article to report.
B. C. and W. J. participated in designing the study. E. R., K. H., A. W., M. L., B. K., C. C., K. K., H. L., I. P., J. P., H. J., K. P., B. C. conducted the study and collected the data. W. J. analysed the data. M. K. and E. R. wrote the manuscript.
Supporting information
Appendix S1. A complete list of the study investigators.
Figure S1. Flow diagram of patients participating in the trial.
Table S1. Baseline characteristics of the study subjects.
Table S2. Adverse events recorded during the 16‐week treatment period (safety summary).
Acknowledgements
This study was funded by Handok Inc., Seoul, Republic of Korea.
References
- 1. Goda M, Kadowaki T. Teneligliptin for the treatment of type 2 diabetes. Drugs Today (Barc) 2013; 49: 615–629. [DOI] [PubMed] [Google Scholar]
- 2. Nabeno M, Akahoshi F, Kishida H et al. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun 2013; 434: 191–196. [DOI] [PubMed] [Google Scholar]
- 3. Eto T, Inoue S, Kadowaki T. Effects of once‐daily teneligliptin on 24‐h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2012; 14: 1040–1046. [DOI] [PubMed] [Google Scholar]
- 4. Scott R, Loeys T, Davies MJ, Engel SS, Sitagliptin Study 801 Group . Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10: 959–969. [DOI] [PubMed] [Google Scholar]
- 5. Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007; 30: 890–895. [DOI] [PubMed] [Google Scholar]
- 6. Cho YM, Wideman RD, Kieffer TJ. Clinical application of glucagon‐like Peptide 1 receptor agonists for the treatment of type 2 diabetes mellitus. Endocrinol Metab (Seoul) 2013; 28: 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetologia 2013; 56: 696–708. [DOI] [PubMed] [Google Scholar]
- 8. Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2013; 4: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled study with an open‐label, long‐term extension. Diabetes Obes Metab 2014; 16: 418–425. [DOI] [PubMed] [Google Scholar]
- 10. Liu Y, Hong T. Combination therapy of dipeptidyl peptidase‐4 inhibitors and metformin in type 2 diabetes: rationale and evidence. Diabetes Obes Metab 2014; 16: 111–117. [DOI] [PubMed] [Google Scholar]
- 11. Yoon KH, Shin JA, Kwon HS et al. Comparison of the efficacy of glimepiride, metformin, and rosiglitazone monotherapy in korean drug‐naïve type 2 diabetic patients: the practical evidence of antidiabetic monotherapy study. Diabetes Metab J 2011; 35: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filippatos TD, Athyros VG, Elisaf MS. The pharmacokinetic considerations and adverse effects of DPP‐4 inhibitors. Expert Opin Drug Metab Toxicol 2014; 10: 787–812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. A complete list of the study investigators.
Figure S1. Flow diagram of patients participating in the trial.
Table S1. Baseline characteristics of the study subjects.
Table S2. Adverse events recorded during the 16‐week treatment period (safety summary).


