Summary
Background
Vonoprazan is a new potassium‐competitive acid blocker for treatment of acid‐related diseases.
Aim
To conduct two randomised‐controlled trials, to evaluate the non‐inferiority of vonoprazan vs. lansoprazole, a proton pump inhibitor, for treatment of gastric ulcer (GU) or duodenal ulcer (DU).
Methods
Patients aged ≥20 years with ≥1 endoscopically‐confirmed GU or DU (≥5 mm white coating) were randomised 1:1 using double‐dummy blinding to receive lansoprazole (30 mg) or vonoprazan (20 mg) for 8 (GU study) or 6 (DU study) weeks. The primary endpoint was the proportion of patients with endoscopically confirmed healed GU or DU.
Results
For GU, 93.5% (216/231) of vonoprazan‐treated patients and 93.8% (211/225) of lansoprazole‐treated patients achieved healed GU; non‐inferiority of vonoprazan to lansoprazole was confirmed [difference = −0.3% (95% CI −4.750, 4.208); P = 0.0011]. For DU, 95.5% (170/178) of vonoprazan‐treated patients and 98.3% (177/180) of lansoprazole‐treated patients achieved healed DU; non‐inferiority to lansoprazole was not confirmed [difference = −2.8% (95% CI −6.400, 0.745); P = 0.0654]. The incidences of treatment‐emergent adverse events were slightly lower for GU and slightly higher for DU with vonoprazan than with lansoprazole. There was one death (subarachnoid haemorrhage) in the vonoprazan group (DU). The possibility of a relationship between this unexpected patient death and the study drug could not be ruled out. In both studies, increases in serum gastrin levels were greater in vonoprazan‐treated vs. lansoprazole‐treated patients; levels returned to baseline after treatment in both groups.
Conclusions
Vonoprazan 20 mg has a similar tolerability profile to lansoprazole 30 mg and is non‐inferior with respect to GU healing and has similar efficacy for DU healing.
Introduction
Peptic ulcer disease is a chronic acid‐related disease that affects up to 20% of the adult Asian population.1 Ulcers usually occur in the stomach [gastric ulcer (GU)] or duodenum [duodenal ulcer (DU)] and, if left untreated, can recur or result in acute gastrointestinal bleeding.2 The two main risk factors for gastrointestinal injury and acid‐related peptic ulcers are Helicobacter pylori infection and the use of nonsteroidal anti‐inflammatory drugs (NSAIDs).3 Both of these factors are becoming increasingly common in Asian populations.1
The treatment strategy for peptic ulcers is to minimise damage to gastrointestinal mucosa by targeting acid secretion. Currently, most patients with GU or DU are treated with proton pump inhibitors (PPIs), such as lansoprazole. PPIs inhibit gastric H+,K+‐ATPase by forming a covalent bond which, in turn, inhibits gastric acid secretion.4 PPIs require the presence of secreted gastric acid to be converted to their active form. Once converted, PPIs limit further secretion by targeting the gastric acid pump.5 While PPIs are generally effective in the suppression of gastric acid, up to one‐third of patients with acid‐related diseases continue to experience symptoms after treatment.6 In addition, PPIs require approximately 2–3 days to exert maximum acid‐inhibitory effects and, due to the mechanism of PPIs, some patients do not experience adequate acid control at night.7 These characteristics of PPIs mean that some patients may continue to experience acid‐related damage to the stomach and duodenum.
Vonoprazan is a potassium‐competitive acid blocker (P‐CAB), which is a class of competitive potassium inhibitors that reversibly inhibit the gastric acid pump in a K+‐competitive manner.4 Vonoprazan was observed to be an effective acid suppressant in laboratory and animal studies and this effect was longer lasting than that observed with lansoprazole.4, 8, 9 In addition, two phase 1 clinical studies have shown that vonoprazan caused rapid and sustained acid suppression in healthy volunteers.10, 11 However, no studies have been conducted to assess the efficacy and safety of vonoprazan compared with lansoprazole in patients with GUs or DUs. The primary objective of the current phase 3 clinical studies was to confirm the efficacy of vonoprazan in patients with GU or DU by verifying the non‐inferiority of vonoprazan with lansoprazole. The secondary objective was to assess the safety of vonoprazan compared with lansoprazole in patients with GU or DU.
Materials and methods
Study design
Two phase 3, non‐inferiority, randomised, double‐blind, double‐dummy, multicentre, parallel group studies were conducted in Japan (GU study: NCT01452711; DU study: NCT01452724) between November 2011 and December 2012 at 83 sites (GU study) and October 2011 to February 2013 at 76 sites (DU study). The studies were conducted in adherence with the Declaration of Helsinki, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, and applicable Japanese regulatory requirements. The Institutional Review Board for each study site approved the clinical study. All patients provided written informed consent before any study procedures were initiated.
Study population
Out‐patients at the study centres were eligible for inclusion if they were aged at least 20 years at the time of informed consent and had at least 1 endoscopically confirmed GU or DU with a white coating that was at least 5 mm wide at the start of the treatment period (Visit 1). An ulcer was defined as a defective mucosa with a white coating, with or without blood clots. Female patients of child‐bearing age were required to agree to use contraception from the date of consent until 4 weeks after study completion.
Patients were excluded from either study for the following reasons: previously received vonoprazan, received any investigational compound within 84 days, or received treatment for an endoscopically confirmed ulcer within 7 days; an endoscopically confirmed linear ulcer (GU) or noncicatrised linear ulcer (DU), post‐operative ulcer, suspected malignant ulcer, acute duodenal or gastric mucosal lesion, or an ulcer for which medicinal therapy in the current studies was not indicated; a history of, or treatment for malignancy within the past 5 years; undergone endoscopic haemostasis for GU or DU within 30 days; been diagnosed with Zollinger–Ellison syndrome or other gastric acid hypersecretion disorders; surgery that could affect gastric acid secretion; or creatinine > 2 mg/dL, alanine aminotransferase or aspartate aminotransferase >2.5 × upper limit of normal (ULN), or total bilirubin >2 × ULN. Patients in the GU study were excluded if they had an endoscopically confirmed DU and, similarly, patients in the DU study were excluded if they had a GU.
Randomisation, treatment and follow‐up
The independent Clinical Study Management Division at Bell Medical Solutions Inc., Tokyo, Japan carried out centralised randomisation and allocation to treatment using a double‐dummy blinding method with key codes kept offsite by an external data manager. Study patients were randomly assigned at a 1:1 ratio to receive either vonoprazan 20 mg (Takeda Pharmaceutical Company Ltd, Osaka, Japan) or lansoprazole 30 mg once daily after eating breakfast for 8 weeks (GU) or 6 weeks (DU). Endoscopy was performed at screening, Week 2, Week 4, and Week 8 (GU) and Week 2, Week 4 and Week 6 (DU). The follow‐up period began when healing of GU or DU was endoscopically confirmed (the white ulcer coating was not visible), or at Week 8 (GU) or Week 6 (DU). After healing was endoscopically confirmed, follow‐up continued for 8 weeks (GU), 6 weeks (DU), or until recovery of the baseline serum gastrin level. Patients whose serum gastrin level was not elevated from baseline were not required to enter the follow‐up period. Each patient who had endoscopically confirmed GU or DU healing at Week 2, 4 or 8 (GU), and at Week 2, 4 or 6 (DU) was discontinued from the study medication and the patient was considered to be a completed case. The primary endpoint was the proportion of patients with endoscopically confirmed GU or DU healing at Week 8 (GU) or Week 6 (DU). Secondary endpoints were the proportion of patients with endoscopically confirmed GU and DU healing at Weeks 2 and 4 and resolution of gastrointestinal symptoms related to GU or DU during the treatment.
Outcome measures
Demographic outcomes
During Visit 1, information on patient demographics, medical history, concomitant medications, CYP2C19 genotype, and history of treatment for H. pylori were documented. Endoscopy was performed to determine the site, number, size and shape of current ulcers. Data on patient ulcers were also collected, including date of onset, use of NSAIDs or low‐dose aspirin, whether the ulcer was a first onset or relapse, and gastrointestinal symptoms related to GU or DU. Endoscopy was not repeated for patients who had endoscopic confirmation of their ulcer within 7 days before Visit 1.
Efficacy outcomes
The proportions of patients with healed GU or DU were measured at Week 2, Week 4, and Week 8 (GU), and Week 2, Week 4 and Week 6 (DU) after the start of treatment. The stomach (GU study) or duodenum (DU study) was endoscopically examined under fasting conditions and the largest diameter of the current ulcer was measured at its white coating using endoscopic forceps. Ulcers were considered healed if the white coating was no longer visible by endoscopy. Gastrointestinal symptoms related to GU or DU [epigastric pain (postprandial and fasting/nocturnal), abdominal distension, nausea and queasiness, heartburn, and anorexia] were recorded at Visit 1 (baseline) and at each clinic visit, and the dates of symptom resolution were noted.
Safety outcomes
Adverse events, clinical laboratory test values, electrocardiogram (ECG) findings, and vital signs were recorded at each visit during the treatment period. Clinical laboratory test values and serum gastrin and pepsinogen I/II levels were measured at Mitsubishi Chemical Medience (Tokyo, Japan) after each visit.
Statistical analysis
Sample size was determined according to the primary endpoint. Data from previous studies showed that the proportion of patients with healed GU with lansoprazole vs. famotidine was 93.4% and 82.5% at Week 8 (point estimate of the difference: 10.9%), and the proportion of patients with healed DU was 97.8% and 91.5% at Week 6 (point estimate of the difference: 6.3%) (data on file, Takeda Pharmaceutical Company Ltd). Therefore, the proportion of patients with healed GU in this study was expected to be 93.4% for both vonoprazan and lansoprazole. The proportion of patients with healed DU was expected to be 97.8%. Based on these assumptions, a sample size of 215 patients per treatment group in the GU study would have a power of more than 90% to detect non‐inferiority for the difference in the proportion of patients with healed GU between the treatment groups [with a non‐inferiority margin of 8% at the lower limit of two‐sided 95% confidence intervals (CIs) in the proportion difference]. Taking into account dropouts after treatment assignment, 240 randomised patients would be required for each treatment group in the GU study. A sample size of 175 patients per treatment group in the DU study would have a power of more than 90% to detect non‐inferiority for the difference in the proportion of patients with healed DU between the treatment groups (with a non‐inferiority margin of 6% at the lower limit of two‐sided 95% CIs in the proportion difference). Taking into account dropouts after treatment assignment, 195 randomised patients would be required for each treatment group in the DU study. The non‐inferiority margins of 8% (GU) and 6% (DU) were selected based on previous studies of lansoprazole (data on file, Takeda Pharmaceutical Company Ltd).
Three analysis sets were defined in these studies: a full analysis set (FAS), a per‐protocol set (PPS), and a safety analysis set. The FAS population included all patients who were randomised to study treatment and received at least one dose of study drug, excluding those patients who were found at baseline to have gastric or duodenal cancer, or did not have evidence of the condition being studied. The PPS population included all FAS patients with an evaluable primary endpoint who were randomised to study treatment, completed study treatment, and had no major protocol deviation. The safety population included all patients who received at least one dose of study drug.
Demographic and other baseline characteristics, ulcer characteristics, serum gastrin and pepsinogen I/II levels, H. pylori history, and CYP2C19 genotype were summarised by treatment group. The proportions of patients with healed GU or DU and gastrointestinal symptoms were analysed by calculating frequency, point estimates, and two‐sided 95% CIs by treatment group. Non‐inferiority was assessed using a Farrington and Manning test.12 One‐sided significance levels were 2.5% for the non‐inferiority tests.
Adverse events were summarised by treatment group. A treatment‐emergent adverse event (TEAE) was defined as an adverse event occurring after receiving the study drug. TEAEs were summarised by treatment group and categorised by severity. All analyses were conducted using sas software version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Demographic and baseline clinical characteristics
A total of 498 individuals consented to participate in the GU Study and 482 were randomised to receive vonoprazan (N = 244) or lansoprazole (N = 238; Figure 1). Of these, 92.2% (225/244) and 93.3% (222/238) completed the treatment phase, respectively; the main reasons for discontinuation from treatment were protocol deviations and adverse events (Figure 1). A total of 217 (vonoprazan) and 212 (lansoprazole) patients with endoscopically healed GU entered the follow‐up phase. Of these, 96.3% (209/217) and 95.8% (203/212) completed the follow‐up phase, respectively; the main reason for discontinuation from follow‐up was adverse events. The most common adverse events leading to study discontinuation were gastrointestinal disorders.
Figure 1.
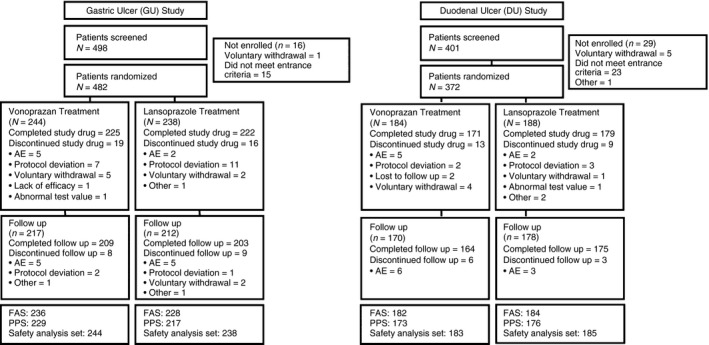
Disposition of patients in the gastric ulcer and duodenal ulcer studies. AE, adverse event; DU , duodenal ulcer; FAS , full analysis set; GU, gastric ulcer; PPS, per protocol set. Note, only patients with endoscopically confirmed healed GU or DU were eligible to enter the follow‐up phase.
A total of 401 individuals consented to participate in the DU study and 92.8% (372/401) were randomised to receive vonoprazan (N = 184) or lansoprazole (N = 188; Figure 1). Of these, 92.9% (171/184) and 95.2% (179/188) completed the treatment phase, respectively; the main reasons for discontinuation from treatment were adverse events and protocol deviations (Figure 1). A total of 170 (vonoprazan) and 178 (lansoprazole) patients with endoscopically healed DU entered the follow‐up phase. Of these, 96.5% (164/170) and 98.3% (175/178) completed the follow‐up phase, respectively; the main reason for discontinuation from follow‐up was adverse events. The most common adverse events leading to study discontinuation were nervous system disorders [vonoprazan group: dizziness (1 patient) and subarachnoid haemorrhage (1 patient); lansoprazole group: headache (1 patient)] and gastrointestinal disorders [vonoprazan group: DU haemorrhage (1 patient); lansoprazole group: acute pancreatitis (1 patient) and vomiting (1 patient)].
No obvious differences in demographic characteristics were observed between treatment groups in either the GU or DU study. However, it was observed that approximately two‐thirds of patients were men, and patients in the DU study were, on average, close to 10 years younger than those in the GU study [mean (s.d.) = 50 (14.7) vs. 58.4 (13.3) years] (Table 1). Most patients were not using NSAIDs or low‐dose aspirin at enrolment, were positive for H. pylori, and had a CYP2C19 EM genotype. Most patients had a single ulcer and the most common size of ulcers was 5–10 mm. Approximately 40% of patients in the GU study and 50% of patients in the DU study had a recurring ulcer.
Table 1.
Baseline characteristic of study patients in the gastric ulcer and duodenal ulcer studiesa
| Characteristic | GU study | DU study | ||||
|---|---|---|---|---|---|---|
| Vonoprazan | Lansoprazole | Total | Vonoprazan | Lansoprazole | Total | |
| (N = 244) | (N = 238) | (N = 482) | (N = 184) | (N = 188) | (N = 372) | |
| Age (years), mean (s.d.) | 58.2 (13.2) | 58.6 (13.5) | 58.4 (13.3) | 49.9 (14.6) | 50.2 (14.8) | 50.0 (14.7) |
| Male, n (%) | 163 (66.8) | 170 (71.4) | 333 (69.1) | 125 (67.9) | 120 (63.8) | 245 (65.9) |
| Height (cm), mean (s.d.) | 163.5 (9.7) | 163.8 (9.0) | 163.7 (9.4) | 164.9 (8.9) | 164.6 (9.8) | 164.8 (9.3) |
| Weight (kg), mean (s.d.) | 59.6 (11.6) | 60.6 (12.1) | 60.1 (11.8) | 63.0 (12.5) | 62.2 (12.8) | 62.6 (12.6) |
| BMI (kg/m2), Mean (s.d.) | 22.2 (3.5) | 22.5 (3.5) | 22.3 (3.5) | 23.1 (3.6) | 22.8 (3.5) | 23.0 (3.6) |
| NSAID/low‐dose aspirin use, n (%) | ||||||
| Yes, stopped before Visit 1 | 16 (6.6) | 17 (7.1) | 33 (6.8) | 14 (7.7) | 11 (5.9) | 25 (6.7) |
| Yes, ongoing | 20 (8.2) | 28 (11.8) | 48 (10.0) | 13 (7.1) | 17 (9.0) | 30 (8.1) |
| No | 208 (85.2) | 193 (81.1) | 401 (83.2) | 156 (85.2) | 160 (85.1) | 316 (85.2) |
| Helicobacter pylori positive, n (%) | 208 (85.2) | 191 (80.3) | 399 (82.8) | 152 (83.1) | 141 (75.8) | 293 (79.4) |
| CYP2C19 genotype, n (%) | ||||||
| Homozygous EM (*1/*1) | 94 (39.7) | 90 (38.0) | 184 (38.8) | 61 (33.9) | 60 (32.4) | 121 (33.2) |
| Heterozygous EM (*1/*2, *1/*3) | 102 (43.0) | 114 (48.1) | 216 (45.6) | 89 (49.4) | 89 (48.1) | 178 (48.8) |
| PM (*2/*2, *2/*3, *3/*3) | 41 (17.3) | 33 (13.9) | 74 (15.6) | 30 (16.7) | 36 (19.5) | 66 (18.1) |
| Current ulcers: number, n (%) | ||||||
| Single | 201 (82.4) | 175 (73.5) | 376 (78.0) | 136 (74.3) | 134 (71.3) | 270 (72.8) |
| Multiple | 43 (17.6) | 63 (26.5) | 106 (22.0) | 47 (25.7) | 54 (28.7) | 101 (27.2) |
| Current ulcers: shape, n (%) | ||||||
| Circular | 82 (33.6) | 106 (44.5) | 188 (39.0) | 69 (37.7) | 63 (33.5) | 132 (35.6) |
| Oval | 136 (55.7) | 106 (44.5) | 242 (50.2) | 95 (51.9) | 93 (49.5) | 188 (50.7) |
| Other | 26 (10.7) | 26 (10.9) | 52 (10.8) | 19 (10.4) | 32 (17.0) | 51 (13.7) |
| Current ulcers: size, n (%) | ||||||
| Smaller than 5 mm | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.5) | 0 (0.00) | 1 (0.3) |
| 5–10 mm | 116 (47.5) | 104 (43.7) | 220 (45.6) | 134 (73.2) | 130 (69.1) | 264 (71.2) |
| 10–20 mm | 86 (35.2) | 95 (39.9) | 181 (37.6) | 43 (23.5) | 52 (27.7) | 95 (25.6) |
| 20–30 mm | 29 (11.9) | 28 (11.8) | 57 (11.8) | 5 (2.7) | 2 (1.1) | 7 (1.9) |
| Larger than 30 mm | 13 (5.3) | 11 (4.6) | 24 (5.0) | 0 (0.0) | 4 (2.1) | 4 (1.1) |
| Ulcer history, n (%) | ||||||
| First episode | 154 (63.1) | 133 (55.9) | 287 (59.5) | 95 (51.9) | 92 (48.9) | 187 (50.4) |
| Recurrence | 90 (36.9) | 105 (44.1) | 195 (40.5) | 88 (48.1) | 96 (51.1) | 184 (49.6) |
BMI, body mass index; CYP2C19, cytochrome P450 2 C19; DU, duodenal ulcer; EM, extensive metaboliser; GU, gastric ulcer; NSAID, nonsteroidal anti‐inflammatory drug, PM, poor metaboliser, s.d., standard deviation.
This analysis includes patients who were randomised to study treatment.
Primary outcome measure
Gastric ulcers
The proportions of patients with healed GU verified by endoscopy at Week 8 were the same between the vonoprazan and lansoprazole groups (Table 2). The proportions of healed patients at Week 8 in the FAS population were 93.5% (216/231) and 93.8% (211/225) in the vonoprazan and lansoprazole groups respectively. The non‐inferiority of vonoprazan to lansoprazole with respect to the proportion of patients with healed GU at Week 8 was verified in the FAS population (difference = −0.3%; 95% CI: −4.750, 4.208; P = 0.0011) (Figure 2). These findings were supported by the secondary analyses in the PPS population. The proportions of patients with healed GU at Week 8 in the PPS population were 93.4% (214/229) and 94.5% (205/217) in the vonoprazan and lansoprazole groups respectively (difference = −1.0%; 95% CI: −5.438, 3.397; P = 0.0024).
Table 2.
Healing rates of gastric ulcers and duodenal ulcers during the 8‐week (GU) or 6‐week (DU) treatment period – FAS
| GU study (8 weeks) | DU study (6 weeks) | |||||
|---|---|---|---|---|---|---|
| Vonoprazan | Lansoprazole | Difference (Von.−Lans.) | Vonoprazan | Lansoprazole | Difference (Von.−Lans.) | |
| Week 2 | ||||||
| N | 231 | 225 | 178 | 180 | ||
| Healed, n (%) | 69 (29.9) | 73 (32.4) | −2.6 | 118 (66.3) | 115 (63.9) | 2.4 |
| 95% CI, lower, upper | 24.044, 36.221 | 26.373, 38.986 | −11.075, 5.926 | 58.839, 73.192 | 56.411, 70.901 | −7.469, 12.276 |
| Week 4 | ||||||
| N | 231 | 225 | 178 | 180 | ||
| Healed, n (%) | 169 (73.2) | 170 (75.6) | −2.4 | 167 (93.8) | 170 (94.4) | −0.6 |
| 95% CI, lower, upper | 66.955, 78.760 | 69.402, 81.023 | −10.407, 5.616 | 89.212, 96.875 | 90.021, 97.304 | −5.494, 4.245 |
| Study end | ||||||
| N | 231 | 225 | 178 | 180 | ||
| Healed, n (%) | 216 (93.5) | 211 (93.8) | −0.3 | 170 (95.5) | 177 (98.3) | −2.8 |
| 95% CI, lower, upper | 89.516, 96.320 | 89.780, 96.557 | −4.750, 4.208 | 91.337, 98.040 | 95.207, 99.655 | −6.400, 0.745 |
| Non‐inferioritya | ||||||
| P‐value | 0.0011 | 0.0654 | ||||
GU, gastric ulcer; DU, duodenal ulcer; FAS, full analysis set.
Farrington and Manning test with a non‐inferiority margin of 8% (GU) or 6% (DU).
Figure 2.
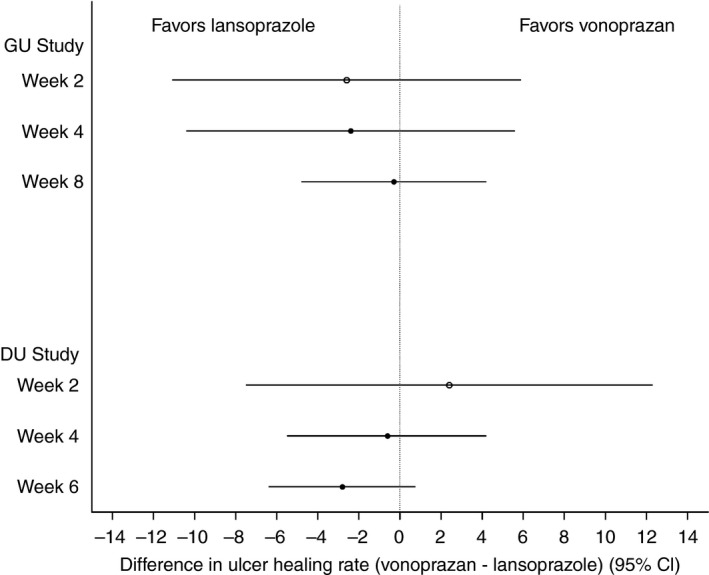
Difference in the proportions of patients with healed GU or DU between treatment groups [vonoprazan−lansoprazole (95% CI)]. Data are calculated using the full analysis set. CI, confidence interval; DU, duodenal ulcer; GU, gastric ulcer.
The proportion of healed patients in the FAS population was similar between the vonoprazan and lansoprazole groups at Week 2 or at Week 4. At Week 2, the proportions of healed patients were 29.9% (69/231) and 32.4% (73/225) in the vonoprazan and lansoprazole groups respectively (difference: −2.6%; 95% CI: −11.075, 5.926) (Table 2, Figure 2). At Week 4 they were 73.2% (169/231) and 75.6% (170/225) in the vonoprazan and lansoprazole groups respectively (difference: −2.4%; 95% CI: −10.407, 5.616) (Table 2, Figure 2). Preplanned subgroup analyses showed similar healing rates among CYP2C19 genotype subgroups and between H. pylori serology subgroups (Table 3).
Table 3.
Subanalyses of ulcer healing rates by CYP2C19 genotype and Helicobacter pylori serology (FAS)
| Variable | GU study | DU study | ||||||
|---|---|---|---|---|---|---|---|---|
| Vonoprazan | Lansoprazole | Vonoprazan | Lansoprazole | |||||
| Healed | Unhealed | Healed | Unhealed | Healed | Unhealed | Healed | Unhealed | |
| CYP2C19 genotype, n (%) | ||||||||
| Homozygous EM (*1/*1) | 84 (92.3) | 7 (7.7) | 81 (93.1) | 6 (6.9) | 57 (93.4) | 4 (6.6) | 57 (96.6) | 2 (3.4) |
| Heterozygous EM (*1/*2, *1/*3) | 96 (97.0) | 3 (3.0) | 101 (95.3) | 5 (4.7) | 84 (96.6) | 3 (3.4) | 87 (100.0) | 0 (0.0) |
| PM (*2/*2, *2/*3, *3/*3) | 36 (90.0) | 4 (10.0) | 29 (90.6) | 3 (9.4) | 29 (96.7) | 1 (3.3) | 33 (97.1) | 1 (2.9) |
| Helicbacter pylori serology, n (%) | ||||||||
| Positive | 189 (95.0) | 10 (5.0) | 176 (95.1) | 9 (4.9) | 142 (95.9) | 6 (4.1) | 133 (97.8) | 3 (2.2) |
| Negative | 27 (84.4) | 5 (15.6) | 35 (87.5) | 5 (12.5) | 28 (93.3) | 2 (6.7) | 44 (100.0) | 0 (0.0) |
CYP2C19, cytochrome P450 2 C19; DU, duodenal ulcer; EM, extensive metaboliser; GU, gastric ulcer; PM, poor metaboliser.
Both treatments had positive effects on gastrointestinal symptoms (Table 4). The resolution of heartburn was higher in the vonoprazan group compared with the lansoprazole group (difference: 14.5%; 95% CI: 2.820, 26.162; Table 4).
Table 4.
Resolution of ulcer‐related symptoms in study patients in the gastric ulcer and duodenal ulcer studiesa
| Symptom | GU study | DU study | ||||
|---|---|---|---|---|---|---|
| Vonoprazan | Lansoprazole | Difference (Von.−Lan.) | Vonoprazan | Lansoprazole | Difference (Von.−Lan.) | |
| Epigastric pain (postprandial) | ||||||
| N | 77 | 65 | 56 | 61 | ||
| Resolved, n (%) | 70 (90.9) | 61 (93.8) | −2.9 | 47 (83.9) | 53 (86.9) | −3.0 |
| 95% CI, lower, upper | 82.162, 96.266 | 84.987, 98.298 | −11.618, 5.744 | 71.672, 92.378 | 75.784, 94.164 | −15.774, 9.861 |
| Epigastric pain (fasting/nocturnal) | ||||||
| N | 120 | 118 | 116 | 123 | ||
| Resolved, n (%) | 109 (90.8) | 106 (89.8) | 1.0 | 98 (84.5) | 107 (87.0) | −2.5 |
| 95% CI, lower, upper | 84.190, 95.335 | 82.910, 94.634 | −6.507, 8.512 | 76.589, 90.536 | 79.737, 92.378 | −11.383, 6.365 |
| Abdominal distension | ||||||
| N | 42 | 44 | 59 | 38 | ||
| Resolved, n (%) | 39 (92.9) | 39 (88.6) | 4.2 | 48 (81.4) | 32 (84.2) | −2.9 |
| 95% CI, lower, upper | 80.517, 98.502 | 75.442, 96.206 | −7.969, 16.411 | 69.085, 90.308 | 68.747, 93.977 | −18.125, 12.415 |
| Nausea | ||||||
| N | 44 | 41 | 39 | 36 | ||
| Resolved, n (%) | 41 (93.2) | 37 (90.2) | 2.9 | 36 (92.3) | 34 (94.4) | −2.1 |
| 95% CI, lower, upper | 81.344, 98.571 | 76.869, 97.277 | −8.808, 14.684 | 79.130, 98.385 | 81.336, 99.320 | −13.359, 9.085 |
| Heartburn | ||||||
| N | 57 | 50 | 50 | 38 | ||
| Resolved, n (%) | 55 (96.5) | 41 (82.0) | 14.5 | 44 (88.0) | 33 (86.8) | 1.2 |
| 95% CI, lower, upper | 87.893, 99.572 | 68.563, 91.424 | 2.820, 26.162 | 75.690, 95.466 | 71.914, 95.586 | −12.865, 15.181 |
| Anorexia | ||||||
| N | 56 | 51 | 38 | 41 | ||
| Resolved, n (%) | 53 (94.6) | 43 (84.3) | 10.3 | 35 (92.1) | 36 (87.8) | 4.3 |
| 95% CI, lower, upper | 85.133, 98.881 | 71.412, 92.976 | −1.264, 21.922 | 78.623, 98.341 | 73.796, 95.919 | −8.884, 17.485 |
CI, confidence interval; DU, duodenal ulcer; GU, gastric ulcer.
This analysis includes all full analysis set patients who had gastrointestinal symptoms at the start of treatment.
Duodenal ulcers
The non‐inferiority of vonoprazan to lansoprazole for the proportion of patients with healed DU confirmed by endoscopy at Week 6 was not verified in the FAS population (P = 0.0654). Proportions of patients with healed DU at Week 6 in the FAS population were 95.5% (170/178) and 98.3% (177/180) in the vonoprazan and lansoprazole groups respectively (difference: −2.8%; 95% CI: −6.400, 0.745) (Table 2). However, the non‐inferiority of vonoprazan to lansoprazole was suggested by the secondary analyses in the PPS population. The proportions of healed patients at Week 6 in the PPS population were 97.1% (168/173) and 98.9% (174/176) in the vonoprazan and lansoprazole groups respectively (difference = −1.8%; 95% CI: −4.701, 1.193; P = 0.0171).
The proportions of patients with healed DU in the FAS population were similar between the vonoprazan and lansoprazole groups at Week 2 or at Week 4. At Week 2, the proportions of healed patients were 66.3% (118/178) and 63.9% (115/180) in the vonoprazan and lansoprazole groups respectively (difference: 2.4%; 95% CI: −7.469, 12.276). At Week 4 they were 93.8% (167/178) and 94.4% (170/180) in the vonoprazan and lansoprazole groups respectively (difference: −0.6%; 95% CI: −5.494, 4.245) (Table 2). Pre‐planned subgroup analyses showed similar healing rates among CYP2C19 genotype subgroups and between H. pylori serology subgroups (Table 3).
Both treatments had positive effects on gastrointestinal symptoms [epigastric pain (postprandial and fasting/nocturnal), abdominal distension, nausea and queasiness, heartburn, and anorexia] (Table 4). The resolution of symptoms during the study was similar between groups with the exception of heartburn and anorexia, which had numerically higher resolution rates in the vonoprazan group compared with the lansoprazole group.
Safety and tolerability measures
Overall, the safety and tolerability profile of vonoprazan for treatment of GU or DU was similar to that of lansoprazole (Table 5).
Table 5.
Treatment‐emergent adverse events and serious adverse events in patients in the gastric ulcer and duodenal ulcer studies (safety analysis set)
| Event | GU study | DU study | ||||||
|---|---|---|---|---|---|---|---|---|
| Vonoprazan (N = 244) | Lansoprazole (N = 238) | Vonoprazan (N = 183) | Lansoprazole (N = 185) | |||||
| Events | Patients (%) | Events | Patients (%) | Events | Patients (%) | Events | Patients (%) | |
| TEAEs | 101 | 65 (26.6) | 111 | 79 (33.2) | 85 | 63 (34.4) | 77 | 53 (28.6) |
| Related to study drug | 19 | 16 (6.6) | 19 | 14 (5.9) | 21 | 17 (9.3) | 11 | 9 (4.9) |
| Mild | 87 | 53 (21.7) | 104 | 73 (30.7) | 68 | 51 (27.9) | 63 | 44 (23.8) |
| Moderate | 10 | 9 (3.7) | 5 | 5 (2.1) | 11 | 8 (4.4) | 14 | 9 (4.9) |
| Severe | 4 | 3 (1.2) | 2 | 1 (0.4) | 6 | 4 (2.2) | 0 | 0 (0.0) |
| Leading to study drug discontinuation | 6 | 5 (2.0) | 2 | 2 (0.8) | 6 | 5 (2.7) | 3 | 2 (1.1) |
| SAEs | 7 | 6 (2.5) | 5 | 4 (1.7) | 8 | 6 (3.3) | 4 | 4 (2.2) |
| Related to study drug | 0 | 0 (0.0) | 3 | 2 (0.8) | 1 | 1 (0.5) | 0 | 0 (0.0) |
| Leading to study drug discontinuation | 2 | 2 (0.8) | 2 | 2 (0.8) | 4 | 3 (1.6) | 1 | 1 (0.5) |
| Deaths | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.5) | 0 | 0 (0.0) |
DU, duodenal ulcer; GU, gastric ulcer; SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
Gastric ulcers
In the GU study, the incidence of TEAEs was slightly lower in the vonoprazan group than in the lansoprazole group and most TEAEs in both groups were mild in severity (Table 5). Drug‐related TEAEs were reported in 6.6% (16/244) and 5.9% (14/238) of patients in the vonoprazan and lansoprazole groups respectively. The most common TEAEs in patients with GUs were gastrointestinal disorders, particularly upper abdominal pain, constipation, GU, abdominal discomfort, diarrhoea, and vomiting [vonoprazan: 16.8% (41/244) of patients; lansoprazole: 18.1% (43/238) of patients] and infections and infestations, particularly nasopharyngitis, bronchitis and oesophageal candidiasis [vonoprazan: 5.3% (13/244) of patients; lansoprazole: 7.1% (17/238) of patients]. Two patients in the lansoprazole group experienced serious adverse events that were considered to be related to the study drug (GU, interstitial lung disease and pulmonary hypertension). No drug‐related serious adverse events were reported in the vonoprazan group and no deaths were reported in either group.
Duodenal ulcers
In the DU study, the incidence of TEAEs was slightly higher in the vonoprazan group than in the lansoprazole group and most TEAEs were mild in severity (Table 5). Drug‐related TEAEs were reported in 9.3% (17/183) and 4.9% (9/185) of patients in the vonoprazan and lansoprazole groups respectively. The most common TEAEs in patients with DUs were gastrointestinal disorders, particularly upper abdominal pain, diarrhoea and DU [vonoprazan: 19.1% (35/183) of patients; lansoprazole: 14.6% (27/185) of patients] and infections and infestations, particularly nasopharyngitis and gastroenteritis [vonoprazan: 9.3% (17/183) of patients; lansoprazole: 8.1% (15/185) of patients].
One patient, a 46 year old Japanese male in the vonoprazan group, died of subarachnoid haemorrhage 12 h after receiving his first dose of study drug. The possibility of a relationship between this unexpected patient death and the study drug could not be ruled out. However, given the general course of subarachnoid haemorrhage, it is possible that the patient had a pre‐existing undiagnosed cerebral aneurysm or cerebral arteriovenous malformation at baseline. The subject's relevant medical history included alcohol use, smoking and high blood pressure readings in the past, which are known risk factors for subarachnoid haemorrhage. He had headache and increased blood pressure at baseline, both symptoms of subarachnoid haemorrhage. Therefore, given the time course of the symptoms, it is possible that the event may have started before the patient received the study drug.
In both the GU and DU studies, serum gastrin and pepsinogen I/II increased after treatment in both treatment groups; this increase was generally greater in the vonoprazan group than in the lansoprazole group. However, mean serum gastrin levels returned to baseline levels after the completion of the treatment period (Table 6) (Tables S1 and S2). No clinically significant changes in laboratory test values, vital signs, or ECG findings were reported in either group during the study.
Table 6.
Gastrin levels from baseline (pg/mL) during treatment and follow‐up periods of the gastric and duodenal ulcer studies (safety analysis set)
| Variable | GU study | DU study | ||||||
|---|---|---|---|---|---|---|---|---|
| Vonoprazan (N = 244) | Lansoprazole (N = 238) | Vonoprazan (N = 183) | Lansoprazole (N = 185) | |||||
| N | Mean (s.d.) | N | Mean (s.d.) | N | Mean (s.d.) | N | Mean (s.d.) | |
| Treatment period | ||||||||
| Baseline | 242 | 156.3 (181.6) | 238 | 147.2 (124.9) | 182 | 117.1 (103.3) | 183 | 118.2 (84.2) |
| Week 2 | 239 | 330.7 (351.9) | 237 | 245.4 (212.0) | 179 | 372.8 (253.1) | 185 | 261.2 (226.3) |
| Week 4 | 159 | 353.4 (274.3) | 151 | 256.3 (173.3) | 53 | 437.3 (272.4) | 64 | 295.6 (255.0) |
| Week 6 | NA | NA | NA | NA | 4 | 514.3 (255.9) | 8 | 245.8 (177.5) |
| Week 8 | 54 | 394.1 (307.3) | 52 | 289.3 (250.3) | NA | NA | NA | NA |
| Follow‐up | ||||||||
| Week 2 | 209 | 134.4 (103.3) | 205 | 153.9 (120.6) | 166 | 104.9 (69.8) | 173 | 123.9 (145.8) |
| Week 4 | 26 | 202.5 (89.9) | 41 | 216.5 (136.4) | 10 | 206.9 (134.4) | 19 | 204.6 (153.1) |
| Week 6 | NA | NA | NA | NA | 4 | 229.8 (148.1) | 5 | 339.4 (239.5) |
| Week 8 | 10 | 199.3 (129.7) | 12 | 199.3 (83.3) | NA | NA | NA | NA |
DU, duodenal ulcer; GU, gastric ulcer; NA, not applicable; s.d., standard deviation.
Discussion
In the GU study, the tolerability of vonoprazan was confirmed and was similar to that of lansoprazole 30 mg, and the non‐inferiority of vonoprazan to lansoprazole with respect to endoscopic healing of GUs during 8 weeks of treatment was verified. The tolerability of vonoprazan was also confirmed in the DU study; although the frequency of adverse events was slightly higher in the vonoprazan group compared with the lansoprazole group, overall, the safety and tolerability profile was similar. Non‐inferiority was not verified with respect to endoscopic healing of DUs during 6 weeks of treatment. These are the first randomised controlled trials investigating the effects of the P‐CAB, vonoprazan, in patients with peptic ulcer disease to be reported. The high healing rates, above 90%, highlight the potential benefits of vonoprazan in patients with peptic ulcer disease.
A total of 93.5% of patients in the vonoprazan group experienced GU healing at Week 8. While this is the first clinical study of vonoprazan in patients with GU, these results are not surprising given the pharmacokinetic and pharmacodynamic profiles of vonoprazan in healthy volunteers.10, 11 In two phase 1 clinical studies, vonoprazan was well tolerated and increased gastric pH within 4 h after a single dose. In one study, the 24 h pH > 4 holding time for vonoprazan 20 mg was 83.4% in Japanese and 85.2% in white healthy volunteers after 7 days of dosing.10 This is important because it is believed that peptic ulcer healing occurs above a pH of 3.13
While the non‐inferiority of vonoprazan with lansoprazole was verified in the GU study, it was not verified in the DU study, though healing rates between the two treatment groups were similar. This difference in non‐inferiority results between the GU and DU studies was unexpected as there are no pronounced differences between GUs and DUs and because both vonoprazan and lansoprazole provide sufficient acid suppression (i.e. both drugs have a sufficient pH3 holding time ratio) to heal peptic ulcers.13 In addition, extremely high healing rates were observed for both treatments, with more than 95% of patients experiencing DU healing with vonoprazan or lansoprazole. There are a number of possibilities as to why the results in patients with DU differed from those in patients with GU. For example, lansoprazole is thought to have an anti‐inflammatory effect,14, 15 which could potentially affect its activity in DU; however, this has not been published in the setting of DUs. The most likely explanation for the differences in non‐inferiority results between the GU and DU studies is the number of patients who discontinued study treatment early, which may have affected the results of the FAS analyses in the DU study. Overall, seven patients in the vonoprazan group and two patients in the lansoprazole group discontinued treatment early without confirmation of healed ulcers, did not receive treatment for the planned duration, and were considered to be non‐healed for the analysis. As all patients except 1 in each group had confirmed endoscopic healing of DU at Week 6, only one patient in each group was available at Week 6 to confirm a failure to heal. The healing status of the nine patients who withdrew from the study early was unknown, assumed to be non‐healed and, therefore, the data may have been skewed as a consequence of this assumption of non‐healing. Had these patients continued treatment and been assessed at Week 6, the non‐inferiority of vonoprazan may have been observed.
In addition to the quantitative endoscopic healing data, qualitative patient‐reported outcome data were also collected in both studies for patients who had symptoms at baseline. The symptom data collected included epigastric pain, abdominal distension, nausea, heartburn and anorexia, which are representative of upper acid‐related disease. Resolution of symptoms was observed by the last study visit in more than 80% of patients in both studies, which is an additional benefit to those patients who had both peptic ulcer disease and associated symptoms.
Overall, vonoprazan was well tolerated in Japanese patients with GU or DU for up to 8 (GU) or 6 (DU) weeks of treatment. The safety and tolerability profile was similar to the profile of lansoprazole in these studies. Most TEAEs were mild in severity and few drug‐related TEAEs were reported. Mean serum gastrin levels returned to baseline levels after patients completed treatment. Eleven per cent of the patients had increased gastrin levels 2 weeks after cessation of treatment, which might indicate that they also had rebound hypersecretion of acid, since gastrin stimulates gastric acid secretion. In fact, ulcer recurrence was observed in 11 patients with GU (seven in the vonoprazan group and four in the lansoprazole group) and in eight patients with DU (six in the vonoprazan group and two in the lansoprazole group) after study completion. Rebound hypersecretion may be the reason for the recurrent ulcers, but another possibility may be that most of these patients were positive for anti‐H. pylori IgG antibody at baseline. However, there was no significant difference between the treatment groups in the incidence of adverse events related to acid secretion. Furthermore, gastrointestinal medications other than mucosal protective agents were prohibited by the protocol during follow‐up to allow monitoring of the recovery of serum gastrin levels. Therefore, the recurrent ulcers were probably caused by H. pylori, not by rebound hypersecretion of acid.
One patient in the vonoprazan group died of subarachnoid haemorrhage. Although the possibility of a relationship between this patient death and the study drug could not be disregarded, it is possible that the event may have occurred before the patient received the study drug, given the time course of the symptoms. The patient had risk factors for (alcohol and tobacco use) and symptoms of (headache and increased blood pressure) subarachnoid haemorrhage at baseline. Although this patient, who developed subarachnoid haemorrhage, had been pointed out to have hypertension, he did not receive treatment with an antihypertensive drug. In general, the blood pressure variation when on a low dose of vonoprazan of 20 mg would be very slight and not likely to cause subarachnoid haemorrhage. As that patient had complained of a headache on the day before taking vonoprazan and took acetaminophen, the development of a subarachnoid haemorrhage was not likely related to vonoprazan.
The strengths of the studies included their randomised, double‐blind, double‐dummy, multi‐site designs; large sample sizes; and their focus on a Japanese population. However, the studies do have some limitations. Due to the study design, only non‐inferiority and not the superiority of vonoprazan to lansoprazole was examined. In the DU study, the early discontinuation of patients from treatment may have resulted in a misrepresentation of the effect of vonoprazan in this population of patients with DU as early withdrawals were categorised as non‐healed cases despite not being endoscoped on withdrawal. In addition, because the main causes of both GU and DU are H. pylori infection and the use of NSAIDs, patients with DU often present with concomitant GU,16 and patients with ulcers at both sites were excluded from this study. The exclusion of DU patients with GU may have affected the characteristics of the patient group in the DU study.
We believe that the patients who were positive for H. pylori received the recommended eradication therapy after completion of the study. All study patients signed an informed consent form stating ‘After healing of the ulcer, you may take treatments with H2 blockers or mucosal protectants, or H. pylori eradication (if H. pylori positive) for preventing the recurrence of ulcers’. In this study, you should transition to the follow‐up period to monitor the recovery of serum gastrin level after healing of the ulcer. In the follow‐up period, you can take mucosal protectants but cannot take H2 blockers and H. pylori eradication therapy. The recurrence of ulcers may occur after treatment, if you feel some kind of abnormality, please immediately tell the investigator, sub‐investigator or study coordinators’.
The Maastricht IV/Florence Consensus Report states that long‐term treatment with PPIs in H. pylori positive patients is associated with the development of a corpus predominant gastritis and accelerates atrophic gastritis.17 However, in this study, the treatment period was only 8 weeks for treatment of GU and 6 weeks for treatment of DU, not long term; therefore, atrophic gastritis in the corpus was unlikely to accelerate even in patients with H. pylori infection.
In conclusion, the findings showed that vonoprazan 20 mg has a similar tolerability profile to lansoprazole 30 mg and is non‐inferior with respect to the proportion of patients with healed GU at Week 8. Although non‐inferiority to lansoprazole 30 mg was not shown with respect to the proportion of patients with healed DU, more than 95% of patients in both groups experienced DU healing, which suggests that vonoprazan may be an effective treatment in patients with DU. The P‐CAB vonoprazan is a novel compound for the treatment of peptic ulcer disease and is a clinically useful alternative to PPIs. Vonoprazan provides a further treatment option for prescribing physicians. Clinical trials.gov registration NCT01452711 (GU study); NCT01452724 (DU study).
Authorship
Guarantor of the article: Yuuichi Sakurai.
Author contributions: All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. YM and YS were involved in the study design and YT was involved in the statistical analyses. HM was a primary investigator in the study. NU was an investigator in the study. All authors have approved the final version of the article, including the authorship list.
Supporting information
Table S1. Summary of gastrin levels by duration of exposure and visit in gastric ulcer patients: safety analysis set.
Table S2. Summary of gastrin levels by duration of exposure and visit in duodenal ulcer patients: safety analysis set.
Acknowledgements
The authors would like to thank the patients in both studies, and Richard Jenkins from Takeda Development Centre, Europe, Göran Hasselgren and Fiona Steinkamp of Takeda Pharmaceuticals International GmbH for reviewing the manuscript.
Declaration of personal interests: Noriya Uedo and Jiro Watari have received research funding from Takeda Pharmaceutical Company Limited. Tomohide Tatsumi and Nobuhiro Sakaki have received support for consulting services from Takeda Pharmaceutical Company Limited. Hiroto Miwa has received funding support for research and fees for lectures from Takeda Pharmaceutical Company Limited. Yuuya Mori, Akira Nishimura, Yuuichi Sakurai, and Yohei Takanami are employees of Takeda Pharmaceutical Company Limited.
Declaration of funding interests: This study was funded in full by Takeda Pharmaceutical Company Limited. The writing of this paper was funded by Takeda Pharmaceutical Company Limited and was provided by Dr Elise Magatova, PhD and Dr Serina Stretton, PhD, CMPP of ProScribe – Envision Pharma Group. ProScribe's services complied with international guidelines for Good Publication Practice (GPP3). Takeda Pharmaceutical Company Limited was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Investigators in the Vonoprazan GU/DU Study Group
Tomoyuki Ohta, Sapporo Higashi Tokushukai Hospital, Sapporo, Hokkaido, Japan; Toshiro Kusakabe, Higashi Sapporo Hospital, Sapporo, Hokkaido, Japan; Kazuhisa Fukuda, Sapporo Tokushukai Medical Corporation, Sapporo, Hokkaido, Japan; Ikuo Mitani, Mitani Clinic, Sapporo, Hokkaido, Japan; Harufumi Oizumi, Oizumi Gastrointestinal and Internal Medicine Clinic, Yamagata, Yamagata, Japan; Shinji Hirai, Hitachi General Hospital, Hitachi, Ibaraki, Japan; Kanho Rai, Hitachinaka General Hospital, Hitachinaka, Ibaraki, Japan; Kentaro Sugano, Jichi Medical University Hospital, Shimotsuke, Tochigi, Japan; Hideyuki Hiraishi, Dokkyo Medical University Hospital, Mibu, Tochigi, Japan; Keiichi Tominaga and Takero Koike, Japanese Red Cross Ashikaga Hospital, Ashikaga, Tochigi, Japan; Hironori Masuyama, Masuyama Gastrointestinal Clinic, Ohtawara, Tochigi, Japan; Toshiro Kubo, Saitama Jikei Hospital, Kumagaya, Saitama, Japan; Kou Nishikawa, Ageo Central General Hospital, Ageo, Saitama, Japan; Etsuro Iwashita, Iwashita Etsuro Shoukakinaika Clinic, Tokorozawa, Saitama, Japan; Ryosaku Azemoto, Kimitsu Chuo Hospital, Kisarazu, Chiba, Japan; Kazuhiro Ono and Jouji Yamamoto, Kamagaya General Hospital, Kamagaya, Chiba, Japan; Shinichi Takahashi, Kyorin University Hospital, Mitaka, Tokyo, Japan; Yasuyoshi Takeo, Hachioji Digestive Disease Hospital, Hachioji, Tokyo, Japan; Yuichi Takeda, Showa Central Hospital, Kodaira, Tokyo, Japan; Hisato Maekawa, Tokyo Takanawa Hospital, Minato‐ku, Tokyo, Japan; Nobuyuki Matsuhashi, NTT Medical Center Tokyo, Shinagawa‐ku, Tokyo, Japan; Tetsuya Sanji, Tokyo Kamata Medical Center, Ota‐ku, Tokyo, Japan; Hironori Kowazaki, Kohsei Chuo General Hospital, Meguro‐ku, Tokyo, Japan; Nobutoshi Watanabe, Tama Medical Clinic, Kokubunji, Tokyo, Japan; Masae Banno, Banno Clinic, Ota‐ku, Tokyo, Japan; Tomofumi Murakami, Shimokitazawa Tomo Clinic, Setagaya‐ku, Tokyo, Japan; Akira Mizuki, Keiyu Hospital, Yokohama, Kanagawa, Japan; Seiji Otsuka, Nippon Koukan Hospital, Kawasaki, Kanagawa, Japan; Daishu Toya, Fukui‐ken Saiseikai Hospital, Fukui, Fukui, Japan; Yuichiro Kojima, Yamanashi Prefectural Central Hospital, Kofu, Yamanashi, Japan; Kei Matsuki, Matsuki Clinic, Shizuoka, Shizuoka, Japan; Takahisa Suzuki, TOYOTA Memorial Hospital, Toyota, Aichi, Japan; Naoki Wakabayashi, Otsu Municipal Hospital, Otsu, Shiga, Japan; Hirozumi Obata, Obata Medical Clinic, Kyoto, Kyoto, Japan; Kozo Kajimura, Kishiwada City Hospital, Kishiwada, Osaka, Japan; Wataru Ono, Kishiwada Tokushukai Hospital, Kishiwada, Osaka, Japan; Shinji Kitamura, Sakai City Hospital, Sakai, Osaka, Japan; Tatsuichi An, Bell Land General Hospital, Sakai, Osaka, Japan; Makoto Ichiba, Toyonaka Municipal Hospital, Toyonaka, Osaka, Japan; Nobuyuki Inoue, Suita Municipal Hospital, Suita, Osaka, Japan; Kunio Suzuki, Saiseikai Senri Hospital, Suita, Osaka, Japan; Makoto Sanomura, Hokusetsu General Hospital, Takatsuki, Osaka, Japan; Takashi Tamada, Takatsuki Red Cross Hospital, Takatsuki, Osaka, Japan; Hitoshi Hongo, Fujita Gastroenterological Hospital, Takatsuki, Osaka, Japan; Masahiro Kido, Hirakata kohsai Hospital, Hirakata, Osaka, Japan; Tamotsu Fujibayashi and Hidemitsu Nakagawa, Nozaki Tokushukai Hospital, Daito, Osaka, Japan; Kiyoshi Ashida, Osaka Saiseikai Nakatsu Hospital, Osaka, Osaka, Japan; Wataru Ueda, Osaka City Juso Hospital, Osaka, Osaka, Japan; Soken Sai, Sai Gastroentric Proctology Clinic, Fujiidera, Osaka, Japan; Yuichi Yasunaga, Hyogo Prefectural Nishinomiya Hospital, Nishinomiya, Hyogo, Japan; Takashi Abe, Takarazuka City Hospital, Takarazuka, Hyogo, Japan; Tetsuro Inokuma, Kobe City Medical Center General Hospital, Kobe, Hyogo, Japan; Mikitaka Iguchi, Wakayama Medical University Hospital, Wakayama, Wakayama, Japan; Masao Yoshioka, Okayama Saiseikai General Hospital, Okayama, Okayama, Japan; Atsuyoshi Hirano, Simonoseki Medical Center, Shimonoseki, Yamaguchi, Japan; Mitsuyasu Yano, Tokushima Prefectural Central Hospital, Tokushima, Tokushima, Japan; Mitsushige Shibatouge, Takamatsu Red Cross Hospital, Takamatsu, Kagawa, Japan; Tomoki Inaba, Kagawa Prefectural Central Hospital, Takamatsu, Kagawa, Japan; Hideyuki Inoue, Kagawa Rosai Hospital, Marugame, Kagawa, Japan; Terufumi Sakai and Tetsuji Akiyama, St. Mary's Hospital, Kurume, Fukuoka, Japan; Ryuichiro Maekawa, Tagawa Hospital, Tagawa, Fukuoka, Japan; Makoto Kohakura, Nagata Hospital, Yanagawa, Fukuoka, Japan; Yasunori Kyoyama, Shinyukuhashi Hospital, Yukuhashi, Fukuoka, Japan; Teppei Kabemura and Toshiaki Ochiai, Saiseikai Fukuoka General Hospital, Fukuoka, Fukuoka, Japan; Yoshiharu Chijiiwa, Harasanshin Hospital, Fukuoka, Fukuoka, Japan; Kyota Higashi and Yashuhiro Ota, Fukuoka Wajiro Hospital, Fukuoka, Fukuoka, Japan; Michio Ando, Fukuoka Kieikai Hospital, Fukuoka, Fukuoka, Japan; Tetsu Yamaguchi, Fukuoka Kinen Hospital, Fukuoka, Fukuoka, Japan; Tetsuo Hisadome, Shin Komonji Hospital, Kitakyushu, Fukuoka, Japan; Suketo Sou, Tobata Kyoritsu Hospital, Kitakyushu, Fukuoka, Japan; Shigeo Nakamura, Steel Memorial Yawata Hospital, Kitakyushu, Fukuoka, Japan; Shigeru Fujii, Fukuoka Shin Mizumaki Hospital, Mizumaki, Fukuoka, Japan; Sadamune Hatakeyama, Hatakeyama Clinic, Fukuoka, Fukuoka, Japan; Kouji Mori, Mori Clinic, Fukuoka, Fukuoka, Japan; Shinichi Ogata, Saga Prefectural Hospital Koseikan, Saga, Saga, Japan; Hidetoshi Oda, Sasebo Chuo Hospital, Sasebo, Nagasaki, Japan; Yutaka Fukuda, Yutaka Fukuda Clinic, Nagasaki, Nagasaki, Japan; Michiharu Mihara, Kumamoto City Hospital, Kumamoto, Kumamoto, Japan; Shinichi Yoshimatsu and Masato Sasaki, Kumamoto Rosai Hospital, Yatsushiro, Kumamoto, Japan; Masaomi Maeda, Hanabata Clinic, Kumamoto, Kumamoto, Japan; Takashi Oribe, Oribe Digestive Clinic, Oita, Oita, Japan; Toru Niihara, Nanpuh Hospital, Kagoshima, Kagoshima, Japan; Hitoshi Uchizono, Kagoshima Medical Association Hospital, Kagoshima, Kagoshima, Japan; Koki Ido, Osumikanoya Hospital, Kanoya, Kagoshima, Japan; Satoshi Tamaki, Nanbutokushukai Hospital, Yaese, Okinawa, Japan.
The Handling Editor for this article was Professor Roy Pounder, and it was accepted for publication after full peer‐review.
The copyright line for this article was changed on 28 November 2016 after original online publication.
References
- 1. Lau JY, Barkun A, Fan DM, et al Challenges in the management of acute peptic ulcer bleeding. Lancet 2013; 381: 2033–43. [DOI] [PubMed] [Google Scholar]
- 2. Tang RS, Wu JC. Managing peptic ulcer and gastroesophageal reflux disease in elderly Chinese patients–focus on esomeprazole. Clin Interven Aging 2013; 8: 1433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sung JJ, Kuipers EJ, El‐Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther 2009; 29: 938–46. [DOI] [PubMed] [Google Scholar]
- 4. Hori Y, Imanishi A, Matsukawa J, et al 1‐[5‐(2‐Fluorophenyl)‐1‐(pyridin‐3‐ylsulfonyl)‐1 h‐pyrrol‐3‐Yl]‐N‐methylmethanamin E monofumarate (TAK‐438), a novel and potent potassium‐competitive acid blocker for the treatment of acid‐related diseases. J Pharm Exp Ther 2010; 335: 231–8. [DOI] [PubMed] [Google Scholar]
- 5. Shin JM, Inatomi N, Munson K, et al Characterization of a novel potassium‐competitive acid blocker of the gastric H, K‐atpase, 1‐[5‐(2‐fluorophenyl)‐1‐(pyridin‐3‐ylsulfonyl)‐1 h‐pyrrol‐3‐Yl]‐N‐methylmethanamin E monofumarate (TAK‐438). J Pharm Exp Ther 2011; 339: 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gisbert JP, Cooper A, Karagiannis D, et al Management of gastro‐oesophageal reflux disease in primary care: a European observational study. Curr Med Res Opin 2009; 25: 2777–84. [DOI] [PubMed] [Google Scholar]
- 7. Simon WA, Herrmann M, Klein T, et al Soraprazan: setting new standards in inhibition of gastric acid secretion. J Pharm Exp Ther 2007; 321: 866–74. [DOI] [PubMed] [Google Scholar]
- 8. Hori Y, Matsukawa J, Takeuchi T, et al A study comparing the antisecretory effect of Tak‐438, a novel potassium‐competitive acid blocker, with lansoprazole in animals. J Pharm Exp Ther 2011; 337: 797–804. [DOI] [PubMed] [Google Scholar]
- 9. Matsukawa J, Hori Y, Nishida H, et al A comparative study on the modes of action of TAK‐438, a novel potassium‐competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol 2011; 81: 1145–51. [DOI] [PubMed] [Google Scholar]
- 10. Jenkins H, Sakurai Y, Nishimura A, et al Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK‐438 (vonoprazan), a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2015; 41: 636–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sakurai Y, Nishimura A, Kennedy G, et al Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK‐438 (vonoprazan) doses in healthy male Japanese/non‐Japanese subjects. Clin Transl Gastroenterol 2015; 6: e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non‐zero risk difference or non‐unity relative risk. Stat Med 1990; 9: 1447–54. [DOI] [PubMed] [Google Scholar]
- 13. Hunt RH, Cederberg C, Dent J, et al Optimizing acid suppression for treatment of acid‐related diseases. Dig Dis Sci 1995; 40(2 Suppl.): 24S–49S. [DOI] [PubMed] [Google Scholar]
- 14. Handa O, Yoshida N, Fujita N, et al Molecular mechanisms involved in anti‐inflammatory effects of proton pump inhibitors. Inflamm Res 2006; 55: 476–80. [DOI] [PubMed] [Google Scholar]
- 15. Iwahi T, Satoh H, Nakao M, et al Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against Helicobacter pylori . Antimicrob Agents Chemother 1991; 35: 490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Take S, Mizuno M, Ishiki K, et al Reinfection rate of Helicobacter pylori after eradication treatment: a long‐term prospective study in Japan. J Gastroenterol 2012; 47: 641–6. [DOI] [PubMed] [Google Scholar]
- 17. Malfertheiner P, Megraud F, O'Morain CA, et al Management of Helicobacter pylori infection—the Maastricht IV/Florence consensus report. Gut 2012; 61: 646–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of gastrin levels by duration of exposure and visit in gastric ulcer patients: safety analysis set.
Table S2. Summary of gastrin levels by duration of exposure and visit in duodenal ulcer patients: safety analysis set.


