Abstract
Rationale: Chronic obstructive pulmonary disease is an independent risk factor for lung cancer, but the underlying molecular mechanisms are unknown. We hypothesized that lung stromal cells activate pathological gene expression programs that support oncogenesis.
Objectives: To identify molecular mechanisms operating in the lung stroma that support the development of lung cancer.
Methods: The study included subjects with and without lung cancer across a spectrum of lung-function values. We conducted a multiomics analysis of nonmalignant lung tissue to quantify the transcriptome, translatome, and proteome.
Measurements and Main Results: Cancer-associated gene expression changes predominantly manifested as alterations in the efficiency of mRNA translation modulating protein levels in the absence of corresponding changes in mRNA levels. The molecular mechanisms that drove these cancer-associated translation programs differed based on lung function. In subjects with normal to mildly impaired lung function, the mammalian target of rapamycin (mTOR) pathway served as an upstream driver, whereas in subjects with severe airflow obstruction, pathways downstream of pathological extracellular matrix emerged. Consistent with a role during cancer initiation, both the mTOR and extracellular matrix gene expression programs paralleled the activation of previously identified procancer secretomes. Furthermore, an in situ examination of lung tissue showed that stromal fibroblasts expressed cancer-associated proteins from two procancer secretomes: one that included IL-6 (in cases of mild or no airflow obstruction), and one that included BMP1 (in cases of severe airflow obstruction).
Conclusions: Two distinct stromal gene expression programs that promote cancer initiation are activated in patients with lung cancer depending on lung function. Our work has implications both for screening strategies and for personalized approaches to cancer treatment.
Keywords: cancer, COPD, translation, secretome, fibroblast
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic obstructive pulmonary disease is an independent risk factor for lung cancer, but how the underlying molecular mechanisms relate to lung function remains undefined.
What This Study Adds to the Field
We show that two distinct stromal gene expression programs that promote cancer initiation are activated in patients with cancer depending on lung function. Our work has major implications for both screening strategies and personalized approaches to cancer treatment.
The stroma includes endothelial cells, pericytes, fibroblasts, and immune cells (1–3). Stromal alterations have been directly linked to oncogenesis. In a mouse model of breast cancer, loss of the tumor suppressor gene PTEN in stromal fibroblasts accelerated cancer initiation and progression (4), partially depending on fibroblast-secreted factors (5). Similarly, secreted factors from senescent fibroblasts promote tumor formation in mice (6, 7). Therefore, mouse models indicate that stromal cells are capable of expressing secreted factors that favor tumor initiation. Chronic obstructive pulmonary disease (COPD) is a risk factor for developing lung cancer independently of smoking (8–10). Moreover, there appears to be a biological link between the lung stroma and lung cancer risk, as lung cancer is more likely to arise in proximity to regions of more severe emphysema (9). Yet, the molecular mechanisms operating in lung stromal cells that promote human lung carcinogenesis are unknown.
Gene expression is dynamically regulated by modulation of one or more mechanisms that control protein levels, including transcription, mRNA stability, and mRNA translation (hereafter referred to as translation) (11). Consequently, protein measurements do not reveal upstream regulatory mechanisms (12–17), and steady-state mRNA measurements provide no insights into translational regulation of gene expression. Critically, translation has emerged as a major mechanism that selectively modulates the proteome (12, 15). Therefore, concurrent measurement of the transcriptome, translatome (by quantifying mRNA associated with polysomes to define the transcriptome-wide pool of efficiently translated mRNA), and proteome is potentially required to elucidate complex molecular mechanisms. We recently applied this strategy to compare tumor tissue, tumor-adjacent stroma, and stroma from control subjects without lung cancer (18). That study indicated that stromal gene expression is primarily regulated via changes in translational efficiency, but we could not determine whether such changes in translation depend on lung function or cancer status (as samples were not matched for lung function). Here, we compared noncancerous stroma from patients with and without lung cancer, who were matched for lung function, to identify molecular mechanisms in stromal cells that favor lung cancer development. Some of the results of these studies have been previously reported in the form of an abstract (19–21).
Methods
Study Population and Lung Tissue Samples
This matched case/control study was approved by the University of Minnesota Institutional Review Board. Surgical lung tissue samples were obtained from the NIH Lung Tissue Research Consortium. The study subjects were current or former smokers who had undergone surgical lung resection and provided consent for tissue storage for future studies (Table 1). Cases were patients with histologically confirmed non–small cell lung cancer; no further differentiation was available. To broadly sample across a spectrum of lung-function values, we chose cases from Global Initiative for Obstructive Lung Disease (GOLD) 0/1 (FEV1 ≥ 80% of the predicted value [FEV1pp]), 2 (FEV1pp 50–80%) and 3/4 (FEV1pp < 50%). We analyzed lung tissue adjacent to, but not containing, lung cancer. The control subjects were individuals without lung cancer, matched on GOLD status. Notably, a majority of the GOLD 0/1 subjects were GOLD 0; however, a proportion of these patients (∼55%) had emphysema on computed tomography imaging. There were 58 samples (distinct from those previously reported [18]) for mass spectrometry (MS) (Table 1) and 32 of these tissue samples were sufficiently large for polysome profiling (Table E1 in the online supplement).
Table 1.
Characteristics of the Subjects for Proteomics
| GOLD Stage | Lung Cancer | Sex | Age (yr) (Mean) | FEV1 (L) (Mean) | FEV1pp (Mean) |
|---|---|---|---|---|---|
| 0/1 | Yes | 3F/6M | 52–83 (71.0) | 1.7–4.5 (2.9) | 84–133 (102.8) |
| 0/1 | No | 2F/7M | 44–68 (61.2) | 2.0–4.9 (3.4) | 83–180 (106.5) |
| 2 | Yes | 3F/7M | 48–84 (72.2) | 0.9–2.9 (1.7) | 51–73 (62.0) |
| 2 | No | 6F/4M | 43–79 (61.7) | 1.5–2.2 (1.8) | 52–76 (66.5) |
| 3/4 | Yes | 6F/4M | 53–82 (67.7) | 0.5–1.5 (1.0) | 19–47 (37.8) |
| 3/4 | No | 2F/8M | 47–64 (56.3) | 0.4–0.8 (0.6) | 10–22 (15.9) |
Definition of abbreviations: FEV1pp = FEV1 percent of the predicted value; GOLD = Global Initiative for Obstructive Lung Disease.
Proteomics
iTRAQ-based MS was used to obtain proteomes from 58 samples (Table 1). The resulting peptide data were normalized using NOMAD (22). A total of 1,259 proteins had a maximum of 12 missing values (i.e., not detected in 12 samples). Protein levels of CAV1 and SFXN3 (16447-1-ap and 15156-1-ap, respectively; Proteintech) were validated using Western blotting. Vinculin (ab18058; Abcam) was used as a loading control along with full-length protein controls for CAV1 and SFXN3 (ag8049 and ag7310, respectively; Proteintech). Additional information is provided in the online supplement.
Polysome-Profiling Sample and Data Processing
RNA sequencing (RNAseq) was performed on both total mRNA and polysome-associated mRNA using Smart-Seq2 (23) (Table E1). The resulting RNAseq reads (on average 55 million reads per sample) were mapped and aligned to hg19 using HISAT (default parameters; on average 54% mapped to the genome) (24). Reads that mapped to multiple locations in the genome were discarded (on average 13% mapped uniquely, as a large proportion mapped to noncoding RNA in a nonunique fashion). Gene expression was quantified as described previously using default settings (25) and refSeq gene annotation (26). Genes with zero counts in more than four samples were removed from the analysis before transformation/normalization (a total of 10,039 genes were analyzed). Raw counts were rlog transformed/normalized using the DESeq2 package (27). Additional information is provided in the online supplement.
Data Analysis
Analysis of covariance (ANCOVA) was applied to study how protein, total mRNA levels, and translation related to age, cancer, FEV1pp, and the cancer status–FEV1pp interaction (CFI). P values were adjusted per analysis (i.e., protein, total mRNA, or translation) and factor (age, cancer, FEV1pp, or CFI) separately using Storey’s q-value approach (28) as implemented in the “q value” bioconductor package. Additional information is provided in the online supplement.
Immunofluorescence
Immunofluorescence was performed on tissue adjacent to non-small cell lung cancer using antibodies for IL-6, BMP1, and α–smooth muscle actin (α-SMA). Additional information is provided in the online supplement.
Results
We collected lung tissue adjacent to, but not containing, lung cancer from 29 patients across a spectrum of lung-function values ranging from normal lung function to severe airflow obstruction. Controls (n = 29) consisted of surgically resected specimens from individuals without lung cancer matched for FEV1pp (Figure 1A). All subjects were between 43 and 84 years of age and were either former or current smokers. Subjects with cancer were slightly older than the matched control subjects (Table 1).
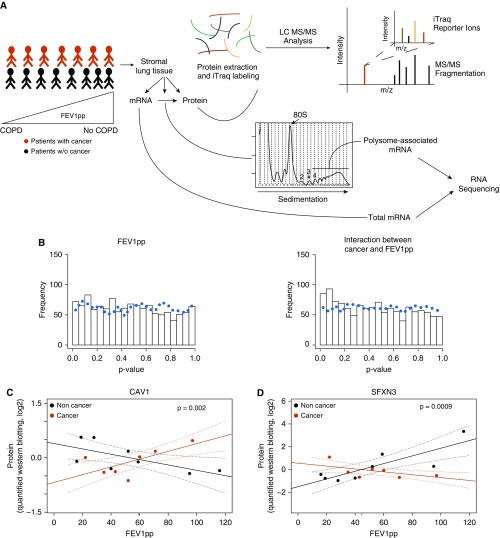
Figure 1.
Cancer-associated lung stromal proteomes depend on FEV1pp. (A) Non–cancer-containing stromal samples collected from donors with or without lung cancer across a range of lung-function values were subjected to quantitative proteomics and polysome profiling. This allowed for characterization of gene expression at multiple levels (total mRNA, translational efficiency [after adjusting changes in polysome-associated mRNA levels for changes in total mRNA], and protein). (B) Histograms of P values for changes in protein levels in an ANCOVA in which protein levels are related to age (not shown), cancer (not shown), FEV1pp, and the CFI (n = 58). The dotted line indicates the frequency of these P values when the same analysis was performed on a randomly sampled data set. (C and D) Quantified Western blots with antibodies that detect CAV1 (C) and SFXN3 (D) normalized to vinculin for a subset of stromal samples with and without cancer across a range of FEV1pp values (n = 15). Shown are regression lines with individual samples indicated by dots. Dotted lines indicate 95% confidence intervals for regressions of protein expression in subjects with cancer (red) and subjects without cancer (black) versus FEV1pp. The P value for the CFI is also indicated together with the FEV1pp intersection (blue tick mark along the x axis). ANCOVA = analysis of covariance; CFI = cancer status–FEV1pp interaction; COPD = chronic obstructive pulmonary disease; FEV1pp = FEV1 percent of the predicted value; LC MS/MS = liquid chromatography with tandem mass spectrometry.
Lung Stroma Proteomes Differ Depending on Both FEV1pp and Lung Cancer Status
Quantitative proteomics consistently yielded expression levels for ∼1,200 proteins. Although emphysema scores were unavailable for a majority of subjects without cancer, when available, the score correlated with FEV1pp (Figure E1A). Initially, we analyzed changes in the proteome using ANCOVA, which allows for concurrent analysis of cancer status and FEV1pp after adjusting for age, but observed no cancer-dependent protein expression. We next examined the CFI in the ANCOVA. Notably, when the CFI is included, statistics for cancer are hard to interpret, and hence we only show results for FEV1pp and CFI. This analysis revealed enrichment of proteins with low P values for the CFI (Figures 1B and E1B), which is consistent with differences in relative stromal protein expression between subjects with and without cancer depending on lung function (FEV1pp). To test the validity of this expression pattern, we performed a Western blot analysis for two representative proteins, CAV1 and SFXN3, with positive and negative interaction coefficients (i.e., the difference between slopes relating gene expression to FEV1pp for subjects with cancer vs. subjects without cancer), respectively. Western blotting confirmed a significant CFI for both CAV1 (Figure 1C) and SFXN3 (Figure 1D) protein expression. Thus, lung stromal protein expression depends on an interaction between cancer status and lung function (FEV1pp).
The Lung Stroma Proteome Is Predominantly Modulated via Changes in the Efficiency of mRNA Translation
To gain insight into the mechanisms underlying the observed changes in stromal proteomes, we performed polysome profiling quantified by RNAseq in 32 samples (17 subjects with cancer and 15 subjects without cancer; Table E1). Polysome profiling involves isolation of polysome-associated mRNA (mRNA associated with more than three ribosomes) to quantify efficiently translated mRNA (23). Changes in polysome-associated mRNA reflect either a change in translational efficiency (i.e., a smaller/larger proportion of all mRNA copies from a gene is associated with more than three ribosomes) or a change in total mRNA levels (29). To distinguish between these possibilities, we applied an adaptation of the anota algorithm (30) for RNAseq data and ANCOVA. The anota algorithm adjusts changes in polysome-associated mRNA levels for changes in total mRNA levels to quantify changes in translational efficiency. We performed the ANCOVA analysis described above using the abundance (total mRNA) or translational efficiency of each mRNA detected. This revealed enrichment of low P values for gene expression depending on FEV1pp and the CFI for the analysis of translational efficiencies but not total mRNA (Figures 2A–2D and E2; File E1 lists genes with a P value < 0.05 for the CFI). One potential confounder we observed was a systematic difference between the number of RNAseq reads generated from total mRNA and polysome-associated mRNA samples. To address this, we randomly sampled RNAseq reads from the total mRNA and polysome-associated mRNA datasets such that all samples had identical RNAseq depths, and reanalyzed the data as described in Figures 2A–2D. This showed that differences in sequencing depths did not explain the observed patterns in Figures 2A–2D (Figures E3 and E4).
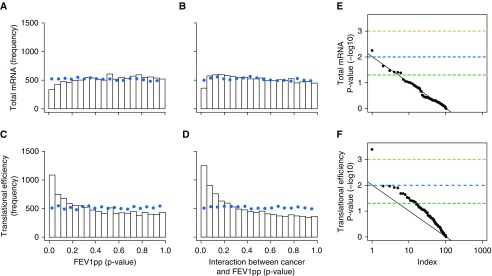
Figure 2.
The lung stromal proteome is predominantly modulated via changes in translational efficiency. (A–D) Histograms of P values for changes in total mRNA levels (A and B) and translational efficiencies (C and D) in ANCOVAs in which expression levels are related to age (not shown), cancer (not shown), FEV1pp (A and C), and the CFI (B and D) (n = 32). The dotted line indicates the frequency of these P values when the same analysis was performed on a randomly sampled data set. (E) Quantile-quantile plot of rank (“Index” plotted on a log10 scale) by P value (−log10) for the CFI analyzed using total mRNA data (i.e., B) and their corresponding P values (for proteins with a low P value [<0.05] CFI according to mass spectrometry, i.e., Figure 1B, right). (F) Plots similar to those shown in E based on analysis of translational efficiency. (E and F) The black line indicates the relationship expected by chance, and data points above the line indicate enrichment of low P values. Dashed lines indicate a P value of 0.05 (green), 0.01 (blue), or 0.001 (orange). ANCOVA = analysis of covariance; CFI = cancer status–FEV1pp interaction; FEV1pp = FEV1 percent of the predicted value.
We next sought to determine whether the observed changes in the proteome resulted from changes in mRNA levels or translational efficiencies. For this purpose, we used the set of proteins identified with a CFI P value < 0.05 and examined the P values for the CFI obtained from analysis of their polysome-profiling data. The distribution of these P values from the analysis of total mRNA data followed the null distribution that would be expected in the absence of regulation via changes in total mRNA. In contrast, the corresponding analysis for translational efficiency showed enrichment of low P values (Figures 2E and 2F). The same pattern was observed for CAV1 and SFXN3 (validated by Western blotting as described above [Figure E5]). Thus, polysome profiling confirmed a strong CFI, and suggests that altered translational efficiency is the predominant mechanism underlying CFI-dependent lung stroma proteomes.
Stromal Gene Expression Depends on FEV1pp
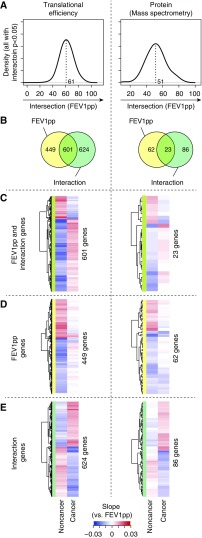
When we related gene expression to FEV1pp in subjects with and without cancer, we found that >99% of genes with P values < 0.05 for the CFI had an intersection between their slopes at an FEV1pp of 20–100 (i.e., similar to CAV1 and SFXN3 in Figures 1C and 1D). To gain further insights, we examined the distribution for such intersections. The peak occurred at FEV1pp ∼ 60 (Figure 3A), which is consistent with the notion that distinct cancer-associated gene expression that depends on FEV1pp manifests as a gradual shift in gene expression between subjects with and without cancer that intersects at FEV1pp ∼ 60 (i.e., similar to Figures 1C and 1D). To further explore the nature of this expression pattern, we examined whether genes with FEV1pp-dependent expression in subjects without cancer (P < 0.05) were the same as those that showed a CFI (P < 0.05). This revealed that a majority of transcripts whose translational efficiency depended on FEV1pp in subjects without cancer showed altered regulation in subjects with cancer, but also indicated a subset of genes specific to malignancy (Figure 3B). To examine the direction (up or down) of gene expression changes in these subsets, we compared regression slopes for the relationship between gene expression and FEV1pp for subjects with and without cancer (separately for genes in each sector of the Venn diagram in Figure 3B). This revealed differences in the slopes of FEV1pp-dependent gene expression between subjects with and without cancer. For many genes, the relationship was reversed in subjects with cancer versus those without cancer (Figures 3C–3E). To illustrate this, we assessed how such gene expression differences would vary in subjects with severe airflow obstruction (FEV1pp = 30) versus normal lung function (FEV1pp = 90) by calculating expected expression levels based on the relationships between gene expression and FEV1pp. A sizable fraction of all mRNAs showed inverted expected expression differences between subjects with cancer and those without cancer under high versus low FEV1pp (Figure 4A and File E1). Thus, FEV1pp-associated stromal gene expression is radically different, and often is even reversed, in subjects with cancer compared with subjects without cancer.
Figure 3.
Distinct FEV1pp-dependent stromal gene expression in samples from patients with or without cancer. (A) Distributions of intersections (i.e., for the regression slopes relating gene expression to FEV1pp for subjects with cancer vs. subjects without cancer) for mRNAs with translational efficiencies or proteins with a low P value (P < 0.05) for the CFI. The dotted black line indicates the peak of the density. (B) Venn diagrams comparing genes that show FEV1pp-dependent expression in subjects without cancer (P < 0.05, FEV1pp) with those that show a CFI (P < 0.05, Interaction). (C–E) Heatmaps of slopes for the relationship between gene expression and FEV1pp for subsets of genes from sectors of the Venn diagram. (A–E) The analysis was performed separately on translational efficiency (n = 32) and protein levels (n = 58). CFI = cancer status–FEV1pp interaction; FEV1pp = FEV1 percent of the predicted value.
Figure 4.
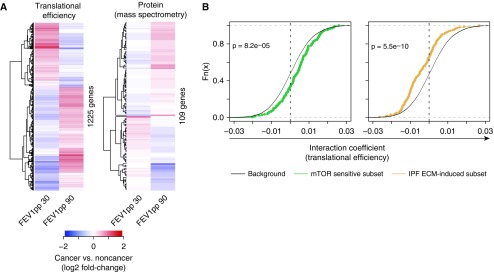
Distinct cancer-associated activation of gene expression programs downstream of mTOR signaling and fibrotic ECM depending on FEV1pp. (A) Heatmaps of estimated fold changes (subjects with cancer vs. subjects without cancer, using slopes and intersections from Figure 3) associated with poor (FEV1pp 30) or relatively normal (FEV1pp = 90) lung function. The analysis was performed separately on translational efficiency and protein levels. (B) Cumulative distributions of coefficients for the CFI as quantified from translational efficiency data (n = 32). Subsets of mRNAs previously identified as showing mTOR-sensitive translation or fibrotic ECM-induced translation are compared with background coefficients (i.e., all detected mRNAs not in a subset). A Wilcoxon P value for a difference between the subset and the background is indicated. A shift toward positive interaction coefficients indicates more efficient translation in adjacent stroma of patients with cancer and a high FEV1pp (i.e., similar to CAV1 in Figure 1C), whereas a shift toward negative interaction coefficients indicates more efficient translation in patients with cancer and a low FEV1pp (i.e., similar to SFXN3 in Figure 1D). CFI = cancer status–FEV1pp interaction; ECM = extracellular matrix; FEV1pp = FEV1 percent of the predicted value; IPF = idiopathic pulmonary fibrosis.
Distinct Cancer-associated Activation of Extracellular Matrix– or mTOR-Dependent Translatomes in the Lung Stroma Depends on FEV1pp
In principle, such distinct pathological FEV1pp-dependent gene expression programs should be activated by different upstream signals and likely target divergent cellular functions. To assess this possibility, we performed gene ontology analyses separately for mRNAs with a positive or negative CFI (P < 0.05). When intersections occur at FEV1pp ∼ 60 (Figure 3A), genes with such positive and negative coefficients are expected to show distinct differences in gene expression between subjects with and without cancer depending on their lung function status (illustrated by CAV1 and SFXN3 in Figures 1C and 1D). To test this possibility, we used polysome-profiling data from independent subjects assessed in our recent study (18) with an FEV1pp < 50 (albeit not matched for FEV1pp or age). Indeed, the transcripts identified in the present study with a CFI (P < 0.05) for translation showed the expected differences in translation between noncancerous stroma from patients with cancer and that obtained from control subjects (Figure E6).
We next explored the potential impact on cellular functions from genes with a CFI for translation. Proteins encoded by mRNAs with positive coefficients were enriched for annotation to processes including mRNA translation and mitochondrial function, whereas proteins encoded by mRNAs with a negative coefficient were enriched for annotation to cell adhesion and extracellular matrix (ECM) signaling (Table 2 and File E1). Strikingly, these functions overlap with functions enriched among proteins encoded by mRNAs whose translational efficiency was previously shown to be sensitive to mammalian target of rapamycin (mTOR) activity (31–36) or fibrotic ECM (17). To explore this in more detail, we compared coefficients for the CFI with background interaction coefficients at the level of translational efficiency for 1) mRNAs whose translational efficiency parallels mTOR activity in MCF7 cells (32) (human breast adenocarcinoma), and 2) mRNAs whose translational efficiency is activated by fibrotic ECM in fibroblasts (17). mTOR-sensitive mRNAs displayed a shift toward positive interaction coefficients (Figures 4B and E7), indicating that they are translated more efficiently in the stroma of patients with cancer and a high FEV1pp. In contrast, mRNAs that were previously shown to exhibit more efficient translation in response to fibrotic ECM showed shifts consistent with increased translational efficiency in the stroma of patients with cancer and a low FEV1pp (Figures 4B and E7). Of note, mRNAs whose translation is suppressed by fibrotic ECM showed interaction coefficients similar to those of the background, indicating that these gene expression programs are uncoupled (Figure E8). In summary, we identified cancer-associated expression of a fibrotic ECM program in low FEV1pp and an mTOR program in normal to mildly impaired FEV1pp.
Table 2.
Selected Biological Processes Enriched among Proteins Encoded by mRNAs with Low CFI P values (<0.05) When Analyzing Translation Using the Hypergeometric Test in GOstats and Functions Annotated by the Gene Ontology Consortium
| Number of Genes | P Value | Benjamini-Hochberg False Discovery Rate | |
|---|---|---|---|
| Proteins encoded by mRNAs with a positive interaction coefficient |
|
|
|
| Translation initiation | 52 | 2.1 × 10−28 | 1.7 × 10−26 |
| Translation | 85 | 6.2 × 10−20 | 1.7 × 10−18 |
| Electron transport chain | 27 | 4.1 × 10−9 | 6.1 × 10−8 |
| Mitochondrial respiratory chain complex assembly | 17 | 9.7 × 10−7 | 1.3 × 10−5 |
| Proteins encoded by mRNAs with a negative interaction coefficient |
|
|
|
| Extracellular matrix organization | 28 | 2.4 × 10−7 | 21.1 × 10−5 |
| Cell–cell adhesion | 41 | 7.2 × 10−6 | 1.7 × 10−4 |
| Blood vessel development | 50 | 6.8 × 10−11 | 1.5 × 10−8 |
Definition of abbreviations: CFI = cancer status–FEV1pp interaction; FEV1pp = FEV1 percent of the predicted value.
The Expression of Two Discrete Procancer Secretomes Is Associated with Lung Cancer Depending on FEV1pp
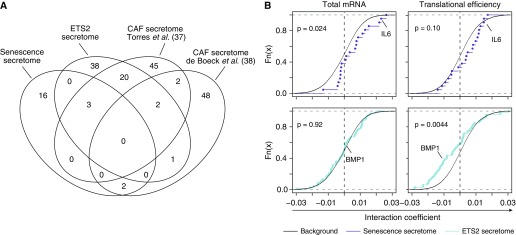
The stroma can support or oppose tumor progression and/or initiation through secretion of key factors. Such secretory programs could be activated upon interaction with the tumor (i.e., tumor induced) or activated in the absence of any tumor (i.e., tumor naive). The latter would have the potential to affect tumor initiation and possibly tumor progression, whereas the former has a role only in tumor progression. To determine whether the identified stromal expression programs included a procancer secretome, we identified two tumor-naive procancer secretomes (which promote tumor initiation in mouse models and were identified in PTEN null fibroblasts, largely depending on ETS2 (5), and senescent fibroblasts (6) [hereafter referred to as the ETS2 and senescence secretomes, respectively]), and two tumor-induced secretomes from cancer-associated fibroblasts (CAFs) (37, 38). These secretomes were largely distinct (Figure 5A). Whereas the senescence-associated secretome was shifted toward positive coefficients for the CFI (for total mRNA levels with a trend for translational efficiency; Figure 5B), the ETS2 secretome was shifted toward negative coefficients (via changes in translational efficiency only; Figure 5B). In contrast to these tumor-naive secretomes, the CAF secretomes were not selectively regulated (Figure E8). These results are consistent with the noncancerous lung stroma acquiring two distinct procancer secretory states, as reflected by activation in a tumor-naive fashion of a senescence secretome in subjects with normal or mildly reduced FEV1pp, and an ETS2 secretome in subjects with severe airflow obstruction.
Figure 5.
Distinct activation of procancer secretomes in cancer-adjacent stroma depending on FEV1pp. (A) Venn diagram comparing two tumor-naive procancer secretomes (senescence and ETS2 secretomes) with two secretomes from CAFs. (B) Same analysis as in Figure 4B on coefficients for the CFI as quantified from total mRNA data and translational efficiency (n = 32). The senescence and ETS2 secretomes were evaluated. Data points corresponding to IL-6 and BMP1 are indicated. CAF = cancer-associated fibroblast; CFI = cancer status–FEV1pp interaction; FEV1pp = FEV1 percent of the predicted value.
Stromal Fibroblasts Express Factors from Procancer Secretomes
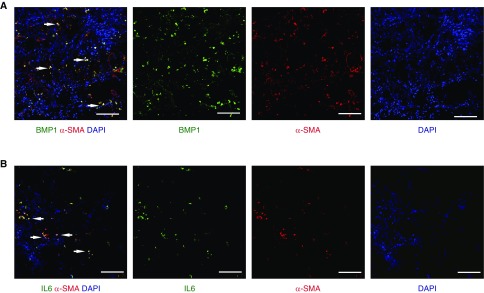
The two secretomes identified above were originally defined in studies of fibroblasts (4–6). This implies that lung fibroblasts establish a procancer microenvironment that favors cancer initiation in noncancerous lung stroma. To assess this, we selected IL-6, a constituent of the senescence secretome (Figure 5B), and BMP1, a constituent of the ETS2 secretome (Figure 5B), for in situ immunofluorescence studies of human lung tissue. We also stained for α-SMA as a marker of activated myofibroblasts (38). In cancer-adjacent stroma from patients with mild airflow obstruction, a subset of α-SMA+ cells also expressed IL-6 (Figure 6A). Similarly, BMP1 was expressed by a subset of α-SMA+ cells in the stroma of cancer patients with severe obstruction (Figure 6B). Thus, factors from the procancer secretomes are present in human lung tissue and originate from activated myofibroblasts.
Figure 6.
Fibroblast subsets express cancer-associated secreted factors. (A and B) Adjacent lung tissue from cancer patients with severe (A) or mild/no (B) chronic obstructive pulmonary disease were stained with antibodies for BMP1 (green) or IL-6 (green), respectively, together with the activated fibroblast marker α–smooth muscle actin (α-SMA, red). Cell nuclei are visualized with DAPI (blue). Arrows point to examples of double-positive cells. Scale bars represent 100 μM.
Discussion
This study demonstrates that cancer signals in lung stroma differ significantly based on lung function as measured by FEV1pp. Individuals with normal lung function or mild airflow obstruction demonstrated a different cancer-promoting repertoire than those with severe airflow obstruction. Furthermore, changes in translational efficiencies largely mediated these differences identified in proteomes in noncancerous lung stroma. Yet, expression of the senescence secretome was activated via changes in total mRNA levels (Figure 5B). Thus, although translational efficiencies accounted for the majority of the observed changes in gene expression, altered total mRNA levels appear to account for some key cancer-promoting aspects. These results highlight the importance of using a multiomics, systems-level approach to understand the relationship between lung function and lung cancer.
We provide multiple mechanistic insights into how such alterations in gene expression emerge. First, in subjects with cancer and normal to mildly reduced FEV1pp, we uncovered selective translational activation of mRNAs whose translation is mTOR sensitive. These findings are consistent with increased mTOR activity in subjects without cancer but with severe COPD, as previously described (39) (i.e., similar to Figure 1C). In contrast, expression of a translational program previously described as depending on fibrotic ECM was selectively activated in the stroma of patients with lung cancer and severe airflow obstruction. Thus, signals emanating from mTOR and ECM appear to differentially coordinate cancer-associated translatomes depending on lung function.
Loss of ECM in the parenchyma is a hallmark of emphysema, whereas pathological ECM deposits characterize airway disease in COPD (40). Pathological ECM activates translation of ECM-encoding mRNAs in primary human lung fibroblasts (17, 41). Enhanced synthesis of ECM proteins will affect remodeling of the microenvironment, cell signaling, and release of ECM-associated growth factors and proteases (17). Moreover, ECM secretion can stiffen the microenvironment, which leads to loss of lung function and promotes tumor initiation and progression (42–44). In individuals with relatively preserved lung function, the upstream signaling that activates the mTOR pathway, which bolsters translation of mTOR-sensitive mRNAs, could include one of several signals that impinge on mTOR (45, 46). mTOR regulates the translation of key factors that promote cancer, including IL-6 (47), which we found to be induced by changes in mRNA levels and translational efficiency in the stroma of patients with cancer and high FEV1pp (Figure 5B).
Further supporting the idea that pathological stromal gene expression facilitates cancer initiation, we unraveled selectively activated expression of two distinct procancer secretory programs that differed depending on lung function as measured by FEV1pp. Importantly, both of these secretomes increase cancer initiation in mouse models and may therefore underlie an increased risk for lung cancer initiation in humans. One of these secretomes was identified in patients with normal or mildly reduced FEV1pp, is induced during cellular senescence, and partially depends on mTOR (47). This raises the possibility that patients with relatively preserved lung function who develop cancer have a higher frequency of senescent cells, even though we found no resemblance between the transcriptomes identified here and those associated with cellular senescence. The second secretome was identified in patients with severe airflow obstruction and was previously described as being regulated by ETS2. Yet, consistent with an alternative mechanism at the level of mRNA translation to induce this secretome, we observed no regulation of ETS2 total mRNA levels or translation (protein was not detected by MS; Figure E9). Thus, although we observe overlaps with previously described cancer-inducing secretomes, their induction cannot be directly linked to previously described signals. Nevertheless, the procancer effects of the secretomes are expected to be maintained as previously delineated (4, 7). In this respect, we demonstrated that a subset of fibroblasts—specifically, the senescence secretome constituent IL-6 and the ETS2 secretome constituent BMP1—is one source of procancer factors. However, our experiments do not exclude the possibility that other cell types contribute to the secretion of IL-6, BMP1, or other factors (indeed, some cells stained only for IL-6 or BMP1 in Figure 6).
The analysis we performed has several limitations. Although all of our subjects were current or former smokers, we could not adjust for differences in smoking, nor could we use emphysema as a parameter during analysis due to incomplete annotation of samples. Moreover, our findings were based on identification of a statistical interaction (i.e., the CFI), which could have resulted from data normalization (48). We view this as unlikely, for several reasons. We identified the CFI in proteomics data (Figure 1B) and in translation data (Figure 2D), which were normalized using different methods. In addition, we did not find a CFI using total mRNA data (Figure 2B), which were normalized similarly to the translation data. Thus, the CFI does not associate with the method for data normalization. Moreover, we validated the CFI using Western blotting (Figures 1C and 1D) and showed that proteins encoded by mRNAs with a CFI were 1) previously described as being coregulated by signals emanating from mTOR or fibrotic ECM (Figure 4B), 2) enriched for specific cellular functions (Table 2), and 3) enriched for secretomes that facilitate cancer initiation (Figure 5B). This makes it likely that the CFI represents an authentic biological finding. Another potential limitation of our work is that although the tissues obtained from patients with cancer did not directly contact the tumor, they may have been affected by factors secreted by the tumor, in which case the results would not reflect the initiation of cancer but rather how the cancer affected adjacent tissues. The fact that the identified regulation of gene expression does not overlap with secretomes from CAFs (Figure S8), but rather from cells that support cancer initiation (Figure 5B), makes it most likely that the tissues examined in our study predominantly reflect the stroma before establishment of the tumor.
Although our study is limited by its cross-sectional design that identifies associations but not causality, to our knowledge, this is the first study to link stromal gene expression that is associated with cancer initiation in a manner that differs significantly depending on FEV1pp. In addition, these gene expression programs activate two unique procancer secretory programs. The exact molecular mechanisms that link the stroma to lung cancer remain to be identified, but they appear to depend on lung function and involve a combination of pathological ECM- and mTOR-mediated selective activation of mRNA translation together with their associated procancer secretomes.
Supplementary Material
Footnotes
Supported by NHLBI grant R01-HL107612, the Swedish Research Council, the Wallenberg Academy Fellows program of the Knut and Alice Wallenberg Foundation, the Swedish Cancer Society, and the Strategic Research Program in Cancer, Karolinska Institute. This material is the result of work supported in part by resources and the use of facilities at the Veterans Affairs Medical Center, Minneapolis. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Author Contributions: Conception and design: B.J.S., P.Y., A.H.L., T.J.G., P.B.B., C.H.W., and O.L. Data acquisition: B.J.S., L.M., M.B., S.A., T.M., M.P., T.J.G., P.B.B., C.H.W., and O.L. Analysis and interpretation: B.J.S., L.M., C.M., M.B., L.H., J.B., C.R., P.B.B., C.H.W., and O.L. Drafting of manuscript: B.J.S., L.M., M.B., C.R., P.B.B., C.H.W., and O.L. Editing of the manuscript: B.J.S., L.M., C.M., M.B., A.H.L., P.B.B., C.H.W., and O.L.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201801-0080OC on February 11, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Xouri G, Christian S. Origin and function of tumor stroma fibroblasts. Semin Cell Dev Biol. 2010;21:40–46. doi: 10.1016/j.semcdb.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21:33–39. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bremnes RM, Dønnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, et al. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol. 2011;6:209–217. doi: 10.1097/JTO.0b013e3181f8a1bd. [DOI] [PubMed] [Google Scholar]
- 4.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H, et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol. 2011;14:159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106:512–518. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- 9.Hohberger LA, Schroeder DR, Bartholmai BJ, Yang P, Wendt CH, Bitterman PB, et al. Correlation of regional emphysema and lung cancer: a lung tissue research consortium-based study. J Thorac Oncol. 2014;9:639–645. doi: 10.1097/JTO.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins RJ, Duan F, Chiles C, Greco EM, Gamble GD, Aberle D, et al. Reduced expiratory flow rate among heavy smokers increases lung cancer risk: results from the National Lung Screening Trial-American College of Radiology Imaging Network Cohort. Ann Am Thorac Soc. 2017;14:392–402. doi: 10.1513/AnnalsATS.201609-741OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccirillo CA, Bjur E, Topisirovic I, Sonenberg N, Larsson O. Translational control of immune responses: from transcripts to translatomes. Nat Immunol. 2014;15:503–511. doi: 10.1038/ni.2891. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen AR, Gsponer J, Foster LJ. Protein synthesis rate is the predominant regulator of protein expression during differentiation. Mol Syst Biol. 2013;9:689. doi: 10.1038/msb.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovanovic M, Rooney MS, Mertins P, Przybylski D, Chevrier N, Satija R, et al. Immunogenetics: dynamic profiling of the protein life cycle in response to pathogens. Science. 2015;347:1259038. doi: 10.1126/science.1259038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 16.Larsson O, Tian B, Sonenberg N. Toward a genome-wide landscape of translational control. Cold Spring Harb Perspect Biol. 2013;5:a012302. doi: 10.1101/cshperspect.a012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandri BJ, Kaplan A, Hodgson SW, Peterson M, Avdulov S, Higgins L, et al. Multi-omic molecular profiling of lung cancer in COPD. Eur Respir J. 2018;52:1702665. doi: 10.1183/13993003.02665-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wendt CH, Sandri BJ, Masvidal L, Murie C, Bartish M, Avdulov S, et al. Chronic obstructive pulmonary disease phenotype dictates cancer-promoting stromal gene expression programs. Ann Am Thorac Soc. 2018;15:S290–S291. [Google Scholar]
- 20.Sandri BJ, Bitterman P, Griffin TJ, Higgins L, Markowski T, Avdulov S, et al. Transcriptomic, translatomic, and proteomic profiling platform discovers a physiological hallmark of COPD-associated lung cancer. Am J Respir Crit Care Med. 2017;195:A2484. [Google Scholar]
- 21.Sandri B, Limper AH, Jagtap P, Avdulov S, Peterson M, Murie C, et al. Large-scale quantitative proteomic analysis identifies multiple pathways in COPD-associated lung cancer. Am J Respir Crit Care Med. 2015;191:A1251. [Google Scholar]
- 22.Murie C, Sandri B, Sandberg AS, Griffin TJ, Lehtiö J, Wendt C, et al. Normalization of mass spectrometry data (NOMAD) Adv Biol Regul. 2018;67:128–133. doi: 10.1016/j.jbior.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang S, Bellato HM, Lorent J, Lupinacci FCS, Oertlin C, van Hoef V, et al. Polysome-profiling in small tissue samples. Nucleic Acids Res. 2018;46:e3. doi: 10.1093/nar/gkx940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsköld D, Wang ET, Burge CB, Sandberg R. An abundance of ubiquitously expressed genes revealed by tissue transcriptome sequence data. PLoS Comput Biol. 2009;5:e1000598. doi: 10.1371/journal.pcbi.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:D733–D745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson O, Sonenberg N, Nadon R. Identification of differential translation in genome wide studies. Proc Natl Acad Sci USA. 2010;107:21487–21492. doi: 10.1073/pnas.1006821107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson O, Sonenberg N, Nadon R. anota: analysis of differential translation in genome-wide studies. Bioinformatics. 2011;27:1440–1441. doi: 10.1093/bioinformatics/btr146. [DOI] [PubMed] [Google Scholar]
- 31.Ingolia NT. Ribosome footprint profiling of translation throughout the genome. Cell. 2016;165:22–33. doi: 10.1016/j.cell.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson O, Morita M, Topisirovic I, Alain T, Blouin MJ, Pollak M, et al. Distinct perturbation of the translatome by the antidiabetic drug metformin. Proc Natl Acad Sci USA. 2012;109:8977–8982. doi: 10.1073/pnas.1201689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandin V, Masvidal L, Hulea L, Gravel SP, Cargnello M, McLaughlan S, et al. nanoCAGE reveals 5′ UTR features that define specific modes of translation of functionally related MTOR-sensitive mRNAs. Genome Res. 2016;26:636–648. doi: 10.1101/gr.197566.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morita M, Gravel SP, Chénard V, Sikström K, Zheng L, Alain T, et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 2013;18:698–711. doi: 10.1016/j.cmet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Miloslavski R, Cohen E, Avraham A, Iluz Y, Hayouka Z, Kasir J, et al. Oxygen sufficiency controls TOP mRNA translation via the TSC-Rheb-mTOR pathway in a 4E-BP-independent manner. J Mol Cell Biol. 2014;6:255–266. doi: 10.1093/jmcb/mju008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres S, Bartolomé RA, Mendes M, Barderas R, Fernandez-Aceñero MJ, Peláez-García A, et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin Cancer Res. 2013;19:6006–6019. doi: 10.1158/1078-0432.CCR-13-1130. [DOI] [PubMed] [Google Scholar]
- 38.De Boeck A, Hendrix A, Maynard D, Van Bockstal M, Daniëls A, Pauwels P, et al. Differential secretome analysis of cancer-associated fibroblasts and bone marrow-derived precursors to identify microenvironmental regulators of colon cancer progression. Proteomics. 2013;13:379–388. doi: 10.1002/pmic.201200179. [DOI] [PubMed] [Google Scholar]
- 39.Houssaini A, Breau M, Kebe K, Abid S, Marcos E, Lipskaia L, et al. mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight. 2018;3:93203. doi: 10.1172/jci.insight.93203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Churg A, Zhou S, Preobrazhenska O, Tai H, Wang R, Wright JL. Expression of profibrotic mediators in small airways versus parenchyma after cigarette smoke exposure. Am J Respir Cell Mol Biol. 2009;40:268–276. doi: 10.1165/rcmb.2007-0367OC. [DOI] [PubMed] [Google Scholar]
- 41.Herrera J, Beisang DJ, Peterson M, Forster C, Gilbertsen A, Benyumov A, et al. Dicer1 deficiency in the idiopathic pulmonary fibrosis fibroblastic focus promotes fibrosis by suppressing microRNA biogenesis. Am J Respir Crit Care Med. 2018;198:486–496. doi: 10.1164/rccm.201709-1823OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmon H, Donnadieu E. Within tumors, interactions between T cells and tumor cells are impeded by the extracellular matrix. OncoImmunology. 2012;1:992–994. doi: 10.4161/onci.20239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laklai H, Miroshnikova YA, Pickup MW, Collisson EA, Kim GE, Barrett AS, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med. 2016;22:497–505. doi: 10.1038/nm.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miroshnikova YA, Mouw JK, Barnes JM, Pickup MW, Lakins JN, Kim Y, et al. Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat Cell Biol. 2016;18:1336–1345. doi: 10.1038/ncb3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong Y, Song F. Intracellular calcium signaling regulates autophagy via calcineurin-mediated TFEB dephosphorylation. Autophagy. 2015;11:1192–1195. doi: 10.1080/15548627.2015.1054594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong Y, Sheen J. Moving beyond translation: glucose-TOR signaling in the transcriptional control of cell cycle. Cell Cycle. 2013;12:1989–1990. doi: 10.4161/cc.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castaldi PJ, Cho MH, Liang L, Silverman EK, Hersh CP, Rice K, et al. Screening for interaction effects in gene expression data. PLoS One. 2017;12:e0173847. doi: 10.1371/journal.pone.0173847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.