Abstract
Rationale: Augmentation therapy with intravenous AAT (alpha-1 antitrypsin) is the only specific therapy for individuals with pulmonary disease from AAT deficiency (AATD). The recommended standard dose (SD; 60 mg/kg/wk) elevates AAT trough serum levels to around 50% of normal; however, outside of slowing emphysema progression, its effects in other clinical outcomes have not been rigorously proven.
Objectives: To evaluate the biological effects of normalizing AAT trough levels with double-dose (DD) therapy (120 mg/kg/wk) in subjects with AATD already receiving SD therapy.
Methods: Clinically stable subjects were evaluated after 4 weeks of SD therapy, followed by 4 weeks of DD therapy, and 4 weeks after return to SD therapy. At the end of each phase, BAL fluid (BALF) and plasma samples were obtained.
Measurements and Main Results: DD therapy increased trough AAT levels to normal and, compared with SD therapy, reduced serine protease activity in BALF (elastase and cathepsin G), plasma elastase footprint (Aα-Val360), and markers of elastin degradation (desmosine/isodesmosine) in BALF. DD therapy also further downregulated BALF ILs and cytokines including Jak-STAT (Janus kinases–signal transducer and activator of transcription proteins), TNFα (tumor necrosis factor-α), and T-cell receptor signaling pathways, cytokines involved in macrophage migration, eosinophil recruitment, humoral and adaptive immunity, neutrophil activation, and cachexia. On restarting SD after DD treatment, a possible carryover effect was seen for several biological markers.
Conclusions: Subjects with AATD on SD augmentation therapy still exhibit inflammation, protease activity, and elastin degradation that can be further improved by normalizing AAT levels. Higher AAT dosing than currently recommended may lead to enhanced clinical benefits and should be explored further.
Clinical trial registered with www.clinicaltrials.gov (NCT 01669421).
Keywords: alpha-1 antitrypsin deficiency, antiinflammatory, dosing, immunomodulation
At a Glance Commentary
Scientific Knowledge on the Subject
AAT (alpha-1 antitrypsin) has antiinflammatory and immunomodulatory functions, in addition to antiprotease properties. Augmentation therapy with intravenous AAT is the only specific therapy for individuals with AAT deficiency. Although the recommended standard dose of 60 mg/kg/wk keeps trough AAT levels around 50% of normal (range, 20–53 μM) and slows radiologic progression of emphysema, its proven clinical benefits have been elusive. This, along with the variability of disease manifestations and progression in individuals on augmentation therapy, suggests that the standard dose may not be optimal for all patients.
What This Study Adds to the Field
This study describes the effects of double-dose AAT therapy (120 mg/kg/wk) on several biological parameters associated with the development and progression of chronic obstructive pulmonary disease. Overall, double-dose therapy was shown to restore serum AAT levels to greater than 25 μM and to further reduce circulating and airway levels of serine proteases, reduce elastin degradation in the lung, and diminish airway inflammation in subjects already receiving standard dose therapy. Therefore, increasing AAT levels into the normal range may provide additional clinical benefits and have a more robust impact on clinical outcomes in subjects with AAT deficiency requiring augmentation therapy.
AAT (alpha-1 antitrypsin) deficiency (AATD) is a genetic disorder characterized by low circulating levels of AAT (also known as alpha-1 protease inhibitor) (1). AATD predisposes the lung to the unopposed action of proteases, such as cathepsin G and proteinase 3 but primarily NE (neutrophil-derived elastase) (2). This results in progressive and irreversible destruction of lung parenchyma (emphysema) usually associated with an accelerated decline in lung function, particularly in the context of exposure to tobacco smoke inhalation (3). Compared with smoke exposure–associated chronic obstructive pulmonary disease (COPD), individuals with AATD seem to have higher levels of inflammatory markers in both BAL fluid (BALF) (4) and sputum (5, 6), and a higher prevalence of airway hyperreactivity (7) and bronchiectasis (8).
Independent of its antiprotease properties, AAT has important antiinflammatory and immunomodulatory functions (3, 9, 10). Accumulating evidence suggests that AAT reduces the activity of proinflammatory cytokines and increases the release of antiinflammatory mediators (11, 12). For example, AAT upregulates the expression of the antiinflammatory cytokine IL-Ra (IL-1 receptor antagonist) and blocks the release of IL-1 and TNFα (tumor necrosis factor-α) in stimulated peripheral blood mononuclear cells (13, 14). In neutrophils, AAT also has broad effects, such as inhibiting superoxide production, induction of IL-1Ra, and decreasing chemotaxis and adhesion (15). AAT inhibits bacterial- and endotoxin-induced proinflammatory responses in vitro and in vivo, such as lowering levels of IL-8 and MCP-1 (monocyte chemotactic protein-1), two major chemokines involved in trafficking inflammatory cells (16, 17). AAT also acts on the lung endothelium, an active participant in the inflammatory response, by assisting in the resolution of chronic inflammation (18).
Intravenous AAT is currently the only specific therapy available for individuals with AATD. The recommended standard dose (SD) of 60 mg/kg/wk aims to increase AAT plasma levels above the putative protective threshold level (>11 μM) (19). Findings from the RAPID clinical trial program confirmed the benefits of SD AAT therapy in slowing progression of emphysema radiologically, providing evidence of a disease-modifying therapy for AATD (20, 21). However, no randomized trial has shown an effect of SD therapy on conventional COPD outcomes, such as exacerbation frequency and severity, quality of life, lung function decline, or mortality, which largely reflects their poor sensitivity to detect change and the natural influence of aging (22). The therapeutic protective threshold goal of 11 μM is still much lower than levels found in nondeficient individuals (20–53 μM) (23). The clinical variability of disease manifestations and progression in individuals on therapy (24), suggests that the SD may not be sufficient for all patients and suggests higher doses may prove more beneficial.
Our hypothesis states that patients with AATD receiving SD AAT therapy (60 mg/kg/wk) may have residual systemic and pulmonary inflammation that could be further improved with a higher dose aimed at increasing AAT to physiologic levels. In this prospective, open-label pilot study, we evaluated the biological effects of 1 month of double-dose (DD) AAT therapy (120 mg/kg/wk) in subjects with AATD previously receiving SD therapy. This was an exploratory study to evaluate the feasibility and justification for further trials of higher-dose AAT therapy. Some of the results of this study have been previously reported in the form of abstracts (25, 26).
Methods
Detailed and expanded methodology is included in the online supplement.
Patients and Medications
Patients aged 18–75 years with a clinically symptomatic diagnosis of COPD and AATD receiving SD AAT therapy (60 mg/kg/wk) for at least 1 month before study entry were included. For detailed inclusion and exclusion criteria please refer to Table E1 in the online supplement. To compare SD and DD augmentation therapy, all study treatments were performed using Zemaira (CSL Behring LLC). Importantly, all patients were on standard COPD medications, including at least one long-acting bronchodilator and an inhaled steroid, for at least a month before entering the study. Patients who experienced an exacerbation during phase 1 (SD therapy) where continued on SD therapy until at least 1 month after resolution of the episode before starting the study protocol. An acute exacerbation requiring antibiotics or systemic steroids after any study bronchoscopy was an indication for study termination.
Written informed consent was obtained from all participants and the study was approved by the institutional review board of the University of Miami, School of Medicine. The protocol was registered in clinicaltrials.gov (NCT 01669421).
Outcomes
The primary outcome was change in pulmonary inflammatory markers measured in BALF; secondary outcomes were improvement in serum/plasma inflammatory markers and elastin degradation markers in serum and BALF.
Safety
All subjects were carefully monitored for adverse events (AEs) and serious AEs (SAEs) characterized by their seriousness, severity, and relationship to the administration of AAT therapy at 120 mg/kg/wk.
Procedures
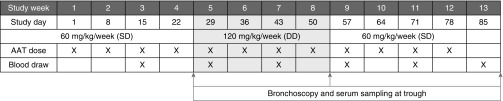
After signing the informed consent, participants already receiving SD therapy entered a three-phase protocol: 4 weeks of SD AAT therapy (60 mg/kg/wk), followed by 4 weeks of open-label DD AAT therapy (120 mg/kg/wk), and a final 4-week phase in which subjects returned to standard dosing (60 mg/kg/wk). At the end of each phase, subjects underwent bronchoscopy and plasma sampling. Serum trough AAT levels were measured at the end of each phase to provide the nadir AAT level with SD and DD therapy; the study was not designed for full pharmacokinetic characterization. All sample collections were obtained during the stable clinical state. The study protocol is outlined in Figure 1. Administration of DD augmentation therapy required an Investigational New Drug designation by the Food and Drug Administration (IND #14636).
Figure 1.
Summary of study procedures. AAT = alpha-1 antitrypsin; DD = double dose; SD = standard dose.
Cytokine, Chemokine, and Growth Factor Measurements
Human cytokines, chemokines, and growth factors were determined in BALF and plasma using bead assays (Bio-Rad Magnetic cytokine, chemokine, and growth factor bead panels; Bio-Rad) performed with the Bio-Rad Bio-Plex 200 system. BALF data were standardized to total BALF protein and urea concentrations. Total protein was determined by bicinchoninic acid assays, according to the manufacturer’s instructions (Thermo Fisher).
Desmosine/Isodesmosine Quantification
HPLC and tandem mass spectrometry were used as previously described (27). Analyses of desmosine/isodesmosine (DES/IDES) levels were performed in triplicate in both BALF and plasma with eight samples per group on subsequent days. In BALF, levels were normalized to BALF protein concentrations. The coefficient of variation for the method is 8%.
Protease Activity Measurement
The NE footprint Aα-Val360 was measured in plasma samples using a highly specific assay as described previously (28). BALF NE activity was determined using 50 μM fluorogenic substrate N-(methoxysuccinyl)-Ala-Ala-Pro-Val-7-amino-4-methylcoumarin (Enzo Life Sciences) in 0.1 M N-2-hydroxyethylpiperazine-N′-ethane sulfonic acid, 0.5 M NaCl, pH 7.5 by excitation at 360 nm, and emission at 460 nm. Experiments were performed with and without NE inhibitor (1 mM N-[methoxysuccinyl]-Ala-Ala-Pro-Val-chloromethyl ketone). NE was also confirmed by performing immunoblots on BALF using an anti-NE polyclonal antibody (ab68672; Abcam). Cathepsin G activity was determined in 50 μl BALF with a colorimetric cathepsin G activity assay kit (ab126780; Abcam), according to the manufacturer’s instructions.
Pathway Analysis
Downregulated proteins were submitted to the Database for Annotation, Visualization and Integrated Discovery to identify biological processes/pathways relevant to lung pathophysiology (29).
Statistical Analysis
For this pilot study, we aimed to enroll a minimum of 10 subjects based on estimating a 30–50% decrease in inflammatory markers. The variability of these markers was calculated based on published data for patients with usual COPD because no BALF data were available for patients with AATD at the time of the study. For example, the levels of IL-8 in BALF from patients with usual COPD are in the range 0.104 ± 0.03 ng/ml (30); assuming that treatment reduced IL-8 by 50%, the calculated N was 8 (α = 0.05; power, 90%) and for a 30% reduction, the calculated N was 18 (α = 0.05; power, 90%).
Statistical analysis was performed using D’Agostino and Pearson normality tests and paired Student’s t tests on normally distributed data and Wilcox matched-pair signed rank testing on data not normally distributed. Two-tailed analysis was performed on all data and P values for significance were set at 0.05. All analyses were performed using GraphPad Prism software version 6.0h for Mac OS X. All samples were collected and analyzed; an intention-to-treat analysis was not performed.
Results
A total of 10 subjects were enrolled in the study; two withdrew after bronchoscopy-related events (one hemoptysis after procedure; one after respiratory distress after procedure). Overall, eight subjects completed all procedures. Baseline demographic and clinical characteristics are provided in Table 1 (see Table E2 for patient pulmonary function test results after therapy).
Table 1.
Baseline Demographic and Clinical Characteristics for the Full Population of Patients Who Entered the Study
| Characteristic | Data (N = 10) |
|---|---|
| Mean age, yr (StD) | 60.5 (8.1) |
| Sex, n (%) | |
| M | 7 (70) |
| F | 3 (30) |
| Genotype, n (%) | |
| ZZ | 9 (90) |
| SZ | 1 (10) |
| Baseline mean (StD) AAT level, μM* | 6.1 (2.5) |
| Mean (StD) post-bronchodilator FEV1, % | 55.0 (11.7) |
| Mean (StD) number of exacerbations in previous year | 1.9 (1.2) |
| Chronic bronchitis, n (%) | 8 (80) |
| Median number of months on SD AAT therapy, median (IQR) | 81 (29–246) |
| St. George’s respiratory questionnaire, median (IQR) | |
| Total score | 48.9 (34–60.2) |
| Symptoms | 63.7 (46.5–73.6) |
| Impacts | 33.3 (24–46) |
| Activity | 63.6 (40.3–72.5) |
| BODE score, median (IQR) | 2 (1–3.5) |
| Smoking history | |
| Ever-smokers, n (%) | 6 (60) |
| Mean (StD) pack-years | 19.08 (14.7) |
| Active smokers, n | 0 |
Definition of abbreviations: AAT = alpha-1 antitrypsin; BODE = body mass index, airflow obstruction, dyspnea, and exercise; IQR = interquartile range; SD = standard dose; StD = standard deviation.
Historical baseline AAT levels were obtained from medical records and correspond to levels recorded before the initiation of AAT therapy.
DD Therapy Restored AAT to Normal Levels
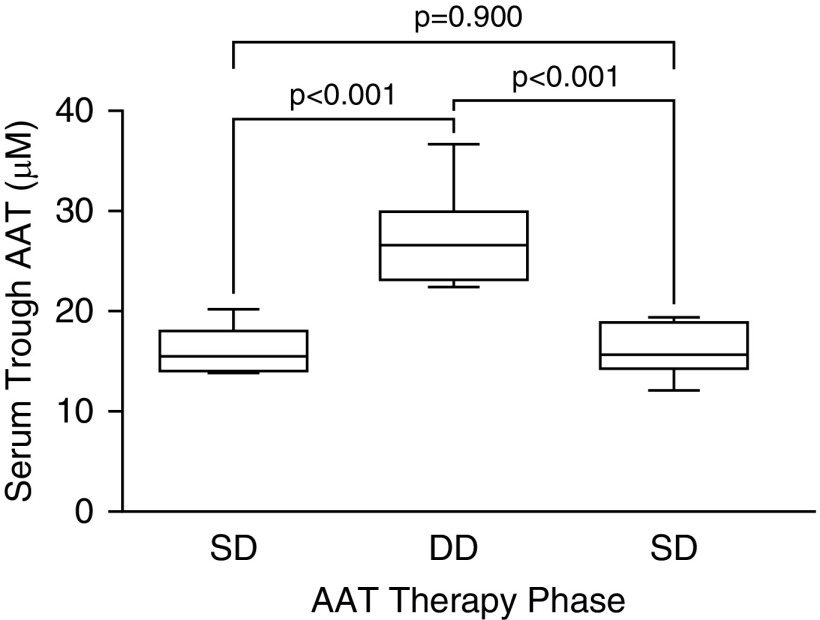
DD AAT therapy results in AAT serum levels that were closer to those for AAT-competent subjects (normal range, 20–53 μM) (23). Trough levels of AAT increased from 16.7 ± 2.3 μM with SD therapy to 27.2 ± 5.0 μM with DD therapy (Figure 2), and returned to baseline (16.0 ± 2.6 μM) after SD therapy reinstitution.
Figure 2.
Trough serum AAT levels. For definition of abbreviations, see Figure 1.
DD Therapy Increased Antiprotease Activity and Decreased Elastin Degradation in Subjects with AATD Receiving SD Therapy
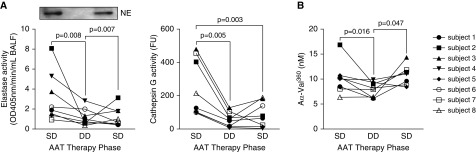
Compared with baseline values while receiving SD therapy, treatment with DD therapy (120 mg/kg/wk) produced significant reductions in serine protease activity in BALF (NE and cathepsin G) (Figure 3A). Levels of NE reduced from 3.32 ± 0.86 nM after SD therapy to 1.61 ± 0.29 nM on DD therapy (P = 0.008), and cathepsin G reduced from 247.00 ± 60.37 nM to 56.32 ± 15.28 nM (P = 0.005), respectively. The activity of these proteases in BALF began to rise (although not significantly) by 4 weeks after resuming SD therapy with levels of NE and cathepsin G of 1.75 ± 0.33 nM and 86.06 ± 26.43 nM, respectively, which suggests a possible carryover effect of the DD therapy (31, 32). Plasma levels of Aα-Val360 were also significantly reduced with DD therapy, from 10.15 ± 1.19 nM after SD therapy to 7.89 ± 0.57 nM on DD therapy (P = 0.016) (Figure 3B). This marker increased again a month after SD therapy was resumed.
Figure 3.
Activity of serine proteases in BAL fluid (BALF) (elastase activity, cathepsin G) and plasma (Aα-Val360). Eight subjects with AAT (alpha-1 antitrypsin) deficiency and chronic obstructive pulmonary disease on standard dose (SD) therapy (60 mg/kg/wk; Zemaira, CSL Behring) underwent BALF and plasma sampling after 4 weeks of SD, 4 weeks of double-dose (DD), and finally 4 weeks of SD therapy. (A) Elastase and cathepsin G activity were measured in BALF. (B) Aα-Val360 was measured in plasma. Representative immunoblot for neutrophil elastase in BALF sample from one subject. Graphs are represented as individual subject response to AAT therapy; each measurement performed three times (n = 8 per group). NE = neutrophil elastase.
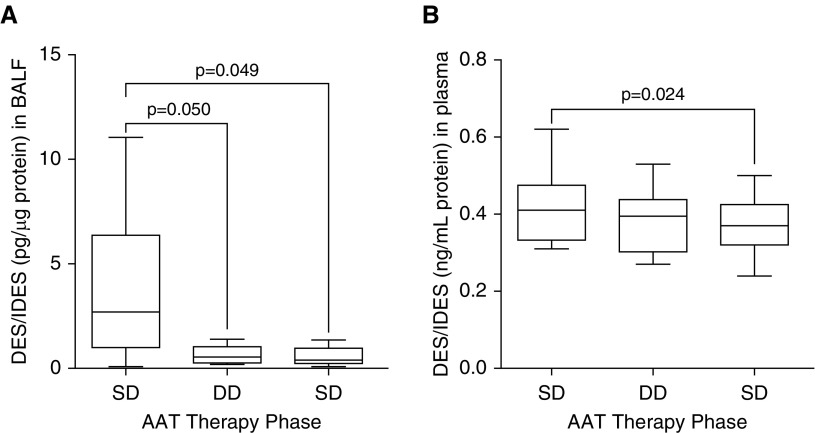
Increasing AAT levels with DD therapy also produced significant reductions in local markers of elastin degradation. In BALF, DES/IDES levels fell after DD therapy (0.65 ± 0.34 pg/μg protein compared with 3.74 ± 3.7 pg/μg protein on SD therapy; P = 0.050) (Figure 4A) and a possible carryover effect was noted, because levels a month after SD was resumed were as low as after a month on DD therapy. In plasma, however, we did not observe changes in DES/IDES with DD therapy compared with baseline (0.38 ± 0.03 ng/ml compared with 0.42 ± 0.03 ng/ml, respectively; P = 0.493) (Figure 4B).
Figure 4.
Changes in desmosine (DES) and isodesmosine (IDES) levels in (A) BAL fluid (BALF) and (B) plasma. Eight subjects with AAT (alpha-1 antitrypsin) deficiency and chronic obstructive pulmonary disease on standard dose (SD) therapy (60 mg/kg/wk; Zemaira, CSL Behring) underwent BALF and plasma sampling after 4 weeks of SD, 4 weeks of double-dose (DD), and finally 4 weeks of SD therapy. DES/IDES levels were measured in (A) BALF and (B) plasma. Graphs are represented as individual subject response to AAT therapy; each measurement performed three times (n = 8 per group). Month 1: SD, 60 mg/kg/wk. Month 2: DD, 120 mg/kg/wk. Month 3: SD, 60 mg/kg/wk.
Normalization of AAT Levels Significantly Reduced Airway Inflammation in Subjects with AATD Receiving SD Therapy
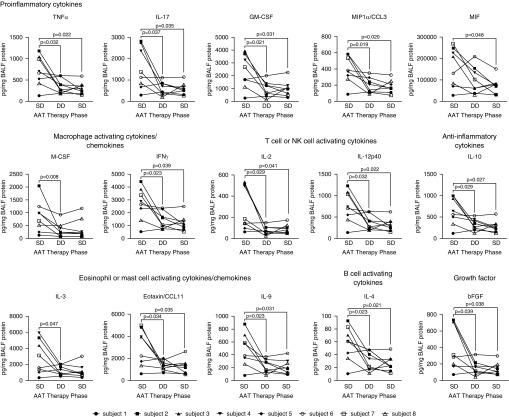
BALF concentrations of multiple cytokines, chemokines, and growth factors were significantly reduced after 120 mg/kg/wk dosing of AAT (Figure 5). For some measures, the levels returned back to those observed with SD therapy (60 mg/kg/wk) at study start after reinstitution; however, in other measures, including CCL3 (chemokine [C-C motif] ligand 3), CCL11, TNFα, IFNγ, and bFGF (basic fibroblast growth factor), a possible carryover effect was observed. In addition, DD therapy significantly downregulated several other inflammatory markers, including ILs and cytokines that affect the Jak-STAT (Janus kinases–signal transducer and activator of transcription proteins), TNFα, and T-cell receptor signaling pathways. DD therapy also affected cytokines involved in macrophage migration, eosinophil recruitment, humoral and adaptive immunity, neutrophil activation, and cachexia (see Table E3). However, no significant differences in levels of some cytokines, chemokines, and growth factors were observed after DD AAT therapy (see Tables E4 and E5).
Figure 5.
BAL fluid (BALF) concentrations of several ILs and related cytokines were significantly reduced after 120 mg/kg/wk dosing of AAT (alpha-1 antitrypsin). Eight subjects with AAT deficiency and chronic obstructive pulmonary disease on standard dose (SD) therapy (60 mg/kg/wk; Zemaira, CSL Behring) underwent BALF and plasma sampling after 4 weeks of SD, 4 weeks of double-dose (DD), and finally 4 weeks of SD therapy. BALF levels were determined by Luminex assays. Graphs are represented as individual subject BALF concentrations; each measurement performed three times (n = 8 per group). bFGF = basic fibroblast growth factor; CCL = chemokine (C-C motif) ligand; GM-CSF = granulocyte–macrophage colony–stimulating factor; M-CSF = macrophage colony–stimulating factor; MIF = macrophage migration inhibitory factor; MIP = macrophage inflammatory protein; NK = natural killer; TNF = tumor necrosis factor.
Safety
During the study, three subjects experienced an SAE that required urgent medical evaluation or hospitalization (see Table E6). The SAEs were all acute respiratory exacerbations or possible bronchoscopy complications and unrelated to the administration of DD augmentation therapy. There were seven minor AEs reported in six patients, none of which were judged to be related to the administration of DD AAT therapy. Of these, three (mild stridor after bronchoscopy, arm bruise, and nausea and anxiety 1 d after bronchoscopy) were judged to be probably or likely related to study procedures.
Discussion
This study describes the impact of “normalizing” AAT levels on several biological parameters implicated in development and progression of COPD in subjects with AATD. Although these subjects were already receiving the currently approved augmentation therapy dose of 60 mg/kg/wk (SD therapy), we show that they still exhibit residual inflammation and elastin degradation that can be further improved by increased dosing. SD therapy aims to increase AAT levels above the “protective” threshold estimated at 11 μM (19). This estimation, which is based on the observation that never-smokers with AATD genotype SZ usually exhibit AAT levels above this level and rarely develop lung disease (33), is approximately 50% of normal. As previously shown, DD therapy (120 mg/kg/wk) leads to trough serum AAT levels similar to those observed in non–AAT-deficient individuals and can be administered without any major AEs (34). We show here that with only 1 month of weekly DD intravenous infusions, significant reductions in serine protease activity in BALF and plasma can be achieved, which coincided with reductions in BALF concentrations of DES/IDES, indicating reduced local elastin degradation in the lung. In combination with diminished airway inflammation, these results suggest that DD therapy may have a more pronounced impact on slowing disease progression in subjects with AATD compared with SD therapy.
Most augmentation therapy scheduling and dosing studies have focused on achieving equivalent AAT levels to SD therapy, the only dose approved for use in AATD more than 25 years ago. For example, dosing at 250 mg/kg every 28 days confers “protective” serum AAT levels and antielastase activity in epithelial lining fluid for “at least” 25 days after the infusion (35), whereas a regimen of 120 mg/kg every 2 weeks could not maintain nadir serum levels above the 11 μM threshold for the entire 14-day dosing interval (36). Our observations highlight that this putative protective value of 11 μM as a goal of therapy should be revisited and that subjects with AATD in need of therapy should preferably be treated with a regimen that normalizes AAT levels. Except for slowing emphysema progression, the clinical effects of SD augmentation therapy on other COPD outcomes has been deemed controversial by many. For example, slowing of lung function decline has been noted in observational studies but not within randomized controlled trials (37, 38). In addition, subjects on SD augmentation therapy exhibit high rates of exacerbations (24), and although augmentation therapy may slow exacerbation rates in observational studies (39, 40) this has not been shown in randomized controlled trials (20, 41). Therefore, to prove the effects of augmentation therapy on lung function decline or exacerbation rates may require trials with larger numbers of patients and of longer duration than is currently feasible, unless patients are specifically recruited to enrich for the outcome in mind (22). However, our results also suggest that in part the lack of clinical effect may be due to suboptimal dosing using SD therapy.
In our group of patients with moderate COPD severity on SD therapy, residual serine protease activity could be significantly reduced in both BALF (elastase activity and cathepsin G) and the footprint of this activity in plasma (Aα-Val360) after DD therapy. This control of serine protease activity is important because mice deficient in all three neutrophil serine proteases (proteinase 3, cathepsin G, and NE) are substantially protected against lung tissue destruction after long-term exposure to cigarette smoke (2). The effect we observed on Aα-Val360 is also important because it represents a potential biomarker of disease activity given its significant correlation to physiologic, radiologic, and symptomatic markers of disease severity in untreated subjects with AATD (32). Overall, because of the protean effects of AAT, DD therapy produces a profound antiproteolytic effect, in addition to significant reductions in airway levels of other important proteases, such as collagenase (MMP1 [matrix metalloproteinase-1]) and gelatinase (MMP9), as we have previously described (42).
The overall reduction in proteolytic activity achieved with DD therapy translated into decreased elastin degradation even when this therapy was of relatively short duration (1 mo). DES and IDES, unique amino acid cross-links in mature elastin fibers, can serve as biomarkers of elastin degradation when measured in body fluids (43). The concentrations of DES/IDES present in body fluids are extremely low and recent methodologic advances have aided their detection. The specificity and sensitivity of DES/IDES measurement has improved with the development of an analytical method using HPLC followed by electrospray ionization (44). This method, in addition to tandem mass spectrometry, has resulted in enhanced detection of DES/IDES levels in plasma and sputum from subjects with usual COPD or AATD (27, 45). Furthermore, data support the use of DES/IDES as biomarkers to monitor emphysema progression and treatment response (46). Despite this enhanced detection method, 1 month of DD therapy did not lead to measurable changes in plasma levels of DES/IDES; however, significant changes were observed in BALF, highlighting the importance of directly studying lung samples to assess the impact of treatments aimed at halting elastin degradation. Overall, our findings reflect the importance of normalizing AAT levels to further decrease elastin degradation.
We assessed changes that occur in 58 different cytokines, chemokines, growth factors, and markers of tissue damage in the BALF and plasma of subjects when transitioning from SD to DD therapy. DD therapy significantly reduced a wide range of these inflammatory markers in BALF (CCL3, CCL11, IL2, IL3, IL4, IL9, IL10, IL12p40, IL17, GM-CSF [granulocyte–macrophage colony–stimulating factor], M-CSF [macrophage colony–stimulating factor], MIF [macrophage migration inhibitory factor], TNFα, IFNγ, and bFGF) compared with SD administration. These biomarkers are primarily linked with immune cell recruitment and activation (47), and these changes further confirm the additional role of AAT as a major immunomodulatory protein. MIF, GM-CSF, IL10, MCP7, CCL3, TNFα, IFNγ, IL2, and IL4 are major activators of macrophages and neutrophils. Equally, several T-cell activators are regulated by AAT, such as IFNγ, IL2, IL4, IL9, IL10, IL12p40, and IL15, and several of these targets are associated with emphysema formation and progression, such as IL-17 (48), IFNγ (49), and CCL11 (50). These changes are not surprising because AAT has been shown to have protean antiinflammatory effects, such as regulation of neutrophil chemotaxis (51), degranulation and autoimmunity (51), neutrophil apoptosis (52), antiinflammatory phosphatase responses (42), caspase activity (53), nitric oxide production (54), HIV type 1 infectivity and reproduction (55), TNFα converting enzyme activity (18), and endoplasmic reticulum stress (56, 57). We also observed a significant decrease in IL-10, an antiinflammatory cytokine that plays a central role in limiting local host immune responses. This decrease is expected because IL-10 is triggered by enhanced inflammatory states (58), now ameliorated with DD therapy.
Overall, further studies are required to determine the functional role of each of the targets affected by AAT level normalization identified in this study. The observation of a possible carryover effect in most of these markers, where the effect of DD therapy lasted at least a month after resuming SD therapy, should be further explored in studies of longer duration to determine when, and if, the potential carryover effect disappears. Clinical studies of DD therapy over a longer time period or the exploration of higher doses, which may yield greater improvements in biological parameters and potentially clinical parameters, should be implemented.
Some important limitations of this study should be mentioned. This was a small pilot study, which reported complete findings in eight patients with AATD. The primary outcome was change in pulmonary inflammatory markers measured in BALF and there are limited previous data on the natural variability of cytokine levels in BALF over such a short time period in healthy individuals, individuals with COPD, and in particular those with AATD. However, the significant changes observed despite this small sample size is a strong hint of the potent effects of DD therapy, at least in the individuals studied here. Unfortunately, we did not include non–AAT-deficient subjects with COPD, healthy control subjects, or a placebo arm to document the variability of inflammatory markers in BALF and better assess the magnitude of changes observed with DD therapy. For this reason, we opted for a three-phase design in which baseline conditions were restored, a design that followed a prior study evaluating the impact of AAT on sputum inflammation (12). We acknowledge that biological changes do not necessarily translate into changes in clinically relevant outcomes and further studies are required to validate the clinical impact of higher AAT dosing. It is important, however, to highlight that our results cannot be extrapolated to the entire AATD population, because the clinical manifestations in this condition are protean, from no lung symptoms to varying levels of COPD severity. We used defined inclusion criteria to enroll symptomatic subjects likely to have increased lung inflammation, such as presence of chronic bronchitis, exacerbations, or poor quality of life. Therefore, the magnitude of the effects observed might not be reflective of a broader population of patients with AATD.
It is important to note that although we standardized inhaler treatment that may affect lung inflammation (all subjects received a long-acting bronchodilator and an inhaled corticosteroid), different patterns of lung inflammation were observed among participants. For example, Subjects 2, 3, and 4 (Figures 2–4) always had high levels of cytokines, proteases, and lung degradation and a marked response to DD therapy, whereas Subject 1 had low levels that were not affected with increasing AAT dosing. This raises the question of applying precision medicine to tailor each subject’s AAT requirement, similar to the personalized approach proposed to decide the initiation of augmentation therapy (59). Further studies are required to assess this, ideally with reliable and accessible biomarkers to adjust therapy (not in BALF). The only plasma marker we observed that changed with DD therapy and followed the changes observed in BALF was Aα-Val360 and should be further explored.
Conclusions
Overall, we have confirmed that DD therapy is well tolerated by subjects with AATD, restores serum AAT levels to more than 25 μM, significantly reduces circulating and airway levels of serine proteases, reduces elastin degradation in the lung, and diminishes airway inflammation when compared with SD therapy. Further work in a larger cohort of patients is required to explore the long-term benefit or adverse effects of DD therapy because it could further slow the loss of lung function in subjects with AATD. Equally, the “protective” threshold concentration of AAT may require reevaluation.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Elio Donna, M.D., Luis Escobar, M.D., and Patricia Rebolledo, R.R.T., for their support in different aspects of the study. Editorial assistance was provided by Meridian HealthComms.
Footnotes
Funded by CSL Behring (investigator-initiated study grant to M.A.C.).
Author Contributions: Conception and design: M.A.C. and E.M. Data acquisition: M.A.C., P.G., G.H., E.M., P.R.N., S.M., L.V.L.-D., G.M.T., and R.A.S. Interpretation of data: M.A.C., P.G., G.H., E.M., P.R.N., S.M., L.V.L.-D., G.M.T., and R.A.S. Intellectual contribution to the manuscript: M.A.C., P.G., G.H., E.M., L.V.L.-D., G.M.T., and R.S. All authors were involved in preparation of the original draft of the manuscript and revising it critically for content. All authors gave final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201901-0010OC on April 9, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stoller JK, Aboussouan LS. A review of α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185:246–259. doi: 10.1164/rccm.201108-1428CI. [DOI] [PubMed] [Google Scholar]
- 2.Guyot N, Wartelle J, Malleret L, Todorov AA, Devouassoux G, Pacheco Y, et al. Unopposed cathepsin G, neutrophil elastase, and proteinase 3 cause severe lung damage and emphysema. Am J Pathol. 2014;184:2197–2210. doi: 10.1016/j.ajpath.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Janciauskiene SM, Bals R, Koczulla R, Vogelmeier C, Köhnlein T, Welte T. The discovery of α1-antitrypsin and its role in health and disease. Respir Med. 2011;105:1129–1139. doi: 10.1016/j.rmed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Morrison HM, Kramps JA, Burnett D, Stockley RA. Lung lavage fluid from patients with alpha 1-proteinase inhibitor deficiency or chronic obstructive bronchitis: anti-elastase function and cell profile. Clin Sci (Lond) 1987;72:373–381. doi: 10.1042/cs0720373. [DOI] [PubMed] [Google Scholar]
- 5.Stockley RA, Hill AT, Hill SL, Campbell EJ. Bronchial inflammation: its relationship to colonizing microbial load and alpha(1)-antitrypsin deficiency. Chest. 2000;117(Suppl 1):291S–293S. doi: 10.1378/chest.117.5_suppl_1.291s. [DOI] [PubMed] [Google Scholar]
- 6.Hill AT, Bayley DL, Campbell EJ, Hill SL, Stockley RA. Airways inflammation in chronic bronchitis: the effects of smoking and alpha1-antitrypsin deficiency. Eur Respir J. 2000;15:886–890. doi: 10.1034/j.1399-3003.2000.15e12.x. [DOI] [PubMed] [Google Scholar]
- 7.McElvaney NG, Stoller JK, Buist AS, Prakash UB, Brantly ML, Schluchter MD, et al. Baseline characteristics of enrollees in the National Heart, Lung and Blood Institute Registry of alpha 1-antitrypsin deficiency: Alpha 1-Antitrypsin Deficiency Registry Study Group. Chest. 1997;111:394–403. doi: 10.1378/chest.111.2.394. [DOI] [PubMed] [Google Scholar]
- 8.Parr DG, Guest PG, Reynolds JH, Dowson LJ, Stockley RA. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2007;176:1215–1221. doi: 10.1164/rccm.200703-489OC. [DOI] [PubMed] [Google Scholar]
- 9.Jonigk D, Al-Omari M, Maegel L, Müller M, Izykowski N, Hong J, et al. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc Natl Acad Sci USA. 2013;110:15007–15012. doi: 10.1073/pnas.1309648110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy C, Reeves EP, McElvaney NG. The role of neutrophils in alpha-1 antitrypsin deficiency. Ann Am Thorac Soc. 2016;13:S297–S304. doi: 10.1513/AnnalsATS.201509-634KV. [DOI] [PubMed] [Google Scholar]
- 11.Lewis EC. Expanding the clinical indications for α(1)-antitrypsin therapy. Mol Med. 2012;18:957–970. doi: 10.2119/molmed.2011.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stockley RA, Bayley DL, Unsal I, Dowson LJ. The effect of augmentation therapy on bronchial inflammation in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2002;165:1494–1498. doi: 10.1164/rccm.2109013. [DOI] [PubMed] [Google Scholar]
- 13.Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993;178:1629–1636. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aldonyte R, Jansson L, Janciauskiene S. Concentration-dependent effects of native and polymerised alpha1-antitrypsin on primary human monocytes, in vitro. BMC Cell Biol. 2004;5:11. doi: 10.1186/1471-2121-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janciauskiene S, Wrenger S, Immenschuh S, Olejnicka B, Greulich T, Welte T, et al. The multifaceted effects of alpha1-antitrypsin on neutrophil functions. Front Pharmacol. 2018;9:341. doi: 10.3389/fphar.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nita I, Hollander C, Westin U, Janciauskiene SM. Prolastin, a pharmaceutical preparation of purified human alpha1-antitrypsin, blocks endotoxin-mediated cytokine release. Respir Res. 2005;6:12. doi: 10.1186/1465-9921-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pott GB, Chan ED, Dinarello CA, Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol. 2009;85:886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lockett AD, Kimani S, Ddungu G, Wrenger S, Tuder RM, Janciauskiene SM, et al. α1-Antitrypsin modulates lung endothelial cell inflammatory responses to TNF-α. Am J Respir Cell Mol Biol. 2013;49:143–150. doi: 10.1165/rcmb.2012-0515OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadek JE, Klein HG, Holland PV, Crystal RG. Replacement therapy of alpha 1-antitrypsin deficiency: reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J Clin Invest. 1981;68:1158–1165. doi: 10.1172/JCI110360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman KR, Burdon JG, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM, et al. RAPID Trial Study Group. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 21.McElvaney NG, Burdon J, Holmes M, Glanville A, Wark PA, Thompson PJ, et al. RAPID Extension Trial Group. Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE) Lancet Respir Med. 2017;5:51–60. doi: 10.1016/S2213-2600(16)30430-1. [DOI] [PubMed] [Google Scholar]
- 22.Stockley RA, Edgar RG, Starkey S, Turner AM. Health status decline in α-1 antitrypsin deficiency: a feasible outcome for disease modifying therapies? Respir Res. 2018;19:137. doi: 10.1186/s12931-018-0844-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brantly ML, Wittes JT, Vogelmeier CF, Hubbard RC, Fells GA, Crystal RG. Use of a highly purified alpha 1-antitrypsin standard to establish ranges for the common normal and deficient alpha 1-antitrypsin phenotypes. Chest. 1991;100:703–708. doi: 10.1378/chest.100.3.703. [DOI] [PubMed] [Google Scholar]
- 24.Campos MA, Alazemi S, Zhang G, Wanner A, Salathe M, Baier H, et al. Exacerbations in subjects with alpha-1 antitrypsin deficiency receiving augmentation therapy. Respir Med. 2009;103:1532–1539. doi: 10.1016/j.rmed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Campos M, Geraghty P, Holt G, Donna E, Mendes E, Escobar LA, et al. The biological effects of double dose augmentation therapy for subjects with alpha-1 antitrypsin deficiency [abstract] Am J Respir Crit Care Med. 2017;195:A6315. [Google Scholar]
- 26.Campos M, Geraghty P, Holt G, Donna E, Mendes E, Escobar L, et al. Effects of double dose alpha 1 antitrypsin (AAT) therapy on cytokine pathways in AAT deficiency (AATD) Eur Respir J. 2018;52:PA571. [Google Scholar]
- 27.Ma S, Lin YY, He J, Rouhani FN, Brantly M, Turino GM. Alpha-1 antitrypsin augmentation therapy and biomarkers of elastin degradation. COPD. 2013;10:473–481. doi: 10.3109/15412555.2013.771163. [DOI] [PubMed] [Google Scholar]
- 28.Carter RI, Mumford RA, Treonze KM, Finke PE, Davies P, Si Q, et al. The fibrinogen cleavage product Aα-Val360, a specific marker of neutrophil elastase activity in vivo. Thorax. 2011;66:686–691. doi: 10.1136/thx.2010.154690. [DOI] [PubMed] [Google Scholar]
- 29.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 30.Hollander C, Sitkauskiene B, Sakalauskas R, Westin U, Janciauskiene SM. Serum and bronchial lavage fluid concentrations of IL-8, SLPI, sCD14 and sICAM-1 in patients with COPD and asthma. Respir Med. 2007;101:1947–1953. doi: 10.1016/j.rmed.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Carter RI, Ungurs MJ, Mumford RA, Stockley RA. Aα-Val360: a marker of neutrophil elastase and COPD disease activity. Eur Respir J. 2013;41:31–38. doi: 10.1183/09031936.00197411. [DOI] [PubMed] [Google Scholar]
- 32.Carter RI, Ungurs MJ, Pillai A, Mumford RA, Stockley RA. The relationship of the fibrinogen cleavage biomarker Aα-Val360 with disease severity and activity in α1-antitrypsin deficiency. Chest. 2015;148:382–388. doi: 10.1378/chest.14-0520. [DOI] [PubMed] [Google Scholar]
- 33.Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, et al. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316:1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- 34.Campos MA, Kueppers F, Stocks JM, Strange C, Chen J, Griffin R, et al. Safety and pharmacokinetics of 120 mg/kg versus 60 mg/kg weekly intravenous infusions of alpha-1 proteinase inhibitor in alpha-1 antitrypsin deficiency: a multicenter, randomized, double-blind, crossover study (SPARK) COPD. 2013;10:687–695. doi: 10.3109/15412555.2013.800852. [DOI] [PubMed] [Google Scholar]
- 35.Hubbard RC, Sellers S, Czerski D, Stephens L, Crystal RG. Biochemical efficacy and safety of monthly augmentation therapy for alpha 1-antitrypsin deficiency. JAMA. 1988;260:1259–1264. [PubMed] [Google Scholar]
- 36.Barker AF, Iwata-Morgan I, Oveson L, Roussel R. Pharmacokinetic study of alpha1-antitrypsin infusion in alpha1-antitrypsin deficiency. Chest. 1997;112:607–613. doi: 10.1378/chest.112.3.607. [DOI] [PubMed] [Google Scholar]
- 37.Chapman KR, Stockley RA, Dawkins C, Wilkes MM, Navickis RJ. Augmentation therapy for alpha1 antitrypsin deficiency: a meta-analysis. COPD. 2009;6:177–184. doi: 10.1080/15412550902905961. [DOI] [PubMed] [Google Scholar]
- 38.Gøtzsche PC, Johansen HK. Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung disease. Cochrane Database Syst Rev. 2016;9:CD007851. doi: 10.1002/14651858.CD007851.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barros-Tizón JC, Torres ML, Blanco I, Martínez MT Investigators of the rEXA Study Group. Reduction of severe exacerbations and hospitalization-derived costs in alpha-1-antitrypsin-deficient patients treated with alpha-1-antitrypsin augmentation therapy. Ther Adv Respir Dis. 2012;6:67–78. doi: 10.1177/1753465812438387. [DOI] [PubMed] [Google Scholar]
- 40.Köhnlein T, Janciauskiene S, Welte T. Diagnostic delay and clinical modifiers in alpha-1 antitrypsin deficiency. Ther Adv Respir Dis. 2010;4:279–287. doi: 10.1177/1753465810376407. [DOI] [PubMed] [Google Scholar]
- 41.Dirksen A, Piitulainen E, Parr DG, Deng C, Wencker M, Shaker SB, et al. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J. 2009;33:1345–1353. doi: 10.1183/09031936.00159408. [DOI] [PubMed] [Google Scholar]
- 42.Geraghty P, Eden E, Pillai M, Campos M, McElvaney NG, Foronjy RF. α1-Antitrypsin activates protein phosphatase 2A to counter lung inflammatory responses. Am J Respir Crit Care Med. 2014;190:1229–1242. doi: 10.1164/rccm.201405-0872OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turino GM, Ma S, Cantor JO, Lin YY. Biomarkers in alpha-1 antitrypsin deficiency chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13:S336–S340. doi: 10.1513/AnnalsATS.201509-574KV. [DOI] [PubMed] [Google Scholar]
- 44.Ma S, Lieberman S, Turino GM, Lin YY. The detection and quantitation of free desmosine and isodesmosine in human urine and their peptide-bound forms in sputum. Proc Natl Acad Sci USA. 2003;100:12941–12943. doi: 10.1073/pnas.2235344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma S, Lin YY, Turino GM. Measurements of desmosine and isodesmosine by mass spectrometry in COPD. Chest. 2007;131:1363–1371. doi: 10.1378/chest.06-2251. [DOI] [PubMed] [Google Scholar]
- 46.Ma S, Lin YY, Cantor JO, Chapman KR, Sandhaus RA, Fries M, et al. The effect of alpha-1 proteinase inhibitor on biomarkers of elastin degradation in alpha-1 antitrypsin deficiency: an analysis of the RAPID/RAPID extension trials. Chronic Obstr Pulm Dis (Miami) 2016;4:34–44. doi: 10.15326/jcopdf.4.1.2016.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bryant VL, Slade CA. Chemokines, their receptors and human disease: the good, the bad and the itchy. Immunol Cell Biol. 2015;93:364–371. doi: 10.1038/icb.2015.23. [DOI] [PubMed] [Google Scholar]
- 48.Lindén A, Hoshino H, Laan M. Airway neutrophils and interleukin-17. Eur Respir J. 2000;15:973–977. doi: 10.1034/j.1399-3003.2000.15e28.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA, Jr, et al. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med. 2000;192:1587–1600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Armiento JM, Scharf SM, Roth MD, Connett JE, Ghio A, Sternberg D, et al. Eosinophil and T cell markers predict functional decline in COPD patients. Respir Res. 2009;10:113. doi: 10.1186/1465-9921-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergin DA, Reeves EP, Meleady P, Henry M, McElvaney OJ, Carroll TP, et al. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest. 2010;120:4236–4250. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurley K, Lacey N, O’Dwyer CA, Bergin DA, McElvaney OJ, O’Brien ME, et al. Alpha-1 antitrypsin augmentation therapy corrects accelerated neutrophil apoptosis in deficient individuals. J Immunol. 2014;193:3978–3991. doi: 10.4049/jimmunol.1400132. [DOI] [PubMed] [Google Scholar]
- 53.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, et al. alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan ED, Pott GB, Silkoff PE, Ralston AH, Bryan CL, Shapiro L. Alpha-1-antitrypsin inhibits nitric oxide production. J Leukoc Biol. 2012;92:1251–1260. doi: 10.1189/jlb.0212071. [DOI] [PubMed] [Google Scholar]
- 55.Shapiro L, Pott GB, Ralston AH. Alpha-1-antitrypsin inhibits human immunodeficiency virus type 1. FASEB J. 2001;15:115–122. doi: 10.1096/fj.00-0311com. [DOI] [PubMed] [Google Scholar]
- 56.Carlson JA, Rogers BB, Sifers RN, Finegold MJ, Clift SM, DeMayo FJ, et al. Accumulation of PiZ alpha 1-antitrypsin causes liver damage in transgenic mice. J Clin Invest. 1989;83:1183–1190. doi: 10.1172/JCI113999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carroll TP, Greene CM, O’Connor CA, Nolan AM, O’Neill SJ, McElvaney NG. Evidence for unfolded protein response activation in monocytes from individuals with alpha-1 antitrypsin deficiency. J Immunol. 2010;184:4538–4546. doi: 10.4049/jimmunol.0802864. [DOI] [PubMed] [Google Scholar]
- 58.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stockley RA, Miravitlles M, Vogelmeier C Alpha One International Registry (A.I.R.) Augmentation therapy for alpha-1 antitrypsin deficiency: towards a personalised approach. Orphanet J Rare Dis. 2013;8:149. doi: 10.1186/1750-1172-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.