Abstract
Objectives
The purpose of this study was to quantify the reduction in patient radiation dose during coronary angiography (CA) by a new X‐ray technology, and to assess its impact on diagnostic image quality.
Background
Recently, a novel X‐ray imaging technology has become available for interventional cardiology, using advanced image processing and an optimized acquisition chain for radiation dose reduction.
Methods
70 adult patients were randomly assigned to a reference X‐ray system or the novel X‐ray system. Patient demographics were registered and exposure parameters were recorded for each radiation event. Clinical image quality was assessed for both patient groups.
Results
With the same angiographic technique and a comparable patient population, the new imaging technology was associated with a 75% reduction in total kerma‐area product (KAP) value (decrease from 47 Gycm2 to 12 Gycm2, P < 0.001). Clinical image quality showed an equivalent detail and contrast for both imaging systems. On the other hand, the subjective appreciation of noise was more apparent in images of the new image processing system, acquired at lower doses, compared to the reference system. However, the higher noise content did not affect the overall image quality score, which was adequate for diagnosis in both systems.
Conclusions
For the first time, we present a new X‐ray imaging technology, combining advanced noise reduction algorithms and an optimized acquisition chain, which reduces patient radiation dose in CA drastically (75%), while maintaining diagnostic image quality. Use of this technology may further improve the radiation safety of cardiac angiography and interventions. © 2015 Wiley Periodicals, Inc.
Keywords: coronary angiography, coronary artery disease, imaging, radiation dose
INTRODUCTION
Cardiovascular disease (CVD) is the most important health problem globally, particularly in the western world. Yet, the decrease in mortality rate for CVD and, especially, coronary heart disease emphasizes the value of prevention, diagnosis and improved treatment in cardiology 1. Although diagnostic accuracy of other less invasive techniques such as computed tomography has substantially improved over the last years, angiography (CA) remains the gold standard for the evaluation of suspected coronary atherosclerosis 2, 3.
Despite radiation‐associated risks, X‐rays remain indispensable for diagnosis in CA. CA is typically ranked amongst medical procedures as an examination delivering a high radiation exposure to the patient 4, 5. In a large US population survey, CA and percutaneous coronary intervention (PCI) were among the 20 imaging procedures that provided the largest contribution to annual cumulative radiation doses 6. Radiation‐induced skin injuries are a real risk in PCI with high cumulative skin doses 7, 8. Both physicians and manufacturers are making efforts to implement new techniques in order to reduce the possible radiation risks, while maintaining an image quality that is sufficient for the respective clinical tasks 5, 9, 10, 11, 12.
The new X‐ray imaging technology tested in this study uses advanced real‐time image noise reduction algorithms combined with an optimized acquisition chain that enables dose reduction and adequate image quality at the same time. The new image processing combines temporal and spatial noise reduction filters with automatic pixel shift functionality. Parameters that control the algorithms are tuned to achieve optimal results, depending on the specific demands for image quality by each clinical application (e.g., neurology, cardiology, electrophysiology) 13, 14, 15. A first study on the application of this new technology in interventional neuroradiology reported non‐inferiority of image quality in a single digital subtraction angiogram at 75% radiation dose reduction 13. A 60% dose reduction for the whole procedure was confirmed in neuroangiography and interventional neuroradiology, without affecting the working habits of the physician 14. In electrophysiological interventions, the new algorithm has proven to significantly reduce both patient and operator dose by 43% and 50%, respectively 15. However, dose reduction and image quality were not yet evaluated in diagnostic CA, in which image quality requirements are more demanding compared to electrophysiology procedures.
The aim of this study was to quantify the reduction in patient radiation dose for coronary angiography (CA) by this new X‐ray imaging technology and to assess its impact on diagnostic image quality.
MATERIALS AND METHODS
Imaging Systems
This study was designed to assess the patient radiation dose and image quality on a reference system (room A: Allura Xper FD10, Philips Healthcare, The Netherlands) versus the new system (room B: Allura Clarity FD20/10, Philips Healthcare, The Netherlands) equipped with the novel ClarityIQ‐technology (ClarityIQ, Philips Healthcare, The Netherlands). The latter has predefined system settings, fine‐tuned for the clinical tasks in diagnostic cardiac catheterization at an X‐ray dose of 100%, 50%, or 30% of the reference system. The powerful X‐ray tube (MRC, Philips Healthcare, Best, The Netherlands) allows the use of smaller focal spot sizes, shorter pulses and additional beam filtration. The image processing algorithm combines several features: motion compensation and temporal averaging of consecutive images to allow for temporal noise reduction; spatial filtering within larger neighborhoods for spatial noise reduction; and subjective image quality enhancements with, e.g., edge enhancement, contrast enhancement, background contrast reduction and brightness control. The manufacturer states that no image quality compromises are made for the reduced radiation exposure settings 13, 14.
Room B is equipped with a biplane angiography system, whereas room A is monoplanar. For comparability reasons, physicians were asked to use only one X‐ray tube in room B for this study. The frame rate for left and right coronary angiography (LCA and RCA) is 12.5 f/s in room A and 15 f/s in room B, and for left ventriculography (LV) 25 f/s and 30 f/s, respectively. For the low‐dose fluoroscopy setting (the only one used in this study) the additional filtration contains 0.4 mm Cu and 1 mm Al in both systems. The beam filtration for cineangiography is equivalent for the reference setting in room A and the 100% dose setting in room B (only inherent filtration). Yet, additional filtration is inserted into the beam for the 50% (0.1 mm Cu and 1 mm Al) and 30% (0.4 mm Cu and 1 mm Al) dose settings in room B. Because of the higher frame rate, no 30% dose setting is available for LV in room B.
Patient Cohort
A total of 70 successive patients were enrolled in the study. The patient population included adult patients referred to the cardiac catheterization laboratory for a diagnostic CA. Patients with previous coronary artery bypass graft were excluded from the study. Patients were randomly divided over both angiographic rooms. Patients' demographics were registered. All patients provided written informed consent and the study protocol was approved by the Ethics Committee of our institution.
Angiographic Procedure
In our facility, a standard acquisition protocol was implemented, with six views for the left coronary arteries and three views for the right coronary artery 12. The acquisition of fewer or additional views was left to the judgment of the interventional cardiologist. Contrast medium injection was dosed by power injection with 7 mL and 6 mL per cinegraphic acquisition for LCA and RCA, respectively. Although radial access is commonly used, the right femoral approach was performed in all procedures as not to confound exposure parameters by access site. Physicians were asked to use the lowest available dose settings of the reference system (room A): fluoroscopy “low dose” and cinegraphy “reference dose,” which are equivalent to the fluoroscopy “low dose” and cinegraphy “100% dose” setting of the new imaging system (room B). For the patients imaged with new imaging system, physicians were asked to adopt the lowest radiation dose setting for LCA and RCA (30% dose). If patient size or image quality required this, the operator could switch to a higher dose setting. Physicians were asked to perform a left ventriculogram for all patients, and to use the reference dose setting in room A (equivalent to the 100% dose setting of room B), and to use the 50% dose setting in room B, unless there were patient‐ or procedure‐related contra‐indications.
Radiation Dose Evaluation
Patient radiation exposure was expressed as cumulative kerma‐area product (KAP) and measured using an integrated KAP‐meter, which was calibrated in situ with a NE2571 Farmer ionization chamber and 33 × 41 cm Kodak X‐Omat V films (Eastman Kodak, USA). The calibration factor was taken as the ratio between the actual DAP, calculated as the dose in the center of the field multiplied by the field size measured from the film, and the DAP reading from the integrated KAP‐meter. The calibration was performed for the used dose settings, with a peak potential ranging from 50 to 120 kVp 16. Total fluoroscopy time and contrast medium consumption (CMC) were registered at the end of each procedure. Next to total KAP, KAP data were registered for each separate fluoroscopic or cinegraphic acquisition. This allows analysis of the dose contribution of several recurring angiographic views (±10°): left‐anterior oblique (LAO) 0° cranial (CRAN) 0° and LAO 90° CRAN 0° for LCA, LAO 90° CRAN 0° for RCA and right anterior oblique (RAO) 35° CRAN 0° for LV.
Image Quality Assessment
Two cinegraphic runs were selected from each patient: one posterior‐anterior view of the left coronary tree (LAO 0 ± 10° CRAN 0 ± 10°) and one lateral view of the right coronary tree (LAO 90 ± 10° CRAN 0 ± 10°). Dynamic images were selected by a medical physicist, solely based on the beam angulation. In total, 140 cinegraphic runs were evaluated in randomized, blinded, offline readings by four interventional cardiologists: two senior cardiologists with 32 (Y.T.) and 17 (B.D.) years of experience, and two junior cardiologists with five (SVP) and four (JDP) years of experience. All of the observers have imaged and treated patients in both cathlabs and were therefore familiar with both imaging systems. ViewDEX software 17 was used to display images and record scores. Before starting the study, the readers underwent a training session to familiarize themselves with the scoring method. The four readers graded the diagnostic quality of each run independently according to the questions defined in Table 1. A scale of 0–5 per question was applied, where 0 = insufficient for diagnosis, 1 = very poor, 2 = poor, 3 = fair, 4 = good, 5 = excellent. Scores for each question of the survey were averaged for the 4 readers and reported as a percentage of the maximum possible score.
Table 1.
Criteria for Image Quality Assessment
| Criterion | Description | Question |
|---|---|---|
| 1 | Rating of image resolution | How would you judge the sharpness of delineation? |
| 2 | Rating of image contrast | How would you judge the contrast with the background? |
| 3 | Rating of image noise | How would you judge the noise content? |
| 4 | General image quality score | How would you judge the overall image quality? |
Data Analysis
Differences between two independent (not normally distributed) populations were tested for significance with the two‐tailed Mann‐Whitney test (95% confidence level) and are represented as median values and interquartile range. Categorical data were compared with a chi‐square test. A probability of P < 0.05 was considered statistically significant. All statistical calculations were performed with IBM SPSS Statistics 22 program (IBM corp, USA).
RESULTS
Patient Characteristics
Patient demographics were comparable between both rooms with no statistical difference in gender (22 and 18 of 35 patients were male for room A and B, respectively, P = 0.254), age (median age 66 (interquartile range 58–76) and 64 (interquartile range 59–73) for room A and B, respectively, P = 0.312), and BMI [median BMI 26.5 (interquartile range 24.1–30.8) and 26.1 (interquartile range 23.8–31.0) kg/m2 for room A and B, respectively, P = 0.737]. Hence, no bias was introduced as a result of differences between groups.
Radiation Exposure Data
Patients' radiation exposure in terms of cumulative KAP‐value for the total procedure was considerably lower (75%) in the novel imaging group compared to the reference system (12.0 vs. 47.4 Gycm2, P < 0.001) (Table 2). Figure 1 shows the distribution of total KAP values for both rooms, indicating a narrower range, shifted towards the lower dose range for the new imaging technology. The cinegraphy contribution to the total dose was 89% versus 72% for room A and B, respectively. With the new imaging system, KAP values for fluoroscopy and cineangiography decreased with 30% and 77%, respectively, compared to the reference system. Reductions in total KAP and KAP for cineangiography were statistically significant (P < 0.001). The number of cineangiographic runs or CMC did not differ between groups. Even though the fluoroscopy time was significantly higher in room B, the KAP arising from fluoroscopy was still lower—although not statistically significant—in room B, compared to room A.
Table 2.
Patient Exposure Data for the Reference (Room A) and the Novel Imaging System (Room B)
| Room A | Room B | P‐value | |
|---|---|---|---|
| (n = 35) | (n = 35) | ||
| KAP for total procedure,a Gycm2 | 47.4 (33.6–66.5) | 12.0 (7.59–20.1) | <0.001 |
| KAP total CA,b Gycm2 | 41.7 (29.6–60.3) | 8.77 (6.33–17.6) | <0.001 |
| KAP fluoro, Gycm2 | 4.62 (3.02–9.18) | 3.22 (2.00–6.62) | 0.064 |
| KAP cineangiography, Gycm2 | 41.5 (28.8–55.3) | 9.48 (5.68–14.0) | <0.001 |
| Cine runs, n | 11 (10–12) | 11 (10–12) | 0.468 |
| Fluoroscopy time, s | 114 (72–196) | 172 (114–301) | 0.015 |
| CMC,c mL | 115 (104–130) | 119 (99.7–136) | 0.492 |
| KAP LCA Lao0° Cran0°, Gycm2 | 1.57 (1.25–1.96) | 0.260 (0.169‐ 0.435) | <0.001 |
| KAP LCA Lao90° Cran0°, Gycm2 | 2.99 (1.88–5.21) | 0.617 (0.379–1.16) | <0.001 |
| KAP RCA Lao90° Cran0°, Gycm2 | 2.65 (1.92–4.48) | 0.733 (0.492–1.23) | <0.001 |
| KAP LV Rao35° Cran0°, Gycm2 | 4.37 (3.11–5.95) | 2.57 (1.55–3.31) | <0.001 |
KAP: kermfa‐area product.
CA: coronafry angiography–here without the left ventriculogram.
CMC: contrast medium consumption. Values represent the median (interquartile range).
Figure 1.
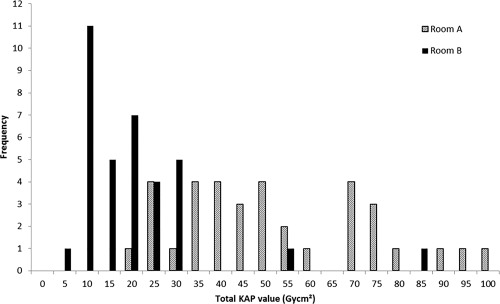
Distribution of total KAP values with the reference system (Room A) and the new imaging system (Room B).
LV was performed in 32 and 33 out of 35 cases in room A and B, respectively (P = 0.667). Three patients where no ventriculography was performed to reduce the contrast load were diabetic, two others had earlier renal failure. All ventriculograms were acquired with the 50% dose setting and KAP values decreased with 41% in room B, compared to room A (P < 0.001).
Median KAP values per exposure of the 4 selected angiographic views are detailed in Table 2. KAP decreased with 83% and 79% for the posterior‐anterior and lateral LCA view, respectively, and with 72% for the lateral RCA view. In 97% and 94% of the images for LCA and RCA, respectively, the 30%‐dose setting was used in room B. The operator switched to the 50% dose setting to improve image quality for the posterior‐anterior view in 1 case (patient BMI of 35 kg/m2) and for the lateral view in two cases (patient BMI of 35 and 34 kg/m2).
Image Quality Assessment
Selected runs of the same angiographic projection were compared for room A and B. An example of still frames of the LCA and RCA of the same patient, acquired with the reference and the novel system, is available in Figs. 2 and 3. The original dynamic images are available in online Supporting Information.
Figure 2.
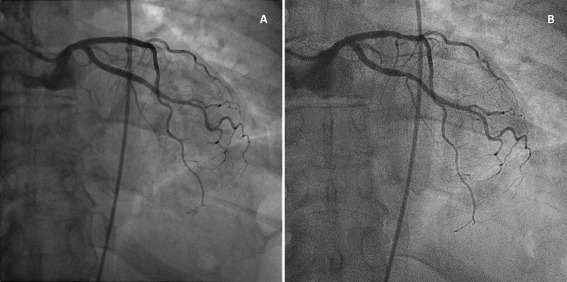
A: Posterior‐anterior view of the LCA of a patient, acquired with the reference system (Room A). B: Posterior‐anterior view of the LCA of the same patient, acquired with the new imaging technology at 30% of the radiation dose (Room B). These images were acquired when the patient returned to the department for therapeutic intervention on a different day. Original moving images are available in online Supporting Information.
Figure 3.
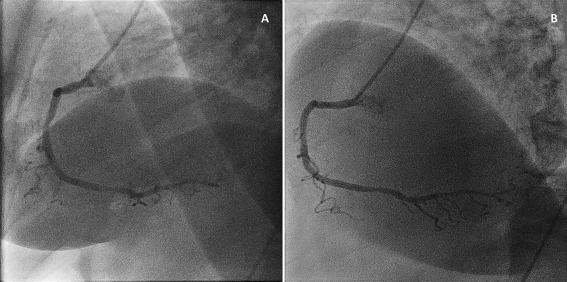
A: Lateral view of the RCA of a patient, acquired with the reference system (Room A). B: Lateral view of the RCA of the same patient, acquired with the new imaging technology at 30% of the radiation dose (Room B). These images were acquired when the patient returned to the department for therapeutic intervention on a different day. Original moving images are available in online Supporting Information.
The mean scores for each question of the LCA and RCA assessment are detailed in Table 3. Images of both systems were evaluated equivalent for general image quality (IQ), resolution and image contrast, resulting in mean scores above 75% for these parameters in both rooms. However, the subjective appreciation of noise (Question 3) is more apparent with the new image processing at 30 or 50% of the reference dose. Yet, this does not represent a limitation for the general image quality appreciation (Question 4). Distributions of the scores for each question of the LCA assessment are detailed in Fig. 4. A comparable score distribution was observed for the RCA assessment, showing a shift toward lower scores for noise appreciation, but not for the general image quality score.
Table 3.
Score for Each Question of the Image Quality Assessment for LCA and RCA, Acquired With the Reference System (Room A) or the Novel System (Room B)
| Room A | Room B | P‐value | |
|---|---|---|---|
| LCA, n | 35 | 35 | |
| Q1: image resolution | 78 ± 7 | 75 ± 10 | 0.168 |
| Q2: image contrast | 82 ± 7 | 80 ± 9 | 0.512 |
| Q3: image noise | 84 ± 7 | 64 ± 9 | <0.001 |
| Q4: general IQ score | 80 ± 8 | 76 ± 11 | 0.172 |
| RCA, n | 35 | 35 | |
| Q1: image resolution | 78 ± 9 | 75 ± 12 | 0.180 |
| Q2: image contrast | 78 ± 8 | 80 ± 10 | 0.293 |
| Q3: image noise | 83 ± 7 | 64 ± 11 | <0.001 |
| Q4: general IQ score | 76 ± 11 | 76 ± 12 | 0.877 |
Scores represent the mean (± standard deviation) of four readers, expressed as a percentage of the maximum possible score.
Figure 4.
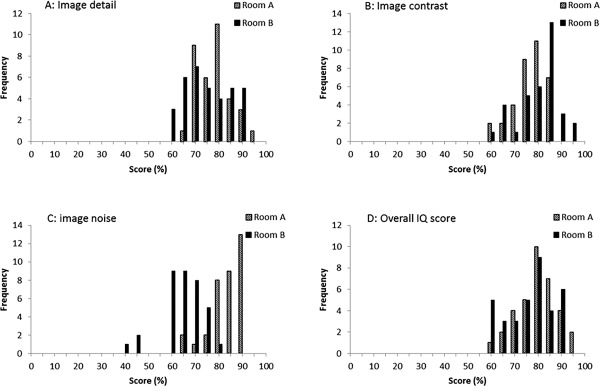
Distribution of the mean image quality score (%) for the images of the LCA assessment. A: Scores for the first question (image detail) for the reference X‐ray system (Room A) and the new X‐ray system (Room B); B: scores for image contrast; C: scores for image noise; D: overall image quality scores.
DISCUSSION
In this study, we report for the first time the impact of a new X‐ray and image processing technology (ClarityIQ, Philips Healthcare, The Netherlands) on radiation dose and image quality during CA procedures. With the same angiographic technique, the new system delivers a 75% lower overall radiation dose to the patient. This drastic reduction is comparable to the reports on this technology in neuroradiology 13, 14. Reported reduction of radiation dose in electrophysiology was lower, since only the 50% dose setting was available there 15. Our study showed that the 30% dose setting could be used for the majority of the patients undergoing CA. Most of the radiation dose reduction is attributable to the altered cinegraphic exposure settings of the new system, which are feasible owing to the new image processing algorithm.
The impact of the diminished radiation exposure on image quality using the new technology is minor: overall image quality scores were higher than 60% (corresponding to rating 3 out of 5, representing the image quality rating “fair”) for both imaging systems, indicating a good perceptual image quality. The subjective appreciation of noise is more distinct with the new image processing (at 30 or 50% of the reference dose), as expected due to the lower dose. However, this does not seem to restrict the diagnostic process.
Assessment of clinical image quality is not straightforward. Evaluation of image quality can be based on detector characteristics such as detective quantum efficiency, modulation transfer function, and contrast‐to‐noise ratio 18. These physical measurements describe the technical performance of the detector but they are difficult to link directly to clinical performance 19. Contrast‐detail phantom studies are well established to compare different radiography systems or acquisition techniques, but are not suited for dynamic images 20. Some phantoms are available for dynamic IQ assessment, yet these phantoms have the disadvantage that they do not incorporate details or features that are directly linked with critical issues in clinical cardiac images (i.e., circulation of contrast agent, anatomical background, lesions, or pulsating arteries) 20, 21, 22. The DIMOND III project defined quality criteria for cardiac angiographic procedures that comprise the assessment of the angiographic technique or visualization levels of different anatomical features, and therefore include the content, but not the quality of the image itself 23, 24. Up to now, no observer models are available for the perceptual quality of clinical cardiac images. Moreover, these models often assess superiority or inferiority of one image to a reference, and not whether the image is acceptable or adequate for diagnosis 25. In this study, the authors chose to adopt a subjective image quality assessment by clinicians that included basic elements of perceptual quality (noise, resolution, and contrast), next to a general diagnostic quality appreciation.
A limitation of our study is the scoring method for the image quality assessment. As not to confound the IQ assessment with the personal image presentation preferences of the observers, the IQ scoring method used general questions, which do not necessarily relate to the clinical observer tasks of an interventional cardiologist in daily practice. An observer study with questions that relate to the detection of lesions, or an analysis of clinically relevant features in the moving images might be a good alternative to this general scoring method.
Optimization in interventional cardiology means to ensure that the lowest practicable dose to the patient is used for obtaining the desired clinical information from a certain imaging procedure 5. The observed total KAP values for room A are within the range reported in literature (10–110 Gycm2), and close to the European reference level for CA of 45 Gycm2 3, 9, 26, 27, 28. The KAP values observed with the new X‐ray technology are at the lower end of this range, especially considering that they include LV, which is not the case in most literature reports. Possibly, the reported KAP values obtained in our interventional cardiology department can be lowered further when applying a modified imaging protocol. Now, an imaging protocol is implemented, which includes lateral views (LAO 90°) of the left and right coronary tree, and, very often, a left ventriculogram. It is well known from literature that steep LAO projections deliver a high relative dose to the patient and the operator, and could possibly be interchanged with less‐irradiating tube angulations to balance the clinical yield with the radiation risk 29, 30, 31. A large multicenter survey in France revealed that in only 58% of CAs a LV was performed 9. The necessity of performing a left ventriculogram can be questioned when other means to assess LV function, like cardiac ultrasonography, are available.
Literature data, including a large scale multicenter study of the research group, shows that a CA procedure contributes to about 38% of the cumulative dose of combined procedures involving PCI 28, 32. Despite significant longer fluoroscopy times, the mean contribution of cineangiography to the total KAP is still 50% for therapeutic procedures 32. Since many patients need cardiac interventions after diagnosis of atherosclerosis, these patients will also benefit from the dose reductions obtained with the new imaging technology reducing KAP values with 30% and 77% for fluoroscopy and cinegraphy, respectively. Moreover, the risk of skin injuries and the proportion of interventional cardiac procedures that exceed the KAP action levels (300 Gycm2, or even the more conservative KAP trigger level of 125 Gycm2) will be significantly lower with the new imaging system 32, 33. Given the direct link between patient exposure and scatter dose, a significant drop in operator occupational dose can be expected with this new X‐ray technology. These effects should be quantified through further research.
CONCLUSION
A new X‐ray imaging technology, combining advanced noise reduction algorithms and an optimized acquisition chain, reduced patient radiation dose in CA by 75%, while maintaining diagnostic image quality. Use of this technology may further improve the radiation safety of cardiac angiography and interventions.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors thank all doctors and nursing staff of the Ghent University Hospital Heart Center that contributed to this study.
Conflict of interest: Nothing to report.
REFERENCES
- 1. Melanie Nichols NT, Scarborough P, et al. Cardiovascular disease in Europe 2014: Epidemiological update. Eur Heart J 2014;2950–2959. [DOI] [PubMed] [Google Scholar]
- 2. Klein AJ, Garcia JA. Rotational coronary angiography. Cardiol Clin 2009;27:395–405. [DOI] [PubMed] [Google Scholar]
- 3. Empen K, Kuon E, Hummel A, et al. Comparison of rotational with conventional coronary angiography. Am Heart J 2010;160:552–563. [DOI] [PubMed] [Google Scholar]
- 4. UNSCEAR . Report of the United Nations scientific committee on the effects of atomic radiaion. Unscear 2008 report Vol I: Sources of ionizing radiation. Annex A: Medical radiation exposure. United Nations Publications. 2010;
- 5. Cousins C, Miller DL, Bernardi G, et al; and International Commission on Radiological P . ICRP PUBLICATION 120: Radiological protection in cardiology. Annals of the ICRP 2013;42:1–125. doi: 10.1016/j.icrp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 6. Fazel R, Krumholz HM, Wang Y, et al. Exposure to low‐dose ionizing radiation from medical imaging procedures. New Engl J Med 2009;361:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun Z, AbAziz A, Yusof AK. Radiation‐induced noncancer risks in interventional cardiology: Optimisation of procedures and staff and patient dose reduction. BioMed Res Int 2013;2013:976–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinberg BD, Vance A, Arbique GM, et al. Evaluation of fluoroscopic cases qualifying as potential fluoroscopic sentinel events. Acad Radiol 2013;20:457–462. [DOI] [PubMed] [Google Scholar]
- 9. Georges JL, Belle L, Ricard C, et al. Patient exposure to X‐rays during coronary angiography and percutaneous transluminal coronary intervention: Results of a multicenter national survey. Catheter Cardiovasc Interv 2014;83:729–738. [DOI] [PubMed] [Google Scholar]
- 10. Fetterly KA, Mathew V, Lennon R, et al. Radiation dose reduction in the invasive cardiovascular laboratory: Implementing a culture and philosophy of radiation safety. JACC Cardiovasc Interv 2012;5:866–873. [DOI] [PubMed] [Google Scholar]
- 11. Wassef AW, Hiebert B, Ravandi A, et al. Radiation dose reduction in the cardiac catheterization laboratory utilizing a novel protocol. JACC Cardiovasc Interv 2014;7:550–557. [DOI] [PubMed] [Google Scholar]
- 12. Eloot L, Bacher K, Steenbeke F, et al. Three‐dimensional rotational X‐ray acquisition technique is reducing patients' cancer risk in coronary angiography. Catheter Cardiovasc Interv 2013;82:E419–E427. [DOI] [PubMed] [Google Scholar]
- 13. Soderman M, Holmin S, Andersson T, et al. Image noise reduction algorithm for digital subtraction angiography: Clinical results. Radiology 2013;269:553–560. [DOI] [PubMed] [Google Scholar]
- 14. Soderman M, Mauti M, Boon S, et al. Radiation dose in neuroangiography using image noise reduction technology: A population study based on 614 patients. Neuroradiology 2013;55:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dekker LR, van der Voort PH, Simmers TA, et al. New image processing and noise reduction technology allows reduction of radiation exposure in complex electrophysiologic interventions while maintaining optimal image quality: A randomized clinical trial. Heart Rhythm 2013;10:1678–1682. [DOI] [PubMed] [Google Scholar]
- 16. Bacher K, Bogaert E, Lapere R, et al. Patient‐specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation 2005;111:83–89. [DOI] [PubMed] [Google Scholar]
- 17. Hakansson M, Svensson S, Zachrisson S, et al. VIEWDEX: An efficient and easy‐to‐use software for observer performance studies. Radiat Prot Dosimetry 2010;139:42–51. [DOI] [PubMed] [Google Scholar]
- 18. Sanchez R, Vano E, Ubeda C, et al. Influence of image metrics when assessing image quality from a test object in cardiac X‐ray systems: Part II. J Digit Imaging 2012;25:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Crop A, Bacher K, Van Hoof T, et al. Correlation of Contrast‐detail analysis and clinical image quality assessment in chest radiography with a human cadaver study. Radiology 2011; 262:298–304. [DOI] [PubMed] [Google Scholar]
- 20. Dragusin O, Bosmans H, Pappas C, et al. An investigation of flat panel equipment variables on image quality with a dedicated cardiac phantom. Phys Med Biol 2008;53:4927–4940. [DOI] [PubMed] [Google Scholar]
- 21. Vano E, Ubeda C, Martinez LC, et al. Paediatric interventional cardiology: Flat detector versus image intensifier using a test object. Phys Med Biol 2010;55:7287–7297. [DOI] [PubMed] [Google Scholar]
- 22. Wondrow MA, Laskey WK, Hildner FJ, et al. Cardiac catheterization laboratory imaging quality assurance program. Catheter Cardiovasc Interv 2001;52:59–66. [DOI] [PubMed] [Google Scholar]
- 23. Bernardi G, Padovani R, Morocutti G, et al. A method based on DIMOND quality criteria to evaluate imaging in diagnostic and interventional cardiology. Radiat Prot Dosimetry 2001;94:167–172. [DOI] [PubMed] [Google Scholar]
- 24. Bernardi G, Bar O, Jezewski T, et al. Quality criteria for cardiac images: An update. Radiat Prot Dosimetry 2008;129:87–90. [DOI] [PubMed] [Google Scholar]
- 25. Richard S, Siewerdsen JH. Comparison of model and human observer performance for detection and discrimination tasks using dual‐energy x‐ray images. Med Phys 2008;35:5043–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bogaert E, Bacher K, Thierens H. A large‐scale multicentre study in belgium of dose area product values and effective doses in interventional cardiology using contemporary X‐ray equipment. Radiat Prot Dosimetry 2008;128:312–323. [DOI] [PubMed] [Google Scholar]
- 27. Vano E, Jarvinen H, Kosunen A, et al. Patient dose in interventional radiology: A European survey. Radiat Prot Dosimetry 2008;129:39–45. [DOI] [PubMed] [Google Scholar]
- 28. Padovani R, Vano E, Trianni A, et al. Reference levels at european level for cardiac interventional procedures. Radiat Prot Dosimetry 2008;129:104–107. [DOI] [PubMed] [Google Scholar]
- 29. Kuon E, Dahm JB, Empen K, et al. Identification of less‐irradiating tube angulations in invasive cardiology. J Am Coll Cardiol 2004;44:1420–1428. [DOI] [PubMed] [Google Scholar]
- 30. Smith IR, Cameron J, Mengersen KL, et al. Evaluation of coronary angiographic projections to balance the clinical yield with the radiation risk. Br J Radiol 2012;85:e722–e728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agarwal S, Parashar A, Bajaj NS, et al. Relationship of beam angulation and radiation exposure in the cardiac catheterization laboratory. JACC Cardiovasc Interv 2014;7:558–566. [DOI] [PubMed] [Google Scholar]
- 32. Bogaert E, Bacher K, Lemmens K, et al. A large‐scale multicentre study of patient skin doses in interventional cardiology: Dose‐area product action levels and dose reference levels. Br J Radiol 2009;82:303–312. [DOI] [PubMed] [Google Scholar]
- 33. Neofotistou V, Vano E, Padovani R, et al. Preliminary reference levels in interventional cardiology. Eur Radiol 2003;13:2259–2263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


