Abstract
Purpose
To assess the impact of human crystalline lens opacification and yellowing, similar to that observed in patients with cataracts, on retinal vessel blood oxygen saturation measurements using custom manufactured soft contact lenses.
Methods
Ten healthy, non‐smoking individuals were enrolled for this study. All subjects underwent digital blood pressure measurements, assessment of non‐contact intra‐ocular pressure, pupil dilation and retinal vessel oximetry using dual‐wavelength photography (Oximetry Module, Imedos Systems). To simulate lens changes, three different contact lenses were inserted, one to simulate opacities followed by two more lenses to simulate different levels of lens yellowing (Cantor & Nissel).
Results
The measurements obtained showed an opposite change in arterial and venous oxygen saturation and optical density ratio across conditions, resulting in a statistically significant difference in arterial minus venous oxygen saturation value (p = 0.003). However, this difference was only significant for the ‘opacity’ condition but not for the ‘yellowing’ conditions.
Conclusion
Lenticular changes such as cataracts can impact on spectrophotometric analysis in particular dual‐wavelength retinal vessel oximetry. Hence, lenticular assessment and cataract grading should be considered when assessing elderly individuals and patient groups developing cataract earlier in life such as those suffering from diabetes mellitus.
Keywords: cataract, dual‐wavelength retinal vessel oximetry, lens opacity, lens yellowing, optical density
Introduction
Retinal vessel oxygen saturation measurements have been used in a number of current research projects examining patients suffering from diabetes mellitus (DM) (Hardarson & Stefánsson 2012; Jørgensen et al. 2014), glaucoma (Olafsdottir et al. 2011; Vandewalle et al. 2014), respiratory disease (Palkovits et al. 2013) and healthy individuals (Geirsdottir et al. 2012). Findings in DM show higher arterial and venous saturations depending on the disease stage as well as reduced oxygen consumption when derived by subtracting venous saturation from arterial saturation. The increased saturation compared to controls is thought to be a consequence of microvascular alterations in DM, which can lead to vessel occlusions and then formation of arterio‐venous shunts. A similar increase in venous saturation was observed in glaucomatous eyes; here, the increase in venous saturation was attributed to a loss in tissue, which explains the decreased consumption values. While there might be both, tissue loss and microvascular changes, involved in reduced oxygen consumption, there is also the error introduced by lenticular changes which needs to be accounted for, in particular in patients with DM who develop lenticular changes earlier in life than non‐diabetics (Sparrow et al. 1990; Pollreisz & Schmidt‐Erfurth 2010). Lenticular changes occur over time due to normal ageing but can be accelerated in systemic disease such as DM (Kinoshita 1974). Lenticular changes are often non‐homogenous across the lens and can include any of the following changes: lens opacities, biometric lenticular changes and brunescence.
Although dual‐wavelength retinal oximetry shows good reproducibility (Lasta et al. 2012; Palsson et al. 2012), it requires clear media to ensure reliable results. Vessel saturation calculations depend on the absorption spectra, which in turn can be affected by light scatter, media opacity and illumination (Heitmar & Cubbidge 2013; Patel et al. 2013). We have previously explored the influence of light scatter using a polybead model (Patel et al. 2013), demonstrating that vessel oxygen parameters are altered depending on the amount of forward light scatter introduced. Unfortunately, this experiment was limited to explore only the effect of light scatter. To gain a better understanding of the influence of lenticular changes, we have conducted the present experiment in which we use contact lenses to simulate lens opacities and lens yellowing separately. The advantage of using contact lenses enables the observation of each change individually as well being able to examine whether different‐sized vessels are affected in the same way.
Materials and Methods
The study adhered to the tenets of the Declaration of Helsinki and was approved by the Aston University institutional review board. Written informed consent was provided. We included 10 healthy participants (mean age 27.7 SD ±7.6 years). All participants were free from systemic disease, ocular abnormalities and had no history of previous ocular surgery.
All measurements were undertaken with the participants having abstained from caffeinated and carbonated beverages, alcohol, chocolate, red meat, vitamin C or participated in any forms of exercise for a minimum of 4 hr prior to testing. Following intra‐ocular pressures (IOP) measurement using non‐contact tonometry, Tropicamide 1% (Minims; Chauvin Pharmaceuticals Ltd., Kingston‐upon‐Thames, UK) was instilled for pupil dilation. After resting in a sitting position and acclimatizing to a temperature of 22 °C for 15–20 min, blood pressure (BP) was measured using a digital BP monitor (UA‐779; PMS Instruments, Maidenhead, UK) adhering to best practice guidelines (Williams et al. 2004).
Artificial cataract model
In order to obtain lenses which possessed the spectral transmission profiles necessary to simulate lens opacities and lens yellowing, we preordered a set of 12 different lenses, six different opacity levels and six different yellowing levels. These lenses underwent an initial testing to ensure the spectral transmission was simulating age‐related lenticular changes as close as possible to those published in the literature (Kessel et al. 2010; Artigas et al. 2012; Sakanishi et al. 2012). Another requirement which needed to be satisfied was the ability to obtain a retinal image with the lenses in situ.
Following this initial selection, we used three lenses (a new lens set for each participant): one to simulate lens opacity without any yellowing added and two lenses with no opacities but different amounts of yellowing. The spectral transmission of each lens (all 30 lenses of the 10 participants) was measured to ensure they all had the same spectral characteristics (Fig. 1 for spectral transmission characteristics).
Figure 1.
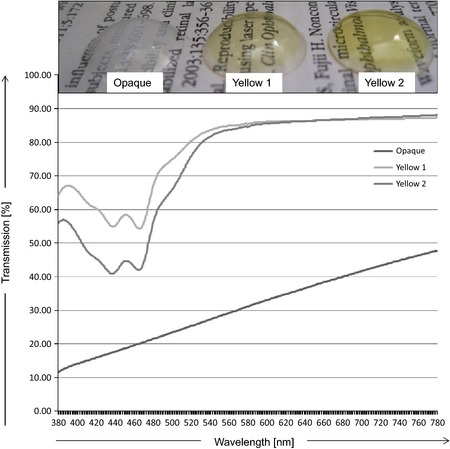
Top: example of one set of lenses used for the study. Bottom: spectral transmission properties of the contact lenses used.
Lens specifications: all lenses were supplied by Cantor & Nissel (Brackley, UK). The lens material is referred to as non‐ionic Filcon II3 [supplied by Contamac (Saffron Waldon, UK)] according to ISO norms. Opacification of the lenses was achieved through barium sulphate exposure.
Image acquisition
Following full pupil dilation, we obtained a minimum of five images per condition, that is no lens, ‘opaque’, ‘yellow 1’ and ‘yellow 2’ with the camera angle set at 30° and the optic nerve head centred. A minimum of 5 min resting time between conditions was given.
Oxygen saturation measurements were performed using the ‘oxygen tool’ and VesselMap software (Imedos Systems; Imedos GmbH, Jena, Germany) as described elsewhere (Hammer et al. 2008). In brief, retinal images were taken with a customized dual‐wavelength filter (transmission bands at 548 and 610 nm; bandwidth 10 nm each) inserted in the illumination pathway of the fundus camera (Zeiss FF450+, Zeiss Meditech, Jena, Germany). Optical densities of retinal vessels were measured as the logarithmic ratio of the fundus reflection at the vessel centre and its surrounding tissue. The optical density ratio (ODR) at 610 and 548 nm has been found to be inversely proportional to the vessel haemoglobin oxygen saturation when compensating for the vessel diameter and fundus pigmentation (Hammer et al. 2008).
Image analysis
For analysis purposes, we selected the three best images per condition. As vessel anatomy and branching is different for every individual, the number of vessels cursing through the measurement zone is variable. To have comparable results, we chose to include the five largest arteries and four largest veins of each individual.
The measurement area consisted of a concentric annulus around the optic nerve head (ONH) which was half a disc diameter (DD) distant from the ONH and of one DD in width (Fig. 2). This distance and length was chosen in order to obtain results, which could be used for comparison to earlier publications using the same device. The individual vessel diameters, ODRs, pigmentation (numerical value output from the software) and oxygen saturations were obtained for all three images (per condition) of each participant, using the software's ‘multimeasurement tool’ [Visualis software (Imedos Systems, Jena, Germany)].
Figure 2.
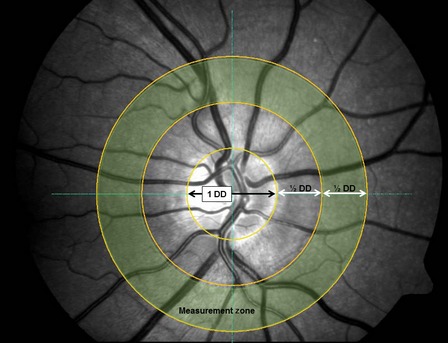
Illustration of the measurement area. Vessels cursing through the measurement zone [½ disc diameter (DD) distant from the optic nerve head rim and ½ DD in width] were included.
Statistical analysis
Statistical analysis was performed using Statistica version 6.0 (StatSoft, Tulsa, OK, USA). Following normality testing with the Shapiro–Wilks test, we conducted a series of analysis of variance (anova) to establish whether the three repeated measures (as obtained per image, per condition) were comparable; following this, for each condition we obtained averaged values for ODR, SO2, pigmentation and vessel diameter for further analyses. Stepwise forward regression analyses were employed to evaluate the influence of the vessel diameter and pigmentary values upon vessel saturation and ODRs and their respective standard deviations.
Across condition testing was performed by one‐way anova, while examining the impact of individual vessel diameter was performed by a factorial anova (with factor one the measurement condition and factor two the vessel diameter). For this analysis, we arbitrary classified each artery and vein according to their size in three distinct groups: S, M and L (Arteries: S = up to 80 μm; M = 80–110 μm; L = over 110 μm; Veins: S = up to 95 μm; M = 95–130 μm; L = over 130 μm). Due to the fact that most arteries and veins measured in the concentric annulus were of the same morphological type and at a similar branching level, we observed the vessel distributions to select the cut‐off value so to ensure a comparable number of vessels in each group was selected.
Results
Our cohort was normotensive (average systolic BP: 119 ± 11 mmHg, average diastolic BP: 71 ± 11 mmHg) and consisted of four Caucasian, two Mediterranean, one South Asian, one Middle Eastern, one East Asian and one Hispanic individual. Iris colour of our subjects was diverse and distributed as follows: three green, three dark brown, two hazel, one blue and one grey. Participants had no lens opacity (as verified by dilated biomicroscopy) and IOP were normal 14.1 (±SD 1.6) mmHg [as measured with a rebound tonometer (iCare, Mainland Instruments Ltd., Birmingham, UK)].
All oximetry data were normally distributed and no differences were found for the measurements obtained of the three separate images per individual, hence for further analyses, average values for single vessels and overall annulus arterial and venular values of ODR, oxygen saturation, pigmentation and vessel diameter were used.
Single vessel analyses
Although the algorithm of the Vesselmap (Imedos GmbH) software has an inbuilt correction factor for arterial and venous saturation output, taking into account the vessel diameter and its surrounding tissue (Hammer et al. 2008), we computed stepwise forward regression analyses for each baseline vessel saturation value as detailed in the Statistical analysis. Results from this analysis showed no relationship of any of the parameters (saturation and ODR) and their respective standard deviation with vessel diameters and pigmentary values.
Across subject averages of single vessel arterial and venous oxygen saturation, ODR, vessel diameter and pigmentation for each condition (baseline, opaque, yellow 1 and yellow 2) can be found in Table 1 (arterial values) and Table 2 (venous values). One‐way anovas computed per vessel across all four conditions revealed no statistically significant differences for vessel saturation and ODRs (Tables 1 and 2). An example of single vessel saturations of two individuals is given in Fig. 3.
Table 1.
Arterial values
| Across subject means (n = 10) | |||||||
|---|---|---|---|---|---|---|---|
| Imaging condition | Oxygen saturation (%) | Optical density ratio | Vessel diameter (μm) | Pigment | |||
| Mean (SD) | anova (per vessel) | Mean (SD) | anova (per vessel) | Mean (SD) | Mean (SD) | ||
| Artery 1 (superior) | Baseline | 86 (13) | 0.993 | 0.070 (0.036) | 0.943 | 97 (21) | 0.170 (0.118) |
| Opaque | 87 (17) | 0.079 (0.040) | 91 (20) | −0.115 (0.028) | |||
| Yellow 1 | 88 (11) | 0.066 (0.031) | 97 (22) | 0.158 (0.109) | |||
| Yellow 2 | 87 (14) | 0.069 (0.036) | 97 (21) | 0.155 (0.115) | |||
| Artery 2 (superior) | Baseline | 86 (10) | 0.334 | 0.73 (0.025) | 0.160 | 108 (16) | 0.180 (0.136) |
| Opaque | 82 (15) | 0.094 (0.035) | 101 (8) | −0.111 (0.035) | |||
| Yellow 1 | 92 (7) | 0.061 (0.017) | 109 (19) | 0.147 (0.120) | |||
| Yellow 2 | 90 (12) | 0.064 (0.027) | 108 (18) | 0.166 (0.123) | |||
| Artery 3 (superior) | Baseline | 88 (11) | 0.937 | 0.067 (0.034) | 0.932 | 101 (17) | 0.181 (0.143) |
| Opaque | 90 (21) | 0.072 (0.056) | 91 (31) | −0.114 (0.038) | |||
| Yellow 1 | 92 (12) | 0.059 (0.036) | 101 (17) | 0.160 (0.123) | |||
| Yellow 2 | 91 (14) | 0.062 (0.036) | 104 (16) | 0.179 (0.131) | |||
| Artery 4 (inferior) | Baseline | 90 (11) | 0.514 | 0.058 (0.030) | 0.225 | 97 (23) | 0.199 (0.160) |
| Opaque | 88 (9) | 0.079 (0.024) | 97 (14) | −0.112 (0.043) | |||
| Yellow 1 | 96 (11) | 0.046 (0.025) | 96 (21) | 0.174 (0.126) | |||
| Yellow 2 | 95 (11) | 0.048 (0.029) | 94 (23) | 0.179 (0.140) | |||
| Artery 5 (inferior) | Baseline | 90 (10) | 0.106 | 0.060 (0.030) | 0.073 | 91 (18) | 0.202 (0.152) |
| Opaque | 83 (13) | 0.088 (0.027) | 86 (26) | −0.119 (0.035) | |||
| Yellow 1 | 96 (12) | 0.047 (0.031) | 91 (16) | 0.182 (0.123) | |||
| Yellow 2 | 97 (8) | 0.044 (0.024) | 91 (19) | 0.184 (0.131) | |||
| Average arteries | Baseline | 89 (7) | 0.379 | 0.064 (0.022) | 0.183 | 96 (10) | 0.189 (0.137) |
| Opaque | 86 (14) | 0.083 (0.033) | 94 (9) | −0.115 (0.037) | |||
| Yellow 1 | 93 (5) | 0.056 (0.016) | 99 (9) | 0.163 (0.113) | |||
| Yellow 2 | 92 (8) | 0.058 (0.020) | 99 (9) | 0.172 (0.123) | |||
Results are shown as group means (n = 10). SD = standard deviation; anova = analysis of variance.
Table 2.
Venous values
| Across subject means (n = 10) | |||||||
|---|---|---|---|---|---|---|---|
| Imaging condition | Oxygen saturation (%) | Optical density ratio | Vessel diameter (μm) | Pigment | |||
| Mean (SD) | anova (per vessel) | Mean (SD) | anova (per vessel) | Mean (SD) | Mean (SD) | ||
| Vein 1 (superior) | Baseline | 49 (14) | 0.036 | 0.169 (0.047) | 0.232 | 113 (40) | 0.174 (0.129) |
| Opaque | 84 (39) | 0.085 (0.103) | 78 (15) | −0.106 (0.010) | |||
| Yellow 1 | 51 (14) | 0.166 (0.059) | 113 (36) | 0.164 (0.123) | |||
| Yellow 2 | 53 (11) | 0.154 (0.042) | 105 (31) | 0.165 (0.136) | |||
| Vein 2 (superior) | Baseline | 40 (11) | 0.252 | 0.199 (0.038) | 0.599 | 126 (35) | 0.177 (0.129) |
| Opaque | 58 (22) | 0.169 (0.223) | 109 (42) | −0.115 (0.035) | |||
| Yellow 1 | 48 (15) | 0.186 (0.036) | 127 (34) | 0.153 (0.121) | |||
| Yellow 2 | 44 (13) | 0.193 (0.035) | 129 (36) | 0.172 (0.128) | |||
| Vein 3 (inferior) | Baseline | 57 (7) | 0.757 | 0.143 (0.29) | 0.848 | 106 (29) | 0.186 (0.150) |
| Opaque | 61 (22) | 0.157 (0.043) | 102 (14) | −0.110 (0.024) | |||
| Yellow 1 | 62 (9) | 0.137 (0.038) | 109 (27) | 0.162 (0.130) | |||
| Yellow 2 | 58 (7) | 0.140 (0.034) | 106 (26) | 0.184 (0.139) | |||
| Vein 4 (inferior) | Baseline | 57 (15) | 0.269 | 0.153 (0.057) | 0.564 | 119 (32) | 0.203 (0.166) |
| Opaque | 74 (12) | 0.109 (0.008) | 81 (28) | −0.083 (0.042) | |||
| Yellow 1 | 54 (15) | 0.162 (0.054) | 119 (29) | 0.180 (0.127) | |||
| Yellow 2 | 51 (14) | 0.167 (0.051) | 119 (31) | 0.179 (0.134) | |||
| Average veins | Baseline | 50 (9) | 0.247 | 0.166 (0.029) | 0.699 | 114 (27) | 0.183 (0.138) |
| Opaque | 62 (19) | 0.147 (0.035) | 91 (15) | −0.118 (0.031) | |||
| Yellow 1 | 53 (8) | 0.163 (0.028) | 118 (19) | 0.165 (0.121) | |||
| Yellow 2 | 52 (8) | 0.164 (0.024) | 116 (19) | 0.175 (0.130) | |||
Results are shown as group means (n = 10). SD = standard deviation; anova = analysis of variance.
Figure 3.
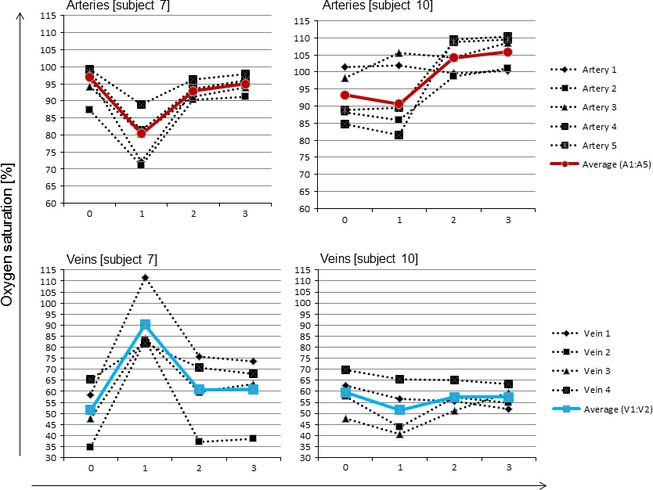
Example showing single vessel and average annulus arterial and venous oxygen saturation values of two individuals (subject 7 and 10) per condition. X‐axis showing the 4 imaging conditions where: 0 = baseline saturation, 1 = opaque saturation, 2 = yellow 1 saturation and 3 = yellow 2 saturation.
Average annulus saturation parameter analyses
Average annulus arterial and venous oxygen saturation was calculated as the mean across all five arteries and all four veins included in the analysis and can be found in Tables 1 and 3 for each of the four conditions. One‐way anova across all four conditions revealed no statistical significant difference of arterial and venous oxygen saturation and ODRs.
Table 3.
Arterio‐venous saturation difference
| Imaging condition | Oxygen saturation (%) | anova p | |
|---|---|---|---|
| Mean (SD) | |||
| Average A−V SO2 | Baseline | 39 (6) | 0.003 |
| Opaque | 24 (11) | ||
| Yellow 1 | 39 (6) | ||
| Yellow 2 | 40 (8) |
Results are shown as group means (n = 10). SD = standard deviation; anova = analysis of variance; A−V SO2 = arterio‐venous saturation difference.
However, average arterial minus venous oxygen saturation (A−V) was statistically significantly different across conditions (one‐way anova p = 0.003). Post hoc testing with Fishers LSD revealed that baseline, yellow 1 and yellow 2 were comparable, while opaque was significantly different from all (all p < 0.001).
Influence of vessel diameter and pigmentary parameters
To evaluate the impact of individual vessel diameter upon saturation values, we computed a factorial anova [with factor one the measurement condition (p1) and factor two the vessel diameter (p2)] (Table 4). For this analysis, we arbitrary classified each artery and vein according to their size in three distinct groups as detailed earlier (see Statistical analysis). This analysis showed no difference across imaging conditions but across diameter classification (p = 0.018). As the vessel grouping is categorical, we employed a Kruskal–Wallis anova, which revealed that the standard deviation of the arterial saturation (average across subjects and per condition) is most variable for the largest vessels (p = 0.011).
Table 4.
Oxygen saturation for different vessel size categories
| Across subject means (n = 10) | ||||
|---|---|---|---|---|
| Imaging condition | Average arterial SO2 (%) | anova p | ||
| Vessel size category | ||||
| S | M | L | ||
| Baseline | 86 (12) | 92 (8) | 83 (12) |
p1 = 0.186 p2 = 0.018 |
| Opaque | 87 (16) | 85 (14) | 86 (16) | |
| Yellow 1 | 91 (7) | 96 (8) | 86 (13) | |
| Yellow 2 | 89 (11) | 94 (10) | 88 (15) | |
| Imaging condition | Average venous SO2 (%) | anova p | ||
|---|---|---|---|---|
| Vessel size category | ||||
| S | M | L | ||
| Baseline | 50 (15) | 53 (12) | 48 (15) |
p1 = 0.879 p2 = 0.049 |
| Opaque | 64 (25) | 69 (24) | 83 (25) | |
| Yellow 1 | 56 (17) | 57 (11) | 47 (15) | |
| Yellow 2 | 56 (13) | 52 (10) | 48 (15) | |
Results are shown as group means (n = 10). S = small, M = medium, L = large, SD = standard deviation; anova = analysis of variance; p1: measurement condition and p2: vessel diameter.
Data are expressed as mean (SD).
Furthermore, average pigmentation for arteries and veins was significantly different for condition ‘opaque’ [see also Tables 1 and 2; arteries: (baseline = 0.189; opaque = −0.115; yellow 1 = 0.163 and yellow 2 = 0.172) one‐way anova p = 0.0009 and veins: (baseline = 0.183; opaque = −0.118; yellow 1 = 0.165 and yellow 2 = 0.175) one‐way anova p = 0.002, respectively].
Discussion
This study was conducted to gain a better understanding of the influence of lenticular changes, namely opacities and lens yellowing, on retinal vessel oximetry measurements in a simulated situation using custom‐made contact lenses with spectral transmission profiles mimicking the effects of cataract.
We previously explored the effects of scatter on dual‐wavelength oximetry (Patel et al. 2013) and were able to demonstrate that simulated light scatter alters retinal vessel oximetry parameters. One of the limitations of this study was the small numbers of participants, vessels assessed and image size due to the optical set‐up. Hence, we wanted to be able to obtain images as similar as those obtained when imaging individuals with early cataract but without having the problem of imaging early cataract candidates and then having to wait until the cataract has progressed to a stage requiring removal, which can take several months or even years.
The key findings of this study illustrate that lens yellowing has very little effect on dual‐wavelength oximetry but changes in opacity can significantly alter some of the parameters introduced to assess oxygen consumption in the eye.
What are the possible factors driving the change in saturation parameters observed?
In contrast to our earlier publication using scatter cells, the diameter of retinal vessels measured rather decreased than increased when introducing the ‘opaque’ condition (Tables 1 and 2), but this was not statistically significant. The most likely explanation for this is that the scatter cells not only act as a neutral density filter but more so as a source of additional intra‐ocular light scatter, resulting in a blurring effect on the vessels overall. Whereas the ‘opaque’ lens has less of a blurring effect as it mainly alters overall image contrast.
Although retinal arterial and venous oxygen saturation assessed separately did not alter with any of the three conditions, the single vessel analysis taking into consideration the vessel calibre illustrated that larger arteries showed more variation than smaller ones. In addition, the relative change in ODR as shown in Fig. 4 is confirming our result from the scatter cell analysis that retinal arterial ODR is changing more than venous ODR. The reason for this has been explained in more detail elsewhere (Patel et al. 2013) but is mainly attributable to changes in contrast, vessel diameter, pigmentation and the wavelength‐dependent nature of scatter.
Figure 4.
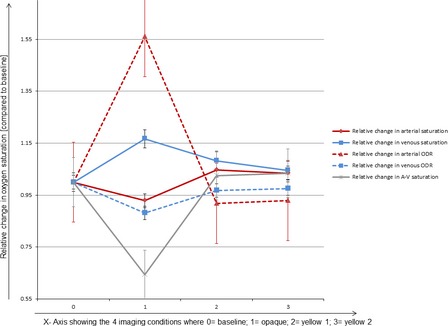
Illustration of the relative change of arterial and venous oxygen saturation and optical density ratio compared to baseline.
Previous publications on lenticular transmission changes in the ageing human crystalline lens show a nonlinear loss of transmission at different wavelengths (Artigas et al. 2012). Although this is different for each individual and can occur at different age/disease stages it could contribute to altered retinal vessel oxygen saturation measurements.
Although the exact mechanisms are unknown, partly due to the limitation of what can be directly measured, we hypothesize that the following factors could be contributories to altered results observed.
Light transmission through a human eye free of cataract, lens brunescence and opacities can be assumed equal across most of the visible light wavelength range (Artigas et al. 2012) which applies also the wavelength range used for measuring oxygen saturation in our case (548 ± 10 nm and 610 ± 10 nm). This can be altered in case of age‐related changes as well as those arising through cataract development.
The Beer–Lambert law defines the absorbance of a material by way of measuring the attenuation of light when travelling through it assuming a linear relationship. In the eye, however, we apply this law different, as we cannot measure the light intensity directly in front and behind the blood vessel. Hence, we make use of the amount of light reflected from the blood vessel itself and its surroundings. While this approximation is suitable to estimate the oxygenation of the blood vessel in ideal conditions: that is where we have less interaction with the tissues the light has to pass through before being absorbed and reflected by the blood vessel.
Also, the Beer–Lambert law does not hold for whole blood, which is due to the scattering properties of erythrocytes which can be altered depending on the haematocrit and the path length (i.e. the depth of the medium) (Harris et al. 2003). Another factor which can contribute to erroneous measurements through cataracts can be found in the light path itself (Kagemann et al. 2007). This is because a beam travelling through a ‘young’ lens will undergo less scatter and absorption on its way, while through an ‘old’ lens, the light will most likely be scattered and absorbed partly by the ocular media; all of which will impact on the amount and direction of the light shone onto the blood vessel under investigation.
Hence, not fulfilling the underpinning criteria to calculate oxygen saturation: a linear relationship between absorbance and reflected light. Apart from changes in light transmittance across the visible light wavelength range, other factors such as scattering around blood vessel edges, retinal pigmentation, absorption and transmission through other ocular media (cornea, aqueous humour and vitreous) can further contribute to erroneous measurements. The wavelength dependency of light scatter (Krishnaswamy et al. 2014) which is exacerbated in the presence of cataract (Cabot et al. 2013) could also offer an explanation as to how the Beer–Lambert Law is not suitable for all imaging conditions as illustrated in this experiment.
Clinical importance of these findings
Current changes in retinal vessel oxygen saturation parameters measured by dual‐wavelength technologies found increased arterial and venous saturation in diabetic individuals, depending on the degree of DR (Hardarson & Stefánsson 2012; Jørgensen et al. 2014). These changes result in lower values for A−V and are thought to be due shunting of blood through preferential channels, bypassing non‐perfused capillaries while parts of the retinal tissue may be hypoxic with blood in larger vessels exhibiting high oxygen saturation. This may be due to occlusions and obliterations in the capillary bed and the formation of arterio‐venous shunt vessels. However, hyperglycaemia‐induced endothelial dysfunction, by suppression of the endothelial NO‐synthase and disturbance of the vascular autoregulation, may also be a contributing factor to retinal tissue hypoxia (Hammer et al. 2012).
In other ocular vascular conditions, such as glaucoma, the decrease in retinal oxygen consumption (A−V) has been attributed to the loss of neural tissue explaining the higher venous saturation (Olafsdottir et al. 2014; Vandewalle et al. 2014). Whether tissue loss is also contributing in the lower consumption measured in diabetics could be a possibility, but without current data on structural retinal and visual function parameters in this patient group this is an area, which needs further exploration. While there is a growing evidence base of how tissue and function loss is reflected and associated with decreased oxygen extraction due to a possible change in demand, dual‐wavelength retinal oximetry is reliant upon clear media to ensure the results reflect only metabolic changes and is not altered by optical properties.
Patients with diabetes develop cataracts earlier in life than those without diabetes (Sparrow et al. 1990; Pollreisz & Schmidt‐Erfurth 2010). In addition, the human crystalline lens changes are non‐uniform and can occur at different locations within the lens (Chylack et al. 1989; Olafsdottir et al. 2012). These lenticular changes are individual and non‐homogenous across the entire crystalline lens, which subsequently lead to images where some areas are more affected by the cataract than others.
The changes observed in the present study indicate that lens yellowing/brunescence has only a limited effect on vessel saturation measurements; however, lens opacities can significantly change some of the outcome measures used to quantify oxygen consumption.
Conclusion
Although we did not find a statistical significant change in ODR measurements for the two yellow conditions, this is mainly due to the transmission profile of the lenses used. Although we aimed to create lenses with transmission criteria close to those reported by Artigas et al. (2012), this was not possible due to the limitations in the manufacturing process of contact lenses. The two lenses used had a decreased transmission at 548 and 610 nm but this decrease was of a similar amount (within 5% of each other). However, in most age‐related transmission changes, there will be a difference of the absolute transmission for these wavelengths, and hence, the opaque lens condition showed that this could affect outcome measures such as oxygen consumption (A−V saturation) as it alters the optical density at the two measured wavelengths at a different magnitude. As we did not measure the transmission of each of our subjects’ crystalline lenses and only used slit lamp observations to rule out lenticular changes, the individual transmission profile of each our participants could have contributed to the presented outcome.
Future projects using dual‐wavelength retinal oximetry should incorporate some form of lens quantification, whether this should be carried out using a photographic, spectrophotometric or statistical method is debatable. Measurement of light scatter across a spectrum of individuals with and without lenticular changes would be a first step to corroborate our results. Because of the problem introduced by lenticular changes upon vessel saturation measurements great care must be taken in patient groups that develop early cataracts. A simple solution could be offered by only including the non‐affected eye or in cases where both eyes require measurement a comparison between the two should be made. In healthy subjects across a large age range, data have been published on right and left eye comparisons which showed good agreement between eyes (Geirsdottir et al. 2012). If there is no pathophysiological reason, which would explain intereye differences, the comparison between both could be a way of ruling out artifactual influences on retinal vessel oximetry measurements.
This work was supported by a summer scholarship from The College of Optometrists (UK).
We would like to thank Dr Richard Armstrong for his advice on the statistical analysis.
References
- Artigas JM, Felipe A, Navea A, Fandiño A & Artigas C (2012): Spectral transmission of the human crystalline lens in adult and elderly persons: color and total transmission of visible light. Invest Ophthalmol Vis Sci 53: 4076–4084. [DOI] [PubMed] [Google Scholar]
- Cabot F, Saad A, McAlinden C, Haddad NM, Grise‐Dulac A & Gatinel D (2013): Objective assessment of crystalline lens opacity lever by measuring ocular light scattering with a double‐pass system. Am J Ophthalmol 155: 629–635. [DOI] [PubMed] [Google Scholar]
- Chylack LT Jr, Leske MC, McCarthy D, Khu P, Kashiwagi T & Sperduto R (1989): Lens opacities classification system II (LOCS II). Arch Ophthalmol 107: 991–997. [DOI] [PubMed] [Google Scholar]
- Geirsdottir A, Palsson O, Hardarson SH, Olafsdottir OB, Kristjansdottir JV & Stefánsson E (2012): Retinal vessel oxygen saturation in healthy individuals. Invest Ophthalmol Vis Sci 53: 5433–5442. [DOI] [PubMed] [Google Scholar]
- Hammer M, Vilser W, Riemer T & Schweitzer D (2008): Retinal vessel oximetry‐calibration, compensation for vessel diameter and fundus pigmentation, and reproducibility. J Biomed Opt 13: 054015. [DOI] [PubMed] [Google Scholar]
- Hammer M, Heller T, Jentsch S, Dawczynski J, Schweitzer D, Peters S, Schmidtke KU & Müller UA (2012): Retinal vessel oxygen saturation under flicker light stimulation in patients with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 53: 4063–4068. [DOI] [PubMed] [Google Scholar]
- Hardarson SH & Stefánsson E (2012): Retinal oxygen saturation is altered in diabetic retinopathy. Br J Ophthalmol 96: 560–563. [DOI] [PubMed] [Google Scholar]
- Harris A, Dinn RB, Kagemann L & Rchtman E (2003): A review of methods for human retinal oximetry. Ophthalmic Surg Lasers Imaging 34: 152–164. [PubMed] [Google Scholar]
- Heitmar R & Cubbidge RP (2013): The impact of flash intensity on retinal vessel oxygen saturation measurements using dual wavelength oximetry. Invest Ophthalmol Vis Sci 54: 2807–2811. [DOI] [PubMed] [Google Scholar]
- Jørgensen CM, Hardarson SH & Bek T (2014): The oxygen saturation in retinal vessels from diabetic patients depends on the severity and type of vision‐threatening retinopathy. Acta Ophthalmol 92: 34–39. [DOI] [PubMed] [Google Scholar]
- Kagemann L, Wollstein G, Wojtkowski M et al. (2007): Spectral oximetry assessed with high‐speed ultra‐high‐resolution optical coherence tomography. J Biomed Opt 12: 041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel L, Lundeman JH, Herbst K, Andersen TV & Larsen M (2010): Age‐related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg 36: 308–312. [DOI] [PubMed] [Google Scholar]
- Kinoshita JH (1974): Mechanisms initiating cataract formation. Proctor lecture. Invest Ophthalmol Vis Sci 13: 713–724. [PubMed] [Google Scholar]
- Krishnaswamy V, Elliott JT, McClatchy DM, Barth RJ, Wells WA, Pogue BW & Paulsen KD (2014): Structured light scatteroscopy. J Biomed Optics 19: 070504. [DOI] [PubMed] [Google Scholar]
- Lasta M, Palkovits S, Boltz A, Schmidl D, Kaya S, Cherecheanu AP, Garhöfer G & Schmetterer L (2012): Reproducibility of retinal vessel oxygen saturation measurements in healthy young subjects. Acta Ophthalmol 90: e616–e620. [DOI] [PubMed] [Google Scholar]
- Olafsdottir OB, Hardarson SH, Gottfredsdottir MS, Harris A & Stefánsson E (2011): Retinal oximetry in primary open‐angle glaucoma. Invest Ophthalmol Vis Sci 52: 6409–6413. [DOI] [PubMed] [Google Scholar]
- Olafsdottir E, Andersson DK & Stefánsson E (2012): The prevalence of cataract in a population with and without type 2 diabetes mellitus. Acta Ophthalmol 90: 334–340. [DOI] [PubMed] [Google Scholar]
- Olafsdottir OB, Vandewalle E, Abegão Pinto L et al. (2014): Retinal oxygen metabolism in healthy subjects and glaucoma patients. Br J Ophthalmol 98: 329–333. [DOI] [PubMed] [Google Scholar]
- Palkovits S, Lasta M, Boltz A et al. (2013): Measurement of retinal oxygen saturation in patients with chronic obstructive pulmonary disease. Invest Ophthalmol Vis Sci 54: 1008–1013. [DOI] [PubMed] [Google Scholar]
- Palsson O, Geirsdottir A, Hardarson SH, Olafsdottir OB, Kristjansdottir JV & Stefánsson E (2012): Retinal oximetry images must be standardized: a methodological analysis. Invest Ophthalmol Vis Sci 53: 1729–1733. [DOI] [PubMed] [Google Scholar]
- Patel SR, Hudson C, Flanagan JG & Heitmar R (2013): The effect of simulated cataract light scatter on retinal vessel oximetry. Exp Eye Res 116: 185–189. [DOI] [PubMed] [Google Scholar]
- Pollreisz A & Schmidt‐Erfurth U (2010): Diabetic Cataract—Pathogenesis, Epidemiology and Treatment. J Ophthalmol 2010. 608752: 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanishi Y, Awano M, Mizota A, Tanaka M, Murakami A & Ohnuma K (2012): Age‐related changes in spectral transmittance of the human crystalline lens in situ . Ophthalmologica 228: 174–180. [DOI] [PubMed] [Google Scholar]
- Sparrow JM, Bron AJ, Brown NA & Neil HA (1990): Biometry of the crystalline lens in early‐onset diabetes. Br J Ophthalmol 74: 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle E, Abegão Pinto L, Olafsdottir OB et al. (2014): Oximetry in glaucoma: correlation of metabolic change with structural and functional damage. Acta Ophthalmol 92: 105–110. [DOI] [PubMed] [Google Scholar]
- Williams B, Poulter NR, Brown MJ et al. (2004): British Hypertension Society Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004‐BHS IV. J Hum Hypertens 18: 139–185. [DOI] [PubMed] [Google Scholar]


