Abstract
We review and synthesize information on invasions of nonnative forest insects and diseases in the United States, including their ecological and economic impacts, pathways of arrival, distribution within the United States, and policy options for reducing future invasions. Nonnative insects have accumulated in United States forests at a rate of ~2.5 per yr over the last 150 yr. Currently the two major pathways of introduction are importation of live plants and wood packing material such as pallets and crates. Introduced insects and diseases occur in forests and cities throughout the United States, and the problem is particularly severe in the Northeast and Upper Midwest. Nonnative forest pests are the only disturbance agent that has effectively eliminated entire tree species or genera from United States forests within decades. The resulting shift in forest structure and species composition alters ecosystem functions such as productivity, nutrient cycling, and wildlife habitat. In urban and suburban areas, loss of trees from streets, yards, and parks affects aesthetics, property values, shading, stormwater runoff, and human health. The economic damage from nonnative pests is not yet fully known, but is likely in the billions of dollars per year, with the majority of this economic burden borne by municipalities and residential property owners. Current policies for preventing introductions are having positive effects but are insufficient to reduce the influx of pests in the face of burgeoning global trade. Options are available to strengthen the defenses against pest arrival and establishment, including measures taken in the exporting country prior to shipment, measures to ensure clean shipments of plants and wood products, inspections at ports of entry, and post‐entry measures such as quarantines, surveillance, and eradication programs. Improved data collection procedures for inspections, greater data accessibility, and better reporting would support better evaluation of policy effectiveness. Lack of additional action places the nation, local municipalities, and property owners at high risk of further damaging and costly invasions. Adopting stronger policies to reduce establishments of new forest insects and diseases would shift the major costs of control to the source and alleviate the economic burden now borne by homeowners and municipalities.
Keywords: disease, forest, insect, invasive, pathogen, policy
Introduction
The most serious and urgent near‐term ecological threat for many United States forests and urban and suburban trees is the recurrent introduction of insects and pathogens from other continents (Liebhold et al. 1995, Lovett et al. 2006, Moser et al. 2009). Invasive forests pests are an undesirable consequence of international trade and travel, and while they are not a new phenomenon, they inflict increasing ecological and economic damage (Aukema et al. 2010, 2011). Many of the invasive insects and diseases are familiar enough to have entered common parlance in the United States: gypsy moth, chestnut blight, and Dutch elm disease are well‐known examples. Many others are more recent arrivals or less widespread, and while the public is largely unaware of them, current and potential impacts can be severe (Table 1).
Table 1.
Eighteen nonnative forest insects and pathogens in North America with current or potential future high impacts
| Common name | Scientific name | Pathway | Hosts | Impacts | Geographic region at risk |
|---|---|---|---|---|---|
| Established species with high impact | |||||
| Chestnut blight | Cryphonectria parasitica (Murrill) Barr. | live plants | American chestnut, chinkapin | virtually eliminated mature chestnuts | eastern deciduous forest |
| White pine blister rust | Cronartium ribicola J. C. Fisch | live plants | five‐needle pines (section Quinquefolia in genus Pinus) | virtually eliminated several western pine species | continent‐wide; greatest impacts in West |
| Phytophthora dieback | Phytophthora cinnamomi Rands | unknown | many hosts including American chestnut, white oak, shortleaf pine, and Fraser fir, fruit trees | high mortality of susceptible trees | continent‐wide |
| Port‐Orford‐cedar root disease | Phytophthora lateralis Tucker & Milbrath | probably live plants | Port‐Orford cedar | virtually eliminated host from lower elevation parts of its range | Klamath Mountains, California and Oregon |
| Beech bark disease (scale insect + fungus) | Cryptococcus fagisuga Lindinger + Nectria coccinea var. faginata (Pers.) Fr. | live plants | American beech | severely reduces mature beech; often replaced by dense thickets of root sprouts | deciduous forests of East and Midwest |
| European gypsy moth | Lymantria dispar dispar L. | deliberate introduction | many hosts includes oaks, aspen, willow, and birch | periodic outbreaks cause defoliations and can sometimes kill hosts | deciduous forests of East and Midwest |
| Hemlock woolly adelgid | Adelges tsugae Annand | live plants | Eastern and Carolina hemlock | 90%+ mortality in most affected stands | Appalachians, Northeast and upper Midwest |
| Sudden oak death | Phytophthora ramorum S. Werres, A.W.A.M. de Cock | live plants | >100 spp., especially tanoak and several western oak species; some eastern oaks vulnerable | vulnerable hosts often succumb, while other hosts show minor impacts | Coastal California and Oregon; could potentially spread to eastern forests |
| Redbay ambrosia beetle + fungus (laurel wilt disease) | Xyleborus glabratus Eichhoff + Raffaelea lauricola Harrington and Fraedrich | wood packaging | numerous probable hosts including redbay and pondberry & pondspice shrubs; | predicted >90% reduction in redbay basal area within 15 yr (25 yr after first detected) | eastern deciduous forests; greatest impacts in southeastern coastal plain |
| Emerald ash borer | Agrilus planipennis Fairmaire | wood packaging | all North American ash species | most ash trees succumb; some species of ash appear to have limited resistance | eastern deciduous forest; riparian areas in Great Plains and West, landscape plantings continent‐wide |
| Dutch elm disease | Ophiostoma ulmi (Buisman) Nannf. & O. novo‐ulmi Brasier; vectored by several insects including Scolytus multistriatus and S. schevyrewi | wood products | American elm; other native elms, e.g., red or slippery elm, are more resistant | severe impacts in urban areas; elms remain, although reduced in number and size, in riparian woodlands | continent‐wide |
| Butternut canker | Sirococcus clavigignenti‐juglandacearum N. B. Niar, Kostichka & Kuntz | unknown | butternut (white walnut) | severe mortality of butternut; over 80% mortality of butternut in the South | deciduous forests of Northeast and Midwest |
| Balsam woolly adelgid | Adelges piceae Ratzeburg | live plants | most true fir species (Abies) in North America | widespread impacts on firs; severe mortality of Fraser fir on Southern Appalachian mountaintops and Christmas tree farms | Northeast; Southern Appalachians; Northwest |
| Established, potential for significant effects in the future | |||||
| Asian longhorned beetle | Anoplophora glabripennis Motschulsky | wood packaging | woody vegetation in 15 families, especially maples, elms, and willows | severe impacts possible in both urban and forest landscapes; eradication being attempted | continent‐wide deciduous forests |
| Winter moth | Operophtera brumata L. | unknown | many species including oaks, maples, cherries | severe impacts on hosts in southeastern New England | Eastern deciduous forest |
| Polyphagous shot hole borer and fusarium fungus | Euwallacea (sp. unknown) + Fusarium euwallacea | unknown | >200 species attacked by insect; >100 support the fungus; hosts killed include box elder, bigleaf maple, coast live oak | high mortality levels in vulnerable hosts | Southern California hardwood forests, riparian and urban; potentially in Southeast |
| European woodwasp | Sirex noctilio | probably wood packaging | many pine species | most important killer of pines in Southern Hemisphere; modest impacts so far in United States | all ecosystems with hard pines: Southeast, Great Lakes States, western United States |
| Not yet established | |||||
| Asian gypsy moth & hybrids | Lymantria dispar asiatica Vinuskovkij | ship super‐structures | >600 species, including common deciduous and coniferous trees | could have more severe impacts than European gypsy moth since has wider host range and females fly | continent‐wide |
Here we summarize the ecological and economic impacts of invasive forest insects and pathogens, characterize the dominant introduction pathways, and consider policy options for preventing establishment of such pests. The major impetus for this synthesis is the publication over the last 5‐10 years of a number of important studies providing new information on the scope of the ecological and economic impacts and the effectiveness of current policies to combat the problem. Our geographic scope for this analysis is the contiguous 48 states of the United States, with a focus on the Northeast and Upper Midwest, where the problem is most severe (Fig. 1, Liebhold et al. 2013). We confine our analysis to natural and managed forests as well as trees in urban and suburban landscapes, but exclude orchards planted for production of crops such as fruits and nuts. We define “nonnative” as any organism whose origin is outside North America. We do not consider native organisms with expanding ranges or increasing impacts due to climate change or other factors (Weed et al. 2013). While these native insects pose severe problems in some areas of the country (e.g., native bark beetles in western United States forests), they are not here as a result of foreign trade, therefore the policy options for addressing the problem are quite different. In general we use the terms “insect” and “pathogen” to distinguish between these two types of organisms, but for the purposes of our discussion we also refer to both with the nonspecific term “pest.”
Figure 1.
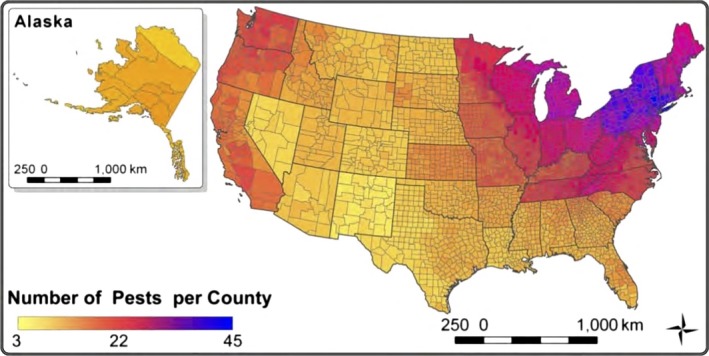
Number of nonnative forest pests per county in the United States in 2012. Reproduced from Liebhold et al. 2013.
Invasions of nonnative species follow a predictable course, beginning with initial arrival at a port of entry and subsequent introduction to the country (Fig. 2, Hobbs and Humphries 1995, Lodge et al. 2006). When the species reach population sizes at which extinction is no longer likely, they are considered “established” (Fig. 2). Over time, established populations may grow and spread, in some cases eventually becoming pervasive. As shown in Fig. 2, along the progression from export to introduction and spread, the management responsibility and associated costs shift from the importer to the federal government, then state governments, and ultimately landowners and municipalities (Aukema et al. 2011). Moreover, as an invasive species advances through these stages, the likelihood of eradication or effective control decreases while ecosystem harm increases, costs increase, and there are increased environmental risks from such techniques as chemical or biological control (Myers et al. 2000, Lodge et al. 2006, Liebhold et al. 2016).
Figure 2.
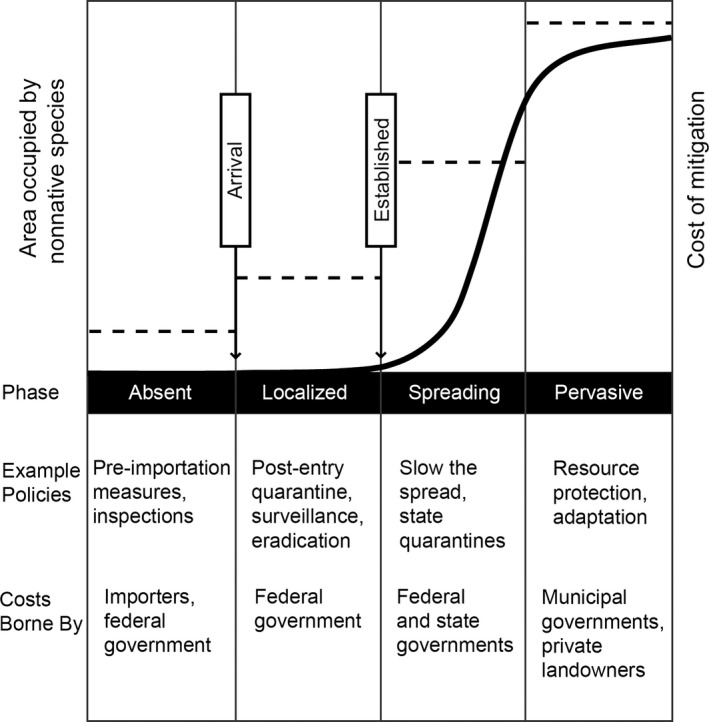
Generalized pattern of spread of an introduced invasive species. The solid curve represents a typical pattern of increasing area occupied by an invasive species, vertical lines delineate the different phases of the invasion, and horizontal dashed lines represent hypothetical costs of mitigation in the different phases. The text below the graph gives examples of policies that are often used in the different phases, and who bears the greatest burden of the costs of those polices. Graph modified from Hobbs and Humphries (1995).
Despite a succession of policies beginning in the early 20th century to reduce the introduction of these pests, the rate of establishment has continued unabated in the face of increasing trade. The United States continues to accumulate nonnative forest insects at the rate of ~2.5 per year, with “high‐impact” insects and pathogens accumulating at 0.43 per year (Aukema et al. 2010), and wood‐boring insects at ~0.23 per year and increasing in recent years (Fig. 3A). However, the cumulative volume of United States global imports is growing faster than the linear trend for insect introductions (Fig. 3A), suggesting that while current policies are having positive effects, they are not enough to reduce the rate of introductions. Nevertheless, the linkage between trade volume and pest establishment (Brockerhoff et al. 2014) suggests that absent more effective policies, continued increase in trade will yield many new establishments of nonnative forest pests, some of which can be expected to become important in terms of their ecological and economic impacts. The continued influx of invasive pests and the spread of those that are already established represent a severe risk to United States forests and urban and suburban landscapes. The following sections summarize the ecological and economic consequences of that risk.
Figure 3.
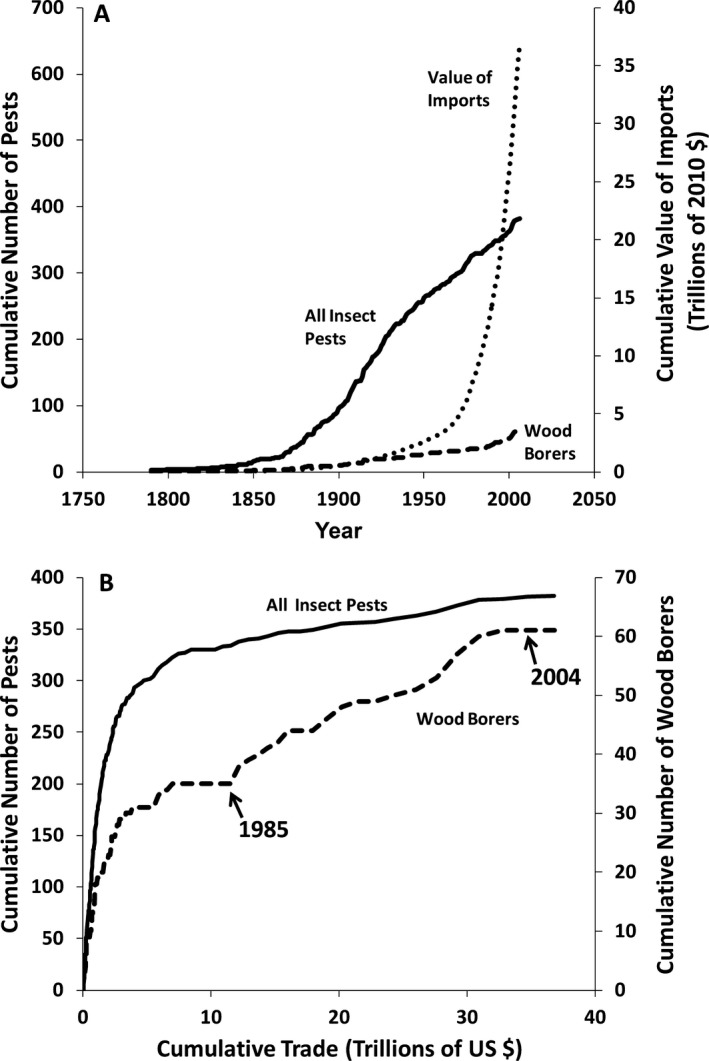
(A) Cumulative detections of all nonnative insect pests (solid line) and wood‐boring insects (dashed line) in the United States. Cumulative value of United States imports (dotted line) in trillions of 2010 US$ is plotted on the right‐hand axis. (B) Same data as (A), plotted as cumulative numbers of total nonnative insect pests (solid line) and nonnative wood‐boring pests (dashed line) vs. cumulative import value. Pest data from Aukema et al. (2010). Trade data from U.S. Census Bureau, Statistical Abstract of the U.S., various years; adjusted to 2010 dollars using the wholesale price index.
Impacts on Trees and Forests
Some nonnative pests are highly destructive and can cause substantial damage to forests and urban/suburban trees (Aukema et al. 2010). Nonnative forest insects and pathogens are present throughout the United States, with the greatest density occurring in the Northeast (Fig. 1). Data from the U.S. Forest Service's National Insect and Disease Forest Risk Assessment (Krist et al. 2014) indicates that 334 million ha, or 63% of the nation's forestland, are at risk for additional basal area mortality of host tree species, and 24.8 million ha are predicted to experience more than 20% loss of host basal area through 2027 (risk assessment tool is available online).13 These estimates are conservative because they are based on projected damage from 13 already‐established pests, whereas more than 60 different pests are currently damaging to United States forests (Aukema et al. 2010) and many new pests are likely to establish in the United States in the coming decades (Leung et al. 2014).
Once a new insect or disease becomes established, the level of impact is determined by a variety of factors such as the virulence of a pathogen and the type of damage from an insect (e.g., phloem‐ or wood‐boring, sap feeding, or defoliation). The severity and extent of the damage can also be affected by other traits of a pest, including its host specificity and its reproductive and dispersal potential, as well as characteristics of the host tree such as its dominance in the forest, its role in productivity and nutrient cycling, and its provisioning of wildlife food and habitat (National Academy of Sciences [NAS] 2002, Lovett et al. 2006). Impacts typically occur in two phases. Initially there is a physical disturbance phase in which trees are damaged or killed by the pest, and which may last for months to years. The second phase occurs for decades to centuries after the initial introduction, and involves the tree species changes that occur when the introduced pest reduces the competitive ability of the host species, allowing competing species to increase and inducing changes that cascade through the ecosystem (Lovett et al. 2006). In this section, we review several key aspects of the functioning of forests and urban systems and illustrate ways in which tree pests and diseases can alter critical ecosystem functions.
Forest species composition and productivity
A highly virulent pest can decimate populations of susceptible tree species; in fact, introduced pests are the only forest disturbance agent that has proved capable of nearly eliminating entire tree species, or in some cases entire genera, within a matter of decades. For example, chestnut blight (see Table 1 for scientific names corresponding to the common names of pests used in this study) effectively eliminated a previously dominant tree species (American chestnut, Castanea dentata) from eastern United States forests (Elliott and Swank 2008, van de Gevel et al. 2012) in the early 20th century. Eastern forests have also been dramatically altered by the hemlock woolly adelgid (HWA), which kills hemlock (Tsuga canadensis and T. caroliniana). In the western United States, the white pine blister rust is a principal cause of the severe decline in populations of whitebark pine (Pinus albicaulis; Keane et al. 2012), an ecologically important high‐elevation species. Whitebark pine has now been listed as a candidate species under the Endangered Species Act, the first widespread tree species to be so listed.
Attack by an introduced pest often leads to significant changes in forest structure and species composition, which in turn lead to changes in ecosystem functions. A well‐studied example is the invasion of the HWA in the eastern United States. Eastern hemlock is a long‐lived, late‐successional species, and its mortality from the HWA can favor early‐successional species (Morin and Liebhold 2015). In the southern Appalachians, HWA‐induced hemlock mortality has opened up streamside canopies and led to release of rhododendron (Rhododendron maximum) understory in some areas (Ford et al. 2012), while in southern New England, declining hemlock stands are often colonized by black birch (Betula lenta; Orwig et al. 2002). Canopy openings caused by HWA can also lead to the establishment of multiple species of invasive plants (Eschtruth et al. 2006). Both the disturbance caused by the death of hemlock trees and the subsequent shifts in species composition reverberate through the ecosystem, causing changes in nutrient cycling (Jenkins et al. 1999), carbon fixation and storage (Nuckolls et al. 2009), and habitat for birds (Tingley et al. 2002) and fish (Siderhurst et al. 2010).
Similar changes in tree species composition result from pest attacks in other forest types. Loss of tanoak (Notholithocarpus densiflorus) due to the disease known as sudden oak death has led to significant structural and compositional changes in coastal California forests (Cobb et al. 2012, Metz et al. 2012). In the Florida Everglades, mortality of swamp bay (Persea palustris) from the laurel wilt disease can cause the loss of this tree species from tree islands, potentially destabilizing their physical structure and leading to colonization by invasive plants (Rodgers et al. 2014). Even a less virulent pest such as beech bark disease can alter species composition by shifting the competitive interactions among trees (Lovett et al. 2010). By preferentially killing larger American beech (Fagus grandifolia) trees, beech bark disease can also change the physical structure of the forest, leading to stands with higher densities of smaller trees and thickets of beech saplings in the understory (Forrester et al. 2003, Griffin et al. 2003, Busby and Canham 2011, Garnas et al. 2011). In addition, many forests are experiencing concurrent invasions by multiple pests, multiplying the local impact.
Primary productivity of a forest is frequently reduced in the initial damage phase after a pest invasion; the magnitude of the reduction depends on the dominance of the host tree and the rapidity of its decline. For instance, ash mortality caused by the emerald ash borer (EAB) has resulted in an average reduction of net primary productivity of ~30% in affected sites in Ohio (Flower et al. 2013), and rapid death of hemlock from HWA reduces productivity of hemlock stands for at least 3 years (e.g., Nuckolls et al. 2009). In the eastern hardwood forests of the United States, beech bark disease has reduced growth rates of American beech and resulted in an overall decline in aboveground tree biomass (Busby and Canham 2011). Long‐term effects on productivity depend on the growth rates of the replacement trees compared to the declining host tree species. For example, eastern hemlock is a slow‐growing, long‐lived species, so nearly any species replacing it will have higher productivity (e.g., Albani et al. 2010). On the other hand, where ash trees killed by EAB are replaced by co‐dominant but slower‐growing competitors such as maples (Lovett et al. 2013, Burr and McCullough 2014), long‐term productivity of the stand may decrease.
Forest nutrient cycles
Both the initial disturbance phase and the long‐term shifts in species composition can lead to major alterations in forest nutrient cycles. Initially, tree mortality produces a pulse of detritus to the forest floor, and the reduction in plant nutrient uptake may result in a loss of plant control over cycling and retention of nutrients in the ecosystem. This can increase leaching of nutrients such as nitrogen (N) to groundwater and streams (Lovett et al. 2002, Cessna and Nielsen 2012). The opening of the canopy and the resulting decline in transpiration can cause increased temperature and moisture in the forest floor, resulting in increased decomposition and N mineralization rates (e.g., Orwig et al. 2013). Outbreaks of foliage‐ or sap‐feeding insects cause large increases in the deposition of insect excreta, which can change the chemistry of throughfall (e.g. Stadler et al. 2006) and alter soil N dynamics (Christenson et al. 2002). In the longer term, pest‐induced changes in tree species composition can profoundly affect productivity, decomposition, carbon storage, and nutrient cycling in forest ecosystems because tree species vary in litter chemistry, growth rates, and nutrient and water use (Albani et al. 2010, Lovett et al. 2010).
Wildlife resources
Forest‐dwelling wildlife can be strongly affected by changes associated with invasive pests. Replacement of one tree species by another will enable some wildlife species to benefit while others decline. For example, woodpeckers that feed on EAB larvae in winter and birds that nest in dead trees may initially benefit from EAB invasion (Koenig et al. 2013, Flower et al. 2014). Hundreds of arthropod species feed on ash, and more than 30 species are thought to require ash. Ash specialists are threatened if highly vulnerable ash species are effectively lost from the forest (Gandhi and Herms 2010, Herms and McCullough 2014). In Connecticut, HWA‐related changes led to local declines in bird species that use hemlock canopies, such as the Black‐throated Green Warbler (Setophaga virens), and increases in other species that prefer hardwood canopies or dead tree habitat (Tingley et al. 2002). Similarly, a model based on data from multiple bird monitoring programs predicted that sudden oak death would reduce populations of multiple bird species in California forests where coast live oak (Quercus agrifolia) occurs (Monahan and Koenig 2006). Ecological interactions that result from pest invasions can be complex and difficult to predict. For example, in the subalpine forests of the Rocky Mountains, whitebark pine, a foundation species (sensu Ellison et al. 2005), depends on a bird, Clark's Nutcracker (Nucifraga columbiana), for dispersal of its large seeds. White pine blister rust can decrease cone production in some whitebark pine stands to the extent that Clark's Nutcrackers no longer forage in them, disrupting the evolved mutualism between the tree and the bird (McKinney et al. 2009). Effects may also extend to aquatic ecosystems within the forested landscape. Fish that prefer shaded, cold water, such as brook trout, are often more abundant in streams with hemlock cover (Ross et al. 2003) and may decrease in streams where hemlock is lost from the streamside canopy due to HWA (Siderhurst et al. 2010). Additionally, the macroinvertebrate and benthic communities inhabiting streams in hemlock forests can change dramatically following hemlock loss (Snyder et al. 2002, Willacker et al. 2009).
Urban and suburban landscapes
Introduced pests attack trees in cities and suburbs, reducing the many benefits that trees provide to residents of densely populated areas. Trees in cities and suburbs moderate climate, provide shade, absorb air pollution, reduce stormwater runoff and soil erosion, provide wildlife habitat, and have important aesthetic value (Dobbs et al. 2014). Recent studies indicate that the presence of urban trees may also improve people's health (Nowak et al. 2014), and the decline in trees due to EAB has been linked to increases in mortality from cardiovascular and respiratory diseases (Donovan et al. 2013, 2015). Potential mechanisms by which trees could affect cardiovascular and respiratory health include improving air quality, reducing stress, increasing physical activity, and moderating temperature (Donovan et al. 2013).
Many cities across the country are investing in “green infrastructure,” natural systems that absorb pollutants from air and water and reduce stormwater overflows and soil erosion, that in many cases require healthy trees and may be at risk from introduced tree pests. Trees along streets and in yards and parks may be particularly attractive and vulnerable to invasive pests because they are often stressed by soil compaction, air pollution, elevated temperatures, and salt exposure (Poland and McCullough 2006). Urban and residential landscapes are often characterized by monospecific aggregations (e.g., multiple individuals of the same species lining a street) that magnify pest impacts.
A notable example of a tree pest with a large impact in urban areas as well as forests is the EAB, which is the most destructive and costliest forest insect to invade North America to date; it has killed hundreds of millions of ash trees and its populations continue to spread (Burr and McCullough 2014, Herms and McCullough 2014). The response to this invasion may cost US$12.7 billion through 2020 (Kovacs et al. 2011a). The high cost is due in large measure to its impacts on urban and suburban landscapes, where planted ash can make up 20% or more of the trees (Poland and McCullough 2006, Kovacs et al. 2010). Ironically, in many neighborhoods, ash trees were planted to replace American elms (Ulmus americana) killed in the mid‐20th century by Dutch elm disease, an introduced pathogen vectored by an invasive beetle.
Economic Value of Impacts
Nonnative insects and pathogens in the United States cause billions of dollars in annual economic damages for timber producers, residential property owners, and governments at the local, state, and federal levels (Aukema et al. 2011). When seen through an economic lens, invasions by nonnative pests are a form of externality resulting primarily from international trade and the domestic movement of commodities and vehicles. While initial calculations were valuable in highlighting the potential magnitude of impacts (e.g., Pimentel et al. 2000, Colautti et al. 2006), this field is now maturing to provide an increasingly robust foundation for economic analyses in policy and management deliberations (Holmes et al. 2009).
The standard approach to analyzing the economic impacts of forest disturbances begins by categorizing impacts into losses and costs (Holmes et al. 2009). Economic losses capture the diminished economic value of forests or trees caused by a pest invasion, while costs are incurred when people take actions designed to prevent or reduce the consequences of the invasion. It is worth noting that some of the actions that people take to reduce costs and losses, such as pre‐emptive harvests or salvage logging, can also be quite damaging ecologically (Foster and Orwig 2006). Here, we briefly describe the constituencies that are impacted by invasive forest pests and give examples of costs and losses from disruptions in ecosystem services.
Timber industry
In addition to direct losses to timber producers from tree mortality, pest outbreaks can also alter timber supply‐and‐demand relationships, resulting in further loss of economic value from price shocks and economic transfers between timber suppliers and buyers (Holmes 1991, Holmes et al. 2014). Aukema et al. (2011) estimated the short‐term (10 yr) value of damage to timber owners by three guilds of invasive forest insects to be about $150 million per year (in 2009 US$). Introduced diseases were not included in this analysis, and the calculations were based on the value of timber mortality due to the insects, assuming timber losses were small enough to not affect timber prices.
Residential property owners
Invasive forest insects and diseases can reduce the value of private properties in urban and residential settings (e.g., Holmes et al. 2010, Kovacs et al. 2011a). In fact, the aggregate economic impacts on residential property value over the past few decades have substantially exceeded impacts on the timber sector (Aukema et al. 2011). Losses in private property values result from a change in the perceived aesthetic quality of the property (Fig. 4), plus any costs associated with homeowner treatments to protect tree health or with the removal and replacement of dead trees. For invasive pests that cause rapidly expanding and spatially extensive tree mortality, such as the EAB, the costs associated with tree removal, protection and replacement by residential property owners may exceed $1 billion annually in urban areas, and if suburban trees are included, costs roughly double (Kovacs et al. 2010).
Figure 4.

A neighborhood in Worcester, Massachusetts, USA (A) before and (B) after removal of trees in an effort to eradicate an outbreak of Asian longhorned beetle. Photo credit: Dermott O'Donnell.
Municipalities
Economic costs associated with treating, removing, and replacing trees on city streets and in parks can place an enormous burden on local governments. An early estimate of the compensatory value of urban trees killed by the Asian longhorned beetle, assuming the worst‐case scenario in which this pest kills all trees in its list of preferred hosts, ranged from $72 million to $2.3 billion per city for nine United States cities (Nowak et al. 2001). Recent estimates show aggregate municipal expenditures associated with nonnative forest insects and pathogens exceeding $2 billion annually in the United States (Aukema et al. 2011).
Public forest land stakeholders
Forests under jurisdiction of state or federal governments belong to the public and impairments to the health of these forests can affect millions of people who benefit from the ecosystem services the forests provide, such as recreational opportunities, wildlife habitat, and water supplies. Several studies have estimated the benefits of protecting federally managed forests from nonnative pests, using nonmarket valuation methods (e.g., Kramer et al. 2003, Rosenberger et al. 2012). In one such study, the value of protecting National Forests in the biologically diverse southern Appalachian Mountains from continued degradation by HWA was found to be orders of magnitude higher than the costs of chemical and biological control programs to control the insect (Moore et al. 2011). The study found that much of the value was derived from protecting forests in remote areas that are difficult for visitors to access, which is consistent with the idea that people value the existence of endangered forest ecosystems even if they never plan on visiting those areas (Kramer et al. 2003).
In summary, a recent analysis indicates that the direct economic impact of nonnative forest insects in the United States is estimated to be at least $2 billion per year in local (e.g., municipal) government expenditures, $1.5 billion per year in lost residential property values, $1 billion per year in homeowner expenditures (e.g., tree removal and replacement), $216 million per year in federal government expenditures, and $150 million in losses to timber owners (Aukema et al. 2011). The study did not sum across cost categories because of the potential for double‐counting. Notably, the majority of this economic burden is borne by municipalities and residential property owners. The Aukema et al. (2011) study, however, likely underestimated the current costs and losses from invasive forest pests because it did not include introduced diseases. For just one disease in one state, Phytophthora ramorum (sudden oak death) in California, the estimated 10‐yr (2010–2020) costs and losses are $7.5 million in tree treatment and removal and $135 million in lost property values (Kovacs et al. 2011b). Also lacking from the Aukema et al. (2011) estimates are the mounting costs to electric utilities that face expanding costs to maintain power lines in treed areas. Further, no economic study to date adequately captures the entire suite of non‐market ecosystem services that are degraded by pests. The value of these services, while real, is very difficult and costly to quantify (Boyd et al. 2013).
Sources, Pathways, and Patterns of Introduction
Forest insects and diseases move around the world via several different modes, but the two most dominant invasion pathways are international movement of wood and live plants. Of the 91 most damaging nonnative species in the United States, 62% are thought to have entered North America with live plants and 30% likely arrived with wood packaging material (WPM) or other wood products (Liebhold et al. 2012).
Live plants
Importation of live plants has facilitated the greatest number of forest pest introductions into the United States (Liebhold et al. 2012) and other countries as well (Kenis et al. 2007, Smith et al. 2007, Roques et al. 2009). Live plants are unfortunately an ideal medium for transporting plant pests because they provide food and habitat that can sustain pest populations during long transit times and upon arrival in the new region.
Live plants are imported for two main reasons. First, the horticultural, agricultural, and forestry industries seek out new plant varieties (frequently nonnative) with favorable properties. Second, low labor costs and better growing conditions in some countries may economically favor producing plants there and shipping them elsewhere for sale and planting. Imports of so‐called “plants for planting” have dramatically increased over the last four decades in both Europe and North America; ~3 billion live plants are now imported annually to the United States (Liebhold et al. 2012).
Before the passage of the Plant Quarantine Act of 1912 and implementation of Quarantine 37 regulations by the U.S. Department of Agriculture (USDA) in 1919, the United States essentially did not regulate plant imports, and a large number of forest pest species entered via imported plants. Many of these species still have considerable impacts on North American forests; notable examples include the chestnut blight fungal pathogen, which arrived with imported chestnut plants in New York near 1900 (Anagnostakis 2001), beech scale, the causal agent of beech bark disease, accidentally introduced with live plants imported to Halifax, Nova Scotia, Canada from Europe, in the 1890s (Houston 1994), and white pine blister rust, introduced to North America on nursery stock in several locations in the early 1900s (Burns et al. 2008). Since the 1920's, nonnative forest pests have continued to enter and establish in the United States, though establishment rates vary among guilds. In recent years, the establishment rate of foliage‐feeding insects has declined but that of wood‐ and phloem‐boring insects has increased, so that the total rate of insect introductions has remained fairly constant (Fig. 3A).
Wood packaging material and other wood products
The form of wood that currently poses the greatest risk for pest invasions is WPM, particularly items made from solid wood such as crating, pallets, spools, and dunnage (Brockerhoff et al. 2006, Haack 2006). With the acceleration of post‐World War II industrial trade, and the increase in containerized shipping beginning around 1980, massive amounts of WPM now move around the world. Low‐quality wood often used as WPM, including slab wood or other pieces that retain patches of bark, can harbor immature life stages of phloem‐ and wood‐boring insects. Increasing worldwide WPM movement since the 1980's has produced a surge of invasions by phloem‐ and wood‐boring insects (Fig. 3A).
Phloem‐ and wood‐boring insects have been intercepted since 1914, when the USDA first implemented inspections at ports, but these species were not historically considered high risk for becoming major pests. Even as late as 1982, a pest risk assessment by the USDA reduced the number of “actionable” wood boring insects, having concluded that such species were not of great concern. It was not until Asian longhorned beetle populations were discovered in Brooklyn, New York in 1996 and Chicago, Illinois in 1998 that the damage potential of exotic wood‐boring insects gained increased attention.
Other pathways
Introduction pathways other than WPM and live plants include roundwood and other wood products, hitch‐hiking on cargo (e.g., insect eggs laid on the outside of ships or shipping containers), intentional introduction, passenger baggage, and mail and parcel post. Trade in roundwood and unprocessed logs is a well‐known pathway for invasions by wood‐boring insects and pathogens. For example, the smaller European elm bark beetle (Scolytus multistriatus Marsham), the primary vector of Dutch elm disease, is believed to have been introduced to North America on imported logs (May 1934). A prominent example of hitch‐hiking is the Asian gypsy moth, which is not yet established in North America but whose egg masses have been repeatedly found on the superstructures of ships arriving at United States ports, especially on the West Coast (Canada‐United States Joint AGM Industry Notice 2014). The European gypsy moth was intentionally imported to Massachusetts in the late 1800s by an amateur entomologist, but soon escaped into the wild and has since spread across much of the eastern United States, where it has been a major pest. Air passenger baggage is well known as an invasion pathway for agricultural pests (Liebhold et al. 2006), but it has also facilitated unregulated importation of live plants, some of which may be infested with forest pests. For example, the chestnut gall wasp, Dryocosumus kuriphilus Yasumatsu, entered the United States in 1974 associated with chestnut plants transported by a private gardener in Georgia (Rieske 2007).
Geographic distribution of pests in the United States
Whereas nonnative forest pests are established throughout the United States, numbers of invasive pests are highest in the Northeast (Fig. 1). Underlying causes for this pattern include high commerce volume, the relatively high tree diversity of eastern forests, and the taxonomic similarity of eastern tree species with those in forests of Asian and European trade partners (Liebhold et al. 2013). High trade volume increases propagule pressure, e.g., the number of arrivals of potential invaders. High tree diversity makes it more likely that a suitable native host is present when a nonnative pest arrives. Taxonomic similarity of tree species between continents increases the likelihood that a newly arrived tree‐feeding pest will recognize American trees as potential hosts and be able to cope with the plants' natural defenses.
Relationship to trade volume
Based on species accumulation theory, one might expect that as the cumulative amount of trade increases, the rate of introduction of nonnative species would slow (Levine and D'Antonio 2003). Broad patterns of pest introductions over the last 200 years are consistent with this expectation (Fig. 3B), but with notable exceptions. The historical accumulation of exotic wood‐boring pests declined with increasing cumulative trade volume as expected until ca. 1985, but then increased until ca. 2004 (Fig. 3B), probably reflecting increases in containerized shipping as well as escalating trade with Asia, especially China, beginning in the 1980s (McCullough et al. 2006). Asia presumably harbors a diverse but relatively little‐known assembly of phloem‐ and wood‐boring insects. Historically, this community was likely undersampled because of limited trade. The reduced slope of this curve after 2004 may reflect United States requirements implemented in 1999 for treatment of wood packing material in shipments from China, given that the discovery of a new introduced species usually does not occur until years after it arrives.
Policies to Reduce Pest Invasions
The current policy system governing activities to prevent the arrival and establishment of invasive species is a patchwork of international, national, state, and local regulations and protocols; for detailed information about specific policies, agreements and governing bodies, see Burgiel et al. (2006). International trade agreements have a dual mission of reducing movement of harmful pests and pathogens while facilitating trade (Burgiel et al. 2006, MacLeod et al. 2010). Because pest introductions increase with trade volume (Brockerhoff et al. 2014), these two objectives can be in opposition.
Evaluations of policy effectiveness are limited, but the available information suggests that while current prevention measures have beneficial effects, they nevertheless leave the nation's trees and forests at risk of future damaging invasions. Once a nonnative pest is established and begins to spread, a cascade of impacts increases the costs borne by local landowners and local governments (Aukema et al. 2011). Further, as infestations spread, the effectiveness of remediation options declines and additional harm to ecosystems from these measures may increase (e.g., tree removal and pesticides; Roy et al. 2014). Therefore, the greatest ecological and economic benefits can be realized from efforts aimed at preventing the arrival and establishment of potentially damaging pests. We focus our discussion of policy options on these front‐line measures.
Efforts to prevent arrival and establishment can be categorized by the point in the trade process at which they are implemented: (1) measures focusing on the point of origin, (2) measures to manage pathways, and (3) measures to strengthen early pest detection and response (e.g., inspection, surveillance, etc.). To compile these policy options, we interviewed scientists, land managers, and policy experts, and reviewed relevant literature on phytosanitary policies. We identified policy options that are supported by existing science and commonly cited as important for reducing arrival and establishment (Table 2). Many of the policy options listed have been implemented to some degree but require strengthening or expansion to be more effective.
Table 2.
Policy options for reducing the importation and establishment of nonnative forest insects and pathogens
| Category and supporting science | Strategy | Options and references |
|---|---|---|
| Point of origin | ||
| (1) Controlling invasives is a “weakest‐link” public good that benefits from strengthening practices in countries of origin | Phytosanitary practices abroad | Strengthen overseas protection measures and establish pre‐clearance partnerships such as clean stock programs for plants (Perrings et al. 2002, Haack et al. 2010, Keller and Perrings 2011) |
| (2) Monitoring native United States plants growing in other countries can help determine susceptibility to pests | Monitoring for new threats | Enhance Sentinel Plant programs (National Academy of Sciences (NAS) 2002, Britton et al. 2010) |
| Arrival pathways | ||
| (1) Interventions to reduce the risk of the wood packaging material (WPM) invasion pathway provide a net economic benefit over time. (2) Historically invasions have increased with trade volume, indicating that pathway risks increase with increasing trade. |
WPM | Require phase‐out of solid WPMs in international shipping (State of NY vs. USDA 2005, Campbell and Schlarbaum 2014) Promote voluntary phase‐out of WPM by retailers (Campbell and Schlarbaum 2014) Strengthen ISPM‐15 measures for treating WPM (Haack et al. 2010) Strengthen enforcement of ISPM‐15 standards and increase penalties for non‐compliance(Campbell and Schlarbaum 2014) |
| (3) Visual inspections are not adequate to detect pests, therefore integrated measures are necessary. (4) Studies indicate that current procedures detect only a fraction of pests imported on live plants and WPM |
Live plant imports | Substantially restrict or eliminate imports of live woody plants for horticultural use (Lodge et al. 2006, Keller and Springborn 2014, Roy et al. 2014) Promote voluntary standards for importing “clean” woody plants and strengthen market demand (Roy et al. 2014) Strengthen enforcement of existing regulations and increase penalties for non‐compliance (Roy et al. 2014) Educate travelers about risks of importing plants and increase penalties (Environmental Law Institute 2002) |
| Pre‐establishment | ||
| (1) Expense of mitigating invasion increases and likelihood of success decreases once a pest becomes established. (2) Quarantine and surveillance have been effective strategies to combat invasion in the United States and other countries. |
Improve inspections | Ensure adequate funding through federal budgets and user fees (Simberloff et al. 2005, Reaser and Waugh 2007, Roy et al. 2014) Enhance evidence‐based inspection by combining robust random sampling with targeted sampling of high‐risk shipments (NAS 2002, GAO 2006, Lodge et al. 2006, Reaser and Waugh 2007, Haack et al. 2014) Accelerate testing, adoption, and sharing of new inspection methods and diagnostic tools (Lodge et al. 2006, Reaser and Waugh 2007) |
| Strengthen early detection and response | Require post‐entry quarantine of all imported trees and shrubs (Campbell and Schlarbaum 2014) Develop coordinated national surveillance system (Lodge et al. 2006, Keller and Perrings 2011) Establish secure funding for pest eradications (Lodge et al. 2006) |
|
| Research and development | ||
| (1) Multiple sources report that current data collection and data sharing protocols are inadequate. (2) Other agencies such as the Center for Disease Control have effectively applied effective data collection, database management, collaboration, data sharing, and timely analysis to combat hazards to the public. |
Improve information management | Improve data quality and data management procedures within APHIS and CBP (NAS 2002, Reaser and Waugh 2007) Revise inspection and data collection methods to support evaluation of policy effectiveness (NAS 2002, Lodge et al. 2006, GAO 2006, Reaser and Waugh 2007, Haack et al. 2014) Improve access to data for researchers and managers (NAS 2002, GAO 2006, Reaser and Waugh 2007, Haack et al. 2014) Institute regular data analysis and reporting by APHIS and CBP on prevention effectiveness, including biennial report to Congress (NAS 2002) Develop and improve global information systems (NAS 2002, Keller and Perrings 2011) Increase collaboration across agencies and with academic researchers (Ricciardi et al. 2000, Herms and McCullough 2014) Establish Scientific Advisory Committee on prevention methods under FACA |
Observations that support the need for new policies are listed (Supporting Science) for each category. References are to literature in which the policy option has been suggested previously.
Prevention measures at the point of origin
The Animal and Plant Health Inspection Service (APHIS, an agency within the USDA) has programs abroad to help prevent the arrival of new pests and to identify possible new threats. Below we describe two opportunities to expand existing efforts in ways that would increase protections for forests and trees.
Options for enhancing measures at the point of origin
Expand pre‐clearance partnerships to include threats to forests and promote clean supply chains from the point of origin. Such partnerships can enforce stringent phytosanitary standards for importers to meet, and in return provide benefits such as expedited processing for partners who comply with these standards. One key challenge is how to monitor for compliance and effectiveness, and periodic inspections would still be needed. Separate pre‐clearance programs could be developed for different pathways such as WPM and live plants. For example, the 2012 international phytosanitary standard (Integrated Measures for Plants for Planting, ISPM‐36) outlines procedures to minimize the presence of pests on nursery plants in exporting countries (FAO 2012). Such “clean stock” programs can be very successful; one good example is provided by the system implemented by APHIS to certify that imported geraniums are free of a serious bacterial disease (Ralstonia) that they can transmit to potatoes (APHIS 2015a).
Expand sentinel tree programs abroad to enhance the identification of possible future forest pests and pathways. Monitoring should include North American trees planted in botanical gardens, arboreta, commercial plantations, and urban plantings. An example is the International Sentinel Plant Network, a public‐private partnership in which woody plant genera from the United States are planted abroad in botanical collections and are monitored for damaging pests and diseases (Britton et al. 2010). When a new threat is discovered, a coalition of researchers, regulatory officials, and stakeholders (e.g., resource managers) should be established to develop a response protocol, similar to the New Pest Advisory Groups organized by APHIS (APHIS 2015b). Species identified as high risk of invasion and potentially damaging should be targeted by surveillance programs.
Managing arrival pathways
We focus here on policy options for managing the two pathways that are most important for nonnative forest pests: WPM and live plants.
Wood packaging material: current policies and effectiveness
The International Standards for Phytosanitary Measures No. 15 (ISPM‐15) is intended to reduce the arrival of wood‐boring and phloem‐feeding pests in WPM (Haack et al. 2014). ISPM‐15 was adopted in 2002 and modified several times since, and it specifies that WPM must be fumigated with methyl bromide or heated for sterilization, then marked with a stamp to certify compliance before transport. The regulation is primarily enforced in the United States through inspections; U.S. Customs and Border Protection (CBP) has responsibility for port‐of‐entry inspections, while APHIS issues phytosanitary guidelines for inspections. To improve compliance with WPM regulations, CBP began using its authority to levy penalties for WPM violations in 2007. CBP may reject a non‐compliant shipment or may assess a penalty if a WPM violation is concealed, or if an importer has received five prior citations for violations in the United States in the previous fiscal year (CBP 2007).
Recent assessments of ISPM‐15 show that the guidelines are beneficial but still allow large numbers of new wood‐boring insects to be imported into the United States (Haack et al. 2014, Leung et al. 2014). Leung et al. (2014) projected that the protocol will yield cumulative net benefits reaching US$11 billion by 2050. Continued implementation through 2050 could reduce pest imports by 36–52% (Leung et al. 2014). Nonetheless, because of the limited effectiveness of the current ISPM‐15 regulations and the growing trade volume, up to three times as many wood‐boring insects may be imported into the United States through 2050 as currently occur there (Leung et al. 2014).
ISPM‐15's effectiveness seems to be limited by several factors: (1) inadequacy of mandated heat and fumigation treatments to ensure potential pests are killed (Zahid et al. 2008, Myers et al. 2009, Haack et al. 2014), (2) post‐treatment colonization of WPM, (3) fraudulent application of the ISPM marking on WPM, and (4) unintentional failure to follow treatment protocols in exporting countries (Haack et al. 2014). A 2009 provision in ISPM‐15 for bark removal on WPM has likely decreased the infestation rate for bark beetles and fungi.
There are potentially large economic benefits from strengthening ISPM‐15 and reducing the importation of wood‐boring insects. Using the risk model described by Leung et al. (2014), we calculated the potential economic benefit (in costs averted) of measures that would increase the efficiency of ISPM‐15 above the 52% reported by Haack et al. (2014). A 25% increase in effectiveness above current levels would eliminate 65% of pests in WPM and provide an estimated $22 billion economic benefit in the United States through 2050. Increases in effectiveness of 50% and 75% would produce economic benefits of $27 billion and $31 billion, respectively. Completely eliminating this pathway by switching to non‐wood packing material would provide an estimated benefit of $36 billion through 2050. These total economic benefits do not consider the costs of implementing the more effective regulations. The cost of implementing the current ISPM‐15 procedures is estimated at about $5 billion through 2050 (Leung et al. 2014), but the cost of improving the procedures or switching to non‐wood packing material has not, to our knowledge, been studied. The environmental impacts of new policies should also be considered, such as increased use of fumigants for wood packaging or the impacts of manufacturing non‐wood packaging. Nonetheless, these calculations suggest that even if the strengthening of the procedures tripled the cost, there would still be a substantial net economic benefit.
Options for strengthening WPM pathway measures
Require packaging materials that are not made from solid wood for international shipping. Banning solid WPM would provide higher levels of protection than, and would reduce the need for, the other WPM policies described here. Such a policy change would need to conform to procedures established in international trade agreements, particularly those of the International Plant Protection Convention (Burgiel et al. 2006), and would be most efficient if it were implemented globally through modification of ISPM‐15 or through a new ISPM.
Promote voluntary substitution of safer alternatives to WPM. Benefits to companies that make this switch include the assurance that goods will not be stalled in transit due to inspections, cost‐savings on shipping fuel when the alternative materials are lighter weight, and additional cost‐savings when the alternative materials take up less space, making room for more merchandise in each shipment. Companies may also be able to gain additional market share from consumers who value green products and sustainable supply chains.
Continue to strengthen the ISPM‐15 requirements to ensure effectiveness for a broader array of pests. For example, additional research is needed on alternative treatments, and on protocols for pallet storage to reduce the likelihood of post‐treatment re‐infestation.
Increase enforcement of existing ISPM‐15 regulations, including stronger penalties for non‐compliance. Examples include ending the practice of allowing each importer five violations each fiscal year before assessing a penalty, instituting temporary bans on specific countries for items that consistently fail to comply, and providing technical assistance to sources in response to unintentional non‐compliance (i.e., faulty equipment or technique). The Standards and Trade Development Facility could support capacity‐building for increasing compliance in developing nations exporting goods to the United States.
Live plant imports: current policies and effectiveness
Current United States federal law mandates inspection of imported live plants to protect against importation of pests and diseases. The relevant regulation, known as Quarantine 37, bars some plants from being imported (a black list), designates some plants for quarantine for specific periods after arrival, and allows most plants into the United States with a phytosanitary certificate from the country of origin and an inspection upon arrival. A 2011 change to the regulation creates a gray list category of plants that are not authorized for import pending pest risk analysis (NAPPRA) by APHIS (USDA‐APHIS 2011).
All shipments of live plants imported into the United States pass through one of 17 APHIS Plant Inspection Stations. Liebhold et al. (2012) reported that standard APHIS inspections at these stations during 2003–2010 found that 2.6% of incoming shipments had reportable pests. However, more thorough inspections of several plant genera revealed much higher infestation rates (Liebhold et al. 2012), indicating that while the current system of inspections has important benefits for intercepting infestations, it still misses many pests imported on live plants.
Options for strengthening live plant measures
Substantially restrict or eliminate imports of live woody plants for horticultural use. This measure would provide greater protection than, and would reduce the need for, the other live plant measures listed below. Bans on other groups of live plants were proposed by Roy et al. (2014) and by an international group of plant pathologists (IUFRO 2011). Such a policy change would need to conform to the procedures established in international trade agreements, such as those of the International Plant Protection Convention. An alternative approach is to ensure that all genera of North American woody plants are included on the NAPPRA list of genera not currently approved for import and awaiting risk assessment.
Work with large retailers to establish voluntary supply chain standards for importing pest‐free woody plants and promote associated markets. This could build on two existing pilot programs: the APHIS United States Nursery Certification Program for nursery plants cultivated for export to Canada, and the National Plant Board program Systems Approach to Nursery Certification (SANC). SANC promotes voluntary phytosanitary certification for United States nurseries in exchange for enhanced opportunities to move live plants domestically and overseas.
Increase enforcement of existing regulations and strengthen penalties for non‐compliance (Roy et al. 2014). Examples include increasing fines for non‐compliant shipments, providing technical assistance to sources in response to unintentional non‐compliance (i.e., faulty equipment or technique), and temporarily freezing import authorizations for specific countries for items that consistently fail to comply.
Enhance education of travelers to foreign countries about risks of bringing live plants back with them, and increase fines for non‐compliance. Plant material that is imported illegally in passenger baggage or in the mail represents a serious pest importation pathway that is difficult to control. While a few individuals may intentionally import plants without a permit to avoid regulation (i.e., smuggling), more of these imports may be carried out by individuals who simply are unaware of regulations and/or dangers association with such practices, and improved education may help.
Preventing pest establishment
If a potentially damaging pest arrives in the United States despite strong pre‐arrival measures, it may be possible to decrease its likelihood of establishment in the broader landscape through effective inspection, surveillance, early detection, and rapid response (GAO 2006).
Inspection
The responsibility for inspection is divided between APHIS, which inspects live plants, and CBP, which inspects all other commercial cargo, mail, packages, and passenger baggage at airports, land‐border crossings, and marine ports. In the United States, visual inspection is currently the primary method for detecting pests on live plant imports. Visual inspection has two major challenges. First, funding constraints limit the number of inspectors available to manage a growing volume of imports. Second, some insects and pathogens are difficult or impossible to detect by sight (Liebhold et al. 2012). While inspections alone are not sufficient to protect the nation's forests from pests, inspections are important for two reasons: (1) they can deter trade partners from violating phytosanitary policy, and (2) they can generate data on the variety, sources, and approach rate of pests, which can be used to improve detection and prevention strategies (e.g., identify commodities that should be targeted for quarantine).
Opportunities to gain more benefits from inspection
Ensure that inspection services are adequately funded and the number of inspectors keeps pace with the trade volume being inspected.
Continue to improve inspection efficiency by using data to identify high‐risk pests or pathways and targeting inspection accordingly. Maximize the value of inspections for generating data to inform pest prevention strategies as discussed in more detail later (see Closing the science–decision gap).
As an alternative to visual inspections, develop and implement additional methods to find pests on plants and in wood, e.g., using trained dogs, sensitive acoustical devices, or air‐sampling techniques to detect sounds or chemical emissions from pests.
Surveillance and eradication
After a pest has entered a port, detecting its presence and reacting quickly can prevent its establishment and spread. These measures include the use of post‐entry quarantine, improved surveillance, and rapid eradication of new pest populations. Within the framework of import regulations, post‐entry quarantine is considered a condition of importation; however since the quarantine activity occurs after the pest has entered the country we discuss it in this section.
Post‐entry quarantine has the potential to stop invasive pests and pathogens at the point of arrival. The value of post‐entry quarantine was demonstrated by the case of the citrus longhorned beetle (CLB; Anoplophora chinensis Forster) infestation discovered during the quarantine of Korean bonsai maple trees in a nursery in Washington, USA (Haack et al. 2010). Immediately after the infestation's discovery, a successful eradication was undertaken, aimed at the five adult CLB that had emerged from the trees and escaped from the nursery. Thus, even a somewhat porous quarantine can provide benefits by enhancing detection likelihood and preventing further dissemination of the pests through shipping of nursery stock. However, in the United States, post‐entry quarantines are currently mandated only for selected species identified as particularly important to food and other high‐value crops.
Nations achieving high levels of phytosanitary protection, such as New Zealand, rely on pathway measures together with strong surveillance and eradication protocols (Bulman 2008). Currently the United States has several independent programs for forest pest surveillance. APHIS coordinates the National Woodborer/Bark Beetle Survey through its Cooperative Agricultural Pest Survey (CAPS), in which APHIS provides organizational, funding, and technical support for states to survey for a list of specific forest pests including woodborers, bark beetles, and defoliators. As part of its national forest inventory, the USDA Forest Service has recently begun an Urban Forest Health Monitoring protocol which has the potential to help with pest detection in urban areas. The Forest Service also runs a surveillance program (the Early Detection Rapid Response program) using traps for bark and ambrosia beetles at urban forests and wooded areas around high‐risk sites such as importers and warehouses. These limited programs each provide valuable services, but the nation currently lacks a comprehensive, centrally coordinated surveillance strategy.
Surveillance for introduced forest pests is inherently difficult because (1) introductions can occur almost anywhere, (2) many potentially damaging insect and pathogen species are unknown, (3) many of those that are known are difficult to detect either because they are inconspicuous or because they live beneath the surface of the tree for part of their life cycle, and (4) only some types of insects, and no diseases, are reliably collected by traps. Nonetheless, our national surveillance system could be improved by establishing a coordinated national program that includes visual inspections and trapping at high‐risk sites. Though potentially expensive, surveillance programs have been shown to provide substantial net economic benefits by reducing the incidence of new infestations (Epanchin‐Niell et al. 2012). Some state regulatory agencies have already adopted a pathway‐centered approach for surveillance activities, often focusing on high‐risk sites such as industrial areas (Colunga‐Garcia et al. 2013) or large import/export nurseries. Targeted surveys can be effectively coupled with specialized training of individuals who work with trees such as extension agents, foresters, utility crews, arborists, and landscapers, increasing the likelihood that unusual pests or symptomatic trees will be noticed and reported. Outreach efforts geared at citizen groups such as neighborhood associations, Master Gardeners, and outdoor recreationists can further increase awareness of invasive forest pests and expand the number of people available to observe and report unusual insects causing tree damage. Previously unknown infestations of the ALB and the EAB, for example, were identified following reports by concerned citizens. Several states are currently undertaking outreach and extension programs to increase citizen awareness of invasive forest pests, and smartphone applications continue to be developed to assist citizens in reporting suspected invasive species. More investment in research and development of new and non‐traditional detection methods, such as is currently under way for EAB (Herms and McCullough 2014), will pay dividends in the long term.
Opportunities to strengthen early detection and response
Apply the same stringent post‐entry quarantine standards now required for fruit trees and grapevines to imports of horticultural trees and shrubs. Under this approach, cuttings and whole plants would be quarantined into certified facilities where they would be monitored closely for infestation while grown to supply the retail market.
Establish a coordinated national surveillance system for forest pests involving three tiers: (1) visual and trapping surveys, carried out by experts from federal or state agencies or universities, that target high‐risk sites including industrial and urban areas and locations that are destinations for imported commodities; (2) improved training for key groups such as extension agents, foresters, arborists, and utility crews who can identify unusual occurrences of tree mortality and pests; and (3) enhanced public education activities at state and local levels to encourage citizens to report unusual tree pest activity in their neighborhoods or parks. Such reporting can be facilitated by increasing support to plant pest diagnostic clinics at land grant universities, telephone hotlines, and development of smartphone applications. Partnerships with university extension personnel, nature centers, local and national NGOs, and related organizations could help mobilize citizen involvement at local, state, and regional levels. The three tiers of this surveillance system would require close vertical and horizontal integration, vertical integration to allow rapid communication among local, state, and federal partners, and horizontal integration to encourage similar protocols and data‐sharing across regions.
Establish a secure funding program that can be accessed to eradicate pests that threaten urban and forest trees. The current funding through the Commodity Credit Corporation is too constrained and short‐term to be effective for this purpose (General‐Accounting‐Office [GAO] 2006, Council on Climate Preparedness and Resilience 2014).
Closing the science–decision gap
Finally, in the age of “big data” and improved information systems, opportunities exist for improving data collection, sharing, and analysis for forest pests in the United States and globally. The following options could lead to improved detection of high‐risk pests and evaluation of the treatment and prevention efforts.
Improve data quality and management. Researchers and stakeholders report that inspection data collection strategies are inconsistent among ports (GAO 2006), and even within ports, and that variability among inspectors makes data use and interpretation challenging (Reaser and Waugh 2007). In one analysis, 5% of pest inspection database entries from 1984 to 2000 contained either incomplete or invalid taxonomic identifications or ambiguous point of entry or origin identifications (McCullough et al. 2006). Increased funding, staffing, and technological resources are needed to overcome persistent data quality issues (Reaser and Waugh 2007).
Revise data collection models. A robust inspection system requires random inspections to identify risk, paired with targeted, non‐random inspections to focus maximum effort on the highest risk commodities and sources (Reaser and Waugh 2007). This pairing of random and targeted inspections is currently used for live plants, but a similar pairing of random and non‐random inspections is needed for WPM. Also, inspection data are currently inadequate to determine the effectiveness of new protocols such as heat treatments for WPM. Surveys should be conducted before and after implementing new phytosanitary policies to aid in policy evaluation (Haack et al. 2014). These changes could be accomplished through increasing funding, personnel, and targeted technology, as well as by increasing collaboration with academic partners to carry out research on risk and effectiveness of new phytosanitary policies.
Enhance access to APHIS and CBP data by personnel from other government agencies and academic researchers. The data should be available in common, accessible formats appropriate for the type of data. Interested parties should be able to access and use inspection data via the APHIS website, in collaboration with APHIS personnel who are familiar with the interpretation of these data.
Provide regular data analysis and reporting. As part of an overall interest in government accountability, a biennial report from APHIS and CBP on the efficacy of existing prevention and inspection programs should be provided to Congress and state regulatory agencies. Similar assessments are required of most federal agency regulatory programs.
Continue to develop global information systems to more effectively collect and share information on known pests. An information clearinghouse that compiles detailed data on species detections or previous invasions, traits that may facilitate introduction or establishment, and habitat and host preferences could help prevent introductions and facilitate early detection (Ricciardi et al. 2000).
Increase collaboration with academic researchers with expertise in forest entomology, pathology, and ecology, and specialists in extension, outreach, and education. This would leverage federal efforts and ensure that the necessary expertise exists to identify pests and pathogens likely to target United States plants, and to develop strategies to prevent such invasions.
Establish a Scientific Advisory Committee under the Federal Advisory Committee Act to annually review the integrity of pest prevention, clean pathway, and surveillance programs and resultant data, including implementation of action items in the National Invasive Species Management Plan (Reaser and Waugh 2007).
Summary
Nonnative forest insects and pathogens are causing significant ecological and economic damage in the United States. The ecological damage has included near‐extirpation of several important tree species, shifts in forest composition and ecosystem function, and disruption of wildlife habitat. The economic damage totals billions of dollars annually, costs borne by timber owners, stakeholders in federal and state forest land, and especially municipalities and residential property owners. There are numerous means by which nonnative pests enter the country, but the two most important pathways are global trade in live plants for horticultural use and WPM such as pallets and crates. Global trade is likely to continue to expand, and current policies place the country at high risk for increasing ecological and economic damage by nonnative pests in the future. Although national and international regulations have reduced the international transport of pests, important opportunities exist to strengthen existing policies and bolster our nation's forest pest defense system.
Acknowledgments
We are grateful to the Doris Duke Charitable Foundation, the F.M. Kirby Foundation, and Northeastern States Research Cooperative of the USDA Forest Service for support of the project that led to this paper. Additional support was provided by the U.S. National Science Foundation's Long Term Ecological Research program through grants DEB 11‐14804 (Hubbard Brook site) and DEB 12‐37491 (Harvard Forest site). The conclusions and opinions in this paper are those of the authors and not the NSRC, the Forest Service, or the USDA. We thank Stas Burgiel, Ann Gibbs, and Rebecca Turner for advice on policy issues, Kurt Gottschalk for a helpful review of the manuscript, and the U.S. Forest Service's Forest Health Technology Enterprise Team for calculations of forest area at risk.
Note
Literature Cited
- Albani, M. , Moorcroft P. R., Ellison A. M., Orwig D. A., and Foster D. R.. 2010. Predicting the impact of hemlock woolly adelgid on carbon dynamics of eastern United States forests. Canadian Journal of Forest Research 40:119–133. [Google Scholar]
- Anagnostakis, S. 2001. The effect of multiple importations of pests and pathogens on a native tree. Biological Invasions 3:245–254. [Google Scholar]
- APHIS, 2015a. USDA Animal and Plant Health Inspection Service (APHIS), Washington, DC. https://www.aphis.usda.gov/aphis/ourfocus/planthealth/sa_domestic_pests_and_diseases/sa_pests_and_diseases/sa_plant_disease/sa_ralstonia/. July 9, 2015. [Google Scholar]
- APHIS, 2015b. New Pest Advisory Group (NPAG). USDA Animal and Plant Health Inspection Service (APHIS), Washington, DC. https://www.aphis.usda.gov/aphis/ourfocus/planthealth/ppq-program-overview/sa_cphst/ct_new_pest_advisory_group. June 26, 2015. [Google Scholar]
- Aukema, J. E. , McCullough D. G., Von Holle B., Liebhold A. M., Britton K., and Frankel S. J.. 2010. Historical accumulation of nonindigenous forest pests in the continental United States. BioScience 60:886–897. [Google Scholar]
- Aukema, J. E. , et al. 2011. Economic impacts of non‐native forest insects in the continental United States. PLoS ONE 9:e24587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, I. L. , Freer‐Smith P. H., Gilligan C. A., and Godfray H. C. J.. 2013. The consequence of tree pests and diseases for ecosystem services. Science 342:823. [DOI] [PubMed] [Google Scholar]
- Britton, K. O. , White P., Kramer A., and Hudler G.. 2010. A new approach to stopping the spread of invasive insects and pathogens: early detection and rapid response via a global network of sentinel plantings. New Zealand Journal of Forestry Science 40:109–114. [Google Scholar]
- Brockerhoff, E. G. , Liebhold A. M., and Jactel H.. 2006. The ecology of forest insect invasions and advances in their management. Canadian Journal of Forest Research 36:263–268. [Google Scholar]
- Brockerhoff, E. G. , Kimberley M., Liebhold A. M., Haack R. A., and Cavey J. F.. 2014. Predicting how altering propagule pressure changes establishment rates of biological invaders across species pools. Ecology 95:594–601. [DOI] [PubMed] [Google Scholar]
- Bulman, L. S. 2008. Pest detection surveys on high‐risk sites in New Zealand. Australian Forestry 71:242–244. [Google Scholar]
- Burgiel, S. , Foote G., Orellana M., and Perrault A.. 2006. Invasive alien species and trade: integrating prevention measures and international trade rules. Center for International Environmental Law, Washington, DC: http://cleantrade.typepad.com/clean_trade/files/invasives_trade_paper_0106.pdf. Published January 2006. [Google Scholar]
- Burns, K. S. , Schoettle A. W., Jacobi W. R., and Mahalovich M. F.. 2008. Options for the management of white pine blister rust in the Rocky Mountain Region. General Technical Report RMRS‐GTR‐206, USDA Forest Service, Rocky Mountain Research Station, Fort Collins, Colorado, USA. [Google Scholar]
- Burr, S. J. , and McCullough D. G.. 2014. Condition of green ash (Fraxinus pennsylvanica) overstory and regeneration at three stages of the emerald ash borer invasion wave. Canadian Journal of Forest Research 44:768–776. [Google Scholar]
- Busby, P. E. , and Canham C. D.. 2011. An exotic insect and pathogen disease complex reduces aboveground tree biomass in temperate forests of eastern North America. Canadian Journal of Forest Research 41:401–411. [Google Scholar]
- Campbell, F. T. , and Schlarbaum S. E.. 2014. Fading forests III: American forests: what choices will we make. The Nature Conservancy, Arlington, Virginia, USA: http://www.nature.org/ourinitiatives/habitats/forests/fading-forests-3-complete-report.pdf [Google Scholar]
- Canada‐United States Joint AGM Industry Notice . 2014. http://www.inspection.gc.ca/plants/plant-protection/insects/gypsy-moth/industry-notice-2014-02/eng/1396111212197/1396111213572
- CBP 2007. Guidelines for Liquidated Damages and Penalties on Wood Packaging Material (WPM). US Customs and Border Protection, Washington, DC. http://www.cbp.gov/sites/default/files/documents/guidelines_wpm_3.pdf
- Cessna, J. F. , and Nielsen C.. 2012. Influences of Hemlock Woolly Adelgid‐induced stand‐level mortality on nitrogen cycling and stream water nitrogen concentrations in southern Pennsylvania. Castanea 77:127–135. [Google Scholar]
- Christenson, L. M. , Lovett G. M., Mitchell M. J., and Groffman P. M.. 2002. The fate of nitrogen in gypsy moth frass deposited to an oak forest floor. Oecologia 131:444–452. [DOI] [PubMed] [Google Scholar]
- Cobb, R. C. , Filipe J. A. N., Meentemeyer R. K., Gilligan C. A., and Rizzo D. M.. 2012. Ecosystem transformation by emerging infectious disease: Loss of large tanoak from California forests. Journal of Ecology 100:712–722. [Google Scholar]
- Colautti, R. I. , Bailey S. A., van Overdijk C. D. A., Amundsen K., and MacIsaac H. J.. 2006. Characterised and projected costs of nonindigenous species in Canada. Biological Invasions 8:45–59. [Google Scholar]
- Colunga‐Garcia, M. , Haack R. A., Magarey R. D., and Borchert D. M.. 2013. Understanding trade pathways to target biosecurity surveillance. NeoBiota 18:103–118. [Google Scholar]
- Council on Climate Preparedness and Resilience . 2014. Priority agenda: enhancing the climate resilience of America's natural resources. Office of the President of the United States, Washington, D.C., USA. [Google Scholar]
- Dobbs, C. , Kendal D., and Nitschke C. R.. 2014. Multiple ecosystem services and disservices of the urban forest establishing their connections with landscape structure and sociodemographics. Ecological Indicators 43:44–55. [Google Scholar]
- Donovan, G. H. , Butry D. T., Michael Y. L., Prestemon J. P., Liebhold A. M., Gatziolis D., and Mao M. Y.. 2013. The relationship between trees and human health evidence from the spread of the Emerald Ash Borer. American Journal of Preventive Medicine 44:139–145. [DOI] [PubMed] [Google Scholar]
- Donovan, G. H. , Michael Y. L., Gatziolis D., Prestemon J. P., and Whitsel E. A.. 2015. Is tree loss associated with cardiovascular‐disease risk in the Women's Health Initiative? A natural experiment. Health and Place 36:1–7. [DOI] [PubMed] [Google Scholar]
- Elliott, K. J. , and Swank W. T.. 2008. Long‐term changes in forest composition and diversity following early logging (1919–1923) and the decline of American chestnut (Castanea dentata). Plant Ecology 197:155–172. [Google Scholar]
- Ellison, A. M. , et al. 2005. Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Frontiers in Ecology and the Environment 3:479–486. [Google Scholar]
- Environmental Law Institute . 2002. Halting the invasion: state tools for invasive species management. Environmental Law Institute, Washington, D.C., USA: https://www.eli.org/sites/default/files/eli-pubs/d12-06.pdf [Google Scholar]
- Epanchin‐Niell, R. S. , Haight R. G., Berec L., Kean J. M., and Liebhold A. M.. 2012. Optimal surveillance and eradication of invasive species in heterogeneous landscapes. Ecology Letters 15:803–812. [DOI] [PubMed] [Google Scholar]
- Eschtruth, A. K. , Cleavitt N. L., Battles J. J., Evans R. A., and Fahey T. J.. 2006. Vegetation dynamics in declining eastern hemlock stands: 9 years of forest response to hemlock woolly adelgid infestation. Canadian Journal of Forest Research 36:1435–1450. [Google Scholar]
- FAO . 2012. Integrated measures for plants for planting. International Standards for Phytosanitary Measures (ISPM) 36. International Plant Protection Convention, FAO, Rome, Italy. [Google Scholar]
- Flower, C. E. , Knight K. S., and Gonzalez‐Meler M. A.. 2013. Impacts of the emerald ash borer (Agrilus planipennis Fairmaire) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biological Invasions 15:931–944. [Google Scholar]
- Flower, C. E. , Long L. C., Knight K. S., Rebbeck J., Brown J. S., Gonzalez‐Meler M. A., and Whelan C. J.. 2014. Native bark‐foraging birds preferentially forage in infected ash (Fraxinus spp.) and prove effective predators of the invasive emerald ash borer (Agrilus planipennis Fairmaire). Forest Ecology and Management 313:300–306. [Google Scholar]
- Ford, C. R. , Elliott K. J., Clinton B. D., Kloeppel B. D., and Vose J. M.. 2012. Forest dynamics following eastern hemlock mortality in the southern Appalachians. Oikos 121:523–536. [Google Scholar]
- Forrester, J. A. , McGee G. G., and Mitchell M. J.. 2003. Effects of beech bark disease on aboveground biomass and species composition in a mature northern hardwood forest, 1985 to 2000. Journal of the Torrey Botanical Society 130:70–78. [Google Scholar]
- Foster, D. R. , and Orwig D. A.. 2006. Preemptive and salvage harvesting of New England forests: when doing nothing is a viable alternative. Conservation Biology 20:959–970. [DOI] [PubMed] [Google Scholar]
- Gandhi, K. J. K. , and Herms D. A.. 2010. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biological Invasions 12:389–405. [Google Scholar]
- Garnas, J. R. , Ayres M. P., Liebhold A. M., and Evans C.. 2011. Subcontinental impacts of an invasive tree disease on forest structure and dynamics. Journal of Ecology 99:532–541. [Google Scholar]
- General‐Accounting‐Office (GAO) . 2006. Invasive forest pests: lessons learned from three recent infestations may aid in managing future efforts. GAO‐06‐053, U.S. General Accounting Office, Washington, D.C., USA. [Google Scholar]
- van de Gevel, S. L. , Hart J. L., Spond M. D., White P. B., Sutton M. N., and Grissino‐Mayer H. D.. 2012. American chestnut (Castanea dentata) to northern red oak (Quercus rubra): forest dynamics of an old‐growth forest in the Blue Ridge Mountains, USA. Canadian Journal of Botany 90:1263–1276. [Google Scholar]
- Griffin, J. M. , Lovett G. M., Arthur M. A., and Weathers K. C.. 2003. The distribution and severity of beech bark disease in the Catskill Mountains, NY. Canadian Journal of Forest Research 33:1754–1760. [Google Scholar]
- Haack, R. A. 2006. Exotic bark‐ and wood‐boring Coleoptera in the United States: recent establishments and interceptions. Canadian Journal of Forest Research 36:269–288. [Google Scholar]
- Haack, R. A. , Herard F., Sun J. H., and Turgeon J. J.. 2010. Managing invasive populations of Asian Longhorned Beetle and Citrus Longhorned Beetle: a worldwide perspective. Annual Review of Entomology 55:521–546. [DOI] [PubMed] [Google Scholar]
- Haack, R. A. , et al. 2014. Effectiveness of the international phytosanitary standard ISPM no. 15 on reducing wood borer infestation rates in wood packaging material entering the United States. PLoS ONE 9:e96611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms, D. A. , and McCullough D. G.. 2014. Emerald Ash Borer invasion of North America: history, biology, ecology, impacts, and management. Annual Review of Entomology 59:13–30. [DOI] [PubMed] [Google Scholar]
- Hobbs, R. J. , and Humphries S. E.. 1995. An integrated approach to the ecology and management of plant invasions. Conservation Biology 9:761–770. [Google Scholar]
- Holmes, T. P. 1991. Price and welfare effects of catastrophic forest damage from southern pine beetle epidemics. Forest Science 37:500–516. [Google Scholar]
- Holmes, T. P. , Aukema J. E., Von Holle B., Liebhold A., and Sills E.. 2009. Economic impacts of invasive species in forests past, present, and future. Year in Ecology and Conservation Biology, Annals of the New York Academy of Sciences 1162:18–38. [DOI] [PubMed] [Google Scholar]
- Holmes, T. P. , Murphy E. A., Bell K. P., and Royle D. D.. 2010. Property value impacts of Hemlock Woolly Adelgid in residential forests. Forest Science 56:529–540. [Google Scholar]
- Holmes, T. P. , Aukema J., Englin J., Haight R. G., Kovacs K., and Leung B.. 2014. Economic analysis of biological invasions in forests pp. 369–386 in Kant S., and Alavalapati J. R. R., editors. Handbook of forest resource economics. Routledge, New York, New York, USA. [Google Scholar]
- Houston, D. R. 1994. Major new tree disease epidemics: beech bark disease. Annual Review of Phytopathology 32:75–87. [Google Scholar]
- IUFRO. 2011. The Montesclaros Declaration. International Union of Forest Research Organizations, Vienna, Austria: http://www.iufro.org/science/divisions/division-7/70000/publications/montesclaros-declaration/ [Google Scholar]
- Jenkins, J. C. , Aber J. D., and Canham C. D.. 1999. Hemlock woolly adelgid impacts on community structure and N cycling rates in eastern hemlock forests. Canadian Journal of Forest Research 29:630–645. [Google Scholar]
- Keane, R. E. , et al. 2012. A range‐wide restoration strategy for whitebark pine (Pinus albicaulis). Gen. Tech. Rep. RMRS‐GTR‐279, USDA Forest Service, Fort Collins, Colorado, USA. [Google Scholar]
- Keller, R. P. , and Perrings C.. 2011. International policy options for reducing the environmental impacts of invasive species. BioScience 61:1005–1012. [Google Scholar]
- Keller, R. P. , and Springborn M. R.. 2014. Closing the screen door to new invasions. Conservation Letters 7:285–292. [Google Scholar]
- Kenis, M. , Rabitsch W., Auger‐Rozenberg M. A., and Roques A.. 2007. How can alien species inventories and interception data help us prevent insect invasions? Bulletin of Entomological Research 97:489–502. [DOI] [PubMed] [Google Scholar]
- Koenig, W. D. , Liebhold A. M., Bonter D. N., Hochachka W. M., and Dickinson J. L.. 2013. Effects of the emerald ash borer invasion on four species of birds. Biological Invasions 15:2095–2103. [Google Scholar]
- Kovacs, K. F. , Haight R. G., McCullough D. G., Mercader R. J., Siegert N. W., and Liebhold A. M.. 2010. Cost of potential emerald ash borer damage in US communities, 2009–2019. Ecological Economics 69:569–578. [Google Scholar]
- Kovacs, K. F. , Mercader R. J., Haight R. G., Siegert N. W., McCullough D. G., and Liebhold A. M.. 2011a. The influence of satellite populations of emerald ash borer on projected economic costs in US communities, 2010–2020. Journal of Environmental Management 92:2170–2181. [DOI] [PubMed] [Google Scholar]
- Kovacs, K. , Vaclavik T., Haight R. G., Pang A., Cunniffe N. J., Gilligan C. A., and Meentemeyer R. K.. 2011b. Predicting the economic costs and property value losses attributed to sudden oak death damage in California (2010–2020). Journal of Environmental Management 92:1292–1302. [DOI] [PubMed] [Google Scholar]
- Kramer, R. A. , Holmes T. P., and Haefele M.. 2003. Contingent valuation of forest ecosystem protection Pages 303–320 in Sills E. O., and Abt K. T., editors. Forests in a market economy. Kluwer, Dordrecht, the Netherlands. [Google Scholar]
- Krist, F. J. , Ellenwood J. R., Woods M. E., McMahan A. J., Cowardin J. P., Ryerson D. E., Sapio F. J., Zweifler M. O., and Romero S. A.. 2014. 2013–2027 National Insect and Disease Forest Risk Assessment. FHTET 14‐01, USDA Forest Service Forest Health Technology Enterprise Team, Fort Collins, Colorado, USA. [Google Scholar]
- Leung, B. , Springborn M. R., Turner J. A., and Brockerhoff E. G.. 2014. Pathway‐level risk analysis: the net present value of an invasive species policy in the US. Frontiers in Ecology and the Environment 12:273–279. [Google Scholar]
- Levine, J. M. , and D'Antonio C. M.. 2003. Forecasting biological invasions with increasing international trade. Conservation Biology 17:322–326. [Google Scholar]
- Liebhold, A. M. , Macdonald W. L., Bergdahl D., and Maestro V. C.. 1995. Invasion by exotic forest pests: a threat to forest ecosystems. Forest Science 41:1–49. [Google Scholar]
- Liebhold, A. M. , Work T. T., McCullough D. G., and Cavey J. F.. 2006. Airline baggage as a pathway for alien insect species invading the United States. American Entomologist 52:48–54. [Google Scholar]
- Liebhold, A. M. , Brockerhoff E. G., Garrett L. J., Parke J. L., and Britton K. O.. 2012. Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Frontiers in Ecology and the Environment 10:135–143. [Google Scholar]
- Liebhold, A. M. , McCullough D. G., Blackburn L. M., Frankel S. J., Von Holle B., and Aukema J. E.. 2013. A highly aggregated geographical distribution of forest pest invasions in the USA. Diversity and Distributions 19:1208–1216. [Google Scholar]
- Liebhold, A. M. , et al. 2016. Eradication of invading insect populations: from concepts to applications. Annual Review of Entomology 61:335–352. [DOI] [PubMed] [Google Scholar]
- Lodge, D. M. , et al. 2006. Biological invasions: recommendations for US policy and management. Ecological Applications 16:2035–2054. [DOI] [PubMed] [Google Scholar]
- Lovett, G. M. , Christenson L. M., Groffman P. M., Jones C. G., Hart J. E., and Mitchell M. J.. 2002. Insect defoliation and nitrogen cycling in forests. BioScience 52:335–341. [Google Scholar]
- Lovett, G. M. , Canham C. D., Arthur M. A., Weathers K. C., and Fitzhugh R. D.. 2006. Forest ecosystem responses to exotic pests and pathogens in eastern North America. BioScience 56:395–405. [Google Scholar]
- Lovett, G. M. , Arthur M. A., Weathers K. C., and Griffin J. M.. 2010. Long‐term changes in forest carbon and nitrogen cycling caused by an introduced pest/pathogen complex. Ecosystems 13:1188–1200. [Google Scholar]
- Lovett, G. M. , Arthur M. A., Weathers K. C., and Griffin J. M.. 2013. Effects of introduced insects and diseases on forest ecosystems in the Catskill Mountains of New York. Annals of the New York Academy of Sciences 1298:66–77. [DOI] [PubMed] [Google Scholar]
- MacLeod, A. , Pautasso M., Jeger M. J., and Haines‐Young R.. 2010. Evolution of the international regulation of plant pests and challenges for future plant health. Food Security 2:49–70. [Google Scholar]
- May, C. 1934. Outbreaks of the Dutch elm disease in the United States. Circular 322, US Department of Agriculture, Washington, D.C., USA. [Google Scholar]
- McCullough, D. G. , Work T. T., Cavey J. F., Liebhold A. M., and Marshall D.. 2006. Interceptions of nonindigenous plant pests at US ports of entry and border crossings over a 17‐year period. Biological Invasions 8:611–630. [Google Scholar]
- McKinney, S. T. , Fiedler C. E., and Tomback D. F.. 2009. Invasive pathogen threatens bird‐pine mutualism: implications for sustaining a high‐elevation ecosystem. Ecological Applications 19:597–607. [DOI] [PubMed] [Google Scholar]
- Metz, M. R. , Frangioso K. M., Wickland A. C., Meentemeyer R. K., and Rizzo D. M.. 2012. An emergent disease causes directional changes in forest species composition in coastal California. Ecosphere 3 (10):86 10.1890/ES12-00107.1 [DOI] [Google Scholar]
- Monahan, W. B. , and Koenig W. D.. 2006. Estimating the potential effects of sudden oak death on oak‐dependent birds. Biological Conservation 127:146–157. [Google Scholar]
- Moore, C. C. , Holmes T. P., and Bell K. P.. 2011. An attribute‐based approach to contingent valuation of forest protection programs. Journal of Forest Economics 17:35–52. [Google Scholar]
- Morin, R. S. , and Liebhold A. M.. 2015. Invasions by two non‐native insects alter regional forest species composition and successional trajectories. Forest Ecology and Management 341:67–74. [Google Scholar]
- Moser, W. K. , et al. 2009. Impacts of nonnative invasive species on US forests and recommendations for policy and management. Journal of Forestry 107:320–327. [Google Scholar]
- Myers, J. H. , Simberloff D., Kuris A. M., and Carey J. R.. 2000. Eradication revisited: dealing with exotic species. Trends in Ecology & Evolution 15:316–320. [DOI] [PubMed] [Google Scholar]
- Myers, S. W. , Fraser I., and Mastro V. C.. 2009. Evaluation of heat treatment schedules for Emerald Ash Borer (Coleoptera: Buprestidae). Journal of Economic Entomology 102:2048–2055. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences (NAS) . 2002. Predicting invasions of nonindigenous plants and plant pests. National Academy Press, Washington, D.C., USA. [PubMed] [Google Scholar]
- Nowak, D. J. , Pasek J. E., Sequeira R. A., Crane D. E., and Mastro V. C.. 2001. Potential effect of Anoplophora glabripennis (Coleoptera: Cerambycidae) on urban trees in the United States. Journal of Economic Entomology 94:116–122. [DOI] [PubMed] [Google Scholar]
- Nowak, D. J. , Hirabayashi S., Bodine A., and Greenfield E.. 2014. Tree and forest effects on air quality and human health in the United States. Environmental Pollution 193:119–129. [DOI] [PubMed] [Google Scholar]
- Nuckolls, A. E. , Wurzburger N., Ford C. R., Hendrick R. L., Vose J. M., and Kloeppel B. D.. 2009. Hemlock declines rapidly with Hemlock Woolly Adelgid infestation: impacts on the carbon cycle of southern Appalachian forests. Ecosystems 12:179–190. [Google Scholar]
- Orwig, D. A. , Foster D. R., and Mausel D. L.. 2002. Landscape patterns of hemlock decline in New England due to the introduced hemlock woolly adelgid. Journal of Biogeography 29:1475–1487. [Google Scholar]
- Orwig, D. A. , Plotkin A. A. B., Davidson E. A., Lux H., Savage K. E., and Ellison A. M.. 2013. Foundation species loss affects vegetation structure more than ecosystem function in a northeastern USA forest. PeerJ 1:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrings, C. , Williamson M., Barbier E. B., Delfino D., Dalmazzone S., Shogren J., Simmons P., and Watkinson A.. 2002. Biological invasion risks and the public good: an economic perspective. Conservation Ecology 6:1. [Google Scholar]
- Pimentel, D. , Lach L., Zuniga R., and Morrison D.. 2000. Environmental and economic costs of nonindigenous species in the United States. BioScience 50:53–65. [Google Scholar]
- Poland, T. M. , and McCullough D. G.. 2006. Emerald ash borer: invasion of the urban forest and the threat to North America's ash resource. Journal of Forestry 104:118–124. [Google Scholar]
- Reaser, J. K. , and Waugh J.. 2007. Denying entry: opportunities to build capacity to prevent the introduction of invasive species and improve biosecurity at US ports. IUCN, Gland, Switzerland. [Google Scholar]
- Ricciardi, A. , Steiner W. W. M., Mack R. N., and Simberloff D.. 2000. Toward a global information system for invasive species. BioScience 50:239–244. [Google Scholar]
- Rieske, L. K. 2007. Success of an exotic gallmaker, Dryocosmus kuriphilus, on chestnut in the USA: a historical account. EPPO Bulletin 37:172–174. [Google Scholar]
- Rodgers, L. , Derksen A., and Pernas T.. 2014. Expansion and impact of laurel wilt in the Florida Everglades. Florida Entomologist 97:1247–1250. [Google Scholar]
- Roques, A. , Rabitsch W., Rasplus J.‐Y., Lopez‐Vaamonde C., Nentwig W., and Kenis M.. 2009. Alien terrestrial invertebrates of Europe Pages 63–79 in Nentwig W., Hulme P., Pysek P., and Vila M., editors. Handbook of alien species in Europe. Springer, Dordrecht, the Netherlands. [Google Scholar]
- Rosenberger, R. S. , Needham M. D., Morzillo A. T., and Moehrke C.. 2012. Attitudes, willingness to pay, and stated values for recreation use fees at an urban proximate forest. Journal of Forest Economics 18:271–281. [Google Scholar]
- Ross, R. M. , Bennett R. M., Snyder C. D., Young J. A., Smith D. R., and Lemarie D. P.. 2003. Influence of eastern hemlock (Tsuga canadensis L.) on fish community structure and function in headwater streams of the Delaware River basin. Ecology of Freshwater Fish 12:60–65. [Google Scholar]
- Roy, B. A. , Alexander H. M., Davidson J., Campbell F. T., Burdon J. J., Sniezko R., and Brasier C.. 2014. Increasing forest loss worldwide from invasive pests requires new trade regulations. Frontiers in Ecology and the Environment 12:457–465. [Google Scholar]
- Siderhurst, L. A. , Griscom H. P., Hudy M., and Bortolot Z. J.. 2010. Changes in light levels and stream temperatures with loss of eastern hemlock (Tsuga canadensis) at a southern Appalachian stream: implications for brook trout. Forest Ecology and Management 260:1677–1688. [Google Scholar]
- Simberloff, D. , Parker I. M., and Windle P. N.. 2005. Introduced species policy, management, and future research needs. Frontiers in Ecology and the Environment 3:12–20. [Google Scholar]
- Smith, R. M. , Baker R. H. A., Malumphy C. P., Hockland S., Hammon R. P., Ostoja‐Starzewski J. C., and Collins D. W.. 2007. Recent non‐native invertebrate plant pest establishments in Great Britain: origins, pathways, and trends. Agricultural and Forest Entomology 9:307–326. [Google Scholar]
- Snyder, C. D. , Young J. A., Lemarie D. P., and Smith D. R.. 2002. Influence of eastern hemlock (Tsuga canadensis) forests on aquatic invertebrate assemblages in headwater streams. Canadian Journal of Fisheries and Aquatic Sciences 59:262–275. [Google Scholar]
- Stadler, B. , Muller T., and Orwig D.. 2006. The ecology of energy and nutrient fluxes in hemlock forests invaded by hemlock woolly adelgid. Ecology 87:1792–1804. [DOI] [PubMed] [Google Scholar]
- Tingley, M. W. , Orwig D. A., and Field R.. 2002. Avian response to removal of a forest dominant: consequences of hemlock woolly adelgid infestations. Journal of Biogeography 29:1505–1516. [Google Scholar]
- USDA‐APHIS . 2011. New pest response guidelines: exotic wood‐boring and bark beetles. USDA‐APHIS‐PPQ–EDP‐Emergency Management, Riverdale, Maryland, USA. [Google Scholar]
- Weed, A. S. , Ayres M. P., and Hicke J. A.. 2013. Consequences of climate change for biotic disturbances in North American forests. Ecological Monographs 83:441–470. [Google Scholar]
- Willacker, J. J. , Sobczak W. V., and Colburn E. A.. 2009. Stream macroinvertebrate communities in paired hemlock and deciduous watersheds. Northeastern Naturalist 16:101–112. [Google Scholar]
- Zahid, M. I. , Grgurinovic C. A., and Walsh D. J.. 2008. Quarantine risks associated with solid wood packaging materials receiving ISPM 15 treatments. Australian Forestry 71:287–293. [Google Scholar]


