Abstract
Aims
Ablation of persistent atrial fibrillation (PsAF) has been performed by many techniques with varying success rates. This may be due to ablation techniques, patient demographics, comorbidities, and trial design. We conducted a meta-regression of studies of PsAF ablation to elucidate the factors affecting atrial fibrillation (AF) recurrence.
Methods and results
Databases were searched for prospective studies of PsAF ablation. A meta-regression was performed. Fifty-eight studies (6767 patients) were included. Complex fractionated atrial electrogram (CFAE) ablation reduced freedom from AF by 8.9% [95% confidence interval (CI) −15 to −2.3, P = 0.009). Left atrial appendage [LAA isolation (three study arms)] increased freedom from AF by 39.5% (95% CI 9.1–78.4, P = 0.008). Posterior wall isolation (PWI) (eight study arms) increased freedom from AF by 19.4% (95% CI 3.3–38.1, P = 0.017). Linear ablation or ganglionated plexi ablation resulted in no significant effect on freedom from AF. More extensive ablation increased intraprocedural AF termination; however, intraprocedural AF termination was not associated with improved outcomes. Increased left atrial diameter was associated with a reduction in freedom from AF by 4% (95% CI −6.8% to −1.1%, P = 0.007) for every 1 mm increase in diameter.
Conclusion
Linear ablation, PWI, and CFAE ablation improves intraprocedural AF termination, but such termination does not predict better long-term outcomes. Study arms including PWI or LAA isolation in the lesion set were associated with improved outcomes in terms of freedom from AF; however, further randomized trials are required before these can be routinely recommended. Left atrial size is the most important marker of AF chronicity influencing outcomes.
Keywords: Persistent atrial fibrillation, Ablation, Lesion set, Outcome, Meta-analysis
What’s new?
Posterior wall isolation (PWI) and left atrial appendage isolation may represent promising lesion sets for persistent AF ablation.
Linear ablation, PWI, and complex fractionated atrial electrogram ablation improves intraprocedural atrial fibrillation (AF) termination, but such termination does not predict better long-term outcomes.
Complex fractionated atrial electrogram ablation is associated with reduced freedom from atrial arrhythmia.
Left atrial size is the most important marker of AF chronicity influencing outcomes.
Introduction
Catheter ablation for atrial fibrillation (AF) is now a well-established therapy recommended in international guidelines. Freedom from AF 1 year after pulmonary vein isolation (PVI) for paroxysmal AF (PAF) ranges between 57.3% and 86% (pooled estimate 78%)1; however, success rates for persistent AF (PsAF) are generally lower.2 Pulmonary vein isolation has long been the cornerstone of AF ablation, however, particularly in the context of PsAF, a wide range of adjunctive ablation strategies have been used, in an attempt to achieve ‘substrate’ modification, with varying success rates.3
The range of success rates may be due to technical differences in the ablation process; however, numerous additional factors including patient demographics, comorbidities, and trial design may have a significant impact.
In this analysis, we aim to quantify the many factors that may affect trial endpoints, by taking all trials studying ablation of PsAF and studying how outcomes associate with the characteristics of the individual study arms via meta-regression analysis. We analyse whether the following factors might impact success rates:
Procedural differences, such as ablation of complex fractionated atrial electrograms (CFAEs), linear, or ganglionated plexi ablation.
Patient demographics and comorbidities.
Markers of AF chronicity [e.g. left atrial (LA) diameter].
Study design characteristics [e.g. blanking period, inclusion of atrial tachycardia (AT) in primary endpoint, and follow-up strategy].
Methods
Search strategy
Two reviewers (A.S. and S.A.) searched the MEDLINE and Cochrane Central Register of Controlled Trials for trials of PAF and PsAF ablation. The searches were conducted between 5 April 2017 and 19 April 2017. Our search string included (persistent or non-paroxysmal or non paroxysmal) AND atrial fibrillation AND ablation AND the Cochrane high sensitive search strategy for MEDLINE.4 Abstracts and relevant full texts were independently screened by the reviewers; disputes were resolved by consensus following discussion with another author (M.S.).
Inclusion and exclusion criteria
Study inclusion criteria were (i) randomized and prospective non-randomized trials published in English, (ii) patient population with PsAF or long-standing persistent atrial fibrillation (LSPAF), (iii) at least one intervention arm including some form of endocardial LA ablation, (iv) minimum follow-up period of 3 months, and (v) outcome measure of freedom from AF or freedom from AF/atrial arrhythmia after a single procedure. Studies of patient cohorts or subsets not representative of the general population with AF (e.g. only patients with heart failure, diabetes, or obesity) were excluded.
Data extraction
Two authors (A.S. and S.A.) extracted the data from the included trials. Where possible, continuous variables were extracted as mean ± standard deviation, and categorical variables were taken as percentages.
Outcomes
The primary outcome was percentage of patients free from AF/AT after a single procedure. In most cases, the 12-month time point was chosen. Where not available the nearest available time point was selected. The 12-month time point was selected as most of the studies reported this value and was felt to be clinically relevant as most recurrences would be expected to occur by this point. Most studies included recurrences of AT in their primary endpoint, where the primary endpoint included freedom from AF only, this endpoint was used.
Data extraction and analysis
Percentage freedom from recurrence (of AF or AF/AT depending on the primary endpoint of the study) was used as the dependent variable, and a meta-regression performed using the restricted maximum likelihood estimator, with study-level heterogeneity factored using a random-effects model. The statistical programming environment R with the metafor package was used for all statistical analysis. The PRISMA guideline was used to report results.
Results
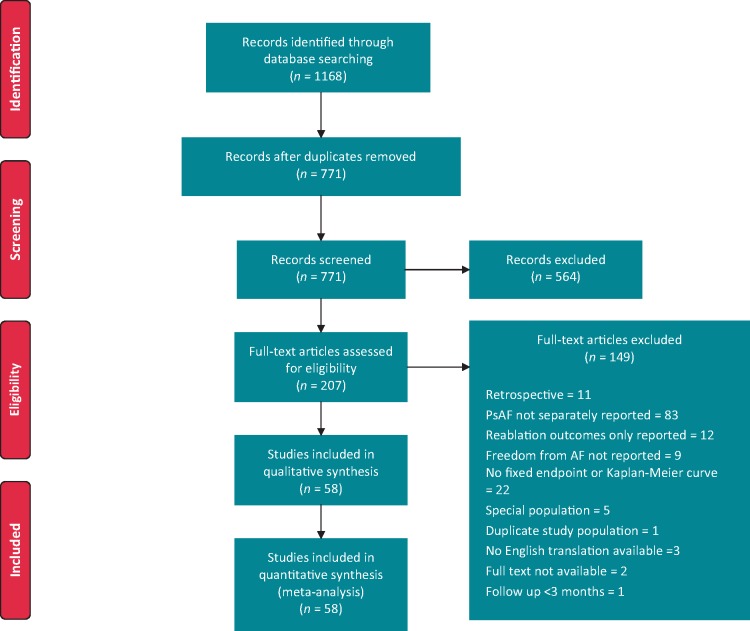
Searches yielded a total of 1168 studies (647 MEDLINE and 521 Cochrane). Screening of studies resulted in 58 studies suitable for analysis (as shown in Figure 1). In total, 106 study arms, 6767 patients were studied with a mean follow-up duration of 16.3 months and a total follow-up duration of 9212 patient-years. About 35 studies were randomized controlled trials (RCTs), and 23 were prospective observational studies.
Figure 1.
Sources of identified studies. AF, atrial fibrillation; PsAF, persistent atrial fibrillation.
For the reader unfamiliar with meta-regression, interpretation of the analyses requires some explanation. Each of Figures 2–8 represents a separate meta-regression analysis in which the outcome (mostly AF-free survival) is explained by a variety of variables used to build a statistical model. The effect of each of these variables is then separated from each of the others in the model. Therefore, where we state that CFAE reduces freedom from AF, this means that within the statistical model, performing CFAE can explain an 8.9% reduction in AF-free survival in those study arms in which it was performed. There is no ‘control’ group since all the data are placed into a single model but the comparison is effectively between ‘CFAE’ vs. ‘no CFAE’ regardless of what other lesion sets are performed.
Figure 2.
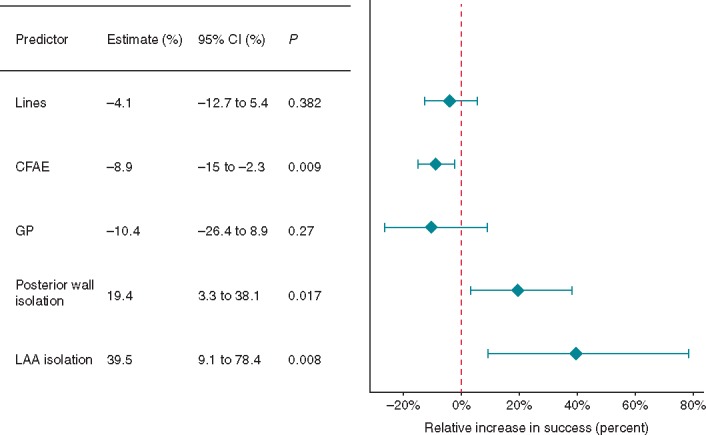
The effect of procedural predictors on freedom from AF. AF, atrial fibrillation; CFAE, complex fractionated atrial electrogram; CI, confidence interval; GP, ganglionated plexi; LAA, left atrial appendage.
Effect of lesion set on freedom from atrial fibrillation
Complex fractionated atrial electrogram ablation (38 study arms) was associated with a worse outcome, reducing freedom from AF by 8.9% (Figure 2, 95% CI −15 to −2.3, P = 0.009). Linear ablation (36 study arms), ganglionated plexi (GP) ablation (three study arms) had no significant impact on freedom from AF. Posterior wall isolation (PWI; eight study arms) increased freedom from AF by 19.4% (95% CI 3.3–38.1, P = 0.007). Left atrial appendage (LAA) isolation (three study arms) greatly increased freedom from AF by 39.5% (95% CI 9.1–78.4, P = 0.008). This result was mostly driven by the BELIEF trial.5 Cryoballoon ablation [as compared to radiofrequency (RF)] did not affect freedom from AF (Supplementary material online, Appendix Figure S3). We performed an additional analysis excluding studies where the primary endpoint was freedom from AF only (i.e. not including freedom from AT, Supplementary material online, Appendix Figure S4). This showed similar results to the primary analysis.
More extensive ablation increases intraprocedural atrial fibrillation termination, increases procedure time, but does not increase freedom from atrial fibrillation
Pulmonary vein isolation has long been the cornerstone of AF ablation. However, given the relatively poorer outcomes in PsAF, more extensive ablation strategies have been developed sometimes with the goal of terminating AF intraprocedurally. We analysed linear ablation, CFAE ablation, GP ablation, and PWI for effect on intraprocedural AF termination. This outcome was reported in 26 studies. Unfortunately, none of the studies including LAA isolation (three study arms) reported intraprocedural AF termination. Linear ablation improved intraprocedural AF termination (23.8%, 95% CI 6.7–43.9%, P = 0.005, Figure 3), as did CFAE ablation (43.6%, 95% CI 19.2–73.1%, P < 0.001), and PWI (95% CI 5–77.1%, P = 0.02). Ganglionated plexi ablation had no significant impact on intraprocedural AF termination.
Figure 3.
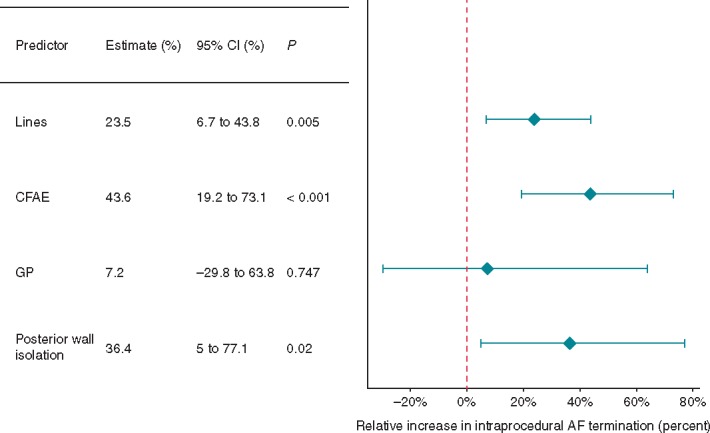
The effect of procedural predictors on intraprocedural AF termination rate. AF, atrial fibrillation; CFAE, complex fractionated atrial electrogram; CI, confidence interval; GP, ganglionated plexi.
Importantly, despite increasing intraprocedural AF termination, neither linear nor CFAE ablation increased freedom from AF (as described above). Similarly, intraprocedural AF termination (achieved by any ablation technique) had no significant impact on freedom from AF (Figure 4). None of the other studied procedural predictors [concomitant anti-arrhythmic drug (AAD) use, general anaesthetic (GA), electroanatomical (EA) mapping] had a significant impact on freedom from AF (Figure 4).
Figure 4.
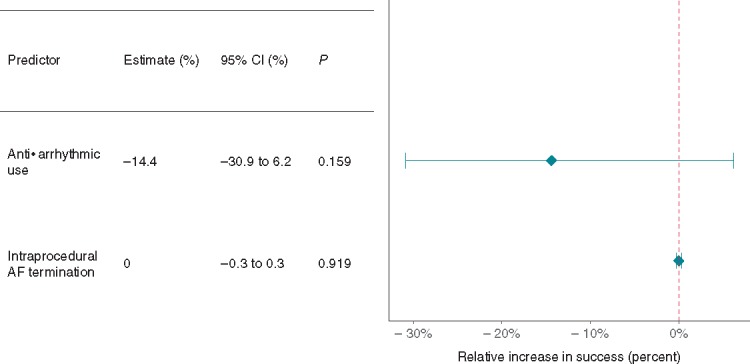
The effect of other procedural predictors on freedom from AF. AF, atrial fibrillation; CI, confidence interval.
As expected, more extensive ablation was associated with increased procedure (Figure 5) and fluoroscopy (Supplementary material online, Appendix Figure S1) times. Despite its lack of efficacy, CFAE imposed a particularly large increase in procedure time (by 61.3 min, 95% CI 58.2–64.5, P < 0.001, Figure 5).
Figure 5.
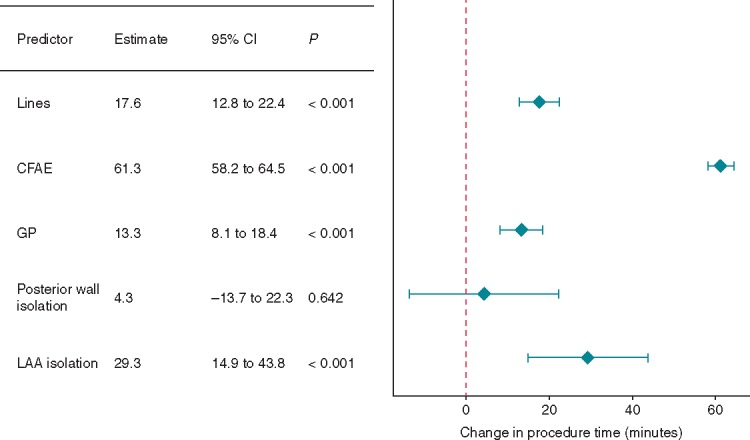
The effect of procedural predictors on procedure time. CFAE, complex fractionated atrial electrogram; CI, confidence interval; GP, ganglionated plexi; LAA, left atrial appendage.
Patient characteristics
We analysed the effect of several patient factors on outcomes following PsAF ablation. Age, gender, left ventricular ejection fraction, and hypertension had no significant impact on outcomes (Figure 6).
Figure 6.
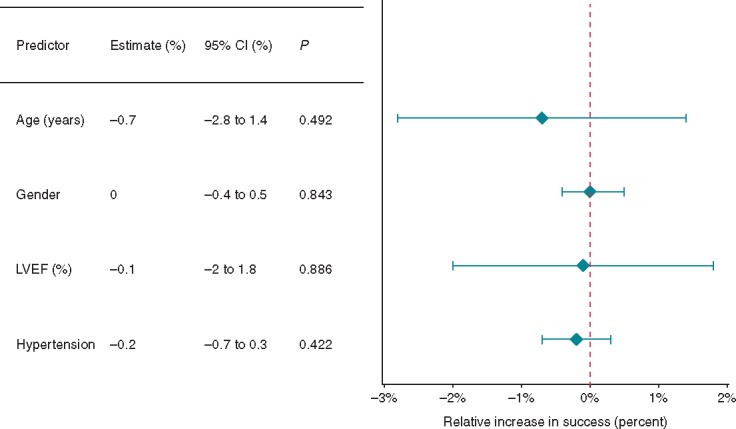
The effect of patient characteristics on freedom from AF. AF, atrial fibrillation; CI, confidence interval; LVEF, left ventricular ejection fraction.
Greater left atrial diameter reduces freedom from atrial fibrillation
We analysed three markers of AF chronicity. Increased LA diameter was the only one associated with a reduction in freedom from AF, by 4% for every 1 mm increase of LA diameter (Figure7, 95% CI −6.8% to −1.1%, P = 0.007). In univariate meta-regression, increased LA diameter of 1 mm was associated with a reduction in freedom from AF by 3.1% (95% CI −5.4 to −0.6, P = 0.014). Study arms which included patients who underwent direct current cardioversion (DCCV) within 7 days of AF onset (early DCCV) showed a trend to increased freedom from AF (11.8%, 95% CI −3.8% to 29.8%, P = 0.145) but did not reach statistical significance. Duration of AF (persistent vs. long-standing persistent) did not affect freedom from AF.
Figure 7.
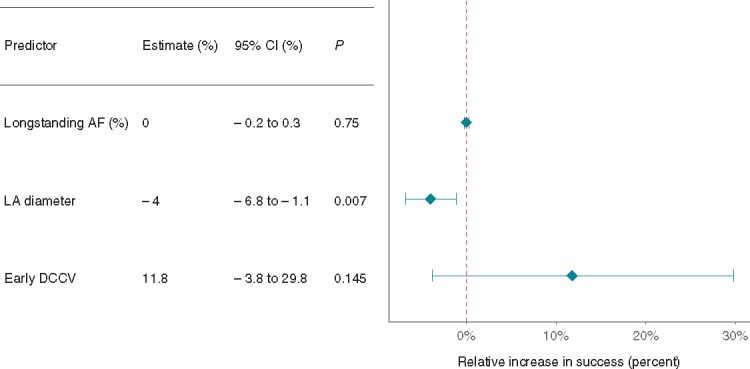
The effect of AF chronicity on freedom from AF. Early DCCV: studies where the definition of PsAF included patients who underwent DCCV within 7 days of AF onset. AF, atrial fibrillation; CI, confidence interval; DCCV, direct current cardioversion; LA, left atrium; PsAF, persistent atrial fibrillation.
Study protocol impacts outcomes
Inclusion of AT in main study endpoint (77 study arms) reduced the freedom from AF by 6.3% (Figure8, 95% CI −10.2 to −2.2, P = 0.003). Study arms with longer blanking periods were associated with a reduced freedom from AF by 6.4% per month blanked (95% CI −8.6 to −4.2, P < 0.001). Use of more stringent monitoring methods [telemetry or internal loop recorder (ILR)] trended towards poorer outcomes but did not reach statistical significance (2.2%, 95% CI −6.9% to 2.7%, P = 0.368). Defining success as being off AAD had no significant impact on freedom from AF.
Figure 8.
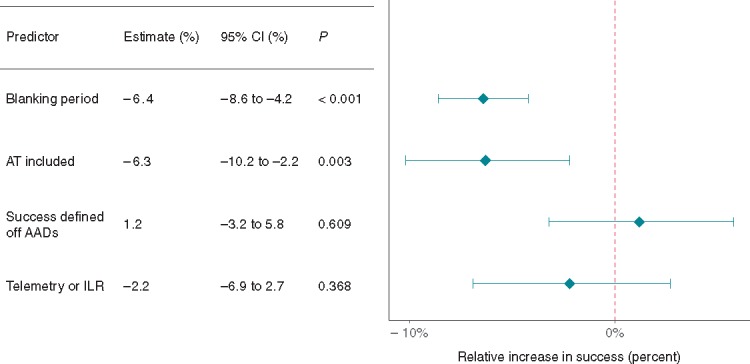
The effect of study characteristics on freedom from AF. AAD, anti-arrhythmic drug; AF, atrial fibrillation; AT, atrial tachycardia; CI, confidence interval; ILR, internal loop recorder.
Discussion
This meta-regression, including data on 6767 patients, has demonstrated several key variables can affect freedom from atrial arrhythmias after catheter ablation of PsAF. Pulmonary vein isolation has long been the cornerstone of AF ablation. Given relatively poor outcomes in PsAF ablation, more extensive ablation has been trialled. Multiple different strategies have been used, both in isolation and combined.
Complex fractionated atrial electrogram ablation reduces freedom from atrial fibrillation
Multiple small studies, and meta-analyses of these studies,6 concluded that CFAE ablation in addition to PVI improved freedom from atrial arrhythmia. New data from STAR AF 27 drastically changed this landscape, with meta-analysis following this showing no significant difference in outcomes, but increased procedure and fluoroscopy times.8
Our analysis adds several additional trials that included CFAE ablation in at least one arm. Together these trials show that study arms which include CFAE have worse outcomes in terms of AF-free survival (Figure 2). This mirrors the data from STAR AF 2 which showed a trend towards reduced freedom from AF in the CFAE group, although this was not statistically significant.
Several previous meta-analyses have shown PVI ablation alone is non-inferior to PVI + CFAE ablation,8–11 with at least two meta-analyses showing non-significant trends towards reduced success rates in the PVI + CFAE group.8,9 A third showed a statistically significant reduction in freedom from AF when PVI + CFAE + linear ablation was performed.10 This study had different inclusion criteria to ours. Most importantly they only included studies with greater than 60 patients in each arm, with at least 12 months of follow-up. They did not exclude retrospective studies. As a result of this their analysis used four RCTs and 109 observational studies, as compared to 35 RCTs and 23 observational studies in this analysis.
Importantly, in our analysis, the vast majority of trials with a CFAE arm included ATs in their primary endpoint (23 out of 27 trials). Complex fractionated atrial electrogram ablation may lead to iatrogenic proarrhythmic areas, particularly if linear block is not achieved,12 increased risk of recurrence in the form of AT may have contributed to reduced success rates in cohorts with CFAE ablation.
Posterior wall isolation
The LA posterior wall has been described as a driver of AF.13 Our study found a 19.4% benefit in study arms including PWI as part of the lesion set (Figure 2). While eight study arms formed this analysis, only three of these were randomized trials investigating the additive effect of PWI. Of these, two studies14,15 showed significant improvements in freedom from AF. The third study16 showed no significant difference with the endpoint of freedom from AF/AT. Lim et al. also report no significant difference when freedom from AF/AT is used as the endpoint. Kim et al. reports only the freedom from AF endpoint. It is possible therefore, that while PWI may reduce recurrences due to AF, AT recurrences remain a problem. This may limit single procedure efficacy. Further studies are needed before PWI can be routinely recommended in PsAF ablation.
Left atrial appendage isolation
The importance of the LAA in AF has been mostly considered in relation to the risk of thrombus formation and stroke. However, the LAA has also been described as a source for triggers of AF in as many as 27% of patients having undergone PVI.17 Left atrial appendage isolation has also been investigated in the context of concomitant LAA closure.18
The BELIEF trial compared extensive ablation with or without LAA isolation in patients with LSPAF, they found a significant additive benefit of LAA isolation.5 Although CFAE ablation was not performed in this study, the ablation of ‘non-PV triggers’ made for an aggressive lesion set.
Our study found a 39.5% benefit in study arms including LAA isolation as part of the lesion set (Figure 2). A degree of scepticism regarding this result is necessary however, given that only three study arms included LAA isolation. The effect size found in our analysis is also unexpectedly large. The BELIEF study was the main driver of this result (n = 173), however, Panikker et al.18 also showed a benefit of electrical LAA isolation with ablation (in addition to LAA occlusion) in 22 patients with LSPAF. The third study was a feasibility study of LAA ligation with no control arm.19 Similar to PWI, LAA isolation may be a beneficial technique in PsAF ablation, but further, larger, randomized trials are required.
Other additive ablation techniques
Linear ablation and GP ablation have also been shown in our analysis to have no impact on AF recurrence (Figure 2), although they do clearly prolong procedure time (Figure 5). The lack of efficacy seen may mean these other targets are not important in the pathogenesis of AF, or that there is a degree of redundancy—e.g. the close association of GPs with the PVs may mean the important GPs are modified by the PVI lesion set; alternatively, as discussed in the case of CFAE ablation, this extra ablation may increase the risk of AT based recurrence. This is particularly a problem with incomplete linear ablation or successful linear ablation with subsequent breakthrough of conduction.
Acute atrial fibrillation termination is not associated with increased freedom from atrial arrhythmia
Acute AF termination has been associated with improved long-term freedom from atrial arrhythmia in several observational studies.20 Aiming for acute AF termination leads to more extensive ablation, often including CFAE.8 In our study, more extensive ablation (in the form of linear ablation, CFAE ablation, or PWI) was associated with increased acute AF termination (Figure 3). However, acute AF termination did not improve long-term freedom from atrial arrhythmia (Figure 4). This could be a limitation of the meta-regression technique since it cannot drill down to the individual patient level and assess whether an individual who has had termination intraprocedurally is less likely to have recurrence. On the other hand, the data were reported in a reasonable portion of study arms (44 study arms), with a wide range of rates reported (from 0% to 100% termination) and so this is very suggestive that aggressive ablation targeting an end-goal of AF termination is not beneficial to a group of patients undergoing ablation. In fact, at the individual level, termination of AF intraprocedurally may simply be indicative that the patient has a less severe AF substrate, and perhaps this confounding contributed to the positive findings in prior studies.20 Alternatively, the benefits of the additional RF delivery needed to affect termination may be offset by the potential pro-arrhythmia of those additional lesions.
Impact of patient characteristics
Increased LA size has been associated with poorer outcomes post-ablation. Clarnette et al.10 showed a 1 mm increase in LA size was associated with a 1.6% decrease in single-procedure success. Our analysis agrees with this finding (Figure 7). This relationship, particularly in the context of a group of patients who already have relatively long-standing AF, shows that LA size may be an independent risk factor for recurrence post AF ablation. Conversely, distinction between persistent and long-standing persistent had no significant impact on predicting AF recurrence. This may mean clinicians should give greater importance to LA size, than duration of AF when considering the likelihood of long-term freedom from AF in a particular patient.
Impact of study factors
Given the significant heterogeneity in the conduct of the many trials and observational studies of AF ablation, it is not surprising that several study characteristics can affect the quoted recurrence rate of a trial. Most importantly, study arms including AT (as well as AF) in the primary endpoint had significantly reduced freedom from AF (by 6.3%) (Figure 8). As our analysis was performed on studies following patients after a single procedure, it is likely that a significant proportion of recurrences were due to an AT. Given that the aim of AF ablation is in most cases symptomatic relief, we feel that inclusion of AT in the endpoint is important, although separation of these modes of recurrence in the secondary endpoints may be important as many clinicians would prefer a patient to return in AT rather than AF.
Surprisingly, a longer blanking period, in our analysis, reduced freedom from AF (Figure 8). It is difficult to understand how including a blanking period could reduce the long-term freedom from AF. Given that the vast majority of studies of AF ablation included a 3-month blanking period, it is possible that there is an unknown confounder present in the few arms that were followed with a reduced blanking period (e.g. more straightforward study patients and hence the more stringent, short, and blanking period). Alternatively, this could be a false positive finding through chance (Type I) error.
More thorough means of detecting AF, such as mobile telemetry and ILR trended towards reduced freedom from AF (Figure 8). While this difference was not statistically significant, this is an important point as studies are often compared against one another, without fully appreciating that differences in methods of detecting recurrence can have a significant impact on quoted outcomes.
Limitations
This meta-regression uses multiple study arms to make observations on associations between variables and outcomes. These observations are therefore not based on randomized comparisons of these variables, and are therefore, vulnerable to the same biases as an observational study. We could only analyse those variables that were disclosed in each manuscript, several important variables were only reported by a few manuscripts; therefore, we could not make meaningful analyses of these variables. The meta-regression analysis only has access to study arm-level data (e.g. the proportion of males, average age and average EF in each study arm) rather than individual-level data. This may reduce the power to detect differences in outcomes, particularly when the variable in question relates to an individual patient (e.g. age, gender, or EF). This is less of an issue with other analyses (such as ablation technique) where there are larger differences between studies and where all individuals in a study arm are treated with the same techniques. Our search only included studies of endocardial (i.e. catheter ablation) of PsAF, therefore, we were unable to analyse or compare the effects of surgical ablation on freedom from PsAF.
Conclusion
The main findings of this meta-regression are that, in agreement with the STAR-AF II trial, linear ablation and CFAE in addition to PVI do not improve outcome in PsAF ablation. In fact, we have shown that CFAE ablation was associated with increased recurrence rates.
Acute AF termination is often the endpoint of extensive ablation techniques; however, higher termination rates did not translate to reduced long-term recurrence, perhaps suggesting that this welcome side effect in some AF ablations should not be viewed as a treatment goal.
Our analyses point towards two lesion sets that may be more promising. We would suggest PWI and LAA isolation (in addition to PVI) should be the subject of future randomized studies of the quality of STAR-AF II. In this study, both of these lesion sets were associated with a significant improvement in AF-free survival at 1 year—posterior wall isolation by 19.4% and LAA isolation by 40%.
Supplementary Material
Acknowledgements
We would like to acknowledge support from the National Institute of Health Research (#2670), Biomedical Research Centre, British Heart Foundation, Wellcome Trust and ElectroCardioMaths Programme of the Imperial Centre for Cardiac Engineering.
Conflict of interest: none declared.
References
- 1. Kis Z, Muka T, Franco OH, Bramer WM, De Vries LJ, Kardos A. et al. The short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Curr Cardiol Rev 2017;13:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nyong J, Amit G, Adler AJ, Owolabi OO, Perel P, Prieto-Merino D. et al. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Heart Group, editor. Cochrane Database Syst Rev 2016;20:CD012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qin M, Liu X, Wu S-H, Zhang X-D.. Atrial substrate modification in atrial fibrillation: targeting GP or CFAE? Evidence from meta-analysis of clinical trials. PLoS One 2016;11:e0164989.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Higgins JP, Green S. The Cochrane Highly Sensitive Search Strategies for identifying randomized trials in MEDLINE [Internet]. Cochrane Handbook for Systematic Reviews of Interventions. http://handbook-5-1.cochrane.org/chapter_6/6_4_11_1_the_cochrane_highly_sensitive_search_strategies_for.htm (1 April 2017, date last accessed).
- 5. Di Biase L, Burkhardt JD, Mohanty P, Mohanty S, Sanchez JE, Trivedi C. et al. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation. J Am Coll Cardiol 2016;68:1929–40. [DOI] [PubMed] [Google Scholar]
- 6. Hayward RM, Upadhyay GA, Mela T, Ellinor PT, Barrett CD, Heist EK. et al. Pulmonary vein isolation with complex fractionated atrial electrogram ablation for paroxysmal and nonparoxysmal atrial fibrillation: a meta-analysis. Heart Rhythm 2011;8:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verma A, Jiang C-Y, Betts TR, Chen J, Deisenhofer I, Mantovan R. et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 8. Scott PA, Silberbauer J, Murgatroyd FD.. The impact of adjunctive complex fractionated atrial electrogram ablation and linear lesions on outcomes in persistent atrial fibrillation: a meta-analysis. Europace 2016;18:359–67. [DOI] [PubMed] [Google Scholar]
- 9. Providencia R, Lambiase PD, Srinivasan N, Babu GG, Bronis K, Ahsan S. et al. Is there still a role for complex fractionated atrial electrogram ablation in addition to pulmonary vein isolation in patients with paroxysmal and persistent atrial fibrillation? Meta-analysis of 1415 patients. Circ Arrhythm Electrophysiol 2015;8:1017–29. [DOI] [PubMed] [Google Scholar]
- 10. Clarnette JA, Brooks AG, Mahajan R, Elliott AD, Twomey DJ, Pathak RK. et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace 2017;14:528.. [DOI] [PubMed] [Google Scholar]
- 11. Wynn GJ, Das M, Bonnett LJ, Panikker S, Wong T, Gupta D.. Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta-analysis of evidence from randomized. Circ Arrhythm Electrophysiol 2014;7:841–52. [DOI] [PubMed] [Google Scholar]
- 12. Sawhney N, Anousheh R, Chen W, Feld GK.. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:243–8. [DOI] [PubMed] [Google Scholar]
- 13. Sueda T, Nagata H, Orihashi K, Morita S, Okada K, Sueshiro M. et al. Efficacy of a simple left atrial procedure for chronic atrial fibrillation in mitral valve operations. Ann Thorac Surg 1997;63:1070–5. [DOI] [PubMed] [Google Scholar]
- 14. Lim TW, Koay CH, See VA, McCall R, Chik W, Zecchin R. et al. Single-ring posterior left atrial (box) isolation results in a different mode of recurrence compared with wide antral pulmonary vein isolation on long-term follow-up: longer atrial fibrillation-free survival time but similar survival time free of any atrial. Circ Arrhythm Electrophysiol 2012;5:968–77. [DOI] [PubMed] [Google Scholar]
- 15. Kim J-S, Shin SY, Na JO, Choi CU, Kim SH, Kim JW. et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation? Int J Cardiol 2015;181:277–83. [DOI] [PubMed] [Google Scholar]
- 16. Tamborero D, Mont L, Berruezo A, Matiello M, Benito B, Sitges M. et al. Left atrial posterior wall isolation does not improve the outcome of circumferential pulmonary vein ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2:35–40. [DOI] [PubMed] [Google Scholar]
- 17. Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R. et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation 2010;122:109–18. [DOI] [PubMed] [Google Scholar]
- 18. Panikker S, Jarman JWE, Virmani R, Kutys R, Haldar S, Lim E. et al. Left atrial appendage electrical isolation and concomitant device occlusion to treat persistent atrial fibrillation: a first-in-human safety, feasibility, and efficacy study. Circ Arrhythm Electrophysiol 2016;9:e003710. [DOI] [PubMed] [Google Scholar]
- 19. Badhwar N, Lakkireddy D, Kawamura M, Han FT, Iyer SK, Moyers BS. et al. Sequential percutaneous LAA ligation and pulmonary vein isolation in patients with persistent AF: initial results of a feasibility study. J Cardiovasc Electrophysiol 2015;26:608–14. [DOI] [PubMed] [Google Scholar]
- 20. Lim HS, Derval N, Komatsu Y, Zellerhoff S, Denis A, Shah AJ. et al. Is ablation to termination the best strategy for ablation of persistent atrial fibrillation? Persistent atrial fibrillation is best ablated by a strategy that terminates the arrhythmia procedural termination is associated with improved long-term outcomes. Circ Arrhythm Electrophysiol 2015;8:963–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.