Abstract
Aims
The aim of our study was to investigate the long-term efficacy and safety of transseptal endocardial left ventricular lead implantation (TELVLI).
Methods and results
Transseptal endocardial left ventricular lead implantation was performed in 54 patients (44 men, median age 69, New York Heart Association III–IV stage) between 2007 and 2017 in a single centre. In 36 cases, the transseptal puncture (TP) was performed via the femoral vein, and in 18 cases, the TP and also the left ventricular (LV) lead placement were performed via the subclavian vein. An electrophysiological deflectable catheter was used to reach the LV wall through the dilated TP hole. The LV lead implantation was successful in all patients. A total of 54 patients were followed up for a median of 29 months [interquartile range (IQR) 8–40 months], the maximum follow-up time was 94 months. Significant improvement in the LV ejection fraction was observed at the 3-month visit, from the median of 27% (IQR 25–34%) to 33% (IQR 32–44%), P < 0.05. Early lead dislocation was observed in three cases (5%), reposition was performed using the original puncture site in all. The patients were maintained on anticoagulation therapy with a target international normalized ratio between 2.5 and 3.5. Four thromboembolic events were noticed during follow-up. A total of 27 patients died, with a median survival of 15 months (IQR 6–40).
Conclusion
The TELVLI is an effective approach for cardiac resynchronization therapy (CRT) however it is associated with a substantial thromboembolic risk (7%).
Keywords: Transseptal, Endocardial, Cardiac resynchronization therapy, Heart failure, Lead
What’s new?
Transseptal endocardial left ventricular (TELV) leads show stable sensing and pacing parameters during long-term follow-up, but the dislodgement rate is higher compared with epicardial passive quadripolar or bipolar active fixation coronary sinus leads.
TELV leads implanted for cardiac resynchronization therapy (CRT) were associated with a significant increase in left ventricular ejection fraction during long-term follow-up.
TELV lead implantation for CRT does not increase the degree of mitral regurgitation during long-term follow-up.
TELV lead implantation for CRT is a feasible, and effective long-term alternative method to surgical approaches, however, it is associated with substantial thromboembolic risk.
Introduction
Cardiac resynchronization therapy (CRT) is a proven, effective therapy in heart failure (HF) patients with left ventricular (LV) dysfunction and asynchronous LV contraction, improving LV function and quality of life,1 as well as reducing morbidity and mortality in those with moderate-to-severe HF, wide QRS and left bundle branch block (BBB).2
Typically, the LV lead is positioned epicardially into a side branch of the coronary sinus (CS). Therefore, ‘transvenous’ CRT is limited by the need to access the LV epicardial surface via the CS and tributaries. Implantation of the LV lead into the CS might be challenging or in some cases impossible,3 and phrenic nerve stimulation could occur in certain locations even with the use of quadripolar leads. According to the literature data, the dislodgement rate of CS lead varies on a large scale, depending on several factors such as follow-up duration, placement site of the LV lead and operator experience.3–5
In addition to the possible lead dislodgement, a remarkable proportion of patients do not benefit from CRT.6 Due to these challenges, novel implantation techniques to implant the LV lead were developed, such as CS lead stenting,7,8 endocardial pacing via the interventricular septum,9 minimal invasive surgical procedures, and some other promising techniques, like the, ‘right ventricular’ permanent His-bundle pacing for CRT10 and leadless LV pacing11.
Another approach, atrial transseptal endocardial LV lead implantation (TELVLI) is relatively less invasive and it has proven to be more physiological than epicardial pacing,12–15 becoming an alternative to conventional surgical lead implantation.16 The TELVLI carries a lower risk of morbidity compared with even less invasive surgical approaches.17,18 However, LV endocardial lead placement carries an increased risk of systemic thromboembolism as it exposes the LV lead to the systemic circulation. There is currently a lack of robust evidence on the long-term benefits and thromboembolic risk of TELVLI.
Therefore, the aim of our retrospective, single-centre study was to evaluate the long-term efficacy and safety of TELVLI, and in particular to determine the long-term changes in LV function, the incidence of thromboembolism and other possible complications.
Patient selection
In this study, we only enrolled patients referred for TELVLI with a guideline-based indication for CRT implantation in HF patients with New York Heart Association (NYHA) functional class III or IV. Patients, who had either a failed previous CS lead implantation (i.e. inability to cannulate the CS, lead dislodgement, unacceptable high pacing thresholds, or phrenic nerve stimulation), or were non-responders to conventional transvenous CRT, who had worsened or unchanged HF status after at least 6 months of optimal CRT therapy, were eligible for enrolment. The patients were referred for TELVLI because in many cases they refused the surgical lead implantation, or because the anaesthesiologists assessed them as high-risk patients for a surgical approach. Exclusion criteria included a contraindication to oral anticoagulants, previous stroke, the presence of intracardiac thrombus as defined by transoesophageal or intracardiac echocardiography, and mild HF patients with NYHA functional class I or II.
Methods
All procedures were performed at a tertiary cardiovascular centre with high volume of CRT implantations, the LV endocardial pacing lead was positioned using an atrial transseptal implantation technique in all cases. Ethics approval was waived by Semmelweis University Regional and Institutional Committee of Science and Research Ethics (No.: 114/2018) due to the retrospective nature of data analysis. Informed consent was obtained before every TELVLI procedure and for data collection.
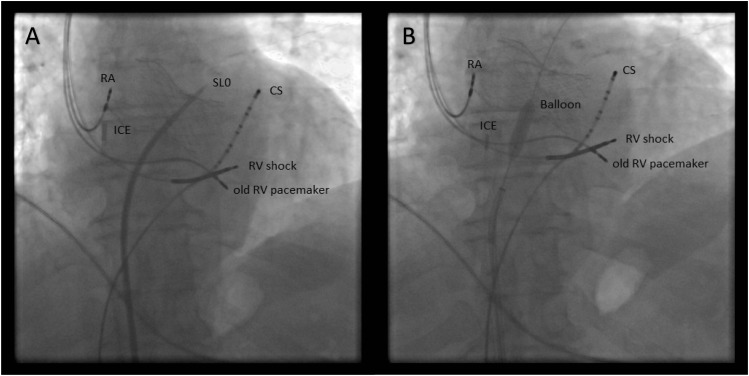
In 36 patients the transseptal puncture (TP) (Figure 1A) was performed via the femoral vein with a Brockenbrough-needle. After the successful puncture, the guidewire was positioned into the left superior pulmonary vein. In four patients a 6 × 40 mm, in the other cases an 8 × 40 mm peripheral balloon (Passeo-18/6/40/90, Passeo-18/8/40/90 Biotronik, Berlin, Germany, respectively) was used to dilate the septum at the puncture site (Figure 1B). A deflectable electrophysiological (EP) catheter (various types) was used to find the dilated puncture site (Figure 2), and via the subclavian vein standard CS sheath (Attain Medtronic, Minneapolis, MN, USA) was advanced on the EP catheter into the cavity of the left ventricle (Figure 3A). Coronary sinus sheath then was pushed gently against the lateral wall of the left ventricle, and an active fixation lead was then screwed into the LV wall through the previously positioned CS sheath (Figures 3B and 4). In 10 cases, CapSureFix Novus 4076 (in 6 cases 65 cm, in 4 cases 58 cm length was used/Medtronic, Minneapolis, MN, USA/), in 20 cases CapSureFix Novus 5076 (in 12 cases 65 cm, in 8 cases 58 cm length was used/Medtronic, Minneapolis, MN, USA/), and in further 6 cases, Tendril 1888–58 cm (St. Jude Medical, Saint Paul, MN, USA) leads were used.
Figure 1.
(A) Transseptal puncture; the transseptal sheath (Swartz SL0 sheath, St. Jude Medical, Saint Paul, MN, USA) is in the left atrium. The patient has previously undergone a TAVI (transcatheter aortic valve implantation) procedure, the frame of the prosthetic valve can be seen. (B) Dilation of the interatrial hole with the peripheral balloon (the distal part of the guidewire is in the left superior pulmonary vein). CS, coronary sinus decapolar catheter; ICE, intracardiac echocardiography catheter; RA, right atrial lead; RV shock, right ventricular shock lead; old RV pacemaker, previously implanted right ventricular pacemaker lead.
Figure 2.
(A) The TP hole was found with the help of a deflectable electrophysiological catheter (Abl). (B) The electrophysiological catheter (Abl) was guided through the interatrial septum into the left atrium. CS, coronary sinus decapolar catheter; ICE, intracardiac echocardiography catheter; RA, right atrial lead; RV shock, right ventricular shock lead; old RV pacemaker, previously implanted right ventricular pacemaker lead; TP, transseptal puncture.
Figure 3.
(A) The coronary sinus sheath was inserted to the left ventricle through an electrophysiological catheter (CS cath). (B) The left ventricular active fixation lead (LV lead) in the CS sheath which is in the cavity of the left ventricle. CS, coronary sinus decapolar catheter; ICE, intracardiac echocardiography catheter; RA, right atrial lead; RV shock, right ventricular shock lead; old RV pacemaker, previously implanted right ventricular pacemaker lead.
Figure 4.
The final picture, an anteroposterior (AP) and a left anterior oblique (LAO) view. The left ventricular lead (LV lead) is in the left lateral region of the left ventricle. The old RV pacemaker lead was explanted successfully by simple traction. RA, right atrial lead; RV shock, right ventricular shock lead.
In the other 18 patients, the TP and the LV lead placement were both performed via the subclavian vein. Venous access was achieved using standard methods. The TP was made with the help of a transseptal radiofrequency powered wire (Model SAK-35–76 Baylis Medical Co., Montreal, Quebec, Canada), delivered by a steerable guide (6227ATS Medtronic, Minneapolis, MN, USA). An LV Select Secure 3830–58 cm (Medtronic, Minneapolis, MN, USA) endocardial active pacing lead was advanced through the interatrial septum with the help of a lead delivery catheter (6248HS, 6248JS, or 6248JL Medtronic, Minneapolis, MN, USA)19, then across the mitral valve the lead tip was fixed to the appropriate LV endocardial site. In the posterolateral region of the left ventricle, approximately two to five lead positions were electrically measured with the help of a pacing system analyser (ERA 300 Biotronik, Berlin, Germany) to find the final best position of the LV lead. The Q-LV distance was measured with callipers, which are aligned with the onset of QRS and peak of the LV electrogram. According to the results, the LV active fixation bipolar lead was fixed in a region where the longest electrical delay was observed: in 40 patients, the lead was screwed into the posterolateral region, and in the remaining patients into the lateral region of the left ventricle. In four patients, the LV lead positioned with the help of an activation map (Figure 5) created with an electroanatomical mapping system (CARTO Biosense Webster Inc., Irvine, CA, USA). In the latter, the activation mapping of the right ventricle, and after the TP, mapping of the left ventricle was performed with a CARTO Quick Star catheter (Biosense Webster Inc., Irvine, CA, USA) which was introduced through the right femoral vein.20 An 11 F-long sheath (SCOUT Pro 8 Fr, Biotronik, Berlin, Germany) was introduced via the left subclavian vein, and the Quick Star catheter was advanced into the sheath and directed to the location of the previously marked TP site applying CARTO map guidance. The Quick Star catheter was used to relocate the LV segment with the latest activation. Then, the Quick Star catheter was withdrawn, and via the sheath, an active fixation LV lead was fixed at the basal or mid-basal lateral or posterolateral portion of the left ventricle in all patients. Acceptable R-wave sensing and pacing thresholds were obtained in all. The TP of the interatrial septum was guided by intracardiac or transoesophageal echocardiography (TOE) or by fluoroscopy. The total procedure time was 112 ± 50 min. Fluoroscopy time was 22 ± 11 min and fluoroscopy dose-area product was 1140 ± 560 (μGym2).
Figure 5.

CARTO image, left lateral projection. Right and the LV activation map: the earliest activation site is the right ventricular anteroseptal region; the latest site is the mid-basal part of the posterolateral wall. LV, left ventricular.
During the procedures, midazolam and fentanyl were used for conscious sedation, and if TOE was necessary, mild sedation was carried out with propofol.
In all patients, immediately after the TP, intraoperative anticoagulation was managed by the administration of intravenous sodium-heparin to maintain a target activated clotting time of 250–300 s until the catheters were removed after lead fixation. Chronic oral anticoagulation with coumarin or warfarin was reinstituted immediately after the procedure. Based on prior recommendations, subcutaneous enoxaparin was used until adequate anticoagulation with oral anticoagulants was obtained with a target international normalized ratio (INR) of 2.5–3.5.
Outcomes
We assessed the safety and efficacy of TELVLI in our single-centre study cohort. Safety was ascertained by reporting the incidence of thromboembolic events, and other complications during long-term follow-up. Efficacy was reported by using the improvement of LV ejection fraction (LVEF) and NYHA functional class changes.
Statistical analysis
Baseline clinical characteristics are presented as number and percentages, and median with inter-quartile range (IQR) for age, or mean ± standard deviation for LVEF, LV end-systolic diameter (LVESD), and LV end-diastolic diameter (LVEDD). Thromboembolic complications were reported using number and percentages during follow-up.
To analyse long-term improvement in LVEF, Wilcoxon signed-rank tests were used. In addition, Kaplan–Meier survival analysis was performed to analyse all-cause mortality during long-term follow-up in the total patient population.
In our study, a P-value of <0.05 was considered significant. Statistical analyses were performed using SPSS for Windows version 22 (IBM Corp. Armonk, New York, USA).
Results
From November 2007 to January 2017, 54 patients underwent TELVLI at our institution. Table 1 summarizes the baseline characteristics of these patients. Most of them were male (n = 44), the median age was 69 years, and the mean LVEF was 29%. The majority of the patients had non-ischaemic cardiomyopathy (n = 33, 61%), the rest presented with ischaemic aetiology of cardiomyopathy. Atrial fibrillation was present in 37% (n = 20). In all cases, TELVLI was carried out successfully, and the LV lead measurements showed stable electrical parameters during the follow-up period (Table 2).
Table 1.
Patient baseline characteristics
| Patient characteristics | All patients (N = 54) |
|---|---|
| General | |
| Age at enrolment (years) | 69 ± 10 |
| Gender (male), n (%) | 44 (81) |
| Failed implant, n (%) | 25 (46) |
| Suboptimal CS anatomy, n (%) | 20 (37) |
| CRT non-responders, n (%) | 9 (17) |
| Cardiovascular history, n (%) | |
| Ischaemic cardiomyopathy | 21 (39) |
| Non-ischaemic cardiomyopathy | 33 (61) |
| Atrial fibrillation | 20 (37) |
| Baseline assessment, n (%) | |
| NYHA class | |
| Class III | 50 (93) |
| Class IV | 4 (7) |
| QRS duration (ms) | 158 ± 28 |
| LVEF (%) | 29 ± 6 |
| LVESD (mm) | 57 ± 10 |
| LVEDD (mm) | 68 ± 8 |
| Mitral regurgitation (moderate/severe), n (%) | 33 (61) |
Data are shown as mean ± SD, or as numbers and percentages.
CRT, cardiac resynchronization therapy; CS, coronary sinus; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter; NYHA, New York Heart Association.
Table 2.
Implantation characteristics
| Device type, n (%) | |
| CRT-P | 19 (35) |
| CRT-D | 35 (65) |
| Lead type, n (%) | |
| Medtronic 4076 | 10 (19) |
| Medtronic 5076 | 20 (37) |
| Medtronic 3830 | 18 (33) |
| St. Jude Medical 1888 | 6 (11) |
| Electrical parameters at implantation | |
| Sensing (mV) | 14 ± 7 |
| Threshold (mV) | 0, 9 ± 0, 4 |
| Impedance (Ω) | 713 ± 256 |
| Lead implantation positions, n (%) | |
| Posterolateral | 40 (74) |
| Lateral | 14 (26) |
Data are shown as mean ± SD, or as numbers and percentages.
CRT-P, cardiac resynchronization therapy pacemaker; CRT-D, cardiac resynchronization therapy defibrillator.
Complications
Thromboembolic events
Table 3 summarizes the thromboembolic events observed during the follow-up period. No thromboembolic events occurred in the early post-operative period until hospital discharge. During longer follow-up, four thromboembolic events (7%) were reported. Two patients had a stroke, and another two patients had a transient ischaemic attack (TIA). In patients with a stroke, the cranial computed tomography scan verified the ischaemic lesion. According to the medical records, the INR value was below the target range in all four cases. All but one patient recovered symptom-free from the thromboembolic events, one of them recovered with minor symptoms.
Table 3.
Thromboembolic complications
| Pt. no. | Sex | Agea | Rhythm | Time to TE event (months) | Type of TE event | Lead type |
|---|---|---|---|---|---|---|
| 1 | M | 69 | Sinus | 46 | stroke | Medtronic 5076 |
| 2 | M | 63 | Sinus | 7 | TIA | St. Jude Medical 1888 |
| 3 | M | 61 | Sinus | 46 | stroke | Medtronic 5076 |
| 4 | M | 69 | AFib | 33 | TIA | Medtronic 4076 |
Age is given at TE event.
F, female; M, male; TE, thromboembolic; TIA, transient ischaemic attack.
Other complications
Device system-related complications occurred in a total of 10 patients during the follow-up period. Among these, there were three patients with LV endocardial lead dislodgement (5%) at 22, 45, and 54 days post-implantation, respectively. In all cases, the LV endocardial lead was repositioned successfully through the original puncture site, without any further complications. Left ventricular endocardial lead dislodgements occurred in two patients who had undergone TELVLI with a 58 cm LV lead (Select Secure 3830–58 cm and Tendril 1888–58 cm) and in one patient when 65 cm lead (CapSureFix Novus 5076–65 cm) was used, we attributed this to the learning curve.
In the first few TELVLI procedures intraoperative anticoagulation and post-operative chronic oral anticoagulation with coumarin or warfarin was used, which was reinstituted immediately after the procedure with subcutaneous enoxaparin bridging until the target INR of 2.5–3.5 was reached. These could have presumably contributed to the formation of a pocket haematoma in three patients (6%), which needed evacuation in two cases. After then, the anticoagulation regime was changed, and TELVLI was carried out with almost therapeutic INR range, followed by anticoagulation only with coumarin or warfarin which was restarted just after the procedure. Following this adjustment, pocket haematoma, or other further minor or major bleeding was not reported during the follow-up period.
In a total of four (7%) patients the CRT system had to be explanted due to pocket infection. Two of these were probably pocket haematoma superinfections, the other two infections occurred probably due to long procedure time. In all cases, the transseptal endocardial leads could be extracted safely by traction, and then reimplanted within 2 months with the original transseptal method, without any complications. No signs of pacing lead endocarditis were diagnosed. During the follow-up period, no CRT system-related complications which affected the mitral valve were confirmed.
All the previously mentioned other complications happened at the beginning of the TELVLI era in our Clinic (2007–2008) in the early phase of the learning curve.
Echocardiography and clinical outcomes
Patients were followed for a median of 29 months (IQR: 8–40 months, the maximum follow-up time was 94 months). During the follow-up period, a total of 27 patients died (Figure 6), 5-year survival was 40%. The cause of death was non-cardiac in three cases, including cancer and chronic renal failure.
Figure 6.

Survival after the transseptal left ventricular lead implantation.
We observed a statistically significant increase in LVEF at the 3-month visit, with an increase from a median of 27% (IQR 25–34%) to 33% (IQR 32–44%), P < 0.05). Both LVEDD and LVESD also showed a statistically significant improvement [median of 67 mm (IQR 62–75 mm) and 58 mm (IQR 50–65 mm), to the median of 62 mm (54–71 mm) and 53.5 mm (42–62 mm), P < 0.05], despite there were no significant changes in QRS duration (158 ± 28 ms vs. 156 ± 29 ms) reported. At the 3-month visit, two (4%) patients proved to be super-responders (EF ≥ 50%) to the CRT therapy. In our study population, from the previously non-responder group (n = 9), six patients showed significant improvement in LVEF, LVEDD, and LVESD.
During the follow-up period, we did not observe worsening of mitral regurgitation (MR) as some reports previously suggested. In fact, our findings suggested a reduced MR, 30% (n = 10) of the patients with previously described severe or moderate MR showed an improvement of MR by ≥1 class, likely due to improvement in LV function.
From our patient population, after 3 months, 48 (89%) patients achieved an improvement of at least one NYHA class. From the remaining six patients, three patients died before the 3-month visit, the other patients were also non-responders to endocardial LV pacing.
Discussion
We have shown in our large volume, single-centre experience of 54 patients undergoing TELVLI due to an unsuccessful CRT implantation or CRT non-response, that the procedure is feasible, and the endocardial LV leads showed excellent electrical parameters. It was previously reported, that a sizable proportion of non-responders to conventional CRT may benefit from endocardial pacing. An ALSYNC study sub-analysis described significant reverse remodelling and functional improvement in half of the prior non-responders to conventional CRT from the study population. The benefit of endocardial pacing was greater in patients with an ischaemic aetiology of HF compared with non-ischaemic ones. The underlying mechanisms in ischaemic patients may comprise not only overriding the limitations of the anatomy of coronary veins, but also a more rapid and more homogeneous ventricular activation by LV endocardial pacing.21
During our long-term follow-up, there was a significant improvement observed in echocardiographic parameters (LVEF, LVESD, and LVEDD), and 89% of the patients reported at least one NYHA class improvement. We did not observe worsening in MR due to the presence of the transmitral LV lead. However, there are some safety issues regarding the procedure, which have to be considered.
In patients with severe LV dysfunction (excluding patients with atrial fibrillation), the estimated incidence of thromboembolic complications range from 1.5 to 3.5 events per 100 patient-years,22 and this risk is further increased in the presence of an LV endocardial lead, which acts as a prosthesis in the left ventricle, therefore lifelong anticoagulation therapy might be necessary.
There is no current consensus regarding the anticoagulation protocols or the type of LV lead used for TELVLI, and there is limited data about the thromboembolic complications regarding the procedure. Jais et al. reported a TIA in 1 of 11 patients, and Pasquie et al.23 reported a TIA in 1 of 6 patients during 85 ± 2 months and 15 ± 12 months follow-up, respectively. Rademakers et al.24 described eight thromboembolic events in 51 patients during the median of 24 months of follow-up time. In both cases, the level of anticoagulation was below the therapeutic range. In our study, the target INR was between 2.5 and 3.5, whereas Rademakers et al.24 defined an INR range between 3.5 and 4.5, similar to that with mechanical prosthetic valves. In our study, four (7%) thromboembolic events were reported. However, thromboembolic event occurrence was always linked to a sub-therapeutic INR level, the event rate was higher than the desirable, and might be preventable with closer follow-up. The prevalence of thromboembolic events was influenced by various factors among different studies, such as NYHA class, LVEF, or the history of atrial fibrillation. According to the different therapeutic INR range in our and other studies, it is not clear, which target INR range should be used in patients with TELVLI. The therapeutic range of novel oral direct thrombin or factor Xa inhibitors may be more predictable than vitamin K antagonists, but currently, there is no experience with novel oral direct thrombin on factor Xa inhibitors with LV endocardial leads. While the type of LV endocardial lead might play a role in thromboembolic events, we could not make any inferences from our cohort, likely due to the small number of events. Two thromboembolic events occurred with the Medtronic CapSureFix Novus 5076, one event with the Medtronic CapSureFix Novus 4076, and one event with the St. Jude Medical Tendril 1888 lead, however, the latter was only used in six cases. The Medtronic lead has polyurethane material, and the St. Jude Medical lead also has polyurethane mixed with silicone insulation. Rademakers et al.24 used only polyurethane coated leads and experienced multiple thromboembolic events with the Medtronic SelectSecure 3830 leads.
In our patient group, the worsening of the mitral valve function was not observed. Previous studies showed similar findings, suggesting that the endocardial LV lead does not interfere with the mitral apparatus.24
The dislodgement rate of the endocardial LV leads in our patient group was 5% (n = 3), which seems to be higher than the dislodgement rate for newer quadripolar leads, however, we could not draw a conclusion as to what the exact causes were, because these happened with different leads, so we attributed this to the learning curve. In 2014, Biffi et al.4 reported a 7.3% dislodgement rate at 6 months, and 9.6% at the 1-year follow-up using passive fixation bipolar CS LV leads, the late lead dislodgements were observed in CRT responder/super-responder patients (with >20% decrease of LV end-systolic volume). A large Dutch study by Ghani et al.5 reported a CS lead dislodgement rate of 1%, using passive fixation CS leads, however, the exact lead types were not reported. In a more recent study, Ziacchi et al.25 reported an overall 9% dislodgement rate with various LV lead types (bipolar, quadripolar, and active fixation leads), with a 4.8% rate for LV lead dislodgement required a re-operation. In 2017, a 2.1% vs. 3.0% dislodgement rate for quadripolar vs. bipolar CS leads was reported by Rijal et al.26 Kassai et al.18 reported a lead dislodgement rate of 10% in transapical LV endocardial paced patients, while Rademakers et al.24 described no lead dislocation during their follow-up period.
During the years, further novel techniques were developed as an alternative approach when conventional CRT fails, such as permanent His-bundle pacing and leadless LV pacing. Permanent His-bundle pacing has recently been shown to be a viable alternative to biventricular pacing in patients requiring CRT. According to a large multi-centre experience, it is a promising alternative for biventricular pacing for HF patients, which can significantly narrow or even normalize the QRS width, increase the LV function and also the functional capacity of the patients. One of the limitations of His-bundle pacing is battery longevity, as it needs usually higher pacing output to overcome BBB. Important to note that about 7% of patients had a late increase in capture threshold with or without loss of BBB recruitment, the mechanism of this phenomenon needs further investigations.10 According to these, permanent His-bundle pacing for CRT can be a promising technique, which carries low-risk rate without the increased risk of systemic thromboembolism.
Leadless LV endocardial pacing offers another alternative to conventional CRT, with good echocardiographic and clinical results, but according to the currently available data, the early complication rate is fairly high. In the SELECT-LV study, serious adverse events occurred in 8.6% within the first 24 h (ventricular fibrillation; electrode embolization to left tibial artery; femoral artery fistula) and in 22.9% in the first month (one death after lead-induced ventricular fibrillation; one stroke; three infections; one pocket haematoma; two femoral pseudoaneurysms). Leadless LV endocardial pacing has several advantages over epicardial pacing, and it can become a promising option in patients requiring CRT in the future, however, it is currently limited by technical, technological, and safety issues.11
Our patients often refused the surgical approaches, and in several cases, the anaesthesiologists refused these fragile advanced HF patients. In the previous instances, TELVLI has been shown a feasible method for failed CRT implants in fragile HF patients. Contrary to the surgical techniques, the TELVLI procedure carries less risk to the patient, and the hospitalization time is shorter.17 If an epicardial CS lead implantation fails, the procedure can be easily converted, and TELVLI can be applied during the same procedure at experienced centres. Over the period of 2007–17, 1520 CRT systems were implanted in our centre. In these cases, TELVLI was used in 4% to establish biventricular pacing. However, over the past years, technical improvement of the LV electrodes led to more successful conventional CRT implantations, thus the number of TELVLIs decreased significantly. Today, TELVLI is reserved for fragile HF patients, when other available alternative techniques (such as His pacing) are not effective, and the patient is not suitable for surgical lead implantation methods.
Conclusions
Our large, single-centre, 10-year experience with TELVLI showed that it is a feasible alternative method to surgical approaches in advanced HF patients when transvenous LV lead implantation cannot be performed or conventional CRT fails. The TELVLI was associated with a significant increase in LVEF that was sustained during long-term follow-up. The endocardial LV lead did not increase MR in our cohort. However, TELVLI is associated with a substantial thromboembolic risk (7%). Based on our experience, TELVLI seems to be a feasible option for failed CRT implantations, with careful follow-up of anticoagulation levels to avoid thromboembolic events.
Conflict of interest: none declared.
References
- 1. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- 2.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013;15:1070–118. [DOI] [PubMed] [Google Scholar]
- 3. Alonso C. In the field of cardiac resynchronization therapy is left ventricular pacing via the coronary sinus a mature technique. Europace 2009;11:544–5. [DOI] [PubMed] [Google Scholar]
- 4. Biffi M, Bertini M, Ziacchi M, Diemberger I, Martignani C, Boriani G.. Left ventricular lead stabilization to retain cardiac resynchronization therapy at long term: when is it advisable? Europace 2014;16:533–40. [DOI] [PubMed] [Google Scholar]
- 5. Ghani A, Delnoy PP, Ramdat Misier AR, Smit JJ, Adiyaman A, Ottervanger JP. et al. Incidence of lead dislodgement, malfunction and perforation during the first year following device implantation. Neth Heart J 2014;22:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saxon LA, Ellenbogen KA.. Resynchronization therapy for the treatment of heart failure. Circulation 2003;108:1044–8. [DOI] [PubMed] [Google Scholar]
- 7. Szilagyi S, Merkely B, Roka A, Zima E, Fulop G, Kutyifa V. et al. Stabilization of the coronary sinus electrode position with coronary stent implantation to prevent and treat dislocation. J Cardiovasc Electrophysiol 2007;18:303–7. [DOI] [PubMed] [Google Scholar]
- 8. Geller L, Szilagyi S, Zima E, Molnar L, Szeplaki G, Vegh EM. et al. Long-term experience with coronary sinus side branch stenting to stabilize left ventricular electrode position. Heart Rhythm 2011;8:845–50. [DOI] [PubMed] [Google Scholar]
- 9. Betts TR, Gamble JH, Khiani R, Bashir Y, Rajappan K.. Development of a technique for left ventricular endocardial pacing via puncture of the interventricular septum. Circ Arrhythm Electrophysiol 2014;7:17–22. [DOI] [PubMed] [Google Scholar]
- 10. Sharma PS, Dandamudi G, Herweg B, Wilson D, Singh R, Naperkowski A. et al. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm 2018;15:413–20. [DOI] [PubMed] [Google Scholar]
- 11. De Maria E, Ziacchi M, Diemberger I, Biffi M.. Leadless left ventricular endocardial pacing: a real alternative or a luxury for a few? Cardiovasc Diagn Ther 2018;8:530–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garrigue S, Jais P, Espil G, Labeque JN, Hocini M, Shah DC. et al. Comparison of chronic biventricular pacing between epicardial and endocardial left ventricular stimulation using Doppler tissue imaging in patients with heart failure. Am J Cardiol 2001;88:858–62. [DOI] [PubMed] [Google Scholar]
- 13. Bracke FA, Houthuizen P, Rahel BM, van Gelder BM.. Left ventricular endocardial pacing improves the clinical efficacy in a non-responder to cardiac resynchronization therapy: role of acute haemodynamic testing. Europace 2010;12:1032–4. [DOI] [PubMed] [Google Scholar]
- 14. Ozcan EE, Szilagyi S, Sallo Z, Molnar L, Zima E, Szeplaki G. et al. Comparison of the effects of epicardial and endocardial cardiac resynchronization therapy on transmural dispersion of repolarization. Pacing Clin Electrophysiol 2015;38:1099–105. [DOI] [PubMed] [Google Scholar]
- 15. Scott PA, Yue AM, Watts E, Zeb M, Roberts PR, Morgan JM.. Transseptal left ventricular endocardial pacing reduces dispersion of ventricular repolarization. Pacing Clin Electrophysiol 2011;34:1258–66. [DOI] [PubMed] [Google Scholar]
- 16. Jais P, Takahashi A, Garrigue S, Yamane T, Hocini M, Shah DC. et al. Mid-term follow-up of endocardial biventricular pacing. Pacing Clin Electrophysiol 2000;23:1744–7. [DOI] [PubMed] [Google Scholar]
- 17. Mair H, Sachweh J, Meuris B, Nollert G, Schmoeckel M, Schuetz A. et al. Surgical epicardial left ventricular lead versus coronary sinus lead placement in biventricular pacing. Eur J Cardiothorac Surg 2005;27:235–42. [DOI] [PubMed] [Google Scholar]
- 18. Kassai I, Friedrich O, Ratnatunga C, Betts TR, Mihalcz A, Szili-Torok T.. Feasibility of percutaneous implantation of transapical endocardial left ventricular pacing electrode for cardiac resynchronization therapy. Europace 2011;13:1653–7. [DOI] [PubMed] [Google Scholar]
- 19. Morgan JM, Biffi M, Geller L, Leclercq C, Ruffa F, Tung S. et al. ALternate Site Cardiac ResYNChronization (ALSYNC): a prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur Heart J 2016;37:2118–27. [DOI] [PubMed] [Google Scholar]
- 20. Kutyifa V, Merkely B, Szilagyi S, Zima E, Roka A, Kiraly A. et al. Usefulness of electroanatomical mapping during transseptal endocardial left ventricular lead implantation. Europace 2012;14:599–604. [DOI] [PubMed] [Google Scholar]
- 21. Biffi M, Defaye P, Jais P, Ruffa F, Leclercq C, Gras D. et al. Benefits of left ventricular endocardial pacing comparing failed implants and prior non-responders to conventional cardiac resynchronization therapy: a subanalysis from the ALSYNC study. Int J Cardiol 2018;259:88–93. [DOI] [PubMed] [Google Scholar]
- 22. Sirajuddin RA, Miller AB, Geraci SA.. Anticoagulation in patients with dilated cardiomyopathy and sinus rhythm: a critical literature review. J Card Fail 2002;8:48–53. [DOI] [PubMed] [Google Scholar]
- 23. Pasquie JL, Massin F, Macia JC, Gervasoni R, Bortone A, Cayla G. et al. Long-term follow-up of biventricular pacing using a totally endocardial approach in patients with end-stage cardiac failure. Pacing Clin Electrophysiol 2007;30Suppl 1:S31–3. [DOI] [PubMed] [Google Scholar]
- 24. Rademakers LM, van Gelder BM, Scheffer MG, Bracke FA.. Mid-term follow up of thromboembolic complications in left ventricular endocardial cardiac resynchronization therapy. Heart Rhythm 2014;11:609–13. [DOI] [PubMed] [Google Scholar]
- 25. Ziacchi M, Diemberger I, Corzani A, Martignani C, Mazzotti A, Massaro G. et al. Cardiac resynchronization therapy: a comparison among left ventricular bipolar, quadripolar and active fixation leads. Sci Rep 2018;8:13262.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rijal S, Wolfe J, Rattan R, Durrani A, Althouse AD, Marroquin OC. et al. Lead related complications in quadripolar versus bipolar left ventricular leads. Indian Pacing Electrophysiol J 2017;17:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]