Abstract
Nanoconjugations have been demonstrated to be a dominant strategy for drug delivery and biomedical applications. In this review, we intend to describe several strategies for drug formulation, especially to improve the bioavailability of poorly water-soluble molecules for future application in the therapy of numerous diseases. The context of current studies will give readers an overview of the conjugation strategies for fabricating nanoparticles, which have expanded from conjugated materials to the surface conjugation of nanovehicles. Moreover, nanoconjugates for theranostics are also discussed and highlighted. Overall, these state-of-the-art conjugation methods and these techniques and applications for nanoparticulate systems of poorly water-soluble drugs will inspire scientists to explore and discover more productive techniques and methodologies for drug development.
Keywords: nanoconjugate, nanotechnology, poorly water-soluble drugs, theranostic, drug delivery, biomedical applications
1. Introduction
New drugs have been studied and developed rapidly worldwide. Unfortunately, these drugs may be limited in their clinical applications due to their poor solubility, adverse effects, or even toxicity. Over 70% of drugs on the current market, as well as recently discovered drugs, have been reported to be poorly water soluble [1,2,3,4,5]. These drugs require extensive research to improve their bioavailability due to low absorption or non-targeted delivery. Therefore, a number of strategies, such as solid dispersions, emulsions, prodrugs, and nanoparticles, have been investigated to improve the therapeutic index of poorly water-soluble drugs [6,7,8,9,10,11]. Nanotechnology applications in medicine have grown enormously, attracting researchers worldwide. Because of their small size and high surface area, nanosized drug particles have achieved encouraging outcomes in terms of improved drug solubility and bioavailability [12]. In addition, available surface modifications using nanotechnology can be applied in nano drug delivery systems for targeting drugs to specific sites, such as cancer tumours, for the development of targeted therapeutics and diagnostics [13].
Although the use of hydrophilic polymers in solid dispersion, that is, the dispersion of a drug molecule in a carrier, has been established for improving drug dissolution and solubility since 1960 [14,15,16,17,18,19,20], hydrophilic–hydrophobic polymer nanoconjugates and hydrophobic drug-hydrophilic polymer nanoconjugates have been studied for loading drugs into nanoparticles for the same purposes in the past few decades of nanotechnological development [21,22,23]. The conjugation can commonly induce the formation of self-assembled polymeric amphiphiles, i.e., the exposure of this structure to aqueous environments results in self-assembled nanoparticles with hydrophobic segments in the inner core and hydrophilic segments towards the aqueous environment [9,24,25]. Consequently, the size of poorly water-soluble drugs is reduced to nanosize, leading to enhanced dissolution. Crystalline drug structure and drug-polymer interactions will be noted in addition to certain recent studies, as these factors may facilitate drug dissolution to improve bioavailability. With regard to theranostics systems for poorly water-soluble drugs, numerous nanoconjugate studies have been performed in cancer research [26,27]. In addition to the preferential accumulation of drug nanosized particles in tumours due to the leaky and porous structure of the tumoural blood vessels, nanoparticles may prolong the half-life of the drug in the blood circulation and specifically target a tumour by their surface decorations. However, conventional conjugations between hydrophilic molecules and hydrophobic molecules often encounter limitations such as low drug solubility, low drug loading, single drug delivery, large particle size, and short half-life, which may lead to unpredictable treatment efficacy. This review summarizes the current conjugation strategies for nanoparticulate formulations, suggesting efficient solutions to overcome these limitations and introducing specific applications of poorly water-soluble drugs (Figure 1).
Figure 1.
Illustration of nanoconjugation for improving drug delivery and therapeutic efficacy of poorly water-soluble drugs.
2. General Hydrophobic-Hydrophilic Nanoconjugates for Poorly Water-Soluble Drug Delivery
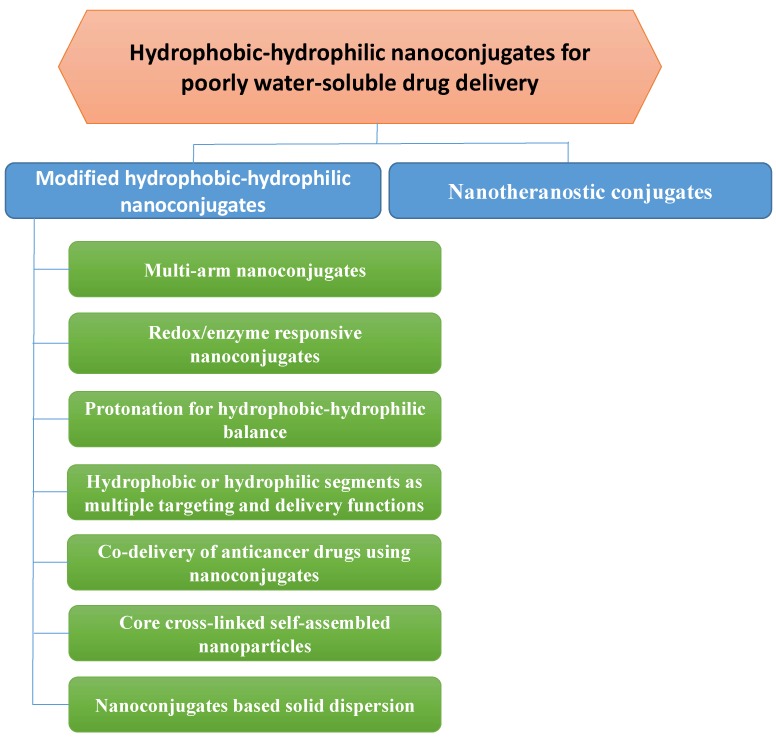
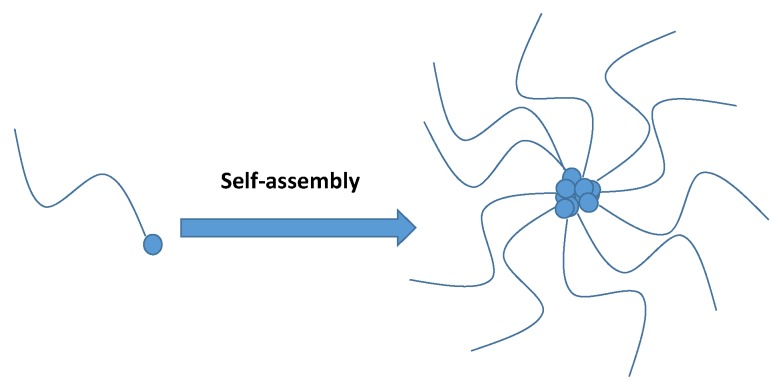
Since the first reported study in 1984 introducing the formation of nanosized polymeric self-assemblies with potential as hydrophobic drug solubilizers [28], amphiphilic polymers have attracted the attention of researchers, especially for studies of nanomedicine in anticancer therapy [29,30]. Typically, conjugation strategies based on multiple interactions such as hydrogen bonding, host–guest interaction, hydrophobic interaction, and electrostatic interaction [31] have been proposed for the attachment of hydrophilic and hydrophobic segments to create an amphiphilic polymer, which is then commonly self-assembled into nanoparticles in an aqueous environment by molecular associations between hydrophobic segments (Figure 2) [32,33,34]. In other words, a hydrophobic segment can significantly affect the formation, drug encapsulation, drug interactions, and stability of nanoconjugates [35]. A poorly water-soluble drug can be loaded into hydrophobic moieties to improve its solubility, increase circulation time, target the tumour environment, and/or prevent drug degradation. In this review, we do not include the general information previously reported in recent prominent review articles on self-assembled nanoparticles such as polymer types, spacers, concentrations, and self-assembly mechanisms [36,37,38,39,40,41,42,43,44,45]. Instead, we focus only on the latest strategies, particularly on how the sophisticated conjugate is modified to improve the capacity of conventional hydrophilic–hydrophobic nanoconjugates to deliver poorly water-soluble drugs.
Figure 2.
Illustration of self-assembled nanoparticles from hydrophobic–hydrophilic nanoconjugates. The blue dot represents the hydrophobic association.
3. Modified Hydrophobic-Hydrophilic Nanoconjugates for the Delivery of Poorly Water-Soluble Drugs
The extensive biomedical applications of nanoparticles arise from their small particle size. Their size enables nanoparticles to accumulate preferentially in tumour sites since tumour blood vessels are generally more heterogeneous in distribution, larger, and more permeable than normal blood vessels. The increased vascular permeability coupled with the impaired lymphatic drainage in rapidly growing tumours allows an enhanced permeability and retention (EPR) effect of the nanoparticles in the tumour. Thus, nanoparticles have been designed and engineered to have sizes and structures that are pertinent to biomedical applications such as targeted drug delivery, biomedical imaging, hyperthermia, and biosensing. However, the small size of nanoparticles might have incidental disadvantages in the context of unexpected drug solubility, particularly drug loading. Therefore, a failure of high drug loading or the achievement of enhanced drug solubility may significantly affect the therapeutic index or toxicity. A summary of works performed to improve those problems using nanoconjuagtes of poorly water-soluble drugs is presented in Table 1.
Table 1.
Examples of studies on nanoconjuagtes of poorly water-soluble drugs for therapeutics.
| Poorly Water-Soluble Drugs | Approaches | Key Results | References |
|---|---|---|---|
| SN38 (the active metabolite of camptothecin) | Multi-arm | Enhanced drug solubility. Significant preclinical therapeutic improvement and a longer half-life of the drug. |
[46,47] |
| Ursolic acid and 10-hydroxycamptothecin | Effective cellular uptake. Higher survival rate of tumour-bearing mice. |
[48] | |
| Docetaxel | Redox/enzyme responsive | Triggering dual-responsive drug release. Facilitating drug release by an on/off switch in the desired environment. |
[49,50] |
| Doxorubicin | Multiple targeting | Synergistic targeting effect. | [51] |
| Paclitaxel Doxorubicin Cytarabine |
Poorly water-soluble drugs as hydrophobic segments in the core-shell structure | High drug loading. | [52,53,54] |
| Fucoidan Paclitaxel Curcumin |
The combination use of a hydrophilic therapeutic agent | Dual functions. | [55,56] |
| Docetaxel | Hydrophobic drug-spacer-hydrophilic drug conjugates | Co-delivery of anticancer drugs. | [57] |
| Chlorambucil | Hydrophobic drug-hydrophilic drug conjugates | Excellent anticancer activity. | [58] |
| Isradipine Prednisolone |
Solid dispersion | Improve drug bioavailability. | [59,60] |
3.1. Multi-Arm Nanoconjugates for Poorly Water-Soluble Drug Delivery
Multi-arm nanoconjugates have recently been investigated to overcome these limitations [61,62,63,64]. For example, in a study of Sapra’s group on developing SN38 (the active metabolite of camptothecin), multi-arm polyethylene glycol was designed to conjugate to the model drug to enhance solubility [46,47]. This proposed delivery system has been demonstrated to confer significant preclinical therapeutic improvement and a longer half-life. More recently, natural pectin was decorated on polyethylene glycol with eight arms, which self-assembled into nanoparticles that were capable of improving drug solubility and controlling drug release [48]. This study showed that the optimal particle size (approximately 90 nm) could deliver two insoluble anticancer drugs, ursolic acid and hydrooxycampothecin, with efficient cellular uptake and cell cytotoxicity [48]. The in vivo test also indicated a higher survival rate in tumour-bearing mice administered the nanoparticles than in those administered the free drugs [48]. The presence of multiple arms on the conjugate structure was hence proposed as a strategy to achieve improved drug encapsulation efficiency, increased drug solubility, and suitable particle size for enhancing drug bioavailability. However, large numbers of conjugate arms may result in large aggregates due to the increased size and complexity of the structure. Therefore, the formulation approach of these multi-arm nanoconjugates should be designed appropriately.
3.2. Redox/Enzyme Responsive Nanoconjugates
To modulate drug release in the tumour microenvironment, redox/enzyme responsive linkage can be inserted into the hydrophobic–hydrophilic conjugate structure. An example of this type of conjugate is the disulfide linkage for redox-responsive drug delivery, which is cleaved by a high intracellular glutathione concentration [65,66,67]. Specifically, in the work of Liu et al. [49], cystamine was conjugated between deoxycholic acid (the hydrophobic segment) and chondroitin sulfate (the hydrophilic portion) for the delivery of docetaxel to treat melanoma. This system was designed as a dual-responsive drug release trigger because chondroitin sulfate was degraded by hyaluronidase-1 in addition to the redox-sensitive cystamine (with disulfide linkage) [49]. Generally, the use of responsive parts in nanoconjugates represents a promising drug delivery strategy with the advantage of facilitating drug release by an on/off switch in the desired environment [49,50]. Nevertheless, a surplus of the responsive part may affect the hydrophobic–hydrophilic balance, which must be optimized for nanoparticle self-assembly and maximum stability [68].
3.3. Protonation for Hydrophobic-Hydrophilic Balance
In addition to hydrophobic–hydrophilic parity, other factors, such as electrostatic and van der Waals interactions, play crucial roles in promoting the formation of self-assembled nanoparticles. For instance, excessive protonation or deprotonation within individual nanoconjugates results in electrostatic repulsion and weakened attractive forces. Dey et al. demonstrated the role of proton balance in the structure of chitosan for self-assembled nanoparticles [69]. Their study indicated that the partial protonation and partial deprotonation of chitosan could aid the self-assembly of nanoparticles for various applications ranging from wound-healing to gene delivery [69].
3.4. Hydrophobic or Hydrophilic Segments as Multiple Targeting and Delivery Functions
Simple hydrophobic–hydrophilic nanoconjugates commonly have limited ability to target tumour sites. Moreover, successful cancer treatment is determined by the ability of the therapeutic to eradicate the tumour while affecting as few healthy cells as possible. Therefore, the nanoconjugates can be actively targeted to tumours for receptor-mediated uptake by specifically recognizing and binding target tissues or cells via a surface-attached specific ligand, such as a “vector” molecule [70,71,72,73]. Interestingly, the hydrophobic core of conjugate-forming nanoparticles can also be used for synergistic targeting effects (in addition to the common functions of hydrophobic segments in self-assembly and the loading of poorly water-soluble drugs) to simplify the multi-step fabrication of the self-assembled nanoparticles [51]. For example, glycyrrhetinic acid, which is a hydrophobic targeting ligand for hepatocytes, was successfully conjugated with hyaluronic acid (as a targeting ligand on the surface of the nanoparticles) to deliver doxorubicin [51,74,75,76]. This study also noted that a conjugate’s biological function may be affected by the binding site and should be considered in polymer conjugate designs [51].
In addition to the conjugation between hydrophobic and hydrophilic segments, self-assembled nanoparticle preparation strategies may use poorly water-soluble drugs as hydrophobic segments in the core-shell structure. This approach can lead to highly stable nanoparticles and high drug loading. For example, Taxol has been proposed to conjugate to a cell-penetrating peptide to yield a high drug loading of 26.4% [52]. This supramolecular nanosphere formation (~130 nm) could also be used as a carrier to deliver doxorubicin. Liu et al. [53] found that cytarabine could be loaded up to 63% into stable self-assembled spherical nanoparticles using this strategy. Currently, the highest loading efficiency is 89.5%, found in a study of a paclitaxel and succinic acid conjugate forming self-assembled nanofibres [54]. In another study, in addition to enhancing oral bioavailability, capsaicin was successfully synthesized and formed self-assembled nanoparticles to reduce mucosa irritation [77].
A hydrophilic therapeutic agent was also used as a carrier, leading to the discovery of its dual functions in nanoconjugates. An example of such a dual-function material is fucoidan, which possesses the properties of a hydrophilic carrier and has also been demonstrated to be a potential anticancer agent [78,79,80,81,82,83]. Fucoidan was conjugated to oleic acid for the loading of paclitaxel and curcumin to maximize efficacy [55]. While the fucoidan and paclitaxel conjugate showed a preference for being released in a physiological environment, the fucoidan and curcumin conjugate showed improved drug release in a tumour environment [55]. For further theranostic development, fucoidan and oleic acid were functionalized on iron oxide nanoparticles [56]. This research showed that fucoidan may stabilize iron oxide nanoparticles as well as deliver poorly water-soluble drugs [56].
3.5. Co-Delivery of Anticancer Drugs using Nanoconjugates
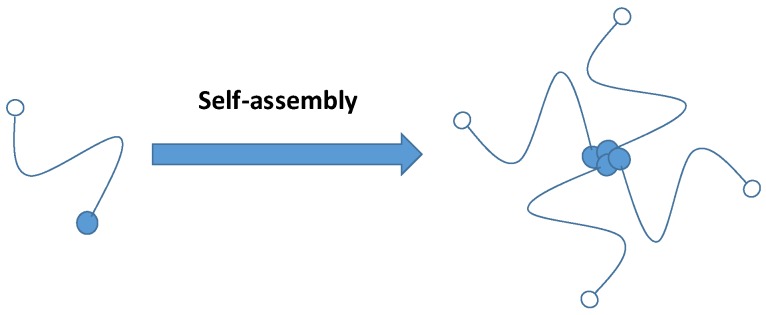
Combination therapy of anticancer drugs has shown potential in therapy due to multiple-mechanism actions, especially for resistant tumours, enabling the reduction of individual dosages and resulting in a synergistic effect and reduced toxicity [57,84,85]. Typically, one hydrophobic drug and one hydrophilic drug are combined and formulated with a hydrophilic carrier (also known as a spacer) for hydrophilic–hydrophobic balance in the structure (Figure 3). For example, docetaxel and gemcitabine were conjugated to polyethylene glycol, demonstrating that their therapeutic efficacy was significantly higher than that of individual drugs [57]. Similarly, Jain et al. bioconjugated gemcitabine and curcumin, likewise demonstrating that the conjugate is more effective than physically combined two drugs or a single drug [84]. Furthermore, Nam et al. [86] developed and compared fucoidan conjugated to curcumin and paclitaxel via an ester linkage. This system showed the dual delivery of two anticancer drugs, hydrophilic (fucoidan) and hydrophobic (curcumin, or paclitaxel) [86]. The curcumin–fucoidan conjugate released more drug in the acidic environment than the conjugate of paclitaxel and fucoidan [86].
Figure 3.
Illustration of drug co-delivery (blue and white dots) using a nanoconjugate through a spacer [57].
Although the strategy of using a spacer to link hydrophobic drugs and hydrophilic drugs yielded impressive therapeutic outcomes, spacer properties such as length, type, and linkage site greatly influence in vitro and in vivo efficacy, which must be taken into account during synthesis [87]. Therefore, direct drug–drug conjugation via a biodegradable bond has been discussed, and researchers have developed self-assembled nanoparticles without using carriers. In such a case, one hydrophobic drug and one hydrophilic drug could self-assemble, combining their own hydrophilic and hydrophobic segments, and form nanostructures with high drug loading, high reproducibility and the potential to improve poor drug solubility [88]. This strategy was used by Huang et al., who conjugated irinotecan (hydrophilic) to chlorambucil (hydrophobic) to achieve excellent anticancer activity [58]. A floxuridine and bendamustine conjugate was also found to overcome multidrug resistance using this strategy [89].
3.6. Core Crosslinked Self-Assembled Nanoparticles
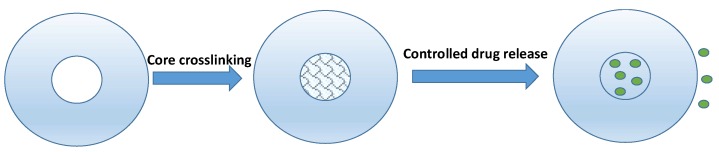
In an effort to control drug release, a core modification in self-assembled nanoparticles could be considered in addition to stimuli-responsive surface modifications, which have been widely reported [90,91]. The crosslinking degree was reported to have a strong effect on drug release in acidic environments [92]. Specifically, the core was crosslinked between furan rings and a crosslinker, resulting in a 10% or 20% degree of crosslinking to remain stable at high pH [92] (Figure 4). The core-crosslinked hyaluronic acid micelle demonstrated a high loading efficiency of a poorly water-soluble drug (>80%) [93]. The combination of lutetium-177-labelled core-crosslinked polymeric micelles and nanoparticles of cyclopamine synergistically delayed tumour growth in chemoradiation therapy [94]. In another recent study, docetaxel-loaded reduction-responsive core-crosslinked hyaluronic acid-b-poly(trimethylene carbonate-co-dithiolane trimethylene carbonate) micelles showed four-fold stronger tumour accumulation compared to free docetaxel [95].
Figure 4.
Illustration of core-crosslinked nanoconjugate for controlled drug release [92]. The green dots represent the drug molecules.
3.7. Nanoconjugate-Based Solid Dispersion
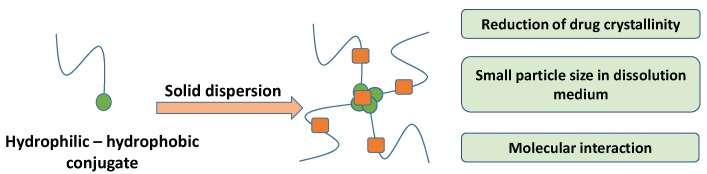
Solid dispersion is a technique in which poorly water-soluble drugs are dispersed in hydrophilic carriers [20,96,97]. Although hydrophilic carriers can prevent drug recrystallization, drug crystals cannot always be transformed into amorphous forms due to the drug’s structure or polymer type. Ngo et al. suggested using hydrophilic–hydrophobic blends to overcome this limitation [98]. Furthermore, Dinh et al. developed a hydrophilic–hydrophobic conjugate using a carrier in solid dispersion to improve drug bioavailability [59] (Figure 5). This conjugate was demonstrated to be a potential carrier because it can induce a molecular interaction, reducing drug particles and changing drug crystals in solid dispersion [59,60].
Figure 5.
Illustration of the use of a hydrophilic–hydrophobic conjugate as a carrier in solid dispersion [59]. The drug molecules (orange dots) are dispersed between hydrophilic parts (blue strings) and hydrophobic parts (green dots).
4. Nanotheranostic Conjugates
Amphiphilic nanoconjugates have been investigated for concomitant therapy and diagnostics to take full advantages of nanoconjugates as single nanomaterials [99,100]. Generally, the imaging agent is incorporated into the hydrophobic core or on the hydrophobic segment for image monitoring. This incorporation would be a simple encapsulation in a conjugate or in complex chemical reactions with a conjugate. Some examples of recent strategies using amphiphilic nanoconjugates as multifunctional nanoparticles are as follows (Table 2):
Table 2.
Example of studies on nanotheranostic conjugates of poorly water-soluble drugs.
| Poorly Water-Soluble Drugs | Imaging Agent | Key Results | References |
|---|---|---|---|
| Paclitaxel | Gadolinium | Significant contrast enhancement. Prolonged accumulation in a tumour. |
[102] |
| Doxorubicin | Rhodamine 6G | Increased fluorescence intensity for cancer cell detection. | [103,104] |
| Doxorubicin | Chlorin e6 | Enhanced fluorescence and photoacoustic imaging. Effective photodynamic therapy. |
[91,109] |
Gadolinium is known for its efficient loading in amphiphiles to combine drug release with enhanced magnetic resonance signals [101]. In a study on an enzyme-sensitive biodegradable conjugate for treating breast cancer with paclitaxel, gadolinium was chelated to the conjugate by reacting it with GdCl3·6H2O in the dark for 24 h [102]. The in vivo magnetic resonance imaging in this study showed significant contrast enhancement and prolonged accumulation in a tumour [102]. In addition, a fluorescence study demonstrated the efficient accumulation of a cyanine 5.5-labelled nanoconjugate in a tumour [102].
In another study, the fluorochrome Rhodamine 6G was bonded to an amphiphile via reversible addition-fragmentation chain transfer (RAFT) polymerization for tumour fluorescence imaging detection [103,104]. Interestingly, the fluorescence intensity of Rhodamine 6G is pH dependent. As the nanoconjugates accumulated in the tumour’s acidic environment, the fluorescence intensity increased to enable cancer cell detection [103,104].
Chlorin e6 has been reported as an NIR fluorescence imaging dye and can be used in photodynamic therapy in biomedical applications [105,106,107,108]. Recently, the coupling of chlorin e6 was to hyaluronic acid via adipic dihydrazide was proposed for dual-modal imaging and phototherapy [109]. This study indicated that a chlorin e6-labelled nanoconjugate enhanced fluorescence and photoacoustic imaging by releasing chlorin e6 in a tumour [109]. Moreover, the accumulation of the nanoconjugate containing chlorin e6 demonstrated effective photodynamic therapy [91,109].
5. Future Prospects of Nanoconjugation for Poorly Water-Soluble Drugs
Harnessing the potential of nanoconjugation for poorly water-soluble drugs would be an effective approach to overcome barriers to the clinical translation of these drugs. However, despite extensive studies on the development of novel nanoconjugate systems with determined structures, the challenges of how to maximize targeting activity and therapeutic efficacy and minimize unwanted side effects remain. Burst drug release, incomplete drug dissolution, drug loading efficiency, and drug resistance all present difficult tasks that must be addressed and solved during the manufacture of these synthesized materials. Additional ongoing challenges include obtaining homogenous structures, achieving reproducible batch-to-batch synthesis, particularly of complex nanoconjugates, decreasing the time consumption of the synthesis, and scaling up product quality control. Nevertheless, a smart design of nanoconjugates in the first stage of a study, including the selection of materials, a rational design approach, formulations, a synthesis approach and efficient characterization techniques, would drive experimental studies to successful outcomes for translational applications.
6. Conclusions
A wide range of nanoconjugates has been developed to improve the bioavailability of poorly water-soluble drugs. Significant efforts in recent studies have demonstrated improved effects of nanoconjugates on drug solubility, particle size, and drug co-delivery, and delivery. Specifically, the strategy of direct conjugation between hydrophilic and hydrophobic drugs could facilitate the generation of nanoconjugates without the use of carriers, resulting in the advantages of high drug loading and limited batch variation. Further investigations of these efficient drug delivery systems should focus on clinical translations. Furthermore, optimization and scale-up procedures should also be attempted and addressed.
Author Contributions
Writing—original draft preparation, T.T.D.T.; writing—review and editing, P.H.L.T.
Funding
Phuong Ha Lien Tran is the recipient of Australian Research Council’s Discovery Early Career Researcher Award (project number DE160100900).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Weerapol Y., Limmatvapirat S., Takeuchi H., Sriamornsak P. Fabrication of spontaneous emulsifying powders for improved dissolution of poorly water-soluble drugs. Powder Technol. 2015;271:100–108. doi: 10.1016/j.powtec.2014.10.037. [DOI] [Google Scholar]
- 2.Hauss D.J. Oral lipid-based formulations. Adv. Drug Delivery. Rev. 2007;59:667–676. doi: 10.1016/j.addr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Ha N.S., Tran T.T.-D., Tran P.H.-L., Park J.-B., Lee B.-J. Dissolution-enhancing mechanism of alkalizers in poloxamer-based solid dispersions and physical mixtures containing poorly water-soluble valsartan. Chem. Pharm. Bull. 2011;59:844–850. doi: 10.1248/cpb.59.844. [DOI] [PubMed] [Google Scholar]
- 4.Sahbaz Y., Williams H.D., Nguyen T.-H., Saunders J., Ford L., Charman S.A., Scammells P.J., Porter C.J. Transformation of Poorly Water-Soluble Drugs into Lipophilic Ionic Liquids Enhances Oral Drug Exposure from Lipid Based Formulations. Mol. Pharm. 2015 doi: 10.1021/mp500790t. [DOI] [PubMed] [Google Scholar]
- 5.Thao T.D.T., Phuong H.L.T. Perspectives on Strategies Using Swellable Polymers in Solid Dispersions for Controlled Drug Release. Curr. Pharm. Des. 2017;23:1639–1648. doi: 10.2174/1381612822666161021152932. [DOI] [PubMed] [Google Scholar]
- 6.Tran P., Tran H., Lee B. Modulation of microenvironmental pH and crystallinity of ionizable telmisartan using alkalizers in solid dispersions for controlled release. J. Control. Release. 2008;129:59–65. doi: 10.1016/j.jconrel.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Tran T.T.-D., Tran P.H.-L., Lee B.-J. Dissolution-modulating mechanism of alkalizers and polymers in a nanoemulsifying solid dispersion containing ionizable and poorly water-soluble drug. Eur. J. Pharm. Biopharm. 2009;72:83–90. doi: 10.1016/j.ejpb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Tuong N.G.N., Van-Thanh T., Wei D., Phuong H.L.T., Tran T.T.D. Nanoprecipitation for Poorly Water-Soluble. Drugs. Curr. Drug Metab. 2017;18:1000–1015. doi: 10.2174/1389200218666171004112122. [DOI] [PubMed] [Google Scholar]
- 9.Tran P.H.-L., Tran T.T.-D., Vo T.V. Polymer Conjugate-Based Nanomaterials for Drug Delivery. J. Nanosci. Nanotechnol. 2014;14:815–827. doi: 10.1166/jnn.2014.8901. [DOI] [PubMed] [Google Scholar]
- 10.Barros A.L.B.d., Mota L.d.G., Soares D.C.F., Souza C.M.M.M.d., Cassali G.D., Oliveira M.C.d., Cardoso V.N. Long-Circulating, pH-Sensitive Liposomes versus Long-Circulating, Non-pH-Sensitive Liposomes as a Delivery System for Tumor Identification. J. Biomed. Nanotechnol. 2013;9:1636–1643. doi: 10.1166/jbn.2013.1649. [DOI] [PubMed] [Google Scholar]
- 11.Nanjwade B.K., Patel J.M., Manvi F.V. Formulation and Evaluation of Paclitaxel Loaded Nanoproliposome. J. Nanopharm. Drug Deliv. 2013;1:165–172. doi: 10.1166/jnd.2013.1003. [DOI] [Google Scholar]
- 12.Khadka P., Ro J., Kim H., Kim I., Kim J.T., Kim H., Cho J.M., Yun G., Lee J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014;9:304–316. doi: 10.1016/j.ajps.2014.05.005. [DOI] [Google Scholar]
- 13.Phillips M.A., Gran M.L., Peppas N.A. Targeted Nanodelivery of Drugs and Diagnostics. Nano today. 2010;5:143–159. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekiguchi K., Obi N. Studies on absorption of eutectic mixture. I. A Comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem. Pharm. Bull. 1961;9:866–872. doi: 10.1248/cpb.9.866. [DOI] [Google Scholar]
- 15.Kaur S., Jena S.K., Samal S.K., Saini V., Sangamwar A.T. Freeze dried solid dispersion of exemestane: A way to negate an aqueous solubility and oral bioavailability problems. Eur. J. Pharm. Sci. 2017;107:54–61. doi: 10.1016/j.ejps.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Parikh T., Sandhu H.K., Talele T.T., Serajuddin A.T. Characterization of solid dispersion of itraconazole prepared by solubilization in concentrated aqueous solutions of weak organic acids and drying. Pharm. Res. 2016;33:1456–1471. doi: 10.1007/s11095-016-1890-8. [DOI] [PubMed] [Google Scholar]
- 17.Brouwers J., Geboers S., Mols R., Tack J., Augustijns P. Gastrointestinal behavior of itraconazole in humans–Part 1: Supersaturation from a solid dispersion and a cyclodextrin-based solution. Int. J. Pharm. 2017;525:211–217. doi: 10.1016/j.ijpharm.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Mazumder S., Dewangan A.K., Pavurala N. Enhanced dissolution of poorly soluble antiviral drugs from nanoparticles of cellulose acetate based solid dispersion matrices. Asian J. Pharm. Sci. 2017;12:532–541. doi: 10.1016/j.ajps.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawicki E., Schellens J., Beijnen J., Nuijen B. Inventory of oral anticancer agents: Pharmaceutical formulation aspects with focus on the solid dispersion technique. Cancer Treat. Rev. 2016;50:247–263. doi: 10.1016/j.ctrv.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Tran T.T.-D., Tran P.H.L., Lim J., Park J.B., Choi S.K., Lee B.J. Physicochemical principles of controlled release solid dispersion containing a poorly water-soluble drug. Ther. Deliv. 2010;1:51–62. doi: 10.4155/tde.10.3. [DOI] [PubMed] [Google Scholar]
- 21.Kopeček J. Polymer–drug conjugates: Origins, progress to date and future directions. Adv. Drug Deli. Rev. 2013;65:49–59. doi: 10.1016/j.addr.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabholkar R.D., Sawant R.M., Mongayt D.A., Devarajan P.V., Torchilin V.P. Polyethylene glycol–phosphatidylethanolamine conjugate (PEG–PE)-based mixed micelles: Some properties, loading with paclitaxel, and modulation of P-glycoprotein-mediated efflux. Int. J. Pharm. 2006;315:148–157. doi: 10.1016/j.ijpharm.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Hoshikawa A., Nagira M., Tane M., Fukushige K., Tagami T., Ozeki T. Preparation of Curcumin-Containing α-, β-, and γ-Cyclodextrin/Polyethyleneglycol-Conjugated Gold Multifunctional Nanoparticles and Their in Vitro Cytotoxic Effects on A549 Cells. Biol. Pharm. Bull. 2018;41:908–914. doi: 10.1248/bpb.b18-00010. [DOI] [PubMed] [Google Scholar]
- 24.Tran P.H.-L., Tran T.T.-D., Vo T.V., Vo C.L.-N., Lee B.-J. Novel Multifunctional Biocompatible Gelatin-Oleic Acid Conjugate: Self-Assembled Nanoparticles for Drug Delivery. J. Biomed. Nanotechnol. 2013;9:1416–1431. doi: 10.1166/jbn.2013.1621. [DOI] [PubMed] [Google Scholar]
- 25.Ke X., Ng V.W.L., Ono R.J., Chan J.M.W., Krishnamurthy S., Wang Y., Hedrick J.L., Yang Y.Y. Role of non-covalent and covalent interactions in cargo loading capacity and stability of polymeric micelles. J. Control. Release. 2014;193:9–26. doi: 10.1016/j.jconrel.2014.06.061. [DOI] [PubMed] [Google Scholar]
- 26.Chechetka S.A., Yu Y., Zhen X., Pramanik M., Pu K., Miyako E. Light-driven liquid metal nanotransformers for biomedical theranostics. Nat. Commun. 2017;8:15432. doi: 10.1038/ncomms15432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta P., Singh M., Kumar R., Belz J., Shanker R., Dwivedi P.D., Sridhar S., Singh S.P. Synthesis and in vitro studies of PLGA-DTX nanoconjugate as potential drug delivery vehicle for oral cancer. Int. J. Nanomed. 2018;13:67–69. doi: 10.2147/IJN.S124995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bader H., Ringsdorf H., Schmidt B. Watersoluble polymers in medicine. Die Angewandte Makromolekulare Chemie. 1984;123:457–485. doi: 10.1002/apmc.1984.051230121. [DOI] [Google Scholar]
- 29.Letchford K., Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007;65:259–269. doi: 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Branco M.C., Schneider J.P. Self-assembling materials for therapeutic delivery. Acta Biomater. 2009;5:817–831. doi: 10.1016/j.actbio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Ma P.X. Host-guest interactions mediated nano-assemblies using cyclodextrin-containing hydrophilic polymers and their biomedical applications. Nano today. 2010;5:337–350. doi: 10.1016/j.nantod.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen K. Structural properties of self-assembled polymeric aggregates in aqueous solutions. Polym. Adv. Technol. 2001;12:2–22. doi: 10.1002/1099-1581(200101/02)12:1/2<2::AID-PAT946>3.0.CO;2-E. [DOI] [Google Scholar]
- 33.Nagasaki Y., Yasugi K., Yamamoto Y., Harada A., Kataoka K. Sugar-Installed Block Copolymer Micelles: Their Preparation and Specific Interaction with Lectin Molecules. Biomacromolecules. 2001;2:1067–1070. doi: 10.1021/bm015574q. [DOI] [PubMed] [Google Scholar]
- 34.Ding J., Chen L., Xiao C., Chen L., Zhuang X., Chen X. Noncovalent interaction-assisted polymeric micelles for controlled drug delivery. Chem. Commun. 2014;50:11274–11290. doi: 10.1039/C4CC03153A. [DOI] [PubMed] [Google Scholar]
- 35.Yang X., Shi X., D’Arcy R., Tirelli N., Zhai G. Amphiphilic polysaccharides as building blocks for self-assembled nanosystems: Molecular design and application in cancer and inflammatory diseases. J. Control. Release. 2018;272:114–144. doi: 10.1016/j.jconrel.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 36.Negahdaripour M., Golkar N., Hajighahramani N., Kianpour S., Nezafat N., Ghasemi Y. Harnessing self-assembled peptide nanoparticles in epitope vaccine design. Biotechnol. Adv. 2017;35:575–596. doi: 10.1016/j.biotechadv.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan P., Chen X., Metavarayuth K., Su J., Wang Q. Self-assembled supramolecular systems for bone engineering applications. Curr. Opin. Colloid Interface Sci. 2018;35:104–111. doi: 10.1016/j.cocis.2018.01.015. [DOI] [Google Scholar]
- 38.Qi W., Zhang X., Wang H. Self-assembled polymer nanocomposites for biomedical application. Curr. Opin. Colloid Interface Sci. 2018;35:36–41. doi: 10.1016/j.cocis.2018.01.003. [DOI] [Google Scholar]
- 39.Xu L., Sun M., Ma W., Kuang H., Xu C. Self-assembled nanoparticle dimers with contemporarily relevant properties and emerging applications. Mater. Today. 2016;19:595–606. doi: 10.1016/j.mattod.2016.05.015. [DOI] [Google Scholar]
- 40.Timmermans S.B.P.E., van Hest J.C.M. Self-assembled nanoreactors based on peptides and proteins. Curr. Opin. Colloid Interface Sci. 2018;35:26–35. doi: 10.1016/j.cocis.2018.01.005. [DOI] [Google Scholar]
- 41.Palao-Suay R., Gómez-Mascaraque L.G., Aguilar M.R., Vázquez-Lasa B., Román J.S. Self-assembling polymer systems for advanced treatment of cancer and inflammation. Prog. Polym. Sci. 2016;53:207–248. doi: 10.1016/j.progpolymsci.2015.07.005. [DOI] [Google Scholar]
- 42.Habibi N., Kamaly N., Memic A., Shafiee H. Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery. Nano Today. 2016;11:41–60. doi: 10.1016/j.nantod.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eskandari S., Guerin T., Toth I., Stephenson R.J. Recent advances in self-assembled peptides: Implications for targeted drug delivery and vaccine engineering. Adv. Drug Delivery. Rev. 2017;110–111:169–187. doi: 10.1016/j.addr.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Acar H., Srivastava S., Chung E.J., Schnorenberg M.R., Barrett J.C., LaBelle J.L., Tirrell M. Self-assembling peptide-based building blocks in medical applications. Adv. Drug Delivery. Rev. 2017;110–111:65–79. doi: 10.1016/j.addr.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi N., Arai R. Design and construction of self-assembling supramolecular protein complexes using artificial and fusion proteins as nanoscale building blocks. Curr. Opin. Biotechnol. 2017;46:57–65. doi: 10.1016/j.copbio.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Sapra P., Zhao H., Mehlig M., Malaby J., Kraft P., Longley C., Greenberger L.M., Horak I.D. Novel Delivery of SN38 Markedly Inhibits Tumor Growth in Xenografts, Including a Camptothecin-11–Refractory Model. Clin. Cancer Res. 2008;14:1888–1896. doi: 10.1158/1078-0432.CCR-07-4456. [DOI] [PubMed] [Google Scholar]
- 47.Zhao H., Rubio B., Sapra P., Wu D., Reddy P., Sai P., Martinez A., Gao Y., Lozanguiez Y., Longley C., et al. Novel Prodrugs of SN38 Using Multiarm Poly(ethylene glycol) Linkers. Bioconjug. Chem. 2008;19:849–859. doi: 10.1021/bc700333s. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y., Liu K., Li X., Xiao S., Zheng D., Zhu P., Li C., Liu J., He J., Lei J., et al. A novel self-assembled nanoparticle platform based on pectin-eight-arm polyethylene glycol-drug conjugates for co-delivery of anticancer drugs. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;86:28–41. doi: 10.1016/j.msec.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 49.Liu M., Du H., Khan A.R., Ji J., Yu A., Zhai G. Redox/enzyme sensitive chondroitin sulfate-based self-assembled nanoparticles loading docetaxel for the inhibition of metastasis and growth of melanoma. Carbohydr. Polym. 2018;184:82–93. doi: 10.1016/j.carbpol.2017.12.047. [DOI] [PubMed] [Google Scholar]
- 50.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12:991. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 51.Wang X., Gu X., Wang H., Yang J., Mao S. Enhanced delivery of doxorubicin to the liver through self-assembled nanoparticles formed via conjugation of glycyrrhetinic acid to the hydroxyl group of hyaluronic acid. Carbohydr. Polym. 2018;195:170–179. doi: 10.1016/j.carbpol.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 52.Tian R., Wang H., Niu R., Ding D. Drug delivery with nanospherical supramolecular cell penetrating peptide–taxol conjugates containing a high drug loading. J. Colloid Interface Sci. 2015;453:15–20. doi: 10.1016/j.jcis.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 53.Liu R., Zhang J., Zhang D., Wang K., Luan Y. Self-assembling nanoparticles based on cytarabine prodrug for enhanced leukemia treatment. J. Mol. Liq. 2018;251:178–184. doi: 10.1016/j.molliq.2017.12.086. [DOI] [Google Scholar]
- 54.Xu H., Lu X., Li J., Ding D., Wang H., Li X., Xie W. Superior antitumor effect of extremely high drug loading self-assembled paclitaxel nanofibers. Int. J. Pharm. 2017;526:217–224. doi: 10.1016/j.ijpharm.2017.04.081. [DOI] [PubMed] [Google Scholar]
- 55.Uyen T.P., Khanh T.N., Toi V.V., Wei D., Phuong H.L.T., Tran T.D. Investigation of Fucoidan-Oleic Acid Conjugate for Delivery of Curcumin and Paclitaxel. Anti-Cancer Agents Med. Chem. 2016;16:1281–1287. doi: 10.2174/1567201810666131124140259. [DOI] [PubMed] [Google Scholar]
- 56.Tran K.N., Tran P.H.L., Van Vo T., Tran T.T.D. Design of fucoidan functionalized - Iron oxide nanoparticles for biomedical applications. Curr. Drug Deliv. 2016;13:774–783. doi: 10.2174/1567201812666151020100921. [DOI] [PubMed] [Google Scholar]
- 57.Kushwah V., Katiyar S.S., Agrawal A.K., Gupta R.C., Jain S. Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2018 doi: 10.1016/j.nano.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Huang P., Wang D., Su Y., Huang W., Zhou Y., Cui D., Zhu X., Yan D. Combination of Small Molecule Prodrug and Nanodrug Delivery: Amphiphilic Drug–Drug Conjugate for Cancer Therapy. J. Am. Chem. Soc. 2014;136:11748–11756. doi: 10.1021/ja505212y. [DOI] [PubMed] [Google Scholar]
- 59.Dinh H.T.T., Tran P.H.L., Duan W., Lee B.-J., Tran T.T.D. Nano-sized solid dispersions based on hydrophobic-hydrophilic conjugates for dissolution enhancement of poorly water-soluble drugs. Int. J. Pharm. 2017;533:93–98. doi: 10.1016/j.ijpharm.2017.09.065. [DOI] [PubMed] [Google Scholar]
- 60.Tran C.T.M., Tran P.H.L., Tran T.T.D. pH-independent dissolution enhancement for multiple poorly water-soluble drugs by nano-sized solid dispersions based on hydrophobic–hydrophilic conjugates. Drug Dev. Ind. Pharm. 2019;45:514–519. doi: 10.1080/03639045.2018.1562466. [DOI] [PubMed] [Google Scholar]
- 61.Larson N., Ghandehari H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012;24:840–853. doi: 10.1021/cm2031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J.Y., Bae K.H., Kim J.S., Nam Y.S., Park T.G. Intracellular delivery of paclitaxel using oil-free, shell cross-linked HSA—Multi-armed PEG nanocapsules. Biomaterials. 2011;32:8635–8644. doi: 10.1016/j.biomaterials.2011.07.063. [DOI] [PubMed] [Google Scholar]
- 63.Nojima Y., Suzuki Y., Yoshida K., Abe F., Shiga T., Takeuchi T., Sugiyama A., Shimizu H., Sato A. Lactoferrin Conjugated with 40-kDa Branched Poly(ethylene Glycol) Has an Improved Circulating Half-Life. Pharm. Res. 2009;26:2125–2132. doi: 10.1007/s11095-009-9925-z. [DOI] [PubMed] [Google Scholar]
- 64.Ramon J., Saez V., Baez R., Aldana R., Hardy E. PEGylated Interferon-α2b: A Branched 40K Polyethylene Glycol Derivative. Pharm. Res. 2005;22:1375–1387. doi: 10.1007/s11095-005-5278-4. [DOI] [PubMed] [Google Scholar]
- 65.Gover Antoniraj M., Angelin Tisha S., Mahesh A., Shanmugarathinam A., Kandasamy R. Synthesis and characterization of cystamine conjugated chitosan-SS-mPEG based 5-Fluorouracil loaded polymeric nanoparticles for redox responsive drug release. Eur. J. Pharm. Sci. 2018;116:37–47. doi: 10.1016/j.ejps.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 66.Russo A., DeGraff W., Friedman N., Mitchell J.B. Selective modulation of glutathione levels in human normal versus tumor cells and subsequent differential response to chemotherapy drugs. Cancer Res. 1986;46:2845. [PubMed] [Google Scholar]
- 67.Torchilin V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discovery. 2014;13:813. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Owen S.C., Chan D.P.Y., Shoichet M.S. Polymeric micelle stability. Nano Today. 2012;7:53–65. doi: 10.1016/j.nantod.2012.01.002. [DOI] [Google Scholar]
- 69.Dey A., Kamat A., Nayak S., Danino D., Kesselman E., Dandekar P., Jain R. Role of proton balance in formation of self-assembled chitosan nanoparticles. Colloids Surf. B. 2018;166:127–134. doi: 10.1016/j.colsurfb.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 70.Amin H.H., Meghani N.M., Park C., Nguyen V.H., Tran T.T.-D., Tran P.H.-L., Lee B.-J. Fattigation-platform nanoparticles using apo-transferrin stearic acid as a core for receptor-oriented cancer targeting. Colloids Surf. B. 2017;159:571–579. doi: 10.1016/j.colsurfb.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 71.Tamura R., Uemoto S., Tabata Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017;57:274–284. doi: 10.1016/j.actbio.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 72.Tian T., Zhang H.-X., He C.-P., Fan S., Zhu Y.-L., Qi C., Huang N.-P., Xiao Z.-D., Lu Z.-H., Tannous B.A., et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 73.Mendes M., Miranda A., Cova T., Gonçalves L., Almeida A.J., Sousa J.J., do Vale M.L.C., Marques E.F., Pais A., Vitorino C. Modeling of ultra-small lipid nanoparticle surface charge for targeting glioblastoma. Eur. J. Pharm. Sci. 2018;117:255–269. doi: 10.1016/j.ejps.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 74.Cai Y., Xu Y., Chan H.F., Fang X., He C., Chen M. Glycyrrhetinic Acid Mediated Drug Delivery Carriers for Hepatocellular Carcinoma Therapy. Mol. Pharm. 2016;13:699–709. doi: 10.1021/acs.molpharmaceut.5b00677. [DOI] [PubMed] [Google Scholar]
- 75.Tian Q., Wang X., Wang W., Zhang C., Liu Y., Yuan Z. Insight into glycyrrhetinic acid: The role of the hydroxyl group on liver targeting. Int. J. Pharm. 2010;400:153–157. doi: 10.1016/j.ijpharm.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 76.Wang X.-H., Tian Q., Wang W., Zhang C.-N., Wang P., Yuan Z. In vitro evaluation of polymeric micelles based on hydrophobically-modified sulfated chitosan as a carrier of doxorubicin. J. Mater. Sci. Mater. Med. 2012;23:1663–1674. doi: 10.1007/s10856-012-4627-1. [DOI] [PubMed] [Google Scholar]
- 77.Feng Y., Zhu Y., Wan J., Yang X., Firempong C.K., Yu J., Xu X. Enhanced oral bioavailability, reduced irritation and increased hypolipidemic activity of self-assembled capsaicin prodrug nanoparticles. J. Funct. Foods. 2018;44:137–145. doi: 10.1016/j.jff.2018.03.006. [DOI] [Google Scholar]
- 78.Senni K., Gueniche F., Foucault-Bertaud A., Igondjo-Tchen S., Fioretti F., Colliec-Jouault S., Durand P., Guezennec J., Godeau G., Letourneur D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006;445:56–64. doi: 10.1016/j.abb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Li B., Lu F., Wei X., Zhao R. Fucoidan: Structure and Bioactivity. Molecules. 2008;13:1671–1695. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tran T.T.-D., Tran P.H.-L., Phan M.-N., Van T.V. Colon specific delivery of fucoidan by incorporation of acidifier in enteric coating polymer. Int. J. Pharma Biosci. Technol. 2013;1:108–117. [Google Scholar]
- 81.Koyanagi S., Tanigawa N., Nakagawa H., Soeda S., Shimeno H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003;65:173–179. doi: 10.1016/S0006-2952(02)01478-8. [DOI] [PubMed] [Google Scholar]
- 82.Aisa Y., Miyakawa Y., Nakazato T., Shibata H., Saito K., Ikeda Y., Kizaki M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005;78:7–14. doi: 10.1002/ajh.20182. [DOI] [PubMed] [Google Scholar]
- 83.Maruyama H., Tamauchi H., Iizuka M., Nakano T. The Role of NK cells in Antitumor Activity of Dietary Fucoidan from Undaria pinnatifida Sporophylls (Mekabu) Planta Med. 2006;72:1415–1417. doi: 10.1055/s-2006-951703. [DOI] [PubMed] [Google Scholar]
- 84.Jain S., Jain R., Das M., Agrawal A.K., Thanki K., Kushwah V. Combinatorial bio-conjugation of gemcitabine and curcumin enables dual drug delivery with synergistic anticancer efficacy and reduced toxicity. RSC Advances. 2014;4:29193–29201. doi: 10.1039/C4RA04237A. [DOI] [Google Scholar]
- 85.Parhi P., Mohanty C., Sahoo S.K. Nanotechnology-based combinational drug delivery: An emerging approach for cancer therapy. Drug Discov. Today. 2012;17:1044–1052. doi: 10.1016/j.drudis.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Nam Hoang P., Thao Thanh L., Minh Nguyet P., Thinh Duc L., Toi Van V., Phuong Ha-Lien T., Thao Truong-Dinh T. A Comparison of Fucoidan Conjugated to Paclitaxel and Curcumin for the Dual Delivery of Cancer Therapeutic Agents. Anti-Cancer Agents Med. Chem. 2017;17:1–7. doi: 10.2174/1871520617666171121125845. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y., Wang X., Deng F., Zheng N., Liang Y., Zhang H., He B., Dai W., Wang X., Zhang Q. The effect of linkers on the self-assembling and anti-tumor efficacy of disulfide-linked doxorubicin drug-drug conjugate nanoparticles. J. Control. Release. 2018;279:136–146. doi: 10.1016/j.jconrel.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 88.Ma W., Su H., Cheetham A.G., Zhang W., Wang Y., Kan Q., Cui H. Synergistic antitumor activity of a self-assembling camptothecin and capecitabine hybrid prodrug for improved efficacy. J. Control. Release. 2017;263:102–111. doi: 10.1016/j.jconrel.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 89.Zhang T., Huang P., Shi L., Su Y., Zhou L., Zhu X., Yan D. Self-Assembled Nanoparticles of Amphiphilic Twin Drug from Floxuridine and Bendamustine for Cancer Therapy. Mol. Pharm. 2015;12:2328–2336. doi: 10.1021/acs.molpharmaceut.5b00005. [DOI] [PubMed] [Google Scholar]
- 90.Park W., Bae B.-C., Na K. A highly tumor-specific light-triggerable drug carrier responds to hypoxic tumor conditions for effective tumor treatment. Biomaterials. 2016;77:227–234. doi: 10.1016/j.biomaterials.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 91.Ha S.E., Chung-Sung L., Kun N. Photomediated Reactive Oxygen Species-Generable Nanoparticles for Triggered Release and Endo/Lysosomal Escape of Drug upon Attenuated Single Light Irradiation. Adv Healthc Mater. 2015;4:2822–2830. doi: 10.1002/adhm.201500622. [DOI] [PubMed] [Google Scholar]
- 92.Galbis E., de-Paz M.V., Iglesias N., Lacroix B., Alcudia A., Galbis J.A. Core cross-linked nanoparticles from self-assembling polyfma-based micelles. Encapsulation of lipophilic molecules. Eur. Polym. J. 2017;89:406–418. doi: 10.1016/j.eurpolymj.2017.02.032. [DOI] [Google Scholar]
- 93.Han H.S., Choi K.Y., Ko H., Jeon J., Saravanakumar G., Suh Y.D., Lee D.S., Park J.H. Bioreducible core-crosslinked hyaluronic acid micelle for targeted cancer therapy. J. Control. Release. 2015;200:158–166. doi: 10.1016/j.jconrel.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 94.You J., Zhao J., Wen X., Wu C., Huang Q., Guan F., Wu R., Liang D., Li C. Chemoradiation therapy using cyclopamine-loaded liquid–lipid nanoparticles and lutetium-177-labeled core-crosslinked polymeric micelles. J. Control. Release. 2015;202:40–48. doi: 10.1016/j.jconrel.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Y., Zhang J., Meng F., Cheng L., Feijen J., Zhong Z. Reduction-responsive core-crosslinked hyaluronic acid-b-poly(trimethylene carbonate-co-dithiolane trimethylene carbonate) micelles: Synthesis and CD44-mediated potent delivery of docetaxel to triple negative breast tumor in vivo. J. Mater. Chem. B. 2018;6:3040–3047. doi: 10.1039/C8TB00094H. [DOI] [PubMed] [Google Scholar]
- 96.Tran P.H.L., Tran T.T.D., Park J.B., Lee B.J. Controlled release systems containing solid dispersions: Strategies and mechanisms. Pharm. Res. 2011;28:2353–2378. doi: 10.1007/s11095-011-0449-y. [DOI] [PubMed] [Google Scholar]
- 97.Tran T.T.D., Tran P.H.L., Khanh T.N., Van T.V., Lee B.J. Solubilization of poorly water-soluble drugs using solid dispersions. Recent Pat. Drug Deliv. Formul. 2013;7:122–133. doi: 10.2174/1872211311307020004. [DOI] [PubMed] [Google Scholar]
- 98.Ngo H., Nguyen P.K., Van Vo T., Duan W., Tran V.T., Tran P.H.L., Tran T.T.D. Hydrophilic-hydrophobic polymer blend for modulation of crystalline changes and molecular interactions in solid dispersion. Int. J. Pharm. 2016;513:148–152. doi: 10.1016/j.ijpharm.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 99.Lammers T., Kiessling F., Hennink W.E., Storm G. Nanotheranostics and Image-Guided Drug Delivery: Current Concepts and Future Directions. Mol. Pharm. 2010;7:1899–1912. doi: 10.1021/mp100228v. [DOI] [PubMed] [Google Scholar]
- 100.Tran T.T.D., Van Vo T., Tran P.H.L. Design of iron oxide nanoparticles decorated oleic acid and bovine serum albumin for drug delivery. Chem. Eng. Res. Des. 2015;94:112–118. doi: 10.1016/j.cherd.2014.12.016. [DOI] [Google Scholar]
- 101.Hu X., Liu G., Li Y., Wang X., Liu S. Cell-Penetrating Hyperbranched Polyprodrug Amphiphiles for Synergistic Reductive Milieu-Triggered Drug Release and Enhanced Magnetic Resonance Signals. J. Am. Chem. Soc. 2015;137:362–368. doi: 10.1021/ja5105848. [DOI] [PubMed] [Google Scholar]
- 102.Cai H., Wang X., Zhang H., Sun L., Pan D., Gong Q., Gu Z., Luo K. Enzyme-sensitive biodegradable and multifunctional polymeric conjugate as theranostic nanomedicine. Appl. Mater. Today. 2018;11:207–218. doi: 10.1016/j.apmt.2018.02.003. [DOI] [Google Scholar]
- 103.Jia X., Tian K., Zhao X., Zhou T., Pei M., Liu P. Fluorescent amphiphilic copolymer-based tumor theranostics for facile DOX-loading and tumor microenvironment-triggered release. Mater. Des. 2016;105:333–340. doi: 10.1016/j.matdes.2016.05.053. [DOI] [Google Scholar]
- 104.Jia X., Zhao X., Tian K., Zhou T., Li J., Zhang R., Liu P. Fluorescent Copolymer-Based Prodrug for pH-Triggered Intracellular Release of DOX. Biomacromolecules. 2015;16:3624–3631. doi: 10.1021/acs.biomac.5b01070. [DOI] [PubMed] [Google Scholar]
- 105.Liu P., Yue C., Sheng Z., Gao G., Li M., Yi H., Zheng C., Wang B., Cai L. Photosensitizer-conjugated redox-responsive dextran theranostic nanoparticles for near-infrared cancer imaging and photodynamic therapy. Polym. Chem. 2014;5:874–881. doi: 10.1039/C3PY01173A. [DOI] [Google Scholar]
- 106.Li Z., Wang C., Cheng L., Gong H., Yin S., Gong Q., Li Y., Liu Z. PEG-functionalized iron oxide nanoclusters loaded with chlorin e6 for targeted, NIR light induced, photodynamic therapy. Biomaterials. 2013;34:9160–9170. doi: 10.1016/j.biomaterials.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 107.Park H., Park W., Na K. Doxorubicin loaded singlet-oxygen producible polymeric micelle based on chlorine e6 conjugated pluronic F127 for overcoming drug resistance in cancer. Biomaterials. 2014;35:7963–7969. doi: 10.1016/j.biomaterials.2014.05.063. [DOI] [PubMed] [Google Scholar]
- 108.Huang P., Lin J., Wang S., Zhou Z., Li Z., Wang Z., Zhang C., Yue X., Niu G., Yang M., et al. Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy. Biomaterials. 2013;34:4643–4654. doi: 10.1016/j.biomaterials.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li W., Zheng C., Pan Z., Chen C., Hu D., Gao G., Kang S., Cui H., Gong P., Cai L. Smart hyaluronidase-actived theranostic micelles for dual-modal imaging guided photodynamic therapy. Biomaterials. 2016;101:10–19. doi: 10.1016/j.biomaterials.2016.05.019. [DOI] [PubMed] [Google Scholar]