Abstract
As the leading causes of human disability and mortality, neurological diseases affect millions of people worldwide and are on the rise. Although the general roles of several signaling pathways in the pathogenesis of neurodegenerative disorders have so far been identified, the exact pathophysiology of neuronal disorders and their effective treatments have not yet been precisely elucidated. This requires multi-target treatments, which should simultaneously attenuate neuronal inflammation, oxidative stress, and apoptosis. In this regard, astaxanthin (AST) has gained growing interest as a multi-target pharmacological agent against neurological disorders including Parkinson’s disease (PD), Alzheimer’s disease (AD), brain and spinal cord injuries, neuropathic pain (NP), aging, depression, and autism. The present review highlights the neuroprotective effects of AST mainly based on its anti-inflammatory, antioxidative, and anti-apoptotic properties that underlies its pharmacological mechanisms of action to tackle neurodegeneration. The need to develop novel AST delivery systems, including nanoformulations, targeted therapy, and beyond, is also considered.
Keywords: neurodegenerative diseases, astaxanthin, pharmacology, neuroprotective agent, oxidative stress, neuroinflammation, apoptosis, drug delivery system
1. Introduction
Neurodegenerative disorder, as a common cause of human disability and death, is a term referring to progressive, symmetric, and selective loss of sensory, motor, and cognitive neuronal structure/function leading to neuronal cell death [1]. The death of neurons underlies the symptoms of several chronic or acute neurological disorders including Parkinson’s disease (PD), Alzheimer’s disease (AD), and brain or spinal cord injuries [2]. Neuronal cell death also affects depression, neuropathic pain (NP), aging, and autism as other neurological disorders [3].
Several causative factors are behind the etiology of neuronal disorders such as oxidative stress, inflammation, and apoptosis, as the main pathological pathways, and they play destructive roles in neuronal cell death and neurodegenerative processes [4]. Microglia activation and cytokines/chemokines release of the inflammatory pathways [5], as well as reactive oxygen species (ROS) and mitochondrial damages in the oxidative stress pathway [6], have destructive effects on neurodegenerative processes, which finally lead to cell death [4].
In spite of many developments in the field of clinical healthcare, neuroprotective therapies of neurodegeneration and neuronal death-related disorders have still remained as clinical challenges with no effective solution. Therefore, the need to develop novel multi-target therapeutics is felt to regulate more involving the signaling pathways and is thought to improve the life quality of individuals with neurodegenerative diseases.
Carotenoids are red-orange lipophilic pigments found in nature [7,8] with protective effects for human health. Thanks to their potential biological activities and health benefits, carotenoids have been receiving growing attention [4,9]. Several reports have attributed positive effects to their antioxidant activities [10,11]. Being the strongest antioxidant between the carotenoids [9], Astaxanthin (AST) is a lipid-soluble keto-carotenoid, belonging to xanthophylls, which has gained attention in experimental methods due to its neuroprotective features [12,13].
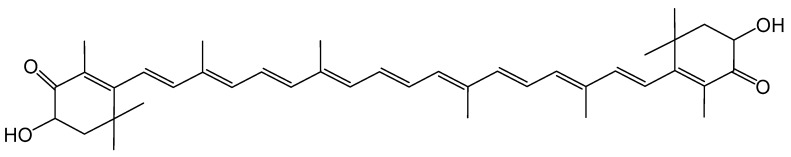
AST can be isolated mostly from microalgae Haematococcus pluvialis. However, shrimp, asteroidean, algae, lobster, crustacean, krill, trout, red sea bream, and salmon as marine animals and seafood are considered as other sources of extraction [4]. AST is chemically known as 3,3′-dihydroxy-β, β′-carotene-4,4′-dione (Figure 1), with the molecular formula of C40H52O4 [14]. It possesses a linear polar-nonpolar-polar structure with keto- and hydroxyl moieties at polar ends and conjugated carbon-carbon double bonds at a non-polar middle part, which allows it to fit specifically into the same span of cell membranes and pass through blood-brain barrier (BBB) [15].
Figure 1.
Chemical structure of Astaxanthin (AST).
It has been shown that AST can block oxidative stress, inflammation, and apoptosis as the key pathways of neurodegeneration [4]. In the inflammatory pathway, AST blocks the macrophage migration inhibitory factor (MIF) as an up-stream cytokine, N-methyl-D-aspartate (NMDA) receptor 2B (NR2B) [12,13], and IκB kinase β (Ikk β). Thus, it inhibits the release of interleukins (ILs), tumor necrosis factor alpha (TNF-α), intercellular adhesion molecule 1 (ICAM1), and monocyte chemoattractant protein-1 (MCP-1) [4]. To tackle the oxidative stress, AST inhibits phosphorylated extracellular regulated protein kinase/extracellular regulated protein kinase ratio (p-ERK/ERK) [12,13], activates Nrf2/antioxidant response elements (Nrf2/ARE), and increases the release of heme oxygenase-1 (HO-1), glutathione S- transferase-α1 (GST-α1), and NAD(P)H quinine oxidoreductase-1 (NQO-1) [4,16]. Indeed, as the major signaling pathway against oxidative stress, Kelch-like ECH-associated protein 1 (Keap1)-Nrf2-ARE plays a key role in the cellular antioxidant response. In the non-stressed situation, Keap1 degrades the Nrf2 protein in the cytoplasm through the proteasome. Upon the oxidative stress, Nrf2 is rapidly degraded by proteasomes via the interaction with Keap1. This modification leads to the cytoplasmic accumulation and the nucleus translocation of newly synthesized Nrf2 in order to bind to the ARE [17,18]. Nrf2-ARE complex, in turn, attenuates the expression of NAD(P)H quinone oxidoreductase-1, superoxide dismutase (SOD), HO-1, and other regulatory enzymes to activate the defense system [18,19]. While glutathione reductase and thioredoxin reductase protect the complexes stability of Keap1-Nrf2 as well as IκB-NF-κB, cytosolic H2O2 cause dissociation of the complexes and allows the nuclear transportation of NF-κB and Nrf2 [20,21]. In this line, the Nrf2-ARE pathway is believed to decrease ROS concentrations in order to keep a balance between ROS and the antioxidant potentials [22,23]. AST also acts against apoptosis by blocking p-ERK/ERK [12,13], cytochrome c, caspase3,9, and the Bax/Bcl2 ratio [4,24].
Nevertheless, considering the unsaturated structure of AST, it is highly susceptible to light, oxygen, and heat stress degradation. In addition, the poor water solubility and bioavailability of AST [25,26,27,28] limit its efficacy in vivo [29,30]. So, investigating novel AST delivery system is necessary in order to solve these drawbacks.
To the best of our knowledge, this is the first review regarding the role of each signaling pathway in the pathogenesis of neurodegenerative disorders, including PD, AD, NP, depression, brain and spinal cord injuries, aging, and autism, and it is the first review regarding the auspicious effects of AST against neurodegeneration pathways as well as clarifying novel AST delivery systems, all together.
2. AST and Neurodegenerative Diseases
2.1. AST and Parkinson’s Disease (PD)
PD is an age-related disorder and the second most common cause of neurodegenerative disorders with a prevalence of 0.1–0.2% worldwide and 3% in people older than 80 years old [31,32]. PD is characterized by midbrain dopaminergic neurons lost, aggregation of α-synuclein called Lewy bodies, and destruction of non-dopaminergic pathways leading to motor and non-motor dysfunction [33,34].
Due to their low efficacy and undesirable adverse effects, conventional treatments of PD are quite challenging. Hence, discovering an effective and safe novel multi-target agent to combat PD is of great importance.
Neuroinflammation and oxidative stress have a major contribution in the pathogenesis of PD [35,36]. The inhibition of the mediators of these pathways plays an important role in preventing the disease progression, which is what AST does as a multi-target drug. In a rat model of homocysteine (Hcy)-induced hippocampal neurotoxicity and apoptosis, AST regulated ROS-mediated oxidative damage and mitochondrial dysfunction. AST also attenuated PI3K/AKT and mitogen-activated protein kinase (MAPK) pathways and thus, was used to tackle these neurological disorders, such as PD [37]. AST acted through the SP1/NR1 and HO-1/NOX2 axis to inhibit MPP+ induced oxidative stress in PC12 cells [38,39]. MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) as a progressive cause of PD in experimental models [40] affected the hydroxylase activity of tyrosine as an enzyme involved in dopamine biosynthesis [41].
AST conserved substantia nigra from MPTP-induced dopaminergic neuronal loss in aged and young mice, but it was not able to protect against the loss of tyrosine hydroxylase induced by MPTP in aged mice. Therefore, Grimmig et al. considered aging as a critical factor in finding novel therapies for PD [42].
In an in vitro study, Lee and colleagues reported that AST ameliorated MPP+-induced production of ROS in SH-SY5Y human neuroblastoma cells. This effect may be attributed to a decrease in α-synuclein, caspase-3, and the Bax/Bcl-2 ratio and the increase of SOD, catalase, and tyrosine hydroxylase [43].
Moreover, Liu et al. indicated that AST pretreatment inhibited 6-hydroxydopamine (6-OHDA) or DHA hydroperoxide (DHA-OOH)-induced apoptosis, intracellular ROS generation, and mitochondrial dysfunctions in dopaminergic SH-SY5Y cells through its antioxidant potential and mitochondria protection [44]. Also, AST inhibited 6-OHDA-induced apoptosis and mitochondrial dysfunction via blocking the phosphorylation of p38 MAPK and reducing caspase 3/9 and poly(ADP-ribose) polymerase [45].
2.2. AST and Alzheimer’s Disease (AD), Cognition, and Memory
The brain has rich irrigation with blood vessels, very high oxygen consumption, and lower antioxidant capability, which it is very susceptible to oxidative damage [46]. AD is an age-related neurodegenerative disease, which is characterized by the overproduction and deposition of beta-amyloid peptide (Aβ) plaques and intracellular neurofibrillary tangles, and by a loss of neurons in the brain [47,48]. One of the main reasons for the development and progression of AD is the oxidative stress [49,50]. AST is one of the few compounds that can cross the blood-brain barrier (BBB) in mammals, and going beyond this barrier could increase their antioxidant properties. The molecular mechanisms still are not elucidated but there are many types of research focused on neuronal apoptosis. Shen et al. reported that AST reduced ischemia-related injury in brain tissue, mainly through the inhibition of oxidative stress. It also protected neuroblastoma cells against Aβ-induced oxidative cell death through induction of the antioxidant enzyme HO-1 expression [51]. Later, Wen et al. investigated the neuroprotective effects of AST on glutamate-induced oxidative ex situ toxicity in a mouse hippocampal HT22 cells through Nrf2-dependent HO-1 expression [52]. The results indicated that AST is a promising biologically active compound for the treatment of neurodegenerative disorders such as AD. The amount of glutathione in the plasma has a correlation with the severity of cognitive dysfunction in AD patients [53]. Another study demonstrated that AST apparently showed a protective effect on L-glutamate-induced PC12 cell death mainly through the Bcl-2/Bax signaling pathway and, therefore, it could be considered a promising agent as prophylactic or remediation against neuronal disorders [54]. An experiment with double transgenic mice administrated with AST and its synthesized variant docosahexaenoic acid-acylated AST diesters (AST-DHA) for 2 months suggested that AST-DHA might be a potential therapeutic agent for AD. In the study, Radial 8-Arm Maze Test, Water Maze Test, Determination of Aβ Concentration, and western blot analysis was carried out [55].
Many studies aimed at exploring the relationship between diet and their effects on cognitive ability [56,57]. Hussein et al. reported the neuroprotective actions of AST and its high potential in human health and nutrition [58]. The contribution of fish oil in the process is an important and significant step in the protection of the nervous system and especially of the brain [59,60]. A protective function of AST in microcirculation and mitochondrial functions was demonstrated [15], which confirms its potential efficacy in several neurodegenerative diseases [61].
2.3. AST and Neuropathic Pain (NP)
NP is caused by a disease or a lesion in the somatosensory nervous system [62], with an estimated cost of 40 billion dollars per year in the U.S. [63]. Several destructive signaling pathways and mechanisms are involved in NP, mostly including neuromodulators (glutamate, and especially NR2B, gamma-aminobutyric acid (GABA), serotonergic, and noradrenergic) and inflammatory agents (cytokines, prostaglandins, and reactive oxygen species) [64], which affect microglia and astrocytes activation, ion currents, and neuronal firing [65,66], as well as apoptosis [67].
Antidepressants and anticonvulsants are among the primary clinical alternatives for the management of NP [68]. Nevertheless, investigating novel multi-target pharmacological therapies for NP, which simultaneously target multiple destructive mediators with acceptable efficacy and safety, is of great importance.
As Sharma et al. reported, AST attenuated biochemical and behavioral alterations using in vivo and in vitro models of NP. They found that AST decreased astrocytic activation. Thereby, glial fibrillary acidic protein (GFAP) afforded suppression and reduced oxido-nitrosative stress in vitro. Also, AST antagonized NR2B in silico and reduced thermal and mechanical allodynia in a rat model of chronic constriction injury (CCI)-induced NP [69]. In the same in vivo model of NP, AST prevented the increase in IL-6, IL-1β, and TNF-α in the spinal cord and hippocampus of mice [70]. Fakhri and colleagues confirmed the neurotoxic role of NR2B as a glutamate-gated channel, as well as the inhibitory effects of AST on NR2B and the glutamate-initiated signaling pathways in a rat model of compression spinal cord injury (SCI). It was also found that, besides improving neuronal damages, AST down-regulated TNF-α and p-p38MAPK, through which neuroinflammation and mechanical allodynia was inhibited [13]. We also confirmed the neuroprotective effects of AST in reducing the cold allodynia passed through the inhibition of p-ERK/ERK and the activation of p-AKT/AKT [12]. In a carrageenan-induced mice model of pain and paw edema, AST decreased thermal and mechanical allodynia, as well as the lipid peroxidation and myeloperoxidase enzyme in the paw [71].
Altogether, AST is introduced as an effective drug to combat NP. Additionally, since patients with chronic NP are at high risk of co-morbid depression [70], the need to investigate the antidepression effects of AST is greatly raised.
2.4. AST and Depression
Depression is a common and complicated psychological condition for human health. As a major cause of mortality and morbidity, depression is predicted to be the main pathogeny of disability by 2030 [72]. Since the complex pathophysiology of depression is not yet completely known, the most suitable treatments are yet to be clarified [73]. In the treatment of depression, monoamine regulation still plays a crucial role and brings up tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and selective–norepinephrine reuptake inhibitors (SNERIs) [74,75], although most of them have low efficacy with potential adverse effects [76]. On the other hand, according to recent evidence, there is a close connection between depression and oxidative stress/inflammation, as non-monoaminergic pathways are involved in depression, which are now areas of active investigation. Besides, destructive intracellular pathways of oxidative stress and inflammation also underlie the etiology of depression and anxiety [77,78,79]. Altogether, investigating novel multi-target therapeutic agents for depression with acceptable safety and efficacy is still a medical need.
Several studies have reported the antidepressant-like effects of AST in different experimental models. As reported by Zhou and colleagues, AST prevented hyperglycemia-induced neuroinflammation contributing to depression. They also found that AST had an antidepressant-like effect by decreasing the level of IL-1β, IL-6, cyclooxygenase-2 (COX-2), cleaved caspase-3, and GFAP, and protecting neurons in the amygdala, hypothalamus, and hippocampus of mice [80]. In this context, Jiang et al. found that trans-AST ameliorated lipopolysaccharide (LPS)-induced depressive-like behaviors through the down-regulation of TNF-α, IL-6, and IL-1β and by antagonizing inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS), and COX-2 expression in mice [81]. Chronic treatment with trans-AST also prevented co-morbid depression in mice, owing to their potent anti-inflammatory effects and involvement in the serotonergic pathway [70]. In this sense, the involvement of the serotonergic pathway in the pathogenesis of depression and related inhibitory effect of trans-AST were raised again [82].
In a rat model of depression, AST reverted the antagonistic and impairing effects of ethanol on cortical spreading depression in a dose-dependent [83], but not age-dependent [84], manner, which was attributed to the antioxidant effects of AST [83]. Moreover, Qiao et al. used a mice model of omethoate-induced depression and found that a combination therapy of AST and lithium chloride efficiently attenuated depressive-like behavior through the Akt/GSK3β/CREB signaling pathway [85].
As another crucial non-monoaminergic mechanism, the NMDA receptor and the glutamatergic pathway play destructive roles in the pathogenesis of depression. A type-specific NMDA receptor antagonist could offer more efficacy and fewer complications related to broader NMDA receptor blockers. Since AST blocks NR2B efficiently [13], it can be suggested as a strong anti-depressant drug.
2.5. AST and Central Nervous System (Brain/Spinal Cord) Injuries
Central nervous system (CNS) injuries, including brain and spinal cord injury, affect millions of individuals worldwide [86,87]. As complex processes of primary and secondary phases, CNS injury phases initiate temporary or permanent neuronal damages. Following the mechanical injury, the primary phase is characterized by direct death of cells followed by the secondary phase, consisting of inflammatory, oxidative, apoptotic, and other molecular pathways that cause further edema and damages to neuronal cells by inciting a breach in the BBB [88,89,90]. There are several destructive mediators that can be targeted by neuroprotective agents to prevent CNS injuries. However, there are still no sufficient data available regarding the improvement of post-CNS injuries.
In the context of brain injury, AST treatment attenuated early brain injury (EBI) after subarachnoidal hemorrhage (SAH) by reducing the brain edema, BBB disruption, and caspase3. This neuroprotective effect of AST has been attributed to its strong antioxidant property by decreasing malondialdehyde (MDA) and increasing glutathione (GSH) and SOD in rodent models [90]. In an in vivo study, AST significantly prevented H2O2-induced apoptosis, improved neurological deficit, and diminished the infarct volume, as well [91]. Also, AST suppressed oxygen-glucose deprivation (OGD)-induced oxidative stress by upregulating the protein expression of HO-1, Hsp32, and Hsp90 in SH-SY5Y cells. Thus, it is confirmed that the neuroprotective effects of AST in CNS damages are also related to its antioxidant effects [92].
Following EBI in SAH model, AST positively attenuated the cortical expression of NAD (P) H: quinone oxidoreductase 1 (NQO-1), HO-1, and glutathione S-transferase-α1 (GST-α1) through the antioxidant pathway named Nrf2-ARE at both mRNA and protein levels. Additionally, AST ameliorated BBB disruption, brain edema, apoptosis, and neurological dysfunction in this context [93].
Considering that apoptosis plays a crucial role in the pathogenesis of EBI, AST considerably increased the phosphorylation of Akt and Bad levels, which led to a reduction in apoptosis and caspase-3 levels following SAH [94]. AST ameliorated mitochondrial membrane potential, cerebral vasospasm, and mitochondria-associated neuronal apoptosis by reducing caspase-3, the Bax/Bcl-2 ratio, and cytochrome c in the prefrontal cortex post-SAH [95].
Zhang et al. found that treatment with AST prevented SAH injury by inhibiting the toll-like receptor 4 signaling pathway and increasing sirtuin 1 and the subsequent inflammatory response, both in vivo and in vitro [96]. This indicated the role of inflammation, besides oxidative stress and apoptosis, in CNS injuries. In a rat model of SAH, AST down-regulated matrix metallopeptidases-9 (MMP-9) which was attributed to a decrement in the level of infiltrating neutrophils, activated microglia, TNF-α, and IL-1β [97].
Recent advancements have also clarified the contribution of Na+/K+/2Cl co-transporters (NKCCs) and aquaporins (AQPs) to brain edema during traumatic brain injury (TBI). Following TBI, AST attenuated AQP4/NKCC1-level in mice brain tissue [98]. According to Zhang and colleagues, AST down-regulated NKCC1 expression through the nuclear factor-kB (NF-κB) pathway, which mediates pro-inflammatory factors, and also protecting astrocytes against TBI [99].
In a rat model of SCI, Fakhri et al. reported that AST down-regulated NR2B, TNF-α, and p-p38MAPK, and it also preserved the tissue and neuronal damages and improved the sensory-motor function following a rat model of compression SCI [13]. We also found that AST increased and decreased the protein expression ratio of p-AKT/AKT and p-ERK/ERK, respectively, following SCI [12].
2.6. AST and Aging
The oxidative stress increases with aging and the brain become significantly more vulnerable to neurodegenerative disease [100,101]. The deficits in memory formation in older individuals caused by oxidative stress affect synaptic plasticity in neural networks in the hippocampus by protecting D-serine-dependent NMDA receptor activation. Hippocampal neurogeneration is associated with learning and memory processes [102]. Another study in mice showed that hippocampal oxidative stress is age dependent [103]. The reaction of young and aged animals to AST treatment on brain oxidative markers showed that there are no significant differences among them, and AST improves all types of oxidative markers in the six studied brain regions—namely the frontal cortex, striatum, parietal cortex, hypothalamus, hippocampus, and cerebellum [104]. The effects of AST on the aging female and male rat brains have been analyzed and the results obtained showed the gender-related differences [105].
Researchers studied the inhibition of oxidative injury of biological membranes by AST and found that its efficacy is higher than vitamin E, which is associated with the mitochondrial theory of aging [106]. This property highlights its unique potential to combat aging [4,107]. Hussein et al. marked the properties of AST for the prevention of age-related macular degeneration [58], and Wu et al. reported its effect on inhibition of apoptosis and alleviation of injury in the brains of aging rats [108].
Topically applied, AST affected aging skin with a visible wrinkle reduction. There are some clinical studies supporting this statement [109]. Yamashita found that the combined use of a dietary supplement containing AST and tocotrienol from palm oil resulted in a significant reduction of fine wrinkles [110]. On the other hand, a single-blind placebo-controlled study showed that dietary supplement containing only AST with a dose of 4 mg per day led to significant improvements in human skin characterized by elasticity during a dermatologist’s visual assessment [109]. The combination of oral supplementation with topical application resulted in significant improvements in skin wrinkles, age spot size, elasticity, skin texture, moisture, the content of the corneocyte layer, and the corneocyte condition [111].
2.7. AST and Autism
As a neurodegenerative disease with an increasing prevalence, autism is characterized by impairments in communication, social interaction, behavior, and regular activities [112,113,114]. Age-related and progressive neuronal pathology and neuronal loss in cerebellar Purkinje [115] or amygdala [116] cells in individuals with autism [117] are all representative of a neurodegenerative process [118,119,120,121]. There is also a close link between autism and the release of proinflammatory cytokines [122,123]. Thus, AST is a multi-target anti-neuroinflammatory drug that can be used against autism.
Besides, given the importance of oxidative stress in the pathogenesis of autism [124,125,126,127], AST as the strongest antioxidant among the carotenoid pigments [9] can be used to combat autism, as Ornoy et al. also reported [128]. On the other hand, the plasma concentrations of exogenous antioxidants, such as carotenoids, are insufficient in autistic adolescents and children, which confirms the role of oxidative stress in the pathogenesis of autism and the inhibitory effects of carotenoids [129].
AST increased the paw withdrawal latency, evaluated by a hot plate test, and improved the behavioral disorders, assessed by the social interaction and open field tests, in a mice model of pre-natally valproic acid-induced autism. In the same context, AST treatment also reduced oxidative stress through the reduction of nitric oxide and lipid peroxidation and the increment of catalase activity [104]. Frutos et al. reported that due to their anti-oxidative and anti-inflammatory properties, carotenoids are introduced as potential foods against autism [130].
Considering the role of oxidative stress and neuroinflammation signaling mediators in the pathogenesis of autism, each of these mediators can be a target to combat autism. Even though there is not enough evidence regarding the effects of AST on autism, it can be suggested as an auspicious neuroprotective agent against autism due to its inhibitory role on neuroinflammation and oxidative stress.
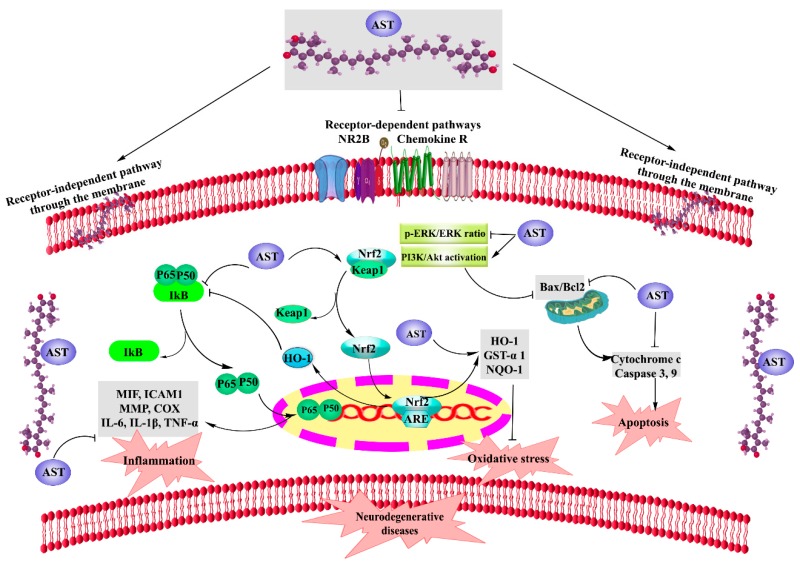
Figure 2 shows the neuroprotective mechanisms of AST for combating neurodegenerative diseases (Figure 2).
Figure 2.
Neuroprotective mechanisms of AST. NR2B: N-methyl-D-aspartate (NMDA) receptor 2B, Chemokine R: Chemokine receptor, HO-1: heme oxygenase-1, GST-α1: glutathione-s-transferase-α1, NQO-1: NAD(P)H quinone oxidoreductase-1, MDA: malondialdehyde, ARE: antioxidant response elements, PI3K/AKT: phosphoinositide 3-Kinase/AKT, p-ERK/ERK: phosphorylated extracellular regulated protein kinase/extracellular regulated protein kinase ratio, MIF: macrophage migration inhibitory factor, ICAM1: Intercellular adhesion molecule 1, MMP: Matrix metallopeptidase, TNF-α: Tumor necrosis factor alpha, NOS: Nitric oxide synthase, COX: Cyclooxygenase, IL: Interlukine, Bax/Bcl2: Bax/Bcl2 ratio, and AST: astaxanthin.
3. AST Novel Delivery Systems: Nanoformulations, Targeted Therapy, and Beyond
As a division of nanotechnology, nanomedicine uses biocompatible and biodegradable nanoaggregates and submicron-sized nanoparticles to target pharmacokinetics, administration routes, and bioavailability of drugs in medicine [131].
AST is a lipid-soluble carotenoid with low bioavailability that is partly absorbed by intestinal cells. It displays poor dispersibility and water solubility in aqueous solutions and is susceptible to light, oxygen, and heat stress degradation [25,26,27,28]. In spite of the strong in vitro neuroprotective effects of AST, the lack of an appropriate AST-delivery system to pass through BBB fails to show the same responses in vivo. Such characteristics of AST have prevented it from being widely used in biomedical or pharmaceutical applications. On the other hand, various approaches have so far been applied to formulate AST into a novel drug delivery system in order to increase its stability, solubility, and bioavailability, and to prolong its shelf life [29,30]. In addition to low bioavailability and lack of an appropriate drug delivery system, high thermolability, instability, and lipophilicity of AST have caused its antioxidant efficacy to be failed in clinical trials. Thus, developing an appropriate drug delivery system capable of overcoming these limitations is of great necessity. However, there are promising ways to overcome these limitations and to improve the performance of pharmaceuticals to prepare a suitable nanoformulation of AST. In this regard, different formulations of AST-loaded lipid-based carriers (LBCs), polymeric systems, and inclusion complexes have been provided to potentiate its effect [132].
As an auspicious delivery system, LBCs offer to enhance the stability and bioavailability of active pharmaceutical ingredients (API) while allowing a controlled release [132,133] achieved by a surface charge or adsorption of a layer of polymer or surfactant. LBCs consist of oil in water (O/W) nano/microemulsions, oil-loaded solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and micelles [134].
As dispersions of small spheroid within an aqueous medium, O/W microemulsions are thermodynamically stable, while nanoemulsions are unstable colloids [134,135]. The bioavailability of AST nanoemulsions has been reported to increase through the elevation of its oxidative and physical stability [136,137]. SLNs are mixtures of O/W nano/microemulsions with the high-ordered inner crystalline structure of lipid phase. SLN-AST is shown to possess unique properties such as large surface area, high drug loading, small size, and a wide spectrum of biodistribution, which, in return, realizes the goals of site-specific and controlled drug delivery as a promising strategy for efficient delivery of hydrophobic drugs. It offers more potential neuronal applications for passing through BBB to protect the brain from oxidative stress and to provide valuable support for brain health [138,139]. As reported by Bhatt et al., SLN-AST was shown to be an effective treatment to tackle oxidative stress-induced neurodegeneration in pheochromocytoma-12 cell line [139]. AST nanoemulsion also plays a momentous role in the stimulation of oxidative stress and mitochondrial-mediated apoptosis in cancer cells [140]. So, mitochondrial-targeted therapy via novel delivery systems could potentiate the effect of AST. Since drug-loaded nanoemulsion is a lipophilic molecule, it is likely to be localized to the membrane, followed by the mitochondria and nucleus [141]. Such drug delivery systems are implemented to selectively target mitochondria, and the targeted therapy was confirmed by apoptotic changes, mitochondrial membrane potential, and intracellular changes of membrane ROS [140].
To compensate for the low bioavailability and capacity of drug loading and release in SLN, NLCs were developed with a less-ordered inner crystalline structure allowing higher bioavailability and loadings of the lipophilic API with easier scale-up [142,143,144,145]. Indeed, NLCs, as more superior options than other colloidal carriers, present a remarkable capability to preserve, stabilize, and amplify the antioxidant capacity of AST [132,133,146,147]. Rodriguez-Ruiz et al. reported that ASTCO2-NLCs could be potentially introduced as excellent candidates for developing new platforms applications for medical devices, as well as antioxidant delivery systems for nutraceuticals [146]. Tamjidi et al. showed no drastic effects of ionic strength, heat, pH, and simulated gastric juice on the chemical stability of AST-loaded NLCs [148]. Several other studies also suggested NLCs as a successful AST-delivery system [29,149]. Although NLCs improve the functionality of AST from different aspects, the key role of emulsifiers in the system composition cannot be neglected [150,151], indicating the strong effect of the emulsifier on the bioavailability of AST-loaded NLCs [152].
Along with SLNs and NLCs, nanoliposomes as nano-scaled colloid systems made from the dispersion of amphiphilic lipids in aqueous solvents are considered to be other successful lipid base carriers in delivery systems [153]. Nanoliposomes benefit from such characteristics as higher membrane penetration and bioavailability, as well as superior potentiality to be used for specific drug targeting systems [154]. Pan et al. revealed that AST-loaded nanoliposomes increased the membrane micropolarity and decreased the membrane fluidity in order to attenuate the membrane structural properties [153]. AST-loaded liposomes functioned as a promising drug delivery system to treat hepatotoxicity [155] with increased bioavailability [155,156]. According to Peng et al., liposome encapsulation also elevated the stability, transportability, and antioxidation properties of AST [156]. The potential of liposomal encapsulation to enhance the radical scavenging effects of AST has been shown by other studies as well [157,158,159].
Polymeric systems have also been recently used as other novel delivery systems to enhance the solubility, protection of the biological activity, physicochemical stability, and the antioxidant effects of AST, and also to control its release. Due to their biodegradability and biocompatibility properties, chitosan [160,161] and alginate [134,162,163,164], as natural polysaccharides, are used in polymeric delivery systems of AST microencapsulation, leading to higher cell uptake and antioxidant effects of AST. The absorption profile of polymeric nanosystems of AST is affected by its shape, particle size, and surface properties, which could be used to handle the release of AST [164].
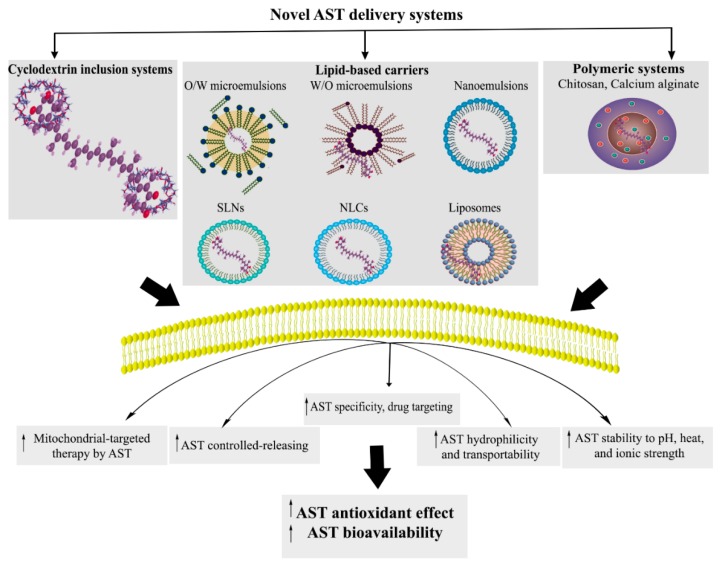
Another upcoming method for AST delivery is to use the inclusion complex formation of cyclodextrin. It is a natural macrocyclic oligosaccharide with a hydrophilic outer surface and a lipophilic cavity used to enclose lipophilic molecules like AST [165]. The involved non-covalent guest–host associations result in the controlled release of the guest, increase of its hydrophilicity, stability against light, heat, and oxygen [166], and bioavailability [167], as well as an improvement in the antioxidation potential of the guest [168]. In several studies, cyclodextrin has shown the potential to increase the stability, hydrophilicity, bioaccessibility [169,170], and antioxidant activity of AST [168]. It is worth mentioning that cyclodextrin inclusion formation gives more hydrophilicity activity to AST, compared to NLCs and polymeric delivery systems. Figure 3 indicates novel AST delivery systems as well as their effects on AST properties (Figure 3).
Figure 3.
Novel AST delivery systems. O/W: oil in water, W/O: water in oil, SLNs: solid lipid nanoparticles, NLCs: nanostructured lipid carriers, and AST: astaxanthin.
From the diagnostic point of view, AST nanoparticles could also be used for diagnostic purposes. Bharathiraja et al. reported the potential of AST gold nanoparticles in the photo-based diagnosis of cancer in the near infra-red range [171].
In general, the novel delivery systems of AST, including lipid carriers, mitochondrial targeted, polymeric, and cyclodextrin inclusion systems, could be considered as new ways to potentiate the effects of AST by improving its stability, hydrophilicity, and antioxidant capacity to be used in several diseases (i.e., neurodegenerative disorders).
4. Conclusions and Future Perspective
AST is an oxy-carotenoid with potential effects on healthcare. Although most of the studies have considered the protective effects of AST on various diseases, recent studies have focused more on their neuroprotective effects. As a multi-target neuroprotective agent, AST affects multiple mechanisms of action to tackle complex pathophysiological mechanisms of neurodegenerative diseases, mainly based on its anti-inflammatory, antioxidant, and antiapoptotic effects. All neuroprotective pharmacological mechanisms of action of AST are highlighted in the current review. On the other hand, the lack of an appropriate drug delivery system of AST has caused its efficacy to be failed in clinical trials. So, investigating an appropriate AST delivery system in order to solve this drawback is of great importance.
Such reports will provide novel applications of AST in the prevention, management, and treatment of neurodegenerative diseases, as well as investigating the most potential novel AST delivery system in clinical trials. Additional studies are needed to elucidate the precise pathophysiological pathways involved in neurodegeneration, and to clarify the potential neuroprotective effects of appropriate AST formulations on humans.
Author Contributions
Conceptualization, S.F., M.H.F., and E.S.-S.; designing the structure of the paper, S.F., M.H.F.; drafting the manuscript, S.F., M.H.F., and I.Y.A.; review and editing the paper: S.F., M.H.F., E.S.-S.; revising the manuscript, S.F., M.H.F., and E.S.-S.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declared no conflicts of interest.
References
- 1.Corrigan J.D., Selassie A.W., Orman J.A.L. The epidemiology of traumatic brain injury. J. Head Trauma Rehabil. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 2.Karnati H.K., Panigrahi M.K., Gutti R.K., Greig N.H., Tamargo I.A. miRNAs: Key players in neurodegenerative disorders and epilepsy. J. Alzheimer’s Dis. 2015;48:563–580. doi: 10.3233/JAD-150395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mamik M.K., Power C. Inflammasomes in neurological diseases: Emerging pathogenic and therapeutic concepts. Brain. 2017;140:2273–2285. doi: 10.1093/brain/awx133. [DOI] [PubMed] [Google Scholar]
- 4.Fakhri S., Abbaszadeh F., Dargahi L., Jorjani M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018;136:1–20. doi: 10.1016/j.phrs.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Shabab T., Khanabdali R., Moghadamtousi S.Z., Kadir H.A., Mohan G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017;127:624–633. doi: 10.1080/00207454.2016.1212854. [DOI] [PubMed] [Google Scholar]
- 6.Bhat A.H., Dar K.B., Anees S., Zargar M.A., Masood A., Sofi M.A., Ganie S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. Biomed. Pharmacother. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Sasso S., Pohnert G., Lohr M., Mittag M., Hertweck C. Microalgae in the postgenomic era: A blooming reservoir for new natural products. FEMS Microbiol. Rev. 2012;36:761–785. doi: 10.1111/j.1574-6976.2011.00304.x. [DOI] [PubMed] [Google Scholar]
- 8.Varela J.C., Pereira H., Vila M., León R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015;125:423–436. doi: 10.1007/s11120-015-0149-2. [DOI] [PubMed] [Google Scholar]
- 9.Gong M., Bassi A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016;34:1396–1412. doi: 10.1016/j.biotechadv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Chuyen H.V., Eun J.-B. Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2017;57:2600–2610. doi: 10.1080/10408398.2015.1063477. [DOI] [PubMed] [Google Scholar]
- 11.Manayi A., Abdollahi M., Raman T., Nabavi S.F., Habtemariam S., Daglia M., Nabavi S.M. Lutein and cataract: From bench to bedside. Crit. Rev. Biotechnol. 2016;36:829–839. doi: 10.3109/07388551.2015.1049510. [DOI] [PubMed] [Google Scholar]
- 12.Fakhri S., Dargahi L., Abbaszadeh F., Jorjani M. Effects of astaxanthin on sensory-motor function in a compression model of spinal cord injury: Involvement of ERK and AKT signalling pathway. Eur. J. Pain. 2019;23:750–764. doi: 10.1002/ejp.1342. [DOI] [PubMed] [Google Scholar]
- 13.Fakhri S., Dargahi L., Abbaszadeh F., Jorjani M. Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury. Brain Res. Bull. 2018;143:217–224. doi: 10.1016/j.brainresbull.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Zajac G., Machalska E., Kaczor A., Kessler J., Bour P., Baranska M. Structure of supramolecular astaxanthin aggregates revealed by molecular dynamics and electronic circular dichroism spectroscopy. Phys. Chem. Chem. Phys. 2018;20:18038–18046. doi: 10.1039/C8CP01742E. [DOI] [PubMed] [Google Scholar]
- 15.Kidd P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. A J. Clin. Ther. 2011;16:355–364. [PubMed] [Google Scholar]
- 16.Wu H., Niu H., Shao A., Wu C., Dixon B., Zhang J., Yang S., Wang Y. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar. Drugs. 2015;13:5750–5766. doi: 10.3390/md13095750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu R.P., Hayashi T., Cottam H.B., Jin G., Yao S., Wu C.C., Rosenbach M.D., Corr M., Schwab R.B., Carson D.A. Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc. Nat. Acad. Sci. USA. 2010;107:7479–7484. doi: 10.1073/pnas.1002890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barros M.P., Rodrigo M.J., Zacarias L. Dietary carotenoid roles in redox homeostasis and human health. J. Agric. Food Chem. 2018;66:5733–5740. doi: 10.1021/acs.jafc.8b00866. [DOI] [PubMed] [Google Scholar]
- 19.Kansanen E., Jyrkkänen H.-K., Levonen A.-L. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic. Biol. Med. 2012;52:973–982. doi: 10.1016/j.freeradbiomed.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 20.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 21.Jones D.P., Sies H. The redox code. Antioxid. Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigo R., Prieto J.C., Castillo R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n− 3 fatty acids: molecular mechanisms and potential clinical applications. Clin. Sci. 2013;124:1–15. doi: 10.1042/CS20110663. [DOI] [PubMed] [Google Scholar]
- 23.De Roos B., Duthie G.G. Role of dietary pro-oxidants in the maintenance of health and resilience to oxidative stress. Mol. Nutr. Food Res. 2015;59:1229–1248. doi: 10.1002/mnfr.201400568. [DOI] [PubMed] [Google Scholar]
- 24.Masoudi A., Dargahi L., Abbaszadeh F., Pourgholami M.H., Asgari A., Manoochehri M., Jorjani M. Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behav. Brain Res. 2017;329:104–110. doi: 10.1016/j.bbr.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Ambati R., Phang S.-M., Ravi S., Aswathanarayana R. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X., McClements D.J., Cao Y., Xiao H. Chemical and Physical Stability of Astaxanthin-Enriched Emulsion-Based Delivery Systems. Food Biophys. 2016;11:302–310. doi: 10.1007/s11483-016-9443-6. [DOI] [Google Scholar]
- 27.Taksima T., Limpawattana M., Klaypradit W. Astaxanthin encapsulated in beads using ultrasonic atomizer and application in yogurt as evaluated by consumer sensory profile. LWT Food Sci. Technol. 2015;62:431–437. doi: 10.1016/j.lwt.2015.01.011. [DOI] [Google Scholar]
- 28.Barańska M., Kaczor A. Carotenoids: Nutrition, Analysis and Technology. Wiley; Blackwell, UK: 2016. [Google Scholar]
- 29.Affandi M., Julianto T., Majeed A. Development and stability evaluation of astaxanthin nanoemulsion. Asian J. Pharm. Clin. Res. 2011;4:142–148. [Google Scholar]
- 30.Ogawa M., Sato M., Suzuki K. Development of astaxanthin nano emulsion with improved shelf life and enhanced absorbability. Fuji Film Res. Dev. 2007;52:26. [Google Scholar]
- 31.Strickland D., Bertoni J.M. Parkinson’s prevalence estimated by a state registry. Mov. Disord. Off. J. Mov. Disord. Soc. 2004;19:318–323. doi: 10.1002/mds.10619. [DOI] [PubMed] [Google Scholar]
- 32.Tysnes O.B., Storstein A. Epidemiology of Parkinson’s disease. J. Neural Transm. (Vienna) 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 33.Archibald N., Miller N., Rochester L. Neurorehabilitation in Parkinson disease. Handb. Clin. Neurol. 2013;110:435–442. doi: 10.1016/B978-0-444-52901-5.00037-X. [DOI] [PubMed] [Google Scholar]
- 34.Shtilbans A., Henchcliffe C. Biomarkers in Parkinson’s disease: An update. Curr. Opin. Neurol. 2012;25:460–465. doi: 10.1097/WCO.0b013e3283550c0d. [DOI] [PubMed] [Google Scholar]
- 35.Stojkovska I., Wagner B.M., Morrison B.E. Parkinson’s disease and enhanced inflammatory response. Exp. Biol. Med. 2015;240:1387–1395. doi: 10.1177/1535370215576313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang T., Sun Q., Chen S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016;147:1–19. doi: 10.1016/j.pneurobio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Wang X.-J., Chen W., Fu X.-T., Ma J.-K., Wang M.-H., Hou Y.-J., Tian D.-C., Fu X.-Y., Fan C.-D. Reversal of homocysteine-induced neurotoxicity in rat hippocampal neurons by astaxanthin: Evidences for mitochondrial dysfunction and signaling crosstalk. Cell Death Dis. 2018;5:50. doi: 10.1038/s41420-018-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye Q., Zhang X., Huang B., Zhu Y., Chen X. Astaxanthin suppresses MPP+-induced oxidative damage in PC12 cells through a Sp1/NR1 signaling pathway. Mar. Drugs. 2013;11:1019–1034. doi: 10.3390/md11041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Q., Huang B., Zhang X., Zhu Y., Chen X. Astaxanthin protects against MPP+-induced oxidative stress in PC12 cells via the HO-1/NOX2 axis. BMC Neurosci. 2012;13:156. doi: 10.1186/1471-2202-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee K.-S., Lee J.-K., Kim H.-G., Kim H.R. Differential effects of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine on motor behavior and dopamine levels at brain regions in three different mouse strains. Korean J. Physiol. Pharmacol. 2013;17:89–97. doi: 10.4196/kjpp.2013.17.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolland A.-S., Kareva T., Kholodilov N., Burke R.E. A quantitative evaluation of a 2.5-kb rat tyrosine hydroxylase promoter to target expression in ventral mesencephalic dopamine neurons in vivo. Neuroscience. 2017;346:126–134. doi: 10.1016/j.neuroscience.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Grimmig B., Daly L., Subbarayan M., Hudson C., Williamson R., Nash K., Bickford P.C. Astaxanthin is neuroprotective in an aged mouse model of Parkinson’s disease. Oncotarget. 2018;9:10388. doi: 10.18632/oncotarget.23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee D.-H., Kim C.-S., Lee Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011;49:271–280. doi: 10.1016/j.fct.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X., Shibata T., Hisaka S., Osawa T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009;1254:18–27. doi: 10.1016/j.brainres.2008.11.076. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda Y., Tsuji S., Satoh A., Ishikura M., Shirasawa T., Shimizu T. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2008;107:1730–1740. doi: 10.1111/j.1471-4159.2008.05743.x. [DOI] [PubMed] [Google Scholar]
- 46.Perluigi M., Sultana R., Cenini G., Di Domenico F., Memo M., Pierce W.M., Coccia R., Butterfield D.A. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer’s disease: Role of lipid peroxidation in Alzheimer’s disease pathogenesis. Proteom. Clin. Appl. 2009;3:682–693. doi: 10.1002/prca.200800161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burns A. Alzheimer’s disease: On the verges of treatment and prevention. Lancet Neurol. 2009;8:4–5. doi: 10.1016/S1474-4422(08)70271-0. [DOI] [PubMed] [Google Scholar]
- 48.Sadigh-Eteghad S., Sabermarouf B., Majdi A., Talebi M., Farhoudi M., Mahmoudi J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Prin. Prac. 2015;24:1–10. doi: 10.1159/000369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim H.A., Miller A.A., Drummond G.R., Thrift A.G., Arumugam T.V., Phan T.G., Srikanth V.K., Sobey C.G. Vascular cognitive impairment and Alzheimer’s disease: Role of cerebral hypoperfusion and oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012;385:953–959. doi: 10.1007/s00210-012-0790-7. [DOI] [PubMed] [Google Scholar]
- 50.Padurariu M., Ciobica A., Lefter R., Lacramioara Serban I., Stefanescu C., Chirita R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr. Danub. 2013;25:401–409. [PubMed] [Google Scholar]
- 51.Wang H.-Q., Sun X.-B., Xu Y.-X., Zhao H., Zhu Q.-Y., Zhu C.-Q. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010;1360:159–167. doi: 10.1016/j.brainres.2010.08.100. [DOI] [PubMed] [Google Scholar]
- 52.Wen X., Huang A., Hu J., Zhong Z., Liu Y., Li Z., Pan X., Liu Z. Neuroprotective effect of astaxanthin against glutamate-induced cytotoxicity in HT22 cells: Involvement of the Akt/GSK-3β pathway. Neuroscience. 2015;303:558–568. doi: 10.1016/j.neuroscience.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 53.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015;24:325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y., Wang W., Hao C., Mao X., Zhang L. Astaxanthin protects PC12 cells from glutamate-induced neurotoxicity through multiple signaling pathways. J. Funct. Foods. 2015;16:137–151. doi: 10.1016/j.jff.2015.04.008. [DOI] [Google Scholar]
- 55.Che H., Li Q., Zhang T., Wang D., Yang L., Xu J., Yanagita T., Xue C., Chang Y., Wang Y. Effects of Astaxanthin and Docosahexaenoic-Acid-Acylated Astaxanthin on Alzheimer’s Disease in APP/PS1 Double-Transgenic Mice. J. Agric. Food Chem. 2018;66:4948–4957. doi: 10.1021/acs.jafc.8b00988. [DOI] [PubMed] [Google Scholar]
- 56.Komaki A., Karimi S.A., Salehi I., Sarihi A., Shahidi S., Zarei M. The treatment combination of vitamins E and C and astaxanthin prevents high-fat diet induced memory deficits in rats. Pharmacol. Biochem. Behav. 2015;131:98–103. doi: 10.1016/j.pbb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Thapa A., Carroll N. Dietary modulation of oxidative stress in Alzheimer’s disease. Int. J. Mol. Sci. 2017;18:1583. doi: 10.3390/ijms18071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hussein G., Sankawa U., Goto H., Matsumoto K., Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006;69:443–449. doi: 10.1021/np050354+. [DOI] [PubMed] [Google Scholar]
- 59.Thomas J., Thomas C.J., Radcliffe J., Itsiopoulos C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. Biomed Res. Int. 2015;2015:172801. doi: 10.1155/2015/172801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Echeverria F., Valenzuela R., Catalina Hernandez-Rodas M., Valenzuela A. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: New dietary sources. Prostaglandins Leukot. Essent. Fat. Acids. 2017;124:1–10. doi: 10.1016/j.plefa.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Barros M., Poppe S., Bondan E. Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients. 2014;6:1293–1317. doi: 10.3390/nu6031293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finnerup N.B., Haroutounian S., Kamerman P., Baron R., Bennett D.L., Bouhassira D., Cruccu G., Freeman R., Hansson P., Nurmikko T., et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain. 2016;157:1599–1606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forner S., Martini A., De Andrade E., Rae G. Neuropathic pain induced by spinal cord injury: Role of endothelin ETA and ETB receptors. Neurosci. Lett. 2016;617:14–21. doi: 10.1016/j.neulet.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Kramer J.L., Minhas N.K., Jutzeler C.R., Erskine E.L., Liu L.J., Ramer M.S. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. J. Neurosci. Res. 2017;95:1295–1306. doi: 10.1002/jnr.23881. [DOI] [PubMed] [Google Scholar]
- 65.Lampert A., Hains B.C., Waxman S.G. Upregulation of persistent and ramp sodium current in dorsal horn neurons after spinal cord injury. Exp. Brain Res. 2006;174:660–666. doi: 10.1007/s00221-006-0511-x. [DOI] [PubMed] [Google Scholar]
- 66.Naseri K., Saghaei E., Abbaszadeh F., Afhami M., Haeri A., Rahimi F., Jorjani M. Role of microglia and astrocyte in central pain syndrome following electrolytic lesion at the spinothalamic tract in rats. J. Mol. Neurosci. 2013;49:470–479. doi: 10.1007/s12031-012-9840-3. [DOI] [PubMed] [Google Scholar]
- 67.D’Angelo R., Morreale A., Donadio V., Boriani S., Maraldi N., Plazzi G., Liguori R. Neuropathic pain following spinal cord injury: What we know about mechanisms, assessment and management. Eur. Rev. Med. Pharmacol. Sci. 2013;17:3257–3261. [PubMed] [Google Scholar]
- 68.Finnerup N.B., Otto M., McQuay H., Jensen T.S., Sindrup S.H. Algorithm for neuropathic pain treatment: An evidence based proposal. Pain. 2005;118:289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Sharma K., Sharma D., Sharma M., Sharma N., Bidve P., Prajapati N., Kalia K., Tiwari V. Astaxanthin ameliorates behavioral and biochemical alterations in in-vitro and in-vivo model of neuropathic pain. Neurosci. Lett. 2018;674:162–170. doi: 10.1016/j.neulet.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 70.Jiang X., Yan Q., Liu F., Jing C., Ding L., Zhang L., Pang C. Chronic trans-astaxanthin treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with chronic pain. Neurosci. Lett. 2018;662:36–43. doi: 10.1016/j.neulet.2017.09.064. [DOI] [PubMed] [Google Scholar]
- 71.Kuedo Z., Sangsuriyawong A., Klaypradit W., Tipmanee V., Chonpathompikunlert P. Effects of astaxanthin from Litopenaeus vannamei on carrageenan-induced edema and pain behavior in mice. Molecules. 2016;21:382. doi: 10.3390/molecules21030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haenisch B., Bönisch H. Depression and antidepressants: Insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol. Ther. 2011;129:352–368. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Kroning M., Kroning K. Teen depression and suicide: A silent crisis. J. Christ. Nurs. 2016;33:78–86. doi: 10.1097/CNJ.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 74.Berton O., Nestler E.J. New approaches to antidepressant drug discovery: Beyond monoamines. Nat. Rev. Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 75.Gabriel M., Sharma V. Antidepressant discontinuation syndrome. CMAJ Can. Med. Assoc. J. J. l’Assoc. Med. Can. 2017;189:E747. doi: 10.1503/cmaj.160991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Riediger C., Schuster T., Barlinn K., Maier S., Weitz J., Siepmann T. Adverse Effects of Antidepressants for Chronic Pain: A Systematic Review and Meta-analysis. Front. Neurol. 2017;8:307. doi: 10.3389/fneur.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller A.H., Raison C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krogh J., Benros M.E., Jørgensen M.B., Vesterager L., Elfving B., Nordentoft M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav. Immun. 2014;35:70–76. doi: 10.1016/j.bbi.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 79.Bakunina N., Pariante C.M., Zunszain P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015;144:365–373. doi: 10.1111/imm.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou X.-Y., Zhang F., Hu X.-T., Chen J., Tang R.-X., Zheng K.-Y., Song Y.-J. Depression can be prevented by astaxanthin through inhibition of hippocampal inflammation in diabetic mice. Brain Res. 2017;1657:262–268. doi: 10.1016/j.brainres.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 81.Jiang X., Chen L., Shen L., Chen Z., Xu L., Zhang J., Yu X. Trans-astaxanthin attenuates lipopolysaccharide-induced neuroinflammation and depressive-like behavior in mice. Brain Res. 2016;1649:30–37. doi: 10.1016/j.brainres.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 82.Jiang X., Zhu K., Xu Q., Wang G., Zhang J., Cao R., Ye J., Yu X. The antidepressant-like effect of trans-astaxanthin involves the serotonergic system. Oncotarget. 2017;8:25552. doi: 10.18632/oncotarget.16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abadie-Guedes R., Santos S.D., Cahú T.B., Guedes R.C.A., de Souza Bezerra R. Dose-dependent effects of astaxanthin on cortical spreading depression in chronically ethanol-treated adult rats. Alcohol. Clin. Exp. Res. 2008;32:1417–1421. doi: 10.1111/j.1530-0277.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 84.Abadie-Guedes R., Guedes R.C., Bezerra R.S. The Impairing Effect of Acute Ethanol on Spreading Depression is Antagonized by Astaxanthin in Rats of 2 Young-Adult Ages. Alcohol. Clin. Exp. Res. 2012;36:1563–1567. doi: 10.1111/j.1530-0277.2012.01766.x. [DOI] [PubMed] [Google Scholar]
- 85.Qiao J., Rong L., Wang Z., Zhang M. Involvement of Akt/GSK3β/CREB signaling pathway on chronic omethoate induced depressive-like behavior and improvement effects of combined lithium chloride and astaxanthin treatment. Neurosci. Lett. 2017;649:55–61. doi: 10.1016/j.neulet.2017.03.048. [DOI] [PubMed] [Google Scholar]
- 86.Rubiano A.M., Carney N., Chesnut R., Puyana J.C. Global neurotrauma research challenges and opportunities. Nature. 2015;527:193–197. doi: 10.1038/nature16035. [DOI] [PubMed] [Google Scholar]
- 87.Thuret S., Moon L.D., Gage F.H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 2006;7:628. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 88.Galgano M., Toshkezi G., Qiu X., Russell T., Chin L., Zhao L.-R. Traumatic brain injury: Current treatment strategies and future endeavors. Cell Transplantat. 2017;26:1118–1130. doi: 10.1177/0963689717714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.David B.T., Ratnayake A., Amarante M.A., Reddy N.P., Dong W., Sampath S., Heary R.F., Elkabes S. A toll-like receptor 9 antagonist reduces pain hypersensitivity and the inflammatory response in spinal cord injury. Neurobiol. Dis. 2013;54:194–205. doi: 10.1016/j.nbd.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 90.Zhang X.-S., Zhang X., Zhou M.-L., Zhou X.-M., Li N., Li W., Cong Z.-X., Sun Q., Zhuang Z., Wang C.-X. Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage. J. Neurosurg. 2014;121:42–54. doi: 10.3171/2014.2.JNS13730. [DOI] [PubMed] [Google Scholar]
- 91.Lu Y.-P., Liu S.-Y., Sun H., Wu X.-M., Li J.-J., Zhu L. Neuroprotective effect of astaxanthin on H2O2-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010;1360:40–48. doi: 10.1016/j.brainres.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 92.Lee D.-H., Lee Y.J., Kwon K.H. Neuroprotective effects of astaxanthin in oxygen-glucose deprivation in SH-SY5Y cells and global cerebral ischemia in rat. J. Clin. Biochem. Nutr. 2010;47:121–129. doi: 10.3164/jcbn.10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu Q., Zhang X.-S., Wang H.-D., Zhang X., Yu Q., Li W., Zhou M.-L., Wang X.-L. Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar. Drugs. 2014;12:6125–6141. doi: 10.3390/md12126125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang X.-S., Zhang X., Wu Q., Li W., Zhang Q.-R., Wang C.-X., Zhou X.-M., Li H., Shi J.-X., Zhou M.-L. Astaxanthin alleviates early brain injury following subarachnoid hemorrhage in rats: Possible involvement of Akt/bad signaling. Mar. Drugs. 2014;12:4291–4310. doi: 10.3390/md12084291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y., Liu Y., Li Y., Liu B., Wu P., Xu S., Shi H. Protective effects of astaxanthin on subarachnoid hemorrhage-induced early brain injury: Reduction of cerebral vasospasm and improvement of neuron survival and mitochondrial function. Acta Histochem. 2019;121:56–63. doi: 10.1016/j.acthis.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 96.Zhang X., Lu Y., Wu Q., Dai H., Li W., Lv S., Zhou X., Zhang X., Hang C., Wang J. Astaxanthin mitigates subarachnoid hemorrhage injury primarily by increasing sirtuin 1 and inhibiting the Toll-like receptor 4 signaling pathway. FASEB J. 2018;33:722–737. doi: 10.1096/fj.201800642RR. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X.-S., Zhang X., Zhang Q.-R., Wu Q., Li W., Jiang T.-W., Hang C.-H. Astaxanthin reduces matrix metalloproteinase-9 expression and activity in the brain after experimental subarachnoid hemorrhage in rats. Brain Res. 2015;1624:113–124. doi: 10.1016/j.brainres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 98.Zhang M., Cui Z., Cui H., Cao Y., Wang Y., Zhong C. Astaxanthin alleviates cerebral edema by modulating NKCC1 and AQP4 expression after traumatic brain injury in mice. BMC Neurosci. 2016;17:60. doi: 10.1186/s12868-016-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang M., Cui Z., Cui H., Wang Y., Zhong C. Astaxanthin protects astrocytes against trauma-induced apoptosis through inhibition of NKCC1 expression via the NF-κB signaling pathway. BMC Neurosci. 2017;18:42. doi: 10.1186/s12868-017-0358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonomini F., Rodella L.F., Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6:109–120. doi: 10.14336/AD.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pereira A.C., Gray J.D., Kogan J.F., Davidson R.L., Rubin T.G., Okamoto M., Morrison J.H., McEwen B.S. Age and Alzheimer’s disease gene expression profiles reversed by the glutamate modulator riluzole. Mol. Psychiatry. 2017;22:296–305. doi: 10.1038/mp.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Al-Amin M.M., Akhter S., Hasan A.T., Alam T., Hasan S.N., Saifullah A., Shohel M. The antioxidant effect of astaxanthin is higher in young mice than aged: A region specific study on brain. Metab. Brain Dis. 2015;30:1237–1246. doi: 10.1007/s11011-015-9699-4. [DOI] [PubMed] [Google Scholar]
- 104.Al-Amin M.M., Rahman M.M., Khan F.R., Zaman F., Reza H.M. Astaxanthin improves behavioral disorder and oxidative stress in prenatal valproic acid-induced mice model of autism. Behav. Brain Res. 2015;286:112–121. doi: 10.1016/j.bbr.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 105.Balietti M., Giannubilo S.R., Giorgetti B., Solazzi M., Turi A., Casoli T., Ciavattini A., Fattorettia P. The effect of astaxanthin on the aging rat brain: Gender-related differences in modulating inflammation. J. Sci. Food Agric. 2016;96:615–618. doi: 10.1002/jsfa.7131. [DOI] [PubMed] [Google Scholar]
- 106.Eren B., Tuncay Tanrıverdi S., Aydın Köse F., Özer Ö. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2019;18:242–250. doi: 10.1111/jocd.12665. [DOI] [PubMed] [Google Scholar]
- 107.Higuera-Ciapara I., Felix-Valenzuela L., Goycoolea F. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 108.Wu W., Wang X., Xiang Q., Meng X., Peng Y., Du N., Liu Z., Sun Q., Wang C., Liu X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2014;5:158–166. doi: 10.1039/C3FO60400D. [DOI] [PubMed] [Google Scholar]
- 109.Seki T., Sueki H., Kohno H., Suganuma K., Yamashita E. Effects of astaxanthin from haematococcus pluvialis on human skin. Fr. J. 2001;12:98–103. [Google Scholar]
- 110.Yamashita E. Cosmetic benefit of the combination of astaxanthin and tocotrienol on human skin. Food Style 21. 2002;6:112–118. [Google Scholar]
- 111.Yamashita E. Let astaxanthin be thy medicine. PharmaNutrition. 2015;3:115–122. doi: 10.1016/j.phanu.2015.09.001. [DOI] [Google Scholar]
- 112.Edition F. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; West Virginia, WV, USA: 2013. [Google Scholar]
- 113.Boyle C.A., Boulet S., Schieve L.A., Cohen R.A., Blumberg S.J., Yeargin-Allsopp M., Visser S., Kogan M.D. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 114.Kern J.K., Geier D.A., Sykes L.K., Geier M.R. Evidence of neurodegeneration in autism spectrum disorder. Transl. Neurodegener. 2013;2:17. doi: 10.1186/2047-9158-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bailey A., Luthert P., Dean A., Harding B., Janota I., Montgomery M., Rutter M., Lantos P. A clinicopathological study of autism. Brain A J. Neurol. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 116.Schumann C.M., Amaral D.G. Stereological analysis of amygdala neuron number in autism. J. Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Courchesne E., Pierce K., Schumann C.M., Redcay E., Buckwalter J.A., Kennedy D.P., Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 118.Kemper T.L., Bauman M. Neuropathology of infantile autism. J. Neuropathol. Exp. Neurol. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 119.Lee M., Martin-Ruiz C., Graham A., Court J., Jaros E., Perry R., Iversen P., Bauman M., Perry E. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain. 2002;125:1483–1495. doi: 10.1093/brain/awf160. [DOI] [PubMed] [Google Scholar]
- 120.Palmen S.J., van Engeland H., Hof P.R., Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- 121.Vargas D.L., Nascimbene C., Krishnan C., Zimmerman A.W., Pardo C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 122.Li X., Chauhan A., Sheikh A.M., Patil S., Chauhan V., Li X.-M., Ji L., Brown T., Malik M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chez M.G., Dowling T., Patel P.B., Khanna P., Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr. Neurol. 2007;36:361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 124.Granot E., Kohen R. Oxidative stress in childhood—In health and disease states. Clin. Nutr. 2004;23:3–11. doi: 10.1016/S0261-5614(03)00097-9. [DOI] [PubMed] [Google Scholar]
- 125.Evans T.A., Siedlak S., Lu L., Fu X., Wang Z., McGinnis W., Fakhoury E., Castellani R., Hazen S., Walsh W. The autistic phenotype exhibits a remarkably localized modification of brain protein by products of free radical-induced lipid oxidation. Am. J. Biochem. Biotechnol. 2008;4:61–72. doi: 10.3844/ajbbsp.2008.61.72. [DOI] [Google Scholar]
- 126.Sajdel-Sulkowska E., Lipinski B., Windom H., Audhya T., McGinnis W. Oxidative stress in autism: Elevated cerebellar 3-nitrotyrosine levels. Am. J. Biochem. Biotechnol. 2008;4:73–84. [Google Scholar]
- 127.Sajdel-Sulkowska E.M., Xu M., McGinnis W., Koibuchi N. Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD) Cerebellum. 2011;10:43–48. doi: 10.1007/s12311-010-0223-4. [DOI] [PubMed] [Google Scholar]
- 128.Ornoy A., Weinstein-Fudim L., Ergaz Z. Prevention or Amelioration of Autism-Like Symptoms in Animal Models: Will it Bring Us Closer to Treating Human ASD? Int. J. Mol. Sci. 2019;20:1074. doi: 10.3390/ijms20051074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krajcovicova-Kudlackova M., Valachovicova M., Mislanova C., Hudecova Z., Sustrova M., Ostatnikova D. Plasma concentrations of selected antioxidants in autistic children and adolescents. Bratisl. Lek. Listy. 2009;110:247–250. [PubMed] [Google Scholar]
- 130.Fernández M.J.F., Valero-Cases E., Rincon-Frutos L. Food Components with the Potential to be Used in the Therapeutic Approach of Mental Diseases. Curr. Pharm. Biotechnol. 2019;20:100–113. doi: 10.2174/1389201019666180925120657. [DOI] [PubMed] [Google Scholar]
- 131.Wu Y., Wu Y., Chen I.-F., Wu Y.-L., Chuang C., Huang H., Kuo S. Reparative effects of astaxanthin-hyaluronan nanoaggregates against retrorsine-CCl4-induced liver fibrosis and necrosis. Molecules. 2018;23:726. doi: 10.3390/molecules23040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tamjidi F., Shahedi M., Varshosaz J., Nasirpour A. Stability of astaxanthin-loaded nanostructured lipid carriers in beverage systems. J. Sci. Food Agric. 2018;98:511–518. doi: 10.1002/jsfa.8488. [DOI] [PubMed] [Google Scholar]
- 133.Tamjidi F., Shahedi M., Varshosaz J., Nasirpour A. Design and characterization of astaxanthin-loaded nanostructured lipid carriers. Innov. Food Sci. Emerg. Technol. 2014;26:366–374. doi: 10.1016/j.ifset.2014.06.012. [DOI] [Google Scholar]
- 134.Zuluaga M., Gueguen V., Letourneur D., Pavon-Djavid G. Astaxanthin-antioxidant impact on excessive Reactive Oxygen Species generation induced by ischemia and reperfusion injury. Chem. Biol. Interact. 2018;279:145–158. doi: 10.1016/j.cbi.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 135.McClements D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012;8:1719–1729. doi: 10.1039/C2SM06903B. [DOI] [Google Scholar]
- 136.Sotomayor-Gerding D., Oomah B.D., Acevedo F., Morales E., Bustamante M., Shene C., Rubilar M. High carotenoid bioaccessibility through linseed oil nanoemulsions with enhanced physical and oxidative stability. Food Chem. 2016;199:463–470. doi: 10.1016/j.foodchem.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 137.Takemoto Y., Hirose Y., Sugahara K., Hashimoto M., Hara H., Yamashita H. Protective effect of an astaxanthin nanoemulsion against neomycin-induced hair-cell damage in zebrafish. Auris Nasus Larynx. 2018;45:20–25. doi: 10.1016/j.anl.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 138.Date A.A., Patravale V. Current strategies for engineering drug nanoparticles. Curr. Opin. Colloid Interface Sci. 2004;9:222–235. doi: 10.1016/j.cocis.2004.06.009. [DOI] [Google Scholar]
- 139.Bhatt P.C., Srivastava P., Pandey P., Khan W., Panda B.P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016;6:10001–10010. doi: 10.1039/C5RA19113K. [DOI] [Google Scholar]
- 140.Shanmugapriya K., Kim H., Kang H.W. In vitro antitumor potential of astaxanthin nanoemulsion against cancer cells via mitochondrial mediated apoptosis. Int. J. Pharm. 2019;560:334–346. doi: 10.1016/j.ijpharm.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 141.Khan I., Bahuguna A., Kumar P., Bajpai V.K., Kang S.C. In vitro and in vivo antitumor potential of carvacrol nanoemulsion against human lung adenocarcinoma A549 cells via mitochondrial mediated apoptosis. Sci. Rep. 2018;8:144. doi: 10.1038/s41598-017-18644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Montenegro L., Lai F., Offerta A., Sarpietro M.G., Micicche L., Maccioni A.M., Valenti D., Fadda A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016;32:100–112. doi: 10.1016/j.jddst.2015.10.003. [DOI] [Google Scholar]
- 143.Weber S., Zimmer A., Pardeike J. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014;86:7–22. doi: 10.1016/j.ejpb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 144.Garcês A., Amaral M., Lobo J.S., Silva A. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018;112:159–167. doi: 10.1016/j.ejps.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 145.Tamjidi F., Shahedi M., Varshosaz J., Nasirpour A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013;19:29–43. doi: 10.1016/j.ifset.2013.03.002. [DOI] [Google Scholar]
- 146.Rodriguez-Ruiz V., Salatti-Dorado J., Barzegari A., Nicolas-Boluda A., Houaoui A., Caballo C., Caballero-Casero N., Sicilia D., Bastias Venegas J., Pauthe E. Astaxanthin-Loaded Nanostructured Lipid Carriers for Preservation of Antioxidant Activity. Molecules. 2018;23:2601. doi: 10.3390/molecules23102601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karimi N., Ghanbarzadeh B., Hamishehkar H., Mehramuz B., Kafil H.S. Antioxidant, antimicrobial and physicochemical properties of turmeric extract-loaded nanostructured lipid carrier (NLC) Colloid Interface Sci. Commun. 2018;22:18–24. doi: 10.1016/j.colcom.2017.11.006. [DOI] [Google Scholar]
- 148.Tamjidi F., Shahedi M., Varshosaz J., Nasirpour A. Stability of astaxanthin-loaded nanostructured lipid carriers as affected by pH, ionic strength, heat treatment, simulated gastric juice and freeze–thawing. J. Food Sci. Technol. 2017;54:3132–3141. doi: 10.1007/s13197-017-2749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anarjan N., Jafarizadeh Malmiri H., Ling T.C., Tan C.P. Effects of pH, ions, and thermal treatments on physical stability of astaxanthin nanodispersions. Int. J. Food Prop. 2014;17:937–947. doi: 10.1080/10942912.2012.685680. [DOI] [Google Scholar]
- 150.Raikos V., Ranawana V. Designing emulsion droplets of foods and beverages to enhance delivery of lipophilic bioactive components—A review of recent advances. Int. J. Food Sci. Technol. 2017;52:68–80. doi: 10.1111/ijfs.13272. [DOI] [Google Scholar]
- 151.Ozturk B., McClements D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016;7:1–6. doi: 10.1016/j.cofs.2015.07.008. [DOI] [Google Scholar]
- 152.Khalid N., Shu G., Holland B.J., Kobayashi I., Nakajima M., Barrow C.J. Formulation and characterization of O/W nanoemulsions encapsulating high concentration of astaxanthin. Food Res. Int. 2017;102:364–371. doi: 10.1016/j.foodres.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 153.Pan L., Wang H., Gu K. Nanoliposomes as Vehicles for Astaxanthin: Characterization, In Vitro Release Evaluation and Structure. Molecules. 2018;23:2822. doi: 10.3390/molecules23112822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sangsuriyawong A., Limpawattana M., Siriwan D., Klaypradit W. Properties and bioavailability assessment of shrimp astaxanthin loaded liposomes. Food Sci. Biotechnol. 2019;28:529–537. doi: 10.1007/s10068-018-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chiu C.-H., Chang C.-C., Lin S.-T., Chyau C.-C., Peng R. Improved hepatoprotective effect of liposome-encapsulated astaxanthin in lipopolysaccharide-induced acute hepatotoxicity. Int. J. Mol. Sci. 2016;17:1128. doi: 10.3390/ijms17071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Peng C.-H., Chang C.-H., Peng R.Y., Chyau C.-C. Improved membrane transport of astaxanthine by liposomal encapsulation. Eur. J. Pharm. Biopharm. 2010;75:154–161. doi: 10.1016/j.ejpb.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 157.Hama S., Uenishi S., Yamada A., Ohgita T., Tsuchiya H., Yamashita E., Kogure K. Scavenging of hydroxyl radicals in aqueous solution by astaxanthin encapsulated in liposomes. Biol. Pharm. Bull. 2012;35:2238–2242. doi: 10.1248/bpb.b12-00715. [DOI] [PubMed] [Google Scholar]
- 158.Hama S., Takahashi K., Inai Y., Shiota K., Sakamoto R., Yamada A., Tsuchiya H., Kanamura K., Yamashita E., Kogure K. Protective effects of topical application of a poorly soluble antioxidant astaxanthin liposomal formulation on ultraviolet-induced skin damage. J. Pharm. Sci. 2012;101:2909–2916. doi: 10.1002/jps.23216. [DOI] [PubMed] [Google Scholar]
- 159.Kamezaki C., Nakashima A., Yamada A., Uenishi S., Ishibashi H., Shibuya N., Hama S., Hosoi S., Yamashita E., Kogure K. Synergistic antioxidative effect of astaxanthin and tocotrienol by co-encapsulated in liposomes. J. Clin. Biochem. Nutr. 2016;59:100–106. doi: 10.3164/jcbn.15-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kittikaiwan P., Powthongsook S., Pavasant P., Shotipruk A. Encapsulation of Haematococcus pluvialis using chitosan for astaxanthin stability enhancement. Carbohydr. Polym. 2007;70:378–385. doi: 10.1016/j.carbpol.2007.04.021. [DOI] [Google Scholar]
- 161.Wang Q., Zhao Y., Guan L., Zhang Y., Dang Q., Dong P., Li J., Liang X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017;227:9–15. doi: 10.1016/j.foodchem.2017.01.081. [DOI] [PubMed] [Google Scholar]
- 162.Zhang X., Yin W., Qi Y., Li X., Zhang W., He G. Microencapsulation of astaxanthin in alginate using modified emulsion technology: Preparation, characterization, and cytostatic activity. Can. J. Chem. Eng. 2017;95:412–419. doi: 10.1002/cjce.22712. [DOI] [Google Scholar]
- 163.Lee J.-S., Park S.-A., Chung D., Lee H.G. Encapsulation of astaxanthin-rich Xanthophyllomyces dendrorhous for antioxidant delivery. Int. J. Biol. Macromol. 2011;49:268–273. doi: 10.1016/j.ijbiomac.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 164.Lin S.-F., Chen Y.-C., Chen R.-N., Chen L.-C., Ho H.-O., Tsung Y.-H., Sheu M.-T., Liu D.-Z. Improving the stability of astaxanthin by microencapsulation in calcium alginate beads. PLoS ONE. 2016;11:e0153685. doi: 10.1371/journal.pone.0153685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Astray G., Gonzalez-Barreiro C., Mejuto J.C., Rial-Otero R., Simal-Gandara J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009;23:1631–1640. doi: 10.1016/j.foodhyd.2009.01.001. [DOI] [Google Scholar]
- 166.Dodziuk H. Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications. Wiley-VCH, GmbH & KGaA; Weinheim, Germany: 2006. Modeling of CyDs and their complexes; pp. 333–355. [Google Scholar]
- 167.Del Valle E.M. Cyclodextrins and their uses: A review. Process. Biochem. 2004;39:1033–1046. doi: 10.1016/S0032-9592(03)00258-9. [DOI] [Google Scholar]
- 168.Dong S., Huang Y., Zhang R., Lian Z., Wang S., Liu Y. Inclusion complexes of astaxanthin with hydroxypropyl-β-cyclodextrin: Parameters optimization, spectroscopic profiles, and properties. Eur. J. Lipid Sci. Technol. 2014;116:978–986. doi: 10.1002/ejlt.201300261. [DOI] [Google Scholar]
- 169.Kim S., Cho E., Yoo J., Cho E., Choi S.J., Son S.M., Lee J.M., In M.-J., Kim D.C., Kim J.-H. β-CD-mediated encapsulation enhanced stability and solubility of astaxanthin. J. Korean Soc. Appl. Biol. Chem. 2010;53:559–565. doi: 10.3839/jksabc.2010.086. [DOI] [Google Scholar]
- 170.Nalawade P., Gajjar A. Assessment of in-vitro bio accessibility and characterization of spray dried complex of astaxanthin with methylated betacyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2015;83:63–75. doi: 10.1007/s10847-015-0541-8. [DOI] [Google Scholar]
- 171.Bharathiraja S., Manivasagan P., Quang Bui N., Oh Y.-O., Lim I., Park S., Oh J. Cytotoxic induction and photoacoustic imaging of breast cancer cells using astaxanthin-reduced gold nanoparticles. Nanomaterials. 2016;6:78. doi: 10.3390/nano6040078. [DOI] [PMC free article] [PubMed] [Google Scholar]