Abstract
Reggio Calabria province (South Italy) is known for being almost the only area of cultivation of the bergamot fruit, grown principally for its essential oil, but today much studied for the health benefits of its juice. The biometrics and physico-chemical properties of the three (Citrus bergamia Risso) existing genotypes namely Castagnaro, Fantastico and Femminello were studied during fruit ripening from October to March. Castagnaro cultivar had the biggest and heaviest fruit during this harvest period. °Brix (7.9–10.0), pH (2.2–2.8) and formol number (1.47–2.37 mL NaOH 0.1 N/100 mL) were shown to be influenced by both the genotype and harvest date. Titratable acidity (34.98–59.50 g/L) and vitamin C (ascorbic acid) (341–867 g/L) decreased during fruit ripening. The evolution of flavonoids such as neoeriocitrin, naringin, neohesperidin, brutieridin and melitidin was studied both in bergamot juice and in the bergamot cloudy juice which is the aqueous extract of bergamot during fruit processing. Bergamot cloudy juice contained a higher quantity of flavonoids compared to the juice. This study gives important information regarding the cultivar and the harvest date for producers who want to obtain the highest juice quantity or the highest juice quality from the bergamot fruit.
Keywords: antioxidants, bergamot, bioactive compound, biometrics, biomolecules, Citrus bergamia Risso, cloudy juice, non-climacteric
1. Introduction
Bergamot (Citrus bergamia, Risso) is an evergreen tree almost exclusively grown on the Ionian and Tyrrhenian Coast of Reggio Calabria Province (South Italy), in a strip of land 1–12 km wide. Given its economic benefits, bergamot is very important to the region where it is cultivated [1]. Three cultivars (cv) (genotypes) are known: Castagnaro, Fantastico and Femminello. In 2017, 1500 hectares of bergamot trees were cultivated in Reggio Calabria, producing 18,750 tons of fruits [2]. Bergamot is a non-climacteric fruit [3] and in the past was picked when the highest essential oil content in the peel was reached. Today the juice is also considered. The fruit was commonly cultivated for its essential oil extracted from the peel, which is used in the cosmetic, perfumery [4] and food industries [5]. Very recently the bioconversion of juice and peel into wines and vinegars was positively conducted [6]. More recently there has been an increasing interest in the use of its juice as a beverage and also in a blend with other fruit juices. This interest is related to the demand for minimally processed foods and functional foods containing antioxidants and biomolecules whose beneficial effects on human health have been widely studied regarding diabetes, cancer, Alzheimer’s disease, insulin resistance and neuro-disease [7]. There is no universally accepted definition of functional food. The following definition could be applied: “Natural or processed foods that contain known or unknown biologically-active compounds; which in defined amounts provide a clinically proven and documented health benefit for the prevention, management, or treatment of chronic disease” [8]. This merges with, and updates, the definitions stated by the National Academy of Sciences Food and Nutrition Board in the United States [9], the Institute of Food Technologists [10], the American Dietetic Association [11] and what De Felice stated for nutraceuticals [12]. The aim of this research was to investigate the evolution of biometrics and the anti-oxidative properties during the fruit ripening (six months) to evaluate the right harvest date to obtain the highest physico-chemical quality of bergamot, bergamot juice and bergamot cloudy juice. In this context, the effect of cultivar was also studied.
2. Materials and Methods
2.1. Plant Material
All three cultivars were grown in the same area, in mono-cultivar plots. The bergamot trees were cultivated by experienced growers on level ground and were planted with a distance of 6 m between each row and the trees were 5 m apart within each row. All the trees (25–30 years old) were irrigated and fertilized in the same way. Fertilization was conducted by using 5 kg per tree of a complex fertilizer (nitrogen, phosphorus and potassium, NPK, 20:10:10) in multiple rates from late winter to early spring and calcium nitrate 200 g/tree/month from July to September. A drip irrigation was conducted from March to late October in relation with the environmental temperature and based on the demand for water by trees (evapotranspiration). The soil was slightly acidic, deep and well drained because bergamot roots suffer water stagnation. Fruits were collected early in the morning and carefully placed in plastic containers commonly used for citrus fruit picking. Thereafter, fruits were immediately transferred to the laboratory for biometric analyses. Thirty kilograms of bergamot fruits were randomly collected from 20 trees at each harvest date for each cultivar (Castagnaro, Fantastico and Femminello) in the middle of each month from October 2016 to March 2017. For each cultivar, two batches (15 kg each) were prepared at each harvest date and two replicates were obtained from each batch. The same experimental design was replicated in the harvest year 2017–2018.
2.2. Juice Extraction
The bergamot fruit is cultivated for essential oil extraction (from peel) and juice extraction. In the present study both the ‘albedo’ and the remaining pulp which are commonly known as ‘pastazzo’ were processed into hammer mills where they were grinded and homogenized with water to solubilize polyphenols. The obtained mixture undergoes various steps in steel tanks to allow the flavonoids to be extracted as much as possible from the ‘pastazzo’ before transferring to the liquid phase. A first rough separation is conducted by a press, which divides the pulp destined for the subsequent recovery of the pectins in another production plant, from the liquid phase which is stored in steel tanks for subsequent processing. The pressed pulp contains a large quantity of pectin, both water soluble and nonsoluble, which makes the separation of pulp from the liquid fraction difficult. For this reason, pectinase was used as a pectolytic enzyme at 50–60 °C to facilitate pectin degradation. The juice obtained by this procedure is called ‘cloudy juice’. Pectinase is commonly used to break up the cell wall and to intensify the phenolic compounds extraction [13,14,15]. The extraction method applied in this work is commonly used for edible juice extraction.
2.3. Chemicals
Chemicals of both analytical grade and chromatographic grade were purchased from Carlo Erba, Milan, Italy. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and pure standards of naringin, neoeriocitrin and neohesperidin, were from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). TPTZ (2,4,6-tripyridyl-s-triazine for FRAP reagent (Ferric Reducing Ability of Plasma) was from Fluka Chemicals Switzerland. Brutieridin and melitidin as pure standards were obtained as described by Di Donna et al. [16].
2.4. Pulp Content
Pulp content is the solid fraction quantified as a percentage in bergamot juice after centrifugation for 10 min at 3500 rpm. Pulp and juice are separated by difference of gravity.
2.5. Turbidity
A 12% bergamot juice in bi-deionized water was prepared, and transmittance was read at 578 nm. Turbidity was expressed as a percentage ratio between the intensity of the incident light and intensity of light emission from the cuvette.
2.6. pH
A Mettler Toledo instrument was used after calibration pH 7.0 and pH 4.0.
2.7. °Brix
The degree Brix was determined by a Mettler Toledo refractometer on a drop of bergamot juice sample after zero-set of the instrument by a drop of bi-deionized water.
2.8. Titratable Acidity
A 10 g aliquot of bergamot juice and 150 mL of bi-deionized water were placed in a glass beaker. The mixture was boiled for 10 min. Thereafter acidity was determined by titration with a 0.1 NaOH aqueous solution up to pH 8.1. Acidity was expressed as g of citric acid monohydrate per liter of juice [17].
2.9. Vitamin C
Vitamin C was quantified by an iodomeric titration. In a glass beaker, 1 mL of bergamot juice and 5 mL of bi-deionized water were mixed and titrated by a 0.01 N iodine solution using a 2% starch solution as an indicator. The result was expressed as mg ascorbic acid/L of juice [17].
2.10. Formol Number
In a glass beaker, 10 mL of bergamot juice, 10 mL of 40% by volume formaldehyde solution (pH 2.8) and 7 drops of phenolphthalein (1% in ethanol) were measured. The mixture was stirred and titrated by a 0.1 NaOH solution (IFUMA 30, method EN 1133) [18].
2.11. Flavonoids
The analysis was carried out using the method suggested by Giuffrè et al. [6] and modified using a HPLC-PDA system (i.e. a liquid chromatograph coupled with a photo diode array detector) and equipped with a column conditioning system at 25 °C. The separation column was a Kinetex 5μ C18 100 Å, 150 mm length, 4.6 mm internal diameter. The mobile phase was 0.1% formic acid in deionized water (A) and methanol (B) with the following conditions: 80% A in isocratic (5 min); from 80% A to 45% A in gradient (42 min); 45% A in isocratic (5 min); from 45 to 20% A (7 min); 20% A in isocratic (5 min); from 20 to 80% A (5 min). The injection volume was 20 μL and the flow rate was set at 1 mL/min. The system was supported by Chromera software version 3.4.0.5712.
The limit of detection and linearity of the detector response were determined by a five points calibration curve of flavonoids. Triplicate standards solutions of neoeriocitrin, naringin and neohesperidin were prepared at 5, 10, 25, 50 and 100 mg/100 mL in methanol, from a solution of 250 mg/100 mL.
2.12. DPPH and FRAP Assays
The analyses were conducted spectrophotometrically as suggested by Panuccio et al. and Sicari et al. [19,20].
2.13. Statistical Analysis
Means and standard deviations were calculated on 8 replicates (4 replicates × 2 harvest years) by Excel 2010 software. Statistical differences were calculated by one-way ANOVA and Tukey test for post hoc analysis at p < 0.05 using the SPSS 17.0 software (SPSS Inc., Chicago, IL, USA); the variables were: the cultivar and the harvest date of bergamots. Principal component analysis (PCA) was carried out using the software XLSTAT version 2009.1.01.
3. Results and Discussion
3.1. Biometrics
The vertical diameter length was longest in the Castagnaro fruit which showed the most constant increase in length, from 6.63 cm in October to more than 9 cm after December. The vertical diameter length of Fantastico and Femminello fruits showed a slight decrease in March at the end of the ripening period (7.15 and 6.50 cm, respectively) (Table 1). The horizontal diameter was greatest in Castagnaro at each monthly sampling and showed a tendency to increase during the ripening of Castagnaro and Fantastico, whereas in Femminello a slightly fluctuating rate was found (Table 1). The pulp in juice content showed very high significant differences (p < 0.001) for each cultivar during fruit ripening but no significant differences were found between cultivars in October, December and January (Table 1). Vertical diameter increased with horizontal diameter (r = 0.958), fruit weight (r = 0.87) and peel weight (r = 0.880), (Table 2). The increase in fruit weight was highly significant (p < 0.001) during fruit ripening of all cultivars. From October to March, the Castagnaro fruit showed both the highest increase in weight during ripening (72%) and the highest weight each month (245 g in October and 421 g in March). Fantastico fruits increased by 49% during ripening (173 g in October and 258 g in March). Femminello fruits showed both the lowest weight on each harvest date and the lowest increase in weight (23%) from October to February (Table 3). The peel weight was always greatest in Castagnaro (44.1 g in October, 55.01 g in December and 74.02 g in February), but a substantial fall in weight was measured in March in all three cultivars (Table 3). The percentage of juice was highest in Fantastico at the first stage of ripening (29.33%–30.50%) and in Fantastico and Femminello at the end of the ripening period (39.97% and 40.01%, respectively) (Table 1). Pulp in juice is a negative parameter because it has to be removed during the industrial process before using or storing the fruit juice. The juice turbidity was very highly significantly different (p < 0.001) during ripening and the same significance of differences was found between cultivars at each monthly sampling (Table 3). In the correlation matrix, fruit weight had a strong positive correlation with the vertical diameter (r = 0.875; p < 0.001; r2 = 0.766; t = 19.33) and a stronger correlation with the horizontal diameter (r = 0.920; p < 0.001; r2 = 0.846; t = 19.25) (Table 2). The increase in peel weight in the three cultivars was strongly correlated with the vertical diameter (r = 0.880; p < 0.001; r2 = 0.774; t = 14.76) and with the horizontal diameter (r = 0.830; p < 0.001; r2 = 0.689; t = 14.62) (Table 2). Fruit weight showed a weak correlation with juice content (r = 0.112; p < 0.001; r2 = 0.013; t = 17.23) but it was strongly correlated with peel weight (r = 0.815; p < 0.001; r2 = 0.664; t = 16.56). This means that the increase in weight during fruit ripening is mainly due to the increase in peel and not that of pulp.
Table 1.
Biometrics of bergamot fruit. Results are presented as the mean value ± standard deviation, n = 8, (2016–2017 and 2017–2018 harvest years).
| Cultivar | October | November | December | January | February | March | Sign. | |
|---|---|---|---|---|---|---|---|---|
|
Vertical
Diameter (cm) |
Castagnaro | 8.63 ± 0.25 cA | 8.67 ± 0.10 bcA | 9.02 ± 0.10 abA | 9.07 ± 0.06 aA | 9.04 ± 0.09 aA | 9.0 ± 0.10 abA | ** |
| Fantastico | 7.33 ± 0.06 cB | 7.3 ± 0.10 cB | 8.12 ± 0.10 aB | 8.11 ± 0.09 aB | 7.65 ± 0.09 bB | 7.15 ± 0.09 cB | *** | |
| Femminello | 6.90 ± 0.1 aC | 6.53 ± 0.25 abC | 6.47 ± 0.15 bC | 6.83 ± 0.06 abC | 6.83 ± 0.06 abC | 6.5 ± 0.17 abC | ** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
| Horizontal Diameter (cm) | Castagnaro | 8.8 ± 0.10 bA | 8.7 ± 0.20 bA | 9.77 ± 0.15 aA | 9.60 ± 0.10 aA | 9.43 ± 0.03 aA | 9.77 ± 0.12 aA | *** |
| Fantastico | 7.13 ± 0.06 cB | 7.2 ± 0.10 cB | 8.40 ± 0.05 aB | 8.36 ± 0.06 aB | 7.98 ± 0.08 bB | 8.04 ± 0.06 bB | * | |
| Femminello | 7.07 ± 0.25 aB | 6.53 ± 0.21 bC | 6.67 ± 0.12 a bC | 7.1 ± 0.10 aC | 7.03 ± 0.25 abC | 6.77 ± 0.15 abC | * | |
| Sign. | ** | *** | *** | *** | *** | *** | ||
|
Pulp in Juice
(%) |
Castagnaro | 10.17 ± 0.29 bA | 10.00 ± 0.50 bB | 7.00 ± 0.50 cA | 9.83 ± 0.29 bA | 10.33 ± 0.58 bA | 11.67 ± 0.58 aAB | *** |
| Fantastico | 10.33 ± 0.58 aA | 10.33 ± 0.58 aA | 7.33 ± 0.58 bA | 10.33 ± 0.58 aA | 10.33 ± 0.58 aA | 10.00 ± 1.0 aB | *** | |
| Femminello | 10.00 ± 0.0 bA | 9.00 ± 0.0 cB | 8.00 ± 0 dA | 10.00 ± 0.0 bA | 7.03 ± 0.06 eB | 12.17 ± 0.29 aA | *** | |
| Sign. | n.s. | * | n.s. | n.s. | *** | ** |
*** significance at p < 0.001; ** significance at p < 0.01; * significance at p < 0.05; n.s., not significant. Means in the same line are distinguished by small letters. Means in the same column are distinguished by capital letters.
Table 2.
The correlation matrix of biometrics is built on the basis of 48 values for each parameter (4 replicates × 2 harvest years × 3 cultivars). In the south-west section of the matrix is the r-value (above) and the significance level (below) with p < 0.001,***; n.s., not significant. In the north-east section of the matrix is the t-value (in italics) with the significance of the t-test calculated at 95% confidence interval and the R2 value (underlined).
| Vertical Diameter | Horizontal Diameter | Pulp in Juice | Fruit Weight | Peel Weight | Juice Content | Fruit Weight/Peel Weight | Fruit Weight/Juice Content | Juice Content/Peel Weight | Turbidity | |
|---|---|---|---|---|---|---|---|---|---|---|
| Vertical Diameter | 1 |
1.38
0.918 |
7.99
0.002 |
19.33
0.766 |
14.76
0.774 |
26.50
0.013 |
4.59
0.028 |
0.28
0.702 |
45.50
0.587 |
37.96
0.000 |
| Horizontal Diameter | 0.958 n.s. |
1 |
6.50
0.001 |
19.25
0.846 |
14.62
0.689 |
26.10
0.002 |
5.47
0.000 |
0.37
0.638 |
41.89
0.426 |
37.44
0.009 |
| Pulp in Juice | 0.048 *** |
0.024 *** |
1 |
19.12
0.001 |
13.78
0.000 |
24.05
0.000 |
10.60
0.031 |
3.98
0.003 |
40.54
0.001 |
34.96
0.034 |
| Fruit Weight | 0.875 *** |
0.920 *** |
0.090 *** |
1 |
16.56
0.664 |
17.23
0.013 |
19.37
0.034 |
19.26
0.619 |
19.84
0.345 |
16.69
0.000 |
| Peel Weight | 0.880 *** |
0.830 *** |
0.000 *** |
0.815 *** |
1 |
2.65
0.006 |
15.27
0.158 |
14.45
0.552 |
18.08
0.619 |
0.35
0.009 |
| Juice Content | −0.114 *** |
0.042 *** |
0.001 *** |
0.112 *** |
–0.075 *** |
1 |
27.33
0.129 |
24.29
0.242 |
34.11
0.360 |
5.52
0.186 |
| Fruit Weight/Peel Weight | −0.166 *** |
0.020 *** |
0.175 *** |
0.185 *** |
–0.397 *** |
0.359 *** |
1 |
2.70
0.003 |
26.13
0.220 |
38.63
0.056 |
| Fruit Weight/Juice content | 0.838 n.s. |
0.799 n.s. |
0.057 *** |
0.787 *** |
0.743 *** |
–0.492 *** |
–0.054 *** |
1 |
16.53
0.682 |
34.16
0.071 |
| Juice content/Peel Weight | −0.766 *** |
–0.653 *** |
0.032 *** |
–0.587 *** |
–0.787 *** |
0.600 *** |
0.469 *** |
–0.826 *** |
1 |
46.66
0.081 |
| Turbidity | 0.022 *** |
0.093 *** |
0.185 *** |
0.010 *** |
–0.093 n.s. |
0.431 *** |
0.236 *** |
–0.266 *** |
0.284 *** |
1 |
Table 3.
Biometrics of bergamot fruit. Results are presented as the mean value ± standard deviation, n = 8, (2016–2017 and 2017–2018 harvest years). *** significance at p < 0.001; ** significance at p < 0.01; * significance at p < 0.05. Means in the same line are distinguished by small letters. Means in the same column are distinguished by capital letters.
| Cultivar | October | November | December | January | February | March | Sign. | |
|---|---|---|---|---|---|---|---|---|
|
Fruit Weight
(g) |
Castagnaro | 245 ± 6 eA | 277 ± 8 dA | 354 ± 14 cA | 363 ± 5 cA | 397 ± 5 bA | 421 ± 7 aA | *** |
| Fantastico | 173 ± 3 dB | 201 ± 2 cB | 241 ± 4 bB | 194 ± 5 cB | 266 ± 3 aB | 258 ± 12 aB | *** | |
| Femminello | 150 ± 2 cC | 174 ± 1 bC | 131 ± 2 dC | 170 ± 2 bC | 185 ± 4 aC | 146 ± 7 cC | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
|
Peel Weight
(g) |
Castagnaro | 44.01 ± 0.26 cdA | 49.77 ± 0.15 bcA | 55.01 ± 0.25 bA | 55.03 ± 0.67 bA | 74.02 ± 5.29 aA | 41.97 ± 0.65 dA | *** |
| Fantastico | 31.43 ± 0.21 dB | 34.81 ± 0.08 cB | 41.13 ± 0.86 bB | 42.23 ± 0.67 bB | 51.58 ± 0.10 aB | 26.53 ± 0.12 eB | *** | |
| Femminello | 25.83 ± 0.12 bC | 22.8 ± 0.17 cC | 20.97 ± 0.06 dC | 20.87 ± 0.15 dC | 26.90 ± 0.10 aC | 19.53 ± 0.50 eC | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
|
Juice
Content (%) |
Castagnaro | 19.73 ± 0.68 fC | 21.73 ± 0.21 eB | 28.77 ± 0.16 dC | 40.08 ± 0.16 aA | 36.07 ± 0.49 bB | 34.73 ± 0.17 cB | *** |
| Fantastico | 29.33 ± 0.25 eA | 30.50 ± 0.36 dA | 31.93 ± 0.23 cB | 33.57 ± 0.21 bB | 30.12 ± 0.03 dC | 39.97 ± 0.42 aA | *** | |
| Femminello | 21.00 ± 0.10 eB | 22.13 ± 0.15 dB | 39.03 ± 0.42 bA | 33.73 ± 0.21 cB | 39.07 ± 0.12 bA | 40.01 ± 0.46 aA | *** | |
| Sign. | *** | *** | *** | ** | *** | *** | ||
| Fruit Weight/Peel Weight | Castagnaro | 5.58 ± 0.13 cAB | 5.57 ± 0.18 cB | 6.44 ± 0.22 bA | 6.60 ± 0.07 bA | 5.38 ± 0.32 cB | 10.04 ± 0.26 aB | *** |
| Fantastico | 5.50 ± 0.09 aB | 5.78 ± 0.06 aB | 5.85 ± 0.13 aB | 4.59 ± 0.14 cC | 5.16 ± 0.05 bB | 9.73 ± 0.51 aA | *** | |
| Femminello | 5.81 ± 0.05 dA | 7.64 ± 0.07 dA | 6.26 ± 0.08 cA | 8.16 ± 0.08 bB | 6.87 ± 0.17 cdA | 7.49 ± 0.32 aA | *** | |
| Sign. | * | *** | ** | *** | *** | *** | ||
| Fruit Weight/Juice Content | Castagnaro | 12.44 ± 0.17 bcA | 12.76 ± 0.29 bA | 12.32 ± 0.52 bA | 9.07 ± 0.14 dA | 11.01 ± 0.25 cdA | 12.13 ± 0.15 aA | *** |
| Fantastico | 5.90 ± 0.14 dC | 6.60 ± 0.15 cC | 7.54 ± 0.15 bB | 5.78 ± 0.18 dB | 8.83 ± 0.10 aB | 6.46 ± 0.31 cB | *** | |
| Femminello | 7.14 ± 0.10 bB | 7.87 ± 0.04 cB | 3.37 ± 0.09 dC | 5.05 ± 0.10 dC | 4.73 ± 0.12 eC | 3.66 ± 0.18 aC | ||
| Sign. | *** | *** | *** | *** | *** | *** | ||
| Juice Content/Peel Weight | Castagnaro | 0.45 ± 0.02 eC | 0.44 ± 0.01 bC | 0.52 ± 0.00 dC | 0.73 ± 0.01 aC | 0.49 ± 0.04 cC | 0.83 ± 0.02 bC | *** |
| Fantastico | 0.93 ± 0.01 bA | 0.88 ± 0.01 aB | 0.78 ± 0.01 eB | 0.79 ± 0.01 cB | 0.58 ± 0.00 dB | 1.51 ± 0.02 eB | *** | |
| Femminello | 0.81 ± 0.01 fB | 0.97 ± 0.00 eA | 1.86 ± 0.02 bA | 1.62 ± 0.02 cA | 1.45 ± 0.00 dA | 2.05 ± 0.03 aA | *** | |
| Sign. | *** | *** | *** | *** | *** | *** |
3.2. pH
Bergamot juice is very acidic and mainly contains ascorbic and citric acid which contribute significantly to the composition of this parameter. Between cultivars, no significant differences were found in November, February and March. A very high significant pH increase (p < 0.001) was found in Castagnaro and Femminello fruit juices and high significant differences were found in Fantastico juice (p < 0.01) (Table 4). pH of juice was negatively and moderately correlated with vitamin C (r = 0.643; p < 0.001; r2 = 0.413; t = 31.32), but strongly and negatively correlated with titratable acidity (r = 0.740; p < 0.001; r2 = 0.548; t = 50.76) (Table 5). The pH of the bergamot juice was lower than grapefruit juice (3.05), orange juice (3.63) and tangerine juice (3.41) but similar or higher than lemon juice (2.43) [21].
Table 4.
Physico-chemical properties of bergamot juice. Results are presented as the mean value ± standard deviation, n = 8, (2016–2017 and 2017–2018 harvest years). *** significance at p < 0.001; ** significance at p < 0.01; * significance at p < 0.05; n.s., not significant. Means in the same line are distinguished by small letters. Means in the same column are distinguished by capital letters.
| Cultivar | October | November | December | January | February | March | Sign. | |
|---|---|---|---|---|---|---|---|---|
|
Turbidity
(%) |
Castagnaro | 33.67 ± 0.25 eB | 36.55 ± 0.13 cA | 34.48 ± 0.28 dB | 48.78 ± 0.30 aB | 36.37 ± 0.15 cB | 39.50 ± 0.17 bB | *** |
| Fantastico | 34.20 ± 0.10 eB | 32.28 ± 0.16 fC | 36.14 ± 0.09 cA | 49.12 ± 0.08 aB | 35.27 ± 0.06 dC | 39.23 ± 0.21 bB | *** | |
| Femminello | 35.27 ± 0.31 dA | 35.33 ± 0.23 dB | 35.7 ± 0.46 dA | 54.03 ± 0.45 aA | 38.50 ± 0.26 cA | 40.03 ± 0.15 bA | *** | |
| Sign. | ** | n.s. | * | * | n.s. | n.s. | ||
| pH | Castagnaro | 2.4 ± 0.06 cA | 2.4 ± 0.06 cA | 2.5 ± 0.06 bcA | 2.7 ± 0.06 abA | 2.7 ± 0.06 abA | 2.8 ± 0.06 aA | *** |
| Fantastico | 2.4 ± 0.0 bA | 2.4 ± 0.06 bA | 2.5 ± 0.10 abAB | 2.5 ± 0.10 abAB | 2.6 ± 0.12 abA | 2.7 ± 0.10 aA | ** | |
| Femminello | 2.2 ± 0.12 dB | 2.3 ± 0.06 dA | 2.3 ± 0.06 dA | 2.4 ± 0.06 bcB | 2.6 ± 0.0 abA | 2.7 ± 0.0 aA | *** | |
| Sign. | ** | n.s. | * | * | n.s. | n.s. | ||
| °Brix | Castagnaro | 9.4 ± 0.06 aA | 9.5 ± 0.06 aB | 8.3 ± 0.06 dB | 8.7 ± 0.10 bB | 8.5 ± 0.06 cA | 8.3 ± 0.06 cdA | *** |
| Fantastico | 9.1 ± 0.06 bB | 10.0 ± 0.10 aA | 8.7 ± 0.10 cA | 9.1 ± 0.10 bA | 8.2 ± 0.20 dAB | 7.9 ± 0.60 eB | *** | |
| Femminello | 9.1 ± 0.12 aB | 8.6 ± 0.06 bC | 8.6 ± 0.12 bA | 9.3 ± 0.06 aA | 8.0 ± 0.06 cB | 8.2 ± 0.06 cA | *** | |
| Sign. | ** | *** | ** | *** | * | *** | ||
| Titratable Acidity (TA) (g/L) | Castagnaro | 53.86 ± 0.29 aB | 51.77 ± 0.06 bB | 49.74 ± 0.26 cA | 42.2 ± 0.26 dC | 40.67 ± 1.42 dC | 34.98 ± 0.2 eC | *** |
| Fantastico | 58.67 ± 0.06 bA | 59.50 ± 0.10 aA | 47.58 ± 0.06 cB | 46.31 ± 0.06 dB | 46.23 ± 0.13 dB | 39.83 ± 0.15 eB | *** | |
| Femminello | 54.28 ± 0.32 aB | 49.87 ± 0.85 bC | 46.63 ± 0.81 cB | 55.37 ± 0.32 aA | 49.0 ± 0.36 bA | 41.90 ± 0.25 dA | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
|
°Brix/TA (%)
(Maturity Index) |
Castagnaro | 1.75 ± 0.02 bcB | 1.84 ± 0.01 bcB | 1.66 ± 0.01 bAB | 2.06 ± 0.02 bB | 2.08 ± 0.07 cB | 2.38 ± 0.01 aA | *** |
| Fantastico | 1.56 ± 0.01 bcB | 1.68 ± 0.02 bB | 1.83 ± 0.02 bB | 1.97 ± 0.02 dC | 1.77 ± 0.05 cdB | 1.97 ± 0.02 aA | *** | |
| Femminello | 1.68 ± 0.03 bA | 1.72 ± 0.02 aA | 1.84 ± 0.01 bA | 1.67 ± 0.00 aA | 1.64 ± 0.00 bA | 1.97 ± 0.02 bB | *** | |
| Sign. | ** | *** | * | *** | *** | *** | ||
|
Formol Number
(mL NaOH 1N/100mL) |
Castagnaro | 2.07 ± 0.06 aA | 1.87 ± 0.06 bB | 1.43 ± 0.06 cC | 2.1 ± 0.10 aA | 2.13 ± 0.06 aA | 2.03 ± 0.06 abB | *** |
| Fantastico | 2.07 ± 0.06 aA | 2.10 ± 0.10 aA | 1.97 ± 0.12 aA | 1.90 ± 0.10 aA | 1.53 ± 0.06 bB | 1.60 ± 0.10 bC | *** | |
| Femminello | 2.03 ± 0.15 bA | 1.47 ± 0.06 cdC | 1.67 ± 0.06 cB | 1.47 ± 0.06 cdB | 1.43 ± 0.06 dB | 2.37 ± 0.06 aA | *** | |
| Sign. | n.s. | *** | *** | *** | *** | *** | ||
|
Vitamin C
(mg/L) |
Castagnaro | 831 ± 7 aB | 593 ± 7 cB | 672 ± 13 bA | 474 ± 12 dC | 498 ± 6 dA | 341 ± 4 eB | *** |
| Fantastico | 867 ± 6 aA | 582 ± 7 bB | 566 ± 3 bB | 571 ± 9 bA | 504 ± 4 cA | 457 ± 5 dA | *** | |
| Femminello | 669 ± 4 aC | 635 ± 7 bA | 556 ± 13 cB | 543 ± 6 cB | 492 ± 4 dA | 349 ± 9 eB | *** | |
| Sign. | *** | *** | *** | *** | n.s. | *** |
Table 5.
The correlation matrix of the physico-chemical properties of bergamot juice and bergamot cloudy juice which is built on the basis of 48 values for each parameter (4 replicates × 2 harvest years × 3 cultivars). In the south-west section of the matrix is the r-value (above) and the significance level (below) with p < 0.05, *. In the north-east section of the matrix there is the t-value (in italics) with the significance of the t-test calculated at 95% confidence interval and the R2 value (underlined).
| pH Juice |
°Brix Juice |
TA Juice |
°Brix/TA/% Juice | Vitamin C Juice |
Formol N Juice |
Neoeriocitrin Juice |
Naringin Juice |
Neohesperidin Juice |
Melitidin Juice |
Brutieridin Juice |
Tot. Floids Juice |
Neoeriocitrin Cloudy Juice |
Naringin Cloudy Juice |
Neohesperidin Cloudy Juice |
Melitidin Cloudy Juice |
Brutieridin Cloudy Juice |
Tot. Floids Cloudy Juice |
DPPH Assay Juice |
FRAP Assay Juice |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Juice | pH | 1 |
76.65
0.326 |
50.76
0.548 |
32.83
0.169 |
31.32
0.413 |
14.31
0.015 |
26.24
0.120 |
36.48
0.251 |
24.92
0.023 |
17.65
0.146 |
21.45
0.285 |
17.55
0.249 |
28.87
0.378 |
53.78
0.048 |
34.16
0.149 |
10.13
0.046 |
24.26
0.047 |
28.44
0.072 |
50.78
0.060 |
50.94
0.330 |
| °Brix | −0.571 *** |
1 |
43.67
0.529 |
24.10
0.153 |
30.98
0.413 |
78.41
0.119 |
12.73
0.309 |
28.34
0.036 |
16.67
0.002 |
0.40
0.072 |
15.18
0.094 |
17.30
0.490 |
19.42
0.184 |
43.33
0.002 |
25.30
0.011 |
16.34
0.180 |
8.36
0.008 |
28.41
0.021 |
49.98
0.272 |
41.07
0.194 |
|
| TA | −0.740 *** |
0.727 *** |
1 |
18.93
0.432 |
28.73
0.588 |
51.46
0.010 |
32.97
0.317 |
14.50
0.309 |
22.54
0.001 |
40.53
0.078 |
17.97
0.377 |
15.75
0.147 |
23.51
0.446 |
11.54
0.003 |
18.49
0.177 |
46.73
0.135 |
40.02
0.016 |
28.24
0.025 |
44.67
0.006 |
11.34
0.453 |
|
| °Brix/TA (%) | 0.411 *** |
−0.391 *** |
−0.657 *** |
1 |
29.97
0.008 |
33.71
0.076 |
13.39
0.003 |
4.34
0.046 |
4.54
0.306 |
21.51
0.252 |
1.88
0.003 |
16.61
0.173 |
4.55
0.006 |
9.78
0.278 |
0.62
0.432 |
28.29
0.209 |
20.18
0.004 |
25.07
0.846 |
47.54
0.017 |
9.55
0.109 |
|
| Vitamin C | −0.643 *** |
−0.643 *** |
0.767 *** |
0.089 *** |
1 |
31.62
0.002 |
30.63
0.280 |
29.71
0.392 |
30.23
0.028 |
30.96
0.168 |
30.08
0.513 |
3.58
0.236 |
30.22
0.415 |
29.46
0.002 |
29.93
0.166 |
31.19
0.124 |
30.85
0.016 |
25.98
0.013 |
8.27
0.000 |
29.45
0.926 |
|
| FormolNumber | 0.124 *** |
0.345 *** |
−0.098 *** |
0.276 *** |
−0.042 *** |
1 |
27.60
0.001 |
37.30
0.161 |
25.77
0.039 |
19.40
0.000 |
22.10
0.030 |
17.57
0.064 |
29.82
0.040 |
54.78
0.078 |
35.06
0.208 |
12.91
0.001 |
36.64
0.001 |
28.44
0.055 |
50.86
0.048 |
51.90
0.002 |
|
| Neoeriocitrin | 0.346 *** |
−0.556 *** |
−0.563 *** |
0.054 *** |
−0.529 *** |
0.032 *** |
1 |
17.80
0.020 |
7.56
0.150 |
9.96
0.004 |
8.35
0.096 |
17.07
0.166 |
8.65
0.303 |
26.99
0.002 |
14.26
0.232 |
19.67
0.003 |
7.38
0.002 |
28.39
0.016 |
49.13
0.014 |
26.06
0.196 |
|
| Naringin | 0.501 *** |
−0.190 *** |
−0.556 *** |
0.214 *** |
−0.626 *** |
0.401 *** |
0.141 *** |
1 |
8.62
0.032 |
25.67
0.080 |
5.47
0.759 |
16.43
0.019 |
8.67
0.202 |
4.71
0.100 |
3.77
0.201 |
32.18
0.121 |
24.57
0.162 |
28.32
0.005 |
46.93
0.023 |
4.61
0.390 |
|
| Neohesperidin | 0.153 *** |
0.045 *** |
−0.024 *** |
−0.553 *** |
−0.166 *** |
−0.197 *** |
−0.387 *** |
0.179 *** |
1 |
14.97
0.599 |
1.95
0.191 |
16.80
0.042 |
0.29
0.006 |
14.36
0.218 |
5.17
0.316 |
20.99
0.100 |
13.30
0.002 |
28.36
0.194 |
48.13
0.022 |
14.05
0.039 |
|
| Melitidin | −0.382 *** |
0.269 n.s. |
0.280 *** |
0.502 *** |
0.410 *** |
0.000 *** |
0.066 *** |
−0.282 *** |
−0.774 *** |
1 |
14.19
0.174 |
17.30
0.163 |
17.01
0.032 |
37.46
0.077 |
22.54
0.085 |
9.54
0.339 |
4.57
0.008 |
28.41
0.182 |
49.91
0.045 |
35.96
0.161 |
|
| Brutieridin | −0.534 *** |
0.306 *** |
0.614 *** |
0.050 n.s. |
0.716 *** |
−0.173 *** |
−0.310 *** |
−0.871 *** |
−0.437 n.s. |
0.417 *** |
1 |
16.69
0.097 |
1.80
0.236 |
9.88
0.001 |
2.40
0.063 |
18.67
0.104 |
12.72
0.047 |
28.35
0.004 |
47.66
0.001 |
9.73
0.488 |
|
| Total Floids | 0.499 *** |
−0.700 *** |
−0.384 *** |
−0.416 *** |
−0.486 *** |
−0.252 *** |
0.407 *** |
0.137 *** |
0.205 *** |
−0.404 *** |
−0.311 *** |
1 |
16.79
0.236 |
16.25
0.111 |
16.58
0.010 |
17.46
0.126 |
17.22
0.016 |
26.37
0.125 |
11.85
0.583 |
16.25
0.242 |
|
| Cloudy Juice | Neoeriocitrin | 0.615 *** |
−0.429 *** |
−0.668 *** |
0.080 *** |
−0.644 *** |
0.200 *** |
0.550 *** |
0.449 *** |
−0.077 n.s. |
−0.178 *** |
−0.486 n.s. |
0.486 *** |
1 |
15.17
0.071 |
5.23
0.370 |
24.07
0.195 |
15.37
0.013 |
28.36
0.026 |
48.14
0.021 |
14.78
0.435 |
| Naringin | −0.219 *** |
−0.048 *** |
0.054 *** |
0.527 *** |
0.048 *** |
0.280 *** |
−0.040 *** |
0.316 *** |
−0.467 *** |
0.278 *** |
0.035 *** |
−0.333 *** |
−0.266 *** |
1 |
9.19
0.187 |
46.96
0.031 |
37.38
0.497 |
28.30
0.045 |
46.42
0.068 |
0.00
0.003 |
|
| Neohesperidin | −0.386 *** |
0.105 *** |
0.421 *** |
−0.657 n.s. |
0.408 *** |
−0.456 *** |
−0.482 *** |
−0.448 *** |
0.562 *** |
−0.291 *** |
0.251 *** |
−0.099 *** |
−0.608 *** |
−0.432 *** |
1 |
29.49
0.038 |
21.27
0.001 |
28.33
0.358 |
47.47
0.000 |
8.97
0.148 |
|
| Melitidin | −0.214 *** |
0.424 *** |
0.367 *** |
0.457 *** |
0.352 *** |
0.032 *** |
−0.052 *** |
−0.348 *** |
−0.316 *** |
0.582 *** |
0.323 *** |
−0.355 *** |
−0.442 *** |
0.175 *** |
−0.195 *** |
1 |
18.21
0.171 |
28.43
0.282 |
50.46
0.002 |
44.75
0.165 |
|
| Brutieridin | 0.217 *** |
0.091 *** |
0.125 *** |
−0.062 *** |
0.127 *** |
0.022 *** |
0.044 *** |
−0.403 *** |
0.047 *** |
0.092 *** |
0.217 *** |
0.128 *** |
0.116 *** |
−0.705 *** |
0.032 *** |
0.414 *** |
1 |
28.40
0.073 |
49.69
0.065 |
35.65
0.029 |
|
| Total Floids | −0.268 *** |
−0.146 *** |
0.158 *** |
−0.920 *** |
−0.114 *** |
−0.235 *** |
−0.126 *** |
−0.071 *** |
0.441 *** |
−0.427 *** |
−0.066 *** |
0.353 *** |
−0.161 *** |
−0.213 *** |
0.598 *** |
−0.531 *** |
−0.271 *** |
1 |
26.73
0.040 |
28.30
0.043 |
|
| Juice | DPPH assay | 0.245 *** |
−0.522 *** |
−0.079 *** |
−0.128 *** |
0.000 *** |
−0.220 *** |
0.116 *** |
−0.151 *** |
0.149 *** |
0.211 *** |
0.024 *** |
0.764 *** |
0.143 *** |
−0.260 *** |
0.017 *** |
−0.048 *** |
0.256 *** |
0.199 *** |
1 |
46.40
0.001 |
| FRAP assay | −0.574 *** |
0.441 *** |
0.673 *** |
−0.330 *** |
0.962 *** |
0.044 *** |
−0.309 *** |
−0.624 *** |
−0.196 *** |
0.402 *** |
0.699 *** |
−0.492 *** |
−0.659 *** |
0.054 n.s. |
0.385 *** |
0.406 *** |
0.171 *** |
−0.206 *** |
0.024 *** |
1 |
3.3. °Brix
The degree Brix is the sugar content expressed as g/100 g juice. It is directly proportional to the sweetness of the fruit and therefore to its organoleptic pleasantness. This value did not exceed 10, which was reached by Fantastico cv in November (Table 4). The analysis of variance showed very highly significant differences during ripening (p < 0.001) in all the cultivars. If the cultivar effect is considered, very high significant differences in November, January and March (p < 0.001) were found, high significant differences in October and December (p < 0.01) and significant differences (p < 0.05) in February (Table 4). The °Brix/titratable acidity (%) is a maturity index and the highest value for all the three cultivars was found in the last month of sampling, with a tendency to increase during ripening (Table 4). The °Brix/titratable Acidity (%) ratio had a strong negative correlation with total flavonoids in juice (r = 0.920; p < 0.001; r2 = 0.846; t = 16.61) (Table 5). Other Authors found different °Brix in other citrus juices: 5.10 (grapefruit), 1.16 (lemon), 4.53 (orange), 6.50 Tangerine [21], and 11.0 °Brix in squeezed blood orange juice cultivated in Calabria [22].
3.4. Titratable Acidity
The titratable acidity is an important parameter to determine the maturity of the fruit and the acidic taste in citrus fruit. The degree of maturity of a fruit is one of the most important factors to determine conservation methods and control quality parameters such as taste and flavor. An immature fruit usually has a low sugar content in relation to acidity, compared to a ripe fruit that has a high level of sugar in relation to acidity. In bergamot juice a very high significant difference in titratable acidity between cultivars (p < 0.001) was observed, from a low 53.86 g/L in Castagnaro to a high 58.67 g/L in Fantastico, measured at the earliest sampling event (Table 4). During fruit ripening a decreasing trend in titratable acidity in all cultivars was observed. At the last sampling event in Castagnaro the lowest content (34.98 g/L) was seen, namely a decrease of 35.05% from October to March, whereas the highest value was not found in Fantastico (as at the earliest sampling event) but in Femminello (41.90 g/L) with a decrease rate of 22.81%.
3.5. Vitamin C
The human body cannot synthesize vitamins; therefore, they have to be part of our diet. Vitamin C (ascorbic acid) is water soluble, has antioxidant potential [23], prevents scurvy [24] and degenerative diseases, particularly those that are ageing-related [25] and has possible protective effects on the bones of older adults. Vitamin C can be oxidized by storage at room temperature, the addition of baking soda, overcooking, contact with copper and over intakes of zinc (cooking tools), alcohol and pectin [26]. In the studied samples, vitamin C decreased dramatically during fruit ripening: Castagnaro (59%), Fantastico (47%) and Femminello (48%) from October to March. In October a very high significant difference in vitamin C between cultivars (p < 0.001) was found: 831 mg/L in Castagnaro, 867 mg/L in Fantastico and 669 mg/L in Femminello (Table 4). Vitamin C content was not influenced by cultivars in February but was very highly affected by this variable (p < 0.001) in all other months (Table 4). Findings of other Authors on vitamin C content in citrus fruit juices revealed 680 mg/L and 455 mg/L, respectively in Marsh and Star Ruby (i.e., two grapefruit cultivars [27]), 680 mg/L in blood orange [17], 220 mg/L in pomelo [28] and 355 mg/L in lemon analyzed by HPLC [21].
3.6. Formol Number
The Formol number can represent, in a normal chemical industrial control, a useful index for the global quantitative evaluation of amino acids present in fruit juices. The Formol number is not influenced by the presence of many natural constituents of fruit juice (sugars, vitamins, flavorings, colorings) and it is applied in the quality determination of fruit juice and beverages because it expresses the total number of amino acids found. In bergamot juice the cultivar did not influence the Formol number in October but very high significant differences (p < 0.001) were found between cultivars from November to March. The harvest date had significant influence (p < 0.001) on the Formol number for the three cultivars (Table 4). The Formol number varied from 23.7 mL NaOH 0.1 N/100 mL (Femminello in March) to 14.3 mL NaOH 0.1 N/100 mL (Castagnaro in December and Femminello in February) and exceeded 20 mL NaOH 0.1 N/100 mL at the same time in all the three cultivars only in October. No correlation was found between Formol number and melitidin in juice (r = 0) (Table 5).
3.7. Flavonoids
Flavonoids are polyphenols with an antioxidant and radical scavenging role and are described by the scientific literature to have many beneficial effects on human health. They are biomolecules that prevent the risk of primary open-angle glaucoma [28], have an antiplatelet effect [29], maintain the anti-inflammatory action of cortisol under pro-oxidant conditions [30], protect vascular endothelial function [31], have an anti-obesity activity [32], reduce the risk of cardiovascular disease [33] and have antimicrobial [34], antiviral [35] and anti-inflammatory effects [36]. Neoeriocitrin was significantly high in Femminello and low in Castagnaro and Fantastico (p < 0.001) at the earliest sampling event. This compound showed a tendency to increase in the bergamot juice of Castagnaro and Fantastico as the fruits ripened from October to February, with a fall in March (Table 6). Naringin, neohesperidin and brutieridin were the major flavonoids in the bergamot juice (Table 6), whereas neoeriocitrin, naringin and neohesperidin prevailed in bergamot cloudy juice (Table 7); this was probably due to a higher water solubility. Naringin was very highly significantly influenced (p < 0.001) by both cultivar and harvest date variables (Table 6 and Table 7). Neoeriocitrin in both bergamot juice and bergamot cloudy juice was highest in the last fruit sample dates (February and March) for all three cultivars (Table 6 and Table 7). In the bergamot juice, naringin content was highest on the last sample date (42.61%, 28.63% and 42.30% of the total flavonoids, respectively, for Castagnaro, Fantastico and Femminello). Neohesperidin in bergamot juice was significantly different at each sample date (p < 0.001) with January being the month in which the highest quantity was measured. Almost always, in both the juice and the cloudy juice of the three cvs of bergamot, neohesperidin content was highest in Fantastico (Table 6 and Table 7). Brutieridin and melitidin are two molecules identified and described in bergamot juice by Di Donna et al. [16,37,38,39] and Fiorillo et al. [40]. The name brutieridin comes from the ancient name of one of the Calabrian cities (Brutium, today Cosenza) where brutieridin and melitidin were studied, whereas melitidin derives from the name of one of the most important towns (Melito Porto Salvo) where the bergamot tree is cultivated. In the samples studied in our work, brutieridin was always greater than melitidin, both in bergamot juice and in bergamot cloudy juice (Table 6 and Table 7). On the first sample dates (October–December), melitidin was higher in Castagnaro and Femminello juice than in Fantastico, and the same situation was found on the last sample date (Table 6). Melitidin in cloudy juice was significantly lower in March when its content was lower than 3% in all the three cultivars, whereas it was double or almost double in the early period of ripening from October to December (Table 7). A significant decreasing tendency in the brutieridin content of bergamot juice was recorded in Castagnaro and Fantastico (p < 0.001), whereas a fluctuating rate was found during fruit ripening in Femminello juice and in the cloudy juice of the three cultivars. Brutieridin detected in both bergamot juice and bergamot cloudy was almost always greatest in Fantastico cv from October to March, except in February when the highest brutieridin content was found in Femminello (26.94%) for bergamot juice and in Castagnaro (14.33%) for bergamot cloudy juice. Harvest date and cultivar variables showed very high significant differences (p < 0.001) between means (Table 6 and Table 7). The correlation between each single flavonoid in juice and its homologous in cloudy juice was between r = 0.217 of brutieridin and r = 0.582 of melitidin (Table 5). In the bergamot juice the total flavonoid content constantly increased with fruit ripening in Fantastico from 361 mg/L in October to 678 mg/L in March (namely an increase rate of 87.81%), and in Femminello from 287 mg/L in October to 824 mg/L in March (namely an increase of 187.11%). Also, in Castagnaro juice the flavonoid content was higher in the last period of fruit ripening compared to October and November (Table 6). Studies on flavonoid content in other citrus juice during storage at 4 °C showed a decreasing trend [41], which indicate fruit should be picked later, and juice should be consumed as soon as possible after picking.
Table 6.
Main flavonoids in bergamot juice. Results are presented as the mean value ± standard deviation, n = 8, (2016–2017 and 2017–2018 harvest years). *** significance at p < 0.001; ** significance at p < 0.01; * significance at p < 0.05. Means in the same line are distinguished by small letters. Means in the same column are distinguished by capital letters.
| Cultivar | October | November | December | January | February | March | Sign. | |
|---|---|---|---|---|---|---|---|---|
|
Neoeriocitrin
(%) |
Castagnaro | 10.16 ± 0.08 eC | 13.85 ± 0.11 cB | 10.14 ± 0.05 eC | 12.64 ± 0.17 dA | 20.58 ± 0.19 aB | 17.95 ± 0.12 bA | *** |
| Fantastico | 12.84 ± 0.07 cB | 12.35 ± 0.05 dC | 12.82 ± 0.08 cB | 11.72 ± 0.09 eB | 22.75 ± 0.05 aA | 16.67 ± 0.06 bC | *** | |
| Femminello | 15.26 ± 0.19 cA | 15.98 ± 0.29 bA | 15.35 ± 0.11 cA | 10.30 ± 0.05 dC | 15.23 ± 0.03 cC | 17.41 ± 0.15 aB | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
|
Naringin
(%) |
Castagnaro | 29.13 ± 0.06 dB | 28.62 ± 0.26 dA | 29.01 ± 0.15 dB | 39.52 ± 0.47 bA | 33.36 ± 0.18 cA | 42.61 ± 0.13 aA | *** |
| Fantastico | 25.15 ± 0.08 dC | 26.35 ± 0.05 cB | 26.11 ± 0.06 cC | 28.42 ± 0.09 aC | 27.21 ± 0.16 bB | 28.63 ± 0.12 aB | *** | |
| Femminello | 30.69 ± 0.19 cA | 23.92 ± 0.20 eC | 30.8 ± 0.05 cA | 37.18 ± 0.03 bB | 26.65 ± 0.03 dC | 42.3 ± 0.26 aA | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
|
Neohesperidin
(%) |
Castagnaro | 17.87 ± 0.06 bC | 16.75 ± 0.20 cC | 17.75 ± 0.13 bB | 33.11 ± 0.10 aA | 13.82 ± 0.12 dC | 17.92 ± 0.07 bC | *** |
| Fantastico | 21.05 ± 0.23 bA | 20.51 ± 0.12 cA | 20.06 ± 0.23 cA | 29.16 ± 0.08 aC | 18.51 ± 0.11 dB | 29.34 ± 0.24 aA | *** | |
| Femminello | 18.35 ± 0.18 eB | 19.51 ± 0.17 cB | 18.01 ± 0.10 eB | 32.62 ± 0.15 aB | 22.91 ± 0.02 bA | 18.78 ± 0.03 dB | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
|
Melitidin
(%) |
Castagnaro | 11.65 ± 0.29 bA | 13.32 ± 0.21 aA | 11.51 ± 0.13 bA | 5.21 ± 0.08 dC | 13.68 ± 0.11 aA | 7.13 ± 0.11 cA | *** |
| Fantastico | 8.13 ± 0.09 cB | 8.36 ± 0.08 bC | 8.19 ± 0.10 b cC | 7.23 ± 0.06 dA | 9.23 ± 0.07 aB | 3.30 ± 0.10 eB | *** | |
| Femminello | 10.56 ± 0.06 aB | 10.43 ± 0.15 aB | 10.51 ± 0.03 aB | 6.91 ± 0.03 dB | 8.27 ± 0.03 bC | 7.22 ± 0.08 cA | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
|
Brutieridin
(%) |
Castagnaro | 31.49 ± 0.05 aB | 27.46 ± 0.12 bC | 31.47 ± 0.15 aB | 9.52 ± 0.08 eC | 18.56 ± 0.07 cC | 14.39 ± 0.16 dB | *** |
| Fantastico | 32.83 ± 0.07 aA | 32.43 ± 0.16 bA | 32.82 ± 0.08 aA | 23.47 ± 0.16 cA | 22.3 ± 0.13 dB | 22.06 ± 0.07 dA | *** | |
| Femminello | 25.50 ± 0.11 cC | 30.16 ± 0.05 aB | 25.33 ± 0.05 cC | 12.99 ± 0.12 eB | 26.94 ± 0.14 bA | 14.29 ± 0.01 dB | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
|
Total Flavonoids
in juice (g/L) |
Castagnaro | 276 ± 3 dC | 212 ± 7 eC | 488 ± 11 aA | 425 ± 7 bB | 419 ± 8 bC | 348 ± 18 cC | *** |
| Fantastico | 361 ± 9 cA | 303 ± 6 dA | 233 ± 17 eC | 509 ± 8 bA | 675 ± 10 aB | 678 ± 4 aB | *** | |
| Femminello | 287 ± 1 eB | 408 ± 4 cB | 364 ± 4 dB | 390 ± 12 cC | 845 ± 11 aA | 824 ± 7 bA | *** | |
| Sign. | *** | *** | *** | *** | *** | *** |
Table 7.
Main flavonoids in the aqueous extract: cloudy juice. Results are presented as the mean value ± standard deviation, n = 8, (2016–2017 and 2017–2018 harvest years). *** significance at p < 0.001; ** significance at p < 0.01; * significance at p < 0.05. Means in the same line are distinguished by small letters. Means in the same column are distinguished by capital letters.
| Cultivar | October | November | December | January | February | March | Sign. | |
|---|---|---|---|---|---|---|---|---|
|
Neoeriocitrin
(%) |
Castagnaro | 17.74 ± 0.09 dB | 25.53 ± 0.10 bA | 17.86 ± 0.11 dA | 18.66 ± 0.09 cC | 25.23 ± 0.15 bA | 28.89 ± 0.14 aB | *** |
| Fantastico | 18.06 ± 0.10 dA | 16.43 ± 0.09 eC | 18.09 ± 0.14 dA | 25.74 ± 0.07 bA | 24.3 ± 0.20 cB | 26.13 ± 0.17 aC | *** | |
| Femminello | 16.23 ± 0.12 eC | 23.36 ± 0.23 bB | 16.33 ± 0.07 eB | 19.11 ± 0.10 dB | 20.16 ± 0.18 cC | 33.49 ± 0.05 aA | *** | |
| *** | *** | *** | *** | *** | *** | *** | ||
|
Naringin
(%) |
Castagnaro | 39.52 ± 0.18 bB | 35.78 ± 0.18 dB | 39.35 ± 0.09 bB | 37.64 ± 0.08 cA | 31.35 ± 0.10 eC | 42.07 ± 0.15 aA | *** |
| Fantastico | 33.44 ± 0.23 cC | 38.98 ± 0.11 aA | 33.50 ± 0.10 cC | 27.18 ± 0.16 eC | 34.38 ± 0.08 bA | 29.12 ± 0.25 dC | *** | |
| Femminello | 42.20 ± 0.11 aA | 33.36 ± 0.07 cC | 42.33 ± 0.06 aA | 31.17 ± 0.06 eB | 32.31 ± 0.04 dB | 37.96 ± 0.06 bB | *** | |
| *** | *** | *** | *** | *** | *** | *** | ||
|
Neohesperidin
(%) |
Castagnaro | 25.71 ± 0.27 bC | 20.76 ± 0.12 dC | 25.64 ± 0.2 bC | 27.08 ± 0.06 aC | 22.05 ± 0.22 cC | 18.91 ± 0.15 eB | *** |
| Fantastico | 32.29 ± 0.25 aA | 24.26 ± 0.12 dB | 32.15 ± 0.07 aA | 31.19 ± 0.16 bB | 22.69 ± 0.13 eB | 30.43 ± 0.15 cA | *** | |
| Femminello | 29.52 ± 0.34 cB | 28.25 ± 0.28 dA | 29.22 ± 0.03 cB | 35.73 ± 0.09 aA | 32.19 ± 0.03 bA | 16.11 ± 0.09 eC | *** | |
| *** | *** | *** | *** | *** | *** | *** | ||
|
Melitidin
(%) |
Castagnaro | 6.22 ± 0.09 cA | 6.52 ± 0.06 bB | 6.24 ± 0.12 b cA | 6.24 ± 0.08 b cA | 7.04 ± 0.18 aA | 2.60 ± 0.05 dA | *** |
| Fantastico | 4.28 ± 0.07 cB | 7.81 ± 0.15 aA | 4.27 ± 0.10 cC | 3.82 ± 0.15 dB | 6.69 ± 0.14 bB | 2.38 ± 0.08 eB | *** | |
| Femminello | 4.45 ± 0.14 aB | 4.3 ± 0.24 aC | 4.55 ± 0.05 aB | 3.37 ± 0.07 bB | 4.32 ± 0.07 aC | 2.13 ± 0.05 cC | *** | |
| *** | *** | *** | *** | *** | *** | *** | ||
|
Brutieridin
(%) |
Castagnaro | 10.81 ± 0.07 cB | 11.61 ± 0.23 bB | 10.91 ± 0.14 cB | 10.38 ± 0.14 dB | 14.33 ± 0.08 aA | 7.53 ± 0.10 eC | *** |
| Fantastico | 11.93 ± 0.15 bA | 12.52 ± 0.15 aA | 11.99 ± 0.06 bA | 12.07 ± 0.16 bA | 11.94 ± 0.15 bB | 11.94 ± 0.24 bA | ** | |
| Femminello | 7.56 ± 0.32 cC | 10.73 ± 0.22 a bC | 7.56 ± 0.06 cC | 10.62 ± 0.08 abB | 11.02 ± 0.06 aC | 10.31 ± 0.07 bB | *** | |
| *** | *** | *** | *** | *** | *** | *** | ||
|
Total Flavonoids
in Cloudy Juice (mg/L) |
Castagnaro | 4272 ± 17 cC | 4027 ± 12 dC | 4778 ± 22 bC | 6546 ± 34 aC | 4066 ± 14 dC | 4273 ± 18 cC | *** |
| Fantastico | 6680 ± 28 cB | 5926 ± 12 dB | 7816 ± 14 bB | 7960 ± 25 aB | 5671 ± 30 eB | 6671 ± 34 cB | *** | |
| Femminello | 8271 ± 82 dA | 7753 ± 42 eA | 8489 ± 12 cA | 8651 ± 9 bA | 9254 ± 10 aA | 8212 ± 23 dA | *** | |
| *** | *** | *** | *** | *** | *** | *** |
3.8. DPPH Assay and FRAP Assay
A citrus juice contains more than one class of antioxidants which have different behaviors. For this reason, many authors suggest applying more than one assay to evaluate antioxidant activity. In the present study we applied DPPH assay and FRAP assay which are two of the most common tests used on many matrices such as the common orange [20], blood orange juice [17], edible vegetable oils and potential industrial vegetable oils [42,43,44], apples, bananas, strawberries, kiwifruit and cauliflower [45]. Vitamin C and flavonoids are the most important antioxidants in bergamot juice and show an inverse ratio during fruit ripening: the vitamin C showed a decreasing trend (Table 4) in opposition to total flavonoid content which increased with harvest date (Table 6). In all the three cultivars DPPH value showed a very high significant difference at each month of sampling (Table 8). The correlation between antioxidant activity of the bergamot juice measured with the DPPH assay was high with total flavonoid content (r = 0.764; p < 0.001; r2 = 0.583; t = 11.87). This was in accordance with results of Roussos [46] which found a strong positive correlation between DPPH and flavonoids in blood orange juice. FRAP assay had an almost strong positive correlation with the titratable acidity (r = 0.673; p < 0.001; r2 = 0.453; t = 29.45) and with brutieridin in juice (r = 0.699; p < 0.001; r2 = 0.488; t = 9.73), and a moderate positive correlation with °Brix (r = 0.441; p < 0.001; r2 = 0.194; t = 41.07) and neohesperidin in cloudy juice (r = 0.385; p < 0.001; r2 = 0.148; t = 8.97). FRAP assay was also moderately correlated with melitidin in both juice (r = 0.402; p < 0.001; r2 = 0.161; t = 35.96) and cloudy juice (r = 0.406; p < 0.001; r2 = 0.165; t = 44.75) showing a very similar Pearson coefficient (Table 8). Lastly, vitamin C was found to be responsible for the antioxidant activity measured by FRAP assay (r = 0.962; p < 0.001; r2 = 0.926; t = 29.45) (Table 5), similar to the findings of other authors on citrus juices [47,48,49], but in partial disagreement with other results [50].
Table 8.
Antioxidant activity of bergamot juice. AAE = ascorbic acid equivalent. Results are presented as the mean value ± standard deviation, n = 8, (2016–2017 and 2017–2018 harvest years). *** significance at p < 0.001; ** significance at p < 0.01; * significance at p < 0.05. Means in the same line are distinguished by small letters. Means in the same column are distinguished by capital letters.
| Cultivar | October | November | December | January | February | March | Sign. | |
|---|---|---|---|---|---|---|---|---|
|
DPPH Assay—Juice
(mg AAE/100mL) |
Castagnaro | 380.8 ± 1.16dC | 320.3 ± 2.30eC | 494.9 ± 2.17aA | 431.2 ± 1.11bA | 386.6 ± 2.21cC | 305.6 ± 1.85fC | *** |
| Fantastico | 423.4 ± 2.17bA | 361.7 ± 3.79cB | 340.7 ± 1.70dB | 416.8 ± 2.41bB | 453.3 ± 4.62aB | 450.9 ± 4.23aB | *** | |
| Femminello | 394.3 ± 4.01cB | 420.6 ± 5.11bA | 330.8 ± 3.53dC | 340.0 ± 7.71dC | 478.4 ± 6.59aA | 473.9 ± 4.10aA | *** | |
| Sign. | *** | *** | *** | *** | *** | *** | ||
|
FRAP Assay—Juice
(mM AAE 100/mL) Formol Number (mL NaOH 0.1N/100mL) |
Castagnaro | 45.21 ± 0.96aA | 34.44 ± 1.93cdB | 40.67 ± 0.56bA | 32.66 ± 0.10dB | 35.77 ± 0.47cA | 28.41 ± 0.08eB | *** |
| Fantastico | 45.84 ± 0.39aA | 36.93 ± 0.64bAB | 35.91 ± 0.64bcB | 35.05 ± 0.64cdA | 34.17 ± 0.75dA | 33.65 ± 0.57dA | *** | |
| Femminello | 39.67 ± 0.28aB | 38.04 ± 0.58aA | 35.24 ± 0.66bB | 33.89 ± 0.78AbcB | 31.82 ± 0.76cB | 26.26 ± 1.35dC | *** | |
| Sign. | *** | * | *** | ** | *** | *** |
3.9. Hierarchical Cluster Analysis and Principal Component Analysis
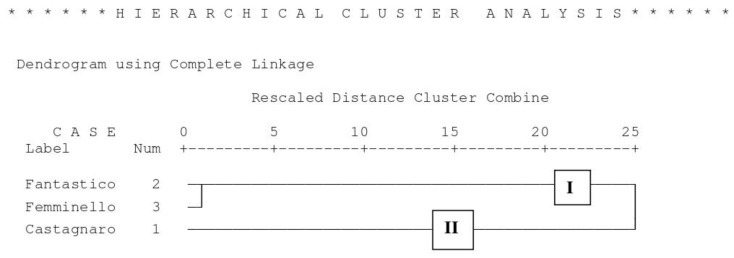
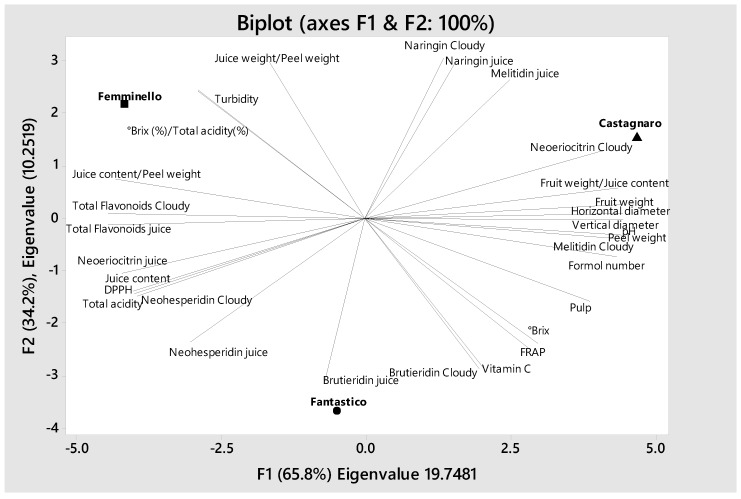
The three cultivars were found to cluster into two clades (Figure 1). Clade 1 contained Fantastico and Femminello which showed a high similarity and were clustered at a distance of 1. The second cluster contained the Castagnaro cv alone, with the highest fruit and peel weights; in particular, Castagnaro showed a peel weight double or more than double that of Femminello. In Castagnaro the highest vertical and horizontal diameters were also found, as well as the lowest flavonoid content in juice and cloudy juice and the lowest titratable acidity. Principal component analysis (PCA) was performed on the three cultivars and 30 parameters were included in the test (Figure 2). Two Eigen values were obtained and together accounted for 100% of the cumulative variability. The Eigen values and the percentage of total variance were 19.7481 (65.8%) and 10.2519 (34.2%). The visualization of the discrimination between the different orange cultivars on the plane of the first two functions led to a distinct separation. The cultivars were split between three sides of the plane which demonstrate the significant difference among the cultivars. The graphic also shows how the parameters are linked or separated from the cultivar factor. The Castagnaro cultivar located in the right corner of the plane is linked to the neoeriocitrin cloudy juice and fruit weight/juice content ratio. The Fantastico cultivar located in the lower center of the plane, is more influenced by the brutieridin in juice and in cloudy juice. Finally, the Femminello cultivar, which is located in the left corner of the plane, is influenced by the °Brix/titratable acidity (%) ratio, turbidity and juice content/peel weight ratio. Some parameters showed an independency from the cultivar factor because of the their location in the plane, such us the naringin in cloudy juice, the naringin in juice, °Brix, FRAP value, DPPH value, titratable acidity, neohesperidin and total flavonoids in juice and cloudy juice. Also, some parameters are correlated negatively, such as neohesperidin and naringin, as these parameters are located in opposite directions in the plane.
Figure 1.
Two-dimensional dendrogram obtained from the cluster analysis of the fruit biometrics and of the physico-chemical properties of juice and cloudy juice of the bergamot fruit (Citrus bergamia, Risso).
Figure 2.
Score plot of the principal component analysis (PCA) performed on the biometrics and the physico-chemical properties of juice and cloudy juice of the three cultivars (Castagnaro, Fantastico and Femminello) of the bergamot fruit (Citrus bergamia, Risso).
4. Conclusions
Bergamot is a tree and fruit with a very strong geographical connotation, growing almost exclusively in Reggio Calabria province (South Italy). Three cultivars of this citrus genus are known (Castagnaro, Fantastico and Femminello) and this study has shown a strong effect of both the cultivar (genotype) and the harvest date. These variables were found to influence the biometrics and the physico-chemical properties of fruits and fruit juice. Castagnaro is the cultivar producing the heaviest fruit, with the highest vertical and horizontal diameter and with the highest peel content during fruit ripening from October to March. Vitamin C content decreases during bergamot fruit ripening and it is close to the mean or in a higher quantity compared to other citrus fruit juice. The findings of this study show that the bergamot fruit is a very good source of flavonoids which can be directly used in food and beverage preparation when obtained from fruit juice and pulp, or which can be extracted from cloudy juice for food, beverages and pharmaceutical purposes. Naringin and neohesperidin were two flavonoids predominating in both the bergamot juice and in the bergamot cloudy juice with brutieridin as one of the two most represented flavonoids in bergamot juice and naringin as the most represented flavonoid in the bergamot cloudy juice. Brutieridin and melitidin are two flavonoids characterizing bergamot juice.
Acknowledgments
This research was conducted in the ambit of a collaboration agreement between the Herbal & Antioxidant Derivatives (H&AD s.r.l.) and the Department AGRARIA (Mediterranea University of Reggio Calabria, Italy), with Angelo Maria Giuffrè as the head of scientific research.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Strano A., Falcone G., Nicolò B.F., Stillitano T., De Luca A.I., Nesci F.S., Gulisano G. Eco-profiles and economic performances of a high-value fruit crop in southern Italy: a case study of bergamot (Citrus bergamia Risso) Agroecol. Sust. Food. 2017;41:1124–1145. [Google Scholar]
- 2.ISTAT. [(accessed on 6 December 2018)];2019 Available online: http://agri.istat.it/jsp/dawinci.jsp?q=plC250000030000193200&an=2017&ig=1&ct=281&id=15A|21A|31A.
- 3.Katz E., Lagunes P.M., Riov J., Weiss D., Goldschmidt E.E. Molecular and physiological evidence suggests the existence of a system II-like pathway of ethylene production in non-climacteric Citrus fruit. Planta. 2004;219:243–252. doi: 10.1007/s00425-004-1228-3. [DOI] [PubMed] [Google Scholar]
- 4.Jin W.F., Shen L.H., Ren J.H., Jin J.M., Shen Y.Y., Zhu J.Q., Liang Z.S., Yang D.F. Research progress on bergamot essential oil and its related product development. Chin. Tradit. Herb. Drugs. 2016;47:857–861. [Google Scholar]
- 5.Pernice R., Borriello G., Ferracane R., Borrelli R.C., Cennamo F., Ritieni A. Bergamot: A source of natural antioxidants Bergamot: A source of natural antioxidants for functionalized fruit juices. Food Chem. 2009;112:545–550. doi: 10.1016/j.foodchem.2008.06.004. [DOI] [Google Scholar]
- 6.Giuffrè A.M., Zappia C., Capocasale M., Poiana M., Sidari R., Di Donna L., Bartella L., Sindona G., Corradini G., Giudici G., et al. Vinegar production to valorise Citrus bergamia by-products. Eur. Food Res. Technol. 2017;245:667–675. doi: 10.1007/s00217-018-3189-y. [DOI] [Google Scholar]
- 7.Qiu T., Wu D., Yang L., Ye H., Wang Q., Cao Z., Tang K. Exploring the mechanism of flavonoids through systematic bioinformatics analysis. Front. Pharmacol. 2018;9:918. doi: 10.3389/fphar.2018.00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martirosyan D.M., Singh J. A new definition of functional food by FFC: what makes a new definition unique? Funct. Foods Health Dis. 2015;5:209–223. [Google Scholar]
- 9.Lee S.C., Foo M.H. “Functional Foods and Its Biomarkers”. In: Richardson T.X., editor. Introduction to Functional Food Science: Textbook. 2nd ed. Functional Food Center; Dallas, TX, USA: 2014. [Google Scholar]
- 10.Clydesdale F. Functional Foods: Opportunities and Challenges. Institute of Food Technologist 58.12: 35-40. [(accessed on 9 April 2019)];2004 Available online: http://www.ift:~/media/Knowledge%20Center/Science%20Reports/Expert%20Reports/Functional%20Foods/Functionalfoods_expertreport_full.pdf.
- 11.American Dietetic Association Position of the American Dietetic Association: functional foods. J. Am. Diet. Assoc. 1999;99:1278–1285. doi: 10.1016/S0002-8223(99)00314-4. [DOI] [PubMed] [Google Scholar]
- 12.DeFelice S.L. ‘The nutraceutical revolution, its impact on food industry research and development’. Trends Food Sci. Technol. 1995;6:59–61. doi: 10.1016/S0924-2244(00)88944-X. [DOI] [Google Scholar]
- 13.Li B.B., Smith B., Hossain M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006;48:182–188. doi: 10.1016/j.seppur.2005.07.005. [DOI] [Google Scholar]
- 14.Laroze L., Soto C., Zúñiga M.E. Phenolic antioxidants extraction from raspberry wastes assisted by-enzymes. Electron. J. Biotech. 2010;13:11–12. doi: 10.2225/vol13-issue6-fulltext-12. [DOI] [Google Scholar]
- 15.Roggia Ruviaro A., de Paula Menezes Barbosa P., Alves Macedo G. Enzyme-assisted biotransformation increases hesperetin content in citrus juice by-products. Food Res. Int. 2019 doi: 10.1016/j.foodres.2018.05.004. in press. [DOI] [PubMed] [Google Scholar]
- 16.Di Donna L., De Luca G., Mazzotti F., Napoli A., Salerno R., Taverna D., Sindona G. Statin-like principles of Bergamot fruit (Citrus bergamia): Isolation of 3-hydroxymethylglutaryl flavonoid glycosides. J. Nat. Prod. 2009;72:1352–1354. doi: 10.1021/np900096w. [DOI] [PubMed] [Google Scholar]
- 17.Giuffrè A.M., Zappia C., Capocasale M. Physico-chemical stability of blood orange juice during frozen storage. Int. J. Food Prop. 2017;20:1930–1943. [Google Scholar]
- 18.IFUMA 30 International Fruit and Vegetable Juice Association (IFUMA) [(accessed on 1 May 2019)];2016 Available online: www.ifu-fruitjuice.com/ifu-methods.
- 19.Panuccio M.R., Papalia T., Attinà E., Giuffrè A.M., Muscolo A. Use of digestate as an alternative to mineral fertilizer: effects on growth and crop quality. Arch. Agron. Soil Sci. 2019;65:700–711. doi: 10.1080/03650340.2018.1520980. [DOI] [Google Scholar]
- 20.Sicari V., Dorato G., Giuffrè A.M., Rizzo P., Albunia A.R. The effect of different packaging on nutritional properties and antioxidant activity of oranges during storage. J. Food Process. Pres. 2017;41:e13168. doi: 10.1111/jfpp.13168. [DOI] [Google Scholar]
- 21.Cserhalmi Z., Sass-Kiss Á., Tóth-Markus M., Lechner N. Study of pulsed electric field treated citrus juices. Innov. Food Sci. Emerg. 2006;7:49–54. doi: 10.1016/j.ifset.2005.07.001. [DOI] [Google Scholar]
- 22.Destani F., Cassano A., Fazio A., Vincken J.P., Gabriele B. Recovery and concentration of phenolic compounds in blood orange juice by membrane operations. J. Food Eng. 2013;117:263–271. doi: 10.1016/j.jfoodeng.2013.03.001. [DOI] [Google Scholar]
- 23.Ryan M.J., Dudash H.J., Docherty M., Geronilla K.B., Baker B.A., Haff G.G., Cutlip R.G., Always S.E. Vitamin E and C supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp. Gerontol. 2010;45:882–895. doi: 10.1016/j.exger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asensi-Fabado A.A., Munné-Bosch S. Vitamins in plants: occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010;15:582–592. doi: 10.1016/j.tplants.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Li Y., Schellhorn H.E. Can ageing-related degenerative diseases be ameliorated through administration of vitamin C at pharmacological levels? Med. Hypotheses. 2007;68:1315–1317. doi: 10.1016/j.mehy.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 26.Sauberlich H.E. Bioavailability of vitamins. Prog. Food Nut. Sci. 1985;9:1–33. [PubMed] [Google Scholar]
- 27.Sicari V., Pellicanò T.M., Giuffrè A.M., Zappia C., Capocasale M., Poiana M. Physical chemical properties and antioxidant capacities of grapefruit juice (Citrus paradisi) extracted from two different varieties. Int. Food Res. J. 2018;25:1978–1984. [Google Scholar]
- 28.Kang J.H., Ivey K.L., Boumenna T., Rosner B., Wiggs J.L., Pasquale L.R. Prospective study of flavonoid intake and risk of primary open-angle glaucoma. Acta Ophthalmol. 2018;96:e692–e700. doi: 10.1111/aos.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan H., Jawad M., Kamal M.A., Baldi A., Xiao J., Nabavi S.M., Daglia S.M. Evidence and prospective of plant derived flavonoids as antiplatelet agents: Strong candidates to be drugs of future. Food Chem. Toxicol. 2018;119:355–367. doi: 10.1016/j.fct.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Veríssimo G., Bast A., Weseler A.R. Monomeric and oligomeric flavanols maintain the endogenous glucocorticoid response in human macrophages in pro-oxidant conditions in vitro. Chem-Biol Interact. 2018;291:237–244. doi: 10.1016/j.cbi.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D., Du M., Wei Y., Wang C., Shen L. A review on the structure–activity relationship of dietary flavonoids for protecting vascular endothelial function: Current understanding and future issues. J. Food Biochem. 2018;42:e12557. doi: 10.1111/jfbc.12557. [DOI] [Google Scholar]
- 32.Hughes L.A., Arts I.C., Ambergen T., Brants H.A., Dagnelie P.C., Goldbohm R.A., van den Brandt P.A., Weijenberg M.P. Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in BMI over time in women: A longitudinal analysis from the Netherlands Cohort Study. Am. J. Clin. Nutr. 2008;88:1341–1352. doi: 10.3945/ajcn.2008.26058. [DOI] [PubMed] [Google Scholar]
- 33.Feliciano R.P., Pritzel S., Heiss C., Rodriguez-Mateos A. Flavonoid intake and cardiovascular disease risk. Curr. Opin. Food Sci. 2015;2:92–99. doi: 10.1016/j.cofs.2015.02.006. [DOI] [Google Scholar]
- 34.Cushnie T.P.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asres K., Seyoum A., Veeresham C., Bucar F., Gibbons S. Naturally derived anti-HIV agents. Phytother. Res. 2005;19:557–581. doi: 10.1002/ptr.1629. [DOI] [PubMed] [Google Scholar]
- 36.Kim H.P., Son K.H., Chang H.W., Kang S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004;96:229–245. doi: 10.1254/jphs.CRJ04003X. [DOI] [PubMed] [Google Scholar]
- 37.Di Donna L., Gallucci G., Malaj N., Romano E., Tagarelli A., Sindona G. Recycling of industrial essential oil waste: Brutieridin and Melitidin, two anticholesterolaemic active principles from bergamot albedo. Food Chem. 2011;125:438–441. doi: 10.1016/j.foodchem.2010.09.025. [DOI] [Google Scholar]
- 38.Di Donna L., Taverna D., Mazzotti F., Benabdelkamel H., Attya M., Napoli A., Sindona G. Comprehensive assay of flavanones in citrus juices and beverages by UHPLC–ESI-MS/MS and derivatization chemistry. Food Chem. 2018;141:2328–2333. doi: 10.1016/j.foodchem.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 39.Di Donna L., Iacopetta D., Cappello A.R., Gallucci G., Martello E., Fiorillo M., Dolce V., Sindona G. Hypocholesterolaemic activity of 3-hydroxy-3-methyl-glutaryl flavanones enriched fraction from bergamot fruit (Citrus bergamia): ‘In vivo’ studies. J. Funct. Foods. 2014;7:558–568. doi: 10.1016/j.jff.2013.12.029. [DOI] [Google Scholar]
- 40.Fiorillo M., Peiris-Pagès M., Sanchez-Alvarez R., Bartella L., Di Donna L., Dolce V., Sindona G., Sotgia G., Cappello A.R., Lisanti M.P. Bergamot natural products eradicate cancer stem cells (CSCs) by targeting mevalonate, Rho-GDI-signalling and mitochondrial metabolism. Biochim. Biophys. Acta Bioenergy. 2018;1859:984–996. doi: 10.1016/j.bbabio.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Del Caro A., Piga A., Vacca V., Agabbio M. Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chem. 2004;84:99–105. doi: 10.1016/S0308-8146(03)00180-8. [DOI] [Google Scholar]
- 42.Giuffrè A.M., Capocasale M., Zappia C., Poiana M. Influence of high temperature and duration of heating on the sunflower seed oil properties for food use and bio-diesel production. J. Oleo Sci. 2017;66:1193–1205. doi: 10.5650/jos.ess17109. [DOI] [PubMed] [Google Scholar]
- 43.Giuffrè A.M., Zappia C., Capocasale M. Effects of high temperatures and duration of heating on olive oil properties for food use and biodiesel production. J. Am. Oil Chem. Soc. 2017;94:819–830. doi: 10.1007/s11746-017-2988-9. [DOI] [Google Scholar]
- 44.Giuffrè A.M., Zappia C., Capocasale M. Tomato seed oil for edible use: cold break, hot break and harvest year effects. J. Food Process. Pres. 2017;41:e13309. doi: 10.1111/jfpp.13309. [DOI] [Google Scholar]
- 45.Szeto Y.T., Tomlinson B., Benzie I.F. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: implications for dietary planning and food preservation. Brit. J. Nut. 2002;87:55–59. doi: 10.1079/BJN2001483. [DOI] [PubMed] [Google Scholar]
- 46.Roussos P.A. Phytochemicals and antioxidant capacity of orange (Citrus sinensis (l.) Osbeck cv. Salustiana) juice produced under organic and integrated farming system in Greece. Sci. Hort. 2011;129:253–258. doi: 10.1016/j.scienta.2011.03.040. [DOI] [Google Scholar]
- 47.Gardner P.T., White T.A.C., McPhail D.B., Duthie G.G. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000;68:471–474. doi: 10.1016/S0308-8146(99)00225-3. [DOI] [Google Scholar]
- 48.Klimczak I., Małecka M., Szlachta M., Gliszczyńska-Świgło A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007;20:313–322. doi: 10.1016/j.jfca.2006.02.012. [DOI] [Google Scholar]
- 49.Sdiri S., Bermejo A., Aleza P., Navarro P., Salvador A. Phenolic composition, organic acids, sugars, vitamin C and antioxidant activity in the juice of two new triploid late-season mandarins. Food Res. Int. 2012;49:462–468. doi: 10.1016/j.foodres.2012.07.040. [DOI] [Google Scholar]
- 50.Ramful D., Tarnus E., Aruoma O.I., Bourdon E., Bahorun T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res. Int. 2011;44:2088–2099. doi: 10.1016/j.foodres.2011.03.056. [DOI] [Google Scholar]