Abstract

The world needs to take urgent action to tackle climate change. The Paris Agreement ([Link]) set a goal of keeping the rise in the global temperature well below 2° Celsius, and to pursue efforts to limit further the temperature increase to 1.5°C. Shell strongly supports this, and our ambition is to make sure the energy we sell is in tune with society as it moves towards that goal. To achieve the goal of the Paris Agreement, Shell believes the world is likely to have to stop adding to the stock of greenhouse gases in the atmosphere by 2070. This is a state known as net zero emissions. But by 2070, the number of people on the planet will have risen by around a third from today to more than 10 billion, and people's living standards will have improved. Together, these trends mean the world will use more and more energy. Even with a huge improvement in energy efficiency, the world is likely to be using 50% more energy by 2070, compared with today. The energy system the world has now cannot supply more energy and, at the same time, reduce greenhouse gas emissions like carbon dioxide. To achieve this, the system must change. Today's energy system is largely tied to fuels that release greenhouse gases into the atmosphere when used. In the future, that system must be made of products which, on average, release much lower levels of greenhouse gases for each unit of energy used. In other words, they must have a lower carbon intensity. The products in the new energy system will include renewable electricity, biofuels and hydrogen, alongside oil and gas.
Shell's ‘Sky’ scenario outlines some possible changes on the road towards net zero ([Link]). Lowering the carbon intensity of the energy products in the energy system is one part of this energy transition. The second part of the energy transition is how those energy products are used: how much is consumed and how efficiently. The third part of the energy transition is dealing with the carbon dioxide emissions that cannot be avoided. These will need to be removed and stored through natural processes like reforestation. In the ‘Sky’ scenario, electric vehicles will come to dominate the passenger vehicle fleet by 2070, whilst advanced biofuels will grow rapidly to displace fossil oil for applications where the energy density of liquid fuels remains a critical factor. The biosphere takes on an increasingly important role in stabilizing the climate through the 21st century, both from its carbon storage potential and from its role in providing a renewable feedstock option for chemicals and materials. Photosynthesis not only provides a mechanism to capture solar energy in molecular form, but also generates molecular building blocks for future bio‐manufacturing industries.
Synthetic biology offers great potential in tackling the energy challenge. Research conducted by Shell in partnership with the University of Exeter's Microbial Biofuels Group used engineered E. coli to produce biodiesel (Howard et al., 2013). Biodiesel is a complex mixture of hydrocarbons with a range of chain lengths and branching. A gene mixture was assembled in E. coli, including fatty acid reductase complex from Photorhabdus luminescens, aldehyde decarbonylase from Nostoc punctiforme, thioesterase from Cinnamomum camphora (camphor tree), plus branched‐chain α‐keto acid dehydrogenase complex and β‐keto acyl‐ACP synthase III from Bacillus subtilis. This study showed that it is possible to use renewable feedstocks to produce a hydrocarbon mixture which is very similar to diesel.
In another project, conducted in collaboration with University of Manchester's Institute of Biotechnology, a new type of biological catalyst was discovered which uses a cycloaddition mechanism to interconvert alkenes with corresponding α,β‐unsaturated carboxylic acids at ambient conditions (Payne et al., 2015; White et al., 2015). This provides a new route to hydrocarbon production and has established proof of principle for renewable production of 2,5‐furandicarboxylic acid (FDCA) from furoic acid (Payne et al., 2019). FDCA is a valuable bioderived compound and potential replacement for petrochemical derived monomers that are used in polymers such as polyethylene terephthalate (PET) plastics. Though the yields of biodiesel and FDCA in each study are low, and there is some way to go before this research is ready for industrial production, it provides an important proof of principle that industrially relevant molecules can be produced using biological catalysts. Challenges ahead include how to scale up the process and which production host organism to use. E.coli is suitable for laboratory studies, but for industrial reality, especially at the scale of the energy industry, we will need a robust industrial host organism that has an associated genetic toolkit for ease of transformation. Ideally, this organism could be tailored to many types of industrial process. Through its academic partnership with Exeter University, Shell has been investigating the feasibility of developing broad‐range, microbial chassis for synthetic biology applied to our own industry's requirements. On the other hand, perhaps this is something the broader community could already be thinking about how to accelerate the industrial deployment of synthetic biology: rather than each industry developing its own host, which is time consuming and costly, could we develop a generic host that is broadly applicable across a number of different applications?
Another hurdle limiting the deployment of industrial biotechnologies is the high capital cost of constructing the bioprocessing plant. Expensive materials, like high grade stainless steel, might be suitable for manufacturing high value products such as pharmaceuticals, but are unaffordable for many bulk chemicals and fuels. Here, innovation is required to identify lower cost materials of construction, such as plastics. Learnings from low‐cost microbial processes, including anaerobic digestion and ensiling, could be relevant. Avoiding contamination in industrial bioprocesses is a further challenge. Steam sterilization carries a capital and operating cost burden and might not even be compatible with plastic bioprocessing. Using thermophilic host organisms, or those that are tolerant to salt, or extremes of pH, might be a strategy for avoiding contamination, or at least to minimize it. Extracting the products from the bioprocess will require different downstream processing options compared to those with which the petrochemical industry is familiar, given that the products tend to be present at rather low concentrations in aqueous media. To speed up developments, synbio researchers should work with biochemical engineers at an early stage to carry out a techno‐economic assessment of their new process. This will help identify which elements of the bioprocess are the most susceptible to difficulties relating to high capital or operating costs and therefore where further improvements to the process might profitably be directed. Equally important, the biochemical engineers can advise on how the technology could be scaled up and which techno‐economic risks need to be addressed at each stage of the development. Shell's technology maturation funnel has 4 distinct stages which we call the ‘4 D's’. It starts with Discovery (laboratory proof of concept) then moves to Development (small‐scale piloting of the technology to identify strengths and weaknesses of the technology and the associated scale‐up risks). If all is well, the project moves to the Demonstration phase. Demonstration is the last time the technology is tested before commercial scale Deployment and usually involves construction and operation of demonstration plant. Since such a plant tends to be expensive to build and run, it is essential to have identified which specific elements of the new process need to be proven, under which operating conditions, and for how long. Each of the four D's leads to a stage gate, at which point the technical, economic and commercial aspects of the development are assessed before moving onto the next stage. At each stage, some options are deselected, but investment in the remaining options necessarily increases as we move into the more capitally intensive pilot and demonstration plant phases. We make conscious decisions on whether to create our own technology, or to buy technology, or co‐create technology in collaboration with others. Being able to provide an optimal mix of proprietary technologies, third party technologies and co‐creation of technologies, we are positioned to create and deliver the most value adding technology solutions. We work back from commercial opportunities to prioritise technology developments and to ensure focus on those developments that really deliver best value.
Looking even further ahead, the new energy future will increasingly rely on solar energy as the primary energy source. In fact, the Sun delivers in one hour the same amount of energy we currently use in a year. Yet sunlight is a dilute form of energy. It needs to be converted into other forms of energy to be useful. Biology does this via photosynthesis, which is of course how crude oil was formed in the first place. The downside is that photosynthesis is rather inefficient in its use of photons.
On the other hand, there have been huge strides in the development of photovoltaics (PVs) and electricity generation via PV's is rapidly coming down in price. In some parts of the world, it is already cost‐competitive with natural gas. But there are challenges relating to solar energy. Obviously, sunlight is not constant: it varies diurnally and seasonally. Moreover, many of the regions of the world with high levels of insolation are remote from the main centres of population where the energy is required. Storing and transporting electricity over long distances is not easy. Whilst transmission and battery storage will be important, we believe there will be a need to produce dense molecular energy carriers, to facilitate the transfer of energy from where it is generated to where it is used.
It is with these future energy challenges in mind that Shell has initiated a Long Range Research programme. One aspect of this addresses the need for converting photons to ‘Dense Energy Carriers’. This is about developing new business models for supplying energy, but it is also about harnessing rapid developments in foundational sciences, such as electrochemistry, materials science and the biosciences to bring about new processes. Figure 1 shows some conceptual routes to Dense Energy Carriers where biology could play a role as shown in these conceptual pathways. Pathway (a) is the basis of the current biofuels industry. Terrestrial plants use light via photosynthesis to fix CO2 and produce biomass, which is converted to a fuel such as ethanol, itself a Dense Energy Carrier. Synthetic biology is already making this conversion process more efficient. Pathway (b) is similar, but we here move from plants to algae or cyanobacteria, relieving pressure on land.
Figure 1.
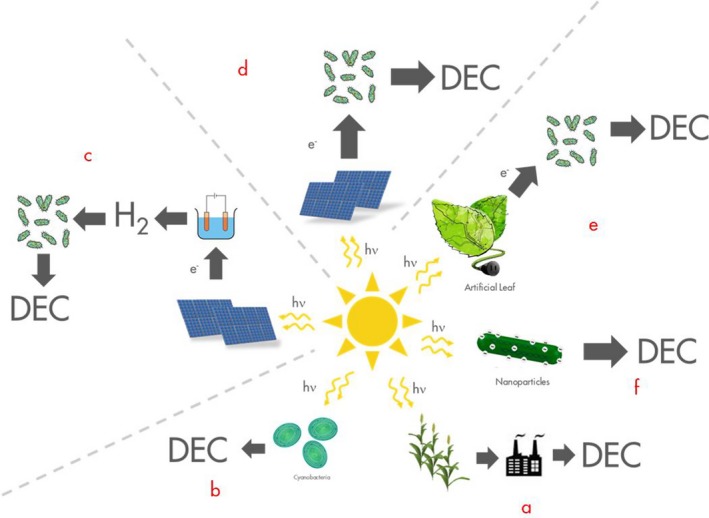
Conceptual pathways of sunlight to Dense Energy Carriers.
However, as noted before, photosynthesis is inherently inefficient in its use of photons. By contrast, solid state systems, such as PV's, are already more efficient. But where photosynthesis does have an advantage is its ability to both fix CO2 at atmospheric concentrations and make molecules. We can therefore ask the question whether it is possible to combine the advantages of solid state systems to gather photons, with biology's CO2 fixation and molecule production capacity. This will need significant breakthroughs in science, but conceptually the elements are there. For example, PVs, or photo‐electrochemical cells, can produce H2, which can in turn be used by organisms as energy sources to capture CO2 and to make molecules. Or, even better, can we use the electrons directly to drive CO2 fixation and molecule synthesis as in pathway (d)? Such, bio‐electrochemical synthesis is already a fruitful field of research (Ganigué et al., 2015).
We will need to address how the electrons interface with the biology – does this involve electrodes and will there be redox intermediates shuttling charge between the electrodes and the biology? How do we get sufficient mass transfer of CO2 from the atmosphere into the biological system? We cannot afford to concentrate the CO2 as it is too energetically costly. Yet, we already know that mass transfer of CO2 can quickly become the rate limiting step. And having tackled these issues, what does a device look like? At this point, these are as much engineering challenges as biological. Therefore, a multi‐disciplinary approach is required: it is essential that the biologists, electrochemists and engineers speak together at an early stage – and that they develop the vocabulary to facilitate such discussions.
Other novel concepts are also gaining traction including the so‐called artificial leaf (Nocera, 2012). Another recent development, pathway (f), is the deposition of photo‐receptive nanodots on the surface of non‐photosynthetic bacteria, such as acetogens, which turns them into photosynthetic organisms, fixing CO2 and making acetate (Müller, 2016). Could the organisms be developed to produce other molecules of interest? And what is their maximum photosynthetic efficiency?
For more than a century, industrial production has been dominated by the conversion of fossil hydrocarbon‐based feedstocks. The development of synthetic chemistry techniques in the 19th and 20th century provided the ‘platform technology’ required to create new industrial processes and products using these feedstocks. Could synthetic biology become the 21st century ‘platform technology’ required to create new industrial processes based on new feedstocks, generating a greater diversity of products? As a recent Royal Society symposium titled ‘[Link]’ observed, synthetic biology certainly has great potential, but we must also be wary of hype and we must also bring society along with us.
Conflict of interest
None declared.
Microbial Biotechnology (2019) 12(5), 824–827
Funding information
No funding information provided.
References
- Ganigué, R. , Puig, S. , Batlle‐Vilanova, P. , Balaguer, M.D. , and Colprim, J. (2015) Microbial electrosynthesis of butyrate from carbon dioxide. Chem Commun 51: 3235–3238. [DOI] [PubMed] [Google Scholar]
- Howard, T.P. , Middelhaufe, S. , Moore, K. , Edner, C. , Kolak, D.M. , Taylor, G.N. , et al (2013) Synthesis of customized petroleum‐replica fuel molecules by targeted modification of free fatty acid. pools in Escherichia coli. PNAS 110: 7636–7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, V. (2016) Microbes in a knight's armor. Science 351: 34. [DOI] [PubMed] [Google Scholar]
- Nocera, D.G. (2012) The Artificial Leaf. Acc Chem Res 45: 767–776. [DOI] [PubMed] [Google Scholar]
- Payne, K.A. , White, M.D. , Fisher, K. , Khara, B. , Bailey, S.S. , Parker, D. , et al (2015) New cofactor supports α, β‐unsaturated acid. decarboxylation via 1,3‐dipolar cycloaddition. Nature 522: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, K.A.P. , Marshall, S.A. , Fisher, K. , Cliff, M.J. , Cannas, D.M. , Yan, C. , et al (2019) Enzymatic Carboxylation of 2‐Furoic Acid Yields 2,5‐Furandicarboxylic Acid (FDCA). ACS Catal. 9: 2854–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shell's ‘Sky’ scenario : www.shell.com/skyscenario, accessed April 2019. Note that Sky is not a business plan or forecast, but a scenario.
- ‘Synthetic biology: does industry get it? ’; conference held on 8 February 2017 at the Royal Society, London. https://royalsociety.org/-/media/events/2017/02/tof-synbio/synthetic-biology-post-conference-report.PDF.
- ‘The Paris Agreement ’, https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement, accessed April 2019.
- White, M.D. , Payne, K.A. , Fisher, K. , Marshall, S.A. , Parker, D. , Rattray, N.J. , et al (2015) UbiX is a flavin prenyltransferase required for bacterial ubiquinone. biosynthesis. Nature 522: 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]


