Summary
The preservation of the viability of microorganisms in probiotic formulations is the most important parameter ensuring the adequate concentration of live microorganisms at the time of administration. The formulation and processing techniques used to produce these probiotic formulations can influence the preservation of the microbial viability. However, it is also required that the bacteria maintain their key probiotic capacities during processing, formulation and shelf life. In this study, we investigated the impact of spray‐drying on different cell wall properties of the model probiotic strain Lactobacillus rhamnosus GG, including its adherence to intestinal epithelial cells. The dltD gene knock‐out mutant, L. rhamnosus GG CMPG5540, displaying modified cell wall lipoteichoic acids, showed significantly increased colony‐forming units after spray‐drying and subsequent storage under standard conditions compared to wild‐type L. rhamnosus GG. In contrast, disruption of the biosynthesis of exopolysaccharides or pili expression did not impact survival. However, spray‐drying did significantly affect the adherence capacity of L. rhamnosus GG. Scanning electron microscopy confirmed that the pili, key surface factors for adherence to intestinal cells and mucus, were sheared off during the spray‐drying process. These data thus highlight that both the functionality and viability of probiotics should be assessed during the spray‐drying process and subsequent storage.
Introduction
Lactobacillus rhamnosus GG is a well‐known probiotic bacterium that is widely applied and investigated as a model probiotic strain (Doron et al., 2005). Its best documented beneficial effects on human health include benefits to the gastrointestinal tract, and various other effects, including immune modulation. Effects such as prevention of antibiotic‐associated diarrhoea, prevention of cow's milk allergy and reduction in respiratory tract infections (have been well substantiated in several meta‐analyses and systemic reviews (Cai et al., 2018; Laursen and Hojsak, 2018; Tan‐lim and Esteban‐ipac, 2018). However, comparative analysis of different clinical trials shows a large heterogeneity as also reflected in the different formulations that are often used (dosage form, probiotic concentration, excipients and combinations with other strains) (Zeilstra et al., 2018). For probiotic formulations, safeguarding the viability of the probiotic bacteria during processing and shelf life is generally the key parameter being monitored, so as to ensure sufficiently high concentrations of live microorganisms at the time of consumption necessary for their efficacy (Broeckx et al., 2016). Indeed, due to cellular injuries caused by heat treatment, mechanical or osmotic stress during production, significant losses of probiotic viability may occur (Pinto et al., 2015). However, recent molecular findings have shown that the effector molecules that mediate the crucial probiotic functions also need to be carefully monitored during processing, formulation and shelf life (Lebeer et al., 2018). For instance, in L. rhamnosus GG, it is well known that the key adhesive pili can be easily sheared off by processing steps, such as centrifugation (Tripathi et al., 2013). Loss of these pili could impair the efficacy of this probiotic strain, even when enough viable cell numbers are administered. Yet, this is currently underexplored.
Spray‐drying is an attractive production process to dry and formulate probiotic bacteria into powders because of its low cost, high process yield and production of good flowing powder particles (Broeckx et al., 2016). In this study, we investigated the importance of specific cell wall molecules of L. rhamnosus GG by comparing the survival, surface properties and adhesion capacity of different cell wall mutants before and after spray‐drying. We have opted to focus on mutants deficient in SpaCBA pili (Lebeer et al., 2012), d‐alanylation of lipoteichoic acids (LTA) (Vélez et al., 2007) or exopolysaccharides (EPS) (Lebeer et al., 2009), as these cellular factors are important for the host–bacterium interaction and are known to promote bacterial survival and/or adhesion. Because pili on the cell wall of L. rhamnosus GG play an important role in the adherence to intestinal epithelial cells (Lebeer et al., 2012), we investigated how these pili were affected by the spray‐drying process. These pili are often shielded by the exopolysaccharides (EPS) of L. rhamnosus GG (Lebeer et al., 2009, 2011). Exopolysaccharides molecules seem to promote survival of L. rhamnosus GG during transit through the gastrointestinal tract and in interaction with complement factors (Lebeer et al., 2011). Furthermore, EPS seems to promote the anti‐Candida activity of L. . rhamnosus GG (Allonsius et al., 2017). Lipoteichoic acid is an abundant molecule anchored in the cytoplasmic membrane reaching out through the peptidoglycan cell wall of Gram‐positive bacteria. LTA has been shown to have an important role in promoting immunostimulatory responses of L. rhamnosus GG (Claes et al., 2010, 2012; Segers and Lebeer, 2014).
Results
Modification of cell wall molecules influences plate counts after spray‐drying
The survival capacity for different L. rhamnosus GG surface mutants after spray‐drying was evaluated under standard conditions by viable plate count analysis before and after spray‐drying (Fig. 1A). Interestingly, the difference in colony‐forming units (CFU) counts of the different surface mutants before and after spray‐drying was similar to the wild‐type L. rhamnosus GG, except for the L. rhamnosus GG mutant with modified lipoteichoic acid [ΔdltD mutant, CMPG 5540, (Vélez et al., 2007)]. For this mutant, a significantly decreased reduction in CFU was observed (1.69 log‐reduction) compared to the wild type (1.99 log‐reduction) (and other investigated mutant strains). The water activity of the spray‐dried powders was approximately 0.1. No significant differences were seen. The evolution of the number of CFU of L. rhamnosus GG wild‐type and its surface mutants was also investigated during storage at 4°C for 26 weeks (Fig. 1B). Again, the surface mutant with modified lipoteichoic acids showed significantly higher count of CFU than wild‐type L. rhamnosus GG after storage over a period of 4 weeks. However, after 13 weeks this difference disappeared, and the viability of the L. rhamnosus GG with modified lipoteichoic acid was similar to that of the wild‐type L. rhamnosus GG. Of note, under the tested conditions, after 26 weeks, the CFU of L. rhamnosus GG and the different surface mutants was below the detection limit (< 103 CFU gspray‐dried powder −1).
Figure 1.
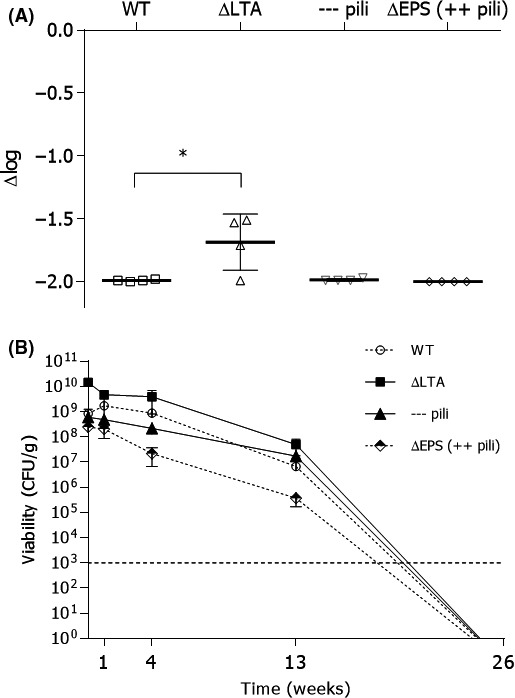
Evaluation of survival and viability of Lactobacillus rhamnosus GG after spray‐drying. A. The Lactobacillus rhamnosus GG wild‐type strain (WT) (ATCC53103), the lipoteichoic acid mutant ΔdltD::TcR (∆LTA) (CMPG 5540), the pili‐deficient ΔspaCBA::TcR mutant (represented as —pili in the graph) (CMPG5357) and the exopolysaccharide deficient ΔwelE::TcR mutant [∆EPS (++pili) (CMPG5351)] were routinely grown and spray‐dried (n = 4). The colony‐forming units before and after spray‐drying were compared, and the data are expressed as a logarithmic reduction of the colony‐forming units per gram powder after spray‐drying (∆log). B. The spray‐dried powders were stored at 4°C, and the viability was determined after 1, 4, 13 and 26 weeks (n = 3). After 26 weeks, the viability was below the detection limit (103 CFU g−1). Values are shown as means ± standard error of the mean. ANOVA test and Tukey's multiple comparisons test were used to determine differences in parametric data. GraphPad Prism, version 6.1 (GraphPad Software Inc) performed the analytical test at a significance level α = 0.05 (indicated with asterisks).
spray‐drying significantly reduces the adhesion capacity of L. rhamnosus GG
Since adhesion to the gastrointestinal mucosa is a key functionality of this probiotic strain (Segers and Lebeer, 2014), the effect of spray‐drying on this functionality was studied (Fig. 2). The adhesion capacity of L. rhamnosus GG wild‐type was more than sixfold reduced after spray‐drying (P < 0.05). These results are comparable to the adhesion of the pili‐deficient mutant (CMPG5357) (Lebeer et al., 2012) under native conditions, suggesting that the pili were removed during the formulation process. The exopolysaccharide deficient mutant strain (CMPG5357) (Lebeer et al., 2012) also showed a two‐ to threefold reduced adherence capacity after spray‐drying, compared to the same mutant strain before spray‐drying (P < 0.05). However, when comparing the adherence capacity of this exopolysaccharide deficient mutant to the other strains after spray‐drying, a significantly higher adherence capacity was observed.
Figure 2.
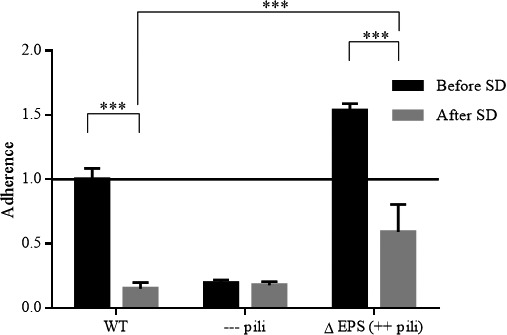
Adhesion capacity of Lactobacillus rhamnosus GG before and after spray‐drying. Adhesion capacity of Lactobacillus rhamnosus GG wild‐type strain (WT) (ATCC53103), the pili‐deficient ΔspaCBA::TcR mutant (—pili) (CMPG5357) and the exopolysaccharide deficient ΔwelE::TcR mutant (∆EPS (++ pili) (CMPG5351) to Caco‐2 intestinal epithelial cells was evaluated after spray‐drying. The line on the ‘1’ mark represents the adhesion capacity of L. rhamnosus GG WT before spray‐drying, which was used as a reference. Values are shown as means ± standard error of the mean (two‐way ANOVA, Tukey's multiple comparisons test). GraphPad Prism, version 6.1 (GraphPad Software Inc, La Jolla, Ca, USA) performed the analytical test at a significance level α = 0.05 (indicated with asterisks). The experiment was repeated three times with three biological repetitions.
Scanning Electron Microscopy (SEM) analysis suggests pili are sheared from the cell surface of L. rhamnosus GG by spray‐drying
To confirm the hypothesis that the pili were removed during the drying process, the surface of the bacteria was visualized by SEM (Fig. 3). Before spray‐drying, a clear distinction between the different bacterial strains was seen, as described below (Fig. 3, top panels), in agreement with our previous transmission electron microscopy (TEM) study on the different presence and exposure in these L. rhamnosus mutants, with more pili exposure in the mutant with deficient exopolysaccharides and their absence in the pili mutant (Lebeer et al., 2009). After spray‐drying (Fig. 3, bottom panels), pili were absent in all the tested mutants. These experiments thus indicate that the pili were removed during the spray‐drying process. Although the L. rhamnosus GG with deficient exopolysaccharides showed strikingly more pili present on the cell wall compared to the wild type before the processing, SEM analysis indicated that these pili were removed after the spray‐drying process. In agreement with what was previously described in Vélez et al. (2007), L. rhamnosus GG cells with altered lipoteichoic acids appeared longer than the wild‐type L. rhamnosus GG before the process. Surprisingly, the SEM analysis also showed that the length of the bacteria with altered lipoteichoic acids was shorter after spray‐drying compared to the length of the cells of this mutant strain before spray‐drying. Also, no pili were present on the cell wall of this mutant after the spray‐drying process. As expected, pili were also absent on the cell wall of the pili‐deficient mutant before or after spray‐drying, because of the introduced mutation in the pili encoding spaCBA genes.
Figure 3.
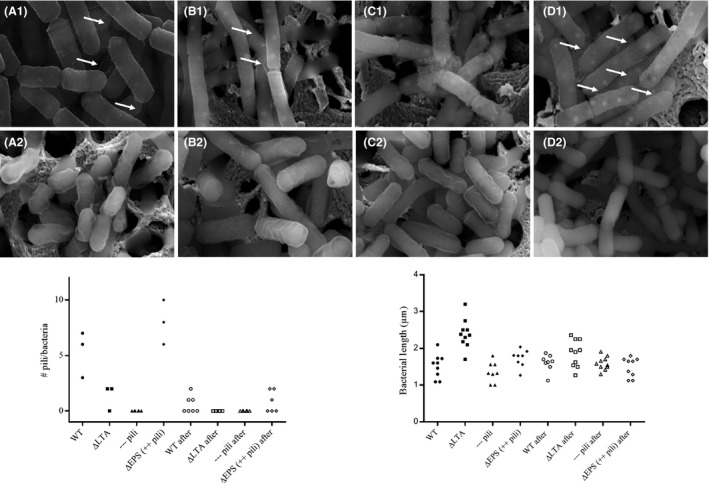
Scanning electron microscopy analysis of cell wall molecules on Lactobacillus rhamnosus GG before and after spray‐drying. SEM pictures showing the surface of the Lactobacillus rhamnosus GG wild‐type strain (WT) (ATCC53103) (A), the lipoteichoic acid mutant ΔdltD::TcR (CMPG5540) (B), the pili‐deficient ΔspaCBA::TcR mutant (CMPG5357) (C) and the EPS‐deficient ΔwelE::TcR mutant (CMPG5351) (D) before (1) and after (2) spray‐drying. The length and the number of pili (marked with an arrow) on each of the bacterium are visualized in the graphs under the SEM pictures.
Discussion
In this study, we explored the impact of spray‐drying on the survival capacity, the presence of pili on the surface and adhesion properties mediated by different cell wall molecules in the model probiotic L. rhamnosus GG. Remarkably, the dltD mutant showed a significantly higher viable plate count after spray‐drying. This is especially noteworthy since previous research had shown that this LTA mutant is more sensitive to stresses, such as gastric juice in vitro and in vivo (Claes et al., 2010), and to antimicrobial peptides such as β‐defensin‐2 and to autolysis (Vélez et al., 2007). The higher resistance to the spray‐drying process could be due to its altered cell wall properties (including altered charge), giving rise to an increased viability and associated higher CFU count. Another explanation could be the almost twofold increased cell length of the dltD mutant [1.87‐fold as determined here by SEM or 2.4‐fold based on TEM (Vélez et al., 2007)], and the morphological alteration at the level of the septum (Vélez et al., 2007). A possible hypothesis could be that larger cells have a larger volume, which may protect the cells against dehydration. The shear forces and other stresses such as heat present during the spray‐drying process could also have split the bacterial chains at the flawed septum region of the LTA mutant, resulting in a higher CFU count. The other cell wall mutations tested (affecting EPS and pili biosynthesis) did not seem to have a significant influence on the survival or viable plate counts of L. rhamnosus GG after spray‐drying. For EPS, this was rather unexpected, since previous research had shown an important role for EPS in promoting survival of L. rhamnosus GG in other stress situations (Lebeer et al., 2011). For example, EPS has been shown to protect L. rhamnosus GG during passage through the gastrointestinal tract of mice and in interaction with human complement and serum (Lebeer et al., 2011). This protective role of EPS has also been described for other bacterial species. For instance, in several strains of Bifidobacterium, the presence of an EPS layer was shown to offer protection during freeze‐drying (Nguyen et al., 2014), in low pH conditions, and in a high bile salt environment (Alp and Aslim, 2010).
The largest impact for the functionality of L. rhamnosus GG found here was that the pili were removed during the spray‐drying process. A key feature of L. rhamnosus GG is its strong adhesiveness to the gastrointestinal mucosa via SpaCBA pili (Lebeer et al., 2012). Interestingly, we found that spray‐drying significantly decreased the adherence properties of L. rhamnosus GG to intestinal epithelial cells. SEM analysis confirmed that the pili were removed from almost all L. rhamnosus GG wild‐type and surface mutant cells after spray‐drying. This finding is in agreement with previous research showing that pili are shear‐sensitive structures that can be easily sheared off, even during centrifugation (Tripathi et al., 2013). Though it is remarkable that the ∆EPS mutant (after spray‐drying) had still a significantly higher (threefold) capacity to adhere to the Caco‐2 cells compared to the wild‐type after spray‐drying, indicating that still some pili are present or re‐merging after shearing. As published previously, EPS molecules have a negative impact on the adherence capacity of L. rhamnosus GG to both caco‐2 cells and mucus. These EPS molecules act like a shield, and thus preventing adhesins to adhere (Lebeer et al., 2009). The SEM analysis is in agreement with previously published TEM analysis that the ∆EPS mutant has a higher expression of pili (Lebeer et al., 2009). Similarly, Golowczyc et al. (2011) investigated different Lactobacillus strains and their resistance to spray‐drying, but did not explore the underlying molecular effects on cell wall properties. Both Lactobacillus kefir 8321 and Lactobacillus kefir 8348 were spray‐dried, and the adhesion capacity was evaluated through a Caco‐2 adhesion assay, whereas L. kefir 8321 did not lose its capacity to adhere to Caco‐2 cells, whereas L. kefir 8348 displayed a significantly reduction capacity to adhere to these colonic adenocarcinoma cells after spray‐drying (Golowczyc et al., 2011). This could be due to the fact that other cell wall molecules could be more important for adhesion than pili in these strains. Also, other techniques to dry bacteria, like freeze‐drying, have been shown to sometimes insufficiently maintain the functionality of bacteria after drying (Meng et al., 2008; Golowczyc et al., 2011; Makinen et al., 2012). For example, freeze‐drying caused a significant decrease in the adhesion ability to human colonic mucus of Lactobacillus rhamnosus GG, Bifidobacterium animalis Bb‐12 and Bifidobacterium animalis IF20/1 (Du Toit et al., 2013). On the other hand, the capacity of Lactobacillus casei Shirota to adhere to the mucus was not decreased after freeze‐drying (Du Toit et al., 2013). Interestingly, the spaCBA and spaFED gene cluster were recently shown to be upregulated after exposure to an acid medium resulting in a higher expression of pili on the surface of Lactobacillus rhamnosus GG (Bang et al., 2018). This indicates that adherence to intestinal cells could be increased after exposure to an acidic conditions, like the stomach, indicating that pili might rapidly re‐emerge in vivo. Generally, drying techniques, like spray‐drying and lyophilization, could impact functionality, but in this study only the adhesion properties were investigated. Future research is needed to visualize the impact on other aspects of the probiotic functionality. A future strategy to protect cell wall structures of microorganisms, and thus reduce or inhibit the loss of pili, during the spray‐drying process could be to add a protectant, like trehalose.
Experimental procedures
Bacterial strains and growth conditions
The used Lactobacillus rhamnosus GG strain comprised the wild‐type strain (ATCC53103) (Kankainen et al., 2009), the pili‐deficient ΔspaCBA::TcR mutant (CMPG5357) (Lebeer et al., 2012), exopolysaccharide deficient ΔwelE::TcR mutant (CMPG5351) (Lebeer et al., 2009) and the lipoteichoic acid mutant ΔdltD::TcR (CMPG5540) (Vélez et al., 2007). Wild‐type L. rhamnosus GG and its surface mutants were routinely grown under non‐shaking conditions in de Man‐Rogosa‐Sharpe (MRS) medium (Carl Roth, Germany) overnight at 37°C.
Spray‐drying process
When wild‐type and mutant L. rhamnosus GG reached the stationary phase, the bacterial cells were harvested by centrifugation at 3893 g for 10 min at 20°C. The cell pellet was subsequently resuspended in its original volume physiological water [0.9% (m/V) NaCl solution]. The resuspended bacteria (approximately 1010 CFU g−1) were immediately dried using a laboratory‐scale spray dryer (B‐290; Büchi, Flawil, Switzerland) in a co‐current configuration. The inlet air was dehumidified with a desiccant air dryer (CD7; Atlas Copco, Nacka, Sweden). The bacterial suspension was atomized into the drying chamber using a two‐fluid nozzle. The inlet air temperature was set at 135°C while the resulting outlet air temperature varied between 48°C and 50°C. The feed flow rate and the drying air flow rate were 7.5 ml min−1 and 32.5 m3 h−1 respectively. The spray‐dried powder was collected from a single cyclone separator at the end of the process.
Enumeration of viable bacteria
The viability of the bacteria before and after spray‐drying was determined by the plate count method as described earlier with minor modifications (Broeckx et al., 2016). Briefly, resuspended bacteria were plated on MRS agar (Carl Roth, Mühlburg, Germany) in duplicate, after a series of 10‐fold dilutions (in triplicate). After an incubation period of 48 h at 37°C, the CFU were counted.
Measurement of the water activity of the spray‐dried powder
The water activity of the powders was measured using a LabSwift water activity instrument (Novasina, AG, Lachen, Switzerland).
Storage of spray‐dried powder
The spray‐dried powder was stored at 4°C. At predefined time intervals, the spray‐dried powder was analysed for its viability. The initial viability after spray‐drying was compared to the viability after 1, 4, 13 and 26 weeks of storage.
Adhesion assay to Caco‐2 cells
The growth conditions of the Caco‐2 cultures and adhesion assays were carried out as described previously (Lebeer et al., 2012). Caco‐2 cells were incubated with 1 ml fresh bacterial culture or rehydrated spray‐dried powder [107 CFU ml−1 in Dulbecco's Modified Eagle Medium (Life Technologies, Waltham, MA, USA) without 10% foetal bovine serum (Thermo Fisher, Asse, Belgium)] for 1 h at 37°C, 5% CO2, 100% humidity. Then, the cells were washed by adding prewarmed phosphate‐buffered saline (PBS), containing 8.2 g l−1 NaCl (Fagron, Waregem, Belgium), 0.3 g l−1 NaH2PO4.2H2O (Merck kGaA, Darmstadt, Germany) and 1.54 g l−1 Na2HPO4.2H2O (Merck kGaA). To determine the number of viable adhered bacteria, appropriated serial dilutions of the remained adhered bacterial cells in PBS were plated n MRS agar in triplicate and the CFU were enumerated after 48 h. The adhesion ratio was calculated by comparing the number of cells present in the bacterial suspension which was added initially to the Caco‐2 cells, to the bacterial colonies counted that adhered to the Caco‐2 cells.
Scanning electron microscopy (SEM)
Scanning electron microscopy was used to visualize the presence or absence of pili on wild‐type L. rhamnosus GG and three surface mutants before and after spray‐drying. Bacteria were spotted on a gold‐coated membrane and fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer for 1 h at room temperature (RT), followed by a further overnight immersion fixation at 4°C. Bacteria were then rinsed three times for 20 min and left overnight in sodium cacodylate buffer (containing 7.5% saccharose) at 4°C. Subsequently, bacteria were dehydrated in an ascending series of ethanol (50%, 70%, 90%, 95% each for 30 min at RT, and three times 30 min in 100%) and critical point dried in a Leica EM CPD030. The membranes were mounted on a stub and coated with 5 nm of carbon in a Leica EM Ace 600 coater. SEM imaging was performed with a Quanta FEG250 SEM system (Thermo Fisher).
Statistics
Values are shown as mean ± standard error of the mean. ANOVA test and Tukey's multiple comparisons test were used to determine differences in parametric data. GraphPad Prism, version 6.1 (GraphPad Software Inc, La Jolla, Ca, USA) was used for statistical analysis at a significance level α = 0.05.
Conflict of interest
None declared.
Acknowledgements
The authors would like to thank the Electron Microscopy for Material Science group (Prof Dr Sara Bals) at the University of Antwerp for the use of the environmental SEM (Quanta 250 FEG). The Quanta 250 FEG microscope was funded by the Hercules foundation of the Flemish Government (Hercules grant AUHA.11.01). We would also like to thank Eline Oerlemans for her assistance with the cell cultures. Work of Shari Kiekens, S. Lebeer and F. Kiekens was funded by UAntwerpen IOF‐SBO and VLAIO IWT‐SBO funding (ProCure). G. Broeckx, C. Allonsius and I. De Boeckx are recipients of an FWO‐SB personal PhD grant.
Microbial Biotechnology (2019) 12(5), 849–855
Funding Information The Quanta 250 FEG microscope was funded by the Hercules foundation of the Flemish Government (Hercules grant AUHA.11.01) and Agentschap voor Innovatie door Wetenschap en Technologie (IWT/50052).
References
- Allonsius, C.N. , Broek, M.F.L. , Van Den Boeck, I. , De Kiekens, S. , Oerlemans, E.F.M. , Kiekens, F. , et al (2017) Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb Biotechnol 10: 1753–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp, G. , and Aslim, B. (2010) Relationship between the resistance to bile salts and low pH with exopolysaccharide (EPS) production of Bifidobacterium spp. isolated from infants feces and breast milk. Anaerobe 16: 101–105. [DOI] [PubMed] [Google Scholar]
- Bang, M. , Yong, C.‐C. , Ko, H.‐J. , Choi, I.‐G. , and Oh, S. (2018) Transcriptional response and enhanced intestinal adhesion ability of Lactobacillus rhamnosus GG after acid stress. Microb Biotechnol 28: 1604–1613. [DOI] [PubMed] [Google Scholar]
- Broeckx, G. , Vandenheuvel, D. , Claes, I.J.J. , Lebeer, S. , and Kiekens, F. (2016) Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int J Pharm 505: 303–318. [DOI] [PubMed] [Google Scholar]
- Cai, J. , Zhao, C. , Du, Y. , Zhang, Y. , Zhao, M. and Zhao, Q. (2018) Comparative efficacy and tolerability of probiotics for antibiotic‐associated diarrhea : systematic review with network meta‐analysis. United European Gastroenterol J: 6, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes, I.J.J. , Lebeer, S. , Shen, C. , Verhoeven, T.L.A. , Dilissen, E. , De Hertogh, G. , et al (2010) Impact of lipoteichoic acid modification on the performance of the probiotic Lactobacillus rhamnosus GG in experimental colitis. Clin Exp Immunol 162: 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes, I.J.J. , Segers, M.E. , Verhoeven, T.L.A. , Dusselier, M. , Sels, B.F. , De Keersmaecker, S.C.J. , et al (2012) Lipoteichoic acid is an important microbe‐associated molecular pattern of Lactobacillus rhamnosus GG. Microb Cell Fact 11: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doron, S. , Snydman, D.R. , and Gorbach, S.L. (2005) Lactobacillus GG: bacteriology and clinical applications. Gastroenterol Clin North Am 34: 483–498. [DOI] [PubMed] [Google Scholar]
- Du Toit, E. , Vesterlund, S. , Gueimonde, M. , and Salminen, S. (2013) Assessment of the effect of stress‐tolerance acquisition on some basic characteristics of specific probiotics. Int J Food Microbiol 165: 51–56. [DOI] [PubMed] [Google Scholar]
- Golowczyc, M.A. , Silva, J. , Teixeira, P. , De Antoni, G.L. , and Abraham, A.G. (2011) Cellular injuries of spray‐dried Lactobacillus spp. isolated from kefir and their impact on probiotic properties. Int J Food Microbiol 144: 556–560. [DOI] [PubMed] [Google Scholar]
- Kankainen, M. , Paulin, L. , Tynkkynen, S. , von Ossowski, I. , Reunanen, J. , Partanen, P. , et al (2009) Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human‐ mucus binding protein. Proc Natl Acad Sci USA 106: 17193–17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen, R.P. and Hojsak, I. (2018) Probiotics for respiratory tract infections in children attending day care centers – a systematic review. Eur J Pediatr, 177: 979–994. [DOI] [PubMed] [Google Scholar]
- Lebeer, S. , Verhoeven, T.L.A. , Francius, G. , Schoofs, G. , Lambrichts, I. , Dufrêne, Y. , et al (2009) Identification of a gene cluster for the biosynthesis of a long, galactose‐rich exopolysaccharide in Lactobacillus rhamnosus GG and functional analysis of the priming glycosyltransferase. Appl Environ Microbiol 75: 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeer, S. , Claes, I.J.J. , Verhoeven, T.L.A. , Vanderleyden, J. , and De Keersmaecker, S.C.J. (2011) Exopolysaccharides of Lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine. Microb Biotechnol 4: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeer, S. , Claes, I. , Tytgat, H.L.P. , Verhoeven, T.L.A. , Marien, E. , von Ossowski, I. , et al (2012) Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl Environ Microbiol 78: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeer, S. , Bron, P.A. , Marco, M.L. , Motherway, M.O.C. , Hill, C. , Pot, B. , et al (2018) ScienceDirect Identification of probiotic effector molecules : present state and future perspectives. Curr Opin Biotechnol 49: 217–223. [DOI] [PubMed] [Google Scholar]
- Makinen, K. , Berger, B. , Bel‐Rhlid, R. , and Ananta, E. (2012) Science and technology for the mastership of probiotic applications in food products. J Biotechnol 162: 356–365. [DOI] [PubMed] [Google Scholar]
- Meng, X.C. , Stanton, C. , Fitzgerald, G.F. , Daly, C. , and Ross, R.P. (2008) Anhydrobiotics: the challenges of drying probiotic cultures. Food Chem 106: 1406–1416. [Google Scholar]
- Nguyen, H.T. , Razafindralambo, H. , Blecker, C. , N'Yapo, C. , Thonart, P. , and Delvigne, F. (2014) Stochastic exposure to sub‐lethal high temperature enhances exopolysaccharides (EPS) excretion and improves bifidobacterium bifidum cell survival to freeze‐drying. Biochem Eng J 88: 85–94. [Google Scholar]
- Pinto, S.S. , Verruck, S. , Vieira, C.R.W. , Prudêncio, E.S. , Amante, E.R. , and Amboni, R.D.M.C. (2015) Influence of microencapsulation with sweet whey and prebiotics on the survival of Bifidobacterium‐BB‐12 under simulated gastrointestinal conditions and heat treatments. LWT – Food Sci Technol 64: 1004–1009. [Google Scholar]
- Segers, M.E. , and Lebeer, S. (2014) Towards a better understanding of Lactobacillus rhamnosus GG – host interactions. Microb Cell Fact 13: S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan‐lim, C.S.C. and Esteban‐ipac, N.A.R. (2018) Probiotics as treatment for food allergies among pediatric patients : a meta‐analysis. World Allergy Organ J, 11: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi, P. , Beaussart, A. , Alsteens, D. , Dupres, V. , Claes, I. , Von Ossowski, I. , et al (2013) Adhesion and nanomechanics of pili from the probiotic Lactobacillus rhamnosus GG. ACS Nano 7: 3685–3697. [DOI] [PubMed] [Google Scholar]
- Vélez, M.P. , Verhoeven, T.L.A. , Draing, C. , Von Aulock, S. , Pfitzenmaier, M. , Geyer, A. , et al (2007) Functional analysis of D‐alanylation of lipoteichoic acid in the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microbiol 73: 3595–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilstra, D. , Younes, J.A. , Brummer, R.J. , and Kleerebezem, M. (2018) Perspective : fundamental limitations of the randomized controlled trial method in nutritional research : the example of probiotics. Adv Nutr 9: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]


